Introduction
In the western industrialised countries one out of
three persons develops some type of cancer during their lifetime
(1) and >50% of them succumb to
the disease (2). For the treatment
of many types of cancer, natural products have been the source of
effective drugs and today >60% of anticancer drugs originate
from natural sources such as plants, marine organisms and
micro-organisms (3). An estimation
conducted by the WHO reveals that even today 80% of the population
of Asia and Africa rely on traditional medicine for primary health
care (Traditional Medicine Fact Sheet No. 134, World Health
Organization, Dec. 2008, http://www.who.int/mediacentre/factsheets/fs134/en/).
Fabricant and Farnsworth (4) describe ethnomedicine as a ‘highly
diversified approach to drug discovery’. It involves observation,
description, and experimental investigation of indigenous remedies
for their possible biological or medicinal activity. The drugs (of
plants mostly) have been used in traditional medicine for hundreds
of years, which is the reason why tolerable toxic effects can be
expected in humans. Traditional medicine is practiced by shamans or
herbalists who keep the healing skills a secret (5) and hence, little is known about their
remedies. Therefore, natural products remain an important source
for the discovery of new drugs. However, the primary extracts of
natural products consist of complex mixtures and this makes the
isolation of the active principles a difficult task. The key
compounds may be unstable, or the activity may be based on two or
more synergistic constituents that may disappear upon separation.
Until 2000 (current data are unavailable) only 6% of higher plant
species had been screened for their biologic, mostly anticancer or
anti-HIV, activity (4).
Important examples of anticancer drugs derived from
an ethnomedicinal plant that are now used in the clinic are the
vinca alkaloids vinblastine and vincristine. Both were isolated
from the Madagascar periwinkle, Catharanthus roseus G. Don
(Apocynaceae). Another agent belonging to the ethno-derived
chemotherapeutic drugs is paclitaxel isolated from the bark of
Taxus brevifolia Nutt. This demonstrates that isolated
compounds from plants traditionally used as home remedies may lead
to the development of novel anticancer agents (3). Owing to their high biodiversity, rain
forests are immensely rich sources for new drugs (6). In particular, ancient civilizations
collected knowledge over many hundreds of years regarding the
natural products that were effective as therapies against several
diseases (7). Combination of these
ethnopharmaceutical benefits and the advantageous biodiversity
through which the medicinal tradition flourished, established the
basis for the present work with the objective of finding new
potential lead compounds against cancer by investigating a healing
plant of the Maya from the Guatemala/Belize lowland rainforest. The
rhizome of Smilax spinosa Miller (Smilacacea) is used by the
indigenous population as a natural remedy against inflammation. We
selected this plant to study potential anti-neoplastic properties,
as similar signalling pathways are upregulated during inflammation
and in cancer cells (8). To date,
only a few pharmacological effects of the Smilax species
have been investigated in clinical trials (9). For example, Smilax regelii
(syn. Sarsaparilla) exhibits antimicrobial activities against
Shigella dysenteria (10)
and a Smilax glabra extract had immuno-modulatory activity
in rats by decreasing the IL-1-, TNF- and NO-release of macrophages
(11). S. regelii is mostly
applied internally against arthritis, rheumatism (both causing
inflammation), psoriasis or dermatitis (skin disorders), impotence,
or as a blood purifier (9). It is
described to be active against snake bites (12) but an excessive dosage of S.
regelii has been reported to cause gastrointestinal irritation
(9). Smilax species are
particularly known to contain saponins and plant steroids that can
be synthesized into human steroids such as estrogen and
testosterone. Also, the majority of S. regelii’s activities
are reported to be caused by these steroids and saponins (9). The methanol extract of S.
spinosa renders DPPH, OH, and O2- radicals innocuous thereby
inhibiting lipid peroxidation. Furthermore, it was effective
against Salmonella typhimurium and Trypanosoma
cruzii. Therefore, the methanol extract has an anti-oxidative
and anti-microbial activity (13).
When used against male impotency, S. spinosa rhizome and
guinweo (a local plant) are soaked in rum and administered twice a
day (14). Already in 1536 a
Smilax root from Mexico was introduced into European
medicine to treat syphilis and rheumatism (9). As S. spinosa has not yet been
investigated for its anti-neoplastic activity, the present study
was conducted to analyse its anti-proliferative effects.
Materials and methods
Antibodies
Antibodies against: cleaved Asp175 caspase3 (no.
9661), cleaved caspase 8 (Asp391, 18C8, no. 9496), cleaved caspase
9 (Asp330, no. 9501), phospho-Stat3 (Tyr705)(D3A7, no. 9145), Stat3
(no. 9132) and phospho-Stat5 (Tyr694)(C11C5, 9359) were from Cell
Signaling (Danvers, MA, USA). PARP-1 (F-2, sc-8007), Cdc25A (F-6,
sc-7389), cyclin D1 (M-20, sc-718), p21 (C-19, sc-397), α-tubulin
(DM1A, sc-32293), β-tubulin (H-235, sc-9104), Stat5 (C-17, sc-835),
c-Jun (H-79, sc-1694) and Jun B (210, sc-73) were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). c-Myc Ab-2 (9E10.3,
no. MS-139-P1) was from Thermo Fisher Scientific (Fremont, CA,
USA), phospho- Ser177 Cdc25A (no. AP3046a) from Abgent (San Diego,
CA, USA), phospho-Ser139 H2AX (DR 1017) from Calbiochem (San Diego,
CA, USA) and β-actin (AC-15, A5441) as well as anti-acetylated
tubulin (clone6-11B-1, T6793) were from Sigma (St. Louis, MO, USA).
The secondary antibodies peroxidase-conjugated anti-rabbit IgG and
anti-mouse IgG were purchased from Dako (Glostrup, Denmark).
Cell culture
HL-60 (human promyelocytic leukaemia) cells and
human MCF-7 and MDA-MB231 breast cancer cell lines were purchased
from American Type Culture Collection (ATCC, Rockville, MD, USA)
and grown in RPMI-1640 medium (HL-60). The other cell lines were
grown in MEM medium, which was supplemented with 10%
heat-inactivated foetal calf serum (FCS), 1% Glutamax and 1%
penicillin/streptomycin (Life Technologies, Carlsbad, CA, USA), 1%
NEAA (Invitrogen, Karlsruhe, Germany). Cells were kept in a
humidified atmosphere at 37°C containing 5% CO2.
Plant material
Parts of the rhizomes of Smilax spinosa
Miller (vernacular name: ‘Kokolmeka roja’) were collected in
Guatemala, Departamento Petén, at the north-western shore of Lago
Petén Itzá, San José, ∼1 km north of the road from San José to La
Nueva San José (16 59′30″ N, 89 54′00″ W). Voucher specimens (leg.
G. Krupitza and R. O. Frisch, Nr. 4-2009, 19. 04. 2009, Herbarium
W, det. B. Wallnöfer (W) 26.1.2010) were archived at the Museum of
Natural History, Vienna, Austria.
Extraction
Rhizomes of S. spinosa were cut, dried by
lyophilisation and then pulverized. Twenty grams of the obtained
powder were mixed with 200 ml solvent (1:10; Table I) extracted in an ultra sonic bath
for 10 min and afterwards under reflux for 1 h in the water bath.
The solution was filtered and the retained plant material (residue)
was dried at room temperature before being re-extracted with the
next more polar solvent. The liquid extract was evaporated under
reduced pressure to give a crude fraction (0.67 mg corresponding to
1 g rhizome; Table I) (15,16).
The extract weights obtained from serial extraction of the dried
rhizomes of S. spinosa with five solvents of increasing
polarity are presented in Table I,
which illustrates that the weight of the methanol extract
corresponds to ∼20.6% of the dried rhizome.
 | Table ISolvents used for extraction of S.
spinosa and extract weights. |
Table I
Solvents used for extraction of S.
spinosa and extract weights.
| Solvent | Extract weight (mg)
corresponding to 1 g dried rhizome |
|---|
| Petroleum
ether | 0.7 |
|
Dichloromethane | 1.1 |
| Ethyl acetate | 11.1 |
| Methanol | 205.5 |
| Water | 147.8 |
Sub-fractionation of the methanol
extract
The methanol extract was the most active of the
S. spinosa extracts, therefore it was further fractionated
by dissolving 4.1 g in 60 ml of a water-methanol mixture (9:1).
After threefold extraction with 60 ml petroleum ether each for the
removal of chlorophyll, waxes and fats, the remaining fraction was
diluted with 60 ml of water. Subsequently, this aqueous solution
was extracted three times with 120 ml chloroform each. The
collected chloroform layers were washed three times with 360 ml
sodium chloride solution (1%). After drying with sodium sulphate,
the solution was filtered and the chloroform was evaporated under
reduced pressure. The weights of the obtained sub-fractions are
listed in Table II. Approximately
10% of the starting extract was lost during the fractionation
process.
 | Table IIObtained amounts of sub-fractions
derived from 4.1 g of methanol extract. |
Table II
Obtained amounts of sub-fractions
derived from 4.1 g of methanol extract.
| Fraction | Potentially
abundant substances | Amount (g) |
|---|
| FI Petroleum
ether | Chlorophyll, wax,
resin less polar compounds | 0.03 |
| F2
Water-methanol | Tannins, more polar
substances | 3.65 |
| F3 Chloroform | Chloroform-soluble
substances | 0.02 |
Thin layer chromatography with chloroform: methanol:
water (70:22:3.5) revealed that the petroleum ether fraction (F1)
exhibited an almost identical banding pattern as the petroleum
ether extract (detection under visible light and UV366 with ASR;
data not shown), but with some additional bands, which seemed to be
responsible for the higher activity of the F1 fraction (data not
shown) compared to the petroleum ether extract.
Proliferation and cytotoxicity
assays
HL-60 cells were seeded in 24-well plates at a
concentration of 1×105 cells/ml allowing logarithmic
growth within the next 48 h. Cells were then incubated with
increasing concentrations of plant extracts (5 μg/ml, 15 μg/ml, 30
μg/ml, 60 μg/ml) for 48 h. After 24 and 48 h, the cell number was
counted using a KX-21 N microcell counter (Sysmex Corporation,
Kobe, Japan) and the percent of cell divisions compared to the
untreated control were calculated as follows: [(C48h + drug - C24h
+ drug)/(C48h- drug - C24h - drug)] x 100=% cell division, whereby
C48h + drug or C48h - drug were the cell numbers after 48 h with or
without extract treatment, respectively. C24h + drug or C24h - drug
were the respective cell numbers after 24 h (17,18).
Apoptosis assay - Hoechst 33258 and
propidium iodide double staining
Hoechst 33258 (HO) and propidium iodide (PI) double
staining (Sigma, St. Louis, MO) determine the type of death the
cell is undergoing, i.e. apoptosis (early or late) or necrosis
(19,20). HL-60 cells were seeded in a 24-well
plate at a concentration of 1×105 cells/ml and treated
with increasing concentrations of fractions F1, F2 and F3. After 24
h, 48 h and 72 h of incubation, 100 μl cell suspension of each well
was transferred into separate wells of a 96-well plate and HO and
PI were added at final concentrations of 5 μg/ml and 2 μg/ml,
respectively. After 1 h of incubation at 37°C, stained cells were
examined and photographed on a fluorescence microscope (Axiovert,
Zeiss, Jena, Germany) equipped with a DAPI filter. Cell death was
evaluated and counted by visual examination of the photographs
according to the morphological characteristics revealed by HOPI
staining. Experiments were performed in triplicate.
Western blotting
HL-60 were seeded in T-75 tissue culture flasks at a
concentration of 1.8×105 cells/ml and treated with the
indicated concentration of methanol extract or fraction F2. Cells
were harvested after 0.5, 2, 4, 8 and 24 h. Then, cells were washed
twice with cold PBS and centrifuged at 1,000 rpm for 5 min at 4°C.
The cell pellet was lysed in a buffer containing 150 mM NaCl, 50 mM
Tris pH 8.0, 1% Triton-X-100, 1 mM phenylmethylsulfonyl fluoride
(PMSF) and 1 mM Protease Inhibitor Cocktail (PIC), (Sigma,
Schnelldorf, Germany). The lysate was centrifuged at 12,000 rpm for
20 min at 4°C. Supernatant was transferred into a 1.5 ml tube and
stored at −20°C until further analysis. Equal amounts of protein
lysate were mixed with SDS (sodium dodecyl sulphate) sample buffer
and loaded onto a 10% polyacrylamide gel. Proteins were separated
by polyacrylamide gel electrophoresis (PAGE) at 120 Volt and
electro-transferred onto a PVDF (polyvinylidene difluoride)
membrane (Hybond, Amersham, Buckinghamshire, UK) at 95 Volt for 80
min. Membranes were allowed to dry for at least 30 min up to 2 h to
provide fixing of the proteins to the membrane. Methanol was used
to remoisten the membranes. Equal sample loading was checked by
staining the membrane with Ponceau S (Sigma, Schnelldorf, Germany).
After removing Ponceau S with PBS or TBS (Tris buffered saline, pH
7.6), membranes were blocked in PBS- or TBS-milk (5% non-fat dry
milk in PBS containing 0.5% Tween-20 or TBS containing 0.1%
Tween-20) for 1 h. Then, membranes were washed with PBS/T (PBS
containing 0.5% Tween-20) or TBS/T (TBS containing 0.1% Tween-20),
changing the washing solution 4–5 times, for at least 20 min. Next,
membranes were incubated with the primary antibody in blocking
solution (according to the data sheet TBS-, PBS-milk or TBS-,
PBS-BSA) diluted 1:500 - 1:1000, gently shaking at 4°C, overnight.
Thereafter, membranes were washed again with PBS/T or TBS/T and
incubated with the secondary antibody (peroxidase conjugated
anti-rabbit IgG or anti-mouse IgG) diluted 1:2000 in PBS- or
TBS-milk at room temperature for 1 h. Chemiluminescence was
developed by the ECL detection kit (Amersham, Buckinghamshire, UK)
and membranes were exposed to Amersham Hyperfilm.
Ethoxyresorufin-O-deethylase (EROD) assay
selective for CYP1A1 activity
MDA-MB-231 and MCF-7 breast cancer cells were grown
in phenol red-free DMEM/F12 culture medium (Invitrogen, Karlsruhe,
Germany) supplemented with 10% FCS and 1% PS (Invitrogen,
Karlsruhe, Germany) under standard conditions at 37°C in a
humidified atmosphere containing 5% CO2 and 95% air.
Twenty-four hours prior to treatment, the cells were transferred to
DMEM/F12 culture medium supplemented with 2.5% charcoal-stripped
FCS (PAN Biotech, Aldenbach, Germany) and 1% PS. The MeOH extract
and F2 were dissolved in DMSO and diluted with medium (final DMSO
concentration <0.1%) to 30 and 60 μg/ml. Experiments under each
set of conditions were carried out in triplicate. Blanks contained
DMSO in the medium of the test compounds. After 18 h of incubation,
ethoxyresorufin (final concentration 5.0 μM, Sigma-Aldrich, Munich,
Germany) was added and 0.4 ml aliquots of the medium were sampled
after 200 min. Subsequently, the formation of resorufin was
analyzed by spectrofluorometry (PerkinElmer LS50B, Waltham, MA,
USA) with an excitation wavelength of 530 nm and an emission
wavelength of 585 nm.
Statistical analysis
For statistical analyses Excel 2003 software and
Prism 5 software package (GraphPad, San Diego, CA, USA) were used.
The values are expressed as the mean ± SEM and the Student’s t-test
was applied to compare differences between control samples and
treatment groups. Statistical significance level was set at
p<0.05.
Results and Discussion
The lyophilized rhizome of S. spinosa was
subjected to sequential extraction with five solvents of increasing
polarity. The obtained extracts were investigated for their
anti-neoplastic potential in HL-60 leukaemia cells, as blood cells
are easily accessible and sensitive to pharmacological compounds,
thereby providing an appropriate system for an initial testing
series.
Inhibition of cell proliferation
To determine the anti-proliferative effects in HL-60
cells, extracts were applied at increasing concentrations (5, 10,
30, 60 μg/ml and partly 90 and 120 μg/ml) for 24, 48, and 72 h
(Fig. 1a–e). The methanol extract
was the most potent and inhibited cell proliferation
dose-dependently with an IC50 (the concentration
inhibiting 50% proliferation) of ∼60 μg/ml. The extract exhibited
the highest activity within the first 48 h, which decreased
thereafter (data not shown). Although the extract concentration at
which proliferation was significantly inhibited was rather high,
the corresponding weight of the rhizome was low, as the methanol
extract was almost one fifth of the whole lyophilized rhizome
substance. This corresponds to 300–500 mg of dried rhizome, or
370–630 mg of fresh rhizome per kg body weight; a person with an
average body weight has to consume the alcoholic extract derived
from only 20 g of rhizome within two days. Inhibition of cell
proliferation was accompanied by a rapid upregulation of Cdc25A
expression within 2 h of treatment (Fig. 1f). It seems that the high Cdc25A
levels were the result of an increased protein synthesis rather
than an inhibited protein degradation, as Ser177 phosphorylation,
which tags Cdc25A for recruitment of the proteasome and subsequent
proteolysis (21), was sustained
throughout 2 and 8 h of treatment, whereas the degradation of the
Cdc25A proto-oncogene below control levels was observed after 24 h.
The increase in Cdc25A was followed by cyclin D1 upregulation
within 4 h. The protooncogene cyclin D1 is necessary for the
transit from early G1 to beginning of S phase and induction of
cyclin D1 expression is indicative for cell cycle activation. p21
inhibits the Cdk2/Cyclin E kinase complex that normally cooperates
with cyclin D1 at a later stage to facilitate G1-S transition and
then S-phase progression (22).
p21 was induced within 2 h of extract treatment and this accurately
counteracted the induction of Cdc25A phosphatase, which causes the
activation of Cdk2/Cyclin E. (23,24)
and therefore, cell cycle progression was blocked. Additionally,
p21 binds to the Cdk4/Cyclin D complex. This results in a
hypo-phosphorylation and activation of pRb and thereby the
suppression of the E2F pathway and cessation of the cell cycle
(25). However, when cyclin D1
became induced, p21 levels were already back to control levels. An
additional band below 21 kD became visible, which was most likely a
degradation product of p21. Expression of p21 transcription is
widely regulated by p53. Since HL-60 cells are p53 deficient
(26) p21 must have been
controlled in a p53-independent manner (27) and it was shown that the
proto-oncogene c-Myc negatively regulates p21 (28). The chaotic expression of prominent
cell cycle protagonists and proto-oncogenes together with p21
induction undoubtedly affected DNA replication and cell duplication
thereby triggering growth arrest.
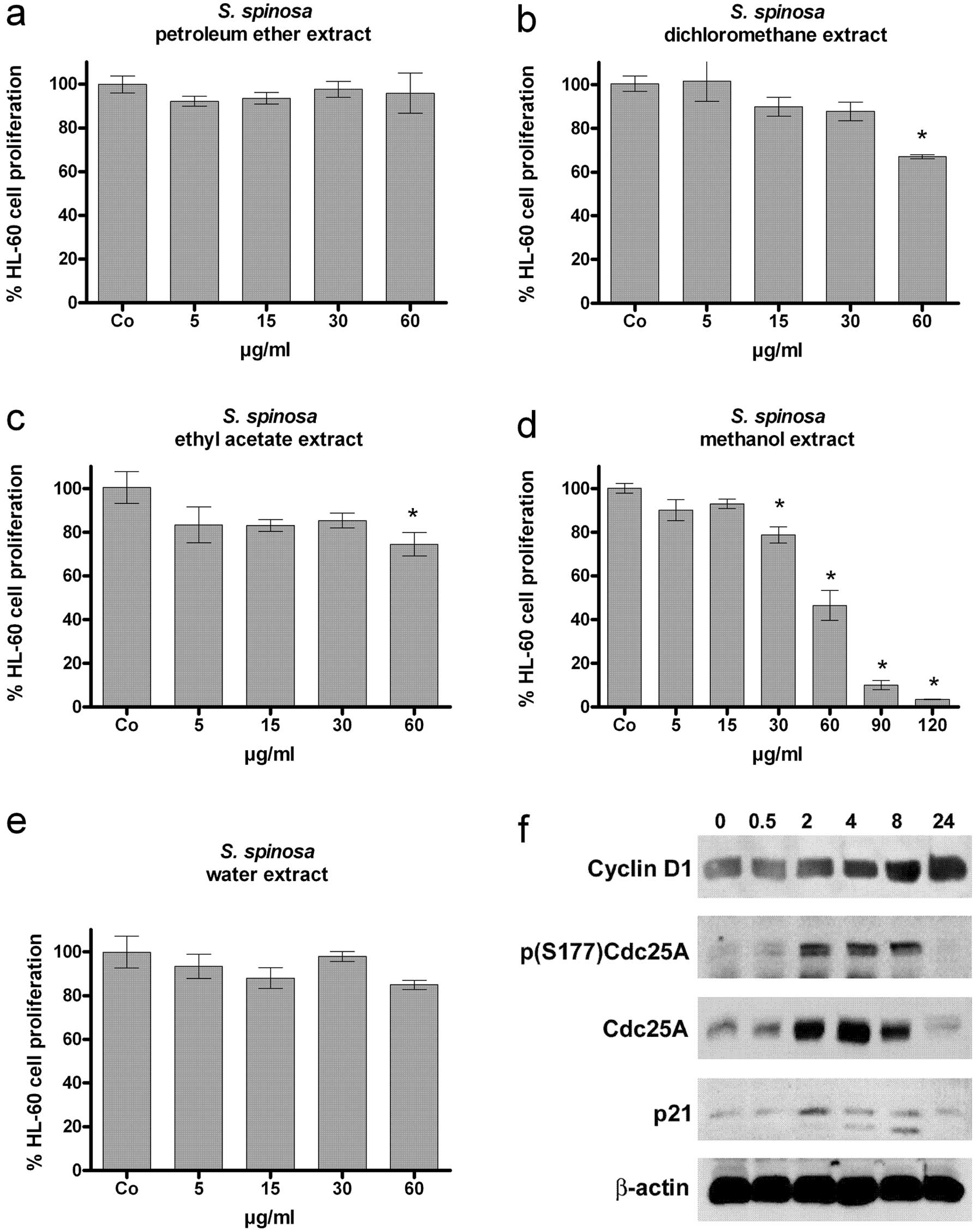 | Figure 1Anti-proliferative effect of
increasingly polar extracts; (a) petroleum ether, (b)
dichloromethane, (c) ethyl acetate, (d) methanol, and (e) water).
HL-60 cells were seeded into 24-well plates (1×105
cells/ml), incubated with 5, 15, 30 and 60 μg/ml of each extract
(the methanol extract also with 90 and 120 μg) for 48 h. Cells were
counted after 24 and 48 h of treatment. The percentage of
proliferation between 24 and 48 h was determined in comparison to
control. Experiments were performed in triplicate. Asterisks
indicate significance compared to untreated control (p<0.05) and
error bars indicate the means ± SEM. (f) Analysis of the expression
of cell cycle regulators. HL-60 cells (1×106 cells/ml)
were incubated with 120 μg/ml of the methanol extract and harvested
after 0.5, 2, 4, 8 and 24 h of treatment. Cells were lysed and the
obtained protein samples were subjected to SDS-gel electrophoresis
and subsequent western blot analysis with the indicated antibodies.
Equal sample loading was confirmed by Ponceau S staining and
β-actin analysis. |
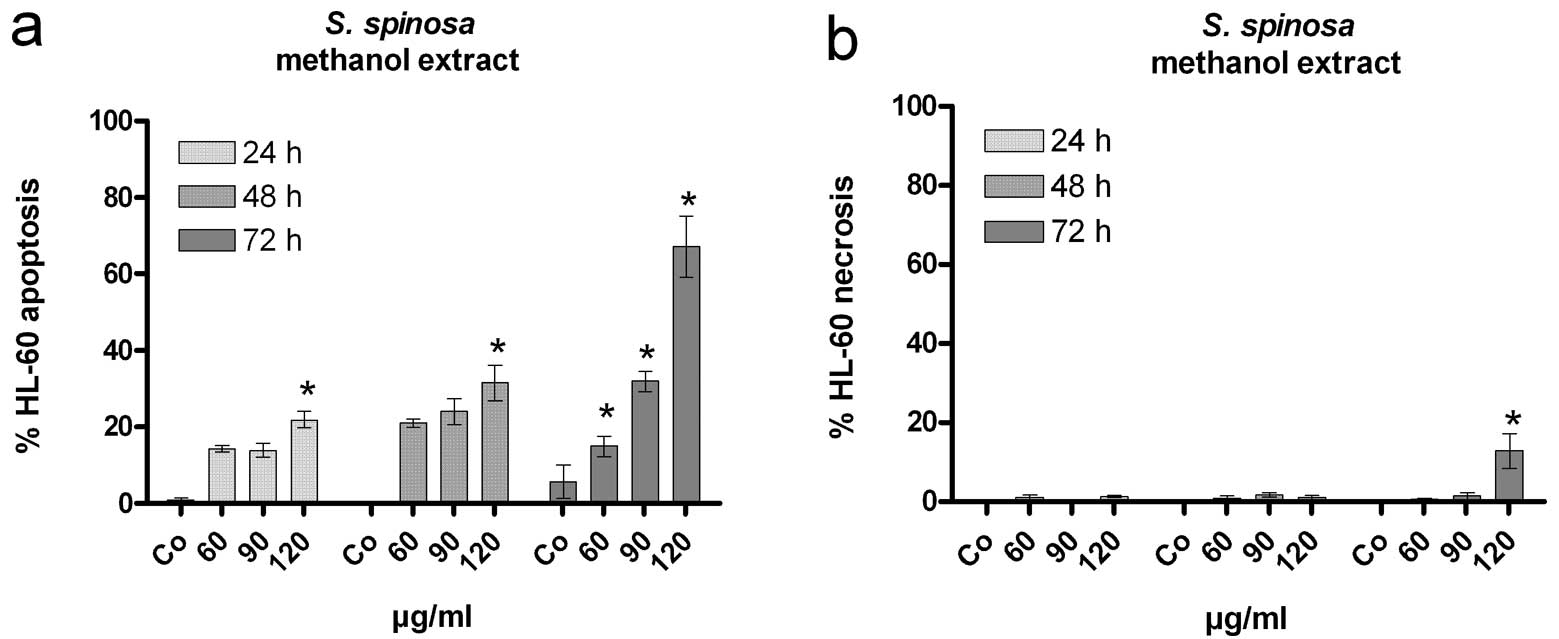
Induction of apoptosis
Growth arrest due to extra-cellular stressors often
elicits apoptosis. Therefore, HL-60 cells were treated with
increasing concentrations (60, 90, 120 μg/ml) of the methanol
extract to analyse cell viability (Fig. 2a). The time- and dose-dependent
increase in the number of dead cells showed a morphology which is
typical for apoptosis, whereas a small number of necrotic cells
were only observed at the highest dose (120 μg/ml) after 72 h
(Fig. 2b). In an attempt to
increase the pro-apoptotic activity the methanol extract was
subjected to a fractionation procedure (as described in Materials
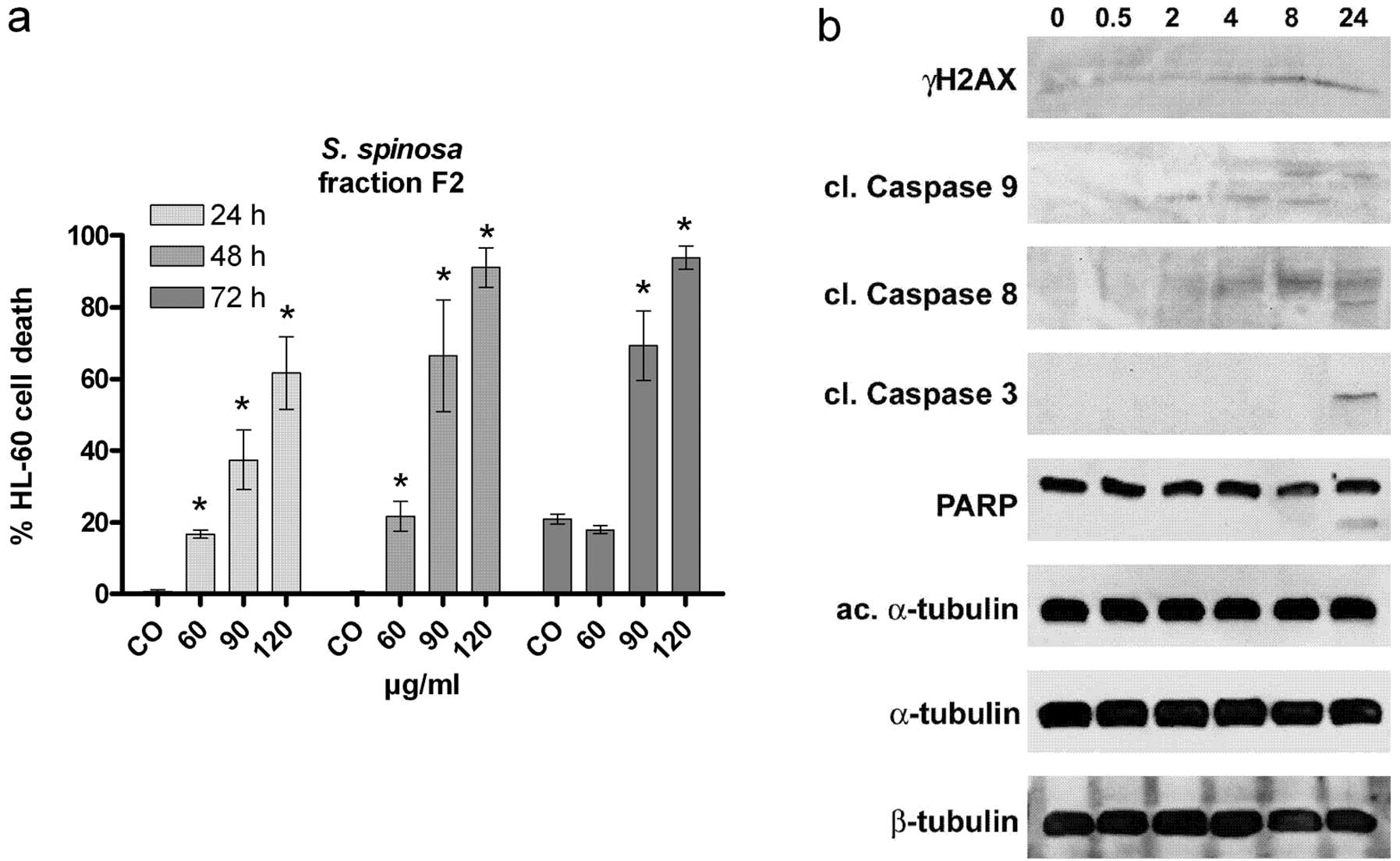
and methods) obtaining three fractions. Fraction 2 (F2) exhibited
the strongest pro-apoptotic effect whereby 40% apoptotic cells were
observed upon treatment with 90 μg/ml F2 for 24 h (Fig. 3a) and this was a ∼2.5 fold increase
compared to the primary methanol extract (<15% apoptotic cells
upon treatment with 90 μg/ml methanol extract for 24 h; Fig. 2a). F2 inhibited cell proliferation
less efficiently than the original methanol extract (data not
shown). Therefore, a cell cycle inhibitory property was separated
from a pro-apoptotic property which was less dependent on the cell
cycle. This is of particular significance as tumour cells, which
are not cycling, could be targeted by this fraction. To get further
insight into the mechanisms of F2, the expression of pro-apoptotic
proteins as well as of markers indicating genotoxicity and
microfilament stress was investigated. The increased cleavage of
caspase 9 was observed after 2 h of incubation, whereas caspase 8
was cleaved after 4 h. Finally the executor, caspase 3, was
activated after 24 h. The caspase cascade i.e. the activation of
caspase 9 and 8, which both cause the cleavage and activation of
caspase 3 and the subsequent induction of apoptosis (2) suggested that F2 induced the intrinsic
pathway. The activity of caspase 3 was reflected by the cleavage of
its target PARP (120 kD) into a smaller 85 kD fragment (29) (Fig.
3b). The phosphorylation of H2AX (γH2AX) is a sensitive and
commonly used marker for the presence of DNA-double-strand breaks
(30). During this experiment, the
phosphorylation of H2AX was induced after 8 and 24 h, whereas
caspase 9 was activated before phosphorylation of H2AX implicating
that the increase of γH2AX levels was the consequence of caspase 3
activation and the subsequent induction of nucleases causing DNA
degradation. Therefore, F2 itself did not induce DNA double strand
breaks but this does not exclude the possibility of a genotoxic
property of F2 triggering DNA single strand breaks or the
generation of DNA adducts.
Several plant compounds were shown to affect the
equilibrium of microtubule polymerization. Tilting this fine-tuned
equilibrium of polymerized-depolymerized microfilaments is
incompatible with normal cell division and causes cell cycle arrest
and apoptosis. The acetylation of α-tubulin reflects the
polymerization status of the microtubule meshwork (31). However, F2 did not alter tubulin
acetylation and therefore did not target the spindle apparatus.
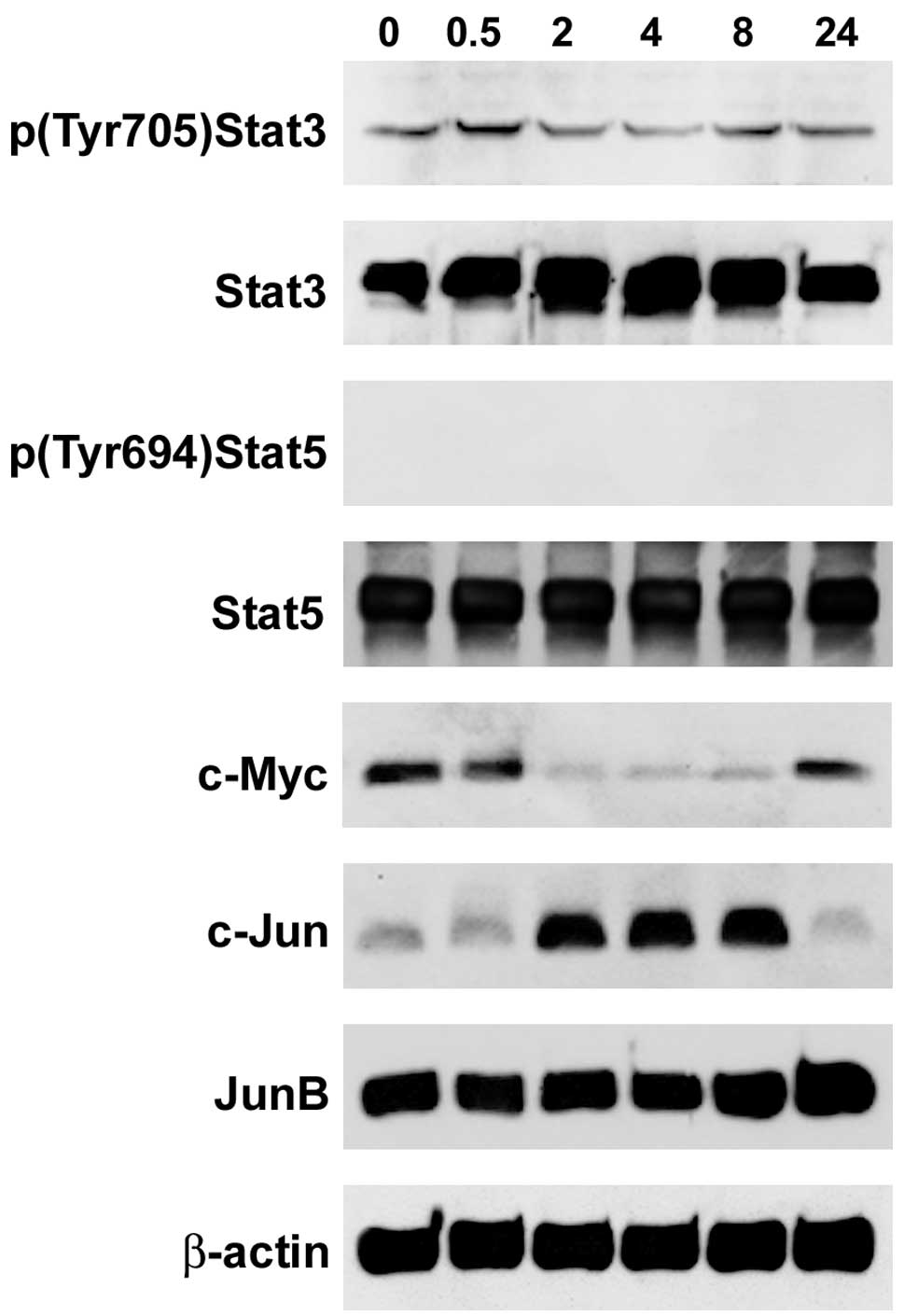
Modulated expression of the
proto-oncogenes Stat3, c-Myc and c-Jun
Stat family proteins are transcription factors
involved in normal and pathological cellular processes. The Stat3
proto-oncogene product is the most activated Stat protein in human
cancers (32) accelerating cell
proliferation, preventing apoptosis (33) and playing a role in angiogenesis
(34). Stat3, which is
phosphorylated at Tyr 705, shifts into the nucleus activating
target genes (35) and
overexpression of Stat3 was found in leukaemia, breast, pancreatic
and prostate cancer, as well as in melanoma (36). In F2-treated HL-60 cells the
constitutive Tyr 705 phosphorylation of Stat3 was downregulated
after 2 h (Fig. 4) which suggests
that the anti-apoptotic activity was also decreased temporally
correlating with the activation of caspase 9. Also, the Stat5
proto-oncogene induces anti-apoptotic genes such as Bcl-X (37), thereby maintaining cell survival
despite drug-induced stresses (38). However, Stat5 was neither
constitutively phosphorylated (activated) nor was the protein level
decreased by F2. By contrast, the expression of c-Myc was strongly
suppressed after 2 h of F2 treatment and was re-expressed (still
below control levels) after 24 h. c-Myc causes an abnormal
proliferation rate and is overexpressed in many tumour types and
influences cell differentiation and apoptosis (39).
c-Jun and JunB belong to the family of Jun
transcription factors, which are components of the activating
protein-1 (AP1) transcription factor complexes. AP1 heterodimers
are important for cell proliferation, differentiation, and
activated c-Jun promotes cell cycle progression and neoplastic
transformation (40). Markedly,
c-Jun was strongly increased between 2 and 8 h of F2 treatment,
whereas JunB expression remained unchanged. The strictly inverse
expression of c-Jun and c-Myc upon F2 treatment was most likely
independent of each other, as it has not been reported that c-Jun
and the AP1 complex suppress c-Myc, nor has it been shown that
c-Myc negatively regulates c-Jun. However, when in complex with
ATF2 and c-Myc, c-Jun binds to the ATF/CRE site of ATF3 and
c-Jun/ATF2/c-Myc induce cell proliferation (41). The dramatic disproportional
expressions of c-Myc and c-Jun upon F2 treatment excluded the
possibility of a transcriptional active c-Myc/ATF2/c-Jun complex
and hence, in case of such a scenario, proliferation was most
likely compromised.
Induction of CYP1A1 activity in MCF-7
cells
Smilacacea species are reported to contain steroidal
compounds with estrogenic and anti-estrogenic effects (42,43).
A subset of cytochrome P450 (CYP) enzymes are important regulators
of estrogen and phyto-estrogen metabolism and CYP1A1 plays a role
in estrogen receptor (ER) pathway-dependent synergic carcinogenic
action of xeno-estrogens (44).
Caucasian individuals with polymorphic CYP1A1 (homozygous for
A2455G) stand an increased risk for breast cancer (45,46).
Therefore, we analysed the activity of CYP1A1 on S. spinosa
extract treatment in ER positive (MCF-7) and ER negative
(MDA-MB231) breast cancer cell lines (Fig. 5). In MCF-7 cells the crude MeOH
extract weakly induced CYP1A1, whereas CYP1A1 was severely induced
by F2. This effect was not observed in MDA-MB231 cells. CYP1A1
inhibition augments LPS-triggered fever, whereas induction of
CYP1A1 controls fever and inhibits inflammation (47,48).
This seems to depend on the expression of ER and suggests that this
remedy is particularly effective in women and could explain its use
against internal haemorrhaging during menstruation or after
childbirth (14). CYPs are phase 1
enzymes and detoxify xenobiotics and contribute to drug clearance
but they also activate pro-carcinogens. Therefore, it has to be
considered that the intake of this remedy can cause unwanted
interactions with other drugs or environmental (nutritional)
compounds.
Conclusion
Among different extracts of increasing polarity the
methanol extract of the rhizome of S. spinosa inhibited cell
proliferation most significantly, which was associated with the
induction of p21. The induction of the proto-oncogenes Cdc25A and
cyclin D1 may have counteracted cell cycle arrest, yet they did not
prevent it. Further fractionation of the methanol extract increased
the apoptotic property (∼2.5 fold at 60 μg/ml), which correlated
with the transient inactivation of the proto-oncogene Stat3 and the
activation of caspase 9, followed by the induction of caspase 8 and
3. Overexpression of c-Jun did not abrogate, but most likely
attenuated apoptosis. Reportedly, the mere overexpression of
proto-oncogenes can trigger apoptosis when other co-operating side
parameters are limited. The traditional use of ‘Kokolmeka roja’ for
many generations proves that the intake of this remedy is safe and
the healing properties prevail over potential adverse effects.
Although it is generally used against inflammatory ailments, in the
present study we have shown that the rhizome of S. spinosa
exhibits significant potential as anti-neoplastic concept and
should therefore be tested in vivo.
Acknowledgements
We thank Toni Jäger who helped prepare
the figures. The Funds for Innovative Interdisciplinary Cancer
Research to G.K. provided financial support. The Austrian Exchange
Service (OeAD) provided a fellowship to K.J.
References
|
1.
|
Pecorino L: Molecular Biology of Cancer.
2nd edition. Oxford University Press; New York, NY: 2008
|
|
2.
|
Stewart BW and Kleihues P: World Cancer
Report. IARC Press; Lyon: 2003
|
|
3.
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.PubMed/NCBI
|
|
4.
|
Fabricant DS and Farnsworth NR: The value
of plants used in traditional medicine for drug discovery. Environ
Health Perspect. 109:69–75. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Rastogi RP and Dhawan BN: Research on
medicinal plants at the Central Drug Research Institute, Lucknow
(India). Indian J Med Res. 76:27–45. 1982.PubMed/NCBI
|
|
6.
|
Cseke LJ: Natural products from plants.
2nd edition. CRC Press Taylor and Francis; Boca Raton, FL: 2006
|
|
7.
|
Shoeb M: Anticancer agents from medicinal
plants. Bangladesh J Pharmacol. 1:35–41. 2006.
|
|
8.
|
Kundu JK and Surh YJ: Inflammation:
gearing the journey to cancer. Mutat Res. 659:15–30. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Taylor L: The Healing Power of Rainforest
Herbs: A Guide to Understanding and Using Herbal Medicinals. Square
One Publishers; New York, NY: 2005
|
|
10.
|
Caceres A, Cano O, Samayoa B and Aguilar
L: Plants used in Guatemala for the treatment of gastrointestinal
disorders. 1 Screening of 84 plants against enterobacteria. J
Ethnopharmacol. 30:55–73. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Jiang J and Xu Q: Immunomodulatory
activity of the aqueous extract from rhizome of Smilax glabra in
the later phase of adjuvant-induced arthritis in rats. J
Ethnopharmacol. 85:53–59. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Alam MI and Gomes A: Adjuvant effects and
antiserum action potentiation by a (herbal) compound
2-hydroxy-4-methoxy benzoic acid isolated from the root extract of
the Indian medicinal plant ‘sarsaparilla’ (Hemidesmus indicus R.
Br.). Toxicon. 36:1423–1431. 1998.PubMed/NCBI
|
|
13.
|
Navarro MC, Montilla MP, Cabo MM, Galisteo
M, Cáceres A, Morales C and Berger I: Antibacterial, antiprotozoal
and antioxidant activity of five plants used in Ibazal for
infectious. Phytother Res. 17:325–329. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Arvigo R and Balick M: Rainforest
Remedies. One Hundred Healing Herbs of Belize. 2nd edition. Lotus
Press; Twin Lakes, WI: pp. 72–73. 1998
|
|
15.
|
Gridling M, Stark N, Madlener S, Lackner
A, Popescu R, Benedek B, Diaz R, Tut FM, Nha Vo TP, Huber D, et al:
In vitro anti-cancer activity of two ethno-pharmacological
healing plants from Guatemala Pluchea odorata and Phlebodium
decumanum. Int J Oncol. 34:1117–1128. 2009.
|
|
16.
|
Stark N, Gridling M, Madlener S, Bauer S,
Lackner A, Popescu R, Diaz R, Tut FM, Vo TP, Vonach C, et al: A
polar extract of the Maya healing plant Anthurium schlechtendalii
(Aracea) exhibits strong in vitro anticancer activity. Int J
Mol Med. 24:513–521. 2009.PubMed/NCBI
|
|
17.
|
Strasser S, Maier S, Leisser C, Saiko P,
Madlener S, Bader Y, Bernhaus A, Gueorguieva M, Richter S, R. Mader
RM, et al: 5-FdUrd-araC heterodinucleoside re-establishes
sensitivity in 5-FdUrd- and AraC- resistant MCF-7 breast cancer
cells overexpressing ErbB2. Differentiation. 74:488–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Maier S, Strasser S, Saiko P, Leisser C,
Sasgary S, Grusch M, Madlener S, Bader Y, Hartmann J, Schott H, et
al: Analysis of mechanisms contributing to AraC-mediated
chemoresistance and re-establishment of drug sensitivity by the
novel heterodinucleoside phosphate 5-FdUrd-araC. Apoptosis.
11:427–440. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Hüttenbrenner S, Maier S, Leisser C,
Polgar D, Strasser S, Grusch M and Krupitza G: The evolution of
cell death programs as prerequisites of multicellularity. Rev Mutat
Res. 543:235–249. 2003.PubMed/NCBI
|
|
20.
|
Grusch M, Fritzer-Szekeres M, Fuhrmann G,
Rosenberger G, Luxbacher C, Elford HL, Smid K, Peters GJ, Szekeres
T and Krupitza G: Activation of caspases and induction of apoptosis
by amidox and didox. Exp Haematol. 29:623–632. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Madlener S, Rosner M, Krieger S, Giessrigl
B, Gridling M, Vo TP, Leisser C, Lackner A, Raab I, Grusch M, et
al: Short 42 degrees C heat shock induces phosphorylation and
degradation of Cdc25A which depends on p38MAPK, Chk2 and 14.3.3.
Hum Mol Genet. 18:1990–2000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kastan MB and Bartek J: Cell-cycle
checkpoints and cancer. Nature. 432:316–323. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Blomberg I and Hoffmann I: Ectopic
expression of Cdc25A accelerates the G(1)/S transition and leads to
premature activation of cyclin E- and cyclin A-dependent kinases.
Mol Cell Biol. 19:6183–6194. 1999.PubMed/NCBI
|
|
24.
|
Kiyokawa H and Ray D: In vivo roles of
CDC25 phosphatases: biological insight into the anti-cancer
therapeutic targets. Anticancer Agents Med Chem. 8:832–836. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Meeran SM and Katiyar SK: Cell cycle
control as a basis for cancer chemoprevention through dietary
agents. Front Biosci. 13:2191–2202. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wolf D and Rotter V: Major deletions in
the gene encoding the p53 tumor antigen cause lack of p53
expression in HL-60 cells. Proc Natl Acad Sci USA. 82:790–794.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Abukhdeir AM and Park BH: P21 and p27:
roles in carcinogenesis and drug resistanc. Expert Rev Mol Med.
10:e192008. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Coller HA, Grandori C, Tamayo P, Colbert
T, Lander ES, Eisenman RN and Golub TR: Expression analysis with
oligonucleotide microarrays reveals that MYC regulates genes
involved in growth, cell cycle, signaling, and adhesion. Proc Natl
Sci USA. 97:3260–3265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Chang C, Zhu YQ, Mei JJ, Liu SQ and Luo J:
Involvement of mitochondrial pathway in NCTD-induced cytotoxicity
in human hepG2 cells. J Exp Clin Cancer Res. 29:145–154. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Paull TT, Rogakou EP, Yamazaki V,
Kirchgessner CU, Gellert M and Bonner WM: A critical role for
histone H2AX in recruitment of repair factors to nuclear foci after
DNA damage. Curr Biol. 10:886–895. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Piperno G, LeDizet M and Chang J:
Microtubules containing acetylated alpha-tubulin in mammalian cells
in culture. J Cell Biol. 104:289–302. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Jackson CB and Giraud AS: Stat3 as a
prognostic marker in human gastric cancer. J Gastroenterol Hepatol.
24:505–507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kanda N, Seno H, Konda Y, Marusawa H,
Kanai M, Nakajima T, Kawashima T, Nanakin A, Sawabu T, Uenoyama Y,
et al: Stat3 is constitutively activated and supports cell survival
in association with survivin expression in gastric cancer cells.
Oncogene. 23:4921–4929. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Gritsko T, Williams A, Turkson J, Kaneko
S, Bowman T, Huang M, Nam S, Eweis I, Diaz N, Sullivan D, et al:
Persistent activation of stat3 signaling induces survivin gene
expression and confers resistance to apoptosis in human breast
cancer cells. Clin Cancer Res. 12:11–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Deng JY, Sun D, Liu XY, Pan Y and Liang H:
Stat-3 correlates with lymph node metastasis and cell survival in
gastric cancer. World J Gastroenterol. 16:5380–5387. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Zhao M, Jiang B and Gao FH: Small molecule
inhibitors of STAT3 for cancer therapy. Curr Med Chem.
18:4012–4018. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Gündogdu MS, Liu H, Metzdorf D, Hildebrand
D, Aigner M, Aktories K, Heeg K and Kubatzky KF: The haematopoietic
GTPase RhoH modulates IL3 signalling through regulation of Stat
activity and IL3 receptor expression. Mol Cancer. 9:225–238.
2010.PubMed/NCBI
|
|
38.
|
Jinawath N, Vasoontara C, Jinawath A, Fang
X, Zhao K, Yap KL, Guo T, Lee CS, Wang W, Balgley BM, et al:
Oncoproteomic analysis reveals co-upregulation of RELA and Stat5 in
carboplatin resistant ovarian carcinoma. PLoS One. 5:e111982010.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Dominguez-Sola D, Ying CY, Grandori C,
Ruggiero L, Chen B, Li M, Galloway DA, Gu W, Gautier J and
Dalla-Favera R: Non-transcriptional control of DNA replication by
c-Myc. Nature. 448:445–451. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Wang H, Birkenbach M and Hart J:
Expression of Jun family members in human colorectal
adenocarcinoma. Carcinogenesis. 21:1313–1317. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Mathiasen DP, Egebjerg C, Andersen SH,
Rafn B, Puustinen P, Khanna A, Daugaard M, Valo E, Tuomela S,
Bøttzauw T, et al: Identification of a c-Jun N-terminal
kinase-2-dependent signal amplification cascade that regulates
c-Myc levels in ras transformation. Oncogene. 31:390–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Ivanova A, Mikhova B, Klaiber I, Dinchev D
and Kostova I: Steroidal saponins from Smilax excelsa rhizomes. Nat
Prod Res. 23:916–924. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Doyle BJ, Frasor J, Bellows LE, Locklear
TD, Perez A, Gomez-Laurito J and Mahady GB: Estrogenic effects of
herbal medicines from Costa Rica used for the management of
menopausal symptoms. Menopause. 16:748–755. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Yu Z, Hu D and Li Y: Effects of
zearalenone on mRNA expression and activity of cytochrome P450 1A1
and 1B1 in MCF-7 cells. Ecotoxicol Environ Saf. 58:187–193. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Sergentanis TN and Economopoulos KP: Four
polymorphisms in cytochrome P450 1A1 (CYP1A1) gene and breast
cancer risk: a meta-analysis. Breast Cancer Res Treat. 122:459–469.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Sergentanis TN and Economopoulos KP:
Erratum to: Four polymorphisms in cytochrome P450 1A1 (CYP1A1) gene
and breast cancer risk: a meta-analysis. Breast Cancer Res Treat.
131:10832012. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Kozak W, Mayfield KP, Kozak A and Kluger
MJ: Proadifen (SKF-525A), an inhibitor of cytochrome P-450,
augments LPS-induced fever and exacerbates prostaglandin-E2 levels
in the rat. J Therm Biol. 25:45–50. 2000. View Article : Google Scholar
|
|
48.
|
Sun J, Sui X, Bradbury JA, Zeldin DC,
Conte MS and Liao JK: Inhibition of vascular smooth muscle cell
migration by cytochrome p450 epoxygenase-derived eicosanoids. Circ
Res. 90:1020–1027. 2002. View Article : Google Scholar : PubMed/NCBI
|