Introduction
Renal cell carcinoma (RCC) is the urologic cancer
with the highest mortality rate. Approximately one third of
patients with RCC exhibit visceral metastasis at the time of
diagnosis and approximately 30% of the patients who undergo
potentially curative radical nephrectomy develop metastatic disease
(1). Cytokine-based immunotherapy
with interferon (IFN)-α or interleukin (IL)-2 produces a response
rate of only 10–15%. The advent of molecular targeted therapy in
the last few years has revolutionized the treatment of metastatic
RCC with benefits in terms of disease stabilization, improvement in
the quality of life and overall survival (2–5).
The discovery of the molecular links underlying the
relationship between Von Hippel-Lindau, hypoxia signaling and VEGF
in the biology of clear cell-RCC has led to the development of
inhibitors against the VEGF signaling pathway. One of these
inhibitors is sorafenib, which inhibits several RTKs including
VEGFR-2 and -3, PDGFR-β, the FLT3 and c-KIT receptors (6,7). A
randomized phase III trial comparing sorafenib vs. placebo
involving approximately 900 patients with clear cell metastatic RCC
showed the median progression free survival (PFS) was 5.5 months in
the sorafenib arm compared to 2.8 months in the placebo group
(3). This study also suggested
overall survival benefits in the sorafenib arm compared to placebo
(3). However, up to 34% of
patients in the sorafenib arm suffered serious adverse events
(3), which led to the dose
reduction or discontinuation of sorafenib treatment (8). Although the availability of sorafenib
is likely to have a considerable clinical impact in RCC, there
remains a need for additional treatment options for patients who
cannot tolerate full dose sorafenib. This need may be covered by
one or more of the novel inhibitors or novel combinations currently
being developed.
In a previous study, we reported that sorafenib
inhibited RCC xenograft lines regardless of histological subtypes,
in a dose-dependent manner. Sorafenib-induced growth suppression
was associated with the inhibition of angiogenic targets p-PDGFR-β
and p-VEGFR-2 and partial reduction in p-Akt and p-ERK1/2 (9). In the present study, we investigated
the effect of a combination of sorafenib and a MEK inhibitor with a
view to achieve sustained antitumor activity with low dose
sorafenib, thus minimizing adverse effects. We used a
patient-derived RCC xenograft model (9) to test the combination of sorafenib
with a MEK inhibitor, AZD6244 (ARRY-142886) (10).
Materials and methods
Drugs and reagents
Research grade Capsitol was purchased from CyDex,
Inc., Lenexa, KS, USA. Sorafenib tosylate (BAY 43-9006, Nexavar™,
Bayer and Onyx Pharmaceuticals) was purchased from Bayer
HealthCare, Leverkusen, Germany. Sorafenib was dissolved in vehicle
(30% Capsitol in water) prior to use. AZD6244 was obtained from
AstraZeneca (Alderley Park, Macclesfield, UK). Antibodies against
p27, S6R, p70S6K, Akt, Rb, VEGFR-2, Raf-1, PDGFR-β, cleaved caspase
3, cleaved PARP, and phosphorylation-specific antibodies against
ERK1/2, Rb Ser780 and Ser807/811, Akt Ser473, p70S6KThr421/Ser424,
S6R Ser235/236, 4EBP1 Thr70, Histone 3 Ser10, and cdk-2 Thr14/Tyr15
were obtained from Cell Signaling Technology, Beverly, MA, USA. The
antibodies against p-PDGFR-β Tyr1021, p-VEGFR-2 Tyr951, ERK1,
cyclin B1, cdk-2 and α-tubulin were obtained from Santa Cruz
Biotechnology Inc, Santa Cruz, CA, USA. Anti-mouse CD31 antibody
was purchased from BioLegend, San Diego, CA, USA.
Cell culture
RCC 08-0910 cells were isolated from RCC 08-0910
tumors as previously described (10). Human RCC 786-0 cells were a gift
from Dr Val Macaulay, University of Oxford. They were maintained as
monolayer cultures in Hi-Gluc-DMEM (DMEM) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin-streptomycin (growth
medium) at 37°C, 5% CO2.
To determine the effects of sorafenib/AZD6244 on
cell number, RCC 786-0 cells were plated at a density of
2×104 cells per dish and treated with either vehicle, 1
μM sorafenib, 0.5 μM AZD6244, or 1 μM sorafenib plus 0.5 μM AZD6244
in DMEM containing 1% FBS for 72 h. Cell number was determined
manually with a hemocytometer. The data are expressed as the mean ±
SE.
To study the effect of sorafenib/AZD6244 on the
basal expression of the mTOR and ERK signaling pathways, RCC
08-0910 cells were plated at a density of 5×106 cells
per 100 mm dish for 24 h. Cells were then treated with either
vehicle, 1 μM sorafenib, 0.5 μM AZD6244, or 1 μM sorafenib plus 0.5
μM AZD6244 in DMEM containing 1% FBS. Twenty-four hours
post-treatment, cells were harvested for protein extraction and
western blot analysis.
Flow cytometry analysis
RCC 768-0 cells were plated at the density of
5×105 and then treated with either vehicle, 1 μM
sorafenib, 0.5 μM AZD6244, or 1 μM sorafenib plus 0.5 μM AZD6244 in
DMEM containing 1% FBS for 24 h. Cells were fixed in 70% ethanol at
0°C for 24 h and stained with propidium iodide. Fluorescence
intensity of the stained cells was measured using FACSCalibur flow
cytometer (BD, San Jose, CA, USA). Data were analyzed using BD
CellQuest Pro software. For every measurement, 10,000 events were
collected, and gating was set to exclude cell doublets. DNA
contents of certain phases were shown as percentages compared to
the total DNA content within the gate.
Wound-healing scratch assay
RCC 786-0 cell monolayers grown to confluence on 100
mm culture dishes were wounded by scratching with a pipette tip and
treated with either vehicle, 1 μM sorafenib, 0.5 μM AZD6244, or 1
μM sorafenib plus 0.5 μM AZD6244 in DMEM containing 10% FBS for 24
h. The wounds were photographed (10× objective) at 24 h. Each
experiment was performed in triplicate.
Xenograft models
This study received ethical approval from our
institutional review board (SingHealth CRIB 2008/094/B). All mice
were maintained according to the Guide for the Care and Use of
Laboratory Animals published by the National Institutes of Health,
USA.
Primary RCCs have previously been used to create
xenograft lines (9), of which the
following four lines (RCC19-0809, RCC02-0908, RCC07-0408 and
RCC05-1109) were used to establish tumors in male SCID mice (Animal
Resources Centre, Canning Vale, Western Australia) aged 9–10 weeks.
The histological phenotype of the xenografts is conventional
clear-cell (RCC07-0408, RCC05-1109, RCC19-0809), and poorly
differentiated clear-cell with sarcomatoid (RCC02-0908) RCC. In
comparison with the histological features of the clinical
specimens, the four established xenografts retain identical
histological characteristics compared to the original tumor
(9).
Testing the efficacy of sorafenib and
AZD6244 on RCC xenografts
To assess the efficacy of sorafenib and AZD6244 in
RCC xenografts, mice bearing indicated xenografts (10 per group)
were orally administered 200 μl of vehicle (30% Capsitol), 20
mg/kg/day sorafenib, 16 mg/kg/day of AZD6244, or a combination
treatment with 20 mg/kg sorafenib and 16 mg/kg of AZD6244. For dose
reduction study, 10 mg sorafenib plus 8 mg/kg AZD6244 was used.
Growth of established xenografts was monitored at least twice
weekly by Vernier caliper measurement of the length (a) and width
(b) of the tumor. Tumor volume was calculated as
(a–b2)/2. Animals were sacrificed at a pre-determined
duration after the last treatment dose, and body and tumor weights
were recorded, with tumors harvested for further molecular analyses
to correlate drug responses with tumor biology. Part of the tumor
harvest was fixed in neutral buffer containing 10% formalin for
immunohistochemistry.
Western blot analysis
To determine the changes in indicated proteins, 3–4
independent tumors from vehicle and sorafenib-treated mice were
homogenized separately in lysis buffer as previously described
(11). Proteins (80 μg per sample)
were analyzed by western blot analysis as previously described
(11). Blots were incubated with
the indicated primary antibodies and 1:7500 horseradish
peroxidase-conjugated secondary antibodies. All primary antibodies
were then visualized with the chemiluminescent detection system
(Amersham, Pharmacia Biotech).
Immunohistochemistry
For cleaved PARP and p-Histone 3 Ser10 stainings,
tumor samples were processed for paraffin embedding and for CD31
staining, they were embedded in Optimal Cutting Temperature (Sakura
Finetek Inc., Torrance, CA, USA). Sections (5 μm) were stained with
CD31, p-Histone 3 Ser10 and cleaved PARP antibodies to assess
microvessel density, cell proliferation, and apoptosis,
respectively, as previously described (10). The number of p-Histone 3 Ser10
positive cells among at least 500 cells per region was counted and
expressed as percentage values. For the quantification of mean
micro-vessel density in sections stained for CD31, 10 random fields
at a magnification of ×100 were captured for each tumor.
Statistical analysis
For quantification analysis, the sum of the density
of bands corresponding to protein blotting with the antibody under
study was calculated and normalized with α-tubulin and expressed as
fold of controls (the expression level in the vehicle-treated
samples). A value greater (lesser) than one indicates that the
expression levels of protein of interest was greater (lesser) that
in controls. Comparisons of tumor growth over time were performed
using ANOVA followed by Student’s t-test. Body weight and tumor
burden of mice at the point of sacrifice, differences in the levels
of protein under study, tumor weight at sacrifice, p-Histone 3
Ser10 index, mean microvessel density, and cleaved PARP-positive
cells were compared using Student’s t-test. P<0.05 was taken to
indicate a statistically significant difference. All the data
analysis was performed using statistical software, the Graph-Pad
Prism version 4 (GraphPad, CA, USA).
Results
Although sorafenib has been shown to improve overall
survival of patients with advanced or metastatic RCC, adverse
events are frequent. For some patients, these led to a dose
reduction or discontinuation of the treatment (3,8). To
search for the signaling pathway that can complement sorafenib, we
first performed western blot analysis of primary RCC cells treated
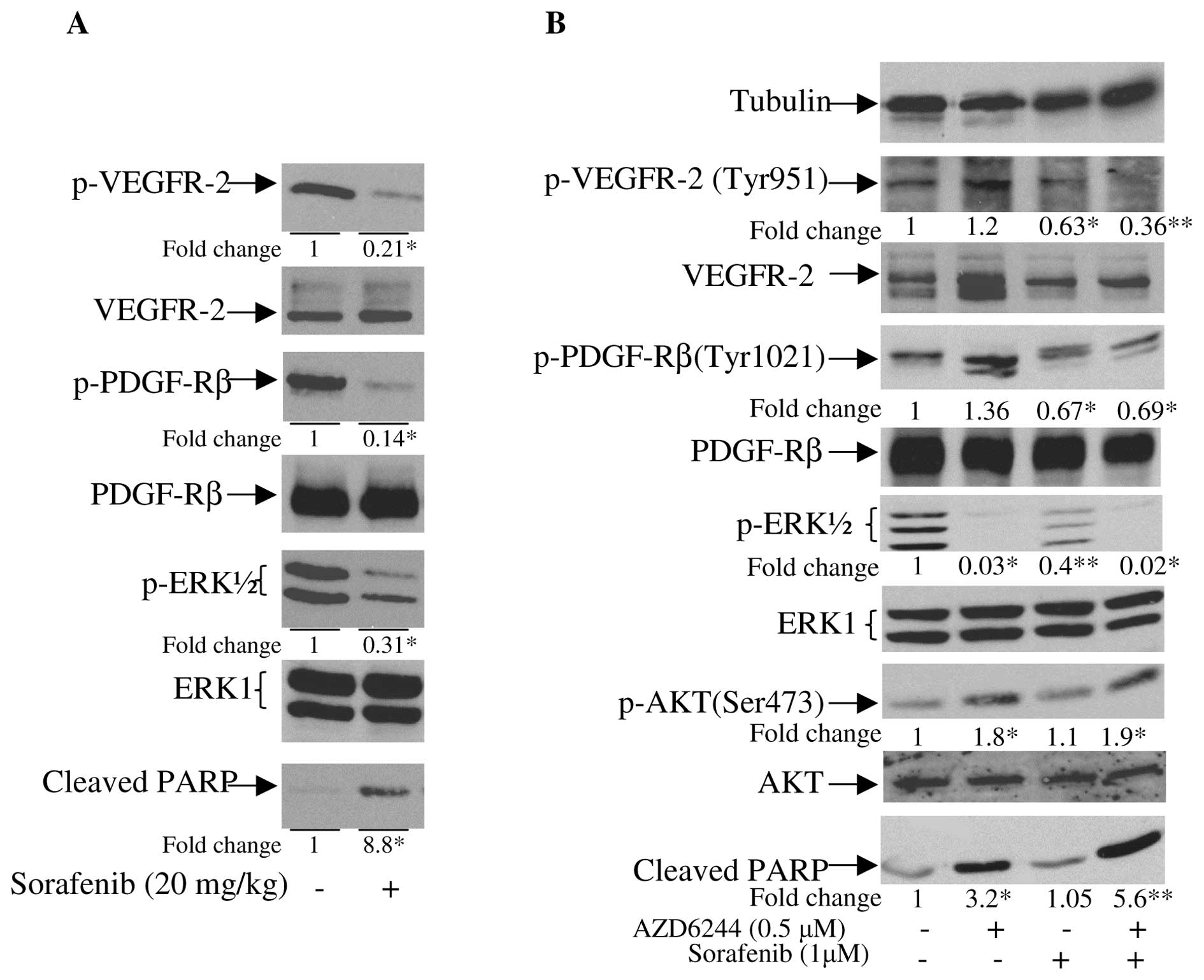
with sorafenib. As shown in Fig.
1A, sorafenib was effective in inhibiting phosphorylation of
its targeted receptors: VEGFR-2 Tyr951, and PDGFR-β Tyr1021.
However, sorafenib only partially inhibited the phosphorylation of
ERK1/2, which is one of the key intracellular kinases that
regulates cell cycle progression, apoptosis resistance,
extracellular matrix remodeling, cellular motility, angiogenesis,
and drug resistance (12). These
findings suggest that the antitumor effect of sorafenib in RCC
could be further improved by combining sorafenib with a MEK
inhibitor that abolishes the remaining activity of MEK/ERK.
To test this hypothesis, we first evaluated the
in vitro activity of sorafenib plus AZD6244 (a MEK
inhibitor) using RCC 08-0910 cells. Treatment of RCC 08-0910 cells
with sorafenib resulted in the inhibition of the phosphorylation of
VEGFR-2 and PDGFR-β (Fig. 1B).
Partial inhibition of p-ERK1/2 and elevation of apoptosis, as
determined by the levels of cleaved PARP, were also observed.
AZD6244 potently inhibited p-ERK1/2 and induced apoptosis. Unlike
sorafenib, which had insignificant effect on p-AKT, AZD6244 caused
elevation of p-AKT. Addition of AZD6244 into sorafenib completely
abolished the activity of p-ERK1/2, and significantly augmented the
apoptotic activity of sorafenib as determined by the levels of
cleaved PARP.
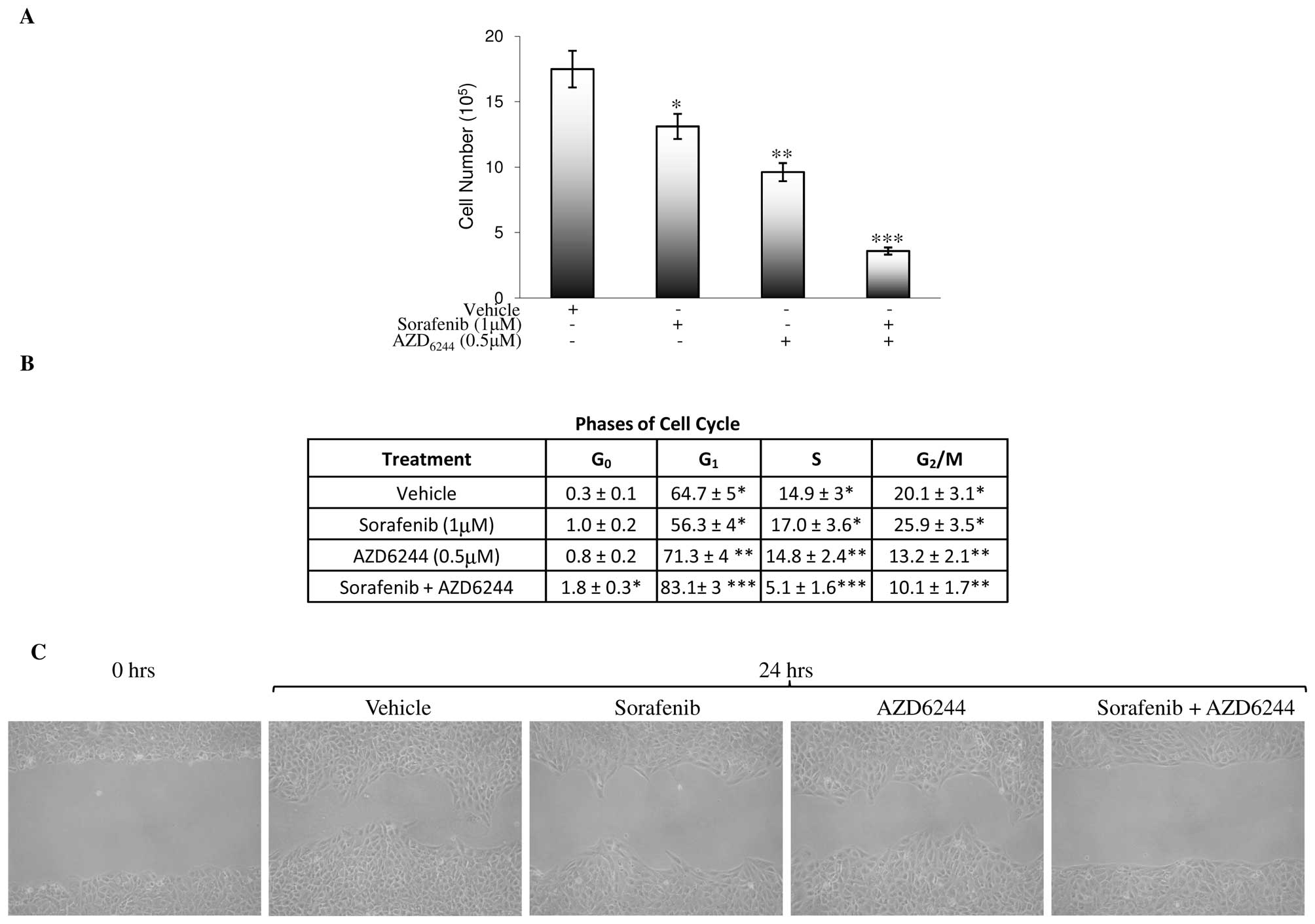
To study the direct effects of sorafenib/AZD6244 on
tumor cell growth and cell cycle, the RCC 786-0 cell line was used.
As shown in Fig. 2A, treatment of
RCC 786-0 cells with vehicle, 1 μM sorafenib, 0.5 μM AZD6244, or 1
μM sorafenib plus 0.5 μM AZD6244 resulted in a 25, 45 and 80%
growth inhibition, respectively, when analyzed on Day 3.
Furthermore, sorafenib treatment also induced more cells to
accumulate in G2/M and S phases with the reduction in G1
phase (Fig. 2B). By contrast,
sorafenib/AZD6244 and AZD6244 caused G1 phase arrest
with reduction in the G2/M and S phases. A modest
increase in G0 phase was also observed in
sorafenib/AZD6244 (Fig. 2B). While
sorafenib partially inhibited serum-induced RCC 786-0 cell
motility, sorafenib/AZD6244 abolished it as determined by the
wound-healing scratch assay (Fig.
2C).
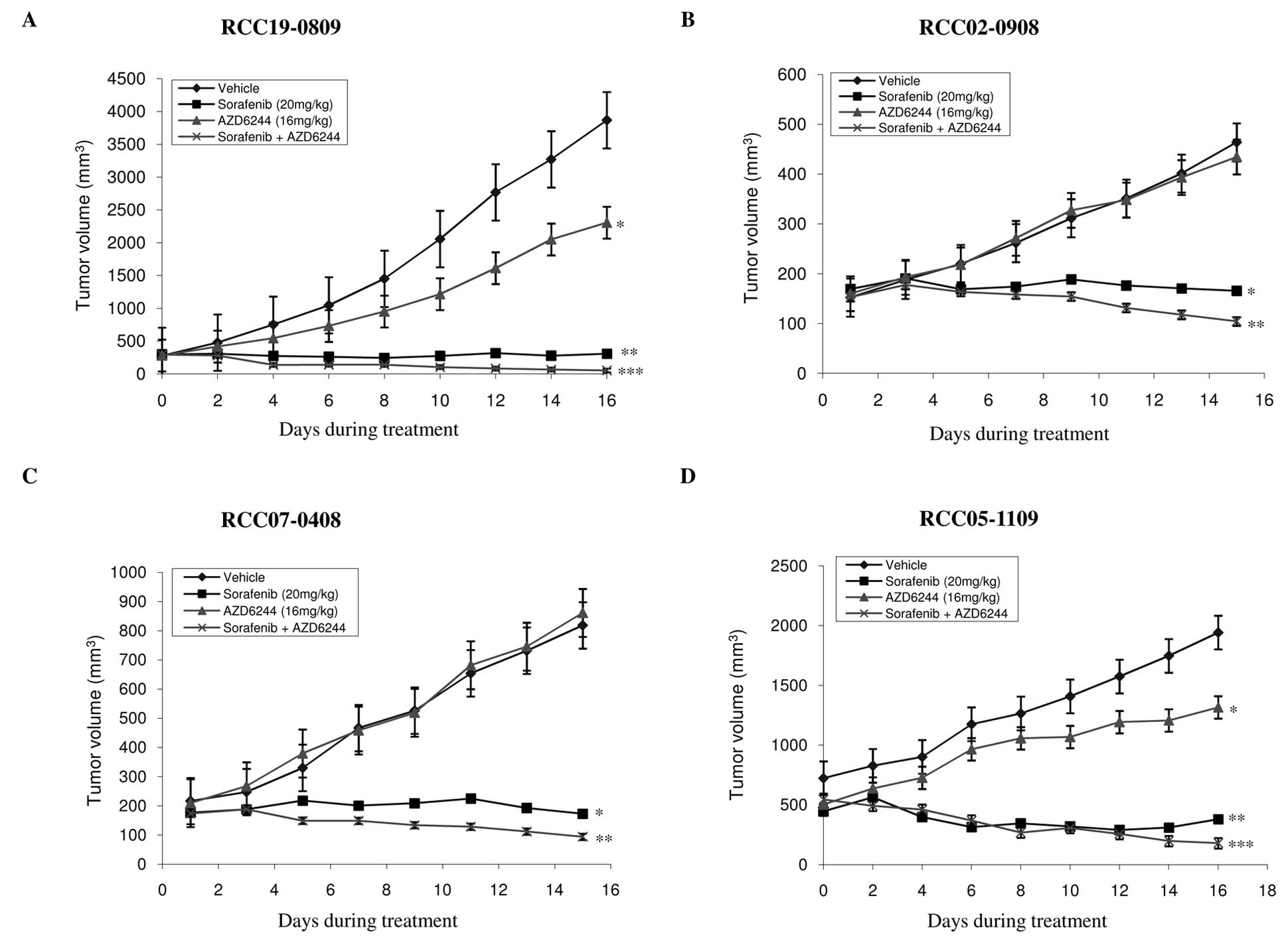
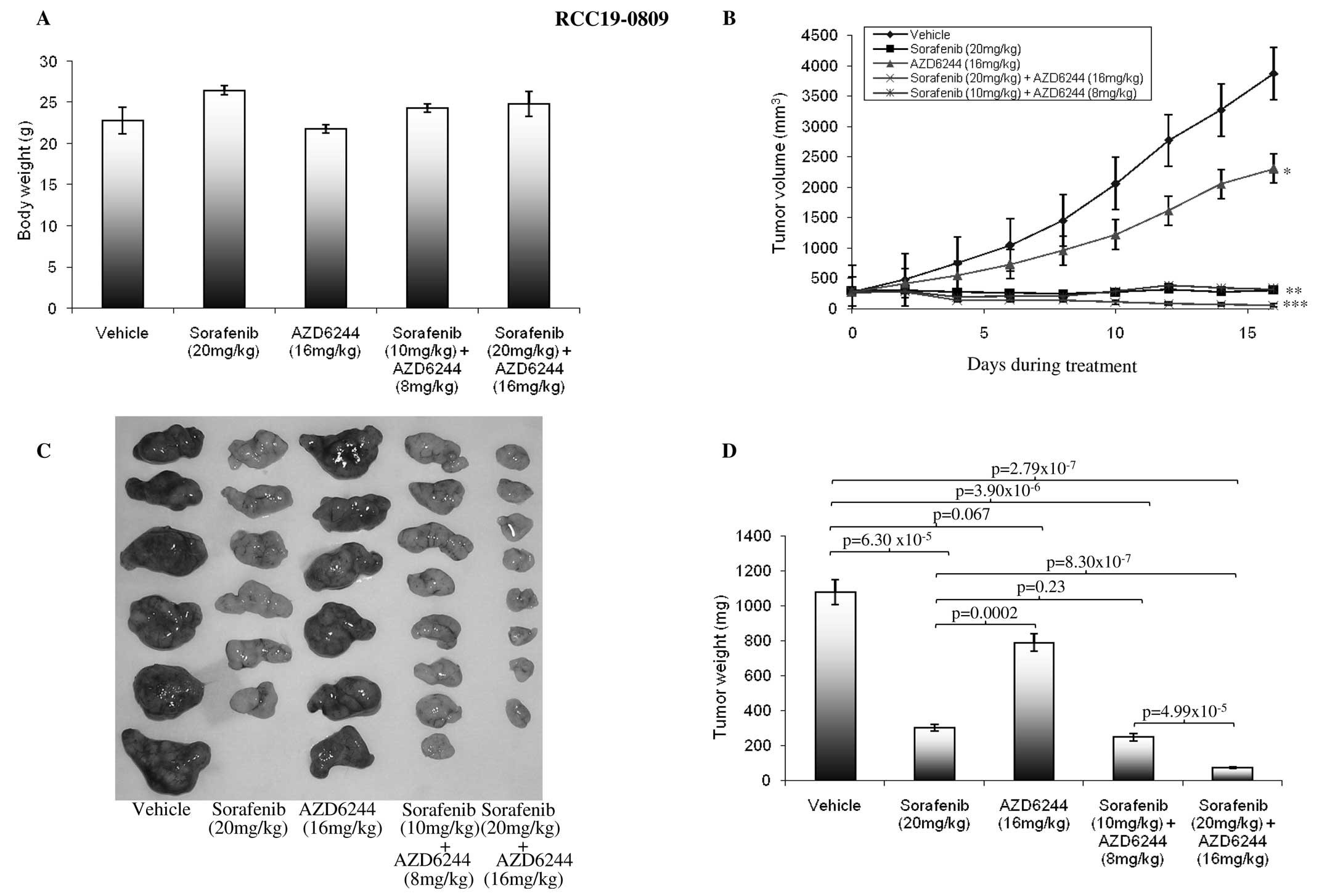
We then studied the antitumor activity of
sorafenib/AZD6244 in four RCC xenografts. As shown in Table I, AZD6244 and sorafenib, alone and
in combination were well tolerated in the dosage used with no
significant weight loss observed in the treated mice. The results
of single agent and combination treatment are presented in Fig. 3 and summarized in Table I. As expected, sorafenib at 20
mg/kg resulted in the significant inhibition of tumor growth in all
the four xenografts compared with control or AZD6244 alone
(P<0.05). By contrast, treatment with AZD6244 alone had either
modest (lines RCC19-0809 and RCC05-1109) or no significant (lines
RCC02-0908 and RCC07-0408) antitumor activity compared to
vehicle-treated mice, which is in agreement with the growth rate
results (Fig. 3). Combinatory
treatment of AZD6244 and sorafenib resulted in a marked inhibition
of tumor growth in all four RCC xenografts tested, compared with
the vehicle-and sorafenib-treated groups. Tumor regression was
observed in sorafebib/AZD6244-treated groups (Fig. 3). Efficacy of each therapy was
further evaluated by comparing the final mean weight of tumors in
the drug-treated arm (T) to that of the control arm (C) (T/C ratio)
with a value of <0.42 considered an active response (NCI
criteria). As shown in Table I,
while sorafenib produced T/C ratios ranging from 0.21 to 0.34,
sorafenib/AZD6244 resulted in T/C ratios <0.154 in all four RCC
lines, namely RCC07-0408 (T/C=0.126), RCC19-0809 (T/C=0.074),
RCC05-1109 (T/C=0.099) and RCC02-0908 (T/C=0.154). The results
suggest that the addition of AZD6244 into sorafenib significantly
improved the antitumor activity of sorafenib.
 | Table IEffects of sorafenib/AZD6244 on tumor
burden, angiogenesis, cell proliferation, and apoptosis of RCC
xenografts. |
Table I
Effects of sorafenib/AZD6244 on tumor
burden, angiogenesis, cell proliferation, and apoptosis of RCC
xenografts.
| Lines of
xenografts | Treatments | Body at sacrifice
(g) | Tumor weight
(mg) | T/C (%) | Microvessel*1 density | p-Histone Ser10
(%) | Cleaved PARP (%) |
|---|
| RCC07-0408 | Vehicle | 25.2±1.2 | 769±65* | 100 | 12.4±5* | 6±1* | 0.5±0.04* |
| Sorafenib | 25.6±1.1 | 182±26** | 31.9 | 1.5±0.8** | 0.8±0.5** | 5.3±0.8** |
| AZD6244 | 23.9±0.8 | 798±80* | 103.7 | 13.4±4* | 4.5±0.7* | 1.3±0.6* |
| Sorafenib +
AZD6244 | 24.1±1.1 | 97±14*** | 12.6 | 0.3±0.2*** | 0.2±0.1*** | 14.2±3*** |
| RCC19-0809 | Vehicle | 21.4±0.9 | 1050±123* | 100 | 9.2±4* | 9.2±3* | 0.2±0.1* |
| Sorafenib | 22.3±1.0 | 317±34** | 30.2 | 2.3±2** | 3.6±2** | 0.4±0.2* |
| AZD6244 | 23.1±0.8 | 787±63*** | 74.9 | 10.3±3* | 7.3±1.1* | 0.6±0.3* |
| Sorafenib +
AZD6244 | 22.4±0.9 | 78±16**** | 7.4 | 0.8±0.4*** | 0.7±0.3*** | 5.3±1.2** |
| RCC05-1109 | Vehicle | 21.7±1.4 | 1823±149* | 100 | 16.4±4* | 11.6±3* | 0.8±0.4* |
| Sorafenib | 20.4±1.1 | 385±58** | 21.1 | 6.8±3** | 2.6±0.9** | 3.3±0.5** |
| AZD6244 | 19.9±0.8 | 1125±174*** | 61.7 | 14.1±3* | 8.5±0.7* | 1.7±0.6* |
| Sorafenib +
AZD6244 | 19.5±1.1 | 180±40**** | 9.9 | 2.1±0.8*** | 2.1±0.5*** | 8.8±1.5*** |
| RCC02-0908 | Vehicle | 23.2±1.0 | 492±52* | 100 | 8.8±1.5* | 6.4±0.9* | 0.2±0.1* |
| Sorafenib | 22.3±0.8 | 167±32** | 33.9 | 2.2±0.5** | 1.9±0.8** | 1.1±0.3** |
| AZD6244 | 21.8±0.9 | 455±68* | 92.4 | 8.6±0.8* | 5.9±0.7* | 0.5±0.3* |
| Sorafenib +
AZD6244 | 20.1±1.1 | 76±16*** | 15.4 | 1.4±0.2** | 0.4±0.3*** | 6.3±1.5*** |
Subsequently, we probed the mechanistic basis for
the synergistic effect of AZD6244 on sorafenib therapy in RCC by
evaluating representative tumor sections from four treatment arms
using immunohistochemistry and western blotting. Representative
staining results for CD31 (a marker of angiogenesis), p-Histone 3
Ser10 (a marker for proliferation) and cleaved PARP (a marker for
apoptosis) for RCC07-0409 tumors are shown in Fig. 4 and Table I. Sorafenib alone and
AZD6244/sorafenib significantly inhibited cell proliferation and
angiogenesis in all four xenograft lines studied compared with the
control (Table I; P<0.05). An
increase in apoptosis was observed in sorafenib-treated tumors.
AZD6244, on the other hand, inhibited p-ERK1/2, modestly reduced
cell proliferation but had an insignificant effect on microvessel
density and apoptosis. As expected, sorafenib/AZD6244 abolished
p-ERK1/2, significantly reduced microvessel density and cell
proliferation but induced apoptosis at the dose studied
(P<0.05). AZD6244/sorafenib also showed a significant reduction
in tumor cell proliferation and elevated levels of apoptosis when
compared with either sorafenib or AZD6244 alone (Table I; P<0.05), suggesting that
sorafenib and AZD6244 complement each other and the addition of
AZD6244 to sorafenib promotes the antitumor, antiangiogenic and
apoptotic activities of either single agent.
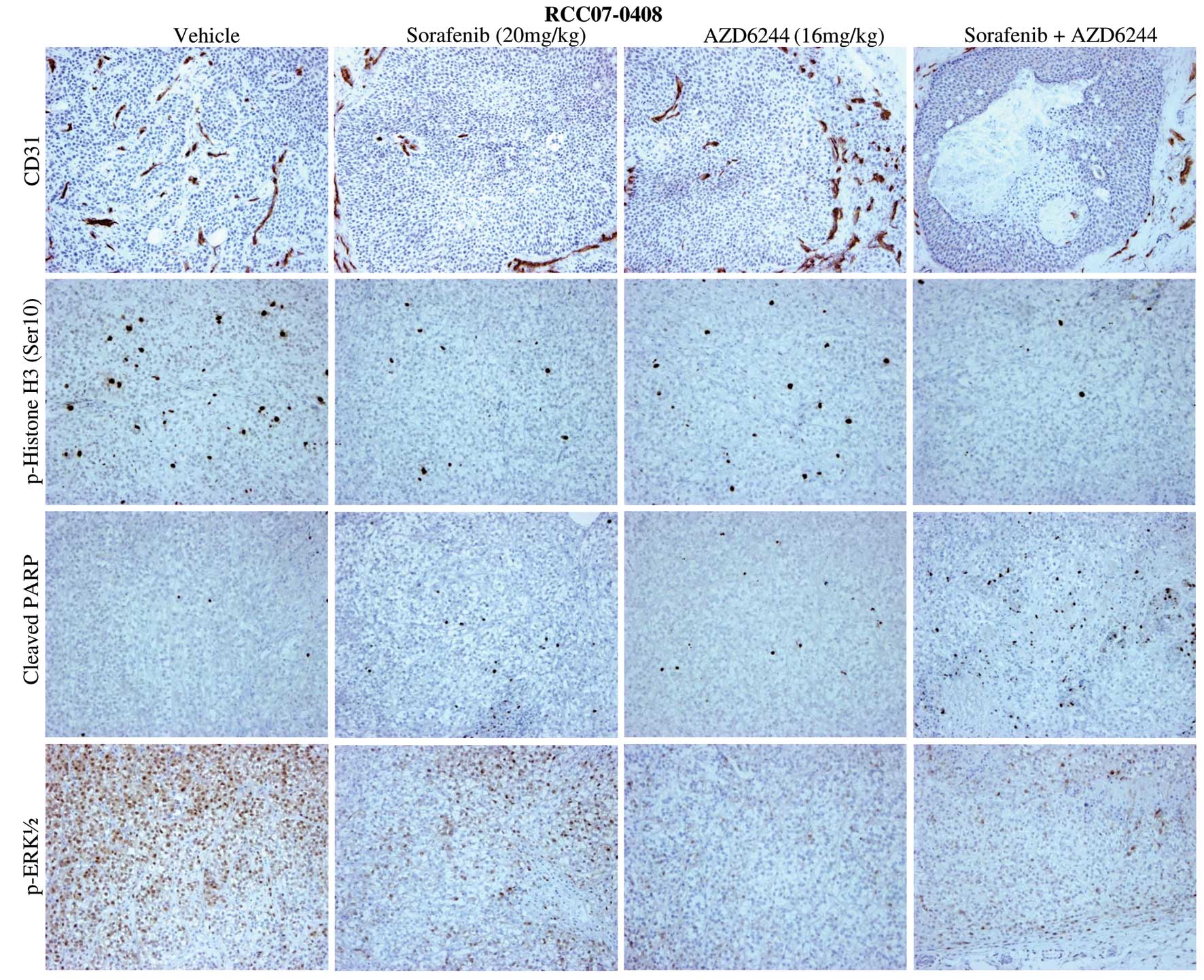 | Figure 4Effects of sorafenib, AZD6244, and
sorafenib/AZD6244 on angiogenesis, cell proliferation and apoptosis
in the RCC07-0408 xenograft. Mice bearing RCC07-0408 tumors (10
mice/group) were treated with vehicle, AZD6244 (16 mg/kg/day),
sorafenib (20 mg/kg/day), or sorafenib/AZD6244 for 16 days, as
described in Materials and methods. Representative pictures of
vehicle- and drug-treated tumors are shown. Proliferative cells are
stained with anti-phospho-Histone Ser10, blood vessels with
anti-CD31, apoptotic cells with anti-cleaved-PARP and p-ERK1/2 are
stained with p-ERK antibodies. Magnification, x200. Experiments
were repeated twice with similar results. |
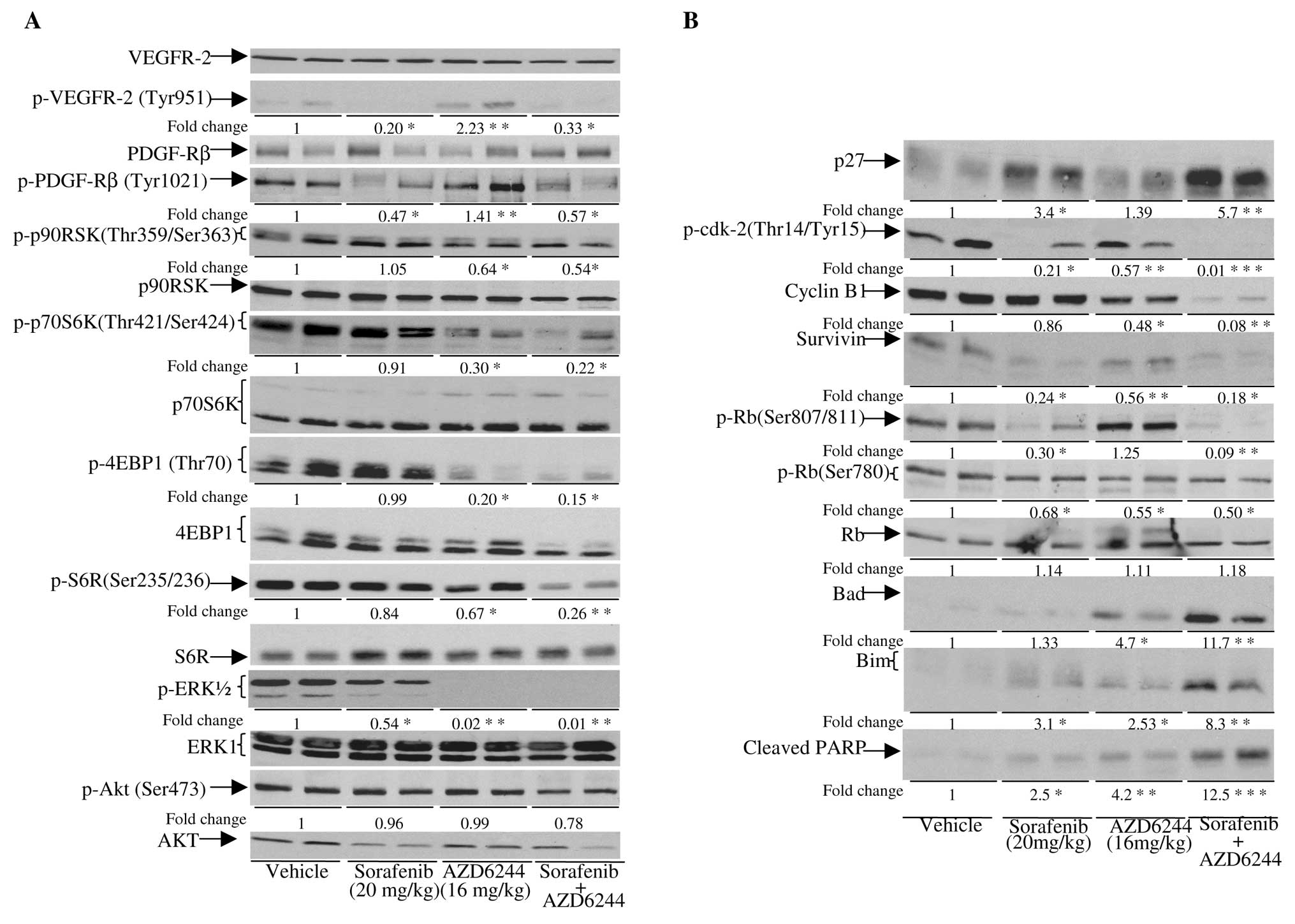
As shown in Fig. 5,
p-VEGFR-2, PDGF-Rβ and, to a lesser extent, p-ERK1/2 in
sorafenib-treated tumors was significantly reduced (P<0.05). A
decrease in p-cdk-2, survivin, p-Rb Ser807/811 and an elevation of
p27 and Bim in sorafenib-treated tumors were also observed.
AZD6244, on the other hand, inhibited phosphorylation of ERK1/2. A
modest elevation of p-VEGFR-2, p-PDGFR-β, Bad and Bim, but a
decrease in cyclin B1, p-70S6K, p-4EBP1 and p-p90RSK were seen in
AZD6244-treated tumors. In sorafenib/AZD6244, the addition of
AZD6244 to the sorafenib treatment not only led to the inhibition
of phosphorylation of VEGFR-2 and PDGFR-β but also resulted in
further reduction of p-ERK and p-p90RSK compared to sorafenib
monotherapy. The levels of Bad, Bim and cleaved PARP were also
elevated in tumors treated with sorafenib/AZD6244 compared to
treatment with sorafenib or AZD6244 alone, suggesting that
sorafenib/AZD6244 treatment caused apoptosis. Elevation of p27, and
hypophosphorylated Rb coupled with a significant reduction in
cyclin B1, survivin and p-cdk-2 were also observed in
sorafenib/AZD6244-treated tumors, supporting the observation that
sorafenib/AZD6244 induced cell cycle arrest. In addition,
sorafenib/AZD6244 also reduced the levels of p-S6R, p-p70S6K and
p-4EBP1, which are downstream targets of the mTOR pathway. These
data suggest that the mTOR pathway is impaired by sorafenib/AZD6244
treatment. Similar results were obtained with the other three
xenografts (data not shown).
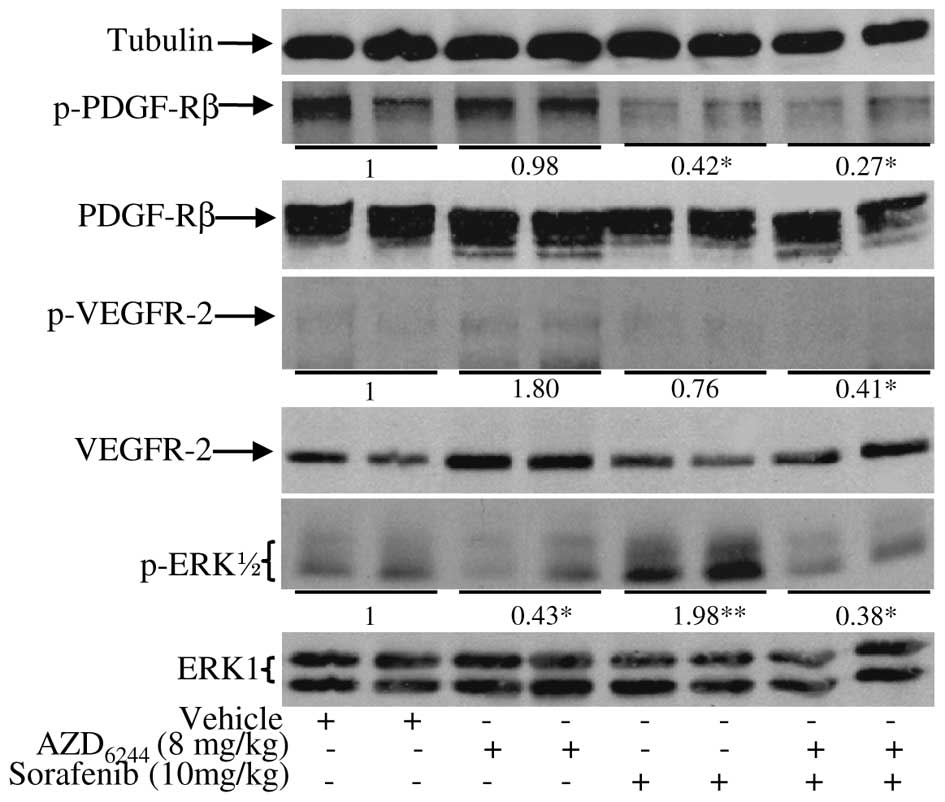
As 20 mg/kg sorafenib plus 16 mg/kg AZD6244 was
quite potent in inhibiting tumor growth and angiogenesis, we sought
to determine if half a dose of each (10 mg/kg of sorafenib plus 8
mg/kg AZD6244) could produce similar antitumor activity as a 20
mg/kg dose of sorafenib. If that is the case then adverse effects
associated with a full dose of sorafenib in some patients (3) could potentially be reduced by
administering a lower dose of sorafenib/AZD6244. To test this
hypothesis, we treated mice bearing RCC19-0809 tumors with the
combination of 10 mg/kg sorafenib and 8 mg/kg AZD6244. As shown in
Fig. 6, sorafenib at the dose of
20 mg/kg produced good antitumor activity with more than 60% tumor
growth inhibition. AZD6244 at the dose of 16 mg/kg had
insignificant antitumor activity. Combination of AZD6244 at 16
mg/kg and sorafenib at 20 mg/kg achieved more than double the
antitumor activity of sorafenib alone at 20 mg/kg. For monotherapy,
40±5% and 5±1.3% tumor growth inhibition were observed for 10 mg/kg
sorafenib and 8 mg/kg AZD6244, respectively. However, combination
sorafenib at 10 mg/kg and AZD6244 at 8 mg/kg produced similar tumor
growth inhibition compared to sorafenib as single-agent therapy at
20 mg/kg (Fig. 6). Although
sorafenib increased p-ERK1/2 at the dose of 10 mg/kg,
sorafenib/AZD6244 still inhibited phosphorylation of VEGFR-2,
PDGFR-β and ERK1/2 (Fig. 7).
Notably, the 10 mg dose of sorafenib used in this study was lower
than the dose given to patients, which is approximately 13 mg/kg.
These findings suggest that AZD6244/sorafenib treatment allows for
the dose reduction of sorafenib without compromising the antitumor
efficacy of the optimal sorafenib dose. Similar results were
obtained for the other RCC xenografts (data not shown). Combination
therapy with low doses of AZD6244 and sorafenib was tolerated well
by the animals with no significant adverse effects observed as
manifested by weight loss, unkempt appearance, mortality or
distress behavior.
Discussion
While the clinical efficacy of tyrosine kinase
inhibitors (TKIs), such as sorafenib, in patients with mRCC is
impressive in some patients, only approximately 60% of patients
with mRCC respond to this agent (3). For those treated with sorafenib, many
adverse effects associated with its use were observed (3). The common adverse effects of
sorafenib include fatigue, weight loss, desquamation, hand-foot
skin reaction, diarrhea, nausea and abdominal pain. Rarely, it can
also cause hematological abnormalities, including leukopenia,
anemia, neutropenia and thrombocytopenia; and severe cardiovascular
adverse event such as hypertensive crisis, myocardial ischemia and
congestive heart failure. It has been reported that almost a third
of the patients on a full dose of sorafenib (400 mg/BD) required a
dose reduction and 10–17% of patients ceased treatment with the
drug due to serious adverse events (8,13).
Based on this, a combination treatment strategy with sorafenib and
an agent that has synergistic effects is being evaluated. In
particular, the addition of a drug that would allow the reduction
of the sorafenib dosage while maintaining its overall antitumor
effects would lead to a decrease in the risk of adverse events,
thus increasing the tolerability of the treatment.
In the present study, we showed that
sorafenib/AZD6244 is considerably more effective than the single
agents in suppressing the growth of human RCC xenografts.
Sorafenib/AZD6244 effectively suppresses tumor growth, induces
apoptosis, and inhibits angiogenesis in vivo. We also
observed that sorafenib inactivates mTOR targets. This combination
is significantly superior to either drug in suppressing tumor
growth with minimal toxicity. Our findings provide further insights
into the limited benefits observed in clinical trials using
sorafenib or MEK inhibitors as single agents and exemplify how the
efficacy of sorafenib or MEK inhibitors could be improved in the
treatment of RCC. Combined sorafenib and ERK inhibition may be a
promising drug combination as one third of RCCs are driven by
signals generated from angiogenesis, the Ras/Raf/ERK, PI3K/Akt/mTOR
signaling pathways and this combination can reduce side effects due
to the high dose of the sorafenib treatment. The addition of
AZD6244 complements well with the effect of sorafenib in that it
allows reducing the optimal dose of sorafenib in half without
reducing the antitumor activity. Markedly, we found that AZD6244
alone, and combination sorafenib/AZD6244 at a full dosage (20 and
16 mg/kg respectively) and half doses (10 and 8 mg/kg) were well
tolerated by the animals. This finding has important clinical
implications as it suggests that the sorafenib/AZD6244 combination
is a potentially more effective clinical treatment strategy for
patients with advanced RCC.
The significance of the molecular inhibition of both
VEGFR-2/PDGFR-β and MEK/ERK pathways by combination
sorafenib/AZD6244 treatment was evidenced by a more profound
inhibition of angiogenesis, cell proliferation and induction of
apoptosis as shown in the immunohistochemical staining of the
treated RCC xenografts. We had previously identified survivin as an
oncogene that plays an important role in the pathogenesis of RCC
(9). Of note, sorafenib/AZD6244
combination treatment leads to marked depletion of survivin, in
addition to more inhibition of cell cycle proteins and the mTOR
signaling pathway compared to sorafenib alone. It is hypothesized
that combination sorafenib/AZD6244 treatment resulted in more
profound inhibition of both VEGFR-2/PDGFR-β and MEK/ERK pathways
compared to sorafenib alone in RCC, leading to significantly more
inhibition of mTOR, survivin, cell cycle proteins and apoptosis
induction, thus enhancing the antitumor activity of sorafenib in
RCC.
Although sorafenib/AZD6244 is effective in
preclinical models of human RCC, the molecular mechanisms
responsible for this cooperativity remain unknown. Due to the fact
that VEGF-induced migration and proliferation of endothelial cells
are in part mediated by the activation of the MEK/ERK signaling
cascades and recruitment of pericytes into tumors involves
VEGFR/PDGFR system, pronounced inhibition of VEGFR-2, PDGFR-β and
the MEK/ERK signaling pathway by sorafenib/AZD6244 would
effectively inhibit tumor angiogenesis and ultimately tumor growth
in vivo. Since cyclin B1 and Cdk-2 are required for cell
cycle progression, inhibition of cyclin B1 and Cdk-2 coupled with
upregulation of p27 by sorafenib/AZD6244 would cause cell cycle
arrest. Our present study shows that the pro-apoptotic activity of
sorafenib is significantly enhanced when combined with AZD6244.
However, the mechanism(s) responsible for this effect has yet to be
fully elucidated. Bim has been implicated in the regulation of
apoptosis (10). It is possible
that upregulation of Bad and Bim by sorafenib/AZD6244 would allow
more Bim and Bad to bind to and antagonize anti-apoptotic effect of
the Bcl-2 and Bcl-xL, leading to Bax-dependent apoptogen release,
caspase activation and cell death.
The clinical effects of sorafenib in advanced RCC is
primarily disease stabilization, thereby leading to the
prolongation of progression-free survival with only marginal
overall survival clinically. In this patient RCC-derived xenograft
model, combination therapy with sorafenib/AZD6244 resulted in more
tumor regression compared to monotherapy with either AZD6422 or
sorafenib monotherapy. The antitumor effects resulting from the
addition of AZD6422 to sorafenib may bring about significant
survival benefit not seen with the sorafenib monotherapy clinically
thus far. Despite favorable in vivo data suggesting the
beneficial effects of combination sorafenib/AZD6244 treatment in
RCC, a study using a murine xenograft model has its inherent
limitations. Firstly, clinical conclusions based on data derived
from a limited number of RCC xenografts should be interpreted with
caution. Secondly, even though we demonstrated that the xenografts
we generated retain identical histological features compared to the
original specimens, tumor behavior in a non-orthotropic environment
in mice may not resemble the micro-environment of RCC in patients.
Nevertheless, our molecular data generated using RCC-derived
xenografts is consistent and reproducible. A recent pilot study of
treatment guided by personalized tumor xenografts in patients with
advanced cancer demonstrated a remarkable correlation between drug
sensitivity in the model and clinical outcome, both in terms of
resistance and sensitivity (14).
Although we caution direct extrapolation of these data to the
clinical setting, these data suggest that the sorafenib/AZD6244
combination is potentially beneficial; the extent of these benefits
require a clinical trial to be verified.
In summary, our study shows that combination
sorafenib/AZD6244 treatment enhances the antitumor activity of
sorafenib and allows for a dose reduction of sorafenib without
compromising its antitumor activity.
Acknowledgements
This work was supported by the
following organizations and research grants: the National Kidney
Foundation (NKFRC/2008/07/23), SingHealth (TDF/CS004/2008) and the
National Medical Research Council (NIG/005/2008), awarded to J.S.P.
Yuen; the Singapore Millennium Foundation, Singapore Cancer
Syndicate (SCS-AS0032, SCS-HS0021, SCS-AMS0086), awarded to H.
Huynh.
References
|
1
|
Zisman A, Pantuck AJ, Wieder J, Chao DH,
Dorey F, Said JW, deKernion JB, Figlin RA and Belldegrun AS: Risk
group assessment and clinical outcome algorithm to predict the
natural history of patients with surgically resected renal cell
carcinoma. J Clin Oncol. 20:4559–4566. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Atkins MB, Avigan DE, Bukowski RM, et al:
Innovations and challenges in renal cancer: consensus statement
from the first international conference. Clin Cancer Res.
10:6277S–6281S. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J
Med. 356:125–134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Heng DY, Chi KN, Murray N, Jin T, Garcia
JA, Bukowski RM, Rini BI and Kollmannsberger C: A population-based
study evaluating the impact of sunitinib on overall survival in the
treatment of patients with metastatic renal cell cancer. Cancer.
115:776–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Overall survival and updated results for sunitinib compared with
interferon alfa in patients with metastatic renal cell carcinoma. J
Clin Oncol. 27:3584–3590. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fabian MA, Biggs WH III, Treiber DK, et
al: A small molecule-kinase interaction map for clinical kinase
inhibitors. Nat Biotechnol. 23:329–336. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilhelm SM, Carter C, Tang L, et al: BAY
43-9006 exhibits broad spectrum oral antitumor activity and targets
the RAF/MEK/ERK pathway and receptor tyrosine kinases involved in
tumor progression and angiogenesis. Cancer Res. 64:7099–7109. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Dong B, Lu JJ, Yao X, Zhang S,
Dai B, Shen Y, Zhu Y, Ye D and Huang Y: Efficacy of sorafenib on
metastatic renal cell carcinoma in Asian patients: results from a
multicenter study. BMC Cancer. 9:249–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuen JS, Sim MY, Siml HG, Chong TW, Lau
WK, Cheng CW and Huynh H: Inhibition of angiogenic and
non-angiogenic targets by sorafenib in renal cell carcinoma (RCC)
in a RCC xenograft model. Br J Cancer. 104:941–947. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huynh H, Ngo VC, Koong HN, et al: AZD6244
enhances the antitumor activity of sorafenib in ectopic and
orthotopic models of human hepatocellular carcinoma (HCC). J Hepat.
52:79–87. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huynh H, Soo KC, Chow PK, Panasci L and
Tran E: Xenografts of human hepatocellular carcinoma: A useful
model for testing drugs. Clin Cancer Res. 12:4306–4314. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huynh H, Ong RW, Li PY, Lee SS, Yang S,
Chong LW, Luu DA, Jong CT and Lam IW: Targeting receptor tyrosine
kinase pathways in hepatocellular carcinoma. Anticancer Agents Med
Chem. 11:560–575. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bukowski RM, Stadler WM, McDermott DF, et
al: Safety and efficacy of sorafenib in elderly patients treated in
the North American advanced renal cell carcinoma sorafenib expanded
access program. Oncology. 78:340–347. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hidalgo M, Bruckheimer E, Rajeshkumar NV,
Garrido-Laguna I, Oliveira ED, Rubio-Viqueira B, Strawn S, Wick MJ,
Martell J and Sidransky D: A pilot clinical study of treatment
guided by personalized tumorgrafts in patients with advanced
cancer. Mol Cancer Ther. 10:1311–1316. 2011. View Article : Google Scholar : PubMed/NCBI
|