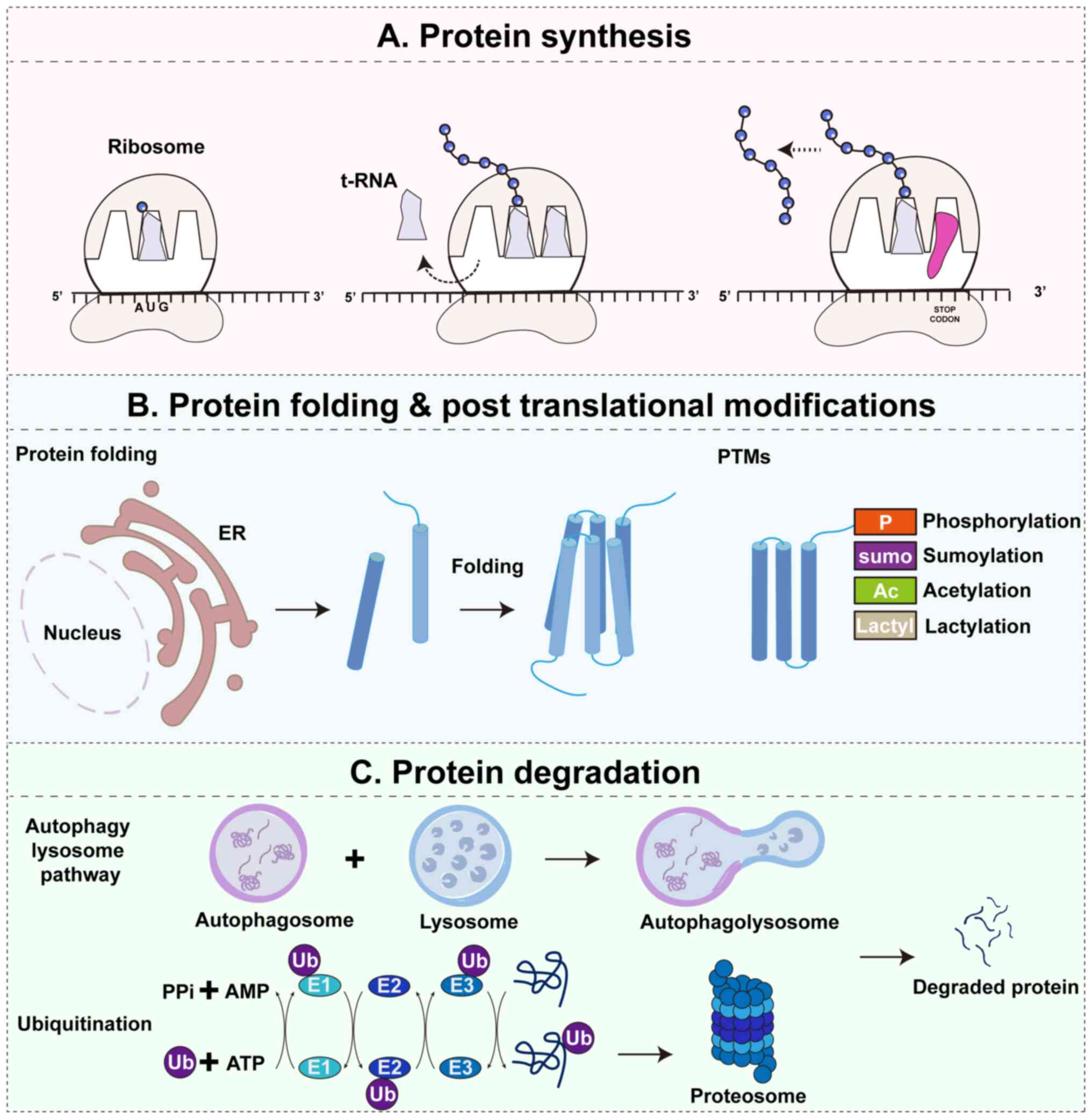
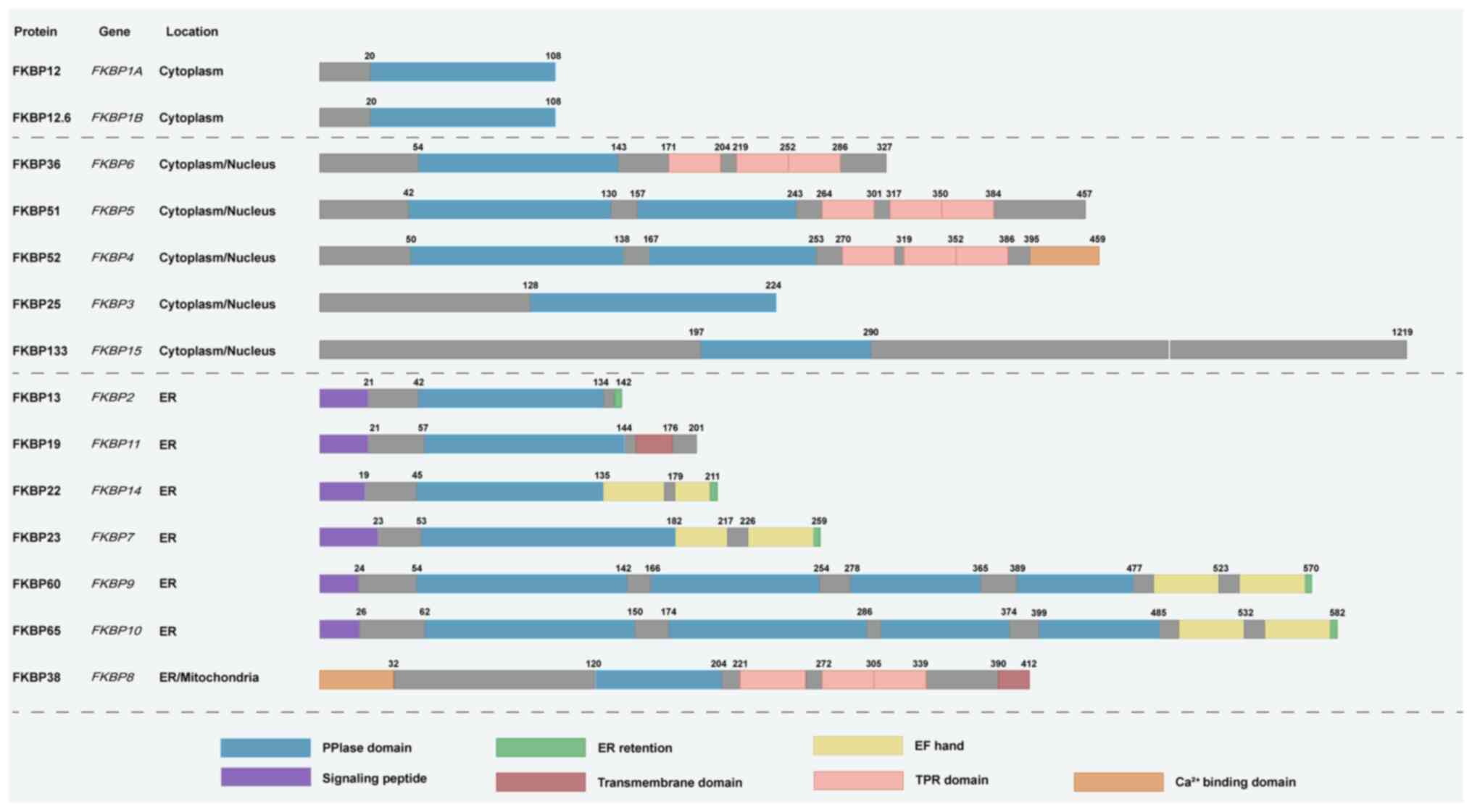
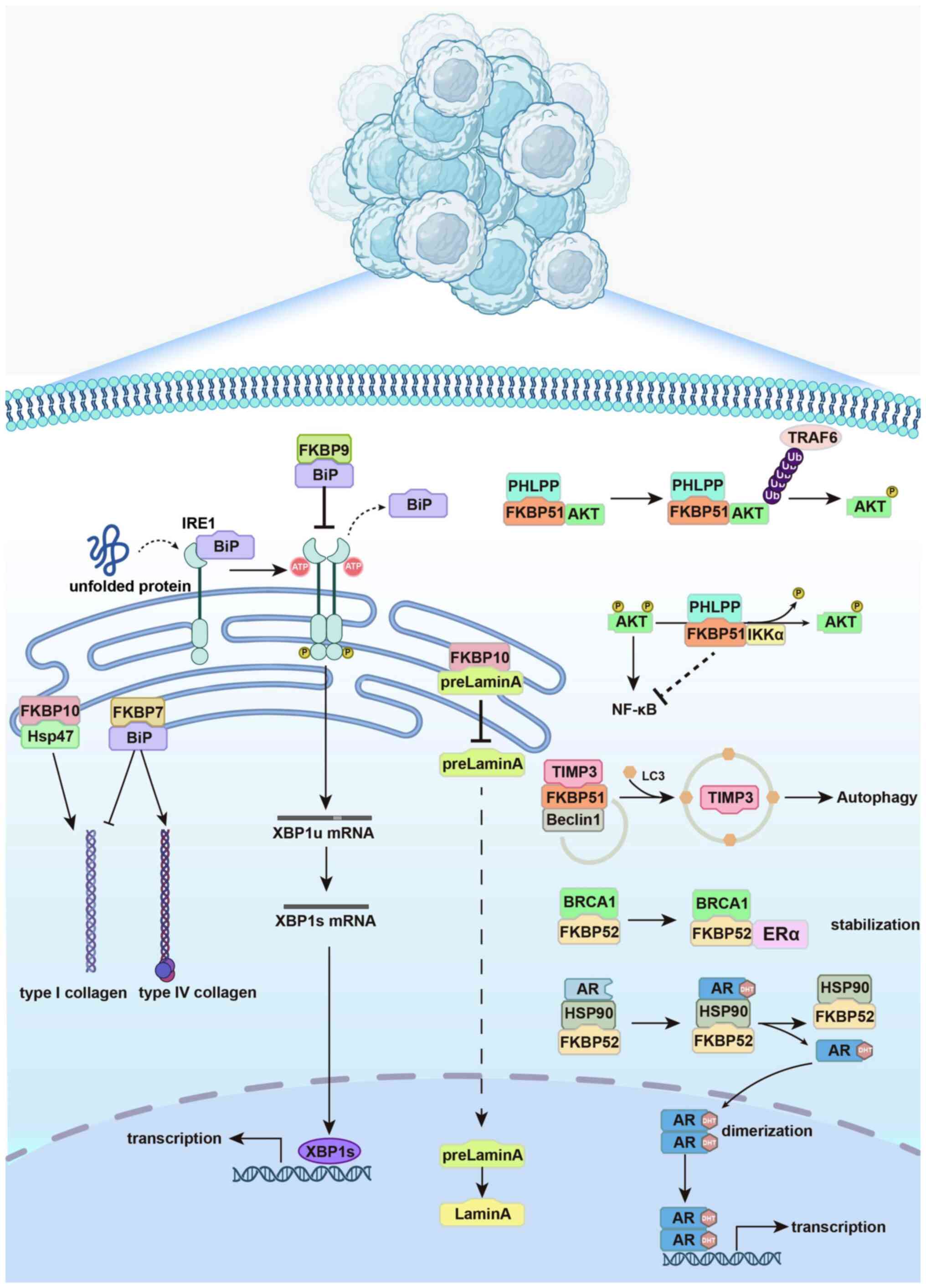
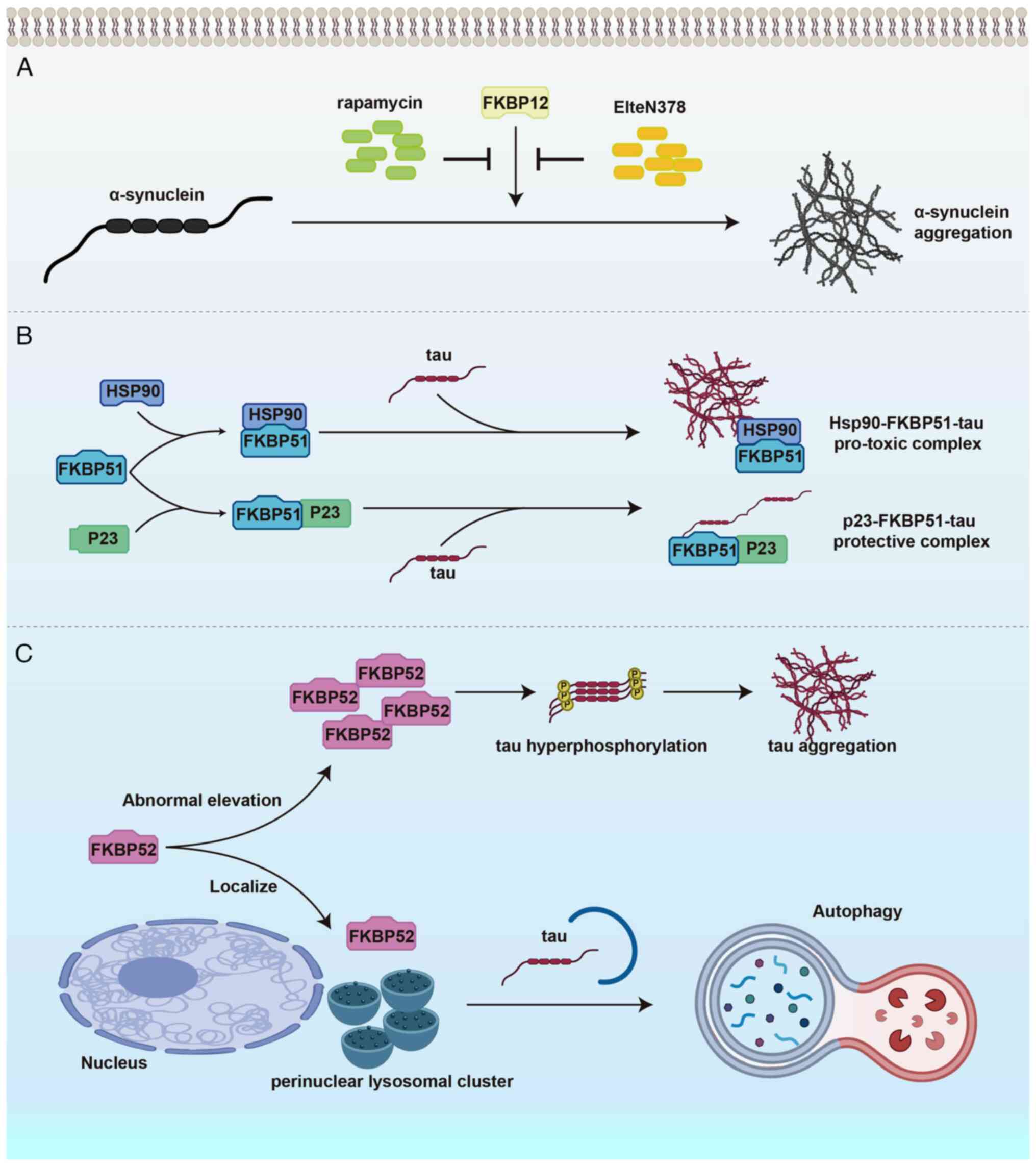
|
1
|
Díaz-Hung ML and Hetz C: Proteostasis and
resilience: On the interphase between individual's and
intracellular stress. Trends Endocrinol Metab. 33:305–317. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hetz C: Adapting the proteostasis capacity
to sustain brain healthspan. Cell. 184:1545–1560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weinberg J, Gaur M, Swaroop A and Taylor
A: Proteostasis in aging-associated ocular disease. Mol Aspects
Med. 88:1011572022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gressler AE, Leng H, Zinecker H and Simon
AK: Proteostasis in T cell aging. Semin Immunol. 70:1018382023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lu Q, Qin X, Chen C, Yu W, Lin J, Liu X,
Guo R, Reiter RJ, Ashrafizadeh M, Yuan M and Ren J: Elevated levels
of alcohol dehydrogenase aggravate ethanol-evoked cardiac
remodeling and contractile anomalies through FKBP5-yap-mediated
regulation of ferroptosis and ER stress. Life Sci. 343:1225082024.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jeanne X, Török Z, Vigh L and Prodromou C:
The role of the FKBP51-Hsp90 complex in Alzheimer's disease: An
emerging new drug target. Cell Stress Chaperones. 29:792–804. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakamura K, Aoyama-Ishiwatari S, Nagao T,
Paaran M, Obara CJ, Sakurai-Saito Y, Johnston J, Du Y, Suga S,
Tsuboi M, et al: Mitochondrial complexity is regulated at
ER-mitochondria contact sites via PDZD8-FKBP8 tethering. Nat
Commun. 16:34012025. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akbar M, Toppo P and Nazir A: Ageing,
proteostasis, and the gut: Insights into neurological health and
disease. Ageing Res Rev. 101:1025042024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bailus BJ, Scheeler SM, Simons J, Sanchez
MA, Tshilenge KT, Creus-Muncunill J, Naphade S, Lopez-Ramirez A,
Zhang N, Lakshika Madushani K, et al: Modulating FKBP5/FKBP51 and
autophagy lowers HTT (huntingtin) levels. Autophagy. 17:4119–4140.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cai HQ, Zhang MJ, Cheng ZJ, Yu J, Yuan Q,
Zhang J, Cai Y, Yang LY, Zhang Y, Hao JJ, et al: FKBP10 promotes
proliferation of glioma cells via activating AKT-CREB-PCNA axis. J
Biomed Sci. 28:132021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
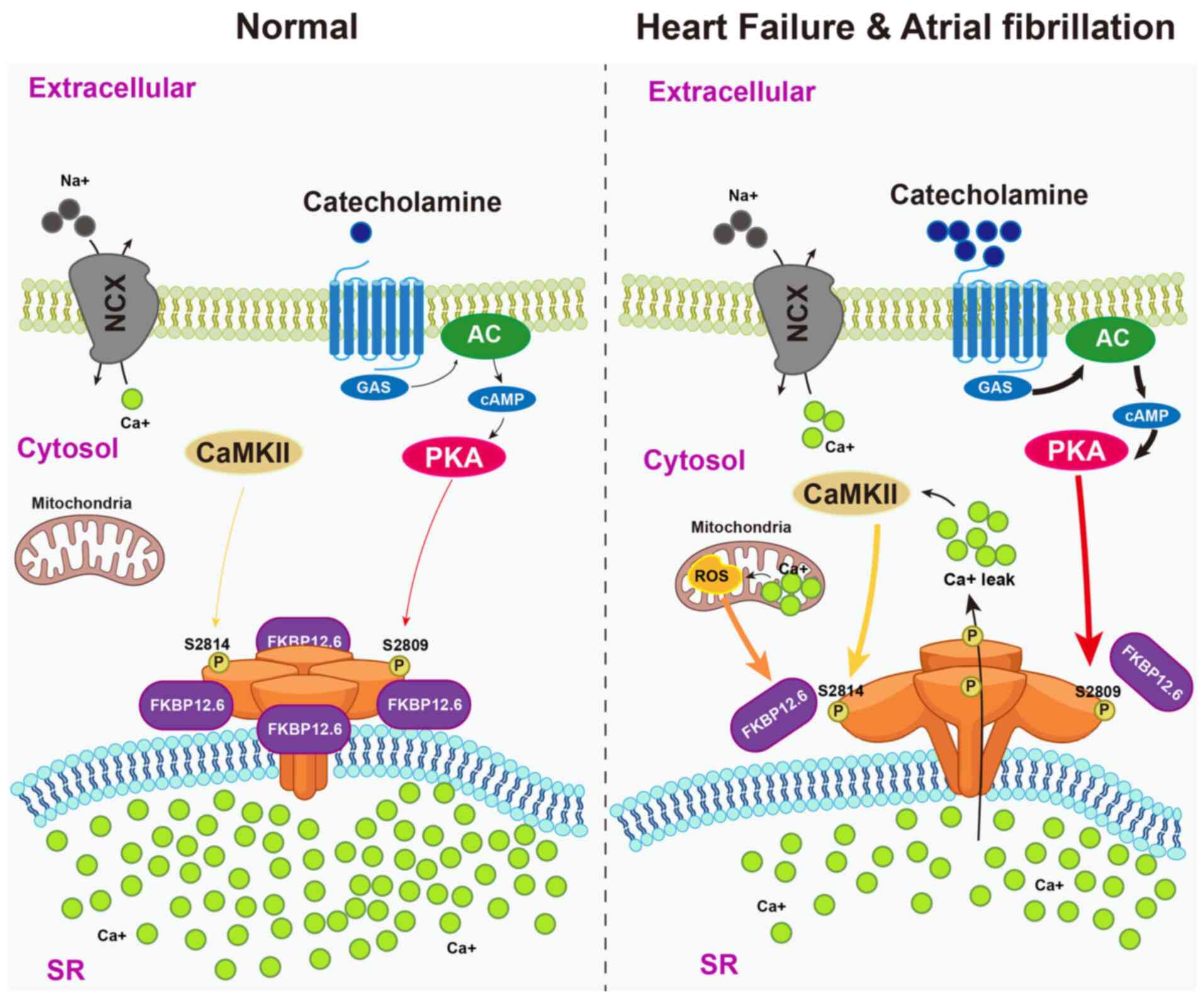
Mei L, Zheng YM, Song T, Yadav VR, Joseph
LC, Truong L, Kandhi S, Barroso MM, Takeshima H, Judson MA and Wang
YX: Rieske iron-sulfur protein induces FKBP12.6/RyR2 complex
remodeling and subsequent pulmonary hypertension through
NF-κB/cyclin D1 pathway. Nat Commun. 11:35272020. View Article : Google Scholar
|
|
12
|
Chambraud B, Daguinot C, Guillemeau K,
Genet M, Dounane O, Meduri G, Poüs C, Baulieu EE and Giustiniani J:
Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal
positioning and MAPT/Tau behavior during MAPT/Tau-induced
proteotoxic stress. Autophagy. 17:3491–3510. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
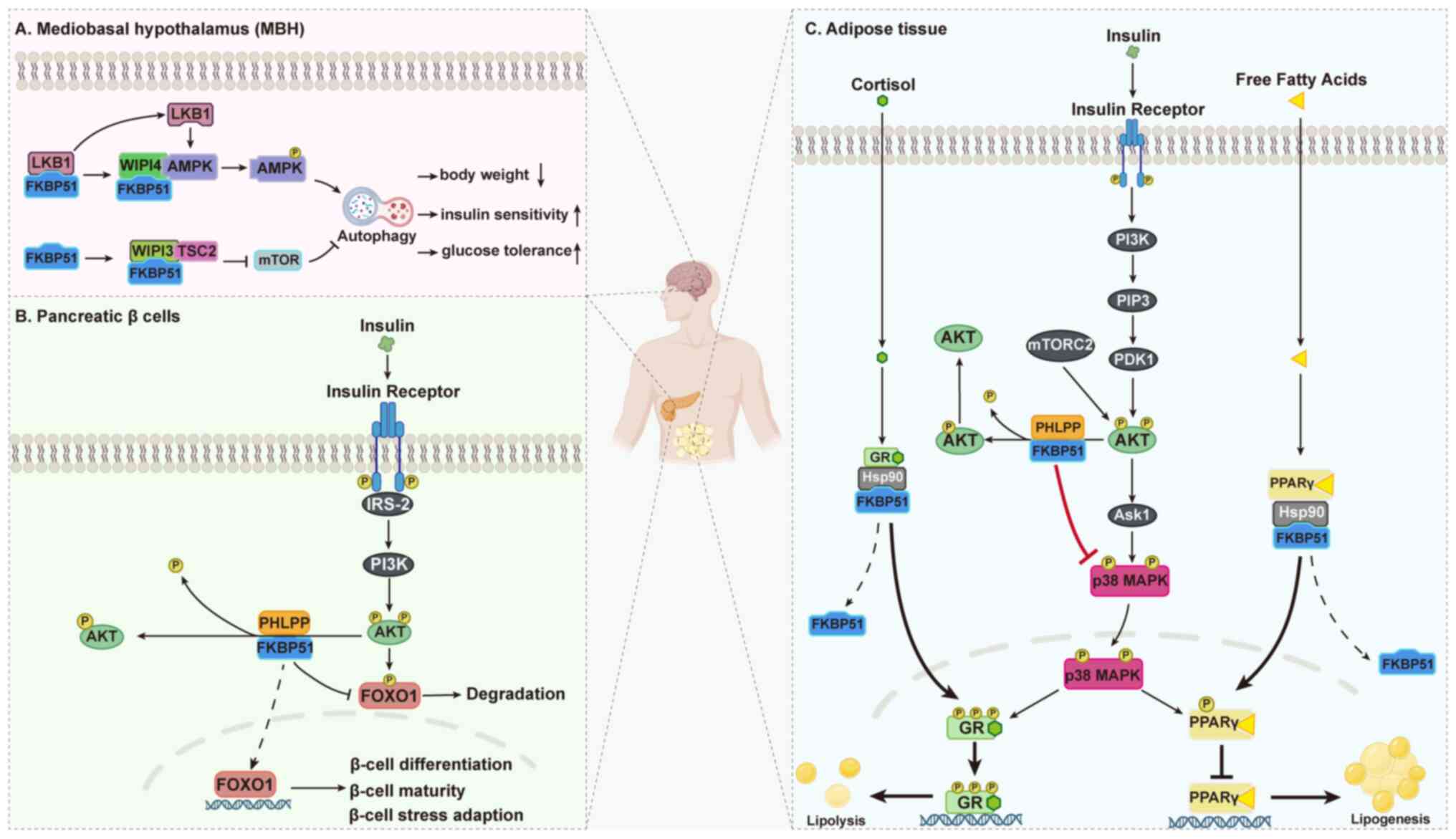
Ke H, Chen Z, Chen L, Zhang H, Wang Y,
Song T, Bi A, Li Q, Sheng H, Jia Y, et al: FK506-binding proteins:
Emerging target and therapeutic opportunity in multiple tumors. Int
J Biol Macromol. 307:1419142025. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jiang L, Chakraborty P, Zhang L, Wong M,
Hill SE, Webber CJ, Libera J, Blair LJ, Wolozin B and Zweckstetter
M: Chaperoning of specific tau structure by immunophilin FKBP12
regulates the neuronal resilience to extracellular stress. Sci Adv.
9:eadd97892023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Richardson SJ, Thekkedam CG, Casarotto MG,
Beard NA and Dulhunty AF: FKBP12 binds to the cardiac ryanodine
receptor with negative cooperativity: Implications for heart muscle
physiology in health and disease. Philos Trans R Soc Lond B Biol
Sci. 378:202201692023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smedlund KB, Sanchez ER and Hinds TD Jr:
FKBP51 and the molecular chaperoning of metabolism. Trends
Endocrinol Metab. 32:862–874. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cozachenco D, Ribeiro FC and Ferreira ST:
Defective proteostasis in Alzheimer's disease. Ageing Res Rev.
85:1018622023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dikic I and Schulman BA: An expanded
lexicon for the ubiquitin code. Nat Rev Mol Cell Biol. 24:273–287.
2023. View Article : Google Scholar
|
|
19
|
Nixon RA and Rubinsztein DC: Mechanisms of
autophagy-lysosome dysfunction in neurodegenerative diseases. Nat
Rev Mol Cell Biol. 25:926–946. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen X, Shi C, He M, Xiong S and Xia X:
Endoplasmic reticulum stress: Molecular mechanism and therapeutic
targets. Signal Transduct Target Ther. 8:3522023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Agam G, Atawna B, Damri O and Azab AN: The
Role of FKBPs in complex disorders: Neuropsychiatric diseases,
cancer, and type 2 diabetes mellitus. Cells. 13:8012024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Stauffer WT, Goodman AZ and Gallay PA:
Cyclophilin inhibition as a strategy for the treatment of human
disease. Front Pharmacol. 15:14179452024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhuang S, Chakraborty P and Zweckstetter
M: Regulation of tau by peptidyl-prolyl isomerases. Curr Opin
Struct Biol. 84:1027392024. View Article : Google Scholar
|
|
24
|
Deutscher RCE, Meyners C, Repity ML,
Sugiarto WO, Kolos JM, Maciel EVS, Heymann T, Geiger TM, Knapp S,
Lermyte F and Hausch F: Discovery of fully synthetic FKBP12-mTOR
molecular glues. Chem Sci. 16:4256–4263. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hanaki S and Shimada M: Impact of FKBP52
on cell proliferation and hormone-dependent cancers. Cancer Sci.
114:2729–2738. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soto OB, Ramirez CS, Koyani R,
Rodriguez-Palomares IA, Dirmeyer JR, Grajeda B, Roy S and Cox MB:
Structure and function of the TPR-domain immunophilins FKBP51 and
FKBP52 in normal physiology and disease. J Cell Biochem.
125:e304062024. View Article : Google Scholar
|
|
27
|
Singh MK, Shin Y, Ju S, Han S, Choe W,
Yoon KS, Kim SS and Kang I: Heat shock response and heat shock
proteins: Current understanding and future opportunities in human
diseases. Int J Mol Sci. 25:42092024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gu J, He Y, He C, Zhang Q, Huang Q, Bai S,
Wang R, You Q and Wang L: Advances in the structures, mechanisms
and targeting of molecular chaperones. Signal Transduct Target
Ther. 10:842025. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pokhrel S, Devi S and Gestwicki JE:
Chaperone-dependent and chaperone-independent functions of
carboxylate clamp tetratricopeptide repeat (CC-TPR) proteins.
Trends Biochem Sci. 50:121–133. 2025. View Article : Google Scholar
|
|
30
|
Baischew A, Engel S, Taubert MC, Geiger TM
and Hausch F: Large-scale, in-cell photocrosslinking at
single-residue resolution reveals the molecular basis for
glucocorticoid receptor regulation by immunophilins. Nat Struct Mol
Biol. 30:1857–1866. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Noddings CM, Johnson JL and Agard DA:
Cryo-EM reveals how Hsp90 and FKBP immunophilins co-regulate the
glucocorticoid receptor. Nat Struct Mol Biol. 30:1867–1877. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jarayseh T, Debaenst S, De Saffel H,
Rosseel T, Milazzo M, Bek JW, Hudson DM, Van Nieuwerburgh F,
Gansemans Y, Josipovic I, et al: Bmpr1aa modulates the severity of
the skeletal phenotype in an fkbp10-deficient Bruck syndrome
zebrafish model. J Bone Miner Res. 40:154–166. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishikawa Y, Bonna A, Gould DB and Farndale
RW: Local net charge state of collagen triple helix is a
determinant of FKBP22 binding to collagen III. Int J Mol Sci.
24:151562023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Herrema H, Guan D, Choi JW, Feng X,
Salazar Hernandez MA, Faruk F, Auen T, Boudett E, Tao R, Chun H and
Ozcan U: FKBP11 rewires UPR signaling to promote glucose
homeostasis in type 2 diabetes and obesity. Cell Metab.
34:1004–1022.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Akintade DD and Chaudhuri B: FK506-binding
protein 2 (FKBP13) inhibit Bax-induced apoptosis in Saccharomyces
cerevisiae (yeast). Cell Biol Toxicol. 39:719–728. 2023. View Article : Google Scholar :
|
|
36
|
Yang Y, Chen X, Yao W, Cui X, Li N, Lin Z,
Zhao B and Miao J: Esterase D stabilizes FKBP25 to suppress mTORC1.
Cell Mol Biol Lett. 26:502021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao RQ, Ren C, Xia ZF and Yao YM:
Organelle-specific autophagy in inflammatory diseases: A potential
therapeutic target underlying the quality control of multiple
organelles. Autophagy. 17:385–401. 2021. View Article : Google Scholar :
|
|
38
|
López-Otín C, Blasco MA, Partridge L,
Serrano M and Kroemer G: Hallmarks of aging: An expanding universe.
Cell. 186:243–278. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li S, Xia W, Sun B, Peng W, Yang D, Gao J,
He S, Yang H, Zhu Y, Zhou H, et al: The stability of FKBP9
maintained by BiP is crucial for glioma progression. Genes Dis.
11:1011232023. View Article : Google Scholar
|
|
40
|
Xu H, Liu P, Yan Y, Fang K, Liang D, Hou
X, Zhang X, Wu S, Ma J, Wang R, et al: FKBP9 promotes the malignant
behavior of glioblastoma cells and confers resistance to
endoplasmic reticulum stress inducers. J Exp Clin Cancer Res.
39:442020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quemerais C, Jean C, Brunel A, Decaup E,
Labrousse G, Audureau H, Raffenne J, Belhabib I, Cros J, Perraud A,
et al: Unveiling FKBP7 as an early endoplasmic reticulum sentinel
in pancreatic stellate cell activation, collagen remodeling and
tumor progression. Cancer Lett. 614:2175382025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao X, Wang J, Tian S, Tang L, Cao S, Ye
J, Cai T, Xuan Y, Zhang X, Li X and Li H: FKBP10 promotes the
muscle invasion of bladder cancer via lamin A dysregulation. Int J
Biol Sci. 21:758–771. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ramadori G, Ioris RM, Villanyi Z, Firnkes
R, Panasenko OO, Allen G, Konstantinidou G, Aras E, Brenachot X,
Biscotti T, et al: FKBP10 regulates protein translation to sustain
lung cancer growth. Cell Rep. 30:3851–3863.e6. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma W, Li X, Yang L, Pan J, Chen Y, Lu Y,
Dong X, Li D and Gan W: High VSX1 expression promotes the
aggressiveness of clear cell renal cell carcinoma by
transcriptionally regulating FKBP10. J Transl Med. 20:5542022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu R, Zou Z, Chen L, Feng Y, Ye J, Deng
Y, Zhu X, Zhang Y, Lin J, Cai S, et al: FKBP10 promotes clear cell
renal cell carcinoma progression and regulates sensitivity to the
HIF2α blockade by facilitating LDHA phosphorylation. Cell Death
Dis. 15:642024. View Article : Google Scholar
|
|
46
|
Fu Y, Chen J, Ma X, Chang W, Zhang X, Liu
Y, Liu Y, Shen H, Hu X and Ren AJ: Subcellular expression patterns
of FKBP prolyl isomerase 10 (FKBP10) in colorectal cancer and its
clinical significance. Int J Mol Sci. 24:114152023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li W, Li F, Zhang X, Lin HK and Xu C:
Insights into the post-translational modification and its emerging
role in shaping the tumor microenvironment. Signal Transduct Target
Ther. 6:4222021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tufano M, Marrone L, D'Ambrosio C, Di
Giacomo V, Urzini S, Xiao Y, Matuozzo M, Scaloni A, Romano MF and
Romano S: FKBP51 plays an essential role in Akt ubiquitination that
requires Hsp90 and PHLPP. Cell Death Dis. 14:1162023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Luo K, Li Y, Yin Y, Li L, Wu C, Chen Y,
Nowsheen S, Hu Q, Zhang L, Lou Z and Yuan J: USP49 negatively
regulates tumorigenesis and chemoresistance through FKBP51-AKT
signaling. EMBO J. 36:1434–1446. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Shang Z, Yu J, Sun L, Tian J, Zhu S, Zhang
B, Dong Q, Jiang N, Flores-Morales A, Chang C and Niu Y: LncRNA
PCAT1 activates AKT and NF-κB signaling in castration-resistant
prostate cancer by regulating the PHLPP/FKBP51/IKKα complex.
Nucleic Acids Res. 47:4211–4225. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tufano M, Cesaro E, Martinelli R, Pacelli
R, Romano S and Romano MF: FKBP51 affects TNF-related apoptosis
inducing ligand response in melanoma. Front Cell Dev Biol.
9:7189472021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mao S, Zhang D, Chen L, Tan J, Chu Y,
Huang S, Zhou W, Qin H, Xia Q, Zhao Y, et al: FKBP51 promotes
invasion and migration by increasing the autophagic degradation of
TIMP3 in clear cell renal cell carcinoma. Cell Death Dis.
12:8992021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Batko J, Antosz K, Miśków W, Pszczołowska
M, Walczak K and Leszek J: Chaperones-A new class of potential
therapeutic targets in Alzheimer's disease. Int J Mol Sci.
25:34012024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Maeda K, Habara M, Kawaguchi M, Matsumoto
H, Hanaki S, Masaki T, Sato Y, Matsuyama H, Kunieda K, Nakagawa H
and Shimada M: FKBP51 and FKBP52 regulate androgen receptor
dimerization and proliferation in prostate cancer cells. Mol Oncol.
16:940–956. 2022. View Article : Google Scholar :
|
|
55
|
Habara M, Sato Y, Goshima T, Sakurai M,
Imai H, Shimizu H, Katayama Y, Hanaki S, Masaki T, Morimoto M, et
al: FKBP52 and FKBP51 differentially regulate the stability of
estrogen receptor in breast cancer. Proc Natl Acad Sci USA.
119:e21102561192022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Xiong H, Chen Z, Lin B, Xie B, Liu X, Chen
C, Li Z, Jia Y, Wu Z, Yang M, et al: Naringenin regulates
FKBP4/NR3C1/NRF2 axis in autophagy and proliferation of breast
cancer and differentiation and maturation of dendritic cell. Front
Immunol. 12:7451112022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Mangé A, Coyaud E, Desmetz C, Laurent E,
Béganton B, Coopman P, Raught B and Solassol J: FKBP4 connects
mTORC2 and PI3K to activate the PDK1/Akt-dependent cell
proliferation signaling in breast cancer. Theranostics.
9:7003–7015. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kim MJ, Choi GE, Chae CW, Lim JR, Jung YH,
Yoon JH, Park JY and Han HJ: Melatonin-mediated FKBP4
downregulation protects against stress-induced neuronal
mitochondria dysfunctions by blocking nuclear translocation of GR.
Cell Death Dis. 14:1462023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zong S, Jiao Y, Liu X, Mu W, Yuan X, Qu Y,
Xia Y, Liu S, Sun H, Wang L, et al: FKBP4 integrates
FKBP4/Hsp90/IKK with FKBP4/Hsp70/RelA complex to promote lung
adenocarcinoma progression via IKK/NF-κB signaling. Cell Death Dis.
12:6022021. View Article : Google Scholar
|
|
60
|
Wilson DM III, Cookson MR, Van Den Bosch
L, Zetterberg H, Holtzman DM and Dewachter I: Hallmarks of
neurodegenerative diseases. Cell. 186:693–714. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Creekmore BC, Watanabe R and Lee EB:
Neurodegenerative disease tauopathies. Annu Rev Pathol. 19:345–370.
2024. View Article : Google Scholar :
|
|
62
|
Weintraub D, Aarsland D, Chaudhuri KR,
Dobkin RD, Leentjens AF, Rodriguez-Violante M and Schrag A: The
neuropsychiatry of Parkinson's disease: Advances and challenges.
Lancet Neurol. 21:89–102. 2022. View Article : Google Scholar :
|
|
63
|
Morris HR, Spillantini MG, Sue CM and
Williams-Gray CH: The pathogenesis of Parkinson's disease. Lancet.
403:293–304. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ye H, Robak LA, Yu M, Cykowski M and
Shulman JM: Genetics and pathogenesis of Parkinson's syndrome. Annu
Rev Pathol. 18:95–121. 2023. View Article : Google Scholar
|
|
65
|
Ding XB, Wang XX, Xia DH, Liu H, Tian HY,
Fu Y, Chen YK, Qin C, Wang JQ, Xiang Z, et al: Impaired meningeal
lymphatic drainage in patients with idiopathic Parkinson's disease.
Nat Med. 27:411–418. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Caminati G and Procacci P: Mounting
evidence of FKBP12 implication in neurodegeneration. Neural Regen
Res. 15:2195–2202. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Caraveo G, Soste M, Cappelleti V, Fanning
S, van Rossum DB, Whitesell L, Huang Y, Chung CY, Baru V, Zaichick
S, et al: FKBP12 contributes to α-synuclein toxicity by regulating
the calcineurin-dependent phosphoproteome. Proc Natl Acad Sci USA.
114:E11313–E11322. 2017. View Article : Google Scholar
|
|
68
|
Zhang Z, Shen Z, Xie S, Li J, Zhang Z,
Zhang S, Peng B and Huang Q, Li M, Ma S and Huang Q: Rapamycin
exerts neuroprotective effects by inhibiting FKBP12 instead of
mTORC1 in the mouse model of Parkinson's disease.
Neuropharmacology. 275:1105042025. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Caminati G, Martina MR, Menichetti S and
Procacci P: Blocking the FKBP12 induced dendrimeric burst in
aberrant aggregation of α-synuclein by using the ElteN378 synthetic
inhibitor. J Enzyme Inhib Med Chem. 34:1711–1715. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Scheltens P, De Strooper B, Kivipelto M,
Holstege H, Chételat G, Teunissen CE, Cummings J and van der Flier
WM: Alzheimer's disease. Lancet. 397:1577–1590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Liu E, Zhang Y and Wang JZ: Updates in
Alzheimer's disease: From basic research to diagnosis and
therapies. Transl Neurodegener. 13:452024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Zheng Q and Wang X: Alzheimer's disease:
Insights into pathology, molecular mechanisms, and therapy. Protein
Cell. 16:83–120. 2025. View Article : Google Scholar
|
|
73
|
Ossenkoppele R, van der Kant R and Hansson
O: Tau biomarkers in Alzheimer's disease: Towards implementation in
clinical practice and trials. Lancet Neurol. 21:726–734. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Carter SF, Herholz K, Rosa-Neto P,
Pellerin L, Nordberg A and Zimmer ER: Astrocyte biomarkers in
Alzheimer's disease. Trends Mol Med. 25:77–95. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Graff-Radford J, Yong KXX, Apostolova LG,
Bouwman FH, Carrillo M, Dickerson BC, Rabinovici GD, Schott JM,
Jones DT and Murray ME: New insights into atypical Alzheimer's
disease in the era of biomarkers. Lancet Neurol. 20:222–234. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Oroz J, Chang BJ, Wysoczanski P, Lee CT,
Pérez-Lara Á, Chakraborty P, Hofele RV, Baker JD, Blair LJ, Biernat
J, et al: Structure and pro-toxic mechanism of the human
Hsp90/PPIase/Tau complex. Nat Commun. 9:45322018. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Chakraborty P and Zweckstetter M:
Interplay of p23 with FKBP51 and their chaperone complex in
regulating tau aggregation. Nat Commun. 16:6692025. View Article : Google Scholar
|
|
78
|
Chambraud B, Byrne C, Meduri G, Baulieu EE
and Giustiniani J: FKBP52 in neuronal signaling and
neurodegenerative diseases: A microtubule story. Int J Mol Sci.
23:17382022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Criado-Marrero M, Gebru NT, Blazier DM,
Gould LA, Baker JD, Beaulieu-Abdelahad D and Blair LJ: Hsp90
co-chaperones, FKBP52 and Aha1, promote tau pathogenesis in aged
wild-type mice. Acta Neuropathol Commun. 9:652021. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Ren J, Bi Y, Sowers JR, Hetz C and Zhang
Y: Endoplasmic reticulum stress and unfolded protein response in
cardiovascular diseases. Nat Rev Cardiol. 18:499–521. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Abdellatif M, Rainer PP, Sedej S and
Kroemer G: Hallmarks of cardiovascular ageing. Nat Rev Cardiol.
20:754–777. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Marks AR: Targeting ryanodine receptors to
treat human diseases. J Clin Invest. 133:e1628912023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Keefe JA, Garber R, McCauley MD and
Wehrens XHT: Tachycardia and atrial fibrillation-related
cardiomyopathies: Potential mechanisms and current therapies. JACC
Heart Fail. 12:605–615. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Do TQ and Knollmann BC: Inhibitors of
intracellular RyR2 calcium release channels as therapeutic agents
in arrhythmogenic heart diseases. Annu Rev Pharmacol Toxicol.
65:443–463. 2025. View Article : Google Scholar
|
|
85
|
Grisorio L, Bongianino R, Gianeselli M and
Priori SG: Gene therapy for cardiac diseases: Methods, challenges,
and future directions. Cardiovasc Res. 120:1664–1682. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Papa A, Kushner J and Marx SO: Adrenergic
regulation of calcium channels in the heart. Annu Rev Physiol.
84:285–306. 2022. View Article : Google Scholar :
|
|
87
|
Shemarova I: The dysfunction of
Ca2+ channels in hereditary and chronic human heart
diseases and experimental animal models. Int J Mol Sci.
24:156822023. View Article : Google Scholar
|
|
88
|
Keefe JA, Moore OM, Ho KS and Wehrens XHT:
Role of Ca2+ in healthy and pathologic cardiac function:
From normal excitation-contraction coupling to mutations that cause
inherited arrhythmia. Arch Toxicol. 97:73–92. 2023. View Article : Google Scholar
|
|
89
|
Fowler ED and Zissimopoulos S: Molecular,
subcellular, and arrhythmogenic mechanisms in genetic RyR2 disease.
Biomolecules. 12:10302022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Do TQ and Knollmann BC: RYR2 as new target
for antiarrhythmic therapy: Harnessing the power of existing
chemical entities for drug discovery. Heart Rhythm. 22:1372–1373.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Szentandrássy N, Magyar Z, Hevesi J,
Bányász T, Nánási PP and Almássy J: Therapeutic approaches of
ryanodine receptor-associated heart diseases. Int J Mol Sci.
23:44352022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Benitah JP, Perrier R, Mercadier JJ,
Pereira L and Gómez AM: RyR2 and calcium release in heart failure.
Front Physiol. 12:7342102021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chi X, Gong D, Ren K, Zhou G, Huang G, Lei
J, Zhou Q and Yan N: Molecular basis for allosteric regulation of
the type 2 ryanodine receptor channel gating by key modulators.
Proc Natl Acad Sci USA. 116:25575–25582. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Dridi H, Kushnir A, Zalk R, Yuan Q,
Melville Z and Marks AR: Intracellular calcium leak in heart
failure and atrial fibrillation: A unifying mechanism and
therapeutic target. Nat Rev Cardiol. 17:732–747. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Steinberg GR and Hardie DG: New insights
into activation and function of the AMPK. Nat Rev Mol Cell Biol.
24:255–272. 2023. View Article : Google Scholar
|
|
96
|
Szwed A, Kim E and Jacinto E: Regulation
and metabolic functions of mTORC1 and mTORC2. Physiol Rev.
101:1371–1426. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Al-Kuraishy HM, Sulaiman GM, Mohsin MH,
Mohammed HA, Dawood RA, Albuhadily AK, Al-Gareeb AI, Albukhaty S
and Abomughaid MM: Targeting of AMPK/MTOR signaling in the
management of atherosclerosis: Outmost leveraging. Int J Biol
Macromol. 309:1429332025. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Zhang D, Lu C and Sang K: Exercise as a
metabolic regulator: Targeting AMPK/mTOR-autophagy crosstalk to
counteract sarcopenic obesity. Aging Dis. Jun 7–2025.Epub ahead of
print.
|
|
99
|
Cong Y, So V, Tijssen MAJ, Verbeek DS,
Reggiori F and Mauthe M: WDR45, one gene associated with multiple
neurodevelopmental disorders. Autophagy. 17:3908–3923. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Almannai M, Marafi D and El-Hattab AW:
WIPI proteins: Biological functions and related syndromes. Front
Mol Neurosci. 15:10119182022. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bajaj T, Häusl AS, Schmidt MV and Gassen
NC: FKBP5/FKBP51 on weight watch: Central FKBP5 links regulatory
WIPI protein networks to autophagy and metabolic control.
Autophagy. 18:2756–2858. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Häusl AS, Bajaj T, Brix LM, Pöhlmann ML,
Hafner K, De Angelis M, Nagler J, Dethloff F, Balsevich G, Schramm
KW, et al: Mediobasal hypothalamic FKBP51 acts as a molecular
switch linking autophagy to whole-body metabolism. Sci Adv.
8:eabi47972022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Campbell IH, Frye MA and Campbell H:
Metabolic plasticity: An evolutionary perspective on metabolic and
circadian dysregulation in bipolar disorder. Mol Psychiatry.
30:5600–5612. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tian S, Wu T, Zhang Z, Lv S, Ji X, Zhao Z,
Ma X, Wang J and Bi Y: Activation of central Angiotensin-(1-7)/Mas
receptor alleviates synaptic damage in diabetes-associated
cognitive impairment via modulating AKT/FOXO1/PACAP axis. Int J
Biol Sci. 21:2824–2842. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Yin B, Qian B, Yu H, Ke S, Li Z, Hua Y, Lu
S, Wang C, Li M, Guo S, et al: NNMT/1-MNA protects against hepatic
ischemia-reperfusion injury through the AKT/FOXO1/ANGPT2/JNK axis.
Nat Commun. 16:47792025. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang H, Bai R and Wang Y, Qu M, Zhou Y,
Gao Z and Wang Y: The multifaceted function of FoxO1 in pancreatic
β-cell dysfunction and insulin resistance: Therapeutic potential
for type 2 diabetes. Life Sci. 364:1233842025. View Article : Google Scholar
|
|
107
|
Lees J, Hay J, Moles MW and Michie AM: The
discrete roles of individual FOXO transcription factor family
members in B-cell malignancies. Front Immunol. 14:11791012023.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu N, Li R, Cao J, Song X, Ma W, Liu T,
Liang R, Zheng R and Wang S: The inhibition of FKBP5 protects
β-cell survival under inflammation stress via AKT/FOXO1 signaling.
Cell Death Discov. 9:2472023. View Article : Google Scholar
|
|
109
|
Salama SA and Elshafey MM: Cross-talk
between PPARγ, NF-κB, and p38 MAPK signaling mediates the
ameliorating effects of bergenin against the iron overload-induced
hepatotoxicity. Chem Biol Interact. 368:1102072022. View Article : Google Scholar
|
|
110
|
Kokkinopoulou I and Moutsatsou P:
Mitochondrial glucocorticoid receptors and their actions. Int J Mol
Sci. 22:60542021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zuo L, Kuo WT, Cao F, Chanez-Paredes SD,
Zeve D, Mannam P, Jean-François L, Day A, Vallen Graham W, Sweat
YY, et al: Tacrolimus-binding protein FKBP8 directs myosin light
chain kinase-dependent barrier regulation and is a potential
therapeutic target in Crohn's disease. Gut. 72:870–881. 2023.
View Article : Google Scholar
|
|
112
|
Dowling AL, Walbridge S, Ertekin C,
Namagiri S, Camacho K, Chowdhury A, Bryant JP, Kohut E, Heiss JD,
Brown DA, et al: FKBP38 regulates self-renewal and survival of GBM
neurospheres. Cells. 12:25622023. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Lee B, Oh Y, Cho E, DiAntonio A, Cavalli
V, Shin JE, Choi HW and Cho Y: FK506-binding protein-like and
FK506-binding protein 8 regulate dual leucine zipper kinase
degradation and neuronal responses to axon injury. J Biol Chem.
298:1016472022. View Article : Google Scholar : PubMed/NCBI
|