Population aging represents a significant social
challenge on a global scale. As human lifespans extend and
fertility rates continue to decline in developed nations,
projections indicate that by 2050, the global population of
individuals aged 65 years and older will reach 2.1 billion
(1). Among the most substantial
social burdens associated with aging is the prevalence of
neurodegenerative diseases (NDDs) (2). NDDs encompass a group of
neurological disorders characterized by the progressive
degeneration of neurons within the central nervous system (CNS)
(3), thereby adversely impacting
the quality of life and well-being of millions worldwide. The
incidence of NDDs increases exponentially with advancing age
(2). Current research indicates
that significant damage to the structure and function of neural
networks, coupled with extensive neuronal loss leading to
heterogeneity and disruption of neural circuit integrity, are key
characteristics of NDDs (3-5).
Nevertheless, due to the intricate biological mechanisms underlying
NDDs and the challenges associated with developing targeted
pharmacological interventions, effective treatment of these
disorders remains a formidable challenge (6,7).
Studies have shown that pathological protein
aggregation, synaptic and neuronal network dysfunction, protein
homeostasis abnormalities, cytoskeletal abnormalities, energy
metabolism changes, DNA and RNA defects, and inflammation are
typical features of NDDs such as Alzheimer's disease (AD),
Parkinson's disease (PD), primary tauopathies, frontotemporal
dementia and amyotrophic lateral sclerosis (ALS) (3). Neuroinflammation is a significant
pathological marker of NDDs, and the activation of
neuroinflammatory signals plays a clear role in neurodegeneration
(8).
The interaction between astrocytes and both resident
and infiltrating cells within the CNS is pivotal in modulating
tissue states under both physiological and pathological conditions.
Research has demonstrated that astrocytes are responsive to
inflammatory signals and can promote inflammation (9,10), thereby regulating various life
processes within the nervous system under these conditions.
Importantly, the pathological microenvironment of the nervous
system can activate astrocytes (11-13), which are essential for preserving
neuronal health and function. Recent studies have indicated that
astrocytes provide critical support to neurons (14), and their phenotypic
transformation is intricately linked to the onset and progression
of NDDs (15). Further
investigations have revealed that astrocytes are involved not only
in the formation of synaptic networks but also in maintaining
glutamate homeostasis between astrocytes and neurons (16,17), a balance crucial for normal
neuronal function. Furthermore, reactive astrocytes are
characteristically activated in NDDs, interacting with various
types of neurons and thereby accelerating the progression of these
disorders. Research has demonstrated that reactive astrocytes have
a substantial impact on synaptic function, energy homeostasis,
protein aggregation and neurodegeneration (18). The rapid advancements in
single-cell sequencing, flow cytometry sorting and metabolomics
have progressively illuminated the critical role of astrocytes in
the risk association of NDDs. However, the precise biological
mechanisms underlying these associations have yet to be fully
elucidated.
Lipid metabolism constitutes a fundamental aspect of
the brain's metabolic processes, with fatty acids serving as the
primary substrates in this context (19-23). LDs function as essential
organelles for the storage of fatty acids and play critical roles
in inflammation, metabolic disorders and cellular communication
(24,25). While the biological functions of
LDs have been extensively investigated in peripheral organs such as
the liver and heart, their roles within the brain, a central organ,
have received comparatively less attention (26). Recently, there has been a growing
recognition among researchers regarding the presence and
significance of LDs as dynamic organelles within the CNS (25,26). LDs are found in various neural
cell types, with a particularly high concentration in astrocytes
(27,28). Nonetheless, the implications of
abnormal astrocytic LDs in the onset and progression of NDDs, as
well as the specific mechanisms involved, remain inadequately
elucidated. This article aims to synthesize and discuss the
functions of astrocytic LDs and the impact of LD abnormalities on
the initiation and progression of NDDs, with the objective of
exploring potential therapeutic strategies and innovative research
directions focused on LDs.
The CNS exhibits considerable cellular
heterogeneity. Its cellular composition encompasses not only
neurons but also a diverse array of glial cells, including
astrocytes, oligodendrocytes, ependymal cells and peripheral glial
cells. Among these, astrocytes are the most prevalent glial cell
type within the CNS, fulfilling essential functions such as
supporting and nourishing neurons (29,30), regulating the integrity of the
blood-brain barrier (BBB) (31),
maintaining the homeostasis of the cellular microenvironment
(32) and mediating immune
responses (33). Recent
investigations have elucidated the heterogeneity among astrocytes,
with single-cell RNA sequencing technology identifying
multitudinous transcriptionally distinct astrocyte states,
including ATPase Na+/K+ transporting subunit
β 2 astrocytes, S100 calcium binding protein A4 astrocytes, G
protein-coupled receptor 84 astrocytes, complement C3/G0/G1 switch
2 astrocytes, glial fibrillary acidic protein/transmembrane 4L6
family member 1 astrocytes, glutathione synthetase/crystallin αB
astrocytes (30,34). In individuals with temporal lobe
epilepsy, as well as in murine models of epilepsy, there is a
notable accumulation of lipids within astrocytes, resulting in the
emergence of lipid-accumulating reactive astrocytes, termed LARAs.
This newly identified subtype of reactive astrocytes is
distinguished by an upregulation of apolipoprotein E (APOE)
expression. Reactive astrocytes are prevalent in a range of human
NDDs, providing valuable insights and potential research pathways
for targeting lipid metabolism in specific astrocyte subtypes
during the development and treatment of NDDs (35). Research suggests that the various
astrocyte subtypes are not only integral to the maintenance of
nervous system homeostasis but also help to find new astrocyte
subtypes as a target for CNS repair (30,34).
Astrocytes play a crucial role in the construction
of neural circuits by engaging in calcium signaling responses
(36), neurotransmitter reuptake
(37,38), maintenance of ion (39) and inorganic phosphate homeostasis
(40), and involvement in
synaptic formation and elimination (41). The membranes of astrocytes are
enriched with glutamate transporters, such as glutamate transporter
1 (42) and glutamate-aspartate
transporter (43), which
facilitate the rapid clearance of glutamate from the synaptic cleft
following neurotransmission, effectively preventing
glutamate-induced excitotoxicity and providing metabolic and
nutritional support to neurons. Research indicates that this
adaptive inflammatory response of astrocytes encompasses both
anti-inflammatory and pro-inflammatory effects (44). In the initial phase of infection,
characterized by acute inflammatory injury, anti-inflammatory
cytokines such as transforming growth factor-β and interleukin 10
(IL-10) play a protective role by mitigating inflammation and
safeguarding neuronal integrity. Conversely, during the chronic
phase of infection, the extensive release of pro-inflammatory
cytokines, including tumor necrosis factor-α (TNF-α) and IL-1β,
significantly contributes to neuronal dysfunction and the
advancement of NDDs. Studies have demonstrated that IL-1β
stimulates over-activated astrocytes to secrete vascular
endothelial growth factor, which increases the permeability of the
BBB and exacerbates inflammation (45).
The intricate coordination between astrocytes and
neurons is essential for the functionality of the CNS. Empirical
evidence indicates that astrocyte-neuron coupling not only
furnishes metabolic support to neurons (46), but also modulates the ionic
milieu of the neuronal microenvironment (47) and facilitates the release and
transmission of synaptic vesicles. Concurrently, under
physiological conditions, astrocytes synthesize and release various
lipids while facilitating their transport to neurons, thereby
supporting synaptic formation and function (48,49). In the context of brain injury and
NDDs, the activation of astrocytes and the presence of aberrant
inflammatory responses can profoundly impact neuronal function and
survival, leading to disruptions in neural network
connectivity.
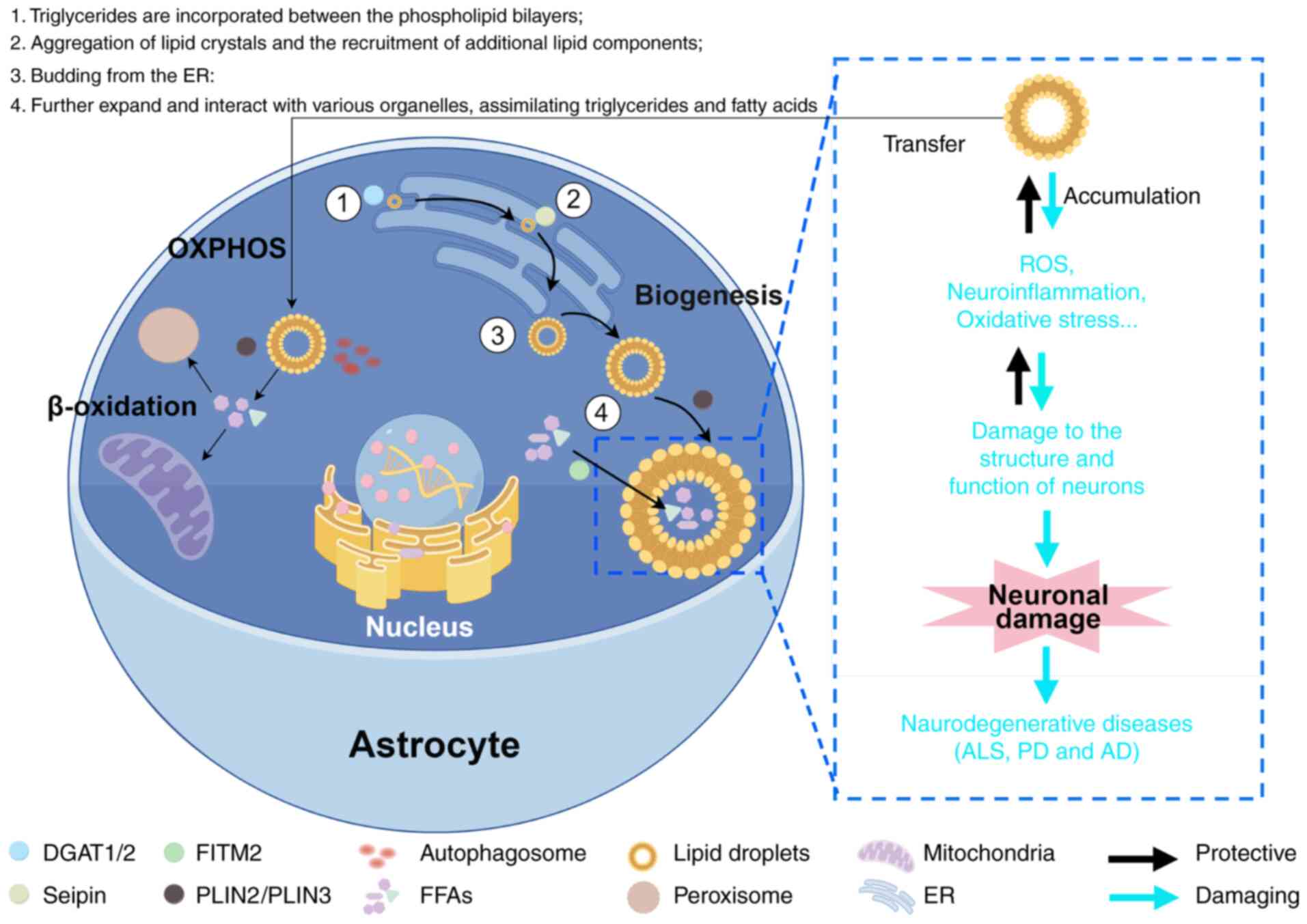
LDs serve as the primary organelles for lipid
metabolism and storage within cells, encapsulated by a monolayer of
phospholipid membrane. These organelles predominantly consist of
neutral lipids and phospholipids. LDs function as energy storage
entities, playing a crucial role in lipid metabolism and energy
homeostasis. They are ubiquitously present across various cell
types, with a pronounced presence in adipocytes, where they occupy
a significant portion of the cytoplasmic volume and exhibit a
unique organelle architecture. Notably, LDs are the sole organelles
characterized by a core of hydrophobic neutral lipids enveloped by
a phospholipid monolayer. The core is primarily composed of
triglycerides and a minor fraction of cholesterol esters. Research
indicates that the expansion of LDs involves the lipid transport
facilitated by APOE and the accumulation and budding of newly
synthesized neutral lipids at the endoplasmic reticulum (ER)
(27). The budding process of
LDs is regulated by alterations in the contact angle between the ER
and LDs, as well as the asymmetry in monolayer tension. LDs
originate in the ER, where acetyl-CoA and glycerol 3-phosphate
facilitate the synthesis of triglycerides (50). Initially, triglycerides are
incorporated between the phospholipid bilayers, subsequently
promoting the localized aggregation of lipid crystals and the
recruitment of additional lipid components, thereby facilitating
the expansion of LDs formation. Upon budding from the ER, LDs
migrate into the cytoplasm, where they can further expand and
interact with various organelles, assimilating triglycerides and
fatty acids, which contributes to their volumetric increase
(51). The elevation of neutral
lipid levels within the ER enhances LD formation, serving to
sequester potentially harmful free fatty acids (FFAs) and mitigate
lipotoxicity (52). Cellular
stress can trigger LD biogenesis, stimulating lipid synthesis,
autophagy, lysosomal phospholipid conversion or an increase in
phospholipase activity, thereby modulating LD formation and
maintaining intracellular lipid homeostasis (Fig. 1).
Proteins associated with LDs are essential for their
functional roles. These proteins are transported to LDs via
distinct mechanisms, influencing the development, maturation and
degradation of LDs. Class I proteins are directly targeted to LDs
from the ER. Examples of Class I LD proteins include the lipid
biosynthesis enzymes glycerol-3-phosphate acyltransferase 4 and
diacylglycerol O-acyltransferase 2 (DGAT2), caveolin-1 and
caveolin-2 (53). An example is
ceramide - the production of acylated ceramides is catalyzed by the
key lipid-synthesizing enzyme DGAT2 on lipid droplets. It relies on
its N-terminal hydrophobic domain to embed itself in the ER
membrane, ensuring stable localization to LDs and efficient
catalysis of triglyceride synthesis, thereby targeting biogenesis
to LDs. By contrast, Class II proteins are directed to the surface
of LDs from the cytoplasm through amphipathic helices or extended
hydrophobic residues. The recruitment of these proteins by LDs is
modulated by developmental requirements or the metabolic state of
the cell. Specific proteins, including recombinant perilipin 3
(PLIN3), reticulon 4 (RTN4), cytoskeletal linker protein 7,
receptor expression-enhancing protein 5, Seipin and peroxisome
biogenesis factor 30 (PEX30), are integral to LD formation. DGAT1/2
catalyzes the final step of triglyceride synthesis and marks the
initiation point of LD biogenesis (54); Seipin promotes the initial
aggregation of neutral lipids through tissue-specific ER domains
and ensures the proper morphology and size of nascent LDs (55); Fat storage-inducing transmembrane
protein 2 facilitates the transfer of neutral lipids into
developing LDs, promoting LD expansion (56). Notably, Seipin influences the
budding and formation of LDs through its impact on the levels of
phosphatidylcholine, phosphatidylinositol and diacylglycerol (DAG).
Newly formed LDs bud from the ER and require the core regulatory
actions of PLIN family proteins to develop into fully functional
LDs. The cell death-inducing DFF45-like effector family proteins
serve as key regulators of LD growth and fusion. They mediate the
directed transfer of neutral lipids from small to large LDs by
forming bridging structures between adjacent LDs, thereby promoting
LD growth and fusion (57). The
PLIN family members (PLIN1 to PLIN5) are representative proteins
found on the surface of LDs, classified as type II proteins that
regulate interactions between LDs and the cytoplasmic environment.
Each PLIN family member exhibits unique expression patterns and
possesses distinct fundamental functions (58). PLIN4 possesses the ability to
interact with the LD membrane and establish a protective barrier,
thereby shielding LDs from degradation by lipases. PLIN3
contributes to the stability and metabolic processes of LDs
(59), while RTN4 is implicated
in their formation and localization. Cytoskeletal linker protein 7
potentially regulates LD formation by modulating the cytoskeletal
architecture. Additionally, PEX30 facilitates LDs formation by
modulating the ER domain (60).
The brain is the organ with the highest cholesterol
content in the body, and maintaining cholesterol homeostasis is
essential for proper brain function. A deficiency in cholesterol
can impair memory formation, while excessive cholesterol
accumulation can damage neuronal synaptic plasticity and induce
neuronal apoptosis (61).
Although cholesterol in the brain is synthesized locally, the
capacity for cholesterol synthesis varies among different cell
types. Astrocytes, for instance, possess a greater ability to
synthesize cholesterol from acetyl-CoA compared to neurons, which
have lower levels of cholesterol synthesis enzymes (62). Astrocytes can supply cholesterol
to surrounding neurons. Research has identified key cholesterol
transporters, including APOE (63) and members of the ATP-binding
cassette transporter family, such as ATP-binding cassette subfamily
A member 1 (ABCA1) (64) and
ABCG4 (65). Astrocytes
facilitate cholesterol transport by synthesizing lipoproteins and
apolipoproteins. Research indicates that astrocytes secrete
cholesterol-rich lipoproteins containing APOE, primarily mediating
neuronal lipid uptake through endocytosis facilitated by
low-density lipoprotein receptor (LDLR) and LDLR-related protein 1
(LRP1) co-receptors. Different APOE homozygous expression states
also determine the efficiency and specificity of lipid shuttles.
Phospholipid-rich, small high-density lipoprotein particles are
readily recognized by APOE2 and APOE3, whereas APOE4 more
frequently binds to triglyceride-rich large very-LDL particles
(66). The presence of the BBB
renders cholesterol metabolism in the brain relatively independent
from systemic cholesterol metabolism. Lipoproteins are exclusively
synthesized within the CNS, where cholesterol sourced from
astrocytes is crucial for the formation of neuronal membranes and
synapses. This astrocytic cholesterol is instrumental in modulating
various biochemical processes within the synaptic membrane, such as
membrane fluidity and ion channel function (67). Furthermore, evidence from
macrophage-specific ABCA1-knockout mice demonstrates that
cholesterol significantly influences multiple regulatory
mechanisms, including membrane protein activity and localization,
lipid raft formation, glucose transport and inflammatory signaling
(61,68). Lipid rafts, which are membrane
microdomains enriched with cholesterol and sphingolipids, are
recognized as pivotal regulators of numerous membrane protein
activities (69). The activation
of pro-inflammatory receptors, such as Toll-like receptor 4 (TLR4),
predominantly occurs within these cholesterol-rich membrane
regions. Cholesterol also impacts the membrane transport and
functionality of the glucose transporter glucose transporter 1
(70). In comparison to the
APOE3 isoform, APOE4 demonstrates reduced efficacy in inhibiting
neuronal cholesterol synthesis and facilitating
acetylation-mediated memory formation (63).
Astrocyte-secreted lipid factors function as
signaling molecules that significantly impact neuronal
proliferation, migration and synapse formation (71). The sterol regulatory
element-binding protein (SREBP) family, particularly SREBP2, is
integral to lipid metabolism, as it regulates the transcription of
numerous genes involved in sterol synthesis (72). Presently, it is yet to be
determined and confirmed whether astrocyte-derived effectors can be
transported to neurons and subsequently influence memory formation.
Furthermore, the degree to which astrocytes adapt to neuronal
demands and sustain brain cholesterol homeostasis warrants further
investigation.
Sphingolipids, a class of lipids containing
sphingosine, encompass ceramides and sphingomyelins and serve as
essential components of cellular membranes, including lipid rafts.
Metabolites derived from sphingolipids have been recognized as
significant regulators of inflammation, autophagy, cellular growth
and survival (73). Notably,
ceramides, which can be further modified to form gangliosides, are
implicated in the pathogenesis of NDDs (74). Given that de novo
synthesis of sphingolipids also occurs in the ER, recent research
has demonstrated that ceramides can be converted into
acylglyceramide through the action of DAG O-acyltransferase and
subsequently stored in LDs (75). Sphingolipids fulfill multiple
roles in astrocyte function, particularly in the context of NDDs,
where alterations in their structure and metabolism are observed.
Astrocytes modulate synaptic architecture and neuronal
functionality through the regulation of sphingolipids, including
the production of gangliosides. In the context of AD, membrane
gangliosides engage in interactions with amyloid proteins, thereby
facilitating plaque formation and influencing the fibrillation
process of α-synuclein (76).
This conclusion has been validated in vitro using human
SH-SY5Y cells and membrane liposome models (77). The aggregation of gangliosides,
mediated by antibodies, has the potential to activate signaling
pathways that suppress neuronal growth. Gangliosides synthesized by
astrocytes contribute to the enhancement of neurite outgrowth, the
regulation of neuronal inflammation and the stabilization of
neuron-astrocyte interactions (78).
Phospholipids constitute essential components of
cellular membranes, forming phospholipid bilayers that are vital
for maintaining neuronal membrane fluidity, facilitating signal
transduction and supporting synaptic function. The brain
synthesizes substantial quantities of phospholipids, including
phosphatidylcholine, phosphatidylserine, phosphatidylethanolamine
and phosphatidylinositol. These phospholipids are conveyed to
neurons via vesicle and protein-mediated transport systems, thereby
influencing neuronal fate (71).
Empirical studies have demonstrated that phosphatidylethanolamine
promotes the differentiation of nerve cells into astrocytes by
interacting with phosphatidylethanolamine binding protein 1 through
the mitogen-activated protein kinase (MAPK)/extracellular
signal-regulated kinase signaling pathway, whereas
phosphatidylcholine exerts an antagonistic effect (79,80).
Neurons possess a restricted capacity for fatty acid
oxidation. Together, neurons and astrocytes constitute a critical
coupled unit for cerebral energy metabolism. Astrocytes supply
metabolic substrates and antioxidant factors to neurons, thereby
enhancing the efficiency of neuronal information transmission
(81). By storing and releasing
lipids via LDs, astrocytes fulfill the metabolic demands of
neurons, which is essential for neuronal function and the
maintenance of brain homeostasis. Lipid metabolism represents a
significant aspect of the interaction between neurons and
astrocytes. Research indicates that lipids recycled from neurons
are transferred to astrocytes, where they are stored in LDs prior
to mitochondrial metabolism (82).
The accumulation of neutral lipids in astrocytes
primarily occurs through two pathways. Firstly, activated neurons
produce FFAs, which are subsequently secreted as APOE4 lipid
particles. These particles are internalized by neighboring
astrocytes via endocytosis and are stored in LDs. Docosahexaenoic
acid (DHA) is an unsaturated fatty acid with neuroprotective
effects on cognitive function and memory, and its reduced levels
are associated with the progression of NDDs (83). In the brain, DHA exists in two
forms: Phospholipid-bound and free. The major facilitator
superfamily domain containing 2A (MFSD2A) is exclusively present in
the endothelial cells forming the BBB and transports
phospholipid-bound DHA in the form of lysophosphatidylcholine (LPC)
in a sodium-dependent manner, enabling its passage across the BBB
into the brain. This process is critical for maintaining brain
lipid composition (84). FFAs
bind to cell membrane proteins, such as the MFSD2A protein that
transports omega-3 DHA into the brain as LPC-DHA (84), and plasma membrane fatty
acid-binding proteins (FABPs) located on the BBB endothelial cell
membrane, which promotes the uptake and activation of long-chain
fatty acids and CD36 (85,86), which mediates cellular uptake of
various lipids including long-chain fatty acids, enabling their
passage through the BBB into cells. Once inside the body, fatty
acids form complexes with FABP7, facilitating their transport from
the cell membrane to various metabolic sites within the cell.
Although FABP3 and FABP5 are present, FABP7 is the predominant
isoenzyme in the brain and is crucial for the regulation of
dendritic morphology and synaptic transmission in neurons (87). Furthermore, in the
astrocyte-specific carnitine palmitoyltransferase-1 (CPT-1) A
knockout mouse model, astrocytes exhibit preferential expression of
CPT-1, an essential enzyme in the β-oxidation of long-chain fatty
acids, thereby providing energy for cellular functions (88). LPC-DHA differs from
non-esterified DHA transport mediated by fatty acid transport
protein 1 and cellular metabolic pathways mediated by CD36 as a
long-chain free fatty acid transporter (89). Lipids acquired through these
distinct pathways can be utilized in astrocytes for energy
production, membrane synthesis or storage within LDs, thereby
participating in the brain-wide lipid distribution network.
The formation of LDs is integral to the regulation
of fatty acid storage and hydrolysis, as well as providing
substrates for β-oxidation, thereby playing a pivotal role in lipid
metabolism. Under conditions of stress, such as starvation,
β-oxidation of LDs serves as an alternative energy generation
pathway for various cell types, including astrocytes. Research has
highlighted the significance of oxidative phosphorylation (OXPHOS)
activity in astrocytes for fatty acid degradation and the
maintenance of lipid homeostasis in the brain. A deficiency in
OXPHOS activity within astrocytes not only leads to the
accumulation of LDs but also precipitates AD-like pathologies,
including synaptic loss, neuroinflammation, demyelination and
cognitive dysfunction (82).
When the fatty acid load surpasses the OXPHOS capacity of
astrocytes, there is an increase in acetyl-CoA levels, which
subsequently promotes the acetylation and activation of signal
transducer and activator of transcription 3 (82). Following lipid overload, these
abnormally activated astrocytes will stimulate neuronal fatty acid
oxidation and oxidative stress responses, which in turn activate
microglia through IL-3 signaling and start inflammatory signaling
cascades (82). Research has
established that the formation of LDs can subsequently impact
mitochondrial function. For instance, astrocytes exposed to lipid
mixtures can initiate the formation of intracellular LDs, which
results in a reduction of mitochondrial membrane potential and an
increase in mature apoptotic inducible factor levels, culminating
in mitochondrial dysfunction (90). The defects in OXPHOS and the
accumulation of LDs exhibit a mutually reinforcing and synergistic
relationship. Further investigations have demonstrated that OXPHOS
defects contribute to abnormal LD accumulation and trigger NDDs
(91). A targeted knockout of
the key OXPHOS gene, mitochondrial transcription factor A, in
astrocytes has shown significantly elevated levels of FFAs,
triacylglycerols, monoglycerides and cholesterol esters in the
brains of mice, accompanied by progressive neurodegeneration
(82). This study confirmed that
mitochondrial dysfunction impairs astrocytic energy metabolism,
with a pronounced enrichment in lipid metabolism pathways.
Consequently, this leads to abnormal LD accumulation and drives
astrocyte-mediated neurotoxicity.
LDs form tight physical connections with
mitochondria and peroxisomes via membrane contact sites, actively
participating in and regulating the network hub of lipid
trafficking. In normal astrocytes, LDs typically cluster near the
nucleus and reside adjacent to mitochondria and the ER. Through
direct connections, these droplets create microenvironments that
direct fatty acid transport to mitochondria. The key mechanism
involves efficient flow from the LD core to mitochondria for
β-oxidation, rapidly generating ATP to fuel astrocyte
energy-consuming processes (28). Very long-chain fatty acids
(VLCFAs), which mitochondria cannot directly β-oxidize, are
primarily degraded by peroxisomes. Research indicates peroxisomes
play a vital role in the oxidation and degradation of long-chain
and VLCFAs, contributing significantly to reactive oxygen species
(ROS) production and clearance, thereby helping maintain cellular
redox balance. VLCFAs require preliminary oxidation within
peroxisomes before entering mitochondria for β-oxidation, a process
facilitated by the LD-peroxisome contact site (92).
In the CNS, neurons rely on autophagy to maintain
synaptic neurotransmission and prevent neurodegeneration (93). Autophagosomes selectively engulf
LDs to promote lysosomal degradation-a process termed lipophagy.
During energy deficiency, phagocytes ensure LD degradation through
this pathway, which is crucial for lipid and energy homeostasis
(94). During lipophagy, a
double-membrane autophagosome engulfs LDs or partial LDs and fuses
with lysosomes containing acid hydrolases, degrading LDs into FFAs
(95). Studies in PD models
reveal that pathological progression correlates with the
PLIN4/LD/mitochondrial autophagy axis, where PLIN4 upregulation
leads to abnormal LD accumulation (96).
Enzymatic lipolysis is the primary mechanism for
fatty acid mobilization. Research indicates that adipose
triglyceride lipase (ATGL) and hormone-sensitive lipase (HSL) are
two key enzymes in lipid mobilization. ATGL hydrolyzes the first
ester bond to produce DAG and fatty acids, followed by HSL
converting DAG into monoglyceride and fatty acids (97). Modulating ATGL expression alters
LD mobilization in cells and tissues (98). Studies reveal that ATGL promotes
LD accumulation in mouse microglia and suppresses
lipopolysaccharides (LPS)-induced neuroinflammation (99). Enzyme-controlled lipolysis is
tightly coupled with the OXPHOS capacity of astrocytes. Research
indicates that knocking out carnitine palmitoyltransferase-1A (a
key enzyme in mitochondrial fatty acid oxidation) in adult mouse
astrocytes inhibits fatty acid oxidation and alters the brain's
metabolomic profile, leading to cognitive impairment (100). Against a backdrop of heightened
synaptic activity, efficient lipolysis provides substrates for
OXPHOS, ensuring astrocytes effectively support neuronal
function.
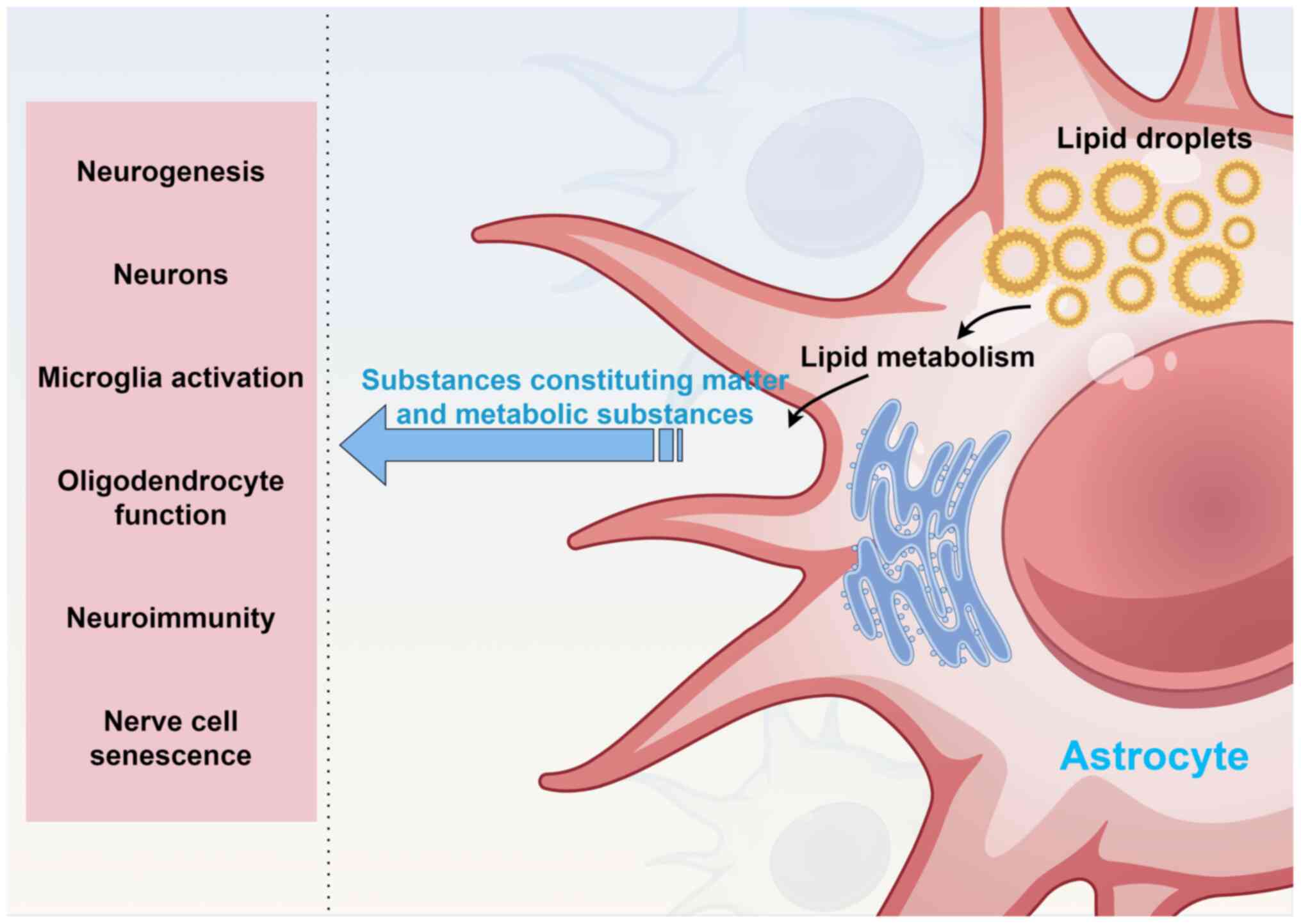
Astrocyte LDs influence numerous neurobiological
processes through lipid metabolism, such as neurogenesis, neuronal
structure and function, microglial activation, oligodendrocyte
function, neuroimmunity and nerve cell senescence (Fig. 2).
Astrocytes play a critical role in regulating
neuronal metabolism and maintaining brain homeostasis through the
storage and release of lipids via LDs. Their lipid metabolism is
essential for supporting energy production, cell membrane synthesis
and neuronal repair, as well as modulating neuronal activity, which
in turn influences neurogenesis (101). Studies using a mouse model of
middle cerebral artery occlusion indicate that the overexpression
of endothelin-1 (ET-1) in astrocytes within the murine brain can
adversely affect hippocampal neurogenesis following cerebral
ischemic injury, with peroxiredoxin 6 implicated in this process
(102). Nonetheless, the impact
of ET-1 overexpression in astrocytes on lipid metabolism and the
underlying pathways requires further elucidation. Given that
neurons possess a limited capacity for lipid synthesis during
development, the provision of lipids by astrocytes is vital for
neuronal growth and differentiation. Lipids, including cholesterol
and fatty acids, are indispensable for the formation of neuronal
membranes and synapses, and lipid factors secreted by astrocytes
also influence neuronal proliferation and migration.
Lipid metabolism is intricately regulated by the
PLINs and SREBPs, which subsequently influence the formation and
functionality of LDs (103,104). Regulatory factors of lipolysis,
such as PLINs, modulate the lipolytic process by governing the
recruitment of lipases and their cofactors to the surface of LDs.
For instance, PLIN1 acts as an inhibitor of lipolysis, and in a
chronic PD mouse model induced by
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine combined with
probenecid, PLIN4 was confirmed to promote the formation of LDs
(105,106). Transcription factors, including
the SREBP family, play a crucial role in regulating the expression
of genes involved in lipid synthesis. The depletion of
astrocyte-specific SREBP2 has significant implications for brain
development and function, manifesting in reduced synaptic growth of
neurons and a decrease in overall brain size and weight (107).
The coordination of lipid metabolism between neurons
and glial cells is essential for the proper functioning of the
nervous system. Astrocytes play a pivotal role in this process by
storing and releasing lipids through LDs, thereby supplying lipids
necessary for energy production, cell membrane synthesis and repair
in neurons. Additionally, these lipids serve as signaling molecules
that modulate neuronal activity. Research has demonstrated that
regulating neuronal glycerolipid metabolism by enhancing the
synthesis of phospholipids, which are integral to cell membrane
composition, while concurrently reducing the synthesis of storage
lipids such as triglycerides, can facilitate the regeneration of
nerve axons following injury (108).
Recent research has increasingly elucidated the
critical role of cholesterol and phospholipids supplied by
astrocytes in neuronal synapse formation and axon growth (109). Initial investigations
established that cholesterol derived from glial cells, secreted by
astrocytes and transported via the lipoprotein APOE, serves as a
potent cofactor in the induction of retinal ganglion cell synapse
formation (110). Subsequent
studies identified additional astrocyte-secreted proteins,
including thrombospondin ½ (111), hevin (112) and glypicans (113), which facilitate the formation
of excitatory synapses and enhance glutamatergic activity. However,
the mechanisms by which developing astrocytes regulate axon growth
remain less understood. Recent investigations have revealed that
astrocyte-derived exosomes, particularly the hepatic and glial cell
adhesion molecule signal present on their surface, play a
developmental role in modulating the axon growth of cortical
pyramidal neurons (114). APOE,
the primary cholesterol transporter secreted by astrocytes, plays a
crucial role in promoting synaptogenesis while markedly inhibiting
the stimulatory impact of astrocyte-derived exosomes on axonal
growth (114). Notably, a
deficiency in APOE during developmental stages has been indicated
to lead to a significant reduction in the spine density of cortical
pyramidal neurons (114). This
study suggests that astrocyte exosomes, through their surface
contact mechanisms, work in opposition to APOE to collectively
regulate axonal growth and dendritic spine formation in pyramidal
neurons during the early postnatal period (114). Consequently, astrocytes are
capable of transferring lipids to neurons via both direct and
indirect pathways, including the active transport of APOE and
lipoprotein particles.
Fatty acids constitute the fundamental building
blocks of all lipid types, rendering the precise regulation of
their metabolic balance in the brain essential for optimal lipid
functionality. Excessive FFA intake can lead to cellular
accumulation and subsequent damage (115); thus, mitigating excessive FFA
intake is an effective strategy to avert lipid toxicity. Neurons
facilitate the release of FFAs via APOE particles, which are
subsequently internalized by astrocytes through endocytosis and
sequestered in LDs as triglycerides, thereby mitigating their
cytotoxic effects (116). Upon
entry into astrocytes, the FFAs released are assimilated by LDs and
utilized as substrates for mitochondrial β-oxidation. This process
enables mitochondria to catabolize stored fatty acids, thereby
generating energy and sustaining normal neurological function. The
formation of astrocytic LDs and the intercellular lipid transport
from neurons to glial cells are modulated by APOE levels.
Astrocytic APOE may play a regulatory role in lipid transfer, while
neuron-derived APOE is implicated in lipid transport during
oxidative stress, collectively contributing to the maintenance of
lipid metabolic equilibrium and neurological function in the brain
(117).
Microglia serve as the principal immune cells within
the CNS, playing a crucial role in the clearance of cellular debris
and the modulation of neuroinflammation. A substantial body of
research suggests that the activation of microglia and their
interactions with astrocytes significantly influence the
inflammatory processes associated with NDDs and brain injury
(9,118,119). Under stress conditions,
including oxidative stress, inflammation and aging, the phagocytic
activity and inflammatory responses of microglia may contribute to
LD accumulation in the brain. This process is predominantly
regulated by the TLR4 and nuclear factor κ-light-chain-enhancer of
nuclear factor κB (NF-κB) signaling pathways. LPS, functioning as
TLR4 ligands, initiate the TLR4 signaling cascade, subsequently
activating downstream signaling molecules such as myeloid
differentiation factor 88 and Toll/IL-1 receptor-domain-containing
adapter-inducing interferon (IFN)-β, and facilitating the formation
of LDs within microglia (120).
The substantial accumulation of LDs in microglia may result from
enhanced lipid synthesis coupled with diminished lipid catabolism.
Fatty acids stored in microglial LDs can be mobilized through the
enzymatic activity of two lipases, ATGL and HSL, when required for
stress responses, thereby contributing to metabolic processes or
inflammatory reactions (121).
Peroxisome proliferator-activated receptors (PPARs)
and NF-κB transcription factors are integral to lipid metabolism
and inflammatory responses. The activation of TLR4 can further
stimulate the NF-κB signaling pathway, resulting in substantial
production of inflammatory cytokines and the onset of
neuroinflammation. Research has identified a strong association
between the accumulation of LDs in microglia and the dysfunction of
the aging brain, as well as the establishment of a pro-inflammatory
state. Astrocytes promote phagocytic activity in microglia by
secreting cytokines. When microglia undergo metabolic changes
characterized by LD accumulation, phagocytosis becomes impaired.
This process is often regarded as an inflammatory marker involved
in NDDs, thereby accelerating neuronal degeneration (122). This accumulation is
significantly implicated in the pathogenesis of various
neuroinflammatory and NDDs. Evidence suggests that LD accumulation
in microglia within the aging mouse brain can induce the production
of ROS and the secretion of pro-inflammatory cytokines, thereby
contributing to neurodegeneration (122). In AD, both postmortem brain
tissue from patients with AD and AD mouse models demonstrate that
amyloid β (Aβ) deposition and hyperphosphorylated tau protein can
activate microglia, leading to chronic inflammation and
neurodegeneration (123). In
PD, studies using human induced pluripotent stem cell
(iPSC)-derived astrocyte models (carrying the APOE4 genotype) and
APOE-targeted replacement mouse brain tissue models reveal that the
degeneration of dopaminergic neurons activates microglia, which
release inflammatory mediators that further damage the remaining
neurons (124). Thus,
microglial activation is a critical factor in the development of
neuroinflammation and NDDs.
Lipid metabolism plays a crucial role in modulating
inflammation and neuroprotection by regulating LD formation and
fatty acid release. As research progresses, genome-wide
transcriptome analyses have elucidated that the human-specific
variant APOE4 disrupts lipid metabolism in astrocytes and microglia
(125). This disruption
elucidates how APOE4-induced dysregulation, both cell-autonomous
and non-cell-autonomous, may elevate the risk of AD. Studies have
demonstrated that the activation of microglia in a male rat spinal
cord injury model can stimulate astrocytes to secrete and release
increased levels of lipocalin 2 (LCN2), resulting in neuronal
damage and extensive neuronal loss within the CNS (126,127). Glial cells exhibit distinct
interactions in lipid metabolism. Aβ-exposed astrocytes secrete
LCN2, which activates the pro-inflammatory function of microglia
and further stimulates astrocytes to secrete and release higher
levels of LCN2 in a bidirectional manner (128). Research indicates that reducing
LCN2 levels can diminish the activation of neurotoxic α1-subtype
astrocytes, offering a more effective pathway for preventing
neuronal cell death (129).
Microglia bridge the gap between lipid dysregulation and
neurodegeneration in PD by sensing metabolic disturbances. Oxidized
lipids and glycosphingolipids activate microglia, which in turn
promote A1-type astrocyte activation through inflammatory cytokine
release, further accelerating PD progression (130). Therefore, modulating the lipid
metabolism interaction pathway between these cells holds promise
for achieving precise interventions against various NDDs.
Furthermore, another research group has highlighted the significant
role of BTB and CNC homolog 1 (BACH1) in maintaining metabolic
homeostasis in microglia during mouse brain development (131). BACH1 influences microglial
metabolism by inhibiting key glycolytic enzymes, hexokinase 2 and
glyceraldehyde 3-phosphate dehydrogenase, thereby reducing lactate
production. This metabolic alteration leads to a decrease in
lactate-dependent histone modifications at the leucine rich repeat
containing 15 (LRRC15) promoter region, consequently affecting the
expression of the LRRC15 gene. LRRC15 is a factor secreted by
microglia, which participates in the JAK/STAT signaling pathway by
binding to the CD248 receptor and regulates astrocyte generation
(131).
The brain primarily relies on glucose for its energy
requirements and lacks alternative forms of energy storage, except
for glycogen present in astrocytes. During conditions such as
starvation, the brain can utilize ketone bodies (KB), which are
derived from the breakdown of fatty acids in the liver, to meet the
energy demands of central neurons. Recent research has identified
that cortical glial cells in the fruit fly brain can synthesize KB
from their own stored LDs and subsequently transport these to
neurons via monocarboxylate transporters for energy provision
(132). Oligodendrocytes, in
addition to their role in myelin formation, contribute to the
energy metabolism of axons by supplying pyruvate and lactate. A
recent study highlighted that the interaction between astrocytes
and oligodendrocytes is crucial for myelin regeneration (133). Persistent activation of the
nuclear factor erythroid 2-related factor 2 (Nrf2) pathway in
astrocytes has been shown to inhibit the cholesterol biosynthesis
pathway, consequently impairing myelin regeneration. The experiment
demonstrated in a mouse model of focal demyelination that
activation of Nrf2 in astrocytes results in the suppression of the
cholesterol biosynthesis pathway, consequently impairing myelin
regeneration (133). In the
CNS, astrocytes and oligodendrocytes collaborate, particularly in
lipid metabolism and the maintenance of myelin, to ensure efficient
nerve conduction. Astrocytes supply oligodendrocytes with essential
lipids, including cholesterol and phospholipids, which are crucial
for myelination. Astrocytes supply lipids to oligodendrocytes via
an SREBP2-dependent pathway to regulate myelin
maintenance/regeneration. Under the regulation of the key
transcription factor SREBP2, astrocytes synthesize large amounts of
cholesterol through their own mevalonate pathway and transport it
to oligodendrocytes via lipoproteins such as APOE. Research
confirms that SREBP controls gene transcription in the cholesterol
biosynthesis pathway, enhancing cholesterol synthesis when SREBP2
is overexpressed (134). By
contrast, phospholipids and fatty acids are conveyed through
vesicular and protein-mediated mechanisms, as well as via
lipoproteins and FABPs. Dysregulation of lipid metabolism can lead
to myelin damage and neurodegeneration (135).
The heterogeneity of astrocyte responses in CNS
pathology involves the roles of astrocytes and recruited peripheral
cells in facilitating disease progression. Research indicates that
in postmortem brain tissue from patients with ischemic stroke and
in mouse models of transient focal cerebral ischemia, astrocytes
express IL-15 upon stimulation. This factor recruits CD8+ T cells
during brain injury and enhances their effector functions (136). Notably, the interaction between
astrocytes and T cells is reciprocal; T cells can also influence
astrocyte responses. Astrocytes express receptors for IL-17 and
granulocyte-macrophage colony-stimulating factor (GM-CSF),
cytokines produced by pro-inflammatory T helper 17 (Th17) cells,
which are implicated in the pathologies of multiple sclerosis (MS),
experimental autoimmune encephalomyelitis (EAE), and other
diseases. Initial findings from in vivo mouse experimental
autoimmune EAE models and in vitro primary mouse astrocyte
models suggested that IL-17 derived from Th17 cells upregulates the
production of pro-inflammatory cytokines GM-CSF and C-X-C motif
chemokine ligand 1, C-X-C motif chemokine ligand 2 and C-C motif
chemokine ligand 20 in an NF-κB activator 1-dependent manner,
thereby promoting leukocyte recruitment to the CNS during EAE
(137,138). Recently, it has been reported
that IFN-γ produced by natural killer (NK) cells can induce
TNF-related apoptosis-inducing ligand expression in syngeneic
astrocytes, enabling pro-inflammatory T cells expressing death
receptor 5 to undergo apoptosis (137,139). Notably, NK cells acquire the
ability to produce IFN-γ in intestinal tissues through the
meningeal circulation in response to signals provided by the
commensal microbiota (140).
These findings identify mechanisms of the gut-CNS axis for
controlling inflammation, which not only reveal the potential role
of the microbiota in the pathologies of neurological diseases but
also provide promising targets for therapeutic intervention.
Astrocyte-derived lipid metabolites play a crucial
role in modulating the activation and differentiation of immune
cells, including T cells and B cells, and in safeguarding neurons
against oxidative stress by activating antioxidant pathways, such
as the Nrf2 signaling pathway. Empirical evidence indicates that
disruptions in astrocytic lipid metabolism can initiate neuroimmune
responses and contribute to the pathogenesis of NDDs (82). Notably, through specific knockout
of genes encoding key components of OXPHOS mitochondrial
dysfunction in mouse astrocytes results in the abnormal
accumulation of LDs within the hippocampus, which subsequently
activates both astrocytes and microglia, thereby eliciting a
neuroimmune response (82). This
aberration in lipid metabolism has also been observed in AD model
mice, implying a potential link between lipid metabolic
disturbances in AD and dysfunctional mitochondrial activity in
astrocytes.
Cell senescence refers to the state where cells
enter an irreversible non-dividing state after undergoing a certain
number of divisions, preventing the excessive proliferation of
damaged cells and inhibiting the occurrence of pathological
reactions (141) such as
cancer. Certain studies have found that glial cells with high
activating protein 1 (AP-1) activity have abnormal morphology,
produce matrix metalloproteinases and promote tau-related aging
pathological reactions (20,142). However, the mechanism of
senescent glial cell generation and how they affect neuronal
senescence remain elusive.
Recent studies have elucidated the origin of
senescent glial cells characterized by abnormally elevated AP-1
activity and their influence on neuronal aging (20,143). It has been discovered that
mitochondrial dysfunction in neurons initiates the formation of
senescent glial cells, which subsequently induce the excessive
accumulation of LDs in non-senescent glial cells. Under chronic
oxidative stress conditions (such as persistent mitochondrial
damage), the excessive accumulation of LDs observed in AD mouse
models becomes maladaptive, impairing phagocytic function and
promoting tau protein aggregation. Chronic inflammation and lipid
metabolism disorders activate inflammatory signaling pathways, such
as NF-κB and MAPK, thereby enhancing the production of inflammatory
mediators and exacerbating neuronal aging. In addition, lipid
metabolites adversely affect mitochondrial function, resulting in
dysfunction and further accelerating neuronal aging.
Abnormal LDs in astrocytes are critical initiators
of NDDs, including AD, PD and ALS. In AD, dysregulated lipid
metabolism impairs Aβ clearance and inflammatory responses, leading
to Aβ accumulation, neuroinflammation and increased neuronal injury
(115). In PD, impaired lipid
metabolism disrupts dopaminergic neurons, accelerating disease
progression. Similarly, in ALS, studies of postmortem spinal cord
tissue from patients and ALS mouse models reveal that astrocyte
dysmetabolism contributes to inflammation and neuronal death, with
aberrant LDs accumulation enhancing ROS production and exacerbating
neuronal damage (144). The
accumulation of glial cell LDs is a prominent feature in motor
neuron diseases such as ALS and may be prevalent across various
NDDs (145). Astrocytic lipid
metabolism is intricately linked to neuronal function, and
oxidative stress can induce LD formation, thereby accelerating
neurodegenerative processes. Dysregulation of lipid metabolism in
astrocytes has been implicated in neuropsychiatric disorders,
affecting neuronal activity regulation, neurotransmitter release
and synaptic pruning processes. Astrocytes facilitate synaptic
pruning through interactions with microglia during developmental
stages. For instance, astrocytes promote synaptic pruning during
development through an IL-33-mediated mechanism related to
microglia-dependent synaptic pruning (146,147). In addition, the complement
produced by astrocytes in an acute epilepsy mouse model has been
shown to trigger microglia-dependent pruning to reshape neural
circuits (148) (Fig. 1).
The formation of LDs in the brains of AD mouse
models is intricately linked to processes such as inflammation,
oxidative stress and aging. A reduction in the OXPHOS capacity of
astrocytes adversely affects the lipid distribution, leading to
issues such as synaptic loss, altered astrocyte reactivity and
heightened oxidative stress (82). These factors are critical
contributors to neuroinflammation and cognitive dysfunction.
Various alleles of APOE, including E2 and E3, exhibit
neuroprotective effects, in contrast to the APOE4 allele, which is
a significant genetic risk factor for AD. Previous studies have
demonstrated that PLIN3, possessing apolipoprotein-like
characteristics, promotes the conversion of liposomes into lipid
rafts. This membrane remodeling function is attributed to the
C-terminal (quadruple helix bundle) domain of PLIN3. The C-terminal
region of PLIN3 shares homology with the N-terminal domain of APOE,
which is known to promote small LD formation. This domain binds to
PLIN3 via a hydrophobic cleft, jointly regulating LD transport and
facilitating droplet-lysosome contact (59,149). This interaction provides a
potential therapeutic target for developing strategies to address
NDDs by specifically modulating the interaction between APOE4 and
LD regulatory proteins. APOE4 affects lipid transfer ability and
reduces the production of LDs. In glial cells expressing APOE4,
there is an increase in LDs, and functional deletion of APOE4
results in fewer lipoprotein particles and lipid accumulation in
astrocytes (150). APOE4 is
characterized by low lipid-binding capacity, reduced protein
stability and diminished affinity for brain lipoprotein particles.
Neuronal lipid transport proteins such as ABCA1 (151) and ABCA7 (152), LRP1 (153), and genes related to endocytosis
are associated with an elevated risk of AD. Astrocytes produce
cytokines that are associated with depression and anxiety-like
behaviors. For instance, adenosine signaling in amygdala astrocytes
promotes anxiety-related phenotypes in regionally and synaptically
specific ways (154). Targeting
astrocyte cytokine production may provide new treatment options for
depression and related disorders. The accumulation of LDs in
astrocytes has the potential to activate inflammatory signaling
pathways, thereby exacerbating neuroinflammation. This process can
disrupt normal metabolic functions, resulting in energy
deficiencies and abnormalities in metabolite production, which may
exert neurotoxic effects. Furthermore, the accumulation of LDs in
astrocytes is associated with mitochondrial dysfunction and can
compromise protein quality control mechanisms, leading to the
accumulation of misfolded proteins and the induction of apoptosis
or necrosis (90). In the early
stages of AD, lipid imbalances and LD accumulation are particularly
pronounced in astrocytes of individuals carrying the APOE4 allele,
who exhibit increased LD accumulation and diminished fatty acid
uptake and oxidation (155).
These phenomena have been confirmed in postmortem brain tissue from
APOE4 carriers and in APOE4 mouse models. Additionally, neuronal
mitochondrial abnormalities that contribute to astrocytic LD
accumulation, as observed in a mouse model of Leigh syndrome with
mitochondrial dysfunction, may represent an early indicator of
neurodegeneration (156).
While the brain primarily utilizes glucose as its
main energy source, lipids play a crucial structural and functional
role in brain activity. Given that excessive FFAs are linked to
lipid toxicity, their concentrations must be meticulously
regulated. Research indicates that OXPHOS in astrocytes is
essential for the catabolism of fatty acids, thereby maintaining
lipid homeostasis (82). A
deficiency in astrocytic OXPHOS can lead to significant damage to
brain lipid structures and activate several critical pathological
mechanisms. These mechanisms include synaptic loss and dysfunction,
reactive astrocyte dysfunction, reactive astrogliosis, microglial
activation, oxidative stress and demyelination. Collectively, these
pathological processes contribute to neuroinflammation,
neurodegeneration and cognitive impairment (157). Consequently, strategies aimed
at preserving or restoring the equilibrium between fatty acid load
and mitochondrial catabolism hold promise for mitigating reactive
astrocyte hyperplasia. Furthermore, such strategies may enhance the
adverse cerebral environment and ameliorate cognitive deficits.
This represents a potentially promising avenue for future research
(Table I).
Astrocytes play a crucial role in maintaining the
normal functioning of the nervous system and safeguarding it
against diseases. The pathophysiology of astrocytes is intricate,
highly heterogeneous and variable, with responses that can differ
based on the specific contexts and environments associated with
disease. Any form of brain injury elicits an astrocytic response
aimed at preserving or restoring homeostasis. In cases of severe
injury, reactive astrogliosis is initiated, which serves to protect
the surrounding neural tissue. A significant mechanism underlying
neurological dysfunction in most neurological disorders is the loss
of astrocytic function. Although the role of lipids in the
distribution of brain regions and NDDs has not been fully
elucidated, advancements in single-cell sequencing technology and
matrix-assisted laser desorption/ionization mass spectrometry
imaging mass spectrometry have enabled the direct detection of
intramembranous LDs and lipid metabolism (158).
The impairment of astrocyte function can result in
the accumulation of detrimental lipid metabolism byproducts and the
progressive loss of neuronal support functions. While there are
currently no specific therapies or drugs that directly target
astrocyte LD storage and lipid metabolism, numerous pharmacological
agents are known to influence astrocyte reactivity and
astroglia-neuron interactions. Observations in adult mouse fasting
models revealed elevated lysophosphatidic acid (LPA) levels in
blood/cerebrospinal fluid following fasting. Upon crossing the BBB
into the cortex, LPA increased cortical neuronal excitability
(159). In a mouse model of
ischemic stroke induced by middle cerebral artery occlusion,
autotaxin (ATX) and LPA levels significantly increased in the
cortex, leading to sustained glutamate-mediated hyperexcitability.
However, when mice were genetically engineered to specifically
knockout ATX or when ATX was pharmacologically inhibited, LPA
levels decreased, the cortical excitatory/inhibitory balance was
restored and neurological function improved (153). Research has demonstrated that
ATX, an enzyme responsible for LPA synthesis and expressed by
astrocytes in excitatory synaptic regions, can modulate glutamate
transmission and is itself regulated by neuronal activity (160). This model proposes that
pharmacologically inhibiting ATX through indirect mechanisms of
neurotransmission may offer a novel therapeutic approach for
neurological disorders such as schizophrenia. Enhancing the
catabolic pathways and mobilizing internal energy reserves can
improve the bioenergetic efficiency of astrocytes and their
capacity to support neurons under stress, representing a promising
metabolic therapeutic strategy. Supplementation with omega-3
polyunsaturated fatty acids, such as DHA, has been demonstrated to
mitigate astrocyte activation and inflammatory responses by
inhibiting inflammatory signaling pathways like NF-κB and AP-1,
enhancing cell membrane fluidity and thereby indirectly improving
astrocyte function (161,162). However, large-scale human
clinical trials have yielded inconsistent results regarding
cognitive enhancement, suggesting that efficacy may depend on
disease stage and APOE genotype. The PPAR-δ agonist GW0742 has
demonstrated potential therapeutic efficacy in preclinical models
of AD by promoting fatty acid β-oxidation capacity in astrocytes
and directly reducing LD accumulation (163). It has been observed to enhance
CPT1A expression and fatty acid oxidation capacity in iPSC-derived
astrocytes from fatty acid oxidation-deficient patients with AD, as
well as to improve memory, increase neurogenesis and reduce
inflammation-related gene expression in Aβ precursor
protein/presenilin 1 AD mouse models. GW0742 can also significantly
reduce BBB leakage and metalloproteinase-9 expression, while
upregulating tight junction protein expression, reducing neutrophil
recruitment to the brain and protecting BBB integrity (164). Nonetheless, GW0742 does not
prevent the pro-inflammatory activation of iPSCs or astrocyte
proliferation and may be insufficient to fully reverse metabolic
damage, particularly in the context of glucose metabolism
deficiencies, which could lead to uncoupled mitochondrial
respiration and elevated oxidative stress (163). However, due to variations in
APOE genotypes, the effects may differ, and this potential risk has
not yet been fully evaluated. Glucose metabolism modulators can
correct the metabolic reprogramming of astrocytes, thereby
significantly reducing the expression of proinflammatory factors
such as TNF-α and IL-6 in IL-1β-stimulated astrocytes (165). In the future, the combination
of 'GW0742 combined with glucose metabolism modulator' holds
promise for reshaping astrocytes into a neuroprotective phenotype.
This approach may positively and indirectly enhance their glutamate
transport function and BBB maintenance capacity, thereby improving
therapeutic outcomes for NDDs. Similarly, liver X receptor (LXR)
agonists can significantly attenuate tau pathology and
neurodegeneration in APOE4 mice by promoting lipid efflux in
astrocytes. These data suggest that enhancing glial lipid efflux
may serve as a therapeutic approach to improve tau and
APOE4-associated neurodegeneration. However, most non-selective LXR
agonists strongly induce lipogenic genes such as SREBP-1c and fatty
acid synthase in the liver, leading to hypertriglyceridemia and
hepatic steatosis (166).
Therefore, the beneficial effects of LXR agonists in AD models must
be weighed against their known systemic side effects. Long-term
regulation of cellular LDs is not without risk. Multiple studies
indicate that inhibiting LD formation weakens the detoxification
capacity of glial cells under acute oxidative stress. Research
reveals that endogenous tau deficiency impairs LD formation in
astrocytes, leading to elevated lipid peroxides, enhanced ROS
production and increased cellular sensitivity to neurotoxicity
(167). Similarly, studies on
glioblastoma indicate that targeted inhibition of DGAT1 disrupts
lipid homeostasis, exacerbating oxidative stress and increasing
cell mortality, suggesting the critical role of LDs in buffering
lipotoxicity (168). Therefore,
future interventions should not simply aim to 'reduce LDs' but
rather focus on restoring lipid homeostasis.
A significant challenge in developing therapies
targeting astrocytes lies in the heterogeneity of these cells in
both healthy and diseased states. Astrocyte subpopulations exhibit
considerable variability in their distribution across different
regions of the CNS, as well as in their roles in various diseases
or disease states. Notably, certain pathogenetic mechanisms
associated with astrocytes are prevalent across numerous
neurological disorders. These mechanisms include neurotoxicity,
dysregulation of extracellular glutamate levels, defects in
potassium ion cycling and impaired lactate shuttling (169). Modulating these commonly
dysregulated pathways in astrocyte activity may offer therapeutic
targets for a range of neurological diseases that are influenced by
astrocytic dysfunction.
Astrocytes are integral to the CNS, where they
contribute to the maintenance of the BBB, facilitate intercellular
communication and uphold metabolic homeostasis. The lipid
metabolism of astrocytes is essential for preserving membrane
fluidity, mitigating inflammation, and influencing the structure
and signaling of organelles. The interplay of these metabolic
pathways is crucial for the metabolic equilibrium of the nervous
system; disruptions in these pathways can result in disorders of
energy production, inflammation, excitotoxicity and the
accumulation of toxic substances, all of which are common causative
factors in various NDDs (170,171). A comprehensive understanding of
these pathways in both health and disease contexts can elucidate
the underlying causes of such diseases and provide a foundation for
the development of novel therapeutic and nutritional strategies
aimed at enhancing patient outcomes. In various disease contexts, a
range of emerging astrocyte-related molecules may serve as
potential therapeutic targets. Consequently, treatment strategies
should focus on modulating specific molecules involved in astrocyte
responses to bolster astrocyte defense mechanisms and improve
astrocyte homeostasis, ultimately addressing CNS diseases through
pathophysiological interventions.
Not applicable.
YKZ and CCZ contributed to the overall
conceptualization of the review. YCW, BXW and JCH drafted and
revised the manuscript. XDH, CLL and RLG generated the figures.
HGC, YZ and JBZ edited the manuscript and figures. All authors read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
Not applicable.
This work was supported by National Natural Science Foundation
of China (grant nos. 82302116, 82020108026 and 82404215), Shaanxi
Outstanding Youth Fund Project (grant no. 2023-JC-JQ-65) and the
Fourth Military Medical University pilotage operation new flight
program project (grant no. 2023-rcjfzyk).
|
1
|
Rudnicka E, Napierala P, Podfigurna A,
Meczekalski B, Smolarczyk R and Grymowicz M: The world health
organization (WHO) approach to healthy ageing. Maturitas. 139:6–11.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch
SG, Croteau DL and Bohr VA: Ageing as a risk factor for
neurodegenerative disease. Nat Rev Neurol. 15:565–581. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilson DM III, Cookson MR, Van Den Bosch
L, Zetterberg H, Holtzman DM and Dewachter I: Hallmarks of
neurodegenerative diseases. Cell. 186:693–714. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dugger BN and Dickson DW: Pathology of
neurodegenerative diseases. Cold Spring Harb Perspect Biol.
9:a0280352017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bhat MA and Dhaneshwar S:
Neurodegenerative diseases: New hopes and perspectives. Curr Mol
Med. 24:1004–1032. 2024. View Article : Google Scholar
|
|
6
|
Temple S: Advancing cell therapy for
neurodegenerative diseases. Cell Stem Cell. 30:512–529. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Singh K, Sethi P, Datta S, Chaudhary JS,
Kumar S, Jain D, Gupta JK, Kumar S, Guru A and Panda SP: Advances
in gene therapy approaches targeting neuro-inflammation in
neurodegenerative diseases. Ageing Res Rev. 98:1023212024.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi FD and Yong VW: Neuroinflammation
across neurological diseases. Science. 388:eadx00432025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwon HS and Koh SH: Neuroinflammation in
neurodegenerative disorders: The roles of microglia and astrocytes.
Transl Neurodegener. 9:422020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guttenplan KA, Weigel MK, Prakash P,
Wijewardhane PR, Hasel P, Rufen-Blanchette U, Munch AE, Blum JA,
Fine J, Neal MC, et al: Neurotoxic reactive astrocytes induce cell
death via saturated lipids. Nature. 599:102–107. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cameron EG, Nahmou M, Toth AB, Heo L,
Tanasa B, Dalal R, Yan W, Nallagatla P, Xia X, Hay S, et al: A
molecular switch for neuroprotective astrocyte reactivity. Nature.
626:574–582. 2024. View Article : Google Scholar :
|
|
12
|
Debom GN, Rubenich DS and Braganhol E:
Adenosinergic signaling as a key modulator of the glioma
microenvironment and reactive astrocytes. Front Neurosci.
15:6484762021. View Article : Google Scholar
|
|
13
|
Acioglu C, Li L and Elkabes S:
Contribution of astrocytes to neuropathology of neurodegenerative
diseases. Brain Res. 1758:1472912021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kozachkov L, Kastanenka KV and Krotov D:
Building transformers from neurons and astrocytes. Proc Natl Acad
Sci USA. 120:e22191501202023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yamagata K: Lactate supply from astrocytes
to neurons and its role in ischemic stroke-induced
neurodegeneration. Neuroscience. 481:219–231. 2022. View Article : Google Scholar
|
|
16
|
Tewari BP, Woo AM, Prim CE, Chaunsali L,
Patel DC, Kimbrough IF, Engel K, Browning JL, Campbell SL and
Sontheimer H: Astrocytes require perineuronal nets to maintain
synaptic homeostasis in mice. Nat Neurosci. 27:1475–1488. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aldana BI, Zhang Y, Jensen P,
Chandrasekaran A, Christensen SK, Nielsen TT, Nielsen JE, Hyttel P,
Larsen MR, Waagepetersen HS and Freude KK: Glutamate-glutamine
homeostasis is perturbed in neurons and astrocytes derived from
patient ipsc models of frontotemporal dementia. Mol Brain.
13:1252020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qian K, Jiang X, Liu ZQ, Zhang J, Fu P, Su
Y, Brazhe NA, Liu D and Zhu LQ: Revisiting the critical roles of
reactive astrocytes in neurodegeneration. Mol Psychiatry.
28:2697–2706. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yin F: Lipid metabolism and Alzheimer's
disease: Clinical evidence, mechanistic link and therapeutic
promise. FEBS J. 290:1420–1453. 2023. View Article : Google Scholar
|
|
20
|
Byrns CN, Perlegos AE, Miller KN, Jin Z,
Carranza FR, Manchandra P, Beveridge CH, Randolph CE, Chaluvadi VS,
Zhang SL, et al: Senescent glia link mitochondrial dysfunction and
lipid accumulation. Nature. 630:475–483. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nakamura A, Sakai S, Taketomi Y, Tsuyama
J, Miki Y, Hara Y, Arai N, Sugiura Y, Kawaji H, Murakami M and
Shichita T: PLA2G2E-mediated lipid metabolism triggers
brain-autonomous neural repair after ischemic stroke. Neuron.
111:2995–3010 e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chausse B, Kakimoto PA and Kann O:
Microglia and lipids: How metabolism controls brain innate
immunity. Semin Cell Dev Biol. 112:137–144. 2021. View Article : Google Scholar
|
|
23
|
Duquenne M, Folgueira C, Bourouh C, Millet
M, Silva A, Clasadonte J, Imbernon M, Fernandois D, Martinez-Corral
I, Kusumakshi S, et al: Leptin brain entry via a tanycytic
LepR-EGFR shuttle controls lipid metabolism and pancreas function.
Nat Metab. 3:1071–1090. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mathiowetz AJ and Olzmann JA: Lipid
droplets and cellular lipid flux. Nat Cell Biol. 26:331–345. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ralhan I, Chang CL, Lippincott-Schwartz J
and Ioannou MS: Lipid droplets in the nervous system. J Cell Biol.
220:e2021021362021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mallick K, Paul S and Banerjee S and
Banerjee S: Lipid droplets and neurodegeneration. Neuroscience.
549:13–23. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Windham IA, Powers AE, Ragusa JV, Wallace
ED, Zanellati MC, Williams VH, Wagner CH, White KK and Cohen S:
Apoe traffics to astrocyte lipid droplets and modulates
triglyceride saturation and droplet size. J Cell Biol.
223:e2023050032024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smolic T, Tavcar P, Horvat A, Cerne U,
Haluzan Vasle A, Tratnjek L, Kreft ME, Scholz N, Matis M, Petan T,
et al: Astrocytes in stress accumulate lipid droplets. Glia.
69:1540–1562. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kim NS and Chung WS: Astrocytes regulate
neuronal network activity by mediating synapse remodeling. Neurosci
Res. 187:3–13. 2023. View Article : Google Scholar
|
|
30
|
de Ceglia R, Ledonne A, Litvin DG, Lind
BL, Carriero G, Latagliata EC, Bindocci E, Di Castro MA, Savtchouk
I, Vitali I, et al: Specialized astrocytes mediate glutamatergic
gliotransmission in the CNS. Nature. 622:120–129. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu D, Liao P, Li H, Tong S, Wang B, Lu Y,
Gao Y, Huang Y, Zhou H, Shi L, et al: Regulation of blood-brain
barrier integrity by dmp1-expressing astrocytes through
mitochondrial transfer. Sci Adv. 10:eadk29132024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jayaram MA and Phillips JJ: Role of the
microenvironment in glioma pathogenesis. Annu Rev Pathol.
19:181–201. 2024. View Article : Google Scholar
|
|
33
|
Giovannoni F and Quintana FJ: The role of
astrocytes in CNS inflammation. Trends Immunol. 41:805–819. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou J, Bi H, Ge Q, Teng H, Wan G, Yu B,
Jiang Q and Gu X: Heterogeneity analysis of astrocytes following
spinal cord injury at single-cell resolution. FASEB J.
36:e224422022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen ZP, Wang S, Zhao X, Fang W, Wang Z,
Ye H, Wang M, Ke L, Huang T, Lv P, et al: Lipid-accumulated
reactive astrocytes promote disease progression in epilepsy. Nat
Neurosci. 26:542–554. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Veiga A, Abreu DS, Dias JD, Azenha P,
Barsanti S and Oliveira JF: Calcium-dependent signaling in
astrocytes: Downstream mechanisms and implications for cognition. J
Neurochem. 169:e700192025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Iovino L, Tremblay ME and Civiero L:
Glutamate-induced excitotoxicity in Parkinson's disease: The role
of glial cells. J Pharmacol Sci. 144:151–164. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang Y, Ding X, Zhang Y, Cai L, Ge Y, Ma
K, Xu R, Li S, Song M, Zhu H, et al: Fluoxetine inhibited the
activation of a1 reactive astrocyte in a mouse model of major
depressive disorder through astrocytic 5-HT(2b)R/β-arrestin2
pathway. J Neuroinflammation. 19:232022. View Article : Google Scholar
|
|
39
|
Chen Z, Yuan Z, Yang S, Zhu Y, Xue M,
Zhang J and Leng L: Brain energy metabolism: Astrocytes in
neurodegenerative diseases. CNS Neurosci Ther. 29:24–36. 2023.
View Article : Google Scholar :
|
|
40
|
Cheng X, Zhao M, Chen L, Huang C, Xu Q,
Shao J, Wang HT, Zhang Y, Li X, Xu X, et al: Astrocytes modulate
brain phosphate homeostasis via polarized distribution of phosphate
uptake transporter PiT2 and exporter XPR1. Neuron.
112:3126–3142.e8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sharma V, Oliveira MM, Sood R, Khlaifia A,
Lou D, Hooshmandi M, Hung TY, Mahmood N, Reeves M, Ho-Tieng D, et
al: mRNA translation in astrocytes controls hippocampal long-term
synaptic plasticity and memory. Proc Natl Acad Sci USA.
120:e23086711202023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Guo F, Fan J, Liu JM, Kong PL, Ren J, Mo
JW, Lu CL, Zhong QL, Chen LY, Jiang HT, et al: Astrocytic alkbh5 in
stress response contributes to depressive-like behaviors in mice.
Nat Commun. 15:43472024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dewa KI, Arimura N, Kakegawa W, Itoh M,
Adachi T, Miyashita S, Inoue YU, Hizawa K, Hori K, Honjoya N, et
al: Neuronal DSCAM regulates the peri-synaptic localization of
GLAST in bergmann glia for functional synapse formation. Nat
Commun. 15:4582024. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Thakur S, Dhapola R, Sarma P, Medhi B and
Reddy DH: Neuroinflammation in Alzheimer's disease: Current
progress in molecular signaling and therapeutics. Inflammation.
46:1–17. 2023. View Article : Google Scholar
|
|
45
|
Zhang S, Li M, Qiu Y, Wu J, Xu X, Ma Q,
Zheng Z, Lu G, Deng Z and Huang H: Enhanced VEGF secretion and
blood-brain barrier disruption: Radiation-mediated inhibition of
astrocyte autophagy via PI3K-AKT pathway activation. Glia.
72:568–587. 2024. View Article : Google Scholar
|
|
46
|
Bonvento G and Bolaños JP:
Astrocyte-neuron metabolic cooperation shapes brain activity. Cell
Metab. 33:1546–1564. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Arbring Sjöström T, Ivanov AI, Kiani N,
Bernacka-Wojcik I, Samuelsson J, Saarela Unemo H, Xydias D, Vagiaki
LE, Psilodimitrakopoulos S, Konidakis I, et al: Miniaturized
iontronic micropipettes for precise and dynamic ionic modulation of
neuronal and astrocytic activity. Small. 21:e24109062025.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ferris HA, Perry RJ, Moreira GV, Shulman
GI, Horton JD and Kahn CR: Loss of astrocyte cholesterol synthesis
disrupts neuronal function and alters whole-body metabolism. Proc
Natl Acad Sci USA. 114:1189–1194. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tomas M, Duran JM, Lazaro-Dieguez F, Babia
T, Renau-Piqueras J and Egea G: Fluorescent analogues of plasma
membrane sphingolipids are sorted to different intracellular
compartments in astrocytes; Harmful effects of chronic ethanol
exposure on sphingolipid trafficking and metabolism. FEBS Lett.
563:59–65. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zadoorian A, Du X and Yang H: Lipid
droplet biogenesis and functions in health and disease. Nat Rev
Endocrinol. 19:443–459. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klemm RW and Carvalho P: Lipid droplets
big and small: Basic mechanisms that make them all. Annu Rev Cell
Dev Biol. 40:143–168. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Salo VT, Belevich I, Li S, Karhinen L,
Vihinen H, Vigouroux C, Magre J, Thiele C, Holtta-Vuori M, Jokitalo
E and Ikonen E: Seipin regulates ER-lipid droplet contacts and
cargo delivery. EMBO J. 35:2699–2716. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Fu L, Zhang J, Wang Y, Wu H, Xu X, Li C,
Li J, Liu J, Wang H, Jiang X, et al: LET-767 determines lipid
droplet protein targeting and lipid homeostasis. J Cell Biol.
223:e2023110242024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Harris CA, Haas JT, Streeper RS, Stone SJ,
Kumari M, Yang K, Han X, Brownell N, Gross RW, Zechner R and Farese
RV Jr: DGAT enzymes are required for triacylglycerol synthesis and
lipid droplets in adipocytes. J Lipid Res. 52:657–667. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Rao MJ and Goodman JM: Seipin: Harvesting
fat and keeping adipocytes healthy. Trends Cell Biol. 31:912–923.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang H, Nikain C, Fortounas KI, Amengual
J, Tufanli O, La Forest M, Yu Y, Wang MC, Watts R, Lehner R, et al:
FITM2 deficiency results in ER lipid accumulation, ER stress, and
reduced apolipoprotein B lipidation and VLDL triglyceride secretion
in vitro and in mouse liver. Mol Metab. 90:1020482024. View Article : Google Scholar
|
|
57
|
Xu L, Li L, Wu L, Li P and Chen F: CIDE
proteins and their regulatory mechanisms in lipid droplet fusion
and growth. FEBS Lett. 598:1154–1169. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Zhang L, Zhou Y, Yang Z, Jiang L, Yan X,
Zhu W, Shen Y, Wang B, Li J and Song J: Lipid droplets in central
nervous system and functional profiles of brain cells containing
lipid droplets in various diseases. J Neuroinflammation. 22:72025.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Khaddaj R, Stribny J, Cottier S and
Schneiter R: Perilipin 3 promotes the formation of membrane domains
enriched in diacylglycerol and lipid droplet biogenesis proteins.
Front Cell Dev Biol. 11:11164912023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ferreira JV, Ahmed Y, Heunis T, Jain A,
Johnson E, Raschle M, Ernst R, Vanni S and Carvalho P:
Pex30-dependent membrane contact sites maintain ER lipid
homeostasis. J Cell Biol. 224:e2024090392025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Li D, Zhang J and Liu Q: Brain cell
type-specific cholesterol metabolism and implications for learning
and memory. Trends Neurosci. 45:401–414. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Feringa FM and van der Kant R: Cholesterol
and Alzheimer's disease; From risk genes to pathological effects.
Front Aging Neurosci. 13:6903722021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Li X, Zhang J, Li D, He C, He K, Xue T,
Wan L, Zhang C and Liu Q: Astrocytic ApoE reprograms neuronal
cholesterol metabolism and histone-acetylation-mediated memory.
Neuron. 109:957–970. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Litvinchuk A, Suh JH, Guo JL, Lin K, Davis
SS, Bien-Ly N, Tycksen E, Tabor GT, Remolina Serrano J, Manis M, et
al: Amelioration of Tau and Apoe4-linked glial lipid accumulation
and neurodegeneration with an LXR agonist. Neuron. 112:384–403 e8.
2024. View Article : Google Scholar :
|
|
65
|
Yang A, Alrosan AZ, Sharpe LJ, Brown AJ,
Callaghan R and Gelissen IC: Regulation of ABCG4 transporter
expression by sterols and LXR ligands. Biochim Biophys Acta Gen
Subj. 1865:1297692021. View Article : Google Scholar
|
|
66
|
Arenas F, Garcia-Ruiz C and
Fernandez-Checa JC: Intracellular cholesterol trafficking and
impact in neurodegeneration. Front Mol Neurosci. 10:3822017.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Adachi C, Otsuka S and Inoue T:
Cholesterol-induced robust Ca oscillation in astrocytes required
for survival and lipid droplet formation in high-cholesterol
condition. iScience. 25:1051382022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhu X, Owen JS, Wilson MD, Li H, Griffiths
GL, Thomas MJ, Hiltbold EM, Fessler MB and Parks JS: Macrophage
abca1 reduces myd88-dependent toll-like receptor trafficking to
lipid rafts by reduction of lipid raft cholesterol. J Lipid Res.
51:3196–3206. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Sapoń K, Mańka R and Janas T and Janas T:
The role of lipid rafts in vesicle formation. J Cell Sci.
136:jcs2608872023. View Article : Google Scholar
|
|
70
|
Cheng C, Tu J, Hu Z, Chen Y, Wang Y, Zhang
T, Zhang C, Li C, Wang Y and Niu C: SREBP2/Rab11s/GLUT1/6 network
regulates proliferation and migration of glioblastoma. Pathol Res
Pract. 240:1541762022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vanherle S, Loix M, Miron VE, Hendriks JJA
and Bogie JFJ: Lipid metabolism, remodelling and intercellular
transfer in the CNS. Nat Rev Neurosci. 26:214–231. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Benatzy Y, Palmer MA, Lütjohann D, Ohno
RI, Kampschulte N, Schebb NH, Fuhrmann DC, Snodgrass RG and Brüne
B: Alox15b controls macrophage cholesterol homeostasis via lipid
peroxidation, erk1/2 and srebp2. Redox Biol. 72:1031492024.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kuo A and Hla T: Regulation of cellular
and systemic sphingolipid homeostasis. Nat Rev Mol Cell Biol.
25:802–821. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Pan X, Dutta D, Lu S and Bellen HJ:
Sphingolipids in neurodegenerative diseases. Front Neurosci.
17:11378932023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Robles-Martinez L, Morin KH and
Nikolova-Karakashian M: Ceramide homeostasis in hepatic lipid
droplets. Biochem Soc Trans. 53:509–518. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Di Scala C, Yahi N, Boutemeur S, Flores A,
Rodriguez L, Chahinian H and Fantini J: Common molecular mechanism
of amyloid pore formation by Alzheimer's beta-amyloid peptide and
alpha-synuclein. Sci Rep. 6:287812016. View Article : Google Scholar
|
|
77
|
Di Scala C, Troadec JD, Lelievre C, Garmy
N, Fantini J and Chahinian H: Mechanism of cholesterol-assisted
oligomeric channel formation by a short Alzheimer β-amyloid
peptide. J Neurochem. 128:186–195. 2014. View Article : Google Scholar
|
|
78
|
Chahinian H, Yahi N and Fantini J:
Glutamate, gangliosides, and the synapse: Electrostatics at work in
the brain. Int J Mol Sci. 25:85832024. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Corbit KC, Trakul N, Eves EM, Diaz B,
Marshall M and Rosner MR: Activation of Raf-1 signaling by protein
kinase C through a mechanism involving Raf kinase inhibitory
protein. J Biol Chem. 278:13061–13068. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Shim M, San TT, Shin B, Lee H, Han SB, Lee
DK and Kim HJ: Histone demethylase inhibitor KDM5-C70 regulates
metabolomic and lipidomic programming during an astrocyte
differentiation of rat neural stem cell. Sci Rep. 15:54092025.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Thieren L, Zanker HS, Droux J, Dalvi U,
Wyss MT, Waag R, Germain PL, von Ziegler LM, Looser ZJ, Hosli L, et
al: Astrocytic GLUT1 deletion in adult mice enhances glucose
metabolism and resilience to stroke. Nat Commun. 16:41902025.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Mi Y, Qi G, Vitali F, Shang Y, Raikes AC,
Wang T, Jin Y, Brinton RD, Gu H and Yin F: Loss of fatty acid
degradation by astrocytic mitochondria triggers neuroinflammation
and neurodegeneration. Nat Metab. 5:445–465. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Astarita G, Jung K, Berchtold NC, Nguyen
VQ, Gillen DL, Head E, Cotman CW and Piomelli D: Deficient liver
biosynthesis of docosahexaenoic acid correlates with cognitive
impairment in alzheimer's disease. PLoS One. 5:e125382010.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nguyen LN, Ma D, Shui G, Wong P,
Cazenave-Gassiot A, Zhang X, Wenk MR, Goh EL and Silver DL: Mfsd2a
is a transporter for the essential omega-3 fatty acid
docosahexaenoic acid. Nature. 509:503–506. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pelerin H, Jouin M, Lallemand MS,
Alessandri JM, Cunnane SC, Langelier B and Guesnet P: Gene
expression of fatty acid transport and binding proteins in the
blood-brain barrier and the cerebral cortex of the rat: Differences
across development and with different DHA brain status.
Prostaglandins Leukot Essent Fatty Acids. 91:213–220. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kim ID, Ju H, Minkler J, Jiang R, Singh A,
Sharma R, Febbraio M and Cho S: Endothelial cell CD36 mediates
stroke-induced brain injury via BBB dysfunction and monocyte
infiltration in normal and obese conditions. J Cereb Blood Flow
Metab. 43:843–855. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Geng Z, Peng F, Cheng Z, Su J, Song J, Han
X, Li R, Li X, Cui R and Li B: Astrocytic FABP7 alleviates
depression-like behaviors of chronic unpredictable mild stress mice
by regulating neuroinflammation and hippocampal spinogenesis. FASEB
J. 39:e706062025. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Su Y and Yuan Q: Mitochondrial fatty acid
oxidase CPT1A ameliorates postoperative cognitive dysfunction by
regulating astrocyte ferroptosis. Brain Res. 1850:1494242025.
View Article : Google Scholar
|
|
89
|
Ochiai Y, Uchida Y, Ohtsuki S, Tachikawa
M, Aizawa S and Terasaki T: The blood-brain barrier fatty acid
transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid
to the brain, and insulin facilitates transport. J Neurochem.
141:400–412. 2017. View Article : Google Scholar
|
|
90
|
Li YC, Fu JT and Tzeng SF: Exposure to
lipid mixture induces intracellular lipid droplet formation and
impairs mitochondrial functions in astrocytes. Neurochem Int.
178:1057922024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Liang KX, Chen A, Kianian A, Kristiansen
CK, Yangzom T, Furriol J, Hoyland LE, Ziegler M, Krakenes T,
Tzoulis C, et al: Activation of neurotoxic astrocytes due to
mitochondrial dysfunction triggered by POLG mutation. Int J Biol
Sci. 20:2860–2880. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kong J, Ji Y, Jeon YG, Han JS, Han KH, Lee
JH, Lee G, Jang H, Choe SS, Baes M and Kim JB: Spatiotemporal
contact between peroxisomes and lipid droplets regulates
fasting-induced lipolysis via PEX5. Nat Commun. 11:5782020.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kuijpers M, Kochlamazashvili G, Stumpf A,
Puchkov D, Swaminathan A, Lucht MT, Krause E, Maritzen T, Schmitz D
and Haucke V: Neuronal autophagy regulates presynaptic
neurotransmission by controlling the axonal endoplasmic reticulum.
Neuron. 110:7342022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Pu M, Zheng W, Zhang H, Wan W, Peng C,
Chen X, Liu X, Xu Z, Zhou T, Sun Q, et al: ORP8 acts as a lipophagy
receptor to mediate lipid droplet turnover. Protein Cell.
14:653–667. 2023.PubMed/NCBI
|
|
95
|
Smolic T, Zorec R and Vardjan N:
Pathophysiology of lipid droplets in neuroglia. Antioxidants
(Basel). 11:222021. View Article : Google Scholar
|
|
96
|
Han X, Liu Y, Dai Y, Xu T, Hu Q, Yi X, Rui
L, Hu G and Hu J: Neuronal SH2B1 attenuates apoptosis in an MPTP
mouse model of Parkinson's disease via promoting PLIN4 degradation.
Redox Biol. 52:1023082022. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Schweiger M, Schreiber R, Haemmerle G,
Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R and
Zimmermann R: Adipose triglyceride lipase and hormone-sensitive
lipase are the major enzymes in adipose tissue triacylglycerol
catabolism. J Biol Chem. 281:40236–40241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Grabner GF, Xie H, Schweiger M and Zechner
R: Lipolysis: Cellular mechanisms for lipid mobilization from fat
stores. Nat Metab. 3:1445–1465. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Li Q, Liu P, Zhu X, Zhou C, Hu Y, Cao S,
Li H, Zou X, Gao S, Cao X, et al: NG-497 alleviates
microglia-mediated neuroinflammation in a MTNR1A-dependent manner.
Inflammation. 48:2663–2676. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Morant-Ferrando B, Jimenez-Blasco D,
Alonso-Batan P, Agulla J, Lapresa R, Garcia-Rodriguez D,
Yunta-Sanchez S, Lopez-Fabuel I, Fernandez E, Carmeliet P, et al:
Fatty acid oxidation organizes mitochondrial supercomplexes to
sustain astrocytic ROS and cognition. Nat Metab. 5:1290–1302. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Yang D, Wang X, Zhang L, Fang Y, Zheng Q,
Liu X, Yu W, Chen S, Ying J and Hua F: Lipid metabolism and storage
in neuroglia: Role in brain development and neurodegenerative
diseases. Cell Biosci. 12:1062022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li J, Jiang W, Cai Y, Ning Z, Zhou Y, Wang
C, Chung SK, Huang Y, Sun J, Deng M, et al: Astrocytic endothelin-1
overexpression impairs learning and memory ability in ischemic
stroke via altered hippocampal neurogenesis and lipid metabolism.
Neural Regen Res. 19:650–656. 2024. View Article : Google Scholar
|
|
103
|
Majchrzak M, Stojanović O, Ajjaji D, Ben
M'barek K, Omrane M, Thiam AR and Klemm RW: Perilipin membrane
integration determines lipid droplet heterogeneity in
differentiating adipocytes. Cell Rep. 43:1140932024. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shimano H and Sato R: SREBP-regulated
lipid metabolism: Convergent physiology-divergent pathophysiology.
Nat Rev Endocrinol. 13:710–730. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Han X, Zhu J, Zhang X, Song Q, Ding J, Lu
M, Sun S and Hu G: Plin4-dependent lipid droplets hamper neuronal
mitophagy in the MPTP/p-induced mouse model of Parkinson's disease.
Front Neurosci. 12:3972018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Kimmel AR and Sztalryd C: The perilipins:
Major cytosolic lipid droplet-associated proteins and their roles
in cellular lipid storage, mobilization, and systemic homeostasis.
Annu Rev Nutr. 36:471–509. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Xu SF, Pang ZQ, Fan YG, Zhang YH, Meng YH,
Bai CY, Jia MY, Chen YH, Wang ZY and Guo C: Astrocyte-specific loss
of lactoferrin influences neuronal structure and function by
interfering with cholesterol synthesis. Glia. 70:2392–2408. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Yang C, Wang X, Wang J, Wang X, Chen W, Lu
N, Siniossoglou S, Yao Z and Liu K: Rewiring neuronal glycerolipid
metabolism determines the extent of axon regeneration. Neuron.
105:276–292.e5. 2020. View Article : Google Scholar :
|
|
109
|
Brandebura AN, Paumier A, Onur TS and
Allen NJ: Astrocyte contribution to dysfunction, risk and
progression in neurodegenerative disorders. Nat Rev Neurosci.
24:23–39. 2023. View Article : Google Scholar :
|
|
110
|
Mauch DH, Nägler K, Schumacher S, Göritz
C, Müller EC, Otto A and Pfrieger FW: CNS synaptogenesis promoted
by glia-derived cholesterol. Science. 294:1354–1357. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Christopherson KS, Ullian EM, Stokes CC,
Mullowney CE, Hell JW, Agah A, Lawler J, Mosher DF, Bornstein P and
Barres BA: Thrombospondins are astrocyte-secreted proteins that
promote CNS synaptogenesis. Cell. 120:421–433. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kucukdereli H, Allen NJ, Lee AT, Feng A,
Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH,
et al: Control of excitatory CNS synaptogenesis by
astrocyte-secreted proteins hevin and SPARC. Proc Natl Acad Sci
USA. 108:E440–E449. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Allen NJ, Bennett ML, Foo LC, Wang GX,
Chakraborty C, Smith SJ and Barres BA: Astrocyte glypicans 4 and 6
promote formation of excitatory synapses via GluA1 AMPA receptors.
Nature. 486:410–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Jin S, Chen X, Tian Y, Jarvis R, Promes V
and Yang Y: Astroglial exosome hepaCAM signaling and ApoE
antagonization coordinates early postnatal cortical pyramidal
neuronal axon growth and dendritic spine formation. Nat Commun.
14:51502023. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zhang X, Chen C and Liu Y: Navigating the
metabolic maze: Anomalies in fatty acid and cholesterol processes
in Alzheimer's astrocytes. Alzheimers Res Ther. 16:632024.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ioannou MS, Jackson J, Sheu SH, Chang CL,
Weigel AV, Liu H, Pasolli HA, Xu CS, Pang S, Matthies D, et al:
Neuron-astrocyte metabolic coupling protects against
activity-induced fatty acid toxicity. Cell. 177:1522–1535.e14.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Lindner K, Beckenbauer K, van Ek LC,
Titeca K, de Leeuw SM, Awwad K, Hanke F, Korepanova AV, Rybin V,
van der Kam EL, et al: Isoform- and cell-state-specific lipidation
of ApoE in astrocytes. Cell Rep. 38:1104352022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Leng F and Edison P: Neuroinflammation and
microglial activation in Alzheimer disease: Where do we go from
here? Nat Rev Neurol. 17:157–172. 2021. View Article : Google Scholar
|
|
119
|
Liu M, Xu Z, Wang L, Zhang L, Liu Y, Cao
J, Fu Q, Liu Y, Li H, Lou J, et al: Cottonseed oil alleviates
ischemic stroke injury by inhibiting the inflammatory activation of
microglia and astrocyte. J Neuroinflammation. 17:2702020.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Doring C, Regen T, Gertig U, van Rossum D,
Winkler A, Saiepour N, Bruck W, Hanisch UK and Janova H: A presumed
antagonistic LPS identifies distinct functional organization of
TLR4 in mouse microglia. Glia. 65:1176–1185. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Robb JL, Boisjoly F, Machuca-Parra AI,
Coursan A, Manceau R, Majeur D, Rodaros D, Bouyakdan K, Greffard K,
Bilodeau JF, et al: Blockage of ATGL-mediated breakdown of lipid
droplets in microglia alleviates neuroinflammatory and behavioural
responses to lipopolysaccharides. Brain Behav Immun. 123:315–333.
2025. View Article : Google Scholar
|
|
122
|
Marschallinger J, Iram T, Zardeneta M, Lee
SE, Lehallier B, Haney MS, Pluvinage JV, Mathur V, Hahn O, Morgens
DW, et al: Lipid-droplet-accumulating microglia represent a
dysfunctional and proinflammatory state in the aging brain. Nat
Neurosci. 23:194–208. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Pan RY, He L, Zhang J, Liu X, Liao Y, Gao
J, Liao Y, Yan Y, Li Q, Zhou X, et al: Positive feedback regulation
of microglial glucose metabolism by histone H4 lysine 12
lactylation in Alzheimer's disease. Cell Metab. 34:634–648.e6.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Zhang X, Zhang Y, Wang B, Xie C, Wang J,
Fang R, Dong H, Fan G, Wang M, He Y, et al: Pyroptosis-mediator
GSDMD promotes Parkinson's disease pathology via microglial
activation and dopaminergic neuronal death. Brain Behav Immun.
119:129–145. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Tcw J, Qian L, Pipalia NH, Chao MJ, Liang
SA, Shi Y, Jain BR, Bertelsen SE, Kapoor M, Marcora E, et al:
Cholesterol and matrisome pathways dysregulated in astrocytes and
microglia. Cell. 185:2213–2233 e25. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Wang X, Li X, Zuo X, Liang Z, Ding T, Li
K, Ma Y, Li P, Zhu Z, Ju C, et al: Photobiomodulation inhibits the
activation of neurotoxic microglia and astrocytes by inhibiting
Lcn2/JAK2-STAT3 crosstalk after spinal cord injury in male rats. J
Neuroinflammation. 18:2562021. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Jung BK, Park Y, Yoon B, Bae JS, Han SW,
Heo JE, Kim DE and Ryu KY: Reduced secretion of LCN2 (lipocalin 2)
from reactive astrocytes through autophagic and proteasomal
regulation alleviates inflammatory stress and neuronal damage.
Autophagy. 19:2296–2317. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Shin HJ, Kim KE, Jeong EA, An HS, Lee SJ,
Lee J and Roh GS: Amyloid β oligomer promotes microglial galectin-3
and astrocytic lipocalin-2 levels in the hippocampus of mice fed a
high-fat diet. Biochem Biophys Res Commun. 667:10–17. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xiao R, Pan J, Yang M, Liu H, Zhang A, Guo
X and Zhou S: Regulating astrocyte phenotype by Lcn2 inhibition
toward ischemic stroke therapy. Biomaterials. 317:1231022025.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Sun Y, Wei K, Liao X, Wang J, Gao L and
Pang B: Lipid metabolism in microglia: Emerging mechanisms and
therapeutic opportunities for neurodegenerative diseases (review).
Int J Mol Med. 56:1392025. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Wang Y, Wang W, Su L, Ji F, Zhang M, Xie
Y, Zhang T and Jiao J: BACH1 changes microglial metabolism and
affects astrogenesis during mouse brain development. Dev Cell.
59:108–124.e7. 2024. View Article : Google Scholar
|
|
132
|
Asadollahi E, Trevisiol A, Saab AS, Looser
ZJ, Dibaj P, Ebrahimi R, Kusch K, Ruhwedel T, Möbius W, Jahn O, et
al: Oligodendroglial fatty acid metabolism as a central nervous
system energy reserve. Nat Neurosci. 27:1934–1944. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Molina-Gonzalez I, Holloway RK, Jiwaji Z,
Dando O, Kent SA, Emelianova K, Lloyd AF, Forbes LH, Mahmood A,
Skripuletz T, et al: Astrocyte-oligodendrocyte interaction
regulates central nervous system regeneration. Nat Commun.
14:33722023. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Birolini G, Verlengia G, Talpo F, Maniezzi
C, Zentilin L, Giacca M, Conforti P, Cordiglieri C, Caccia C, Leoni
V, et al: SREBP2 gene therapy targeting striatal astrocytes
ameliorates Huntington's disease phenotypes. Brain. 144:3175–3190.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Tiwari V and Simons M: Lipid metabolism
and neuroinflammation: What is the link? J Exp Med.
222:e202412322025. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Li M, Li Z, Yao Y, Jin WN, Wood K, Liu Q,
Shi FD and Hao J: Astrocyte-derived interleukin-15 exacerbates
ischemic brain injury via propagation of cellular immunity. Proc
Natl Acad Sci USA. 114:E396–E405. 2017.
|
|
137
|
Lee HG, Lee JH, Flausino LE and Quintana
FJ: Neuroinflammation: An astrocyte perspective. Sci Transl Med.
15:eadi78282023. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Morkholt AS, Trabjerg MS, Oklinski MKE,
Bolther L, Kroese LJ, Pritchard CEJ, Huijbers IJ and Nieland JDV:
CPT1A plays a key role in the development and treatment of multiple
sclerosis and experimental autoimmune encephalomyelitis. Sci Rep.
9:132992019. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Castellani G, Croese T, Peralta Ramos JM
and Schwartz M: Transforming the understanding of brain immunity.
Science. 380:eabo76492023. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Sanmarco LM, Wheeler MA, Gutierrez-Vazquez
C, Polonio CM, Linnerbauer M, Pinho-Ribeiro FA, Li Z, Giovannoni F,
Batterman KV, Scalisi G, et al: Gut-licensed IFNү(+) NK cells drive
LAMP1(+)TRAIL(+) anti-inflammatory astrocytes. Nature. 590:473–479.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Miwa S, Kashyap S, Chini E and von
Zglinicki T: Mitochondrial dysfunction in cell senescence and
aging. J Clin Invest. 132:e1584472022. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Byrns CN, Saikumar J and Bonini NM: Glial
AP1 is activated with aging and accelerated by traumatic brain
injury. Nat Aging. 1:585–597. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Sheng L, Shields EJ, Gospocic J, Sorida M,
Ju L, Byrns CN, Carranza F, Berger SL, Bonini N and Bonasio R:
Ensheathing glia promote increased lifespan and healthy brain
aging. Aging Cell. 22:e138032023. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Madji Hounoum B, Mavel S, Coque E, Patin
F, Vourc'h P, Marouillat S, Nadal-Desbarats L, Emond P, Corcia P,
Andres CR, et al: Wildtype motoneurons, ALS-linked SOD1 mutation
and glutamate profoundly modify astrocyte metabolism and lactate
shuttling. Glia. 65:592–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zelic M, Blazier A, Pontarelli F, Lamorte
M, Huang J, Tasdemir-Yilmaz OE, Ren Y, Ryan SK, Shapiro C, Morel C,
et al: Single-cell transcriptomic and functional studies identify
glial state changes and a role for inflammatory RIPK1 signaling in
ALS pathogenesis. Immunity. 58:961–979. e82025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
He D, Xu H, Zhang H, Tang R, Lan Y, Xing
R, Li S, Christian E, Hou Y, Lorello P, et al: Disruption of the
IL-33-ST2-AKT signaling axis impairs neurodevelopment by inhibiting
microglial metabolic adaptation and phagocytic function. Immunity.
55:159–173.e9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zengeler KE, Hollis A, Deutsch T, Samuels
JD, Ennerfelt H, Moore KA, Steacy EJ, Sabapathy V, Sharma R, Patel
MK, et al: Inflammasome signaling in astrocytes modulates
hippocampal plasticity. Immunity. 58:1519–1535 e11. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Chen ZP, Zhao X, Wang S, Cai R, Liu Q, Ye
H, Wang MJ, Peng SY, Xue WX, Zhang YX, et al: GABA-dependent
microglial elimination of inhibitory synapses underlies neuronal
hyperexcitability in epilepsy. Nat Neurosci. 28:1404–1417. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Faustino AF, Martins IC, Carvalho FA,
Castanho MA, Maurer-Stroh S and Santos NC: Understanding dengue
virus capsid protein interaction with key biological targets. Sci
Rep. 5:105922015. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Haney MS, Palovics R, Munson CN, Long C,
Johansson PK, Yip O, Dong W, Rawat E, West E, Schlachetzki JCM, et
al: APOE4/4 is linked to damaging lipid droplets in Alzheimer's
disease microglia. Nature. 628:154–161. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Wang S, Li B, Cai Z, Hugo C, Li J, Sun Y,
Qian L, Remaley AT, Tcw J, Chui HC, et al: Cellular senescence
induced by cholesterol accumulation is mediated by lysosomal ABCA1
in APOE4 and AD. Res Sq [Preprint] rs.3.rs-4373201. 2024.
|
|
152
|
Wang N, Pan Y, Starling SC, Haskell DH,
Quintero AC, Kawatani K, Inoue Y, Shue F, Ma X, Aikawa T, et al:
Neuronal ABCA7 deficiency aggravates mitochondrial dysfunction and
neurodegeneration in Alzheimer's disease. Alzheimers Dement.
21:e701122025. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Wang X, Chen S, Xia X, Du Y, Wei Y, Yang
W, Zhang Y, Song Y, Lei T, Huang Q and Gao H: Lysosome-targeting
protein degradation through endocytosis pathway triggered by
polyvalent nano-chimera for AD therapy. Adv Mater. 37:e24110612025.
View Article : Google Scholar
|
|
154
|
Xu X, Xuan S, Chen S, Liu D, Xiao Q and Tu
J: Increased excitatory amino acid transporter 2 levels in
basolateral amygdala astrocytes mediate chronic stress-induced
anxiety-like behavior. Neural Regen Res. 20:1721–1734. 2025.
View Article : Google Scholar
|
|
155
|
Stephenson RA, Sepulveda J, Johnson KR,
Lita A, Gopalakrishnan J, Acri DJ, Beilina A, Cheng L, Yang LG,
Root JT, et al: Triglyceride metabolism controls inflammation and
microglial phenotypes associated with APOE4. Cell Rep.
44:1159612025. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Liu L, Zhang K, Sandoval H, Yamamoto S,
Jaiswal M, Sanz E, Li Z, Hui J, Graham BH, Quintana A and Bellen
HJ: Glial lipid droplets and ROS induced by mitochondrial defects
promote neurodegeneration. Cell. 160:177–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Jernberg JN, Bowman CE, Wolfgang MJ and
Scafidi S: Developmental regulation and localization of carnitine
palmitoyltransferases (CPTs) in rat brain. J Neurochem.
142:407–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Dressman JW, Bayram MF, Angel PM, Drake RR
and Mehta AS: Single-cell multiomic MALDI-MSI analysis of lipids
and N-glycans through affinity array capture. Anal Chem.
97:12493–12502. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Endle H, Horta G, Stutz B, Muthuraman M,
Tegeder I, Schreiber Y, Snodgrass IF, Gurke R, Liu ZW, Sestan-Pesa
M, et al: AgRP neurons control feeding behaviour at cortical
synapses via peripherally derived lysophospholipids. Nat Metab.
4:683–692. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Bitar L, Uphaus T, Thalman C, Muthuraman
M, Gyr L, Ji H, Domingues M, Endle H, Groppa S, Steffen F, et al:
Inhibition of the enzyme autotaxin reduces cortical excitability
and ameliorates the outcome in stroke. Sci Transl Med.
14:eabk1352022. View Article : Google Scholar
|
|
161
|
Xia J, Yang L, Huang C, Deng S, Yang Z,
Zhang Y, Zhang C and Song C: Omega-3 polyunsaturated fatty acid
eicosapentaenoic acid or docosahexaenoic acid improved
ageing-associated cognitive decline by regulating glial
polarization. Mar Drugs. 21:3982023. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zgorzynska E, Stulczewski D, Dziedzic B,
Su KP and Walczewska A: Docosahexaenoic fatty acid reduces the
pro-inflammatory response induced by IL-1β in astrocytes through
inhibition of NF-ĸb and AP-1 transcription factor activation. BMC
Neurosci. 22:42021. View Article : Google Scholar
|
|
163
|
Konttinen H, Gureviciene I, Oksanen M,
Grubman A, Loppi S, Huuskonen MT, Korhonen P, Lampinen R, Keuters
M, Belaya I, et al: PPARβ/δ-agonist GW0742 ameliorates dysfunction
in fatty acid oxidation in PSEN1δE9 astrocytes. Glia. 67:146–159.
2019. View Article : Google Scholar
|
|
164
|
Chehaibi K, le Maire L, Bradoni S, Escola
JC, Blanco-Vaca F and Slimane MN: Effect of PPAR-β/δ agonist GW0742
treatment in the acute phase response and blood-brain barrier
permeability following brain injury. Transl Res. 182:27–48. 2017.
View Article : Google Scholar
|
|
165
|
Spencer M, Kulbe JR, Venkatesh V, Laird A,
Ford M, O'Brien S, Boustani A, Schlachetzki JCM and Fields JA:
Caloric restriction mimetic 2-deoxyglucose alters metabolic and
transcriptomic phenotype in association with changes in chromatin
accessibility in human astrocytes. Sci Rep. 15:193682025.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Kratzer A, Buchebner M, Pfeifer T, Becker
TM, Uray G, Miyazaki M, Miyazaki-Anzai S, Ebner B, Chandak PG,
Kadam RS, et al: Synthetic LXR agonist attenuates plaque formation
in apoE−/− mice without inducing liver steatosis and
hypertriglyceridemia. J Lipid Res. 50:312–326. 2009. View Article : Google Scholar :
|
|
167
|
Goodman LD, Ralhan I, Li X, Lu S, Moulton
MJ, Park YJ, Zhao P, Kanca O, Ghaderpour Taleghani ZS, Jacquemyn J,
et al: Tau is required for glial lipid droplet formation and
resistance to neuronal oxidative stress. Nat Neurosci.
27:1918–1933. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Cheng X, Geng F, Pan M, Wu X, Zhong Y,
Wang C, Tian Z, Cheng C, Zhang R, Puduvalli V, et al: Targeting
DGAT1 ameliorates glioblastoma by increasing fat catabolism and
oxidative stress. Cell Metab. 32:229–242 e8. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Lee H, Wheeler MA and Quintana FJ:
Function and therapeutic value of astrocytes in neurological
diseases. Nat Rev Drug Discov. 21:339–358. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
van Deijk AF, Camargo N, Timmerman J,
Heistek T, Brouwers JF, Mogavero F, Mansvelder HD, Smit AB and
Verheijen MH: Astrocyte lipid metabolism is critical for synapse
development and function in vivo. Glia. 65:670–682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Lee JA, Hall B, Allsop J, Alqarni R and
Allen SP: Lipid metabolism in astrocytic structure and function.
Semin Cell Dev Biol. 112:123–136. 2021. View Article : Google Scholar
|