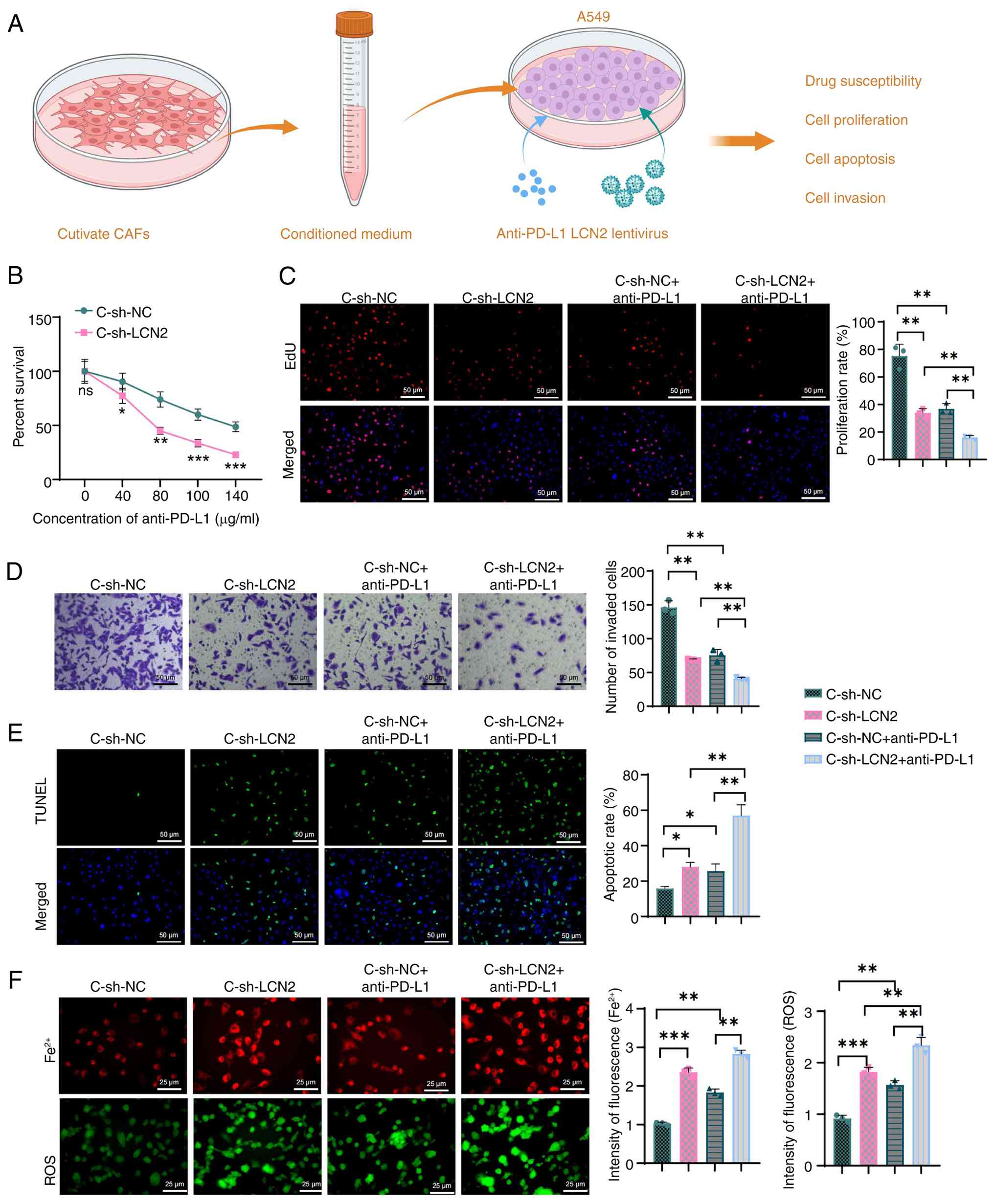
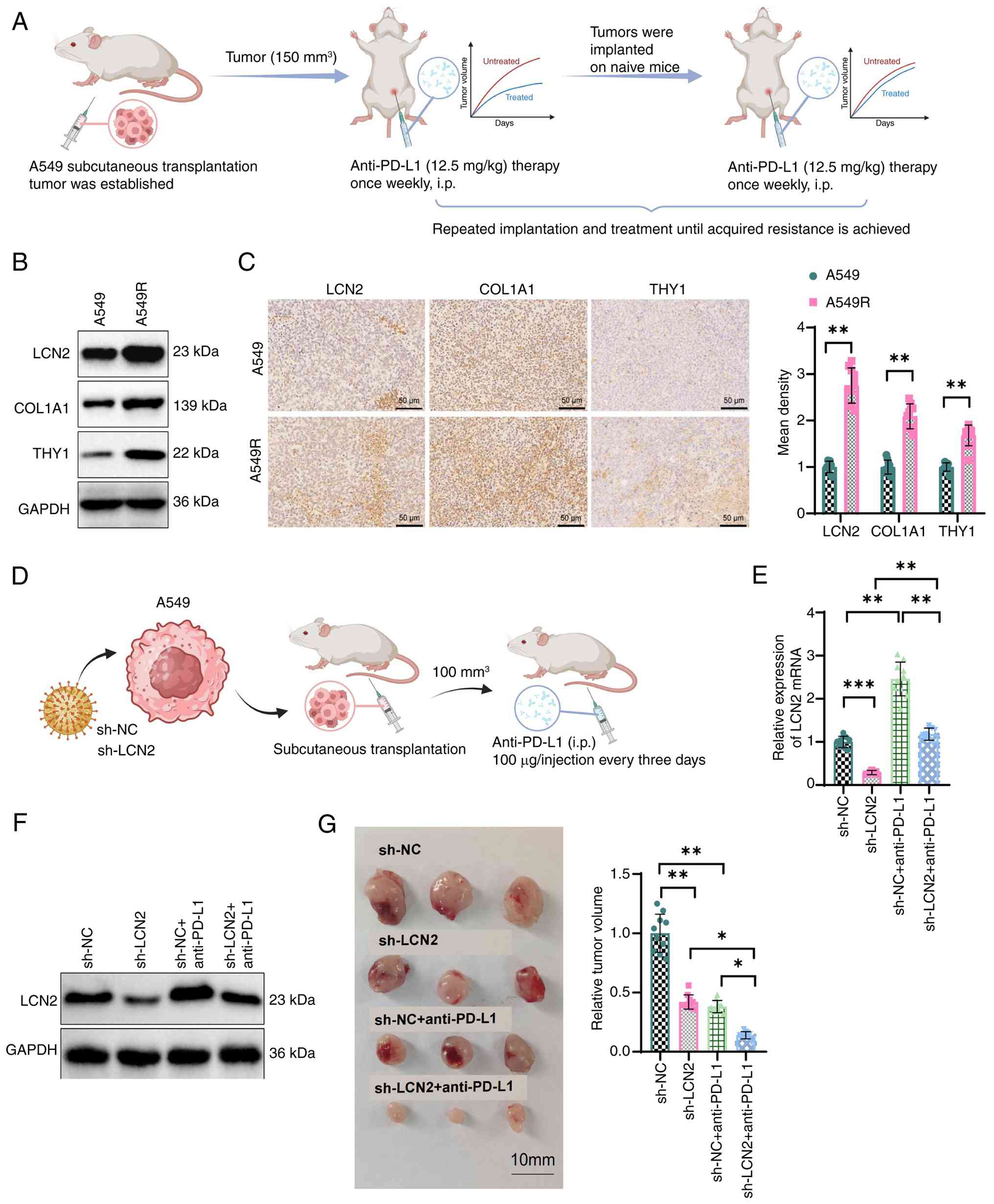
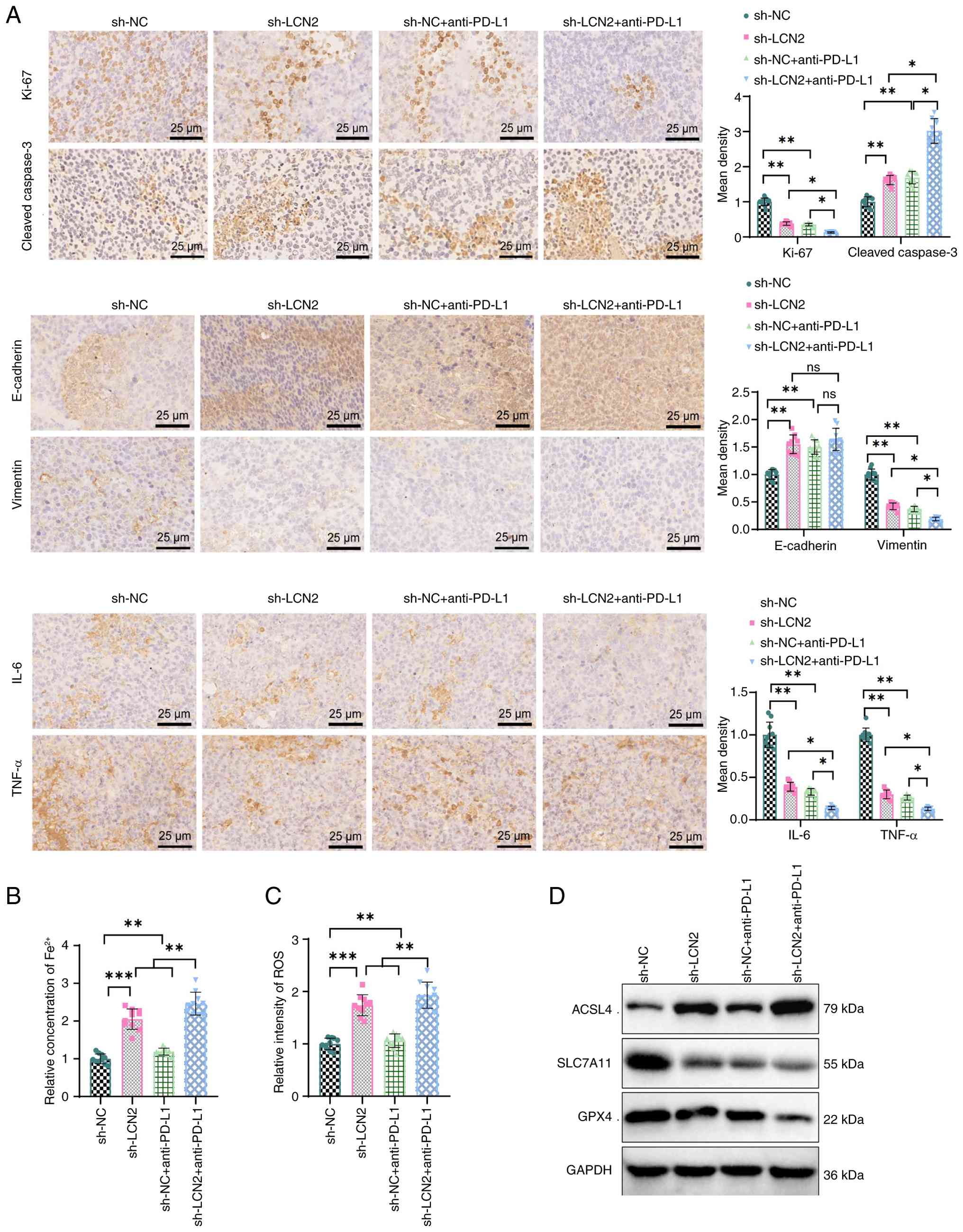
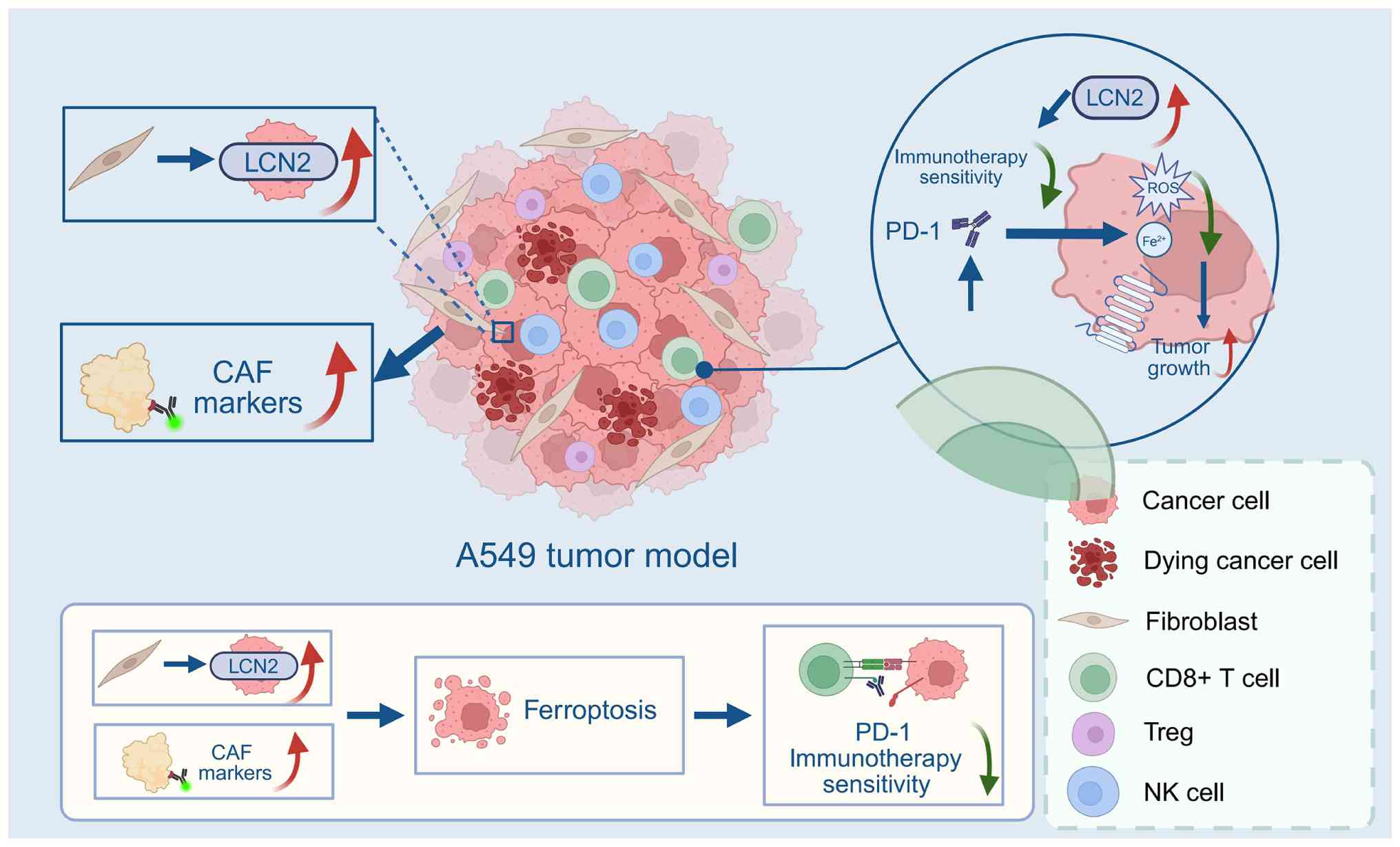
|
1
|
Nasim F, Sabath BF and Eapen GA: Lung
cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu F, Wang L and Zhou C: Lung cancer in
China: Current and prospect. Curr Opin Oncol. 33:40–46. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hoy H, Lynch T and Beck M: Surgical
treatment of lung cancer. Crit Care Nurs Clin North Am. 31:303–313.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou J, Xu Y, Liu J, Feng L, Yu J and Chen
D: Global burden of lung cancer in 2022 and projections to 2050:
Incidence and mortality estimates from GLOBOCAN. Cancer Epidemiol.
93:1026932024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herbst RS, Wu YL, John T, Grohe C, Majem
M, Wang J, Kato T, Goldman JW, Laktionov K, Kim SW, et al: Adjuvant
osimertinib for resected egfr-mutated stage IB-IIIA non-small-cell
lung cancer: Updated results from the phase III randomized ADAURA
trial. J Clin Oncol. 41:1830–1840. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Smith CEP and Prasad V: Targeted cancer
therapies. Am Fam Physician. 103:155–163. 2021.PubMed/NCBI
|
|
8
|
Paz-Ares LG, Ramalingam SS, Ciuleanu TE,
Lee JS, Urban L, Caro RB, Park K, Sakai H, Ohe Y, Nishio M, et al:
First-Line nivolumab plus ipilimumab in advanced NSCLC: 4-year
outcomes from the randomized, open-label, phase 3 CheckMate 227
part 1 trial. J Thorac Oncol. 17:289–308. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Heritage S, Sundaram S, Kirkby NF, Kirkby
KJ, Mee T and Jena R: An update to the malthus model for
radiotherapy utilisation in England. Clin Oncol (R Coll Radiol).
35:e1–e9. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shao J, Wang C, Ren P, Jiang Y, Tian P and
Li W: Treatment- and immune-related adverse events of immune
checkpoint inhibitors in advanced lung cancer. Biosci Rep.
40:BSR201923472020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hartsell WF, Simone CB II, Godes D,
Maggiore J, Mehta MP, Frank SJ, Metz JM and Choi JI: Temporal
evolution and diagnostic diversification of patients receiving
proton therapy in the United States: A ten-year trend analysis
(2012 to 2021) from the national association for proton therapy.
Int J Radiat Oncol Biol Phys. 119:1069–1077. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Niu X, Chen L, Li Y, Hu Z and He F:
Ferroptosis, necroptosis, and pyroptosis in the tumor
microenvironment: Perspectives for immunotherapy of SCLC. Semin
Cancer Biol. 86:273–285. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ito T, Nagashima H, Akiyama M, Utsumi Y,
Sato H, Chiba S, Sugai M, Ube K, Mori Y, Watanabe K, et al:
Treatment with immune checkpoint inhibitors after EGFR-TKIs in
EGFR-mutated lung cancer. Thorac Cancer. 13:386–393. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rangamuwa K, Leong T, Weeden C,
Asselin-Labat ML, Bozinovski S, Christie M, John T, Antippa P,
Irving L and Steinfort D: Thermal ablation in non-small cell lung
cancer: A review of treatment modalities and the evidence for
combination with immune checkpoint inhibitors. Transl Lung Cancer
Res. 10:2842–2857. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lahiri A, Maji A, Potdar PD, Singh N,
Parikh P, Bisht B, Mukherjee A and Paul MK: Lung cancer
immunotherapy: Progress, pitfalls, and promises. Mol Cancer.
22:402023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantovani A, Allavena P, Marchesi F and
Garlanda C: Macrophages as tools and targets in cancer therapy. Nat
Rev Drug Discov. 21:799–820. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sharma P, Goswami S, Raychaudhuri D,
Siddiqui BA, Singh P, Nagarajan A, Liu J, Subudhi SK, Poon C, Gant
KL, et al: Immune checkpoint therapy-current perspectives and
future directions. Cell. 186:1652–1669. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu Y, Xun Z, Ma K, Liang S, Li X, Zhou S,
Sun L, Liu Y, Du Y, Guo X, et al: Identification of a tumour immune
barrier in the HCC microenvironment that determines the efficacy of
immunotherapy. J Hepatol. 78:770–782. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Timperi E, Gueguen P, Molgora M, Magagna
I, Kieffer Y, Lopez-Lastra S, Sirven P, Baudrin LG, Baulande S,
Nicolas A, et al: Lipid-associated macrophages are induced by
cancer-associated fibroblasts and mediate immune suppression in
breast cancer. Cancer Res. 82:3291–3306. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li C, Teixeira AF, Zhu HJ and ten Dijke P:
Cancer associated-fibroblast-derived exosomes in cancer
progression. Mol Cancer. 20:1542021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paul M, Carrara E, Retamar P, Tängdén T,
Bitterman R, Bonomo RA, de Waele J, Daikos GL, Akova M, Harbarth S,
et al: European society of clinical microbiology and infectious
diseases (ESCMID) guidelines for the treatment of infections caused
by multidrug-resistant Gram-negative bacilli (endorsed by European
society of intensive care medicine). Clin Microbiol Infect.
28:521–547. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Park JJ: Epidemiology, pathophysiology,
diagnosis and treatment of heart failure in diabetes. Diabetes
Metab J. 45:146–157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Blassel L, Zhukova A, Villabona-Arenas CJ,
Atkins KE, Hué S and Gascuel O: Drug resistance mutations in HIV:
new bioinformatics approaches and challenges. Curr Opin Virol.
51:56–64. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He F, Zhang P, Liu J, Wang R, Kaufman RJ,
Yaden BC and Karin M: ATF4 suppresses hepatocarcinogenesis by
inducing SLC7A11 (xCT) to block stress-related ferroptosis. J
Hepatol. 79:362–377. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Wang S, Ke A and Guo K:
Ferroptosis and its interaction with tumor immune microenvironment
in liver cancer. Biochim Biophys Acta. 1878:1888482023.PubMed/NCBI
|
|
26
|
Wen R, Dong X, Zhuang H, Pang FX, Ding SC,
Li N, Mai YX, Zhou ST, Wang JY and Zhang JF: Baicalin induces
ferroptosis in osteosarcomas through a novel Nrf2/xCT/GPX4
regulatory axis. Phytomedicine. 116:1548812023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye Y, Chen A, Li L, Liang Q, Wang S, Dong
Q, Fu M, Lan Z, Li Y, Liu X, et al: Repression of the antiporter
SLC7A11/glutathione/glutathione peroxidase 4 axis drives
ferroptosis of vascular smooth muscle cells to facilitate vascular
calcification. Kidney Int. 102:1259–1275. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li Y, Xu B, Ren X, Wang L, Xu Y, Zhao Y,
Yang C, Yuan C, Li H, Tong X, et al: Inhibition of CISD2 promotes
ferroptosis through ferritinophagy-mediated ferritin turnover and
regulation of p62-Keap1-NRF2 pathway. Cell Mol Biol Lett.
27:812022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henning Y, Blind US, Larafa S, Matschke J
and Fandrey J: Hypoxia aggravates ferroptosis in RPE cells by
promoting the Fenton reaction. Cell Death Dis. 13:6622022.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei X, Li X, Hu S, Cheng J and Cai R:
Regulation of ferroptosis in lung adenocarcinoma. Int J Mol Sci.
24:146142023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Sun Y, Bai L, Zhi L, Yang Y, Zhao
Q, Chen C, Qi Y, Gao W, He W, et al: RBMS1 regulates lung cancer
ferroptosis through translational control of SLC7A11. J Clin
Invest. 131:e1520672021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan S, Xi S, Weng H, Guo MM, Zhang JH, Yu
ZP, Zhang H, Yu Z, Xing Z, Liu MY, et al: YTHDC1 as a tumor
progression suppressor through modulating FSP1-dependent
ferroptosis suppression in lung cancer. Cell Death Differ.
30:2477–2490. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yu J, Zhong B, Zhao L, Hou Y, Ai N, Lu JJ,
Ge W and Chen X: Fighting drug-resistant lung cancer by induction
of NAD(P)H:quinone oxidoreductase 1 (NQO1)-mediated ferroptosis.
Drug Resist Updat. 70:1009772023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang D, Li X, Jiao D, Jiao D, Cai Y, Qian
L, Shen Y, Lu Y, Zhou Y, Fu B, et al: LCN2 secreted by
tissue-infiltrating neutrophils induces the ferroptosis and wasting
of adipose and muscle tissues in lung cancer cachexia. J Hematol
Oncol. 16:302023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shi C, Wang C, Fu Z, Liu J, Zhou Y, Cheng
B, Zhang C, Li S and Zhang Y: Lipocalin 2 (LCN2) confers acquired
resistance to almonertinib in NSCLC through LCN2-MMP-9 signaling
pathway. Pharmacol Res. 201:1070882024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li Z, Zhang J, Yin J, Ma W, Liao H, Ling
L, Zou Q, Cao Y, Song Y, Zheng G, et al: Targeting MYOF suppresses
pancreatic ductal adenocarcinoma progression by inhibiting
ILF3-LCN2 signaling through disrupting OTUB1-mediated
deubiquitination of ILF3. Redox Biol. 84:1036652025. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu Y, Qian X, Cai G, Lin Z, Huang W, Wang
C, Wu H, Zhang Y, Sun J and Zhang Q: WTX-L/β-arrestin2/LCN2 axis
controls vulnerability to ferroptosis in gastric cancer. iScience.
28:1119642025. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Feng M, Wu X, Zhang J, Chen P, Qian S and
Chang C: Loss of Lipocalin2 confers cisplatin vulnerability through
modulating NF-ĸB mediated ferroptosis via ferroportin. Am J Cancer
Res. 14:2088–2102. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Martínez-Ruiz C, Black JRM, Puttick C,
Hill MS, Demeulemeester J, Cadieux EL, Thol K, Jones TP, Veeriah S,
Naceur-Lombardelli C, et al: Genomic-transcriptomic evolution in
lung cancer and metastasis. Nature. 616:543–552. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Litchfield K, Reading JL, Puttick C,
Thakkar K, Abbosh C, Bentham R, Watkins TBK, Rosenthal R, Biswas D,
Rowan A, et al: Meta-analysis of tumor- and T cell-intrinsic
mechanisms of sensitization to checkpoint inhibition. Cell.
184:596–614.e14. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Caushi JX, Zhang J, Ji Z, Vaghasia A,
Zhang B, Hsiue EH, Mog BJ, Hou W, Justesen S, Blosser R, et al:
Transcriptional programs of neoantigen-specific TIL in
anti-PD-1-treated lung cancers. Nature. 596:126–132. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo D, Wang X and Feng W: Comprehensive
analysis of cuproptosis and copper homeostasis genotyping and
related immune land scape in lung adenocarcinoma. Sci Rep.
13:165542023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Augustin RC, Newman S, Li A, Joy M, Lyons
M, Pham MP, Lucas P, Smith K, Sander C, Isett B, et al:
Identification of tumor-intrinsic drivers of immune exclusion in
acral melanoma. J Immunother Cancer. 11:e0075672023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Weng W, Meng T, Zhao Q, Shen Y, Fu G, Shi
J, Zhang Y, Wang Z, Wang M, Pan R, et al: Antibody-exatecan
conjugates with a novel self-immolative moiety overcome resistance
in colon and lung cancer. Cancer Discov. 13:950–973. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Huang J, Liu D, Wang Y, Liu L, Li J, Yuan
J, Jiang Z, Jiang Z, Hsiao WW, Liu H, et al: Ginseng
polysaccharides alter the gut microbiota and kynurenine/tryptophan
ratio, potentiating the antitumour effect of antiprogrammed cell
death 1/programmed cell death ligand 1 (anti-PD-1/PD-L1)
immunotherapy. Gut. 71:734–745. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cheng Y, Mo F, Li Q, Han X, Shi H, Chen S,
Wei Y and Wei X: Targeting CXCR2 inhibits the progression of lung
cancer and promotes therapeutic effect of cisplatin. Mol Cancer.
20:622021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hanley CJ, Waise S, Ellis MJ, Lopez MA,
Pun WY, Taylor J, Parker R, Kimbley LM, Chee SJ, Shaw EC, et al:
Single-cell analysis reveals prognostic fibroblast subpopulations
linked to molecular and immunological subtypes of lung cancer. Nat
Commun. 14:3872023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Hwang S, Kwon AY, Jeong JY, Kim S, Kang H,
Park J, Kim JH, Han OJ, Lim SM and An HJ: Immune gene signatures
for predicting durable clinical benefit of anti-PD-1 immunotherapy
in patients with non-small cell lung cancer. Sci Rep. 10:6432020.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wang C, Yu Q, Song T, Wang Z, Song L, Yang
Y, Shao J, Li J, Ni Y, Chao N, et al: The heterogeneous immune
landscape between lung adenocarcinoma and squamous carcinoma
revealed by single-cell RNA sequencing. Sig Transduct Target Ther.
7:2892022. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Heo H, Kim JH, Lim HJ, Kim JH, Kim M, Koh
J, Im JY, Kim BK, Won M, Park JH, et al: DNA methylome and
single-cell transcriptome analyses reveal CDA as a potential
druggable target for ALK inhibitor-resistant lung cancer therapy.
Exp Mol Med. 54:1236–1249. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Q, Wang R, Yang Z, Li W, Yang J, Wang
Z, Bai H, Cui Y, Tian Y, Wu Z, et al: Molecular profiling of human
non-small cell lung cancer by single-cell RNA-seq. Genome Med.
14:872022. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen B, Song Y, Zhan Y, Zhou S, Ke J, Ao
W, Zhang Y, Liang Q, He M, Li S, et al: Fangchinoline inhibits
non-small cell lung cancer metastasis by reversing
epithelial-mesenchymal transition and suppressing the cytosolic
ROS-related Akt-mTOR signaling pathway. Cancer Lett.
543:2157832022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu Y, Paris S, Barsoumian H, Abana CO, He
K, Sezen D, Wasley M, Masrorpour F, Chen D, Yang L, et al: A
radioenhancing nanoparticle mediated immunoradiation improves
survival and generates long-term antitumor immune memory in an
anti-PD1-resistant murine lung cancer model. J Nanobiotechnol.
19:4162021. View Article : Google Scholar
|
|
54
|
Liu T, Han C, Fang P, Ma Z, Wang X, Chen
H, Wang S, Meng F, Wang C, Zhang E, et al: Cancer-associated
fibroblast-specific lncRNA LINC01614 enhances glutamine uptake in
lung adenocarcinoma. J Hematol Oncol. 15:1412022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jiang Y, Wang K, Lu X, Wang Y and Chen J:
Cancer-associated fibroblasts-derived exosomes promote lung cancer
progression by OIP5-AS1/ miR-142-5p/ PD-L1 axis. Mol Immunol.
140:47–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li H, Xu H, Wen H, Liu T, Sun Y, Xiao N,
Bai C, Ge J, Wang X, Song L, et al: Overexpression of LH3 reduces
the incidence of hypertensive intracerebral hemorrhage in mice. J
Cereb Blood Flow Metab. 39:547–561. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tang W, Ma J, Gu R, Lei B, Ding X and Xu
G: Light-induced lipocalin 2 facilitates cellular apoptosis by
positively regulating reactive oxygen species/bim signaling in
retinal degeneration. Invest Ophthalmol Vis Sci. 59:6014–6025.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ries A, Flehberger D, Slany A, Pirker C,
Mader JC, Mohr T, Schelch K, Sinn K, Mosleh B, Hoda MA, et al:
Mesothelioma-associated fibroblasts enhance proliferation and
migration of pleural mesothelioma cells via c-Met/PI3K and WNT
signaling but do not protect against cisplatin. J Exp Clin Cancer
Res. 42:272023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun Y, Shen W, Hu S, Lyu Q, Wang Q, Wei T,
Zhu W and Zhang J: METTL3 promotes chemoresistance in small cell
lung cancer by inducing mitophagy. J Exp Clin Cancer Res.
42:652023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ding K, Jiang X, Wang Z, Zou L, Cui J, Li
X, Shu C, Li A and Zhou J: JAC4 inhibits EGFR-driven lung
adenocarcinoma growth and metastasis through CTBP1-mediated
JWA/AMPK/NEDD4L/EGFR axis. Int J Mol Sci. 24:87942023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhang LX, Gao J, Long X, Zhang PF, Yang X,
Zhu SQ, Pei X, Qiu BQ, Chen SW, Lu F, et al: The circular RNA
circHMGB2 drives immunosuppression and anti-PD-1 resistance in lung
adenocarcinomas and squamous cell carcinomas via the
miR-181a-5p/CARM1 axis. Mol Cancer. 21:1102022. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shi L, Zhu W, Huang Y, Zhuo L, Wang S,
Chen S, Zhang B and Ke B: Cancer-associated fibroblast-derived
exosomal microRNA-20a suppresses the PTEN/PI3K-AKT pathway to
promote the progression and chemoresistance of non-small cell lung
cancer. Clin Transl Med. 12:e9892022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Su S, Chen J, Yao H, Liu J, Yu S, Lao L,
Wang M, Luo M, Xing Y, Chen F, et al: CD10+GPR77+ Cancer-associated
fibroblasts promote cancer formation and chemoresistance by
sustaining cancer stemness. Cell. 172:841–856.e16. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Bao Z, Zeng W, Zhang D, Wang L, Deng X,
Lai J, Li J, Gong J and Xiang G: SNAIL induces EMT and lung
metastasis of tumours secreting CXCL2 to promote the invasion of
M2-type immunosuppressed macrophages in colorectal cancer. Int J
Biol Sci. 18:2867–2881. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zheng Y, Wang Y, Lu Z, Wan J, Jiang L,
Song D, Wei C, Gao C, Shi G, Zhou J, et al: PGAM1 inhibition
promotes HCC ferroptosis and synergizes with anti-PD-1
immunotherapy. Adv Sci (Weinh). 10:e23019282023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Guo Y, Zhang Q, Zhu Q, Gao J, Zhu X, Yu H,
Li Y and Zhang C: Copackaging photosensitizer and PD-L1 siRNA in a
nucleic acid nanogel for synergistic cancer photoimmunotherapy. Sci
Adv. 8:eabn29412022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Denis M, Grasselly C, Choffour PA,
Wierinckx A, Mathé D, Chettab K, Tourette A, Talhi N, Bourguignon
A, Birzele F, et al: In vivo syngeneic tumor models with acquired
resistance to Anti-PD-1/PD-L1 therapies. Cancer Immunol Res.
10:1013–1027. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Bernardo M, Tolstykh T, Zhang Y, Bangari
DS, Cao H, Heyl KA, Lee JS, Malkova NV, Malley K, Marquez E, et al:
An experimental model of anti-PD-1 resistance exhibits activation
of TGFß and Notch pathways and is sensitive to local mRNA
immunotherapy. Oncoimmunology. 10:18812682021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhang J, Lu S, Lu T, Han D, Zhang K, Gan
L, Wu X, Li Y, Zhao X, Li Z, et al: Single-cell analysis reveals
the COL11A1+ fibroblasts are cancer-specific fibroblasts that
promote tumor progression. Front Pharmacol. 14:11215862023.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Ma L, Hernandez MO, Zhao Y, Mehta M, Tran
B, Kelly M, Rae Z, Hernandez JM, Davis JL, Martin SP, et al: Tumor
cell biodiversity drives microenvironmental reprogramming in liver
cancer. Cancer Cell. 36:418–430.e6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Hou W, Ji Z, Chen Z, Wherry EJ, Hicks SC
and Ji H: A statistical framework for differential pseudotime
analysis with multiple single-cell RNA-seq samples. Nat Commun.
14:72862023. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen J, Gao D, Chen J, Hou S, He B, Li Y,
Li S, Zhang F, Sun X, Jin Y, et al: Pseudo-temporal analysis of
single-cell RNA sequencing reveals trans-differentiation potential
of greater epithelial ridge cells into hair cells during postnatal
development of cochlea in rats. Front Mol Neurosci. 15:8328132022.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ferone G, Lee MC, Sage J and Berns A:
Cells of origin of lung cancers: Lessons from mouse studies. Genes
Dev. 34:1017–1032. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Czekay RP, Cheon DJ, Samarakoon R, Kutz SM
and Higgins PJ: Cancer-associated fibroblasts: Mechanisms of tumor
progression and novel therapeutic targets. Cancers (Basel).
14:12312022. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Lee H, Hwang M, Jang S and Um SW: Immune
regulatory function of cancer- associated fibroblasts in non-small
cell lung cancer. Tuberc Respir Dis (Seoul). 86:304–318. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Yamamoto Y, Kasashima H, Fukui Y, Tsujio
G, Yashiro M and Maeda K: The heterogeneity of cancer-associated
fibroblast subpopulations: Their origins, biomarkers, and roles in
the tumor microenvironment. Cancer Sci. 114:16–24. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shintani Y, Kimura T, Funaki S, Ose N,
Kanou T and Fukui E: Therapeutic targeting of cancer-associated
fibroblasts in the non-small cell lung cancer tumor
microenvironment. Cancers (Basel). 15:3352023. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhang H, Yue X, Chen Z, Liu C, Wu W, Zhang
N, Liu Z, Yang L, Jiang Q, Cheng Q, et al: Define cancer-associated
fibroblasts (CAFs) in the tumor microenvironment: new opportunities
in cancer immunotherapy and advances in clinical trials. Mol
Cancer. 22:1592023. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Tang PC, Chung JY, Xue VW, Xiao J, Meng
XM, Huang XR, Zhou S, Chan AS, Tsang AC, Cheng AS, et al: Smad3
promotes cancer-associated fibroblasts generation via
macrophage-myofibroblast transition. Adv Sci (Weinh).
9:e2021012352021.
|
|
81
|
Galbo PM Jr, Zang X and Zheng D: Molecular
features of cancer-associated fibroblast subtypes and their
implication on cancer pathogenesis, prognosis, and immunotherapy
resistance. Clin Cancer Res. 27:2636–2647. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Li H, Han H, Li C, Wu R, Wang Z, Wang Y,
Zhan P, Lv T, Zhang F, Song Y and Liu H: Efficacy and safety of
first-line PD-1/PD-L1 inhibitor combinations for extensive-stage
small-cell lung cancer: A Bayesian network meta-analysis. Ther Adv
Med Oncol. 15:175883592311894302023. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Davies J: PD-1/PD-L1 Inhibitors for
non-small cell lung cancer: Incorporating care step pathways for
effective side-effect management. J Adv Pract Oncol. 10:21–35.
2019.PubMed/NCBI
|
|
84
|
Pathak R, Pharaon RR, Mohanty A, Villaflor
VM, Salgia R and Massarelli E: Acquired Resistance to PD-1/PD-L1
blockade in lung cancer: Mechanisms and patterns of failure.
Cancers (Basel). 12:38512020. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lei Q, Wang D, Sun K, Wang L and Zhang Y:
Resistance mechanisms of Anti-PD1/PDL1 therapy in solid tumors.
Front Cell Dev Biol. 8:6722020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Guardado S, Ojeda-Juárez D, Kaul M and
Nordgren TM: Comprehensive review of lipocalin 2-mediated effects
in lung inflammation. Am J Physiol Lung Cell Mol Physiol.
321:L726–L733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Liu J, Kang R and Tang D: Signaling
pathways and defense mechanisms of ferroptosis. FEBS J.
289:7038–7050. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li N, Wang W, Zhou H, Wu Q, Duan M, Liu C,
Wu H, Deng W, Shen D and Tang Q: Ferritinophagy-mediated
ferroptosis is involved in sepsis-induced cardiac injury. Free
Radic Biol Med. 160:303–318. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Society of Cancer Precision of Chinese
Anti-Cancer Association, Lung Cancer Expert Group of Chinese
Medical Journal; Chinese society of clinical oncology, expert
committee on non-small cell lung cancer, . Chinese expert consensus
on the diagnosis and treatment of advanced RET fusion-positive
non-small cell lung cancer (2023 edition). Zhonghua Zhong Liu Za
Zhi. 45:991–1002. 2023.(In Chinese). PubMed/NCBI
|
|
90
|
Le Pape G and Lassalle JM: Behavioral
development in mice: Effects of maternal environment and the albino
locus. Behav Genet. 16:531–541. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
The Lancet: Lung cancer: Some progress,
but still a lot more to do. Lancet. 394:18802019. View Article : Google Scholar
|
|
92
|
Borghaei H, Gettinger S, Vokes EE, Chow
LQM, Burgio MA, de Castro Carpeno J, Pluzanski A, Arrieta O,
Frontera OA, Chiari R, et al: Five-year outcomes from the
randomized, phase III trials CheckMate 017 and 057: Nivolumab
versus docetaxel in previously treated non-small-cell lung cancer.
J Clin Oncol. 39:723–733. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Krishnamurthy N, Goodman AM, Barkauskas DA
and Kurzrock R: STK11 alterations in the pan-cancer setting:
Prognostic and therapeutic implications. Eur J Cancer. 148:215–229.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Nyein AF, Bari S, Hogue S, Zhao Y, Maller
B, Sha S, Gomez MF, Rollison DE and Robinson LA: Effect of prior
antibiotic or chemotherapy treatment on immunotherapy response in
non-small cell lung cancer. BMC Cancer. 22:1012022. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Rosell R, Jain A, Codony-Servat J,
Jantus-Lewintre E, Morrison B, Ginesta JB and González-Cao M:
Biological insights in non-small cell lung cancer. Cancer Biol Med.
28:1–19. 2023. View Article : Google Scholar
|
|
96
|
Zhou W, Zhang J, Yan M, Wu J, Lian S, Sun
K, Li B, Ma J, Xia J and Lian C: Orlistat induces ferroptosis-like
cell death of lung cancer cells. Front Med. 15:922–932. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Lan Y, Yang T, Yue Q, Wang Z, Zhong X, Luo
X, Zuo B, Zhang M, Zeng T, Liu B and Guo H: IRP1 mediated
ferroptosis reverses temozolomide resistance in glioblastoma via
affecting LCN2/FPN1 signaling axis depended on NFKB2. iScience.
26:1073772023. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Yao F, Deng Y, Zhao Y, Mei Y, Zhang Y, Liu
X, Martinez C, Su X, Rosato RR, Teng H, et al: A targetable
LIFR-NF-κB-LCN2 axis controls liver tumorigenesis and vulnerability
to ferroptosis. Nat Commun. 12:73332021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bao Y, Yan Z, Shi N, Tian X, Li J, Li T,
Cheng X and Lv J: LCN2: Versatile players in breast cancer. Biomed
Pharmacother. 171:1160912024. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Jaberi SA, Cohen A, D'Souza C, Abdulrazzaq
YM, Ojha S, Bastaki S and Adeghate EA: Lipocalin-2: Structure,
function, distribution and role in metabolic disorders. Biomed
Pharmacother. 142:1120022021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Tyagi A, Sharma S, Wu K, Wu SY, Xing F,
Liu Y, Zhao D, Deshpande RP, D'Agostino RB Jr and Watabe K:
RETRACTED ARTICLE: Nicotine promotes breast cancer metastasis by
stimulating N2 neutrophils and generating pre-metastatic niche in
lung. Nat Commun. 12:4742021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Di Gioacchino M, Della Valle L, Allegra A,
Pioggia G and Gangemi S: AllergoOncology: Role of immune cells and
immune proteins. Clin Transl Allergy. 12:e121332022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Ziebart A, Breit C, Ruemmler R, Hummel R,
Möllmann C, Jungmann F, Kamuf J, Garcia-Bardon A, Thal SC, Kreitner
KF, et al: Effect of fluid resuscitation on cerebral integrity: A
prospective randomised porcine study of haemorrhagic shock. Eur J
Anaesthesiol. 38:411–421. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Chaudhary N, Choudhary BS, Shah SG,
Khapare N, Dwivedi N, Gaikwad A, Joshi N, Raichanna J, Basu S,
Gurjar M, et al: Lipocalin 2 expression promotes tumor progression
and therapy resistance by inhibiting ferroptosis in colorectal
cancer. Int J Cancer. 149:1495–1511. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Liu R, Wang J, Chen Y, Collier JM, Capuk
O, Jin S, Sun M, Mondal SK, Whiteside TL, Stolz DB, et al: NOX
activation in reactive astrocytes regulates astrocytic LCN2
expression and neurodegeneration. Cell Death Dis. 13:3712022.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Guo J, Xu B, Han Q, Zhou H, Xia Y, Gong C,
Dai X, Li Z and Wu G: Ferroptosis: A novel anti-tumor action for
cisplatin. Cancer Res Treat. 50:445–460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Shibata Y, Yasui H, Higashikawa K,
Miyamoto N and Kuge Y: Erastin, a ferroptosis-inducing agent,
sensitized cancer cells to X-ray irradiation via glutathione
starvation in vitro and in vivo. PLoS One. 14:e02259312019.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Xiong R, He R, Liu B, Jiang W, Wang B, Li
N and Geng Q: Ferroptosis: A new promising target for lung cancer
therapy. Oxid Med Cell Longev. 2021:84575212021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Jiang J, Zhu J, Qiu P, Ni J, Zhu W and
Wang X: HNRNPA2B1-mediated m6A modification of FOXM1 promotes drug
resistance and inhibits ferroptosis in endometrial cancer via
regulation of LCN2. Funct Integr Genomics. 24:32023. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Santiago-Sánchez GS, Noriega-Rivera R,
Hernández-O'Farrill E, Valiyeva F, Quiñones-Diaz B, Villodre ES,
Debeb BG, Rosado-Albacarys A and Vivas-Mejía PE: Targeting
lipocalin-2 in inflammatory breast cancer cells with small
interference RNA and small molecule inhibitors. Int J Mol Sci.
22:85812021. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Mertens C, Schnetz M, Rehwald C, Grein S,
Elwakeel E, Weigert A, Brüne B and Jung M: Iron-Bound lipocalin-2
from tumor-associated macrophages drives breast cancer progression
independent of ferroportin. Metabolites. 11:1802021. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Pan Z, Li B, Lu P, Rong G and Wang X:
Inhibiting LCN2 can suppress the development of NSCLC by promoting
ferroptosis. Gene. 894:1480262024. View Article : Google Scholar : PubMed/NCBI
|