Introduction
Although the incidence of gastric cancer is
declining worldwide, in 2008 gastric cancer remained the third
leading cause of cancer-related mortality in men and the fifth in
women (1). In Japan, gastric
cancer is one of the most common causes of mortality, despite
advances in diagnosis and treatment. In particular, unresectable or
recurrent gastric cancer is associated with extremely poor
prognosis even when treated with novel therapeutic agents,
including taxanes [paclitaxel (2,3) and
docetaxel (4,5)], irinotecan (6,7),
oxaliplatin (8,9), S-1 (10), and capecitabine (11), which are known to be efficacious in
gastric cancer. A multi-center randomized controlled trial (SPIRITS
trial) performed in Japan reported that the median overall survival
(OS) and progression-free survival in patients with advanced
gastric cancer treated with S-1 plus cisplatin were significantly
longer in those treated with S-1 alone (10). Therefore, the Gastric Cancer
Treatment Guidelines 2010 issued by the Japanese Gastric Cancer
Association recommended an S-1 plus cisplatin combination regimen
as a standard first-line treatment for unresectable and recurrent
gastric cancer (12). However,
even with this treatment, the median overall survival was 13 months
and progression-free survival time was 6 months, suggesting the
need for novel therapeutic modalities.
Immunotherapies, such as tumor antigen vaccination
to induce antitumor T cells or antibodies, has been proposed as a
novel treatment modality (13,14).
To date, a number of cancer-specific immunotherapies, particularly
peptide vaccine therapies with cancer-testis antigens such as MAGE
and NY-ESO-1, have been attempted (15,16).
Clinical trials have indicated that cancer vaccination with
immunogenic epitope peptide derived from these antigens can induce
specific T cell responses in cancer patients (17), however, the clinical response is
considered to be limited due to possible immune evasion of tumor
cells caused by downregulation or loss of human leukocyte antigen
(HLA) and/or antigen proteins during tumor progression (18,19).
Inhibition of angiogenesis is another promising
strategy for cancer treatment (20,21),
and clinical trials have shown that administration of anti-vascular
endothelial growth factor (VEGF) antibody combined with
chemotherapy significantly prolonged the survival of colorectal
cancer patients (22) and the
progression-free survival in gastric cancer patients (23). Therefore, vaccine therapies
targeting VEGF receptor 1 (VEGFR-1, also known as Flt-1) (24) and VEGFR-2 (25), both of which are overexpressed in
endothelial cells of newly formed vessels in various types of
primary and metastatic tumors (26,27),
are potentially effective anti-angiogenic cancer vaccines. In fact,
we have already reported the efficacy of vaccine therapies with
HLA-A*2402-restricted epitope peptides derived from
VEGFR1 and VEGFR2 in a mouse model (28,29).
In this study, we conducted a clinical trial to
evaluate the safety and efficacy of vaccine therapy with
VEGFR1-1084 and/ or VEGFR2-169 combined with S-1 plus cisplatin in
patients with unresectable or recurrent gastric cancer.
Materials and methods
Patient eligibility
Patients diagnosed with gastric adenocarcinoma
considered unresectable or recurrent were enrolled in this trial at
the Department of Gastroenterological Surgery, Osaka University
Hospital (Japan). The following were the other main inclusion
criteria: i) Eastern Cooperative Oncology (ECOG) performance status
of 0 or 1; ii) age between 20 and 74 years; iii) adequate
bone-marrow, cardiac, pulmonary, hepatic and renal functions
including leukocyte count 2500–12000/mm3, neutrophil
count ≥1500/mm3, platelet count ≥100,000/mm3,
hemoglobin level ≥9.0g/dl, aspartate aminotransferase and alanine
aminotransferase ≤2.5x the institutional normal upper limits, total
bilirubin ≤1.5x the institutional normal upper limits, creatinine
equal or less the institutional normal upper limits, creatinine
clearance using the Cockcroft-Gault formula ≥50 ml/min; iv) life
expectancy >3 months; v) no prior chemotherapy or one adjuvant
regimen that did not include S-1 or cisplatin and that was
completed >4 weeks before entry to the study; vi) positive
genomic DNA typing test for HLA-A*2402 (SRL, Tokyo,
Japan); vii) signature of an informed consent. The main exclusion
criteria were: i) the presence of another serious disease such as
uncontrolled diabetes, hepatic disorder, cardiac disease,
hemorrhage/bleeding; ii) pregnant or breast-feeding women; iii)
patients who planned to become pregnant during the study period;
iv) symptomatic infectious disease; v) concurrent treatment with
steroids or immunosuppressive agents; vi) other uncontrolled
malignant diseases; vii) unhealed wound; viii) intestinal
obstruction or interstitial pneumonia; ix) decision of
unsuitability by the principal investigator or the physician in
charge.
Patient characteristics
Thirty patients were considered in this trial and 22
patients (73%) were found to be HLA-A*2402 positive by
DNA typing of HLA genomic variations and enrolled in this study
between April 2008 and March 2010.
Table I shows the
patient characteristics at study entry; patients included 19 males
and 3 females. Sixteen patients had unresectable gastric cancer and
6 had recurrent disease after surgery. The tumor was considered
unresectable for the following reasons: i) peritoneal dissemination
and malignant ascites in 9 patients; ii) other distant organ
metastasis in 4 patients; iii) distant nodal metastasis in 1
patient; and iv) more than one reason including i), ii) and iii) in
2 patients. Recurrent sites were as follows: i) peritoneal
dissemination and malignant ascites in 3 patients; ii) liver or
lung metastases with distal nodal metastasis in 2 patients; iii)
local recurrence and pleural dissemination in 1 patient. Eleven
patients had HLA-A*2402 homo type, and the other 11
patients had HLA-A*2402 and another HLA-A type such as
A*0201. The patients received at least one cycle of
combination therapy with chemotherapy plus peptide vaccination
(1–18 cycles; median, 9 cycles).
 | Table IClinicopathological data. |
Table I
Clinicopathological data.
| S1/CDDP+VEGFR
vaccine |
|---|
| Number | 22 |
| Age (years) (mean ±
SD) | 60.5±10.3 |
| Sex | |
| M | 19 |
| F | 3 |
| Disease
progression | |
| Unresectable | 16 |
| Recurrence | 6 |
| Histological
type | |
|
Differentiated | 7 |
|
Undifferentiated | 15 |
| HLA-A type | |
| A*2402
homo | 11 |
| A*2402
hetero | 11 |
Study design
This study was a non-randomized, open label, phase I
and II clinical trial with VEGFR1-1084 and/or VEGFR2-169 vaccines
combined with standard chemotherapy, S-1 plus cisplatin, for
advanced unresectable or recurrent gastric cancer. The primary
endpoints were the safety of the combination therapy and the median
time to disease progression (TTP). The secondary endpoints were
immunological response, clinical response, accomplishment rate, 1
and 2 year survival rates, and the median survival time (MST).
Toxicities were assessed by the Common Terminology Criteria for
Adverse Events version 4.0 (CTCAE ver4.0). The dose-limiting
toxicity was defined as a hematological toxicity of grade 4 and
non-hematologic toxicity of grade 3 or greater. To assess the
clinical response, computed tomography imaging was performed within
a month before starting the first cycle and within 2 weeks after
every two cycles. Every measurable region such as liver, lung or
lymph node metastasis was evaluated by the Response Evaluation
Criteria in Solid Tumors (RECIST) (30). Peptide-specific immunological
responses were analyzed by IFN-γ enzyme-linked immunospot (ELISPOT)
assay. Stage classification and the assessment of resected
specimens were performed according to the 14th edition of the
Japanese Classification of Gastric Cancer (12). This trial was approved by the Osaka
University Ethics Committee, and registered at UMIN (http://www.umin.ac.jp; Trial registration ID:
UMIN000005007), and carried out in accordance with the Helsinki
declaration on experimentation on human subjects.
Peptides
HLA-A*2402-restricted CMV peptide (QYDPV
AALF), GMP-graded VEGFR1-1084 peptide (SYGVLLWEIF) (28), and GMP-graded VEGFR2-169 peptide
(RFVPDGNRI) (29) were synthesized
by the American Peptide Company (Sunnyvale, CA) according to a
standard solid-phase synthesis method and purified by
reversed-phase high-performance liquid chromatography (HPLC). The
purity (>90%) and the identity of the peptides were determined
by analytical HPLC and mass spectrometry, respectively.
Treatment protocol
The S-1 plus cisplatin regimen was based on that
previously reported in the multicenter phase III SPIRITS trial
(10). S-1 was administered orally
twice daily for the first 3 weeks of a 5-week cycle. The dose of
S-1 administered each time was calculated according to the body
surface area as follows: <1.25 m2, 40 mg; 1.25–1.5
m2, 50 mg; >1.5 m2, 60 mg/day. Cisplatin
was administered by intravenous infusion at 60 mg/m2 on
Day 8 of each cycle. Furthermore, 1 mg VEGFR1-1084 and VEGFR2-169
were emulsified together with 1 ml of incomplete Freund’s adjuvant
(Montanide ISA-51 VG, SEPPIC, Paris) and injected subcutaneously at
inguen from side to side every week 5 times. Patients with more
than one cycle of this treatment were enrolled. Toxicities within 2
cycles, the clinical response within 2 cycles and peptide-specific
immunological response within 6 cycles were evaluated. S-1 and
cisplatin were repeatedly administered until disease progression
was considered to have occurred. Administration of peptide vaccines
was continued after discontinuation of the regimen of S-1 plus
cisplatin after consultation with the patient.
Isolation and stock of peripheral blood
mononuclear cells
Peripheral blood cells were obtained from patients
at the end of every cycle of the treatment. Peripheral blood
mononuclear cells (PBMCs) were isolated immediately by Ficoll-Paque
Plus density gradient solution (GE Healthcare, Little Chalfont,
UK), suspended in Cell Banker (Juji Field, Tokyo), and frozen and
stored in liquid nitrogen.
Enzyme-linked immunospot (ELISPOT)
assay
To assess the specific CTL response, ELISPOT assay
was performed following in vitro expansion. Frozen PBMCs
derived from the same patient were thawed at the same time, and
their viability was confirmed to be >90%. PBMCs
(5x105/ml) were cultured with 10 μg/ml of the
respective peptide and 100 IU/ml of IL-2 (Novartis, Emeryville, CA)
at 37°C. The peptide was added to the culture at Day 0 and 7 (final
concentration 10 μg/ml), and cells were harvested after 2
weeks. Following CD4+ cell depletion by Dynal
CD4-positive isolation kit (Invitrogen, Carlsbad, CA), the cells
were used as responder cells in the ELISPOT assay. IFN-γ ELISPOT
assay was performed using the Human IFN-γ ELISpot PLUS kit
(MabTech, Cincinnati, OH) according to the instructions supplied by
the manufacturer. Briefly, HLA-A*2402-positive
B-lymphoblast TISI cells (IHWG Cell and Gene Bank, Seattle, WA)
were incubated with 20 μg/ml of VEGFR1-1084 peptide or
VEGFR2-169 peptide overnight, then the residual peptide in the
media was washed out to prepare peptide-pulsed TISI cells as the
stimulator cells. Prepared CD4− cells were cultured with
peptide-pulsed TISI cells (2×104 cells/ well) at 1/1,
1/2, 1/4, and 1/8 mixture ratio of responder cells and stimulator
cells (R/S ratio) on 96-well plate (Millipore, Bedford, MA) at 37°C
overnight. Non-peptide-pulsed TISI cells were used as negative
control stimulator cells. All ELISPOT assays were performed in
triplicate wells. The plates were analyzed by the automated ELISPOT
reader, ImmunoSPOT S4 (Cellular Technology, Cleveland, OH) and
ImmunoSpot Professional Software version 5.0 (Cellular Technology).
The number of peptide-specific spots was calculated by subtracting
the spot number in the control well from the spot number of wells
with peptide-pulsed TISI cells. The CTL response was considered
positive when the average of peptide-specific spot number of three
wells was >15/well and the significant difference (p<0.05)
was demonstrated between the average spot numbers. The sensitivity
of our ELISPOT assay was periodically estimated as approximately
average by the ELIPOT panel of Cancer Immunotherapy Consortium
(CIC).
Statistical analysis
Statistical analysis was performed using the
Student’s t-test and Fisher’s exact test. TTP and OS curves were
estimated using the Kaplan-Meier methodology. All statistical
analyses were performed with JMP 8.0.2 (SAS Institute, Cary,
NC).
Results
Toxicity
Table II lists the
adverse effects recorded during the first two cycles of the
combination therapy. Grade 3 or 4 neutropenia and anemia were
observed in approximately 20% of the patients. Anorexia of grade 2
or more was reported by 70% of the patients. During the vaccination
therapy, 6 patients developed a reaction at the injection site and
2 patients developed an ulcer at the injection sites. No delayed
wound healing or gastrointestinal bleeding was seen during the
therapy. The dosage of chemotherapeutic agents, cisplatin and/or
S-1, was reduced in 10 patients, however none of the patients
dropped out of this study due to the above adverse effects. One
patient discontinued chemotherapy with S-1 plus cisplatin after 1
cycle but this was not related to any adverse effect. The same
patient continued the peptide vaccination with the 2nd line
chemotherapy.
 | Table IIComplications observed during the two
cycles of therapy. |
Table II
Complications observed during the two
cycles of therapy.
| S-1/CDDP+VEGFR
vaccine (n=22) |
|---|
| G1 | G2 | G3 | G4 | G3-4 (%) |
|
| Blood/bone
marrow | | | | | |
| Leukopenia | 4 | 10 | 1 | 2 | 3 (14) |
| Neutropenia | 1 | 9 | 3 | 2 | 5 (23) |
| Lymphopenia | 1 | 8 | 4 | 1 | 5 (23) |
| Anemia | 5 | 13 | 4 | 0 | 4 (18) |
|
Thrombocytopenia | 10 | 2 | 1 | 1 | 2 (9) |
|
|
| G1 | G2 | G3 | G4 | G2-4 (% |
|
| Hepatic | | | | | |
| Increase in
AST | 8 | 0 | 0 | 0 | 0 (0) |
| Increase in
ALT | 7 | 0 | 0 | 0 | 0 (0) |
| Increase in
bilirubin | 5 | 1 | 0 | 0 | 1 (5) |
| Renal | | | | | |
| Increase in
Cr | 6 | 0 | 0 | 0 | 0 (0) |
| Hyperkalemia | 9 | 0 | 0 | 0 | 0 (0) |
| Non hematological
complications | | | | | |
| Anorexia | 7 | 13 | 2 | 0 | 15 (68) |
| Cheilitis | 9 | 1 | 0 | 0 | 1 (5) |
| Vomiting | 8 | 1 | 0 | 0 | 1 (5) |
| Diarrhea | 10 | 2 | 0 | 0 | 2 (9) |
| Dysgeusia | 5 | 0 | 0 | 0 | 0 (0) |
| Fever | 2 | 3 | 0 | 0 | 3 (14) |
| Alopecia | 5 | 0 | 0 | 0 | 0 (0) |
| Flu-like
symptoms | 2 | 1 | 0 | 0 | 1 (5) |
| Maculopapular
rash | 4 | 0 | 0 | 0 | 0 (0) |
| Reaction at
injection site | 4 | 0 | 2 | 0 | 2 (9) |
Clinical response and overall
survival
Patients received a median of 9 cycles (range 1–18)
of the combination therapy with S-1 plus cisplatin and peptide
vaccination. Table III shows the
clinical response date of the 22 patients. Twenty-one of the 22
patients received 2 or more cycles of the therapy. The clinical
responses were classified as partial response (PR) in 12 patients
(54.5%); stable disease (SD) in 10 patients (45.5%); and none of
the patients showed progressive disease (PD). The response rate
(RR) was 54.5% and disease control rate (PR + SD) was 100% after
two cycles. One patient achieved disappearance of peritoneal
dissemination and malignant ascites after 4 cycles of the treatment
and underwent complete resection (R0 resection).
 | Table IIITreatment response after the first
two cycles. |
Table III
Treatment response after the first
two cycles.
| S-1/CDDP+VEGFR
vaccine |
|---|
| Number | 22 |
| Achievement rate of
2 cycle therapy | 21 (95%) |
| Dose down of
chemotherapy | 10 (45%) |
| Response
evaluation | |
| PR | 12 |
| SD | 10 |
| PD | 0 |
| Response rate | 12 (55%) |
| Disease control
rate | 22 (100%) |
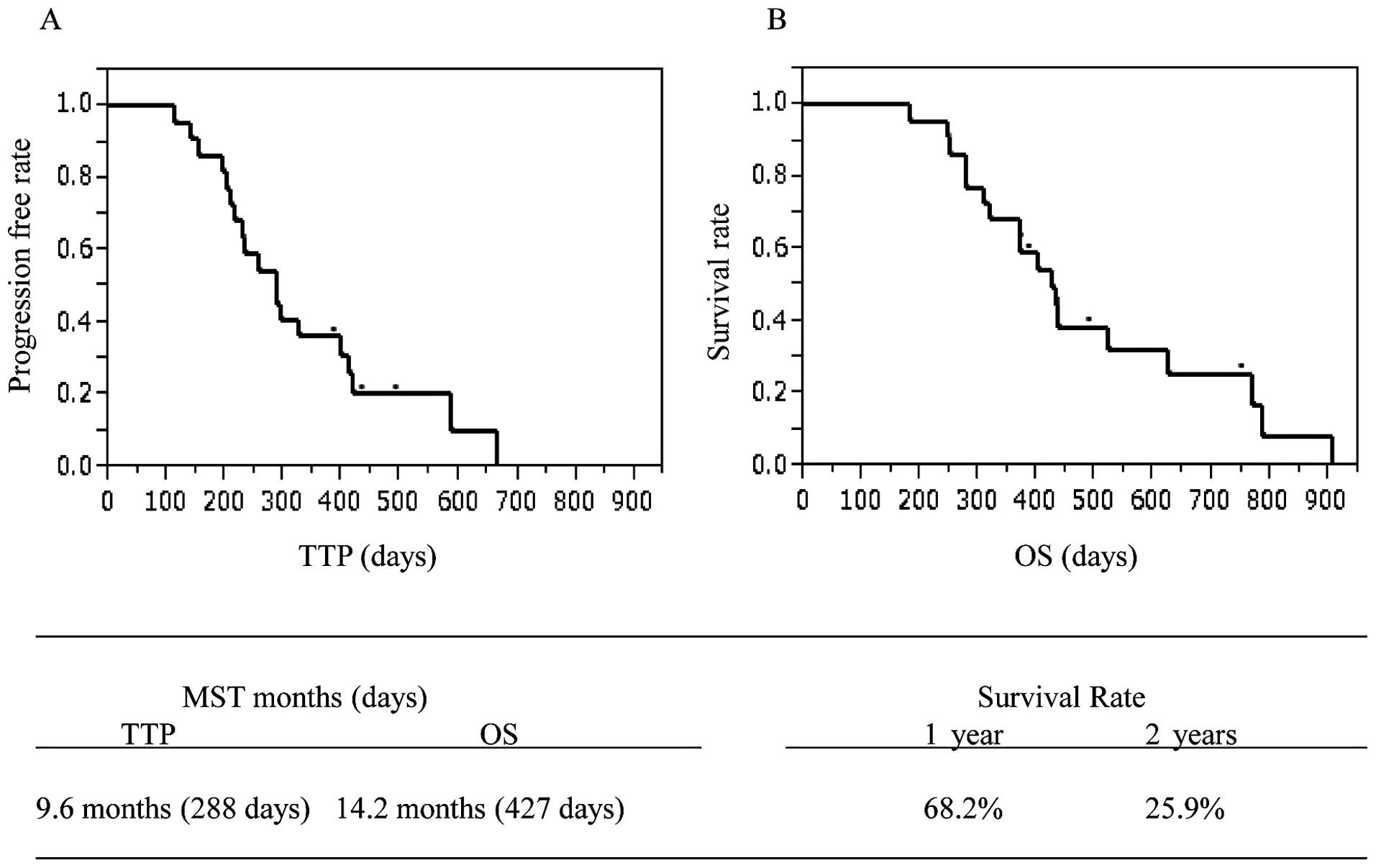
Eighteen of the patients succumbed to the disease
and none of the mortalities were due to other causes. The median
TTP was 288 days (9.6 months), and MST was 427 days (14.2 months).
Seven patients died within one year with a 1-year survival rate of
68.2%. The other 8 patients died in the second year, with a 2-year
survival rate of 25.9% (Fig.
1).
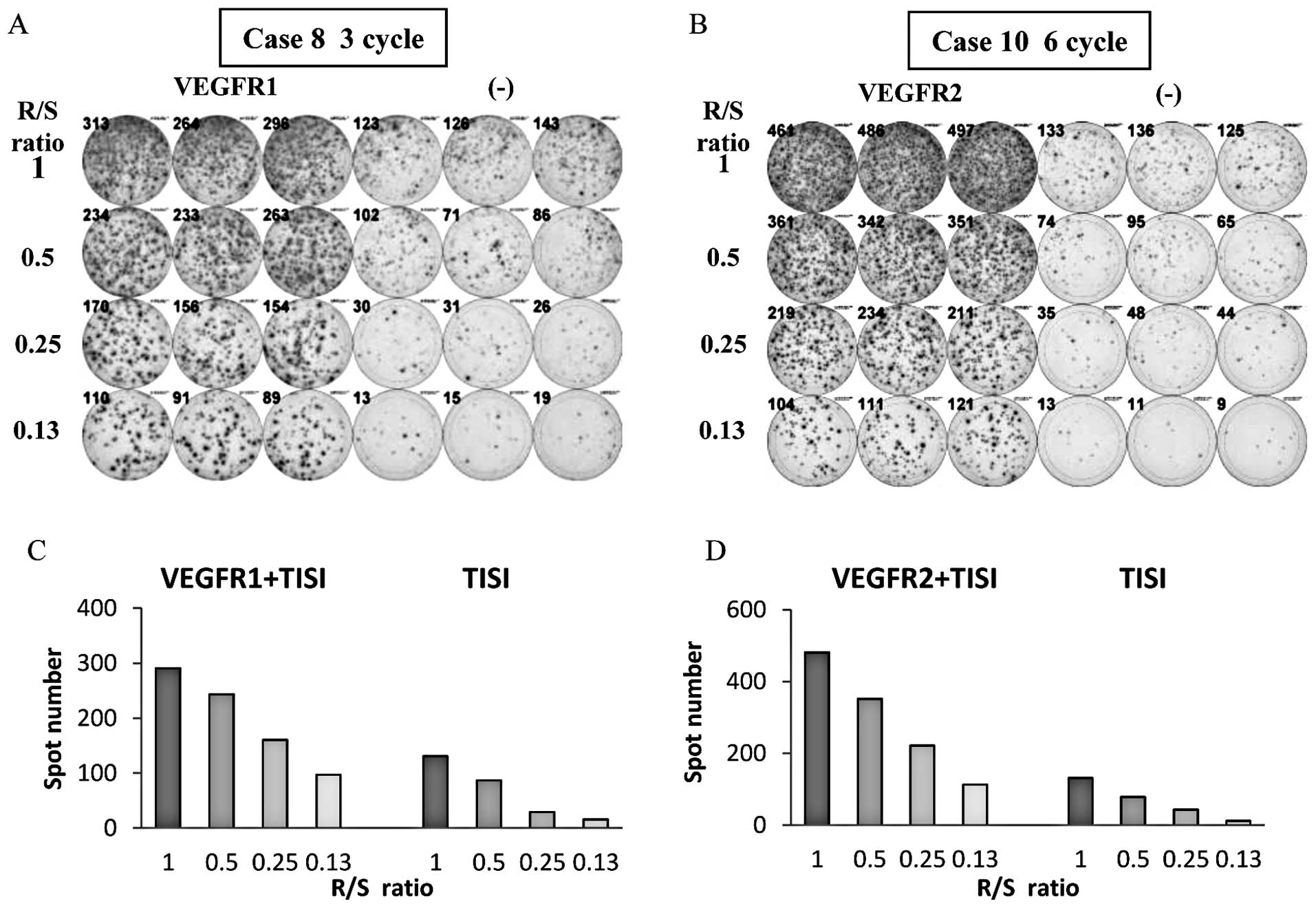
Immunological monitoring
The CTL response against VEGFR1-1084 after 3 cycles
of the treatment in Patient 8 and the CTL response against
VEGFR2-169 after 6 cycles of the treatment in Patient 10 are
presented in Fig. 2 as
representative results of positive immune responses. Eighteen (82%)
of the 22 patients who received at least one course of the
vaccination showed positive CTL response against VEGFR1-1084, and
18 patients (82%) showed positive CTL response against VEGFR2-169).
Sixteen patients showed response to both peptides and only two
patients showed no response to the vaccination.
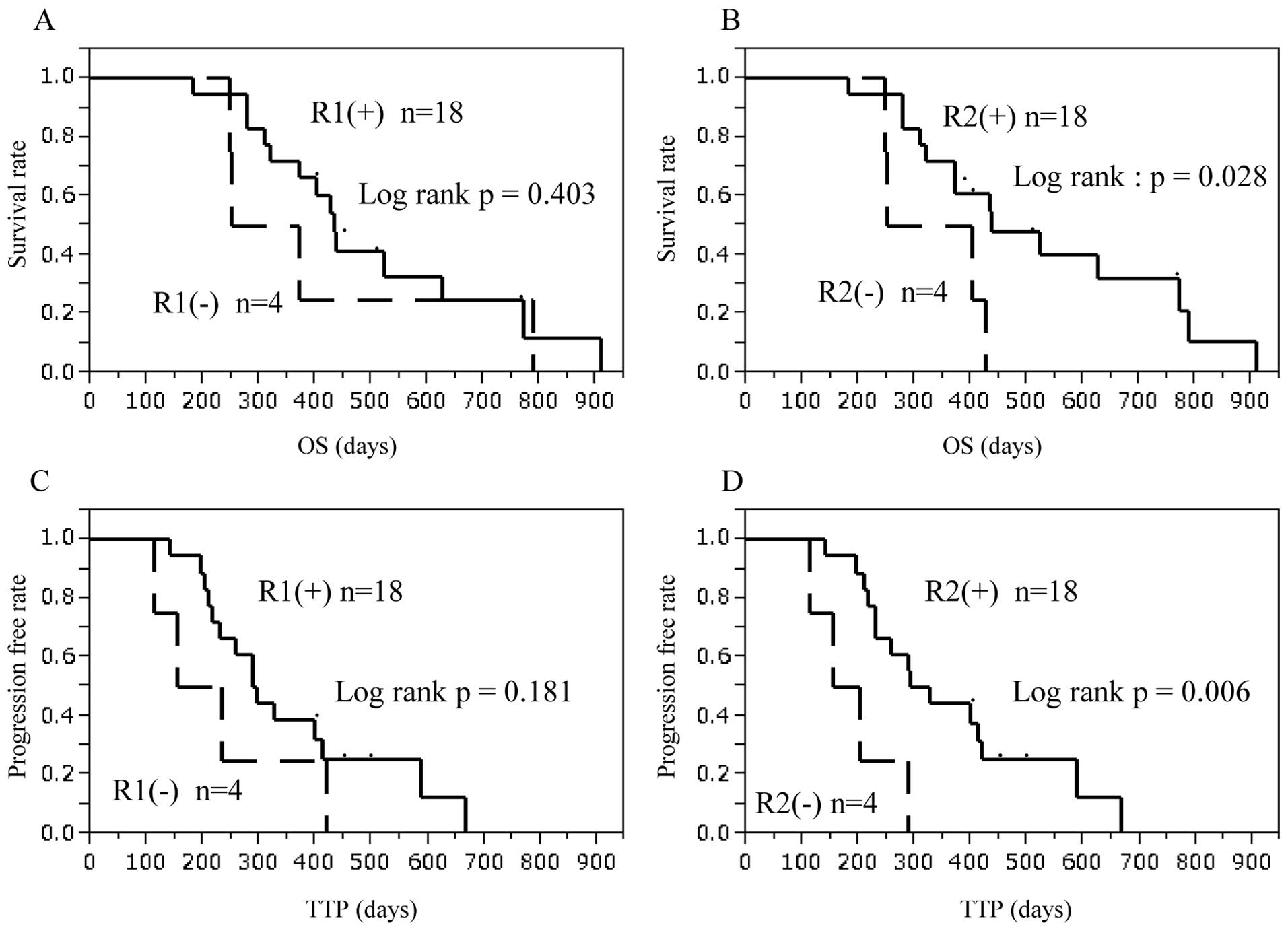
Patients with a positive response to VEGFR2-169
peptide had significantly better prognosis compared with those with
no response in OS (p=0.028) and TTP (p=0.006, Fig. 3). Patients with induced CTL
response to VEGFR1-1084 peptide tended to show better prognosis,
but this was not statistically significant.
Table IV shows all
patient characteristics and results of combination therapy with
peptide vaccine and chemotherapy.
 | Table IVPatient characteristics and results
of combination therapy with peptide vaccine and chemotherapy. |
Table IV
Patient characteristics and results
of combination therapy with peptide vaccine and chemotherapy.
| S-1/CDDP+VEGFR
vaccine (n=22) |
|---|
| Case no. | Age | Sex | HLA-Atype
A*2402 | Disease
progression | Unresectable or
recurrence site | Histological
type | R1 response in
ELISPOT | R2 response in
ELISPOT | Response
evaluation | TTP | OS | Survival |
|---|
| 01 | 70 | M | hetero type | unresectable | cancerous
ascites |
undifferentiated | + | + | SD | 667 | 910 | deceased |
| 02 | 72 | M | hetero type | recurrence | peritoneal
dissemination |
undifferentiated | - | + | SD | 421 | 789 | deceased |
| 03 | 54 | M | hetero type | unresectable | liver | differentiated | + | + | PR | 399 | 771 | deceased |
| 04 | 58 | M | hetero type | unresectable | liver | differentiated | + | + | SD | 141 | 181 | deceased |
| 05 | 46 | F | homo type | unresectable | para-aortic lymph
node |
undifferentiated | + | + | PR | 197 | 311 | deceased |
| 06 | 45 | M | homo type | unresectable | liver, lung | differentiated | + | + | PR | 218 | 627 | deceased |
| 07 | 58 | F | homo type | unresectable | liver |
undifferentiated | + | - | PR | 288 | 427 | deceased |
| 08 | 70 | M | homo type | recurrence | peritoneal
dissemination |
undifferentiated | + | + | SD | 259 | 279 | deceased |
| 09 | 68 | M | homo type | recurrence | liver |
undifferentiated | + | + | PR | 288 | 437 | deceased |
| 10 | 58 | M | hetero type | recurrence | peritoneal
dissemination, local recurrence |
undifferentiated | + | + | PR | 588 | 749 | alive |
| 11 | 57 | M | homo type | unresectable | cancerous
ascites |
undifferentiated | + | + | PR | 211 | 279 | deceased |
| 12 | 69 | M | homo type | unresectable | liver | differentiated | + | - | SD | 204 | 401 | deceased |
| 13 | 38 | F | homo type | unresectable | peritoneal
dissemination |
undifferentiated | + | + | SD | 413 | 524 | deceased |
| 14 | 73 | M | homo type | unresectable | peritoneal
dissemination | differentiated | - | - | SD | 113 | 251 | deceased |
| 15 | 59 | M | hetero type | unresectable | peritoneal
dissemination | differentiated | + | + | SD | 231 | 433 | deceased |
| 16 | 60 | M | hetero type | unresectable | peritoneal
dissemination |
undifferentiated | + | + | PR | 328 | 372 | deceased |
| 17 | 75 | M | homo type | unresectable | cancerous
ascites | differentiated | + | + | PR | 294 | 320 | deceased |
| 18 | 44 | M | homo type | unresectable | cancerous
ascites |
undifferentiated | + | + | PR | - | 490 | alive |
| 19 | 70 | M | hetero type | recurrence | liver, peritoneal
dissemination |
undifferentiated | - | + | SD | 232 | 371 | alive |
| 20 | 61 | M | hetero type | unresectable | peritoneal
dissemination |
undifferentiated | + | + | PR | - | 434 | alive |
| 21 | 62 | M | hetero type | recurrence | liver, lung |
undifferentiated | + | + | PR | - | 386 | alive |
| 22 | 64 | M | hetero type | unresectable | peritoneal
dissemination |
undifferentiated | - | - | SD | 155 | 246 | deceased |
Discussion
In this clinical trial, antiangiogenic vaccination
therapy combined with standard chemotherapy with S-1 plus cisplatin
was well tolerated without any major side-effects in patients with
advanced or recurrent gastric cancer. Furthermore, the combination
therapy achieved promising results for overall response rate (PR,
55%) and the disease control rate (PR + SD) was 100% after two
cycles of the treatment, with MST of 14.2 months, and TTP of 9.6
months. Non-hematological toxicities were generally mild and none
was greater than grade 3. Grade 3 toxicities were anorexia,
observed in only 9%, and skin reaction, in 9% of the patients. The
incidence of grade 3 or 4 anorexia and nausea was also the most
common in the group assigned to the S-1 plus cisplatin (30 and 11%)
in the SPIRITS trial (10). The
frequencies of the observed grade 3 or 4 hematological side-effects
were 23% for neutropenia, 18% for anemia, and 2% for
thrombocytopenia, which were less than those in the group assigned
to the S-1 plus cisplatin in the SPIRITS trial (40, 26, and 5%,
respectively) (2). The only
specific side-effect caused by the vaccine treatment was reaction
at the injection sites in 6 patients. Grade 3 skin ulceration was
observed in 2 patients, although it did not cause discontinuation
of the vaccine treatment. Therefore, this protocol is considered to
be safe and well tolerable.
Both VEGFR1-1084-specific and VEGFR2-169-specific
CTL responses were observed in as many as 82% of the patients even
under the combination therapy with standard chemotherapy in
patients with gastric cancer. This finding is consistent with the
results of a previous study, which reported that administration of
the standard dose of S-1 did not impede immunological responses to
peptide vaccination in patients with gastrointestinal tract cancer
(31). Furthermore, promising
therapeutic effects as well as safety and tolerance in a clinical
phase I trial of the combination of VEGFR2-169 and gemcitabine have
been reported for patients with advanced pancreatic cancer
(32). Gemcitabine, a
chemotherapeutic agent specific for pancreatic cancer, is reported
to reduce the quantity of myeloid-derived suppressor cells and
possibly augmented CTL-mediated antitumor immune responses in
vivo (33–35). Moreover, a randomized clinical
trial of cancer vaccine (ALVAC-CEA/B7.1 vaccine) combined with
fluorouracil, leucovorin, and irinotecan showed that
vaccine-mediated immunity was not affected by chemotherapy
(36). Thus, some chemotherapeutic
reagents do not seem to inhibit, but rather augment, the antitumor
immune responses when combined with immunotherapy, although
suitable dose and treatment schedules should be carefully analyzed
in further studies. In this study, patients treated with the
vaccine and standard chemotherapy showed prolonged median TTP (9.6
months) and MST (14.2 months), compared with the results of the
multicenter phase III trial (SPIRITS trial) in which the median
progression-free survival (PFS) was 6 months and MST was 13 months
(10). Previous clinical trials
showed that vaccine therapy improved OS but not TTP (32,37).
The remarkable improvement of TTP in our study indicates the
additional effectiveness of peptide vaccine therapy in combination
with chemotherapy. Furthermore, patients with induced specific CTL
against VEGFR2-169 peptide showed significantly better prognosis
(OS and TTP) than those without such response, emphasizing the
beneficial effects of immunotherapy with regard to patient
prognosis.
In conclusion, VEGFR1-1084 and VEGFR2-169 vaccine
therapy combined with standard chemotherapy is promising and
warrants further clinical development of the strategy.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Narahara H, Fujitani K, Takiuchi H, et al:
Phase II study of a combination of S-1 and paclitaxel in patients
with unresectable or metastatic gastric cancer. Oncology. 74:37–41.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Iwase H, Shimada M, Tsuzuki T, et al: A
phase II multi-center study of triple therapy with paclitaxel, S-1
and cisplatin in patients with advanced gastric cancer. Oncology.
80:76–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sulkes A, Smyth J, Sessa C, et al:
Docetaxel (Taxotere) in advanced gastric cancer: results of a phase
II clinical trial. EORTC Early Clinical Trials Group. Br J Cancer.
70:380–383. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and fluorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin
Oncol. 24:4991–4997. 2006.PubMed/NCBI
|
|
6
|
Boku N, Ohtsu A, Shimada Y, et al: Phase
II study of a combination of irinotecan and cisplatin against
metastatic gastric cancer. J Clin Oncol. 17:319–323.
1999.PubMed/NCBI
|
|
7
|
Bang YJ, Van Cutsem E, Feyereislova A, et
al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|
|
8
|
Cunningham D, Starling N, Rao S, et al:
Capecitabine and oxaliplatin for advanced esophagogastric cancer. N
Engl J Med. 358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amarantidis K, Xenidis N, Chelis L, et al:
Docetaxel plus oxaliplatin in combination with capecitabine as
first-line treatment for advanced gastric cancer. Oncology.
80:359–365. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Koizumi W, Narahara H, Hara T, et al: S-1
plus cisplatin versus S-1 alone for first-line treatment of
advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet
Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hong YS, Song SY, Lee SI, et al: A phase
II trial of capecitabine in previously untreated patients with
advanced and/or metastatic gastric cancer. Ann Oncol. 15:1344–1347.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Japanese Gastric Cancer Association:
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boon T, De Plaen E, Lurquin C, et al:
Identification of tumour rejection antigens recognized by T
lymphocytes. Cancer Surv. 13:23–37. 1992.PubMed/NCBI
|
|
14
|
Rosenberg SA, Yang JC and Restifo NP:
Cancer immunotherapy: moving beyond current vaccines. Nat Med.
10:909–915. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mackensen A, Herbst B, Chen JL, et al:
Phase I study in melanoma patients of a vaccine with peptide-pulsed
dendritic cells generated in vitro from CD34(+) hematopoietic
progenitor cells. Int J Cancer. 86:385–392. 2000.PubMed/NCBI
|
|
16
|
Bender A, Karbach J, Neumann A, et al: LUD
00-009: phase 1 study of intensive course immunization with
NY-ESO-1 peptides in HLA-A2 positive patients with
NY-ESO-1-expressing cancer. Cancer Immun. 7:16–23. 2007.PubMed/NCBI
|
|
17
|
Nestle FO, Alijagic S, Gilliet M, et al:
Vaccination of melanoma patients with peptide- or tumor
lysate-pulsed dendritic cells. Nat Med. 4:328–332. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: from immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khong HT and Restifo NP: Natural selection
of tumor variants in the generation of ‘tumor escape’ phenotypes.
Nat Immunol. 3:999–1005. 2002.
|
|
20
|
Fong TA, Shawver LK, Sun L, et al: SU5416
is a potent and selective inhibitor of the vascular endothelial
growth factor receptor (Flk-1/KDR) that inhibits tyrosine kinase
catalysis, tumor vascularization, and growth of multiple tumor
types. Cancer Res. 59:99–106. 1999.
|
|
21
|
Gerber HP, Kowalski J, Sherman D, Eberhard
DA and Ferrara N: Complete inhibition of rhabdomyosarcoma xenograft
growth and neovascularization requires blockade of both tumor and
host vascular endothelial growth factor. Cancer Res. 60:6253–6258.
2000.
|
|
22
|
Hurwitz H, Fehrenbacher L, Novotny W, et
al: Bevacizumab plus irinotecan, fluorouracil, and leucovorin for
metastatic colorectal cancer. N Engl J Med. 350:2335–2342. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang Y, Ohtsu A, Van Cutsem E, et al:
AVAGAST: A randomized, double-blind, placebo-controlled, phase III
study of first-line capecitabine and cisplatin plus bevacizumab or
placebo in patients with advanced gastric cancer (AGC). ASCO Annual
Meeting. 2010
|
|
24
|
Shibuya M, Yamaguchi S, Yamane A, et al:
Nucleotide sequence and expression of a novel human receptor-type
tyrosine kinase gene (flt) closely related to the fms family.
Oncogene. 5:519–524. 1990.PubMed/NCBI
|
|
25
|
Matthews W, Jordan CT, Gavin M, Jenkins
NA, Copeland NG and Lemischka IR: A receptor tyrosine kinase cDNA
isolated from a population of enriched primitive hematopoietic
cells and exhibiting close genetic linkage to c-kit. Proc Natl Acad
Sci USA. 88:9026–9030. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wada S, Tsunoda T, Baba T, et al:
Rationale for antiangiogenic cancer therapy with vaccination using
epitope peptides derived from human vascular endothelial growth
factor receptor 2. Cancer Res. 65:4939–4946. 2005. View Article : Google Scholar
|
|
29
|
Ishizaki H, Tsunoda T, Wada S, Yamauchi M,
Shibuya M and Tahara H: Inhibition of tumor growth with
antiangiogenic cancer vaccine using epitope peptides derived from
human vascular endothelial growth factor receptor 1. Clin Cancer
Res. 12:5841–5849. 2006. View Article : Google Scholar
|
|
30
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
31
|
Sato Y, Fujiwara T, Mine T, et al:
Immunological evaluation of personalized peptide vaccination in
combination with a 5-fluorouracil derivative (TS-1) for advanced
gastric or colorectal carcinoma patients. Cancer Sci. 98:1113–1119.
2007. View Article : Google Scholar
|
|
32
|
Miyazawa M, Ohsawa R, Tsunoda T, et al:
Phase I clinical trial using peptide vaccine for human vascular
endothelial growth factor receptor 2 in combination with
gemcitabine for patients with advanced pancreatic cancer. Cancer
Sci. 101:433–439. 2010. View Article : Google Scholar
|
|
33
|
Suzuki E, Kapoor V, Jassar AS, Kaiser LR
and Albelda SM: Gemcitabine selectively eliminates splenic
Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and
enhances antitumor immune activity. Clin Cancer Res. 11:6713–6721.
2005.PubMed/NCBI
|
|
34
|
Dauer M, Herten J, Bauer C, et al:
Chemosensitization of pancreatic carcinoma cells to enhance T
cell-mediated cytotoxicity induced by tumor lysate-pulsed dendritic
cells. J Immunother. 28:332–342. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Correale P, Cusi MG, Del Vecchio MT, et
al: Dendritic cell-mediated cross-presentation of antigens derived
from colon carcinoma cells exposed to a highly cytotoxic multidrug
regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and
leucovorin, elicits a powerful human antigen-specific CTL response
with antitumor activity in vitro. J Immunol. 175:820–828. 2005.
|
|
36
|
Kaufman HL, Lenz HJ, Marshall J, et al:
Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients
with metastatic colorectal cancer. Clin Cancer Res. 14:4843–4849.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kantoff PW, Higano CS, Shore ND, et al:
Sipuleucel-T immunotherapy for castration-resistant prostate
cancer. N Engl J Med. 363:411–422. 2010. View Article : Google Scholar : PubMed/NCBI
|