Introduction
Oral squamous cell carcinoma (OSCC) is the most
common head and neck cancer which exhibits frequent lymph node
metastasis and local invasion, causing poor prognosis (1,2). The
addiction to betel, tobacco, and alcohol is found to be highly
correlated with the risk of HNSCC (3) and the studies in this area might lead
to new approaches in the prevention and treatment of this important
group of human cancers (4).
Apoptosis involves a cascade of molecular changes
such as morphology changes, chromatin condensation, and DNA
fragmentation (5,6). Abnormal regulation of apoptosis leads
to many human disorders including autoimmune disease and cancer.
Thus, understanding the mechanisms of apoptosis is an important
strategy for treatment of cancer (6,7).
Several gene products have been demonstrated to be critical in the
regulation of apoptosis (8,9). For
example, caspases are synthesized as proenzymes and become
activated by cleavage. Caspase activation is often regulated by
various cellular factors, including members of the Bcl-2. The Bcl-2
protein family is divided into two functional subfamilies:
pro-apoptotic proteins (Bax and Bid) and anti-apoptotic proteins
(Bcl-2 and Bcl-xL) (8,10). The family members translocate to
the mitochondria and mediate the membrane potential to induce
cytochrome c release. Cytosolic cytochrome c is
further involved in caspase activation. The caspase cascade is a
key pathway in apoptotic signal transduction, and can be divided
into two types of subfamilies: upstream initiator caspases
(caspase-8 and -9) and downstream effector caspases (caspase-3, -6
and -7), which directly induce the final events of apoptosis
(6,11,12).
Bufalin, a cardioactive C-24 steroid, is the major
component of the traditional Chinese medicine Chan-Su obtained from
the skin and parotid venom glands of the toad (13–15).
A previous study has shown that bufalin is applied for a treatment
of heart failure and used for a variety of biological activities,
such as blood pressure stimulation and antineoplastic activities
(14). In addition, bufalin
processed biological functions as inhibitors of
Na+/K+-ATPase and topoisomerase II, leading
to protein-linked DNA double-strand breaks (14,16).
It has been reported that the topoisomerase inhibitor, etoposide
and adriamycin are widely prescribed anticancer drugs which inhibit
cell proliferation and induce apoptosis in numerous cancer cell
lines (17,18). Our earlier study showed that
bufalin is found to inhibit cell growth at
G0/G1 phase of the cell cycle and to induce
apoptosis in a dose-dependent manner, which was associated with
both mitochondria-regulated and death receptor-initiated pathways
(19). Furthermore, bufalin has
been found to inhibit Bcl-2 and c-myc in human leukemia cells
(20) and to induce apoptosis of
human prostate cancer cells in part with Fas stimulation,
cytochrome c release and caspase activation (15). Additionally, bufalin has also been
found to induce apoptosis by upregulating the expression of Bax
(21) and suppressing orthotopic
transplantation tumor in nude mice in human hepatocellular
carcinoma cells (22). Therefore,
bufalin is thought to be a valuable anticancer drug.
There is limited information, however, on the
cellular and molecular mechanisms underlying the bufalin-induced
apoptosis in human oral cancer cells. The purpose of this study was
to define the biological and therapeutic effects of bufalin-treated
human oral cancer cells for the first time. This study was designed
to: i) evaluate the cytotoxicity effects of bufalin; ii)
characterize the effect of bufalin on the cell cycle; iii)
investigate the apoptotic effects of bufalin by analyzing the
protein expression in CAL 27 human oral cancer cells in
vitro.
Materials and methods
Chemicals and reagent
Dulbecco’s modified Eagle’s medium (DMEM),
L-glutamine, fetal bovine serum (FBS), penicillin/streptomycin and
Trypsin-EDTA were purchased from Gibco/Life Technologies (Carlsbad,
CA, USA). 4,6-diamidino-2-phenylindole dihydrochloride (DAPI),
dimethyl sulfoxide (DMSO), propidium iodide (PI), bufalin, Triton
X-100 and anti-β-actin antibody were obtained from Sigma-Aldrich
Corp. (St. Louis, MO, USA). Caspase-3 inhibitor (z-DEVD-fmk, Cat.
264155), caspase-9 inhibitor (z-LEHD-fmk, Cat. 218761), anti-p-AKT
(Ser473) (Cat. 07-310), anti-AKT (Cat. 05-591) and Immobilon
Western Chemiluminescent HRP substrate (Cat. WBKLS0500) were bought
from Merck Millipore (Billerica, MA, USA). Tdt-mediated
deoxyuridine triphosphate nick end labeling (TUNEL) assay kit
(in situ Cell Death Detection Kit, Fluorescein) was
purchased from Roche Diagnostics (Boehringer Mannheim, Mannheim,
Germany). These primary antibodies (anti-cyclin D1, anti-p-BAD,
anti-BAD, anti-Bcl-2, anti-cytochrome c, anti-Apaf-1 and
anti-AIF) and horseradish peroxidase (HRP) conjugated second
antibodies for western blot analysis were obtained from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). The primary antibodies
(anti-caspase-9 and anti-caspase-3) were obtained from Cell
Signaling Technology (Danvers, MA, USA).
Cell culture and bufalin treatment
CAL 27 (CRL-2095) cell line was purchased from the
American Type Culture Collection (Manassas, VA, USA). Cells were
grown in DMEM supplemented with 10% FBS, 100 U/ml penicillin and
100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5%
CO2 atmosphere incubator. Cells were treated with
bufalin for indicated concentrations as in each experiment.
Equivalent volume of 0.1% DMSO was used as vehicle control.
DNA construct and transfection
Constitutively active AKT was a gift from Dr Way
(Department of Biological Science and Technology, China Medical
University) and subcloned into pcDNA3 (Invitrogen/Life
Technologies). CAL 27 cells were transfected with either an empty
vector (pcDNA3), or a constitutively active AKT construct
(pcDNA3-CA-AKT) using Arresti-In transfection reagent and performed
according to the manufacturer’s instructions (GenDiscovery
Biotechnology, Taipei, Taiwan). Cells were selected in neomycin 48
post-transfection (23,24). Viable stably transfected cells
expressing constitutively active AKT were analyzed by western
blotting as described elsewhere (5,25).
Cell viability assay
The effects of bufalin on cell viability were
determined using the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,
Sigma-Aldrich Corp.) assay. Briefly, 1x104 cells per
well were seeded in 96-well culture plates. After overnight
incubation, the cells were treated with different concentrations of
bufalin (0, 50, 100, 150 or 200 nM) for 24 h. The cells were
treated with 50 μl of 5 mg/ml MTT for 4 h at 37°C and the resulting
formazan crystals were dissolved in dimethyl sulfoxide (DMSO). The
absorbance was measured by microplate spectrophotometer (Bio-Tek
Instruments Inc., Winooski, VT, USA) at 570 nm. Results were
expressed as percentage of the controls, which were arbitrarily
assigned 100% viability. Viability assays were performed in
triplicate from three independent experiments. The 50% inhibitory
concentration (IC50) of bufalin was calculated as
described previously (26,27).
Determination for cell morphology
Cells (2x105 cells/well) were maintained
in 24-well plates and then were treated with 0, 50, 100 and 200 nM
of bufalin. Cell morphological examination was determined utilizing
a phase-contrast microscope as previously described (19). Chromatin condensation was detected
using the DAPI staining method as previously described (19,28).
CAL 27 cells were incubated with 100 nM bufalin for 0, 12, 24 and
48 h. After that, cells were fixed gently by putting 70% ethanol,
stained with DAPI, and then photographed using a fluorescence
microscope.
Analysis for cell cycle progression by
flow cytometry
Cells (2x105 cells/well) in 24-well
plates were exposed to different concentrations of bufalin for 24
h. For determination of cell cycle phase and apoptosis, cells were
then collected, fixed in 70% ice-cold ethanol overnight, washed in
PBS once, and resuspended in PBS containing 40 μg/ml PI, 0.1 mg/ml
RNase A and 0.1% Triton X-100 in dark room for 30 min. Cell cycle
distribution and apoptotic nuclei were determined by flow cytometry
(BD Biosciences, FACSCalibur flow cytometer, San Jose, CA, USA).
The percentages of cells in G1, S, and G2/M
phases were analyzed using CellQuest Pro Software (BD Biosciences)
as described previously (29,30).
Assessment of apoptotic cells by TUNEL
staining
TUNEL staining was performed according to the
manufacturer’s instructions (Roche Diagnostics). Cells
(2x105 cells/ml) in 24-well plates were treated without
or with 125 nM bufalin for 24 h. Cells were harvested and
immediately incubated with terminal deoxynucleotidyl transferase
(TdT) enzyme at 37°C for 1 h. Following TUNEL staining, all samples
were washed once with PBS and resuspended in 0.5 ml of PBS
containing PI (10 μg/ml) and DNase free-RNase A (200 μg/ml). TUNEL
positive cells were analyzed by flow cytometry. The fluorescence
intensity was quantified by BD Pro CellQuest software. TUNEL assays
were performed in triplicate from three independent experiments as
described previously (19,27).
Assay for caspase-3 activity
Caspase-3 colorimetric assay kit (R&D Systems,
Inc., Minneapolis, MN, USA) was aped according to the
manufacturer’s recommendations. In brief, cells (1x107
cells/flask) in T75 flasks were incubated with 125 nM bufalin for
24 h. Cells were harvested and lysed in a cold Lysis buffer
(provided in the kit). Cell lysates (50 μg protein) were
incubated with caspase-3 specific substrate (Ac-IETD-pNA) for 1 h
at 37°C. The caspase activity was determined by measuring cleavage
of chromogenic caspase substrates (pNA) at OD405 in a
microplate spectrophotometer (BioTek Instruments Inc.) (27,31).
Western blot analysis
Cells (1x107 cells/flask) were seeded in
T75 flasks and incubated with or without 125 nM bufalin for 24 h.
Total proteins were prepared and determined as previously described
(25,28). Briefly, the protein concentration
was measured using a BCA assay kit (Pierce Chemical, Rockford, IL,
USA). Equal amounts (40 μg) of proteins were boiled for 5
min, separated by 12% SDS-PAGE, and then electro-transferred to
Immobilon-P transfer membrane PVDF. The transferred membranes were
blocked for 1 h with Tris-buffered saline/Tween-20 containing 5%
non-fat dry milk and incubated with primary antibodies at 4°C
overnight. Membranes were washed three times with Tris-buffered
saline/Tween-20 for 10 min and incubated with secondary
HRP-conjugated antibody (5,25).
Signals of the blots were detected using an enhanced
cheniluminescence (ECL) kit and developed in Kodak Bio-MAX MR film
(Eastman Kodak, Rochester, NY, USA). The band density was
quantified using National Institute of Health (NIH) ImageJ 1.45
program (28). Blots were probed
with β-actin antibody used as the loading control.
Statistical analysis
All the statistical results were expressed as the
mean ± SD of triplicate samples. Statistical analyses of data were
done using one-way ANOVA followed by Student’s t-test, and
p<0.05 was considered significant.
Results
Effects of bufalin on the proliferation
and viability of CAL 27 cells
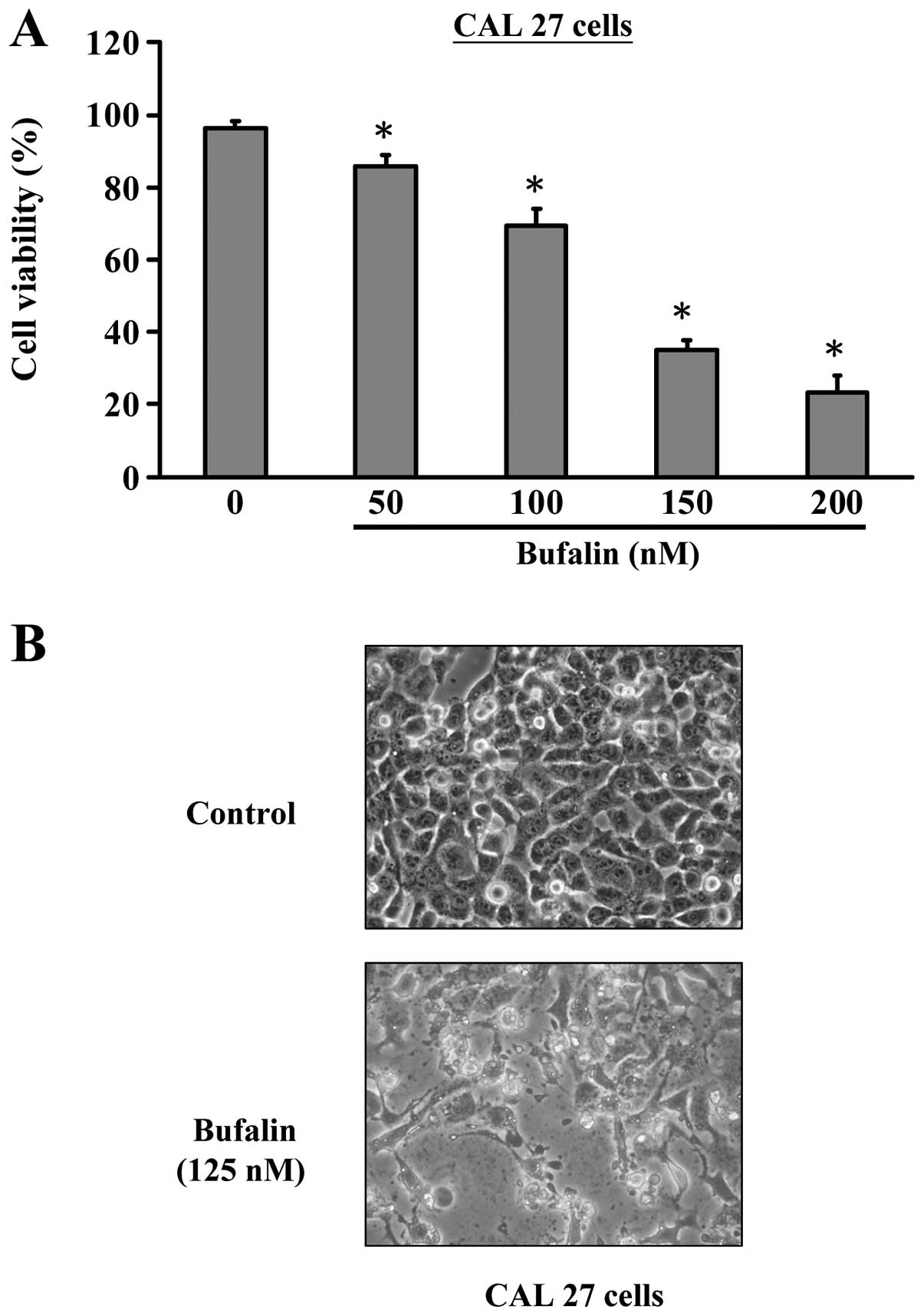
Firstly, we investigated the effects of bufalin
treatment on the growth of CAL 27 cells. As shown in Fig. 1, bufalin significantly reduced the
cell viabilities of CAL 27 cells after 24 h exposure in a
concentration-dependent manner. IC50 values were
calculated to be about 125 nM for 24 h. Thus, 125 nM was applied
for all subsequent experiments. To determine whether the observed
decrease in cell viability is associated with apoptosis, we
examined the nuclear morphology under a phase-contrast microscope.
In the control cells no significant changes were seen in cell
nuclei or cell membrane integrity, whereas CAL 27 cells treated
with 125 nM of bufalin for 24 h showed various extent of cell
shrinkage, volume reduction, apoptotic body formation and cell
blebbing (Fig. 1B). These results
suggest that bufalin exhibited a significant apoptosis-inducing
effect on CAL 27 cells.
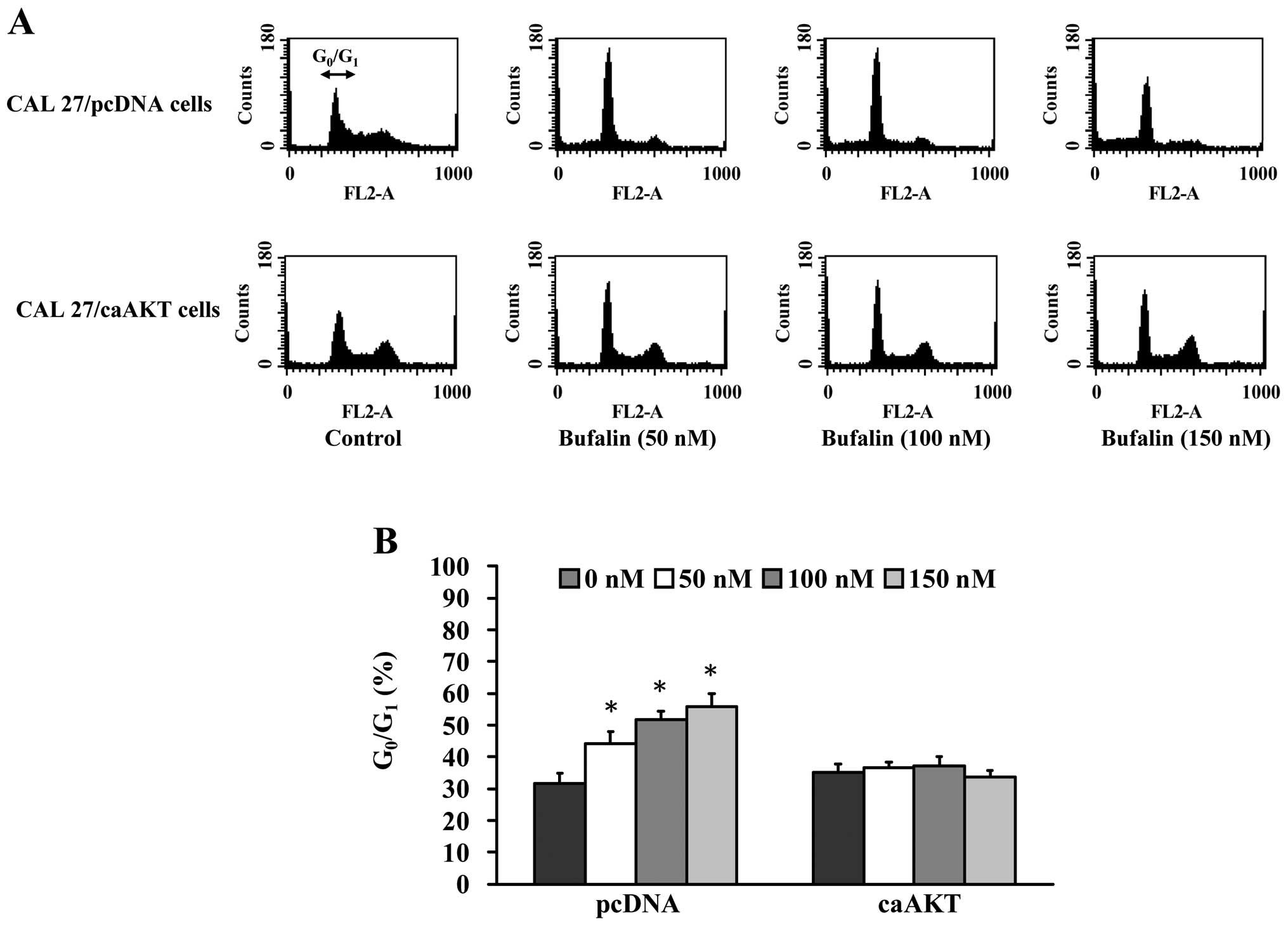
Cell cycle analysis of CAL 27 cells after
exposure to bufalin
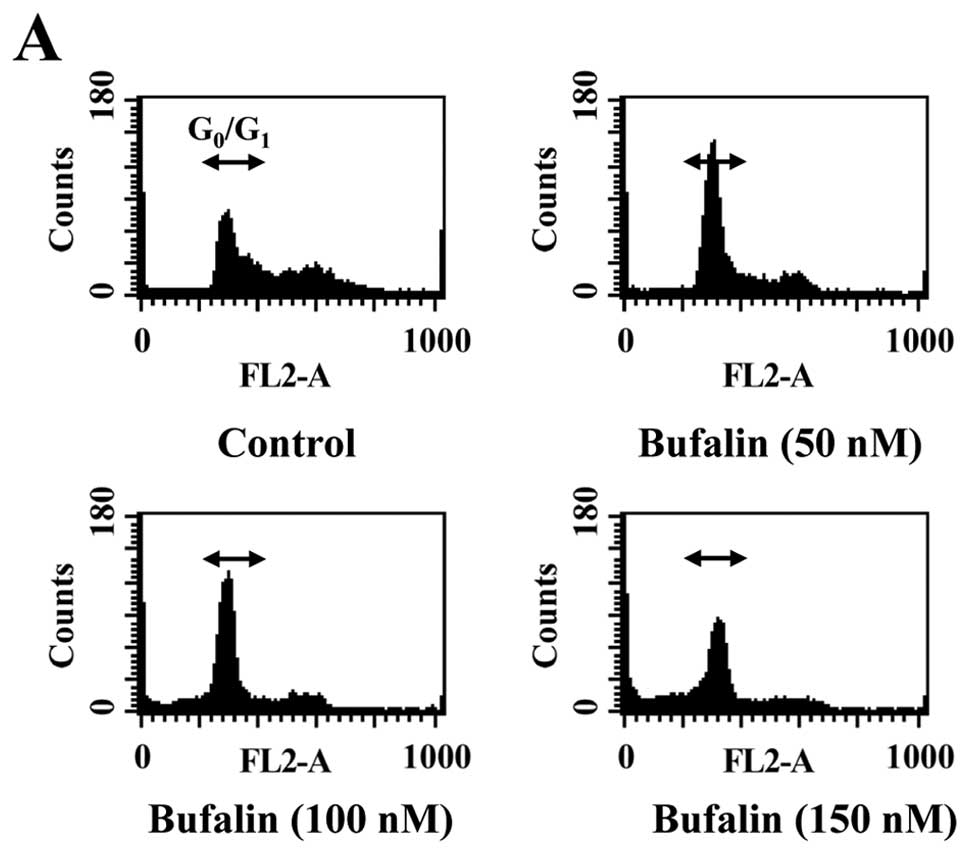
To elucidate whether growth inhibition by bufalin is
associated with apoptosis, we determined apoptotic features by
measurement of the amount of cells in the sub-G1 phase
in CAL 27 cells in vitro. The results from flow cytometric
assay using PI staining revealed that treatment with bufalin
resulted in increased accumulation of G0/G1
phase in CAL 27 cells and this effect is dose-dependent (Fig. 2A and B). Also, we found that
bufalin increased sub-G1 population (apoptosis) in CAL
27 cells (Fig. 2A). These results
suggest that bufalin inhibited proliferation of CAL 27 cells
through G0/G1 phase arrest and induction of
apoptotic cell death.
Apoptotic feathers in bufalin-treated CAL
27 cells
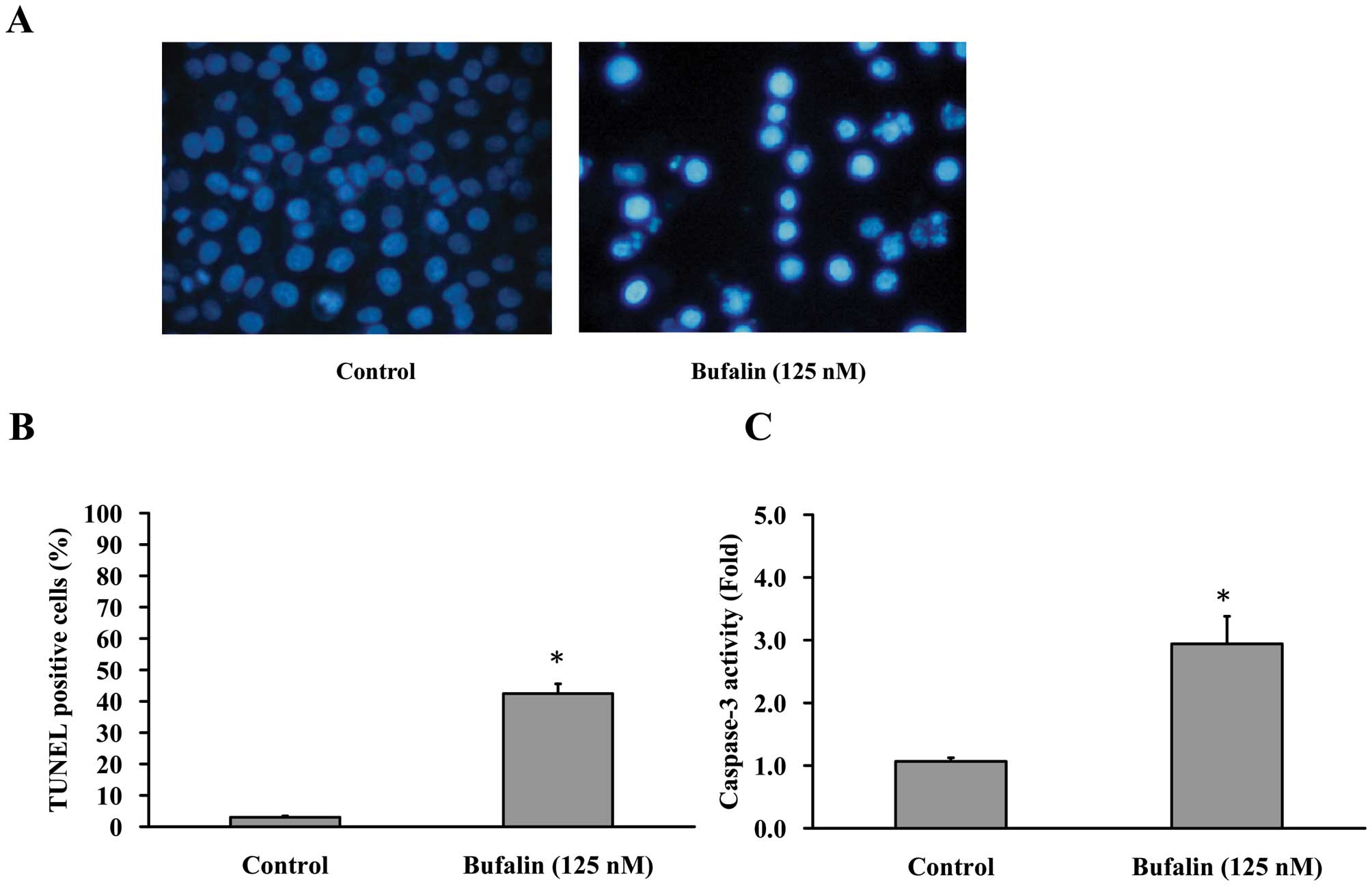
Cells were treated with 125 nM bufalin for 24 h,
then stained with DAPI and analyzed under a fluorescence microscope
(Fig. 3A). In addition, the
average percentage of apoptotic cells (TUNEL positive cells)
increased from 5% of the control to 40% by DAPI/TUNEL double
staining (Fig. 3B). In the present
study, we investigated the possible mechanisms of bufalin-induced
apoptosis in CAL 27 cells. Since caspases are known to play a
pivotal role in mediating various apoptotic signaling (8,10),
we measured the activity of effector caspase (caspase-3) in
bufalin-treated cells. Fig. 3C
shows that exposure of CAL 27 cells to 125 nM of bufalin led to
increased levels of activated caspase-3. Taken together, we
concluded that 125 nM bufalin decreased the percentage of viable
CAL 27 cells through apoptotic cell death. Moreover, caspases are
central regulators of the apoptotic pathway.
Bufalin inhibits the growth of CAL 27
cells via the AKT signaling pathway
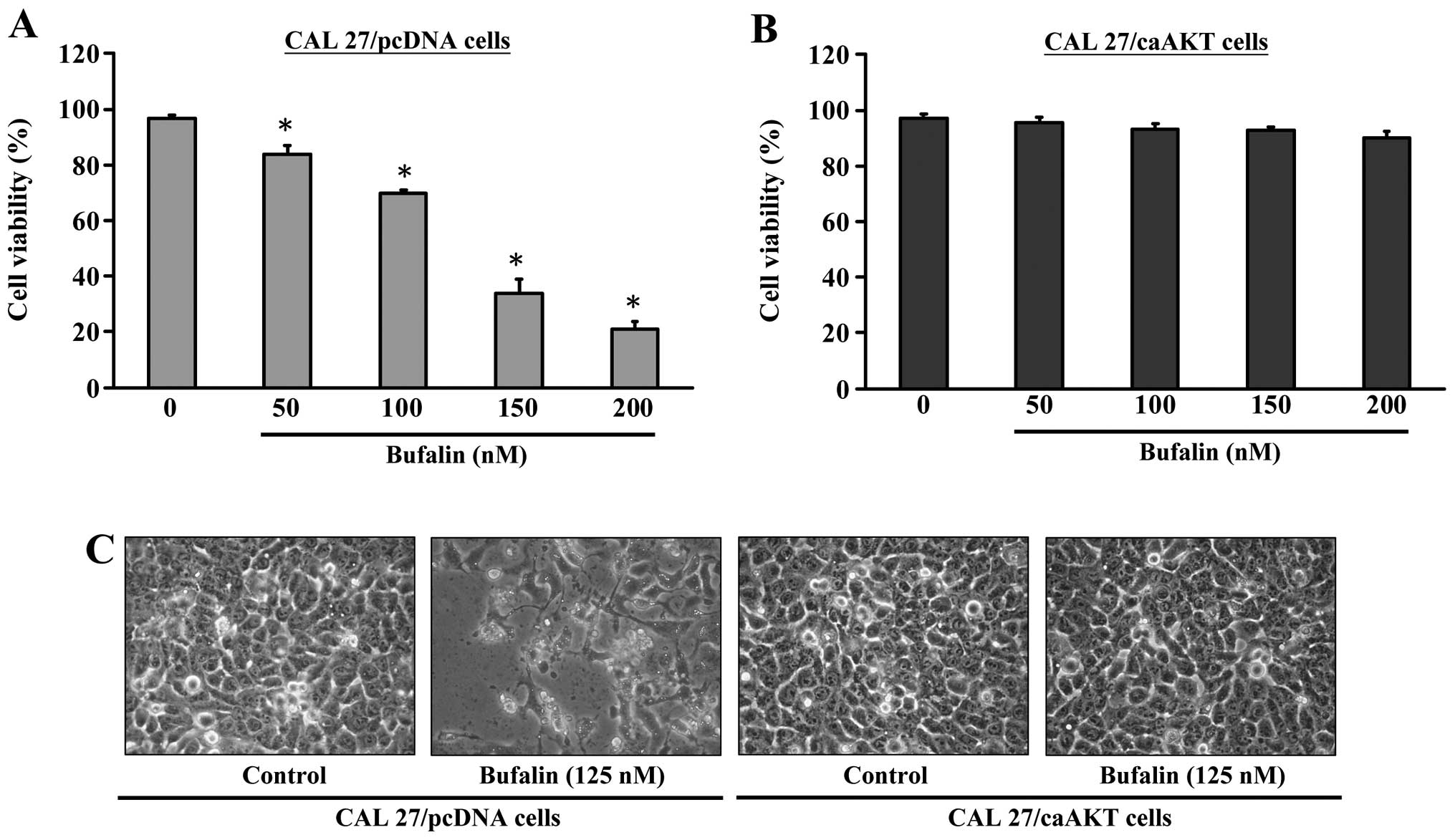
We investigated the effects of bufalin treatment on
the growth of CAL 27/pcDNA or CAL 27/CA-AKT cells. Results in
Fig. 4A indicated that CAL
27/pcDNA cells were inhibited in growth and viability was reduced
by bufalin in a concentration-dependent manner. To further
determine if the observed decrease in cell viability is associated
with apoptosis, we investigated the nuclear morphological changes
CAL 27/pcDNA cells. Fig. 4C
demonstrates that the control cells were not significantly changed
in cell nuclei and cell membrane integrity, whereas bufalin-treated
CAL 27/pcDNA cells showed various extent of chromatin condensation,
nuclear fragmentation and destruction of cell membrane integrity
after a 24-h incubation particularly with 125 nM bufalin. Typical
apoptotic nuclei were observed as early as 24 h in CAL 27/ pcDNA
cells after treatment with bufalin. Characteristically
morphological changes of apoptosis were also observed under a
microscope, including cell shrinkage, volume reduction, chromatin
condensation, cell blebbing and formation of membrane embedded
apoptotic bodies (Fig. 4C).
Strikingly, bufalin had minimal apoptotic effects (alteration of
cell viability) on CAL 27/CA-AKT cells (Fig. 4B and C).
Bufalin causes cell cycle arrest at
G0/G1 phase in CAL 27/ pcDNA but not in CAL
27/CA-AKT cells
To elucidate whether growth inhibition by bufalin is
associated with apoptosis via the AKT signaling pathway, we
explored the amount of cells in the G0/G1
phase in CAL 27/pcDNA and CAL 27/CA-AKT cells. The results from
flow cytometric assay using PI staining revealed that treatment
with 50–150 nM bufalin resulted in increased accumulation of
G0/G1 phase in CAL 27/pcDNA cells and this
effect is dose-dependent (Fig. 5A and
B). We also observed the sub-G1 population in CAL
27/pcDNA cells (Fig. 5A). In
contrast, bufalin did not significantly affect the cell cycle
arrest and had minimal apoptotic effects on CAL 27/ CA-AKT
(Fig. 5A and B). Based on these
observations, we propose that bufalin-induced
G0/G1 phase arrest and apoptotic death in CAL
27 cells is carried out through the AKT signaling pathway.
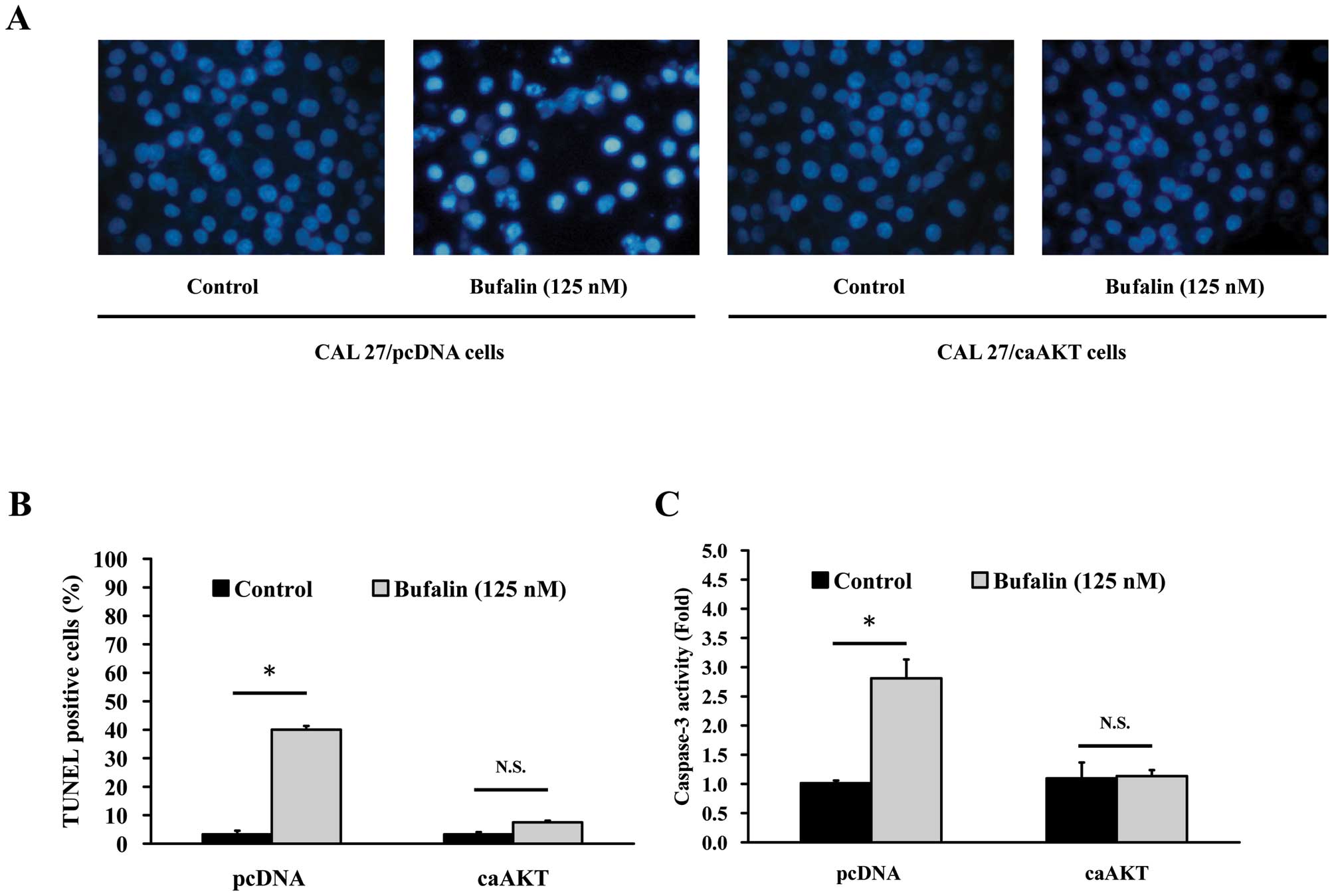
Bufalin triggers apoptosis in CAL
27/pcDNA but not in CAL 27/CA-AKT cells
To confirm whether AKT expression-modulated
bufalin-induced apoptosis in CAL 27 cells, CAL 27/pcDNA and CAL
27/CA-AKT cells were used to investigate DAPI staining, TUNEL assay
and caspase-3 activity. Cells were treated with 125 nM bufalin for
24 h and then stained with DAPI. Data in Fig. 6A show that bufalin stimulated
chromatin condensation in CAL 27/pcDNA but no effect was found in
CAL 27/pcDNA cells. In addition, the average percentage of
apoptotic cells (TUNEL positive cells) increased from 5% of the
control to 40% (Fig. 6B). In the
present study, we investigated the possible mechanisms of
bufalin-induced apoptosis in CAL 27 cells and found that AKT
signaling might be involved in this event. Caspase-3 is known to
play a pivotal role in mediating various apoptotic signaling
(8,10), and then we measured the activity of
effector caspase-3 in bufalin-treated cells. As illustrated in
Fig. 6C, exposure of CAL 27 cells
to 125 nM bufalin led to increased caspase-3 activity, but no
significant effect occurred in CAL 27/CA-AKT cells after bufalin
incubation.
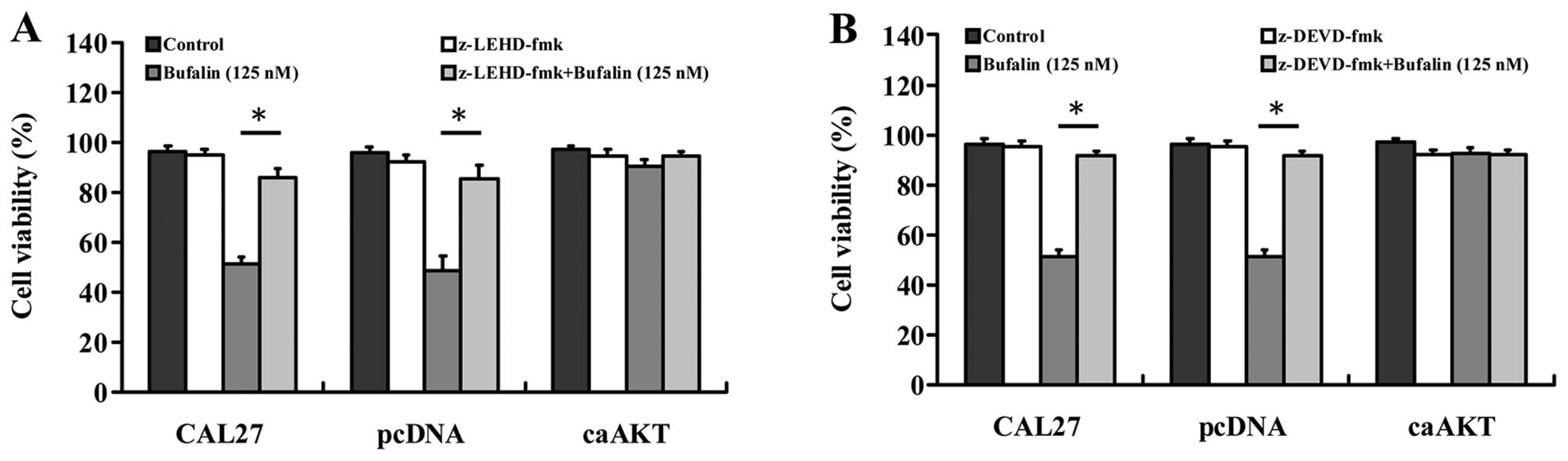
Effects of caspase-9 and caspase-3
inhibitors on bufalin-induced apoptosis in CAL 27, CAL 27/pcDNA and
CAL 27/CA-AKT cells
To further investigate the involvement of intrinsic
caspase signals in bufalin-induced apoptosis, both of z-DEVD-fmk (a
caspase-9 inhibitor) and z-LEHD-fmk (a caspase-9 inhibitor) blocked
intracellular apoptotic proteases and attenuated bufalin-reduced
viability and caused cell death in CAL 27 and CAL 27/pcDNA cells
(Fig. 7A and B). Importantly,
cells overexpressing CA-AKT had minimal effect on bufalin-induced
cell death as can be seen in Fig. 7A
and B. These results suggest that bufalin-induced apoptosis in
CAL 27 cells was associated with the mitochondria-mediated
caspase-9 and caspase-3-dependent pathway.
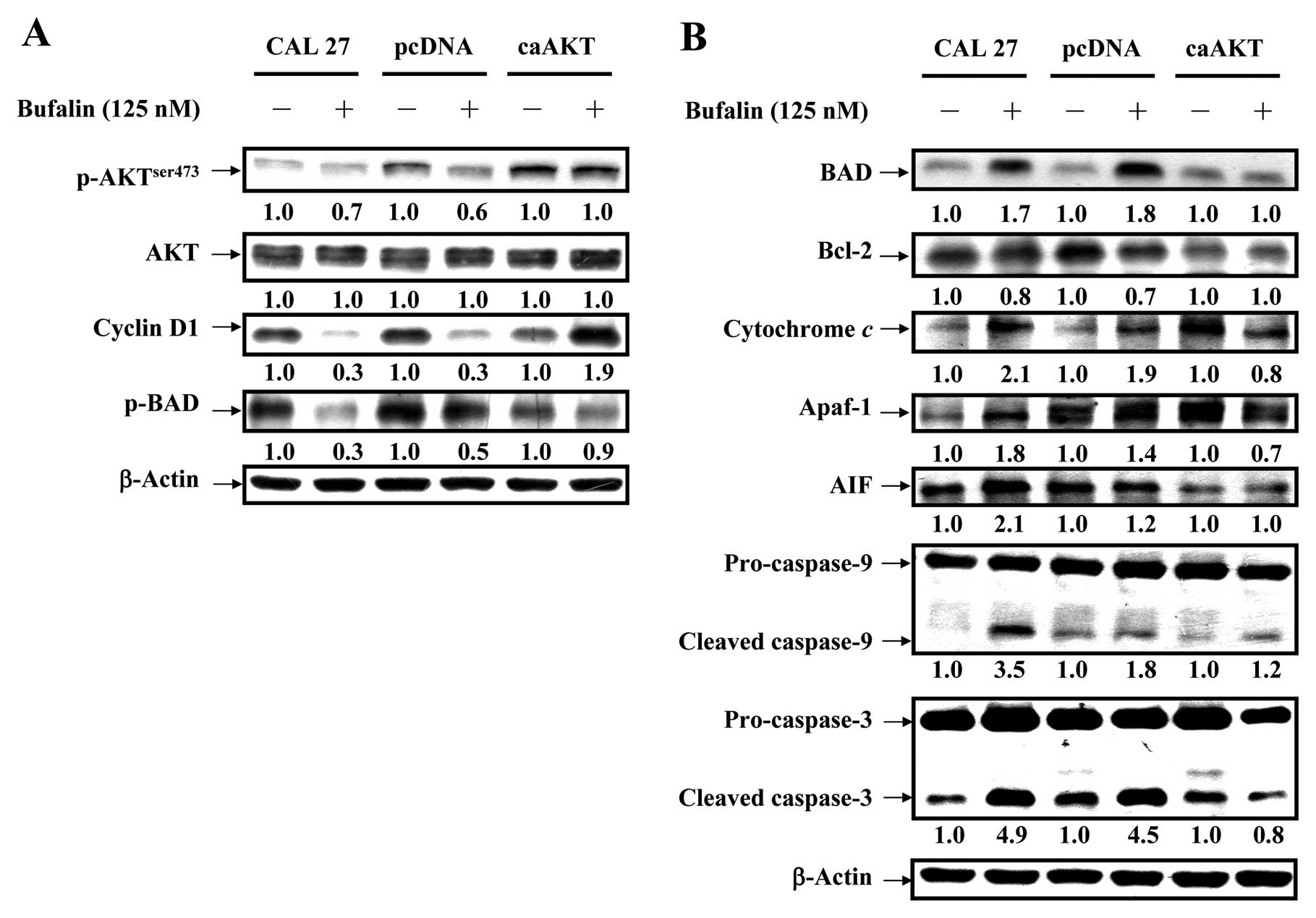
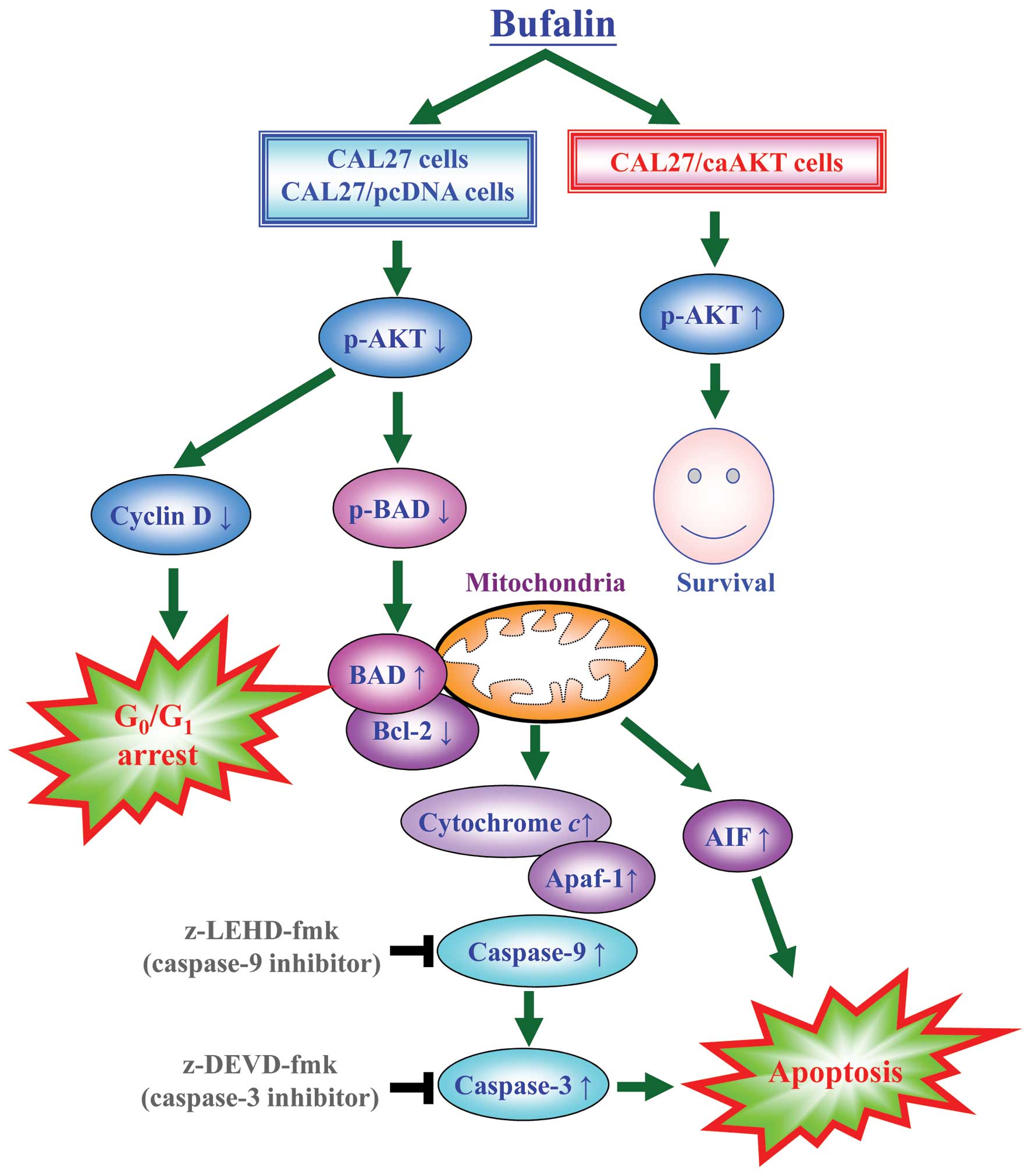
Bufalin activates the mitochondrial
apoptotic pathway involving the regulation of Bcl-2 family members
through AKT signaling in CAL 27 cells
To confirm if AKT signal was overexpressed in CAL 27
cells, our data (Fig. 8A) showed
that bufalin down-regulated level of AKT only when phosphorylated
at threonine 308 but no alteration was observed in AKT expression
in treated cells. Phosphorylated serine/ threonine protein kinase
AKT has been reported to modulate Bad phosphorylation at Ser136
(p-Bad) and cyclin D1 (32,33).
We found that the decreased expression of cyclin D1 and p-Bad
occurred in CAL 27 and pcDNA/CAL 27 cells after exposure to
bufalin. However, no dramatic effect on the levels of cyclin D1 and
p-Bad was observed in caAKT/CAL 27 cells (Fig. 8A). Our results demonstrated the
downstream of AKT signaling (cyclin D1 and p-BAD) was decreased in
bufalin-treated CAL 27 cells. Upon apoptotic signals, pro-apoptotic
Bcl-2 members, such as BAD, was activated; in contrast, Bcl-2 can
prevent this occurrence. The imbalance of expression of pro- and
anti-apoptotic proteins is associated with the ultimate fate of
cells (33,34). To assess whether mitochondrial
pathway is involved in bufalin-induced apoptosis, we evaluated the
expression levels of BAD and Bcl-2 by western blot analysis. As
shown in Fig. 8B, BAD protein
levels increased, whereas Bcl-2 protein levels decreased after
exposure of CAL 27 cells to 125 nM bufalin for 24 h. Thus, bufalin
treatment increased the ratio of BAD/Bcl-2, which is in favor of
the occurrence of apoptosis and leads to the release of cytochrome
c from mitochondria. In contrast, CAL 27 cells
overexpressing constitutively active AKT (CA-AKT) had minimal
effect on bufalin-induced cell death. Once released, cytochrome
c combines with apoptotic protease activating factor-1
(Apaf-1) and procaspase-9 to form the apoptosome in the presence of
ATP, resulting in the activation of caspase-9 and caspase-3
(8,10). Next, we detected the expression of
cytochrome c, Apaf-1 and AIF in bufalin-treated cells. The
expression of cytochrome c, Apaf-1 and AIF significantly
increased after 24 h bufalin treatment (Fig. 8B). These results suggest that
bufalin treatment-induced mitochondria-dependent apoptosis is
mainly through AKT-regulated signaling (Fig. 9).
Discussion
Bufalin, a digitalis-like molecule from an animal
source, functions as a Na+-K+-ATPase
inhibitor and causes an increase in intracellular calcium in cancer
cells (13–16). Just like several antitumor drugs
including etoposide, adriamycin, and genistein, bufalin is also
known as an inhibitor of topoisomerase II (17,18).
In addition, bufalin has been shown to induce apoptosis in a
variety of human tumors, including colorectal carcinoma, melanoma,
hepatoma, breast carcinoma, and gastric carcinoma (19–22).
However, the molecular mechanisms responsible for the pro-apoptotic
effects of bufalin in human oral cancer cells remain elusive.
Apoptotic characteristics include the appearance of
the sub-G1 population and chromatin condensation and
elevated pro-apoptotic protein expression levels, the cytochrome
c release and caspase cascade activation (6,10,12).
In the current study, we provided evidence that bufalin induced
apoptosis in CAL 27 cells in a dose-dependent manner through cell
cycle arrest at G0/G1 phase (Fig. 2), enhanced chromatin condensation
by DAPI staining (Fig. 3A),
up-regulation of pro-apoptotic proteins (Fig. 8A), the cytochrome c
(Figure 8B), and intrinsic caspase
activation (Fig. 3C). Significant
apoptotic death was observed using TUNEL assay, by bufalin in CAL
27 cells (Fig. 3B). Our results
demonstrated that bufalin also serves as an apoptotic inducer in
vitro.
Caspases participate in the execution of apoptosis
(8,11). There are two major
caspase-dependent pathways. One is the death receptor induced
caspase activation pathway, which results in caspase-8 activation.
The other is the mitochondrial apoptotic pathway, dependent on the
release of cytochrome c from mitochondria to the cytosol.
Released cytochrome c binds with Apaf-1 and activates
caspase-9, which then activates caspase-3 (6,12).
Our study revealed that bufalin failed to activate the death
receptor-mediated caspase-8 pathway in CAL 27 cells (data not
shown). In contrast, we observed that the process of
bufalin-induced apoptosis is involved the activation of caspase-9
and -3, and that the treatment with specific inhibitors of
caspase-9 and -3 significantly prevented the bufalin-induced cell
apoptotic effects (Figs. 7 and
8B). Thus, our results
demonstrated that the bufalin-induced apoptosis is carried out
through the mitochondria-dependent response. Additionally,
cytochrome c-mediated apoptosis is controlled prominently by
the members of Bcl-2 family. BAD and Bcl-2 have been identified as
major regulators. BAD possesses proapoptotic ability, while Bcl-2
blocks apoptosis. Therefore, the balance between the levels of
Bcl-2 and BAD is important in determining cell survival or death
(8,10). Our finding shown in the present
study demonstrated that bufalin-treatment increased the ratio of
BAD/Bcl-2, suggesting that the increase of the ratio of BAD/Bcl-2
might be the key factor of bufalin-induced apoptosis (Fig. 8A).
Next, we investigated whether the possible mechanism
of bufalin-induced apoptosis is through activation of the AKT
signaling pathway in CAL 27 cells (Figs. 4–8). AKT is a serinethreonine kinase and
regulates cancer cell progression. It has been demonstrated that
AKT is over-activated or over-expressed in many human malignancies
(5,25). Understanding the control of the Akt
signaling pathway is potentially important for developing
therapeutic inhibitors. CAL 27 cells overexpressing constitutively
active AKT (CA-AKT) is used for evaluating bufalin-induced cell
death. Data demonstrated that CA-AKT diminished bufalin-induced
cell apoptosis. Hence, our study is the first report regarding AKT
signaling contributed to bufalin-triggered mitochondrial apoptotic
death in CAL 27 cells in vitro.
In summary, our results provided further insight
into bufalin-induced apoptosis and deepen our knowledge on the
mechanisms of anticancer activity of bufalin in CAL 27 cells.
Bufalin causes cell cycle arrest at the G0/G1
phase. The bufalin-induced apoptosis is dependent on the
mitochondria-mediated caspase activation and involvement of the
regulation of Bcl-2 and BAD (Fig.
8). An exciting finding in this study is that constitutively
active AKT had no significant effect on bufalin-induced apoptosis.
These data provide a hint toward clarification of the mechanisms of
bufalin-induced apoptosis, but it might be a long way before
unveiling the complete mechanisms underlying bufalin-induced
apoptosis in tumor cells. Moreover, other signaling components such
as FOXO3a, p27KIP1, and c-Myc might be also involved in
bufalin-induced apoptosis (35,36).
We believe that bufalin has important antitumor properties and is a
promising chemotherapeutic agent for the treatment of human oral
cancer in the future. Continued examination of AKT and other
signaling pathways will be important in further delineating the
cell death mechanisms. Work ongoing in our laboratory is addressing
these issues.
Acknowledgements
This study was supported in part by a
research grant from the National Science Council of the Republic of
China (NSC-101-2313-B-039-008) awarded to Dr Jai-Sing Yang and
partly by the grant from Cancer Research Center of Excellence,
China Medical University Hospital, Taiwan Department of Health,
(DOH101-TD-C-111-005) awarded to Dr Sheng-Chu Kuo.
References
|
1.
|
Chien MH, Ying TH, Hsieh YS, et al:
Dioscorea nipponica Makino inhibits migration and invasion
of human oral cancer HSC-3 cells by transcriptional inhibition of
matrix metalloproteinase-2 through modulation of CREB and AP-1
activity. Food Chem Toxicol. 50:558–566. 2012. View Article : Google Scholar
|
|
2.
|
Spiro RH, Alfonso AE, Farr HW and Strong
EW: Cervical node metastasis from epidermoid carcinoma of the oral
cavity and oropharynx. A critical assessment of current staging. Am
J Surg. 128:562–567. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Liu SY, Lu CL, Chiou CT, et al: Surgical
outcomes and prognostic factors of oral cancer associated with
betel quid chewing and tobacco smoking in Taiwan. Oral Oncol.
46:276–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Funk GF, Karnell LH, Robinson RA, Zhen WK,
Trask DK and Hoffman HT: Presentation, treatment, and outcome of
oral cavity cancer: a National Cancer Data Base report. Head Neck.
24:165–180. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Chung JG, Yang JS, Huang LJ, et al:
Proteomic approach to studying the cytotoxicity of YC-1 on U937
leukemia cells and antileukemia activity in orthotopic model of
leukemia mice. Proteomics. 7:3305–3317. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sanjiv K, Su TL, Suman S, et al: The novel
DNA alkylating agent BO-1090 suppresses the growth of human oral
cavity cancer in xenografted and orthotopic mouse models. Int J
Cancer. 130:1440–1450. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Sun Q, Sakaida T, Yue W, Gollin SM and Yu
J: Chemosensitization of head and neck cancer cells by PUMA. Mol
Cancer Ther. 6:3180–3188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kroemer G, Galluzzi L and Brenner C:
Mitochondrial membrane permeabilization in cell death. Physiol Rev.
87:99–163. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lai E, Teodoro T and Volchuk A:
Endoplasmic reticulum stress: Signaling the unfolded protein
response. Physiology (Bethesda). 22:193–201. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Orrenius S: Reactive oxygen species in
mitochondria-mediated cell death. Drug Metab Rev. 39:443–455. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lu CC, Yang JS, Chiang JH, et al: Novel
quinazolinone MJ-29 triggers endoplasmic reticulum stress and
intrinsic apoptosis in murine leukemia WEHI-3 cells and inhibits
leukemic mice. PLoS One. 7:e368312012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Lavrik IN, Golks A and Krammer PH:
Caspases: Pharmacological manipulation of cell death. J Clin
Invest. 115:2665–2672. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Takai N, Kira N, Ishii T, et al: Bufalin,
a traditional oriental medicine, induces apoptosis in human cancer
cells. Asian Pac J Cancer Prev. 13:399–402. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Krenn L and Kopp B: Bufadienolides from
animal and plant sources. Phytochemistry. 48:1–29. 1998.PubMed/NCBI
|
|
15.
|
Yu CH, Kan SF, Pu HF, Jea Chien E and Wang
PS: Apoptotic signaling in bufalin- and cinobufagin-treated
androgen-dependent and -independent human prostate cancer cells.
Cancer Sci. 99:2467–2476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Bagrov AY, Roukoyatkina NI, Fedorova OV,
Pinaev AG and Ukhanova MV: Digitalis-like and vasoconstrictor
effects of endogenous digoxin-like factor(s) from the venom of
Bufo marinus toad. Eur J Pharmacol. 234:165–172. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Bandele OJ and Osheroff N: The efficacy of
topoisomerase II-targeted anticancer agents reflects the
persistence of drug-induced cleavage complexes in cells.
Biochemistry. 47:11900–11908. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Baldwin EL and Osheroff N: Etoposide,
topoisomerase II and cancer. Curr Med Chem Anticancer Agents.
5:363–372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Huang WW, Yang JS, Pai SJ, et al: Bufalin
induces G(0)/G(1) phase arrest through inhibiting the levels of
cyclin D, cyclin E, CDK2 and CDK4, and triggers apoptosis via
mitochondrial signaling pathway in T24 human bladder cancer cells.
Mutat Res. 732:26–33. 2012. View Article : Google Scholar
|
|
20.
|
Masuda Y, Kawazoe N, Nakajo S, Yoshida T,
Kuroiwa Y and Nakaya K: Bufalin induces apoptosis and influences
the expression of apoptosis-related genes in human leukemia cells.
Leuk Res. 19:549–556. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Qi F, Inagaki Y, Gao B, et al: Bufalin and
cinobufagin induce apoptosis of human hepatocellular carcinoma
cells via Fas- and mitochondria-mediated pathways. Cancer Sci.
102:951–958. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Han KQ, Huang G, Gu W, Su YH, Huang XQ and
Ling CQ: Anti-tumor activities and apoptosis-regulated mechanisms
of bufalin on the orthotopic transplantation tumor model of human
hepatocellular carcinoma in nude mice. World J Gastroenterol.
13:3374–3379. 2007.
|
|
23.
|
Aoki M, Batista O, Bellacosa A, Tsichlis P
and Vogt PK: The akt kinase: Molecular determinants of
oncogenicity. Proc Natl Acad Sci USA. 95:14950–14955. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Brognard J, Clark AS, Ni Y and Dennis PA:
Akt/protein kinase B is constitutively active in non-small cell
lung cancer cells and promotes cellular survival and resistance to
chemotherapy and radiation. Cancer Res. 61:3986–3997.
2001.PubMed/NCBI
|
|
25.
|
Lu CC, Yang JS, Huang AC, et al:
Chrysophanol induces necrosis through the production of ROS and
alteration of ATP levels in J5 human liver cancer cells. Mol Nutr
Food Res. 54:967–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Wu PP, Liu KC, Huang WW, et al: Triptolide
induces apoptosis in human adrenal cancer NCI-H295 cells through a
mitochondrial-dependent pathway. Oncol Rep. 25:551–557.
2011.PubMed/NCBI
|
|
27.
|
Yang JS, Hour MJ, Huang WW, Lin KL, Kuo SC
and Chung JG: MJ-29 inhibits tubulin polymerization, induces
mitotic arrest, and triggers apoptosis via cyclin-dependent kinase
1-mediated BCL-2 phosphorylation in human leukemia U937 cells. J
Pharmacol Exp Ther. 334:477–488. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Chiang JH, Yang JS, Ma CY, et al:
Danthron, an anthraquinone derivative, induces DNA damage and
caspase cascades-mediated apoptosis in SNU-1 human gastric cancer
cells through mitochondrial permeability transition pores and
Bax-triggered pathways. Chem Res Toxicol. 24:20–29. 2011.
View Article : Google Scholar
|
|
29.
|
Wu SH, Hang LW, Yang JS, et al: Curcumin
induces apoptosis in human non-small cell lung cancer NCI-H460
cells through ER stress and caspase cascade- and
mitochondria-dependent pathways. Anticancer Res. 30:2125–2133.
2010.PubMed/NCBI
|
|
30.
|
Ji BC, Hsu WH, Yang JS, et al: Gallic acid
induces apoptosis via caspase-3 and mitochondrion-dependent
pathways in vitro and suppresses lung xenograft tumor growth in
vivo. J Agric Food Chem. 57:7596–7604. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Huang WW, Chiu YJ, Fan MJ, et al:
Kaempferol induced apoptosis via endoplasmic reticulum stress and
mitochondria-dependent pathway in human osteosarcoma U-2 OS cells.
Mol Nutr Food Res. 54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Weng LP, Brown JL and Eng C: PTEN
coordinates G(1) arrest by down-regulating cyclin d1 via its
protein phosphatase activity and up-regulating p27 via its lipid
phosphatase activity in a breast cancer model. Hum Mol Genet.
10:599–604. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kuo CT, Hsu MJ, Chen BC, et al: Denbinobin
induces apoptosis in human lung adenocarcinoma cells via akt
inactivation, bad activation, and mitochondrial dysfunction.
Toxicol Lett. 177:48–58. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Qin J, Xie LP, Zheng XY, et al: A
component of green tea, (−)-epigallocatechin-3-gallate, promotes
apoptosis in T24 human bladder cancer cells via modulation of the
PI3K/Akt pathway and Bcl-2 family proteins. Biochem Biophys Res
Commun. 354:852–857. 2007.
|
|
35.
|
Li D, Qu X, Hou K, et al: PI3K/Akt is
involved in bufalin-induced apoptosis in gastric cancer cells.
Anticancer Drugs. 20:59–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Kawazoe N, Watabe M, Masuda Y, Nakajo S
and Nakaya K: Tiam1 is involved in the regulation of
bufalin-induced apoptosis in human leukemia cells. Oncogene.
18:2413–2421. 1999. View Article : Google Scholar : PubMed/NCBI
|