Introduction
The treatment of soft tissue and bone sarcoma
remains challenging because higher-grade sarcomas are associated
with higher local treatment failure rates and increased metastatic
potential. An estimated 10,520 cases of soft tissue sarcoma and
2,650 cases of malignancy of the bones and joints were diagnosed in
the United States in 2010 (1).
There are more than 50 different histological types of soft tissue
sarcoma, and the histological diagnosis of the rare sarcoma is
confusing. Typically, low-grade sarcoma demonstrates local invasion
with a lower propensity to metastasize, while high-grade sarcomas
have a greater likelihood of distant spread to the lungs by a
hematogenous route (2).
Surgical resection remains the most effective
therapeutic approach for the management of soft tissue sarcoma,
especially small (<5 cm), superficial high-grade and low-grade
sarcoma (3). Although local
control of high-grade soft tissue sarcoma can be obtained through
the use of surgery and radiotherapy, recurrence was reported to
occur in more than 50% of such patients (4). In most patients with soft tissue
sarcoma that is of a high histological grade, large size (>5
cm), has invaded deep into the fascia, or is a local recurrence, a
combined modality approach comprising preoperative or postoperative
chemotherapy is used in addition to the radical surgical procedures
(5). However, the role of
chemotherapy for adult-type soft tissue sarcoma remains less well
defined. According to the results from a meta-analysis of 14 trials
performed in 1997 (6), the overall
survival at 10 years improved from 50% to 54% with adjuvant
chemotherapy, but this difference was not statistically
significant. Moreover, according to the results from a randomized
clinical trial with neoadjuvant chemotherapy (7), the disease-free survival at 5 years
improved from 52% to 56%, but this difference was also not
statistically significant. Therefore, the development of a novel
therapeutic strategy is required to better treat patients with
refractory bone and soft tissue sarcomas.
Oncolytic virotherapy, the selective killing of
tumor cells by viruses, is a promising experimental treatment for
cancer. The first report of oncolytic virotherapy was due to an
unintentional exposure to naturally-occurring viruses or the
administration of live attenuated vaccine strains, such as
Newcastle disease virus and reovirus (8). Clinical trials of virotherapy for
cancer started early in the 20th century. Despite encouraging
results in case reports, the overall clinical results have
disappointed clinicians because of the weak therapeutic effects if
such treatments (9). Recently, a
better understanding of the viral tropism for the cells allowed for
the development of new strategies to enhance the specificity of
viruses for cancer cells and to improve the viral replication in
cancer cells (10). In addition to
DNA viruses, such as adenovirus and herpes simplex virus, that are
molecularly engineered to replicate specifically in tumor cells,
RNA viruses with inherent tumor specificity have been developed as
oncolytic agents for cancer treatment. This group of viruses
includes reovirus (11), Newcastle
disease virus (12), measles virus
(13), vesicular stomatitis virus
(14) and poliovirus (15).
The poliovirus is a non-enveloped plus-strand RNA
virus belonging to the Picornaviridae, and is the causative agent
of paralytic poliomyelitis. The vast majority of poliovirus
infections remain asymptomatic, but 1–2% of cases result in
neurologic complications (16).
The restriction of poliovirus cell tropism to motor neurons
resident within the spinal cord and brainstem gives rise to a
highly characteristic clinical syndrome dominated by flaccid
paralysis. Selective targeting of motor neurons by poliovirus is
most likely determined by the distribution of its cellular
receptor, the Ig superfamily molecule CD155 (also known as
poliovirus receptor: PVR and nectin-like molecule-5: Necl-5). This
assumption is supported by the observation that mice transgenic for
human CD155 develop a polio-like syndrome after poliovirus
infection (17–19). In addition, intracellular
conditions favoring viral replication have also been reported to
contribute to poliovirus cell-type specificity (20).
Recently, the biological functions of CD155 have
become clearer. It is now known that CD155 plays an important role
in cell adhesion, migration, polarization and proliferation
(21). CD155 interacts in trans
with nectin-3, a member of the Ig-like nectin family, a
Ca2+-independent cell-cell adhesion molecule, and
cooperatively forms adherence junctions with cadherin. When the
cells contact other cells, CD155 is removed from the cell surface
by clathrin-dependent endocytosis, due to its trans-interaction
with nectin-3. When the cells do not come in contact with other
cells, CD155 is upregulated by growth factor-induced signaling,
while thus assembling the leading edge of moving cells (22).
Previous reports indicated that malignant tumors
originating from the neural crest, including malignant glioma
(23) and neuroblastoma (24) could be experimentally treated with
various types of live attenuated poliovirus (LAPV), suggested its
potential for clinical applications. However, there have been no
reports which have so far examined whether bone and soft tissue
sarcomas also represent targets of LAPV. In the present study, we
first investigated the CD155 expression in bone and soft tissue
sarcomas. We next investigated the oncolytic effects of a LAPV on 6
human bone and soft tissue sarcoma cells in vitro. Finally,
we examined whether LAPV have oncolytic effects on soft tissue
sarcomas using a subcutaneous xenograft animal model.
Materials and methods
Cell lines
Twelve human bone and soft tissue sarcoma cell lines
were used in this study. HT1080 human fibrosarcoma, HS-SY-II human
synovial sarcoma, MFH-ino human malignant fibrous histiocytoma,
HS-PSS human malignant peripheral nerve sheath tumors (MPNST),
HS-Sch-2 human MPNST, NMS-2 human MPNST, 143B human osteosarcoma,
Saos-2 human osteosarcoma, and HOS human osteosarcoma cell lines
were obtained from Riken Cell Bank (Ibaraki, Japan). HuO9,
HuO9-M112, and HuO9-M132 human osteosarcoma cell lines were a kind
gift from Dr Yasuo Beppu (National Cancer Center, Tokyo, Japan).
HT1080 and 143B cells were maintained in MEM with 10% fetal bovine
serum (FBS) (Invitrogen, Tokyo, Japan). HOS cells were additionally
supplemented with 0.1 mM non-essential amino acids (Invitrogen).
HS-SY-II, HS-PSS, HS-Sch-2 cells were maintained in DMEM
(Invitrogen) with 10% FBS. NMS-2, HuO9, HuO9-M112 and HuO9-M132
cells were maintained in RPMI-1640 (Invitrogen) supplemented with
10% FBS. MFH-ino cells were maintained in DMEM/HamF12 (Invitrogen)
with 10% FBS. Saos-2 cells were maintained in McCoy’s (Invitrogen)
with 10% FBS. HeLa and mouse osteosarcoma cell line LM8 cells were
grown in MEM and DMEM, respectively, with 10% FBS.
A live attenuated poliovirus (LAPV)
A LAPV vaccine containing the Sabin 1 strain (Japan
Poliomyelitis Research Institute, Tokyo, Japan) was used as an
oncolytic virus. The virus titer was determined by measuring the
50% tissue culture infectious dose (TCID50) in HeLa
cells.
One-step viral growth curves
The growth of poliovirus in the fibrosarcoma cell
line, HT1080, was measured as previously described (19). Briefly, cell monolayers were washed
with medium, and then were treated with medium containing LAPV at a
multiplicity of infection of 0.2 and 2 TCID50,
respectively. After slowly stirring the dish for 30 min at room
temperature, the cells were thoroughly washed with medium to remove
unbound virus, and then incubated in serum-free medium at 37°C for
different intervals. At 2, 4, 6, 8, 12, 24, and 48 h after virus
inoculation, the extracellular virus and the corresponding
cell-associated virus were recovered after three consecutive
freeze-thaw cycles. The infectivity of the clarified virus
suspension was determined by a TCID50 assay.
Viability assay and morphological
features
The effects of LAPV on the viability of sarcoma
cells and the morphological changes induced by viral infection were
determined. HT1080, MFH-ino, HS-PSS, HuO9-M112, Saos-2, HOS and LM8
cells were seeded at 1.0×104 per well on 96-well plates
and treated with LAPV at a multiplicity of infection (MOI) of 2,
0.2, 2.0×10−2, 2.0×10−3 or
2.0×10−4 TCID50/cell, respectively. At
different intervals, the cell viability was assessed by the MTS
assay (CellTiter 96® AQueous One Solution Cell
Proliferation Assay, Promega, Madison, WI, USA). The morphology of
cells was evaluated under a phase-contrast microscope.
Apoptosis assay
After 6 h of exposure to LAPV at a MOI of 2
TCID50/cell or to vehicle, apoptotic HT1080 cells were
detected by the TUNEL (terminal
deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling)
assay (ApopTag® Peroxidase In Situ Apoptosis
Detection Kit, Millipore, Billerica, MA, USA). In addition, the
characterization of cell death in HT1080 cells exposed to LAPV was
determinated. The HT1080 cells were plated in 96-well plates at
1.0×104 per well. After overnight incubation, the cells
were exposed to LAPV at a MOI of 2, 0.2, 2.0×10−2
TCID50/cell, or the vehicle for different intervals.
After 0, 6, 12, 24, 36 or 48 h of incubation, 20 μl of
Viability/Cytotoxicity Reagent containing GF-AFC Substrate
(ApoTox-GloTM Triplex Assay, Promega) was added. After
30 min of incubation at 37°C, the fluorescence was recorded at 400
nm excitation/505 nm emission using a microplate reader for
fluorescence and luminescence (Promega) to assess the cell
viability. After 100 μl of Caspase-Glo 3/7 Regent
(ApoTox-Glo Triplex Assay, Promega) was added to the cells and
incubated for 30 min at room temperature, the luminescence was
recorded. The evaluation of apoptotic cells was performed by
measuring the activity of caspases 7 and 3 using a luminogenic
substrate. The induction of apoptosis was defined as a decrease in
cell viability with a concomitant increase in the caspase 7 and 3
activity.
Total-RNA extraction and quantitative
real-time polymerase chain reaction
RNA was isolated (Isogen, Nippon Gene, Tokyo,
Japan), reverse transcribed using the 1st Strand cDNA Synthesis Kit
(Roche Applied Science, Mannheim, Germany) and subjected to
real-time quantitative PCR with ABI PRISM® 7000 Sequence
Detection System (Applied Biosystems, Carlsbad, CA, USA). Primer
are purchased from Applied Biosystems. GAPDH was used as an
endogenous ‘house-keeping’ gene for normalization. Standard curves
were generated using cDNA samples from HeLa cells. The relative
expression levels of each target gene were indicated by calculating
the ratio to those in HeLa cells. All assays were performed in
triplicate and repeated three times.
Immunofluorescence microscopy
Cells were fixed with methanol, and blocked with 3%
BSA in PBS. Cells were stained with primary monoclonal antibody
against recognizes the poliovirus binding site of CD155 (mouse
D171, Neomarkers, Union City, CA, USA) (25), and secondary antibody
Alexa®488-conjugated goat anti-mouse IgG (H+L)
(Invitrogen). The nucleus of each sample was detected by
bisBenzimie Hoechst 33342 trihydrochloride staining (Sigma-Aldrich,
St. Louis, MO, USA). Microscopic signals were observed with an
Olympus BX50 epifluorescence microscope (Tokyo, Japan), and the
images were captured with an Olympus DP70 digital camera and
processed with an Olympus DPController with the DPManager software
program.
Western blot analyses
The expression of CD155 protein was determinated by
a western blot analysis as previously described (26). The primary antibody was a goat
anti-CD155 antibody, sc-27754 (1:200, Santa Cruz Biotechnology,
Santa Cruz, CA, USA) (27), and
the secondary antibody reaction was performed using a
peroxidase-conjugated secondary antibody (Dako, Carpinteria, CA,
USA) and visualized using the ECL substrates (GE Healthcare,
Piscataway, NJ, USA).
In vivo xenograft model
Four-week-old BALB/c nu/nu mice were maintained in a
humidity- and temperature-controlled laminar flow room. For
xenografting, 1×107 HT1080 cells in 0.1 ml of PBS were
subcutaneously injected into the right flanks of nude mice using a
26-gauge needle. In all the mice, enlargement of the tumors was
observed within 1–2 weeks after inoculation. The tumor size was
measured with calipers two times a week, and the tumor volume was
calculated using the ellipsoid formula: length × width2
× 0.52 (28). When the tumor
volume increased to 0.20–0.25 cm3, LAPV
(1×106 TCID50) or vehicle alone (for control
animals) was injected into the right flank tumor once a day for 3
days, and the tumor size was monitored every 3–4 days for 2
weeks.
The mice were sacrificed for histopathological
analyses and viral preparation assays. To investigate the
histopathological findings, paraffin-embedding samples were
observed after hematoxylin and eosin and TUNEL staining. For virus
propagation assays, tumor samples were immediately frozen and kept
at −80°C until use. After homogenization of the tumor samples with
additional PBS up to 20% weight/volume, the tumor tissue
homogenates were centrifuged at 2,000 rpm for 20 min at 4°C, and
the viral titer in the supernatants was determined by the
TCID50 in HeLa cell culture. All experimental procedures
using mice were approved by the Institutional Committees on Animal
Welfare.
Statistical analyses
The data were expressed as the means ± SE. The
statistical difference between groups was analyzed by Student’s
t-test or the Mann-Whitney U test. Differences were considered
statistically significant for p<0.05 or p<0.01.
Results
Expression of CD155 in bone and soft
tissue tumor cells
Since CD155 is required for the poliovirus to infect
cells, we first examined the expression of CD155 mRNA by
quantitative real-time PCR. The expressions level of CD155
mRNA in HT1080, MFH-ino, HS-Sch-2, HS-SY-II, HuO9-M112 Saos-2, HOS,
and 143B cells were, respectively, 2.3-, 6.7-, 2.5-, 1.9-, 1.7-,
4.4-, 2.4-and 5.8-fold higher than those in HeLa cells. In NMS-2,
HS-PSS, HuO9 and HuO9-M132 cells, the expression levels of
CD155 were almost equal to that of HeLa cells (Fig. 1A).
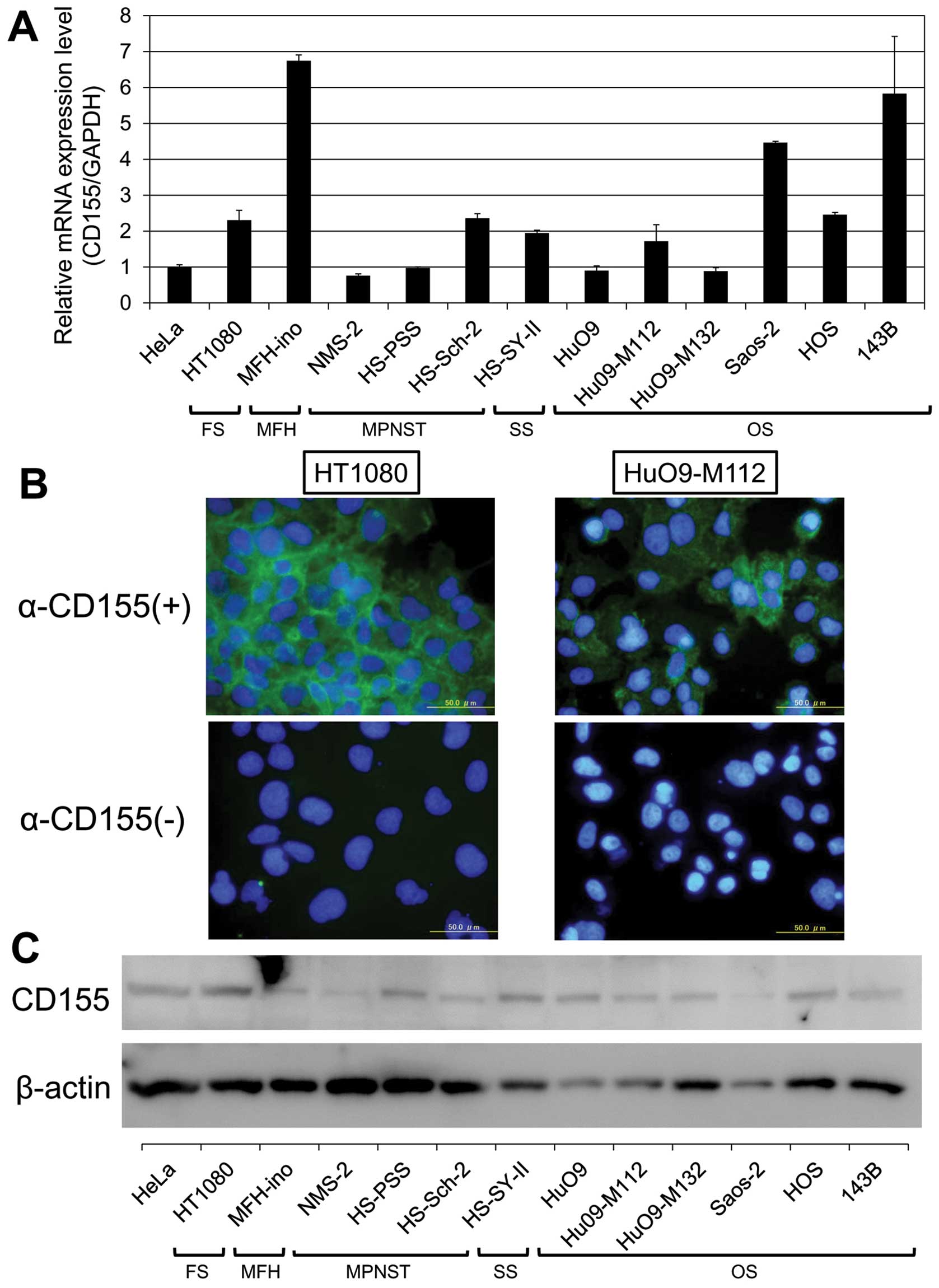 | Figure 1The expression of CD155 in human bone
and soft tissue sarcoma cells. (A) The expression of CD155 mRNA in
human bone and soft tissue sarcoma cells as determined by
quantitative real-time PCR. The relative levels of CD155
mRNA were calculated after normalization with reference to the
expression of GAPDH mRNA. The expression levels of CD155
mRNA in HT1080, MFH-ino, HS-Sch-2, HS-SY-II, HuO9-M112 Saos-2, HOS
and 143B cells were higher than those in HeLa cells. In the NMS-2,
HS-PSS, HuO9 and HuO9-M132 cells, the expression levels of
CD155 were almost equal to that of HeLa cells. (B) The
immunofluorescence of HT1080 (left) and HuO9-M112 cells (right).
CD155 was observed on the cell membrane of these cells. (C) The
results of the western blot analysis of CD155 protein expression in
human bone and soft tissue sarcoma cells lines. The expression of
CD155 protein was observed in all of the bone and soft tissue
sarcoma lines that were examined. FS, fibrosarcoma; MFH, malignant
fibrous histiocytoma; MPNST, malignant peripheral nerve sheath
tumor; SS, synovial sarconma; OS, osteosarcoma. |
Next, we confirmed the CD155 expression using an
immunofluorescence technique. In all of the human cell lines, CD155
was definitely identified in the cytoplasm and at the intercellular
junctions, although the distribution and signal intensity differed
among the cell lines (Fig.
1B).
In addition, we performed a western blot analysis to
demonstrate the expression level of the CD155 protein. As shown in
Fig. 1C, the expression of CD155
protein was observed in all of the bone and soft tissue sarcoma
cell lines (molecular weight ∼80 kDa).
LAPV induces apoptosis in bone and soft
tissue sarcoma cells in vitro
To examine whether LAPV induces cell death in
sarcoma cell lines, we investigated the morphological changes in
the HT1080 cells after LAPV exposure. Under a phase-contrast
microscope, the infected HT1080 cells showed morphological changes
such as rounding, shrinkage, detachment, and floating in the
culture medium within 48 h (Fig.
2A).
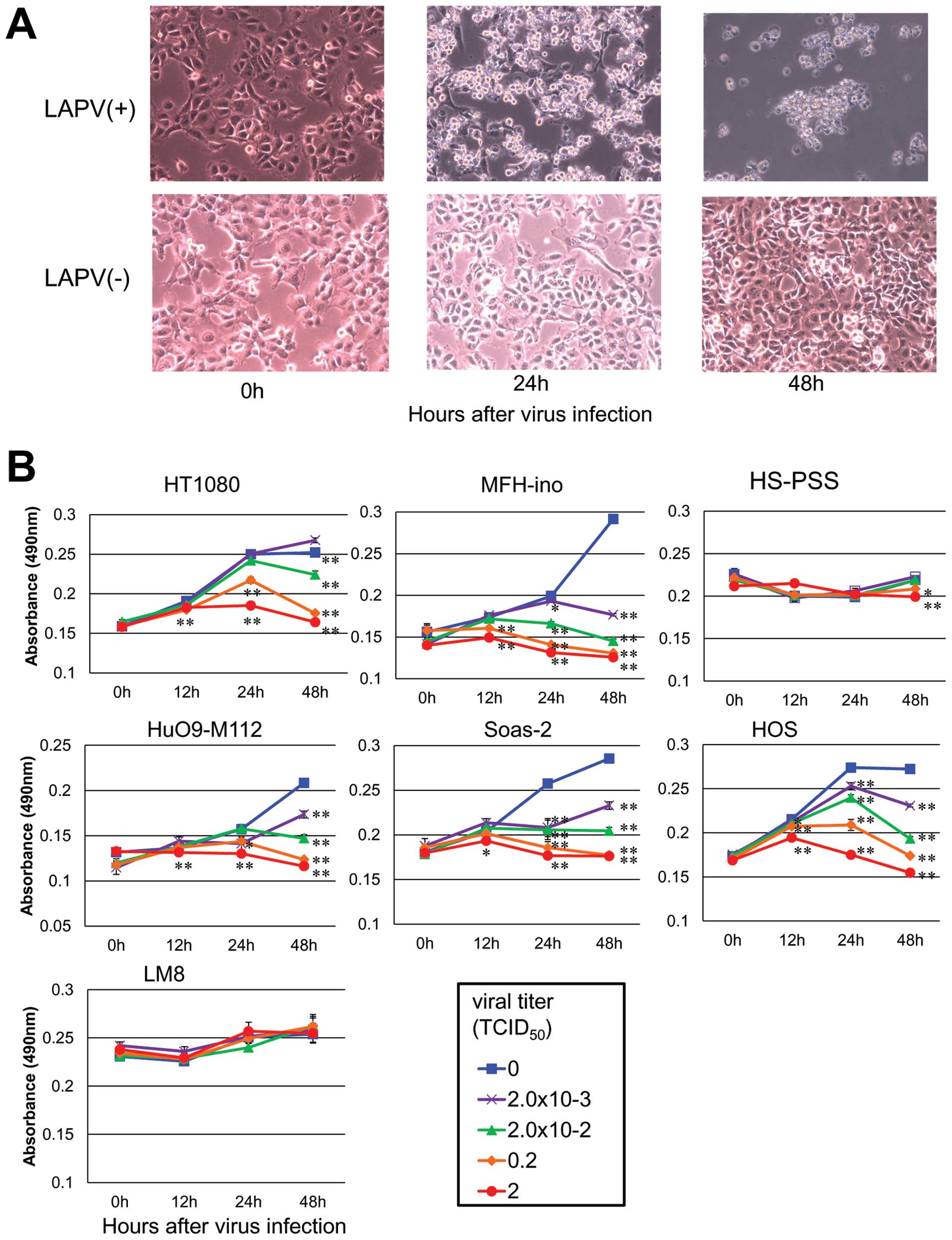 | Figure 2The effect of LAPV. (A) HT1080 cells
were photographed before, 24 and 48 h after LAPV infection. (B) The
human fibloblastoma cell line, HT1080, human malignant fibrous
histiocytoma cell line, MFH-ino, human MPNST cell line, HS-PSS,
human osteosarcoma cell lines, HuO9-M112, Saos-2 and HOS, and the
mouse osteosarcoma cell line, LM8, were incubated with LAPV for 12,
24 or 48 h at the indicated MOI (▪, 0 TCID50; x,
2×10−3 TCID50; ▴, 2×10−2
TCID50; ♦, 0.2 TCID50; •, 2
TCID50). At different intervals, the cell viability was
assessed by the MTS assay. LAPV strongly induced cell death in a
time- and dose-dependent manner in 5 out of the 6 human bone and
soft tissue sarcoma cell lines. The viability of mouse osteosarcoma
LM8 cells did not differ significantly at any of the time points.
The results are expressed as the means ± SE. *P<0.05
and **P<0.01 vs. the control (vehicle, 0
TCID50) group as determined by Student’s t-test. |
To measure the cell viability after LAPV exposure, 6
human bone and soft tissue tumor cell lines; HT1080, MFH-ino,
HS-PSS, HuO9-M112, Saos-2, HOS and one mouse osteosarcoma cell
line, LM8, were incubated in the presence of LAPV at a MOI from 2
to 2.0×10−4 TCID50/cell and their viability
was measured by the MTS assay. LAPV strongly induced cell death in
a time- and dose-dependent manner in 5 out of the 6 human bone and
soft tissue sarcoma cell lines (Fig.
2B).
At first, the HS-PSS cells seemed to be resistant to
LAPV exposure. But, because the HS-PSS cells infected by the LAPV
showed morphological changes such as rounding, shrinkage,
detachment, and floating in the culture medium, we supposed that
the slow growth of the cells minimized the effect of the poliovirus
exposure in the MTS assay. Thus, we next observed the effect of
LAPV exposure for 7 days, and found that the viability of the
HS-PSS cells exposed to the poliovirus was significantly lower than
that of the control cells at 72 h or more after infection (data not
shown). The viability of the mouse osteosarcoma cell line, LM8, did
not differ significantly at any of the time points, and did not
exhibit any morphological changes. We considered that the LM8 cell
line was completely resistant to LAPV, because mouse cells have no
human CD155, which is necessary for the establishment of a
poliovirus infection, as was previously known (29).
Our next question was whether the cell growth
suppression observed in various sarcoma cell lines was due to
apoptotic cell death induced by LAPV. First, we examined the
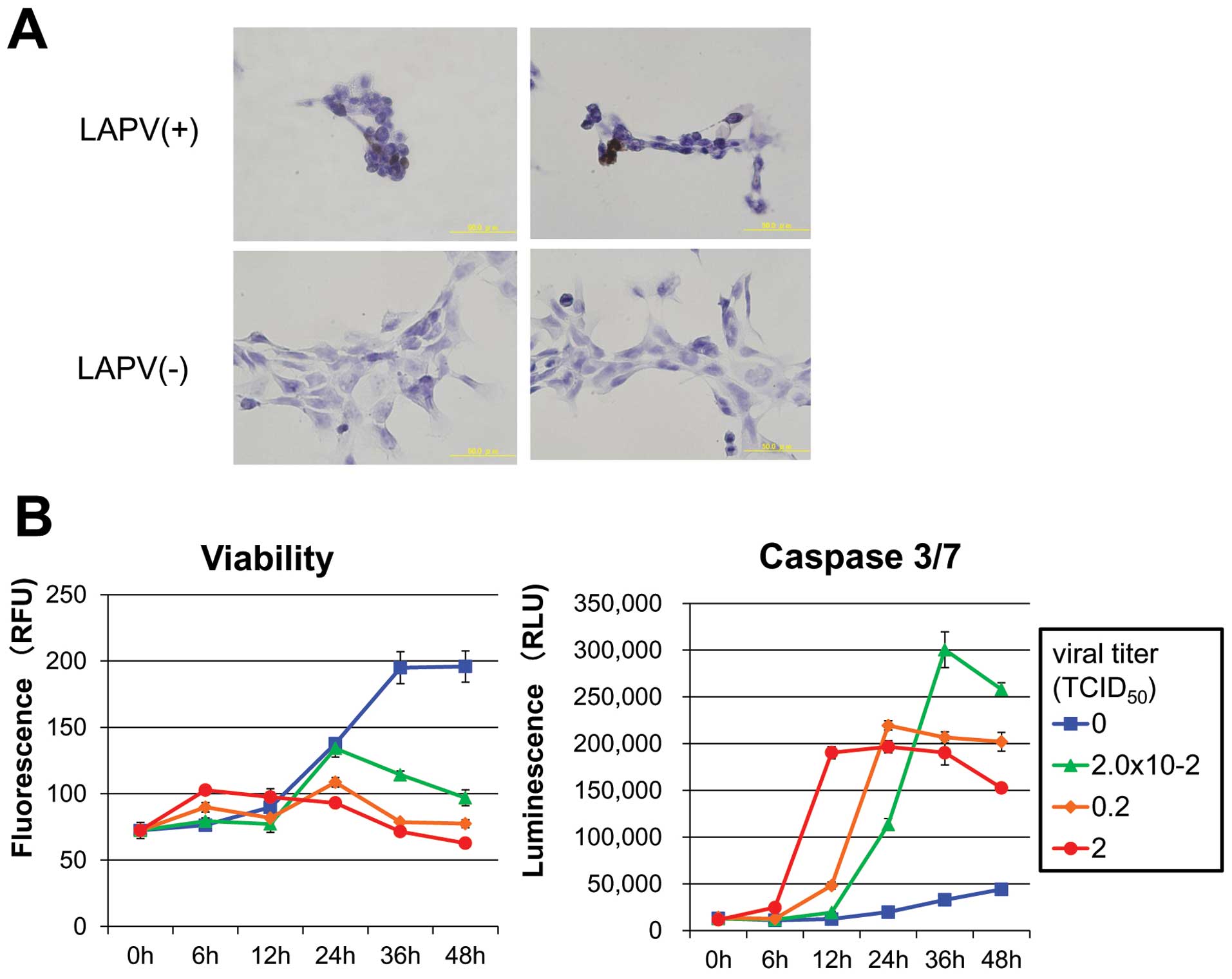
occurrence of apoptotic cell death using the TUNEL assay. We found
that the HT1080 cells treated with LAPV had positive staining for
the TUNEL reagent (Fig. 3A).
To confirm whether LAPV actually induced apoptotic
cell death, the caspase 3/7 activity was measured (Fig. 3C). Our results showed that the
poliovirus induced the activation of caspases 3 and 7 in a time-
and dose-dependent manner that was consistent with the decrease in
cell viability. Hence, we concluded that LAPV induces apoptosis in
bone and soft tissue sarcoma cells in vitro.
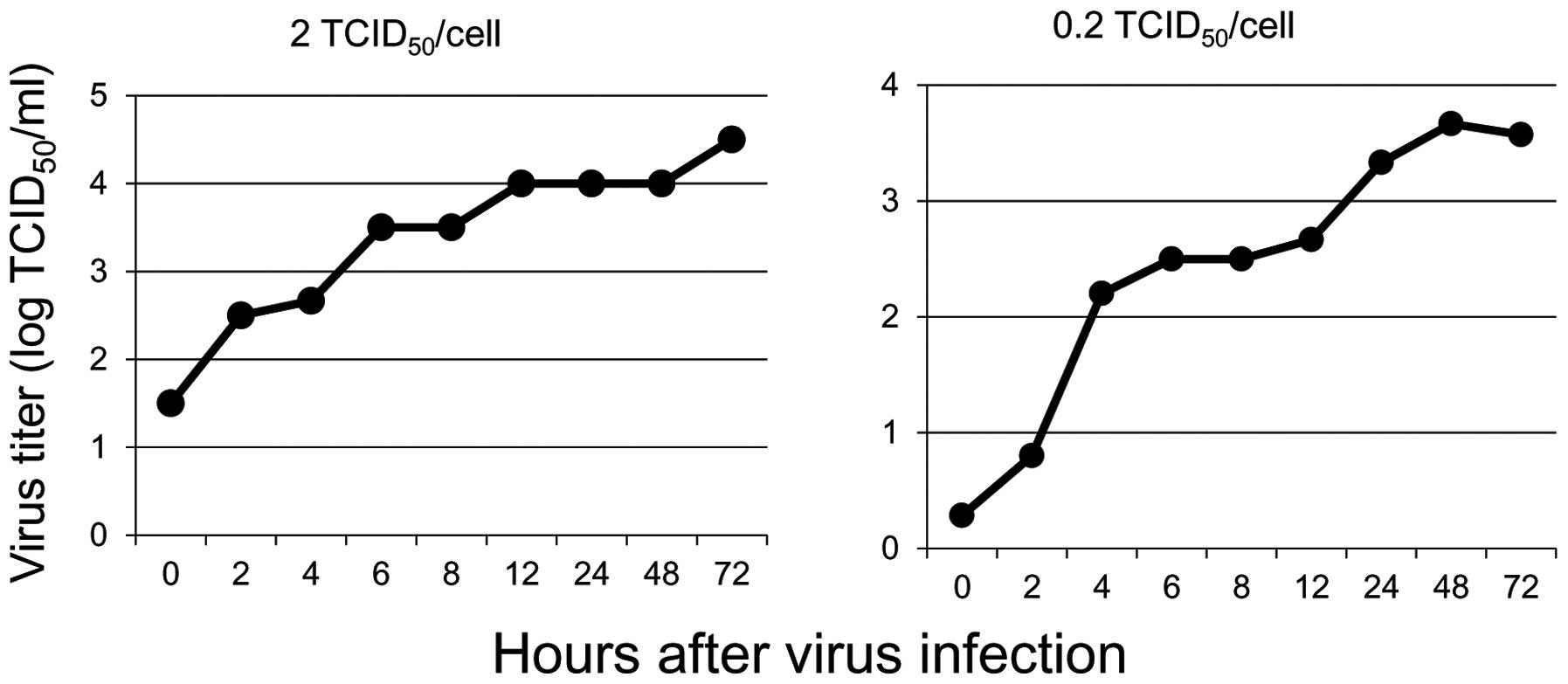
Propagation of live attenuated poliovirus
in soft tissue sarcoma cells
Since LAPV was found to induce apoptosis in the bone
and soft tissue sarcoma cells, we next examined the propagation of
the poliovirus in sarcoma cells. After exposure to LAPV for 30 min
at a titer of 0.2 and 2 TCID50/cell, the cell-associated
and extracellular viral yields were determined by the
TICD50 assay. We found that one-step viral growth curves
showed the propagation of LAPV in monolayer cultures (Fig. 4).
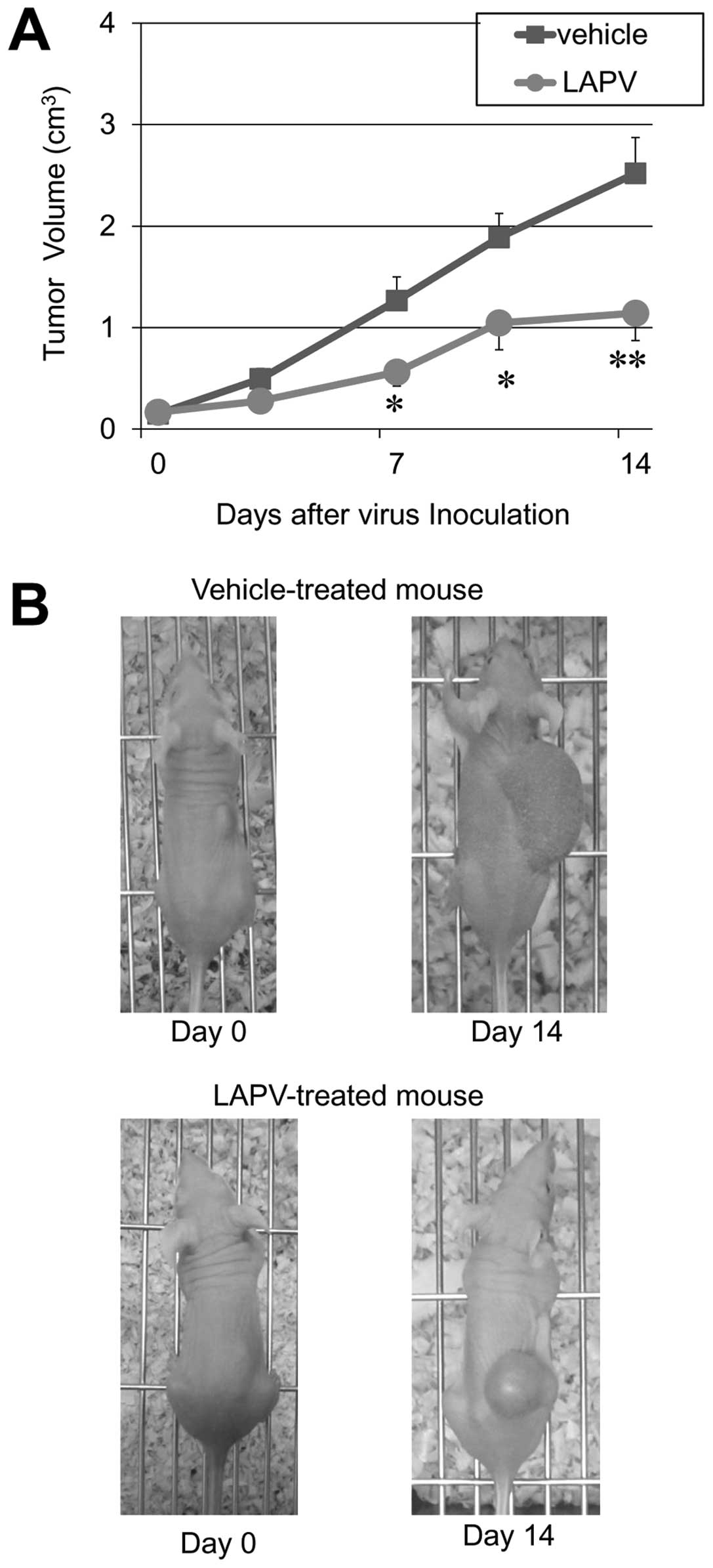
Live attenuated poliovirus kills soft
tissue sarcoma cells in vivo
The inherent capacity for LAPV to kill bone and soft
tissue sarcoma cells in vitro suggested that the virus might
be therapeutically useful. To test the oncolytic properties of LAPV
in vivo, mice bearing subcutaneous HT1080 xenograft tumors
were treated with an intratumoral inoculation of LAPV
(1×106 TICD50) once a day for 3 days. The
size of the xenotransplants in control mice increased by 16.9-fold
two weeks after treatment. However, three injections of LAPV
inhibited the tumor growth by nearly 40% (p<0.05 at 7 days or
more, Mann-Whitney U test) (Fig.
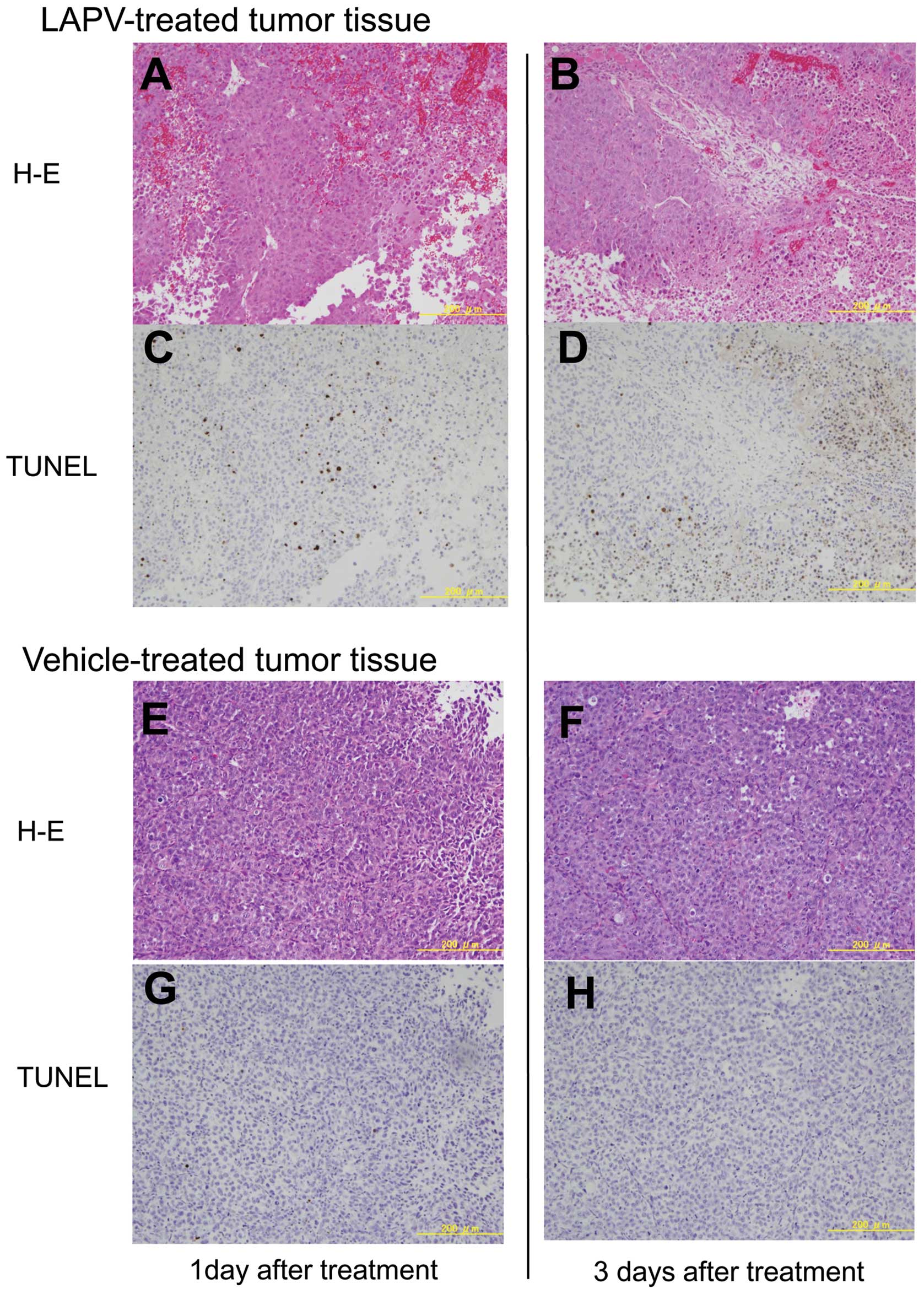
5). Histopathologically, many TUNEL positive cells were
scattered in the xenotransplants on day 1 after treatment with LAP,
and the areas of TUNEL positive degenerated tissue were obviously
increased on day 3 (Fig. 6).
TUNEL-positive cells were rarely observed in the xenotransplants
treated with the vehicle.
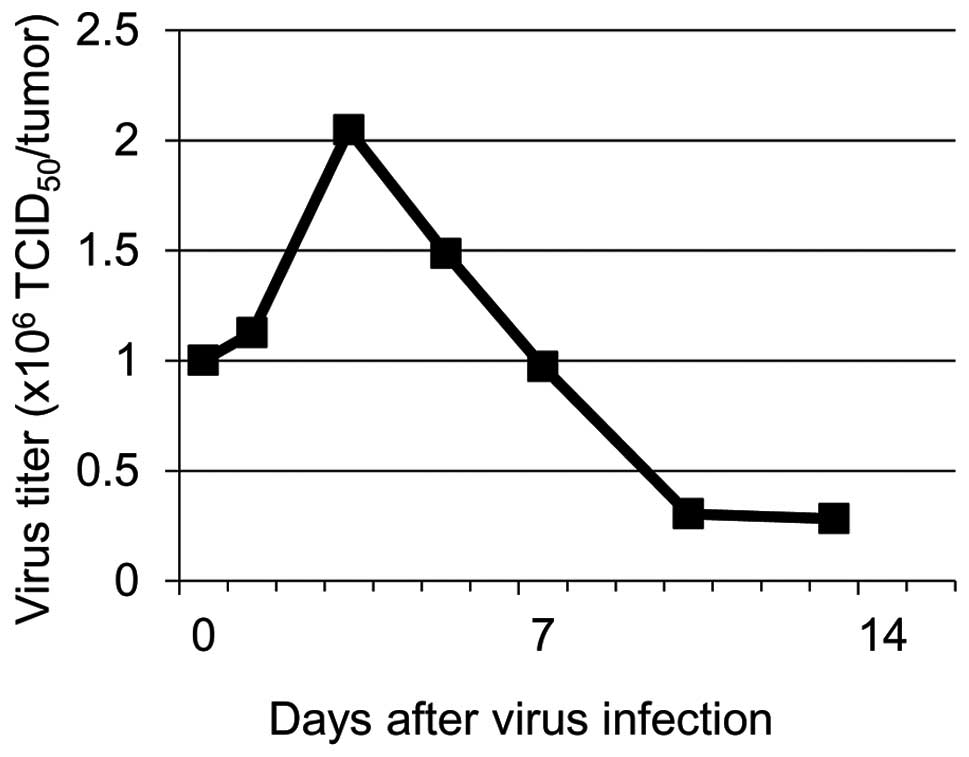
To investigate whether the LAPV is propagated in the
xenotransplants, the viral titer was determined using the
supernatants of tumor tissue homogenates by measuring the
TCID50 in HeLa cell culture. The maximum virus titer was
observed on day 3 after inoculation into the tumor (Fig. 7). This indicated that intratumoral
replication of LAPV induced the release of a number of infectious
viral particles, which lead to the apoptotic cell death of the
xenografts.
Discussion
The clinical outcome of patients with advanced bone
and soft tissue sarcomas is still unsatisfactory, despite the use
of multidisciplinary treatments including surgery, chemotherapy,
and radiotherapy (5,30). Therefore, the development of a
novel therapeutic agent is necessary to improve the prognosis of
these patients. In this study, we showed that LAPV possess an
inherent capacity to induce apoptosis in bone and soft tissue
sarcoma cells in vitro and to suppress the growth of
implanted fibrosarcoma cells in vivo.
The poliovirus receptor CD155, is a key molecule for
oncolytic virotherapy using LAPV, because the expression of CD155
is essential for poliovirus binding and infection (29). We initially supposed that CD155
might be expressed in neurogenic sarcomas, such as MPNST, because
the upregulation of CD155 expression was observed in
neuroectodermal malignancies (e.g. glioblastoma multiforme,
medulloblastoma, or neuroblastoma) (31–33).
Surprisingly, however, CD155 expression was observed in all 12 bone
and soft tissue sarcoma cell lines examined. This is the first
report to show that CD155 is widely expressed in various types of
bone and soft tissue sarcoma cell lines. Solecki et al
previously showed that the expression of the CD155 gene is
transcriptionally activated through the Sonic Hedgehog (SHH)
signaling pathway (34). Recently,
activation of the hedgehog signaling pathway was reported in
certain sarcoma types (35,36).
Upregulated expression of CD155 was demonstrated in NIH3T3 cells
transformed by the V12-Ki-Ras oncogene, and it contributes to the
loss of contact inhibition in transformed cells (37). Further investigation will be needed
to clarify the mechanism of upregulation of CD155 gene
expression in sarcoma cells.
We showed that LAPV can kill various types of
sarcoma cells, and that there is viral propagation. Gromeier et
al have exploited this feature of LAPV to kill glioma cells
(15). Poliovirus uniquely depends
on CD155 for host cell binding and entry. Based on all available
empirical evidence, CD155 is sufficient for all binding and entry
functions leading to uncoating of the viral genome (29,38).
At first, the HS-PSS cells seemed to be resistant to LAPV exposure.
However, the HS-PSS cells infected with the LAPV showed
morphological changes such as rounding, shrinkage, detachment, and
floating. Since HS-PSS cells have a long cell cycle, we observed
the effect of LAPV exposure for 7 days, and confirmed that the
HS-PSS cells were also susceptible to LAPV infection. Because of
the lack of human type of CD155 expression, the poliovirus could
not infect mouse LM8 cells. We concluded that all human bone and
soft tissue sarcoma cell lines were susceptible to LAPV infection,
and that this was dependent on their CD155 expression.
We showed that the poliovirus infection triggered
apoptosis in bone and soft tissue sarcoma cells expressing CD155 by
TUNEL staining and the ApoTox-Glo Triplex assay. The ApoTox-Glo
Triplex assay showed that the poliovirus induced cell death due to
activation of the apoptosis pathway in a time- and dose-dependent
manner. In addition, LAPV was propagated in HT1080 cells, as
indicated by the one-step viral growth curves analysis. These
results suggest that sarcoma cells are targets of the poliovirus.
In previous studies, poliovirus infection triggered apoptosis in
neuroblastoma cells expressing CD155, as shown by DNA
fragmentation, activation of effector caspase activity, and
mitochondrial dysfunction (24,39).
The replication of poliovirus is restricted to neurons in the
spinal cord and brainstem, although CD155 expression is observed in
both the target and non-target tissues in humans. This tissue
tropism results in a distinct disease pattern unique for poliovirus
(40). Ida-Hosonuma et al
showed that α/β interferon (IFN) determine the tissue tropism by
comparing the pathogenesis of the virulent Mahoney strain in
CD155-transgenic mice and CD155-transgenic mice deficient in the
α/β IFN receptor gene (CD155-transgenic/Ifnar knockout mice)
(41).
CD155-transgenic/Ifnar knockout mice showed increased
susceptibility to poliovirus. They subsequently examined the
expression of IFN and IFN-stimulated genes (ISGs) in the
CD155-transgenic mice. In the non-target tissues, ISGs were
expressed even in the non-infected state, and the expression level
increased soon after poliovirus infection. On the contrary, in the
target tissues, ISG expression was low in the non-infected state
and a sufficient response after poliovirus infection was not
observed. Although we did not observe the expression of IFN and
ISGs in the present study, the low IFN response to the poliovirus
may be one of the important determinants of the good susceptibility
of sarcoma cells.
We demonstrated that there is efficient oncolysis of
subcutaneous xenografted sarcoma in nude mice after intratumoral
injection of LAPV. The intratumoral replication of LAPV induced the
release of a number of infectious viral particles, which lead to
the apoptotic cell death of the xenografts. Therefore, LAPV could
be useful for the treatment of bone and soft tissue sarcoma.
The inherent neuropathogenicity of poliovirus is a
point of major concern with regard to its potential therapeutic
applications. Typically, poliovirus infects the gastrointestinal
tract, causing mild symptoms or no symptoms at all. In 1 to 2% of
infections, the poliovirus invades the central nervous system,
where it uniquely targets motor neurons for destruction, resulting
in flaccid paralysis (42).
Paralytic poliomyelitis is considered to result from an invasion by
circulating poliovirus into the central nervous system, probably
via the blood-brain barrier. This notion is supported by a previous
study using a mouse model (43).
However, an accumulation of patients with vaccine-associated
paralytic poliomyelitis following vaccination with orally
administered, attenuated poliovirus (OPV) in Romania has been
linked to multiple intramuscular (i.m.) injections of various
therapeutic or preventive agents administered to OPV recipients (a
phenomenon known as ‘provocation’ poliomyelitis) (44). Recently, the mechanism of
provocation poliomyelitis has been proven using
poliovirus-sensitive transgenic mice produced by introducing the
human CD155 gene into the mouse genome. Poliovirus particles
exist on vesicle structures in the nerve terminals of neuromuscular
junctions (45). Skeletal muscle
injury induces retrograde axonal transport of poliovirus, and
thereby facilitates the viral invasion of the central nervous
system and the progression of spinal cord damage (46). In addition, the direct interaction
between the cytoplasmic domain of CD155 and Tctex-1 is essential
for the efficient retrograde transport of PV-containing vesicles
along microtubules in vivo (45).
In our study, LAPV was injected into the right flank
tumor once a day for 3 days. It remains unclear whether the
intratumoral injection of the LAPV through the muscle surrounding
the tumor would be a safe procedure in human subjects. To treat
patients with bone and soft tissue sarcomas, it will be necessary
to use a highly attenuated phenotype of poliovirus which shows
exceedingly poor infectivity in normal neuronal cells while
retaining its oncolytic effects against bone and soft tissue
sarcomas.
In infected cells, the translation of the
plus-strand poliovirus RNA genome is directed by the internal
ribosome entry site (IRES), a cis-acting RNA element that
facilitates the cap-independent binding of ribosomes to an internal
site of the viral RNA. In each Sabin vaccine strain, a single point
mutation in the IRES secondary-structure domain V is a major
determinant of the neurovirulence attenuation. This point mutation
is an A-to-G exchange in the Sabin 1 vaccine strain (A480G
according to the Sabin 1 nucleotide numbering) (47). However, because of insufficient
genetic stability, the Sabin strain may mutate and convert to a
virulent virus during replication in bone and soft tissue sarcomas.
To eliminate the neuropathogenic potential, many types of
intergeneric poliovirus recombinants were constructed and utilized
for the treatment of malignant glioma (14,15,23)
and neuroblastoma (48). To treat
patients with bone and soft tissue sarcomas, it will be necessary
to develop a recombinant poliovirus with a highly attenuated
phenotype which minimizes the genetic instability as much as
possible.
In the present study, LAPV was injected into the
right flank tumor of 4-week-old BALB/c nu/nu mice with an inhibited
immune system due to their greatly reduced number of T cells, which
leads to impaired antibody formation that requires CD4+
helper T cells. If this treatment strategy is applied to human
subjects, the treatment efficacy may be reduced by the neutralizing
antibodies against poliovirus which were generated in response to a
previous vaccination. However, Toyoda et al (48) showed that neuroblastoma tumors
xenografted into CD155 transgenic mice that had previously been
immunized with poliovirus still remarkably regressed after
intratumoral injection of poliovirus without any side effects.
Other previous investigations have also shown that treatment with
oncolytic viruses can result in enhancement of the antitumor immune
response in vivo (49,50).
Thus, we presume that previous poliovirus vaccination might not
inhibit the oncolytic effect of LAPV, but actually intensify the
oncolysis.
Another potential pitfall is that during a
persistent poliovirus infection in a previous study, specific
mutations were selected in the first extracellular domain of the
CD155 of human neuroblastoma cells, and these mutations
increased the resistance of cells to poliovirus-induced lysis
(51). The same phenomenon may be
observed during the clinical application for bone and soft tissue
sarcoma.
In brief, we demonstrated the expression of both
CD155 mRNA and protein in bone and soft tissue sarcoma cell lines,
and LAPV has oncolytic effects on bone and soft tissue sarcoma
cells in vitro and in vivo. The results of our study
suggest that LAPV has potential for the clinical treatment of bone
and soft tissue sarcomas, but further in vitro and in
vivo studies will be required to evaluate the safety of this
strategy.
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics. CA Cancer J Clin. 60:277–300. 2010.
|
|
2.
|
Billingsley KG, Burt ME, Jara E, Ginsberg
RJ, Woodruff JM, Leung DH and Brennan MF: Pulmonary metastases from
soft tissue sarcoma: analysis of patterns of diseases and
postmetastasis survival. Ann Surg. 229:602–610. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Gilbert NF, Cannon CP, Lin PP and Lewis
VO: Soft-tissue sarcoma. J Am Acad Orthop Surg. 17:40–47.
2009.PubMed/NCBI
|
|
4.
|
Clark MA, Fisher C, Judson I and Thomas
JM: Soft-tissue sarcomas in adults. N Engl J Med. 353:701–711.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Grimer R, Judson I, Peake D and Seddon B:
Guidelines for the management of soft tissue sarcomas. Sarcoma: May
31, 2010 (Epub ahead of print). doi: 10.1155/2010/506182.
|
|
6.
|
No authors listed. Adjuvant chemotherapy
for localised resectable soft-tissue sarcoma of adults:
meta-analysis of individual data. Sarcoma Meta-analysis
Collaboration. Lancet. 350:1647–1654. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Gortzak E, Azzarelli A, Buesa J, et al: A
randomised phase II study on neo-adjuvant chemotherapy for
‘high-risk’ adult soft-tissue sarcoma. Eur J Cancer. 37:1096–1103.
2001.
|
|
8.
|
Sinkovics JG and Horvath JC: New
developments in the virus therapy of cancer: a histrogical review.
Intervirology. 36:193–214. 1993.PubMed/NCBI
|
|
9.
|
Sinkovics JG and Horvath JC: Natural and
genetically engineered viral agents for oncolysis and gene therapy
of human cancers. Arch Immunol Ther Exp (Warsz). 56(Suppl 1):
3s–59s. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Parato KA, Senger D, Forsyth PA and Bell
JC: Recent progress in the between oncolytic viruses and tumors.
Nat Rev Cancer. 5:965–976. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Coffey MC, Storong JE, Forsyth PA and Lee
PW: Reovirus therapy of tumors with activated Ras pathway. Science.
282:1332–1334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Sinkovics JG and Horvath JC: Newcastle
disease virus (NDV): brief history of its oncolytic strain. J Clin
Virol. 16:1–15. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Springfeld C, Fielding AK, Peng KW,
Galanis E, Russell SJ and Cattaneo R: Measles virus: improving
natural oncolytic properties by genetic engineering. Viral Therapy
of Human Cancers. Sinkovics JG and Horvath JC: Marcel Dekker Inc;
New York, NY: pp. 459–480. 2005
|
|
14.
|
Stojdl DF, Litchy B, Knowles S, Marius R,
Atkins H, Sonenberg N and Bell JC: Exploiting tumor-specific
defects in interferon pathway with a previously unknown oncolytic
virus. Nat Med. 6:821–825. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Gromeier M, Lachmann S, Rosenfeld MR,
Gutin PH and Wimmer E: Intergenic poliovirus recombinants for
treatment of malignant glioma. Proc Natl Acad Sci USA.
97:6803–6808. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bodian D: Poliomyelitis. Pathology of the
Nervous System. Minckler J: McGraw-Hill; New York, NY: pp.
2323–2344. 1972
|
|
17.
|
Ren R, Costantini F, Gorgacz EJ, Lee JJ
and Racaniello VR: Transgenic mice expressing a human poliovirus
receptor: a newmodel for poliomyelitis. Cell. 63:353–362. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Koike S, Taya C, Kurata T, Abe S, Ise I,
Yonekawa H and Nomoto A: Transgenic mice susceptible to poliovirus.
Proc Natl Acad Sci USA. 88:951–955. 1991. View Article : Google Scholar
|
|
19.
|
Gromeier M, Alexander L and Wimmer E:
Internal ribosomal entry site substitution eliminates
neurovirulence in intergenic poliovirus recombinants. Proc Natl
Acad Sci USA. 93:2370–2375. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Kauder SE and Racaniello VR: Internal
ribosomal entry site substitution eliminates neurovirulence in
intergenic poliovirus recombinants. Proc Natl Acad Sci USA.
93:2370–2375. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Takai Y, Miyoshi J, Ikeda W and Ogita H:
Nectins and nectin-like molecules: roles in contact inhibition of
cell movement and proliferation. Nat Rev Mol Cell Biol. 9:603–615.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Amano H, Ikeda W, Kawano S, et al:
Interaction and localization of Necl-5 and PDGF receptor beta at
the leading edges of moving NIH3T3 cells: implications for
directional cell movement. Genes Cells. 13:269–284. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Merrill MK, Bernhardt G, Sampson JH,
Wikstrand CJ, Bigner DD and Gromeier M: Poliovirus receptor
CD155-targeted oncolysis of glioma. Neuro Oncol. 6:208–217. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Toyoda H, Hayashi T, Gabbazza EC, et al:
Experimental treatment of human neuroblastoma using live-attenuated
poliovirus. Int J Oncol. 24:49–58. 2004.PubMed/NCBI
|
|
25.
|
Nobis P, Zibirre R, Meyer G, Kuhne J,
Warnecke G and Koch G: Production of a monoclonal antibody against
an epitope on HeLa cells that is the functional poliovirus binding
site. J Gen Virol. 66:2563–2569. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wakabayashi T, Matsumine A, Nakazora S, et
al: Fibulin-3 negatively regulates chondrocyte differentiation.
Biochem Biophys Res Commun. 391:1116–1121. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Kono T, Imai Y, Yasuda S, et al: The
CD155/poliovirus receptor enhances the proliferation of ras-mutated
cells. Int J Cancer. 122:317–324. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tomayko MM and Reynald CP: Determination
of subcutaneous tumor size in athymic (nude) mice. Cance Chemother
Pharmacol. 24:148–154. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Mendelsohn CL, Wimmer E and Racaniello VR:
Cellular receptor for poliovirus: molecular cloning, nucleotide
sequence, and expression of a new member of the immunoglobulin
superfamily. Cell. 56:855–865. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Grimer R, Athanasou N, Gerrand C, et al:
UK Guidelines for the Management of Bone Sarcomas. Sarcoma: Dec 29,
2010 (Epub ahead of print). doi: 10.1155/2010/317462.
|
|
31.
|
Solecki D, Wimmer E, Lipp M and Bernhardt
G: Identification and characterization of the cis-acting elements
of the human CD155 gene core promoter. J Biol Chem. 274:1791–1800.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Solecki D, Bernhardt G, Lipp M and Wimmer
E: Identification of nuclear respiratory factor-1 binding site
within the core promoter of polio virus receptor/ CD155 gene. J
Biol Chem. 275:12453–12462. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Marsson D, Jarry A, Baury B, Blanchardie
P, Laboisse C, Lustenberger P and Denis MG: Overexpression of CD155
gene in human colorectal cartinoma. Gut. 49:236–240. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Solecki DJ, Gromeier M, Mueller S,
Bernhardt G and Wimmer E: Expression of the human poliovirus
receptor/ CD155 gene is activated by sonic hedgehog. J Biol Chem.
277:25697–25702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Li F, Shi W, Capurro M and Filmus J:
Glypican-5 stimulates rhabdomyosarcoma cell proliferation by
activating Hedgehog signaling. J Cell Biol. 192:691–704. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Oue T, Yoneda A, Uehara S, Yamanaka H and
Fukuzawa M: Increased expression of the hedgehog signaling pathway
in pediatric solid malignancies. J Pediatr Surg. 45:387–392. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Minami Y, Ikeda W, Kajita M, Fujito T,
Monden M and Takai Y: Involvement of up-regulated
Necl-5/Tage4/PVR/CD155 in the loss of contact inhibition in
transformed NIH3T3 cells. Biochem Biophys Res Commun. 352:856–860.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Toyoda H, Franco D, Fujita K, Paul AV and
Wimmer E: Replication of poliovirus requires binding of the
poly(rC) binding protein to cloverleaf as well as to the adjacent
C-rich spacer sequence between the cloverleaf and the internal
ribosomal entry site. J Virol. 81:10017–10028. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Gosselin AS, Simonin Y, Guivel-Benhassine
F, et al: Poliovirus-induced apoptosis is reduced in cell
expressing a mutant CD155 selected during presistant poliovirus
infection in neuroblastoma cells. J Virol. 77:790–798. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Whitton JL, Cornell CT and Feuer R: Host
and virus determinants of picornavirus pathogenesis and tropism.
Nat Rev Microbiol. 3:765–776. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ida-Hosonuma M, Iwasaki T, Yoshikawa T, et
al: The alpha/beta interferon response controls tissue tropism and
pathogenicity of poliovirus. J Virol. 79:4460–4469. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Melnick JL: Enteroviruses: polioviruses,
coxsackieviruses, echoviruses, and newer enteroviruses. Virology.
Fields BN, Knipe DM, Howley PM, et al: Raven Press; New York, NY:
pp. 549–605. 1995
|
|
43.
|
Yang WX, Terasaki T, Shiroki K, et al:
Efficient delivery of circulating poliovirus to the central nervous
system independently of poliovirus receptor. Virology. 229:421–428.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Strebel PM, Ion-Nedelcu N, Baughman AL,
Sutter RM and Cochi SL: Intramuscular injections within 30 days of
immunization with oral poliovirus vaccine - a risk factor for
vaccine-associated paralytic poliomyelitis. N Engl J Med.
332:500–506. 1995. View Article : Google Scholar
|
|
45.
|
Ohka S, Matsuda N, Tohyama K, Oda T,
Morikawa M, Kuge S and Nomoto A: Receptor(CD155)-dependent
endocytosis of poliovirus and retrograde axonal transport of the
endosome. J Virol. 78:7186–7198. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Gromeier M and Wimmer E: Mechanism of
injury-provoked poliomyelitis. J Virol. 72:5056–5060.
1998.PubMed/NCBI
|
|
47.
|
Ochs K, Zeller A, Saleh L, Bassili G, Song
Y, Sonntag A and Niepmann M: Impaired binding of standard
initiation factors mediates poliovirus translation attenuation. J
Virol. 77:115–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Toyoda H, Yin J, Mueller S, Wimmer E and
Cello J: Oncolytic treatment and care of neuroblastoma by a novel
attenuated poliovirus in a novel poliovirus-susceptible animal
model. Cancer Res. 67:2857–2864. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Porosnicu M, Mian A and Barber GN: The
oncolytic effect of recombinant vesicular stomatitis virus in
enhanced by expression of the fusion cytosine deaminase/uracil
phosphoribosyltransferase suicide gene. Cancer Res. 63:8366–8376.
2003.
|
|
50.
|
Obuchi M, Fernandez M and Barber GN:
Development of recombinant vesicular stomatitis virus that exploit
defects in host defense to augment specific oncolytic activity. J
Virol. 77:8843–8856. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Pavio N, Couderc T, Girard S, Sgro JY,
Blondel B and Colbère-Garapin F: Expression of mutated poliovirus
receptors in human neuroblastoma cells persistently infected with
poliovirus. Virology. 274:331–342. 2000. View Article : Google Scholar : PubMed/NCBI
|