As a crucial trace element in the human body, copper
serves an essential role in human pathophysiology. Copper is not
only involved in hemoglobin synthesis, supporting the immune system
and neurotransmitter biosynthesis, but also has powerful
antioxidant properties, as well as being involved in the formation
of bone and connective tissue (1). These physiological functions render
copper vital for disease prevention and the maintenance of health.
Copper exhibits dose-dependent dual effects on the cardiovascular
system: i) Copper prevents cardiovascular diseases (CVDs) through
antioxidant effects, protecting endothelial function and
maintaining vascular connective tissue structure (2); ii) however, in CVDs, copper
overload interferes with lipid metabolism, and leads to oxidative
stress, mitochondrial dysfunction and endothelial cell dysfunction
(3). Therefore, maintaining an
appropriate intake of copper is essential to protect the heart and
blood vessels.
In recent years, the discovery of cuproptosis, a
newly identified form of copper-induced cell death, has provided
novel perspectives for resolving the molecular associations between
copper dyshomeostasis and CVDs. Tsvetkov et al (4) revealed that treatment of human cell
lines (such as the lung carcinoma line A549 and the immortalized
kidney line 293T) with the copper ionophore elesclomol induced cell
death and that pharmacologically inhibiting all other known cell
death pathways failed to inhibit elesclomol-induced cell death;
this novel form of cell death was named cuproptosis. Furthermore,
it has been demonstrated that copper ions can induce aberrant
aggregation by targeting mitochondrial lipoylated proteins and,
alongside the loss of iron-sulfur (Fe-S) clusters, trigger
uncoupling of oxidative phosphorylation, ultimately leading to the
collapse of cellular energy metabolism (4). Bioinformatics studies have revealed
the extensive involvement of cuproptosis in the progression of CVDs
(5-7).
The present review aimed to elucidate the dynamic
balance of copper absorption-transportation-storage-excretion in
the human body, and to outline the mechanism underlying the
regulation of cuproptosis. Furthermore, the review summarizes the
molecular pathways linking copper to CVDs, including
atherosclerosis (AS) and heart failure (HF), and critically
appraises the roles of copper chelators [such as tetrathiomolybdate
(TTM) and triethylenetetramine (TETA)], copper ionophores
(including elesclomol) and therapeutics developed based on
cuproptosis in the treatment of CVDs, according to the progress of
current research. The aim was to clarify the causal relationship
between copper and CVD, and to inform the development of prevention
and treatment strategies based on precise regulation of copper
homeostasis.
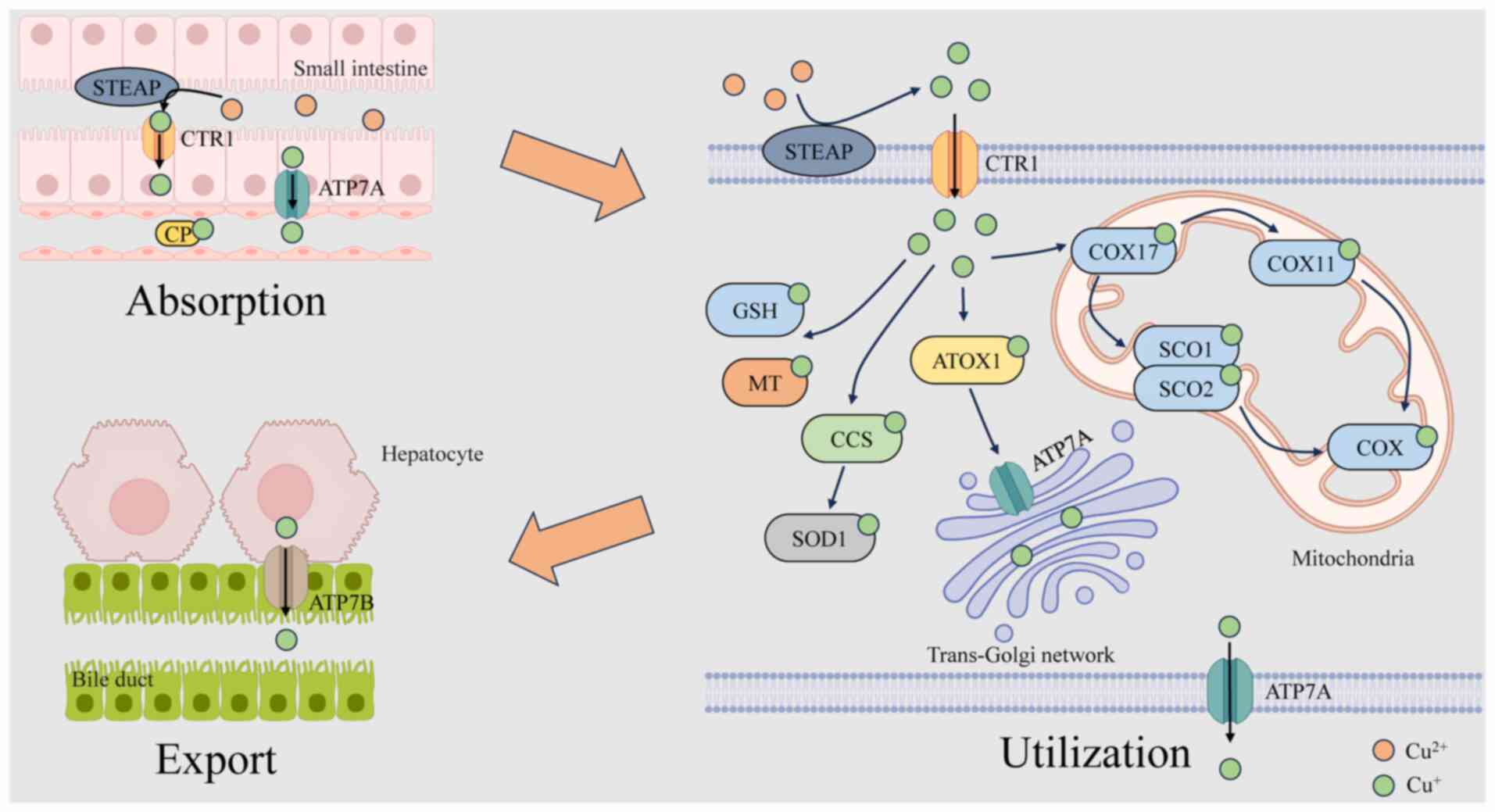
Copper metabolism encompasses the absorption,
distribution, storage, utilization and excretion of copper. These
processes are essential for maintaining copper homeostasis, thereby
supporting the physiological functions of copper. As shown in
Fig. 1, copper metabolism
involves multiple organs and systems, primarily including
intestinal absorption, hepatic storage and release, and excretion
through bile and urine. Abnormalities in any of these processes can
result in disturbances in copper metabolism, thereby affecting
health.
In the cytoplasm, the transportation of copper is
precisely regulated by a network of high-affinity copper chaperones
(16). Copper enters the cell
and forms complexes with reduced glutathione (GSH), metallothionein
(MT), ATOX1 and copper chaperone for superoxide dismutase (CCS)
(11,18). ATOX1, which is conserved across
species, acquires Cu+ from CTR1 through its classical
metal-binding site (19) and
subsequently transports Cu+ via ATP7A and
copper-transporting ATPase β (ATP7B) to the trans-Golgi network
(TGN) for the synthesis of tyrosinase, lysyl oxidase and
ceruloplasmin (CP), thereby sustaining copper homeostasis inside
the cell (20-23). CCS, a copper chaperone protein,
facilitates the transport of Cu to certain proteins, including
superoxide dismutase 1 (SOD1), located in the cytoplasm and
mitochondrial intermembrane space (IMS), thereby neutralizing
mitochondria-derived superoxide radicals (24,25). Cellular copper levels inversely
regulate CCS expression (26).
Moreover, CCS modulates the localization of SOD1 between the IMS
and cytosol in an oxygen-dependent manner, preserving the stability
of reactive oxygen species (ROS) in vivo, thereby preventing
oxidative damage caused by copper overload (27). In addition, CCS facilitates the
transport of Cu+ to mitogen-activated protein kinase 1,
hence modulating its activity and influencing cellular
proliferation (28). The
delivery of Cu+ to mitochondria primarily relies on the
cytochrome c oxidase (COX) copper chaperone 17 (COX17), which
facilitates copper transport into the IMS for COX synthesis.
Additionally, the literature has indicated that copper can be
transported to mitochondria through a non-proteinaceous anionic
ligand (CuL) (29). COX
comprises two core subunits, COX1 and COX2, which bind copper at
the conserved CuB and CuA sites, respectively
(16,30). Within the IMS, COX17 transfers
Cu+ to synthesis of COX (SCO)1 and SCO2 for the assembly
of COX subunits. Cu+ is subsequently conveyed to COX2,
contributing to its structure; alternatively, it is transported by
COX17 to the COX copper chaperone 11 (COX11) and then to COX1 for
use in its assembly (12,31).
The liver serves as the main organ responsible for
copper metabolism and storage within the human body. Upon reaching
the liver, copper in the bloodstream is delivered to designated
structures by copper chaperone proteins, including ATOX1, CCS and
COX17. Excess copper is sequestered by MT and GSH for hepatic
storage, thereby preventing cytotoxicity (16,32).
Biliary excretion represents the principal pathway
for hepatic copper elimination. ATP7A and ATP7B are the primary
transporters of Cu+ within cells. Their regulatory roles
in copper absorption and excretion depend largely on their
subcellular localization (33,34). Under physiological copper
concentrations, these ATPases predominantly localize to the TGN,
where they transfer Cu+ into the TGN lumen (23). When intracellular copper rises,
these transporters relocalize from the TGN to vesicular
compartments, subsequently fusing with the plasma membrane to
facilitate Cu+ export. Once homeostatic copper levels
are restored, the transporters are recycled back to the TGN
(35). ATP7A is widely expressed
in most tissues and organs, excluding the liver, whereas ATP7B is
primarily expressed in the liver (23). After being absorbed by the
intestinal epithelium, copper is released into the bloodstream by
ATP7A and thereafter enters the portal circulation, where it is
absorbed by hepatocytes. It is then pumped into the TGN by ATP7B
and delivered to the plasma membrane to form CP, and then secreted
into the bloodstream through exocytosis. In cases of excessive
copper accumulation, ATP7B is relocated from the TGN to the
canalicular membrane of hepatocytes, facilitating the excretion of
surplus copper from the cytoplasm into the bile and ultimately out
of the body. Copper excreted into bile forms complexes with bile
salts, preventing its intestinal reabsorption (34,36). Copper homeostasis in the body is
maintained through absorption in the duodenum and small intestine,
as well as excretion via the biliary system (37,38).
Copper homeostasis refers to the tightly regulated
balance among copper absorption, transport, storage and excretion
(39). It is crucial for
appropriate physiological processes, as it participates in enzyme
activity, cellular respiration, iron metabolism, and the
development and maintenance of nervous system function. Copper
dyshomeostasis can result in oxidative stress, cellular damage and
even illness, such as Wilson disease (caused by ATP7B mutations
leading to copper accumulation) and Menkes disease (due to ATP7A
deficiency causing systemic copper deficiency). An imbalance in
copper homeostasis is a well-established clinical characteristic of
these inherited disorders. Moreover, copper levels can serve as a
predictive indicator for certain conditions; for example, elevated
copper levels are associated with advanced stage and poor prognosis
in prostate, bladder and renal cancers (11).
Copper homeostasis, largely governed by the
transporters ATP7A and ATP7B, is essential for human health.
Dysfunction of these two transporters may result in severe
illnesses. Menkes disease (MD) results from mutations in the ATP7A
gene. The dysfunction of ATP7A in intestinal epithelial cells
results in copper accumulation inside these cells and a reduced
transfer of copper into the bloodstream, culminating in a notable
systemic copper deficit. Patients present with characteristic
clinical features, such as intellectual disability, hypothermia,
neuronal degeneration, bone fractures, and anomalies of the hair,
skin and vasculature (16).
Wilson's disease (WD) results from mutations in the ATP7B gene.
Impaired ATP7B function hinders the efficient elimination of
Cu+ from cells, causing persistent copper accumulation
in the liver, brain and other tissues, which results in copper
toxicity and organ damage in patients. Patients may present with
hepatic manifestations, including acute liver failure, jaundice and
chronic hepatitis, as well as neurological conditions, such as
tremors, Parkinsonism, ataxia, dystonia, dysarthria, spasticity and
a lack of motor coordination (16).
Copper dyshomeostasis is closely associated with the
development and progression of numerous diseases. Emerging evidence
has suggested that copper dyshomeostasis contributes to the
pathogenesis of coronary heart disease. Furthermore, copper
deficiency may result in myocardial diastolic dysfunction and a
diminished β-adrenergic response, and can lead to HF. By contrast,
copper overload disrupts the mitochondrial electron transport chain
(mtETC), which contributes to HF by impairing the antioxidant
defense, ROS accumulation and activation of ischemic/hypoxic
signaling pathways via CP (40).
Copper overload may result in vascular dysfunction by elevating
nitric oxide generation and endothelial oxidative stress (41). Copper dyshomeostasis in
neurological illnesses is associated with the etiology of
Alzheimer's disease. Copper can facilitate the aggregation of
amyloid-β peptides and induce neurotoxicity (16). Abnormal alterations in copper
levels, specifically elevated serum copper and increased urinary
copper excretion, have been observed in patients with diabetic
nephropathy (42). In addition,
cancer cells have a markedly higher demand for copper compared with
normal cells (43); notably,
copper levels in tumor tissue and/or serum are significantly
elevated in patients with various types of cancer, including
prostate, bladder and renal cell carcinoma, compared with in
healthy controls, and are associated with tumor growth and
progression (39). Furthermore,
copper can promote cancer metastasis by activating enzymes, such as
lysyl oxidase, and signaling pathways, including MEK-ERK, both of
which drive tumor invasion and dissemination (16). These findings provide new
insights into the diagnosis and management of disorders associated
with copper homeostasis.
As research advances on copper dysregulation and
associated diseases, an increasing number of pharmaceuticals are
being identified and utilized. In instances of severe systemic
copper deficiency in patients with MD, Cu-histidine may be injected
into the blood to circumvent intestinal absorption, facilitating
the distribution of Cu2+ to various tissues and organs,
thereby aiding in restoring systemic copper levels in affected
individuals. Conversely, to mitigate the continuous copper
accumulation in the brain, liver and other tissues of individuals
with WD, oral zinc may be employed to decrease copper absorption,
while copper chelators such as D-penicillamine and TTM can be
utilized to lower copper levels in the body. Free α-lipoic acid
(α-LA) is a cuproptosis enhancer; however, it has been shown that
α-LA can mitigate cytotoxicity caused by copper overload through
chelation and improve the copper-induced oxidative stress
environment (44).
Serum and tumor tissue copper levels in patients
with cancer are notably higher than those in healthy individuals,
and copper serves a crucial role in tumor angiogenesis (45). Consequently, the accumulation of
copper in tumor tissue may serve as a target for the formulation of
anticancer agents. Two main methods exist for triggering apoptosis
through copper targeting. The first strategy involves employing
copper chelators, such as D-penicillamine and TTM, to bind Cu
directly, hence diminishing its bioavailability, which may inhibit
tumor proliferation in both cellular and animal tumor models
(46). Copper chelators may
inhibit collagen fiber cross-linking by blocking the
copper-dependent lysyl oxidase family, therefore preventing renal
fibrosis (47,48). Notably, copper chelators also
exhibit anti-metastatic effects, primarily by inhibiting the
recruitment of endothelial progenitor cells (EPCs), which are
essential for angiogenesis and the establishment of metastatic
niches (49). Copper chelators
are also used to treat Alzheimer's disease, amyotrophic lateral
sclerosis and Huntington's disease (16). The alternative approach involves
elevating intracellular copper levels through the use of copper
ionophores, such as disulfiram (DSF), to generate ROS, inhibit
proteasomes and trigger apoptosis (50). Nonetheless, some studies have
suggested that copper ionophores eliminate cancer cells by a
distinct process rather than through apoptosis, necrosis or
oxidative stress (46,51,52). Furthermore, cuprous oxide
nanoparticles can destabilize the copper chaperones ATOX1 and CCS,
consequently producing an anticancer impact (11).
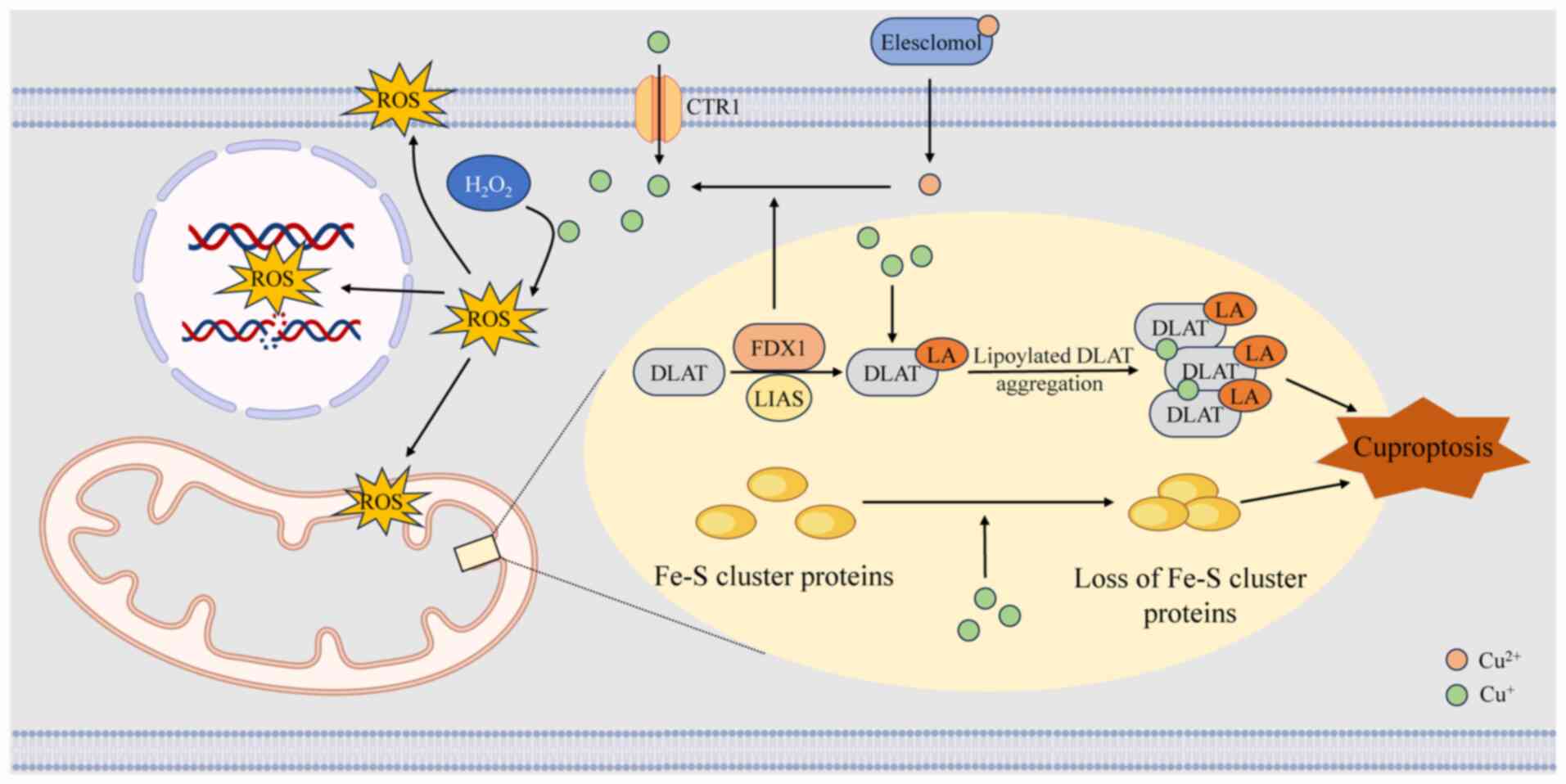
In cells treated with the copper ionophore
elesclomol, the levels of tricarboxylic acid (TCA) cycle
intermediates increase over time, highlighting a robust association
between cuproptosis and mitochondrial metabolism. In mitochondria,
ferredoxin 1 directly binds to lipoic acid synthetase, promoting
the post-translational lipoylation of dihydrolipoamide
S-acetyltransferase (DLAT), a key E2 subunit of the pyruvate
dehydrogenase complex. This lipoylation is essential for the
structural integrity and enzymatic activity of DLAT within the TCA
cycle (54,55). It subsequently facilitates the
dissociation of Cu2+ from elesclomol, and reduces
Cu2+ to Cu+ (56). Cu+ exhibits a high
affinity for the disulfide pentane ring structure within the lipoyl
group (57). When excess
Cu+ binds to lipoylated proteins such as DLAT, it
disrupts their native conformational stability, inducing abnormal
cross-linking and irreversible aggregation in these enzymes that
should normally assemble in an ordered, polymeric form. The
resulting protein aggregation leads to inactivation of the pyruvate
dehydrogenase complex, suppressing acetyl-CoA and α-ketoglutarate
oxidation, and causing a sharp decline in ATP synthesis.
Additionally, copper can replace iron in Fe-S clusters or disrupt
their synthesis, leading to the inactivation of Fe-S-dependent
enzymes, and further disturbing metabolic and redox balance. These
events collectively trigger protein toxicity stress, ultimately
resulting in cell death (4).
Drug inhibition of the electron transport chain and pyruvate uptake
can reverse elesclomol-induced cell death, providing compelling
evidence for this process (4).
Furthermore, excessive copper promotes ROS formation via the Fenton
reaction, damages mitochondrial and plasma membranes, disrupts DNA
integrity and broadly impairs cellular functions (58). The process of cuproptosis is
illustrated in Fig. 2.
Cuproptosis and ferroptosis are two metal
ion-dependent modes of regulated cell death that have attracted
considerable attention, particularly in cancer research. Although
their mechanisms are distinct, ferroptosis being driven by
iron-dependent lipid peroxidation, the two processes are
metabolically interconnected.
During cuproptosis, degradation of Fe-S clusters
releases free iron ions, which elevate intracellular oxidative
stress and can subsequently promote ferroptosis. Conversely, during
ferroptosis, depletion of GSH and mitochondrial injury compromise
cellular copper chelation and export, resulting in copper
accumulation and the induction of cuproptosis. This
self-reinforcing feedback loop between copper and iron metabolism
has been exploited in several nanotherapeutic approaches to enhance
anticancer efficacy (59-61).
Copper participates in the Fenton reaction,
continuously generating hydroxyl radicals during the transformation
of Cu2+ to Cu+. This potent oxidant can
directly induce DNA strand breakage and base oxidation (62). Excessive copper-induced systemic
oxidative stress can disrupt lipid metabolic balance and facilitate
lipid accumulation in the intima of vessels; this mechanism
markedly propels the progression of AS (3). Copper-induced oxidative stress also
increases the oxidation of GSH (63). Experimental evidence clarifying
the toxic mechanism of copper oxide nanoparticles (CuO NPs) has
been demonstrated through studies involving animal models and
cellular experiments. These studies have revealed that CuO NPs
induce oxidative stress in a stepwise manner, encompassing ROS
burst, compensatory upregulation of heme oxygenase-1, mtETC
dysfunction, and aberrant secretion of proinflammatory and
profibrogenic cytokines (64,65). The copper chelator TTM can
markedly alleviate cardiovascular damage associated with CuO NPs by
chelating excess Cu (66). This
multilayered oxidative damage represents a key pathophysiological
mechanism in copper-related CVD.
Mitochondria serve as the energy-producing
organelles in eukaryotic cells and regulate cellular metabolic
activities, including oxidative phosphorylation. Cuproenzymes in
mitochondria are essential to this process. COX, the major
mitochondrial cuproenzyme, utilizes most of the mitochondrial
copper pool, accounting for 20-25% of the total copper content of
the cell. Another important cuproenzyme, SOD1, is situated in the
IMS of the mitochondria and its primary role is to facilitate the
breakdown of superoxide generated by the mitochondrial respiratory
chain (29). Copper deficiency
impairs the delivery of Cu to SCO1/SCO2 and COX11 through COX17,
which in turn reduces the synthesis of COX. Copper deficiency can
also induce the production of the peroxisome proliferator-activated
receptor-γ coactivator-1α protein, thereby altering mitochondrial
ultrastructure, and leading to abnormal mitochondrial proliferation
and dysfunction. This series of changes is closely associated with
myocardial dysfunction (3).
Hypoxia-inducible factor 1 (HIF-1) functions as a
crucial transcription factor in the regulation of angiogenesis
(3). Copper can regulate the
HIF-1 signaling pathway in multiple ways. Copper can enhance the
stability of HIF-1α in the nucleus under normoxic conditions,
causing the expression of HIF-1-dependent genes (67). CCS mediates the effect of copper
on HIF-1 activity (68).
Furthermore, copper-dependent lysyl oxidases are
vital for maintaining the structural integrity of blood vessel
walls by catalyzing the covalent cross-linking of elastin and
collagen, which confer tensile strength and elasticity to
connective tissues (69).
Abnormal lysyl oxidase expression levels or enzyme activity are
associated with pathological processes such as CVD. Under copper
deficiency, reduced lysyl oxidase synthesis and activity cause
abnormal collagen degradation, which may cause serious
complications such as destruction of the vascular matrix and
rupture of the intima (3,69).
AS is a chronic cardiovascular disorder
characterized by the progressive formation of atherosclerotic
plaques, leading to luminal narrowing and impaired vascular
function. Accumulation of the oxidized form of low-density
lipoprotein (LDL) in cells in the subendothelial space is a key
factor in AS formation (70). In
parallel, in silico screening has demonstrated that natural
compounds derived from Chlorella vulgaris and
Boesenbergia rotunda possess the potential to inhibit key
early steps of AS, such as foam cell formation, by targeting
proteins such as cholesteryl ester transfer protein, lectin-like
oxidized LDL receptor-1, CD36 and acyl-CoA:cholesterol
acyltransferase 1 (71,72). Separately, accumulating evidence
has implicated copper dyshomeostasis in AS pathogenesis, with
increased levels of copper being strongly linked to AS (73) and blood copper levels positively
linked with subclinical carotid plaques (74). Furthermore, increased urinary
levels of copper have been reported to be associated with a
heightened 10-year risk of atherosclerotic CVD (75). Copper-related DNA methylation
alterations are also associated with an elevated risk of acute
coronary syndrome, potentially resulting from aberrant lipid
metabolism and inflammation (76), which are associated with
copper-mediated ROS formation, elevated CP, expansion of arterial
endocardium and narrowing of the arterial lumen (40).
Currently, stroke ranks as the second most common
cause of death worldwide, following ischemic heart disease. Stroke
disrupts cerebral perfusion, leading to ischemic brain injury and
subsequent neurological impairment (84). A large case-control study in the
USA indicated that elevated dietary copper consumption was
associated with a reduced risk of stroke (84). However, dietary copper intake
does not necessarily reflect systemic or circulating copper levels,
which complicates the interpretation of these findings. Several
studies have shown that while plasma copper levels are not notably
associated with the first hemorrhagic stroke, there is a positive
association between plasma copper levels and the risk of the first
ischemic stroke (85,86). A case-control study further
reported that elevated plasma copper, along with certain trace
metals such as molybdenum and titanium, was associated with higher
ischemic stroke risk (87).
Similarly, multiple meta-analyses have revealed a favorable
association between elevated circulating copper levels and stroke
incidence (88,89). This apparent contradiction may be
explained by the marked elevation of serum non-CP-bound copper
observed in patients with acute ischemic stroke. As free copper
unbound to CP, it exhibits strong pro-oxidant properties. The
increase in blood copper during the acute stroke phase may also
partially stem from a stress response, meaning elevated blood
copper serves both as a risk factor and potentially as a
pathological consequence. Collectively, the impact of copper
appears to be context-dependent, varying with inflammatory status
and disease stage, and may follow a non-linear dose-response
relationship.
The precise biological pathway by which copper
impacts stroke remains unidentified. Notably, copper contributes to
the synthesis of SOD, which mitigates ROS generated by ischemic
stroke and diminishes cellular damage following such events
(90). Conversely, copper can
exacerbate ischemic stroke. EPCs can improve endothelial function
and promote angiogenesis in ischemic brain tissue, whereas
thrombospondin-1 serves as a principal inhibitor of EPC activity.
Research findings have suggested that copper exposure in mice can
lead to elevated thrombospondin-1 levels, hence exacerbating
ischemic stroke in these subjects (3). Copper may influence stroke risk
indirectly through its modulation of lipid metabolism, a hypothesis
that warrants confirmation in prospective cohort studies (91).
I/R injury refers to tissue damage caused by the
restoration of blood flow after a period of ischemia, primarily
driven by oxidative stress (92). This process generates excessive
ROS and provokes a robust inflammatory response, a major pathogenic
mechanism underlying various diseases, such as acute kidney injury,
particularly in the context of kidney transplantation (93). Following I/R-induced myocardial
infarction (MI), substantial remodeling of the extracellular matrix
(ECM) occurs, leading to interstitial fibrosis, scarring and
cardiac dysfunction. The increased copper-dependent lysyl oxidase
subtype following MI may facilitate cardiac ECM remodeling and
dysfunction (40).
The copper-dependent enzyme Cu/Zn-SOD mitigates
oxidative stress-induced cellular edema during reperfusion
(94). When Cu/Zn-SOD is
upregulated in coronary vascular cells, the heart becomes more
resistant to I/R injury (95,96). A meta-analysis showed that
regular exercise can elevate levels of GSH peroxidase and
Cu/Zn-SOD, and enhance cardiac function after myocardial I/R injury
(97). Nonetheless, the
mechanistic links between copper dyshomeostasis, cuproptosis and
myocardial I/R injury remain to be fully elucidated.
HF is prevalent among adults, and has high morbidity
and mortality rates worldwide. Both acute HF (AHF) and chronic HF
(CHF) is linked to heightened oxidative stress resulting from the
elevated generation of ROS and compromised clearance mechanisms
(98). Furthermore, the Cu:Zn
ratio is important in HF. A prospective study conducted in 2022
demonstrated that for every 1-unit increase in serum Cu:Zn ratio,
the risk of HF was increased by 63%, and this association exhibited
a linear dose-response relationship. Incorporating the Cu:Zn ratio
into traditional HF risk prediction models may improve risk
stratification efficacy (99).
However, the Cu:Zn ratio as a single biomarker may be driven by
confounding factors, such as inflammation, liver function or
inadequate zinc intake, and may not necessarily reflect a causal
relationship. In a study of 125 patients, serum copper levels were
elevated and serum zinc levels were decreased in patients with both
AHF and CHF (100). This has
been confirmed in several studies (99,101-103). Although elevated serum copper
is frequently observed in inflammatory states, it does not
necessarily indicate copper overload. Conversely, evidence
regarding the impact of copper deficiency or excess on HF remains
limited and inconsistent. Patients with ischemic and non-ischemic
HF showed differences in dietary zinc and copper intake, but the
median zinc and copper status biomarkers did not show a notable
difference between the two groups (104), suggesting that the serum Cu:Zn
ratio may be regulated by homeostasis during the chronic stable
phase of HF and thus lose the ability to distinguish between
ischemic and non-ischemic etiologies. A Mendelian randomization
investigation analysis also indicated that dietary copper was not
markedly associated with the risk of cardiovascular and metabolic
diseases (105). Although
observational studies have repeatedly reported elevated serum Cu:Zn
ratios in patients with HF (99,106,107), their clinical significance is
highly dependent on disease stage, inflammatory status and
zinc-copper balance. Single-cell transcriptome sequencing of immune
cells in epicardial adipose tissue from patients with HF has
revealed disturbed zinc and copper metabolism (108). Prior studies have also shown
that elevated blood copper levels in patients with HF are linked to
higher mortality and morbidity rates (99,100,109). Nonetheless, certain studies
have indicated that shortages in zinc and copper may elevate the
risk of coronary heart disease, valvular regurgitation, myocardial
lesions and myocardial hypertrophy (110). Supplementation with several
nutrients, including copper, may serve as a viable therapeutic
method for individuals with HF (111,112). Some experiments have even shown
that the serum copper levels of patients with CHF are similar to
those in a healthy control group (102). In summary, the current evidence
linking zinc and copper status to CVD remains fragmented and
inconsistent. Neither zinc nor copper levels should be used alone
for diagnosing HF or guiding supplementation therapy. Additional
research is needed to determine whether the detection of diseases
relies on the degree of association between zinc and copper content
and CVD (110).
Antioxidant mechanisms serve a regulatory role in HF
pathology. In end-stage HF, increased oxidative stress may
specifically upregulate catalase gene expression (113), while copper dyshomeostasis
exacerbates this pathological process. In a study involving
diabetes-induced HF, patients with diabetes had impaired cardiac
mitochondrial copper regulation, with reduced COX17, COX11, CCS and
SCO1 expression, and mitochondrial translocation in the myocardium,
thus affecting copper transport to the mitochondria and COX
assembly, resulting in a decrease in COX activity of ~27%. Notably,
TETA treatment could restore COX activity to normal, improving
cardiac function (114).
Non-pharmacological interventions also have regulatory potential.
In patients with CHF, 12 weeks of aerobic exercise training
(cycling at 50% of peak oxygen uptake for 45 min, four times/week)
has been shown to increase the expression of Cu/Zn-SOD and GSH
peroxidase, and thus improve cardiac function (115). A previous transcriptome
analysis has indicated that genes associated with cuproptosis
regulate the immunological microenvironment in ischemic HF
(116). However, the mechanism
of cuproptosis in HF is currently unclear.
Copper chelators are agents capable of binding
copper ions, thereby preventing their intracellular accumulation,
or depleting excessive copper to modulate redox homeostasis and
induce cell death (117). An
increasing body of preclinical and clinical evidence has suggested
that copper chelation therapy holds therapeutic potential in CVDs.
As shown in Table I (114,118-132), various copper chelators have
been evaluated in different animal models and patient
populations.
TTM exhibits a high affinity for copper and has the
ability to selectively bind to copper ions. Notably, it is
extensively employed in treating WD. In comparison to conventional
therapies, such as D-penicillamine and trientine, which increase
urinary copper excretion but may provoke paradoxical neurological
worsening, TTM may prevent copper-induced harm to the blood-brain
barrier and demonstrate an improved safety profile (133,134). TTM can specifically form a
TTM-copper-ATOX1 complex with ATOX1, thus obstructing copper
transport to the TGN and its subsequent synthesis of cuproprotein,
or diminishing copper bioavailability and vascular inflammation to
prevent AS in apolipoprotein E-deficient mice (135). The second-generation TTM analog
(ATN-224; choline TTM) inhibits SOD1 within both tumor cells and
endothelial cells, thus decreasing endothelial cell proliferation
in vitro (136), and TTM
can reduce the proliferation of human pulmonary microvascular
endothelial cells (121).
Intravenous injection of ammonium TTM (ATTM) has been shown to
protect the heart in an MI-reperfusion model in a
drug-exposure-dependent manner (118). Notably, ATTM may function as an
inorganic sulfide-releasing molecule (119). Intervention with TTM has been
reported to markedly inhibit the aggregation of DLAT oligomers and
enhance ATP7A expression in the hearts of spontaneously
hypertensive rats, thereby diminishing cuproptosis and
mitochondrial damage (120).
TETA acts as a selective copper chelator and has a
bidirectional regulatory function in the cardiovascular system. In
copper-deficient myocardial hypertrophy model tissues, TETA can
serve as a copper chaperone to promote the transport of copper ions
to cardiomyocytes. Under copper overload conditions, excess copper
ions are removed by chelation (40). TETA may inhibit the rise in serum
copper levels and efficiently reduce the heightened CP activity
during MI (137). Abnormal ECM
Cu2+ accumulation and a defect in intracellular
Cu+ supply can lead to copper dyshomeostasis in the
myocardium. TETA can restore the balance of copper distribution in
the myocardium and effectively maintain the integrity of the heart
structure (127). In addition,
TETA has been reported to enhance myocardial function in the hearts
of diabetic rats by reinstating myocardial copper transport routes
(114,126), and it may serve as a possible
therapeutic agent for diabetic heart disease (125). However, neither TTM nor TETA
has been systematically evaluated in large-scale clinical trials or
meta-analyses.
EDTA can bind to a range of metals. The results of
the TACT randomized trial showed that individuals with type 1 and
type 2 diabetes who had previously suffered from MI experienced a
reduction in the recurrence of cardiovascular events following
EDTA-based injections (129).
However, EDTA chelation proved ineffective in reducing
cardiovascular events among stable patients with coronary artery
disease who also had a history of diabetes and MI (128). Therefore, current evidence does
not support its routine clinical use in CVD management. A
meta-analysis demonstrated that there is currently insufficient
evidence to determine whether chelation therapy is effective in
improving clinical outcomes in patients with atherosclerotic CVD
(138). More high-quality
randomized controlled trials are needed to evaluate the effects of
EDTA chelation therapy on CVD.
Although several copper chelators have shown promise
in preclinical models and selected patient populations, their
clinical efficacy remains limited, largely due to poor cellular
permeability. Moreover, these agents pose risks of off-target
toxicity and oxidative stress (139). Prolonged administration may
further result in copper deficiency, myelosuppression,
nephrotoxicity or imbalances in trace elements (140). Therefore, indicators of copper
metabolism should be carefully monitored throughout treatment.
Copper ionophores are compounds that bind copper
ions and promote their transport across cellular membranes, thereby
increasing intracellular copper concentrations. Among these agents,
elesclomol and DSF are the most extensively studied (141). Elesclomol is an antineoplastic
agent that targets mitochondrial metabolism. It can inhibit cancer
by facilitating the delivery of copper ions into cells, thereby
triggering copper-mediated apoptosis (142). DSF can markedly elevate
intracellular copper levels and inhibit angiogenesis through a
number of routes, thereby exerting an anticancer impact (143). Despite these promising findings
in oncology, no copper ionophore has yet been applied to CVD
therapy. Moreover, carrier-mediated copper delivery may aggravate
cardiovascular injury due to its pro-oxidative effects, and its
safety and tissue specificity remain notable challenges.
Dietary copper supplementation serves as a
non-specific approach to restore systemic copper homeostasis. Among
Chinese adults participating in the China Health and Nutrition
Survey, a U-shaped association exists between dietary copper
consumption and the incidence of new-onset hypertension; the
inflection point is ~1.57 mg/day (144). Furthermore, it has been
suggested that dietary copper supplementation may reverse pressure
overload-induced cardiac hypertrophy (145). Prolonged supplementation with
multiple micronutrients, including copper, can enhance cardiac
function in older individuals with HF (112). Furthermore, a notable
association exists between lower copper intake in the diet and a
higher occurrence of abdominal aortic calcification (146). Longitudinal and cross-sectional
studies in the US population have suggested that moderately
increasing dietary copper intake may confer cardiovascular
protective effects (147,148). By contrast, a prospective
cohort study in the Chinese population indicated that high copper
intake (>2.45 mg/day) may increase the risk of all-cause and CVD
mortality (149). These
findings indicate that the effects of copper follow a U-shaped or
J-shaped curve, both excessively low and excessively high levels
are detrimental, with the optimal range varying depending on the
individual, dietary patterns and baseline health status.
Nevertheless, Mendelian randomization analyses have not
demonstrated a marked causal relationship between dietary copper
intake and CVDs or metabolic diseases (105). This may be due to
methodological limitations that failed to detect such complex
relationships. Currently, there is a lack of precise copper status
assessment tools for patients with CVD, and indiscriminate copper
supplementation carries potential risks. Further studies are
warranted to clarify the role of dietary copper in CVD prevention
and management.
Although research on the molecular mechanisms of
cuproptosis in cardiovascular cells is still at an early stage,
current evidence has indicated a high degree of tissue specificity.
Compared with other tissues, cardiomyocytes, which are highly
dependent on mitochondrial energy supply, are markedly sensitive to
the stability of lipoylated proteins (150). Cuproptosis in the
cardiovascular system does not occur in isolation; instead, it
reflects systemic copper homeostasis dysregulation involving
coordinated interactions among the liver, kidneys and heart.
Elevated blood copper levels, often associated with hepatic or
renal dysfunction, may increase copper uptake by cardiomyocytes. At
the molecular regulatory level, sirtuin 3 maintains intracellular
copper homeostasis by modulating the interaction between copper
transporters and the autophagy-related protein LC3B, which
suppresses cuproptosis in cardiomyocytes (151). In vascular endothelial cells,
MT2A preserves mitochondrial and vascular function by chelating
free copper, effectively blocking the cuproptosis pathway (152). In cardiomyocytes, TTM reverses
cuproptosis caused by copper overload (153). In a diabetic cardiomyopathy
model, advanced glycation end products have been shown to markedly
upregulate CTR1 mRNA and protein levels, while downregulating the
copper efflux transporters ATP7A and ATP7B; this leads to
intracellular copper accumulation, thereby inducing or exacerbating
cuproptosis (154). As shown in
Table II (5-7,116,155-162), bioinformatics and multi-omics
analyses have suggested that cuproptosis serves a broad role in CVD
pathogenesis. Nevertheless, experimental validation of the
functional relevance of these genes remains limited. For example,
although ATP7B has been proposed as a cuproptosis-associated
biomarker in ischemic cardiomyopathy (6), its direct participation in
copper-induced cardiomyocyte death remains to be experimentally
verified. Furthermore, several genes appear to have dual or
context-dependent roles, underscoring the need for further
mechanistic studies. Future studies should move beyond correlative
findings toward causal validation.
CTR1, SOD1 and ATOX1 are the most common
copper-related biomarkers. In human tumor research, substantial
evidence has supported the clinical value of CTR1. Although animal
models have revealed the role of CTR1 in CVDs (163), human clinical studies remain
extremely limited (164,165).
In a model of I/R injury, dysfunction of CTR1 in cardiomyocytes can
lead to excessive copper ion accumulation, inducing cuproptosis
(166). In addition to
potentially serving as a biomarker and target in cancer treatment,
CTR1-induced regulation of intracellular copper levels to influence
cuproptosis offers novel therapeutic approaches for various
diseases. However, the complexity of its regulation poses
challenges for clinical translation (167). SOD1 has demonstrated clear
clinical value in neurodegenerative diseases (168). Bioinformatics research has
indicated that SOD1 may serve as a potential diagnostic biomarker
for AS (7), but evidence
directly linking it to cuproptosis remains lacking in the field of
CVD. Furthermore, its application is constrained by functional
complexity and insufficient tissue specificity (169). ATOX1, as a copper chaperone and
potential transcription factor, occupies a pivotal position in the
cuproptosis-regulated network. In oncology, ATOX1 expression is
upregulated across multiple types of cancer, with its upregulation
associated with poor patient prognosis (170). However, high-quality human
clinical evidence remains scarce in the CVD field. Its potential
value lies in reflecting copper metabolic status and serving as a
prognostic indicator in both cancer and CVDs. The primary
limitations stem from its complex regulatory network, which may
compromise the specificity of ATOX1 as a standalone biomarker and
complicate activity assessment (171). As key molecules in copper
metabolism and oxidative stress pathways, as well as biomarkers for
specific disease subtypes, these three substances require unified
detection standards and large-scale prospective clinical validation
in the future to prove their predictive or prognostic value.
Precision medicine should be achieved by constructing precision
classification models based on copper homeostasis pathways through
multi-omics integration, and drugs for targeted therapy should also
be developed.
Reagents for CVDs based on cuproptosis have been
formulated. When exposed to aging conditions, copper and iron atoms
accumulate in their lower-oxidized states, triggering a cascade of
cuproptosis and ferroptosis. D-handed PtPd2CuFe can
alleviate the effects of aging in multiple aging models and has
notable therapeutic capabilities in AS, a disease that involves
multiple types of senescent cells (172). Conversely, the
mitochondrial-targeted triphenylphosphonium-modified
Cu2+ bis(diethyldithiocarbamate) can markedly increase
copper accumulation in mitochondria, and severely impair
mitochondrial morphology and functions. Therefore, it may serve as
a mitochondrial-targeted cuproptosis inducer to selectively
eliminate diseased cells (173).
Studies in animal models have shown that targeting
CTR1 during I/R injury reduces intracellular copper accumulation,
thereby suppressing cuproptosis. This intervention also alleviates
inflammation, oxidative stress and mitochondrial injury in
cardiomyocytes, ultimately providing protection against I/R-induced
damage (166). Serum copper
levels are often elevated in patients with CHF, potentially
enhancing copper deposition in cardiomyocytes. This may lead to
cardiomyocyte loss via cuproptosis and contribute to ventricular
remodeling. However, the clinical relevance of this association is
complicated by multiple confounding factors. In arterial walls,
copper catalyzes Fenton-like reactions that promote oxidized LDL
formation. Moreover, studies have linked copper dysregulation to
the progression of AS, implying that cuproptosis may participate in
AS pathogenesis (82,122). The molecular pathways
underlying cuproptosis in cardiomyocytes, endothelial cells and
vascular smooth muscle cells have not yet been fully elucidated. In
addition, both direct clinical evidence and clinically applicable
copper ionophores are still lacking. Nevertheless, targeting
cuproptosis holds promise as a novel therapeutic strategy for
refractory conditions such as AS and cardiomyopathy.
Copper modulation strategies have demonstrated
therapeutic potential in animal models of atherosclerosis (in
apolipoprotein E-deficient mice fed a high-fat, high-cholesterol
Western-type diet) (122),
ischemia/reperfusion injury (in rats subjected to surgical
induction of myocardial, cerebral or systemic ischemia/reperfusion)
(119) and myocardial
infarction (in female Large White pigs subjected to surgical
induction) (118), yet their
translation to clinical applications remains challenging. Major
obstacles include interspecies differences in copper metabolism,
toxicity risks arising from the dual biological roles of copper,
the absence of reliable biomarkers and clearly defined clinical
endpoints, and variability due to individual genetic and
environmental factors. Collectively, these limitations hinder the
clinical validation of safety and efficacy. Future research should
focus on developing specific inhibitors targeting cuproptosis
pathways to reduce toxic side effects, utilizing biomarkers to
assess disease risk or treatment response, and establishing
reliable technologies for monitoring copper homeostasis
dynamics.
Cuproptosis and ferroptosis, as two novel forms of
programmed cell death, display distinct yet overlapping molecular
mechanisms, regulatory pathways and therapeutic implications.
Simultaneously inducing cuproptosis and ferroptosis can produce
synergistic cytotoxic effects, and multiple nanotherapeutic
strategies (such as DSF@HMCIS-PEG-FA nanoparticles, which enable a
self-accelerating ferroptosis-cuproptosis cycle; HA@ CuCo-NC
nanoconjugates, which hijack Fe-S clusters; and dual-responsive
NSeMON-P@CuT/LipD platforms that disrupt copper metabolism) have
been designed to exploit this effect (59-61). Their combined use can overcome
single-pathway drug resistance in tumors and enhance tumor
targeting; however, the mechanisms underlying cuproptosis are not
yet fully elucidated, and potential clinical applications must
carefully address toxicity and translational limitations.
The present review provides an overview of copper
metabolism, highlights the mechanisms linking copper dysregulation
to CVDs, and summarizes therapeutic strategies that target copper
homeostasis. The recently identified form of regulated cell death,
termed cuproptosis, provides novel insights into the role of copper
in CVD. However, the mechanisms through which copper dysregulation
drives the onset and progression of CVD remain to be elucidated and
warrant validation through well-designed experimental studies.
Current evidence linking cuproptosis to CVD is largely derived from
bioinformatics and multi-omics analyses, with limited experimental
validation in cellular and animal models. The development of safe
and effective modulators of copper homeostasis represents a key
focus for future research aimed at restoring copper homeostasis in
patients with CVD. Furthermore, therapies targeting cuproptosis and
other copper-mediated cell death pathways may provide promising new
options for treating CVDs.
Not applicable.
PL and YL wrote the manuscript, and made
substantial contributions to the conception and design, literature
search, and analysis and interpretation of the literature. QM and
JW drew the figures and tables, and contributed to the literature
search and the analysis and interpretation of the literature. SY
and KW revised the manuscript. Data authentication is not
applicable. All authors read and approved the final manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by the Qingdao Science and Technology
Benefiting the People Demonstration Project (grant no.
24-1-8-smjk-7-nsh); the Open Project Program of State Key
Laboratory of Frigid Zone Cardiovascular Diseases, Harbin Medical
University (grant no. HDHY2024007); the National Natural Science
Foundation of China (grant no. 82370291); the Major Basic Research
Projects in Shandong Province (grant no. ZR2024ZD46); and the
Taishan Scholar Distinguished Expert, Guiding Fund of Government's
Science and Technology (grant no. YDZX2021004).
|
1
|
Myint ZW, Oo TH, Thein KZ, Tun AM and
Saeed H: Copper deficiency anemia: Review article. Ann Hematol.
97:1527–1534. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mohammadifard N, Humphries KH, Gotay C,
Mena-Sánchez G, Salas-Salvadó J, Esmaillzadeh A, Ignaszewski A and
Sarrafzadegan N: Trace minerals intake: Risks and benefits for
cardiovascular health. Crit Rev Food Sci Nutr. 59:1334–1346. 2019.
View Article : Google Scholar
|
|
3
|
Chen X, Cai Q, Liang R, Zhang D, Liu X,
Zhang M, Xiong Y, Xu M, Liu Q, Li P, et al: Copper homeostasis and
copper-induced cell death in the pathogenesis of cardiovascular
disease and therapeutic strategies. Cell Death Dis. 14:1052023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsvetkov P, Coy S, Petrova B, Dreishpoon
M, Verma A, Abdusamad M, Rossen J, Joesch-Cohen L, Humeidi R,
Spangler RD, et al: Copper induces cell death by targeting
lipoylated TCA cycle proteins. Science. 375:1254–1261. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Yang X, Li W, Lin Y, Lin R, Cai X,
Yan B, Xie B and Li J: Potential molecular and cellular mechanisms
of the effects of cuproptosis-related genes in the cardiomyocytes
of patients with diabetic heart failure: A bioinformatics analysis.
Front Endocrinol (Lausanne). 15:13703872024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tan X, Xu S, Zeng Y, Qin Z, Yu F, Jiang H,
Xu H, Li X, Wang X, Zhang G, et al: Identification of diagnostic
signature and immune infiltration for ischemic cardiomyopathy based
on cuproptosis-related genes through bioinformatics analysis and
experimental validation. Int Immunopharmacol. 138:1125742024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen YT, Xu XH, Lin L, Tian S and Wu GF:
Identification of three cuproptosis-specific expressed genes as
diagnostic biomarkers and therapeutic targets for atherosclerosis.
Int J Med Sci. 20:836–848. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Miao J: An emerging role of
defective copper metabolism in heart disease. Nutrients.
14:7002022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mason KE: A conspectus of research on
copper metabolism and requirements of man. J Nutr. 109:1979–2066.
1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pierson H, Yang H and Lutsenko S: Copper
transport and disease: What can we learn from organoids? Annu Rev
Nutr. 39:75–94. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu J, He J, Liu Z, Zhu X, Li Z, Chen A and
Lu J: Cuproptosis: Mechanism, role, and advances in urological
malignancies. Med Res Rev. 44:1662–1682. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu WQ, Lin WR, Yan L, Xu WH and Yang J:
Copper homeostasis and cuproptosis in cancer immunity and therapy.
Immunol Rev. 321:211–227. 2024. View Article : Google Scholar
|
|
13
|
Lee J, Peña MM, Nose Y and Thiele DJ:
Biochemical characterization of the human copper transporter Ctr1.
J Biol Chem. 277:4380–4387. 2002. View Article : Google Scholar
|
|
14
|
Arredondo M, Muñoz P, Mura CV and Nùñez
MT: DMT1, a physiologically relevant apical Cu1+ transporter of
intestinal cells. Am J Physiol Cell Physiol. 284:C1525–C1530. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross MO, Xie Y, Owyang RC, Ye C, Zbihley
ONP, Lyu R, Wu T, Wang P, Karginova O, Olopade OI, et al: PTPN2
copper-sensing relays copper level fluctuations into EGFR/CREB
activation and associated CTR1 transcriptional repression. Nat
Commun. 15:69472024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen L, Min J and Wang F: Copper
homeostasis and cuproptosis in health and disease. Signal Transduct
Target Ther. 7:3782022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Ma J, Wang R, Luo Y, Zheng S and
Wang X: Zinc transporter 1 functions in copper uptake and
cuproptosis. Cell Metab. 36:2118–2129.e6. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krężel A and Maret W: The bioinorganic
chemistry of mammalian metallothioneins. Chem Rev. 121:14594–14648.
2021. View Article : Google Scholar
|
|
19
|
Yang D, Xiao P, Qiu B, Yu HF and Teng CB:
Copper chaperone antioxidant 1: Multiple roles and a potential
therapeutic target. J Mol Med (Berl). 101:527–542. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Perkal O, Qasem Z, Turgeman M, Schwartz R,
Gevorkyan-Airapetov L, Pavlin M, Magistrato A, Major DT and
Ruthstein S: Cu(I) controls conformational states in human Atox1
metallochaperone: An EPR and multiscale simulation study. J Phys
Chem B. 124:4399–4411. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xue Q, Kang R, Klionsky DJ, Tang D, Liu J
and Chen X: Copper metabolism in cell death and autophagy.
Autophagy. 19:2175–2195. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris ED: Cellular copper transport and
metabolism. Annu Rev Nutr. 20:291–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lutsenko S, Barnes NL, Bartee MY and
Dmitriev OY: Function and regulation of human copper-transporting
ATPases. Physiol Rev. 87:1011–1046. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wong PC, Waggoner D, Subramaniam JR,
Tessarollo L, Bartnikas TB, Culotta VC, Price DL, Rothstein J and
Gitlin JD: Copper chaperone for superoxide dismutase is essential
to activate mammalian Cu/Zn superoxide dismutase. Proc Natl Acad
Sci USA. 97:2886–2891. 2000. View Article : Google Scholar
|
|
25
|
Suzuki Y, Ali M, Fischer M and Riemer J:
Human copper chaperone for superoxide dismutase 1 mediates its own
oxidation-dependent import into mitochondria. Nat Commun.
4:24302013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bertinato J and L'Abbé MR: Copper
modulates the degradation of copper chaperone for Cu,Zn superoxide
dismutase by the 26 S proteosome. J Biol Chem. 278:35071–35078.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sturtz LA, Diekert K, Jensen LT, Lill R
and Culotta VC: A fraction of yeast Cu,Zn-superoxide dismutase and
its metallochaperone, CCS, localize to the intermembrane space of
mitochondria. A physiological role for SOD1 in guarding against
mitochondrial oxidative damage. J Biol Chem. 276:38084–38089. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Grasso M, Bond GJ, Kim YJ, Boyd S, Matson
Dzebo M, Valenzuela S, Tsang T, Schibrowsky NA, Alwan KB, Blackburn
NJ, et al: The copper chaperone CCS facilitates copper binding to
MEK1/2 to promote kinase activation. J Biol Chem. 297:1013142021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garza NM, Swaminathan AB, Maremanda KP,
Zulkifli M and Gohil VM: Mitochondrial copper in human genetic
disorders. Trends Endocrinol Metab. 34:21–33. 2023. View Article : Google Scholar
|
|
30
|
Maxfield AB, Heaton DN and Winge DR: Cox17
is functional when tethered to the mitochondrial inner membrane. J
Biol Chem. 279:5072–5080. 2004. View Article : Google Scholar
|
|
31
|
Sailer J, Nagel J, Akdogan B, Jauch AT,
Engler J, Knolle PA and Zischka H: Deadly excess copper. Redox
Biol. 75:1032562024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Boyd SD, Ullrich MS, Skopp A and Winkler
DD: Copper sources for Sod1 activation. Antioxidants (Basel).
9:5002020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Polishchuk EV, Concilli M, Iacobacci S,
Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D, van
IJzendoorn SC, Chan J, et al: Wilson disease protein ATP7B utilizes
lysosomal exocytosis to maintain copper homeostasis. Dev Cell.
29:686–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Doguer C, Ha JH and Collins JF:
Intersection of iron and copper metabolism in the mammalian
intestine and liver. Compr Physiol. 8:1433–1461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
La Fontaine S and Mercer JF: Trafficking
of the copper-ATPases, ATP7A and ATP7B: Role in copper homeostasis.
Arch Biochem Biophys. 463:149–167. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Festa RA and Thiele DJ: Copper: An
essential metal in biology. Curr Biol. 21:R877–R883. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Turnlund JR, Keyes WR, Anderson HL and
Acord LL: Copper absorption and retention in young men at three
levels of dietary copper by use of the stable isotope 65Cu. Am J
Clin Nutr. 49:870–878. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
van den Berghe PVE and Klomp LWJ:
Posttranslational regulation of copper transporters. J Biol Inorg
Chem. 15:37–46. 2010. View Article : Google Scholar
|
|
39
|
Ge EJ, Bush AI, Casini A, Cobine PA, Cross
JR, DeNicola GM, Dou QP, Franz KJ, Gohil VM, Gupta S, et al:
Connecting copper and cancer: From transition metal signalling to
metalloplasia. Nat Rev Cancer. 22:102–113. 2022. View Article : Google Scholar :
|
|
40
|
Yang L, Yang P, Lip GYH and Ren J: Copper
homeostasis and cuproptosis in cardiovascular disease therapeutics.
Trends Pharmacol Sci. 44:573–585. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chang W and Li P: Copper and diabetes:
Current research and prospect. Mol Nutr Food Res. 67:e23004682023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiayi H, Ziyuan T, Tianhua X, Mingyu Z,
Yutong M, Jingyu W, Hongli Z and Li S: Copper homeostasis in
chronic kidney disease and its crosstalk with ferroptosis.
Pharmacol Res. 202:1071392024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shanbhag VC, Gudekar N, Jasmer K,
Papageorgiou C, Singh K and Petris MJ: Copper metabolism as a
unique vulnerability in cancer. Biochim Biophys Acta Mol Cell Res.
1868:1188932021. View Article : Google Scholar :
|
|
44
|
Lin CH, Chin Y, Zhou M, Sobol RW, Hung MC
and Tan M: Protein lipoylation: Mitochondria, cuproptosis, and
beyond. Trends Biochem Sci. 49:729–744. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Gupte A and Mumper RJ: Elevated copper and
oxidative stress in cancer cells as a target for cancer treatment.
Cancer Treat Rev. 35:32–46. 2009. View Article : Google Scholar
|
|
46
|
Wang Y, Chen Y, Zhang J, Yang Y, Fleishman
JS, Wang Y, Wang J, Chen J, Li Y and Wang H: Cuproptosis: A novel
therapeutic target for overcoming cancer drug resistance. Drug
Resist Updat. 72:1010182024. View Article : Google Scholar
|
|
47
|
Han J, Luo J, Wang C, Kapilevich L and
Zhang XA: Roles and mechanisms of copper homeostasis and
cuproptosis in osteoarticular diseases. Biomed Pharmacother.
174:1165702024. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen W, Yang A, Jia J, Popov YV, Schuppan
D and You H: Lysyl oxidase (LOX) family members: Rationale and
their potential as therapeutic targets for liver fibrosis.
Hepatology. 72:729–741. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Doñate F, Juarez JC, Burnett ME, Manuia
MM, Guan X, Shaw DE, Smith EL, Timucin C, Braunstein MJ, Batuman OA
and Mazar AP: Identification of biomarkers for the antiangiogenic
and antitumour activity of the superoxide dismutase 1 (SOD1)
inhibitor tetrathiomolybdate (ATN-224). Br J Cancer. 98:776–783.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tsui KH, Hsiao JH, Lin LT, Tsang YL, Shao
AN, Kuo CH, Chang R, Wen ZH and Li CJ: The cross-communication of
cuproptosis and regulated cell death in human pathophysiology. Int
J Biol Sci. 20:218–230. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han X, Zhang X, Liu Z, Liu H, Wu D, He Y,
Yuan K, Lyu Y and Liu X: Copper-based nanotubes that enhance
starvation therapy through cuproptosis for synergistic cancer
treatment. Adv Sci (Weinh). 12:e041212025. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yan C, Lv H, Feng Y, Li Y and Zhao Z:
Inhalable nanoparticles with enhanced cuproptosis and cGAS-STING
activation for synergistic lung metastasis immunotherapy. Acta
Pharm Sin B. 14:3697–3710. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang D, Tian Z, Zhang P, Zhen L, Meng Q,
Sun B, Xu X, Jia T and Li S: The molecular mechanisms of
cuproptosis and its relevance to cardiovascular disease. Biomed
Pharmacother. 163:1148302023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dreishpoon MB, Bick NR, Petrova B, Warui
DM, Cameron A, Booker SJ, Kanarek N, Golub TR and Tsvetkov P: FDX1
regulates cellular protein lipoylation through direct binding to
LIAS. J Biol Chem. 299:1050462023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rowland EA, Snowden CK and Cristea IM:
Protein lipoylation: An evolutionarily conserved metabolic
regulator of health and disease. Curr Opin Chem Biol. 42:76–85.
2018. View Article : Google Scholar :
|
|
56
|
Kuang J, Liu A, Xu L, Wang G, Zhang Z,
Tian C and Yu L: Electron paramagnetic resonance insights into
direct electron transfer between FDX1 and
elesclomol-Cu2+ complex in cuproptosis. Chemistry.
31:e2025011452025. View Article : Google Scholar
|
|
57
|
Hu HT, Zhang ZY, Luo ZX, Ti HB, Wu JJ, Nie
H, Yuan ZD, Wu X, Zhang KY, Shi SW, et al: Emerging regulated cell
death mechanisms in bone remodeling: Decoding ferroptosis,
cuproptosis, disulfidptosis, and PANoptosis as therapeutic targets
for skeletal disorders. Cell Death Discov. 11:3352025. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang Z, Feng R and Zhao H: Cuproptosis and
Cu: A new paradigm in cellular death and their role in
non-cancerous diseases. Apoptosis. 29:1330–1360. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Huang L, Zhu J, Wu G, Xiong W, Feng J, Yan
C, Yang J, Li Z, Fan Q, Ren B, et al: A strategy of 'adding fuel to
the flames' enables a self-accelerating cycle of
ferroptosis-cuproptosis for potent antitumor therapy. Biomaterials.
311:1227012024. View Article : Google Scholar
|
|
60
|
Liu G, Tang R, Wang C, Yu D, Wang Z, Yang
H, Wei J, Zhu S, Gao F, Yuan F and Pan B: Bimetallic nanoconjugate
hijack Fe-S clusters to drive a closed-loop cuproptosis-ferroptosis
strategy for osteosarcoma inhibition. J Colloid Interface Sci.
703:1390522025.Epub ahead of print. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang M, Xu H, Wu X, Chen B, Gong X and He
Y: Engineering dual-responsive nanoplatform achieves copper
metabolism disruption and glutathione consumption to provoke
cuproptosis/ferroptosis/apoptosis for cancer therapy. ACS Appl
Mater Interfaces. 17:20726–20740. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Husain N and Mahmood R: Copper(II)
generates ROS and RNS, impairs antioxidant system and damages
membrane and DNA in human blood cells. Environ Sci Pollut Res Int.
26:20654–20668. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Alqarni MH, Muharram MM, Alshahrani SM and
Labrou NE: Copper-induced oxidative cleavage of glutathione
transferase F1-1 from Zea mays. Int J Biol Macromol. 128:493–498.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Wang K, Ning X, Qin C, Wang J, Yan W, Zhou
X, Wang D, Cao J and Feng Y: Respiratory exposure to copper oxide
particles causes multiple organ injuries via oxidative stress in a
rat model. Int J Nanomedicine. 17:4481–4496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Piret JP, Jacques D, Audinot JN, Mejia J,
Boilan E, Noël F, Fransolet M, Demazy C, Lucas S, Saout C and
Toussaint O: Copper(II) oxide nanoparticles penetrate into HepG2
cells, exert cytotoxicity via oxidative stress and induce
pro-inflammatory response. Nanoscale. 4:7168–7184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He H, Zou Z, Wang B, Xu G, Chen C, Qin X,
Yu C and Zhang J: Copper oxide nanoparticles induce oxidative DNA
damage and cell death via copper ion-mediated P38 MAPK activation
in vascular endothelial cells. Int J Nanomedicine. 15:3291–3302.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Martin F, Linden T, Katschinski DM, Oehme
F, Flamme I, Mukhopadhyay CK, Eckhardt K, Tröger J, Barth S,
Camenisch G and Wenger RH: Copper-dependent activation of
hypoxia-inducible factor (HIF)-1: Implications for ceruloplasmin
regulation. Blood. 105:4613–4619. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Xiao Y, Wang T, Song X, Yang D, Chu Q and
Kang YJ: Copper promotion of myocardial regeneration. Exp Biol Med
(Maywood). 245:911–921. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Martínez-González J, Varona S, Cañes L,
Galán M, Briones AM, Cachofeiro V and Rodríguez C: Emerging roles
of Lysyl oxidases in the cardiovascular system: New concepts and
therapeutic challenges. Biomolecules. 9:6102019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang K, Li Y, Luo C and Chen Y: Dynamic
AFM detection of the oxidation-induced changes in size, stiffness,
and stickiness of low-density lipoprotein. J Nanobiotechnology.
18:1672020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Widyananda MH, Grahadi R, Dinana IA,
Ansori ANM, Kharisma VD, Jakhmola V, Rebezov M, Derkho M, Burkov P,
Scherbakov P and Zainul R: Anti-atherosclerotic potential of fatty
acids in Chlorella vulgaris via inhibiting the foam cell formation:
An in silico study. Adv Life Sci. 12:296–303. 2025. View Article : Google Scholar
|
|
72
|
Widyananda MH, Kurniasari CA, Alam FM,
Rizky WC, Dings TGA, Ansori ANM and Antonius Y: Exploration of
potentially bioactive compounds from Fingerroot (Boesenbergia
rotunda L.) as inhibitor of atherosclerosis-related proteins (CETP,
ACAT1, OSC, sPLA2): An in silico study. Jordan J Pharm Sci.
16:550–564. 2023. View Article : Google Scholar
|
|
73
|
Kciuk M, Gielecińska A, Kałuzińska-Kołat
Ż, Yahya EB and Kontek R: Ferroptosis and cuproptosis:
Metal-dependent cell death pathways activated in response to
classical chemotherapy-significance for cancer treatment? Biochim
Biophys Acta Rev Cancer. 1879:1891242024. View Article : Google Scholar
|
|
74
|
Zhou D, Mao Q, Sun Y, Cheng H, Zhao J, Liu
Q, Deng M, Xu S and Zhao X: Association of blood copper with the
subclinical carotid atherosclerosis: An observational study. J Am
Heart Assoc. 13:e0334742024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhu C, Wang B, Xiao L, Guo Y, Zhou Y, Cao
L, Yang S and Chen W: Mean platelet volume mediated the
relationships between heavy metals exposure and atherosclerotic
cardiovascular disease risk: A community-based study. Eur J Prev
Cardiol. 27:830–839. 2020. View Article : Google Scholar
|
|
76
|
Long P, Wang Q, Zhang Y, Zhu X, Yu K,
Jiang H, Liu X, Zhou M, Yuan Y, Liu K, et al: Profile of
copper-associated DNA methylation and its association with incident
acute coronary syndrome. Clin Epigenetics. 13:192021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kuzan A, Wujczyk M and Wiglusz RJ: The
study of the aorta metallomics in the context of atherosclerosis.
Biomolecules. 11:9462021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li H, Zhao L, Wang T and James Kang Y:
Dietary Cholesterol supplements disturb copper homeostasis in
multiple organs in rabbits: Aorta copper concentrations negatively
correlate with the severity of atherosclerotic lesions. Biol Trace
Elem Res. 200:164–171. 2022. View Article : Google Scholar
|
|
79
|
Bügel S, Harper A, Rock E, O'Connor JM,
Bonham MP and Strain JJ: Effect of copper supplementation on
indices of copper status and certain CVD risk markers in young
healthy women. Br J Nutr. 94:231–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Diaf M and Khaled MB: Associations between
dietary antioxidant intake and markers of atherosclerosis in
middle-aged women from north-western Algeria. Front Nutr. 5:292018.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kärberg K, Forbes A and Lember M: Raised
dietary Zn:Cu ratio increases the risk of atherosclerosis in type 2
diabetes. Clin Nutr ESPEN. 50:218–224. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Sudhahar V, Shi Y, Kaplan JH, Ushio-Fukai
M and Fukai T: Whole-transcriptome sequencing analyses of nuclear
antixoxidant-1 in endothelial cells: Role in inflammation and
atherosclerosis. Cells. 11:29192022. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Das A, Sudhahar V, Ushio-Fukai M and Fukai
T: Novel interaction of antioxidant-1 with TRAF4: Role in
inflammatory responses in endothelial cells. Am J Physiol Cell
Physiol. 317:C1161–C1171. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Yang L, Chen X, Cheng H and Zhang L:
Dietary copper intake and risk of stroke in adults: A case-control
study based on national health and nutrition examination survey
2013-2018. Nutrients. 14:4092022. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Hu L, Bi C, Lin T, Liu L, Song Y, Wang P,
Wang B, Fang C, Ma H, Huang X, et al: Association between plasma
copper levels and first stroke: A community-based nested
case-control study. Nutr Neurosci. 25:1524–1533. 2022. View Article : Google Scholar
|
|
86
|
Lai M, Wang D, Lin Z and Zhang Y: Small
molecule copper and its relative metabolites in serum of cerebral
ischemic stroke patients. J Stroke Cerebrovasc Dis. 25:214–219.
2016. View Article : Google Scholar
|
|
87
|
Xiao Y, Yuan Y, Liu Y, Yu Y, Jia N, Zhou
L, Wang H, Huang S, Zhang Y, Yang H, et al: Circulating multiple
metals and incident stroke in Chinese adults. Stroke. 50:1661–1668.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Zhao H, Mei K, Hu Q, Wu Y, Xu Y, Qinling,
Yu P, Deng Y, Zhu W, Yan Z and Liu X: Circulating copper levels and
the risk of cardio-cerebrovascular diseases and cardiovascular and
all-cause mortality: A systematic review and meta-analysis of
longitudinal studies. Environ Pollut. 340:1227112024. View Article : Google Scholar
|
|
89
|
Zhang M, Li W, Wang Y, Wang T, Ma M and
Tian C: Association between the change of serum copper and ischemic
stroke: A systematic review and meta-analysis. J Mol Neurosci.
70:475–480. 2020. View Article : Google Scholar
|
|
90
|
Peng Y, Ren Q, Ma H, Lin C, Yu M, Li Y,
Chen J, Xu H, Zhao P, Pan S, et al: Covalent organic framework
based cytoprotective therapy after ischemic stroke. Redox Biol.
71:1031062024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xu J, Xu G and Fang J: Association between
serum copper and stroke risk factors in adults: Evidence from the
national health and nutrition examination survey, 2011-2016. Biol
Trace Elem Res. 200:1089–1094. 2022. View Article : Google Scholar
|
|
92
|
Yang F and Smith MJ: Metal profiling in
coronary ischemia-reperfusion injury: Implications for KEAP1/NRF2
regulated redox signaling. Free Radic Biol Med. 210:158–171. 2024.
View Article : Google Scholar
|
|
93
|
Ding C, Wang B, Zheng J, Zhang M, Li Y,
Shen HH, Guo Y, Zheng B, Tian P, Ding X and Xue W: Neutrophil
membrane-inspired nanorobots act as antioxidants ameliorate
ischemia reperfusion-induced acute kidney injury. ACS Appl Mater
Interfaces. 15:40292–40303. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kokubo Y, Matson GB, Derugin N, Hill T,
Mancuso A, Chan PH and Weinstein PR: Transgenic mice expressing
human copper-zinc superoxide dismutase exhibit attenuated apparent
diffusion coefficient reduction during reperfusion following focal
cerebral ischemia. Brain Res. 947:1–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen Z, Oberley TD, Ho Y, Chua CC, Siu B,
Hamdy RC, Epstein CJ and Chua BH: Overexpression of CuZnSOD in
coronary vascular cells attenuates myocardial ischemia/reperfusion
injury. Free Radic Biol Med. 29:589–596. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Tanaka M, Mokhtari GK, Terry RD, Balsam
LB, Lee KH, Kofidis T, Tsao PS and Robbins RC: Overexpression of
human copper/zinc superoxide dismutase (SOD1) suppresses
ischemia-reperfusion injury and subsequent development of graft
coronary artery disease in murine cardiac grafts. Circulation.
110(11 Suppl 1): II200–II206. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Song W, Tang Q, Teng L, Zhang M, Sha S, Li
B and Zhu L: Exercise for myocardial ischemia-reperfusion injury: A
systematic review and meta-analysis based on preclinical studies.
Microvasc Res. 147:1045022023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Kuster GM, Nietlispach F, Kiowski W,
Schindler R, Bernheim A, Schuetz P, Mueller B, Morgenthaler NG,
Rüter F, Riesen W, et al: Role of RAS inhibition in the regulation
of Cu/Zn-SOD in the cardiac and peripheral arterial beds in humans.
Clin Pharmacol Ther. 87:686–692. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kunutsor SK, Voutilainen A, Kurl S and
Laukkanen JA: Serum copper-to-zinc ratio is associated with heart
failure and improves risk prediction in middle-aged and older
Caucasian men: A prospective study. Nutr Metab Cardiovasc Dis.
32:1924–1935. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Alexanian I, Parissis J, Farmakis D,
Athanaselis S, Pappas L, Gavrielatos G, Mihas C, Paraskevaidis I,
Sideris A, Kremastinos D, et al: Clinical and echocardiographic
correlates of serum copper and zinc in acute and chronic heart
failure. Clin Res Cardiol. 103:938–949. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Liu R, Yao J, Chen K and Peng W:
Association between biomarkers of zinc and copper status and heart
failure: A meta-analysis. ESC Heart Fail. 11:2546–2556. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Ghaemian A, Salehifar E, Jalalian R,
Ghasemi F, Azizi S, Masoumi S, Shiraj H, Mohammadpour RA and
Bagheri GA: Zinc and copper levels in severe heart failure and the
effects of atrial fibrillation on the zinc and copper status. Biol
Trace Elem Res. 143:1239–1246. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hammadah M, Fan Y, Wu Y, Hazen SL and Tang
WH: Prognostic value of elevated serum ceruloplasmin levels in
patients with heart failure. J Card Fail. 20:946–952. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
de Andrade Freire FL, Dantas-Komatsu RCS,
de Lira NRD, Diniz RVZ, Lima SCVC, Barbosa F Jr, Pedrosa LFC and
Sena-Evangelista KCM: Biomarkers of zinc and copper status and
associated factors in outpatients with ischemic and non-ischemic
heart failure. J Am Nutr Assoc. 41:231–239. 2022.
|
|
105
|
Niu YY, Aierken A and Feng L: Unraveling
the link between dietary factors and cardiovascular metabolic
diseases: Insights from a two-sample Mendelian randomization
investigation. Heart Lung. 63:72–77. 2024. View Article : Google Scholar
|
|
106
|
Cimen YA, Taslidere B, Sarikaya U, Demirel
M, Acikgoz N and Selek S: Assessment of oxidative stress and trace
element dynamics in acute myocardial infarction and heart failure:
A focus on zinc, copper, and thiol dynamics. Clinics (Sao Paulo).
80:1007552025. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Gorący I, Rębacz-Maron E, Korbecki J and
Gorący J: Concentrations of Mg, Ca, Fe, Cu, Zn, P and
anthropometric and biochemical parameters in adults with chronic
heart failure. PeerJ. 9:e122072021. View Article : Google Scholar
|
|
108
|
Zou R, Zhang M, Zou Z, Shi W, Tan S, Wang
C, Xu W, Jin J, Milton S, Chen Y, et al: Single-cell
transcriptomics reveals zinc and copper ions homeostasis in
epicardial adipose tissue of heart failure. Int J Biol Sci.
19:4036–4051. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Guo Q, Cai J, Qu Q, Cheang I, Shi J, Pang
H and Li X: Association of blood trace elements levels with
cardiovascular disease in US adults: A cross-sectional study from
the national health and nutrition examination survey 2011-2016.
Biol Trace Elem Res. 202:3037–3050. 2024. View Article : Google Scholar
|
|
110
|
Malekahmadi M, Firouzi S, Rezayi M,
Ghazizadeh H, Ranjbar G, Ferns GA and Mobarhan MG: Association of
zinc and copper status with cardiovascular diseases and their
assessment methods: A review study. Mini Rev Med Chem.
20:2067–2078. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Bomer N, Pavez-Giani MG, Grote Beverborg
N, Cleland JGF, van Veldhuisen DJ and van der Meer P: Micronutrient
deficiencies in heart failure: Mitochondrial dysfunction as a
common pathophysiological mechanism? J Intern Med. 291:713–731.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Witte KK, Nikitin NP, Parker AC, von
Haehling S, Volk HD, Anker SD, Clark AL and Cleland JG: The effect
of micronutrient supplementation on quality-of-life and left
ventricular function in elderly patients with chronic heart
failure. Eur Heart J. 26:2238–2244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Dieterich S, Bieligk U, Beulich K,
Hasenfuss G and Prestle J: Gene expression of antioxidative enzymes
in the human heart: Increased expression of catalase in the
end-stage failing heart. Circulation. 101:33–39. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Zhang S, Liu H, Amarsingh GV, Cheung CCH,
Wu D, Narayanan U, Zhang L and Cooper GJS: Restoration of
myocellular copper-trafficking proteins and mitochondrial copper
enzymes repairs cardiac function in rats with diabetes-evoked heart
failure. Metallomics. 12:259–272. 2020. View Article : Google Scholar
|
|
115
|
Ennezat PV, Malendowicz SL, Testa M,
Colombo PC, Cohen-Solal A, Evans T and LeJemtel TH: Physical
training in patients with chronic heart failure enhances the
expression of genes encoding antioxidative enzymes. J Am Coll
Cardiol. 38:194–198. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chen Z, Zhu Y, Chen S, Li Z, Fu G and Wang
Y: Immune patterns of cuproptosis in ischemic heart failure: A
transcriptome analysis. J Cell Mol Med. 28:e181872024. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Hao D, Meng Q, Li C, Lu S, Xiang X, Pei Q,
Jing X and Xie Z: A paclitaxel prodrug with copper depletion for
combined therapy toward triple-negative breast cancer. ACS Nano.
17:12383–12393. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Johnson TW, Holt J, Kleyman A, Zhou S,
Sammut E, Bruno VD, Gaupp C, Stanzani G, Martin J, Arina P, et al:
Development and translation of thiometallate sulfide donors using a
porcine model of coronary occlusion and reperfusion. Redox Biol.
73:1031672024. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Dyson A, Dal-Pizzol F, Sabbatini G, Lach
AB, Galfo F, Dos Santos Cardoso J, Pescador Mendonça B, Hargreaves
I, Bollen Pinto B, Bromage DI, et al: Ammonium tetrathiomolybdate
following ischemia/reperfusion injury: Chemistry, pharmacology, and
impact of a new class of sulfide donor in preclinical injury
models. PLoS Med. 14:e10023102017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Chen YF, Qi RQ, Song JW, Wang SY, Dong ZJ,
Chen YH, Liu Y, Zhou XY, Li J, Liu XY and Zhong JC: Sirtuin 7
ameliorates cuproptosis, myocardial remodeling and heart
dysfunction in hypertension through the modulation of YAP/ATP7A
signaling. Apoptosis. 29:2161–2182. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Bogaard HJ, Mizuno S, Guignabert C, Al
Hussaini AA, Farkas D, Ruiter G, Kraskauskas D, Fadel E, Allegood
JC, Humbert M, et al: Copper dependence of angioproliferation in
pulmonary arterial hypertension in rats and humans. Am J Respir
Cell Mol Biol. 46:582–591. 2012. View Article : Google Scholar :
|
|
122
|
Wei H, Zhang WJ, McMillen TS, Leboeuf RC
and Frei B: Copper chelation by tetrathiomolybdate inhibits
vascular inflammation and atherosclerotic lesion development in
apolipoprotein E-deficient mice. Atherosclerosis. 223:306–313.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ambi A, Stanisavljevic A, Victor TW,
Lowery AW, Davis J, Van Nostrand WE and Miller LM: Evaluation of
copper chelation therapy in a transgenic rat model of cerebral
amyloid angiopathy. ACS Chem Neurosci. 14:378–388. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Mandinov L, Moodie KL, Mandinova A, Zhuang
Z, Redican F, Baklanov D, Lindner V, Maciag T, Simons M and de
Muinck ED: Inhibition of in-stent restenosis by oral copper
chelation in porcine coronary arteries. Am J Physiol Heart Circ
Physiol. 291:H2692–H2697. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Cooper GJ, Young AA, Gamble GD, Occleshaw
CJ, Dissanayake AM, Cowan BR, Brunton DH, Baker JR, Phillips AR,
Frampton CM, et al: A copper(II)-selective chelator ameliorates
left-ventricular hypertrophy in type 2 diabetic patients: A
randomised placebo-controlled study. Diabetologia. 52:715–722.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Zhang S, Liu H, Amarsingh GV, Cheung CC,
Hogl S, Narayanan U, Zhang L, McHarg S, Xu J, Gong D, et al:
Diabetic cardiomyopathy is associated with defective myocellular
copper regulation and both defects are rectified by divalent copper
chelation. Cardiovasc Diabetol. 13:1002014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang L, Ward ML, Phillips ARJ, Zhang S,
Kennedy J, Barry B, Cannell MB and Cooper GJS: Protection of the
heart by treatment with a divalent-copper-selective chelator
reveals a novel mechanism underlying cardiomyopathy in diabetic
rats. Cardiovasc Diabetol. 12:1232013. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Lamas GA, Anstrom KJ, Navas-Acien A,
Boineau R, Nemeth H, Huang Z, Wen J, Rosenberg Y, Stylianou M,
Jones TLZ, et al: Edetate disodium-based chelation for patients
with a previous myocardial infarction and diabetes: TACT2
randomized clinical trial. JAMA. 332:794–803. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Lamas GA, Goertz C, Boineau R, Mark DB,
Rozema T, Nahin RL, Lindblad L, Lewis EF, Drisko J and Lee KL; TACT
Investigators: Effect of disodium EDTA chelation regimen on
cardiovascular events in patients with previous myocardial
infarction: The TACT randomized trial. JAMA. 309:1241–1250. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Knudtson ML, Wyse DG, Galbraith PD, Brant
R, Hildebrand K, Paterson D, Richardson D, Burkart C and Burgess E;
Program to Assess Alternative Treatment Strategies to Achieve
Cardiac Health (PATCH) Investigators: Chelation therapy for
ischemic heart disease: A randomized controlled trial. JAMA.
287:481–486. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Anderson TJ, Hubacek J, Wyse DG and
Knudtson ML: Effect of chelation therapy on endothelial function in
patients with coronary artery disease: PATCH substudy. J Am Coll
Cardiol. 41:420–425. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Escolar E, Lamas GA, Mark DB, Boineau R,
Goertz C, Rosenberg Y, Nahin RL, Ouyang P, Rozema T, Magaziner A,
et al: The effect of an EDTA-based chelation regimen on patients
with diabetes mellitus and prior myocardial infarction in the trial
to assess chelation therapy (TACT). Circ Cardiovasc Qual Outcomes.
7:15–24. 2014. View Article : Google Scholar
|
|
133
|
Kirk FT, Munk DE, Swenson ES, Quicquaro
AM, Vendelbo MH, Larsen A, Schilsky ML, Ott P and Sandahl TD:
Effects of tetrathiomolybdate on copper metabolism in healthy
volunteers and in patients with Wilson disease. J Hepatol.
80:586–595. 2024. View Article : Google Scholar
|
|
134
|
Borchard S, Raschke S, Zak KM, Eberhagen
C, Einer C, Weber E, Müller SM, Michalke B, Lichtmannegger J,
Wieser A, et al: Bis-choline tetrathiomolybdate prevents
copper-induced blood-brain barrier damage. Life Sci Alliance.
5:e2021011642021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Wang YM, Feng LS, Xu A, Ma XH, Zhang MT
and Zhang J: Copper ions: The invisible killer of cardiovascular
disease (Review). Mol Med Rep. 30:2102024. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Juarez JC, Betancourt O Jr, Pirie-Shepherd
SR, Guan X, Price ML, Shaw DE, Mazar AP and Doñate F: Copper
binding by tetrathiomolybdate attenuates angiogenesis and tumor
cell proliferation through the inhibition of superoxide dismutase
1. Clin Cancer Res. 12:4974–4982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Yang D, Wang T, Liu J, Wang H and Kang YJ:
Reverse regulation of hepatic ceruloplasmin production in rat model
of myocardial ischemia. J Trace Elem Med Biol. 64:1266862021.
View Article : Google Scholar
|
|
138
|
Villarruz-Sulit MV, Forster R, Dans AL,
Tan FN and Sulit DV: Chelation therapy for atherosclerotic
cardiovascular disease. Cochrane Database Syst Rev.
5:Cd0027852020.PubMed/NCBI
|
|
139
|
Steinbrueck A, Sedgwick AC, Brewster JT
II, Yan KC, Shang Y, Knoll DM, Vargas-Zúñiga GI, He XP, Tian H and
Sessler JL: Transition metal chelators, pro-chelators, and
ionophores as small molecule cancer chemotherapeutic agents. Chem
Soc Rev. 49:3726–3747. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Ranucci G, Polishchuck R and Iorio R:
Wilson's disease: Prospective developments towards new therapies.
World J Gastroenterol. 23:5451–5456. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Zhang C, Huang T and Li L: Targeting
cuproptosis for cancer therapy: Mechanistic insights and clinical
perspectives. J Hematol Oncol. 17:682024. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Zheng P, Zhou C, Lu L, Liu B and Ding Y:
Elesclomol: A copper ionophore targeting mitochondrial metabolism
for cancer therapy. J Exp Clin Cancer Res. 41:2712022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Li Y, Chen F, Chen J, Chan S, He Y, Liu W
and Zhang G: Disulfiram/copper induces antitumor activity against
both nasopharyngeal cancer cells and cancer-associated fibroblasts
through ROS/MAPK and ferroptosis pathways. Cancers (Basel).
12:1382020. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
He P, Li H, Liu C, Liu M, Zhang Z, Zhang
Y, Zhou C, Li Q, Ye Z, Wu Q, et al: U-shaped association between
dietary copper intake and new-onset hypertension. Clin Nutr.
41:536–542. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Li R, Bourcy K, Wang T, Sun M and Kang YJ:
The involvement of vimentin in copper-induced regression of
cardiomyocyte hypertrophy. Metallomics. 7:1331–1337. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Liu Y, Tan L, Kuang Y, Zhang Y, Wang P,
Liu C and Ma Q: A national cross-sectional analysis of dietary
copper intake and abdominal aortic calcification in the US adults:
NHANES 2013-2014. Nutr Metab Cardiovasc Dis. 33:1941–1950. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Hu S, Wei B and Zhang A: Association of
multiple dietary metal intake with cardiovascular-kidney-metabolic
syndrome: A cross-sectional study based on NHANES 2003-2018. Front
Nutr. 12:16124582025. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Xu H, Liu Z, Yao B and Xu Z: The impact of
dietary copper intake on cardiovascular morbidity and mortality
among hypertensive patients: A longitudinal analysis from NHANES
(2001-2018). BMC Public Health. 25:9362025. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Li X, Dehghan M, Tse LA, Lang X,
Rangarajan S, Liu W, Hu B, Yusuf S, Wang C and Li W: Associations
of dietary copper intake with cardiovascular disease and mortality:
Findings from the Chinese Perspective Urban and Rural epidemiology
(PURE-China) study. BMC Public Health. 23:25252023. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Cai L, Tan Y, Holland B and Wintergerst K:
Diabetic cardiomyopathy and cell death: Focus on metal-mediated
cell death. Cardiovasc Toxicol. 24:71–84. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Kong B, Zheng X, Hu Y, Zhao Y, Hai J, Ti Y
and Bu P: Sirtuin3 attenuates pressure overload-induced
pathological myocardial remodeling by inhibiting cardiomyocyte
cuproptosis. Pharmacol Res. 216:1077392025. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Mo N, Tai C, Yang Y, Ling C, Zhang B, Wei
L, Yao C, Wang H and Chen C: MT2A promotes angiogenesis in
chronically ischemic brains through a copper-mitochondria
regulatory mechanism. J Transl Med. 23:1622025. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Huang XP, Shi ZH, Ming GF, Xu DM and Cheng
SQ: S-Allyl-L-cysteine (SAC) inhibits copper-induced apoptosis and
cuproptosis to alleviate cardiomyocyte injury. Biochem Biophys Res
Commun. 730:1503412024. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Huo S, Wang Q, Shi W, Peng L, Jiang Y, Zhu
M, Guo J, Peng D, Wang M, Men L, et al: ATF3/SPI1/SLC31A1 signaling
promotes cuproptosis induced by advanced glycosylation end products
in diabetic myocardial injury. Int J Mol Sci. 24:16672023.
View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Lin Y, Chen K, Guo J, Chen P, Qian ZR and
Zhang T: Identification of cuproptosis-related genes and immune
infiltration in dilated cardiomyopathy. Int J Cardiol.
399:1317022024. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Zhou S, Wang L, Huang X, Wang T, Tang Y,
Liu Y and Xu M: Comprehensive bioinformatics analytics and in vivo
validation reveal SLC31A1 as an emerging diagnostic biomarker for
acute myocardial infarction. Aging (Albany NY). 16:8361–8377.
2024.PubMed/NCBI
|
|
157
|
Wang B, Zhou J and An N: Investigating
molecular markers linked to acute myocardial infarction and
cuproptosis: Bioinformatics analysis and validation in the AMI mice
model. PeerJ. 12:e172802024. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Fang C, Sun S, Chen W, Huang D, Wang F,
Wei W and Wang W: Bioinformatics analysis of the role of
cuproptosis gene in acute myocardial infarction. Minerva Cardiol
Angiol. 72:595–606. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhang J, Yue Z, Zhu N and Zhao N:
Identification of potential biomarkers associated with cuproptosis
and immune microenvironment analysis in acute myocardial
infarction: A diagnostic accuracy study. Medicine (Baltimore).
104:e408172025. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Wang M, Xu X, Li J, Gao Z, Ding Y, Chen X,
Xiang Q and Shen L: Integrated bioinformatics and experiment
revealed that cuproptosis is the potential common pathogenesis of
three kinds of primary cardiomyopathy. Aging (Albany NY).
15:14210–14241. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Wang Y, Wang Q, Liu P, Jin L, Qin X and
Zheng Q: Construction and validation of a cuproptosis-related
diagnostic gene signature for atrial fibrillation based on ensemble
learning. Hereditas. 160:342023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zhang B and He M: Identification of
potential biomarkers for coronary artery disease based on
cuproptosis. Cardiovasc Ther. 2023:59961442023. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Tu B, Song K, Zhou ZY, Lin LC, Liu ZY, Sun
H, Zhou Y, Sha JM, Shi Y, Yang JJ, et al: SLC31A1 loss depletes
mitochondrial copper and promotes cardiac fibrosis. Eur Heart J.
46:2458–2474. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Lv X, Zhao L, Song Y, Chen W and Tuo Q:
Deciphering the role of copper homeostasis in atherosclerosis: From
molecular mechanisms to therapeutic targets. Int J Mol Sci.
25:114622024. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Shen Z, Liu Z, Cai S, Fu H, Gan Y, Li X,
Wang X, Liu C, Ma W, Chen J and Li N: Copper homeostasis and
cuproptosis in myocardial infarction: Molecular mechanisms,
treatment strategies and potential therapeutic targets. Front
Pharmacol. 16:15255852025. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Chen G, Wei M, Zhao CY, Zhang AY, Su JB,
Ni ZR, Cai WW, Hou B, Du B, Liu MH, et al: Phillygenin ameliorates
myocardial ischemia-reperfusion injury by inhibiting cuproptosis
via the autophagy-lysosome degradation of CTR1. Free Radic Biol
Med. 237:542–557. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Lutsenko S, Roy S and Tsvetkov P:
Mammalian copper homeostasis: Physiological roles and molecular
mechanisms. Physiol Rev. 105:441–491. 2025. View Article : Google Scholar :
|
|
168
|
Goutman SA, Hardiman O, Al-Chalabi A, Chió
A, Savelieff MG, Kiernan MC and Feldman EL: Emerging insights into
the complex genetics and pathophysiology of amyotrophic lateral
sclerosis. Lancet Neurol. 21:465–479. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Meng K, Jia H, Hou X, Zhu Z, Lu Y, Feng Y,
Feng J, Xia Y, Tan R, Cui F and Yuan J: Mitochondrial dysfunction
in neurodegenerative diseases: Mechanisms and corresponding
therapeutic strategies. Biomedicines. 13:3272025. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Suwara J and Hartman ML: Balancing between
cuproplasia and copper-dependent cell death: Molecular basis and
clinical implications of ATOX1 in cancer. J Exp Clin Cancer Res.
44:2222025. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Chen J, Jiang Y, Shi H, Peng Y, Fan X and
Li C: The molecular mechanisms of copper metabolism and its roles
in human diseases. Pflugers Arch. 472:1415–1429. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Ding C, Min J, Tan Y, Zheng L, Ma R, Zhao
R, Zhao H, Ding Q, Chen H and Huo D: Combating atherosclerosis with
chirality/phase dual-engineered nanozyme featuring
microenvironment-programmed senolytic and senomorphic actions. Adv
Mater. 36:e24013612024. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Lu Y, Fan X, Pan Q, He B and Pu Y: A
mitochondria-targeted anticancer copper dithiocarbamate amplifies
immunogenic cuproptosis and macrophage polarization. J Mater Chem
B. 12:2006–2014. 2024. View Article : Google Scholar : PubMed/NCBI
|