Introduction
Adenoviral vectors are frequently used for gene
transfer, in spite of their shortcomings, such as increased
immunogenicity (which may occasionally prove beneficial in cancer
treatment), prevalence of pre-existing anti-Ad immunity and lack of
specific targeting. However, they also possess significant
advantages, such as efficient transgene delivery, expression in
both dividing and non-dividing cells and ease of propagation to
high titers (1–3).
Although adenoviral vectors offer these advantages,
poor tumor transduction efficiency in several types of tumor, dense
stromal tissue impeding intratumoral virus spread and
immune-mediated viral clearance, remain the key factors limiting
cancer gene therapy and virotherapy (1,4,5).
Over a number of years, extensive research has been directed toward
overcoming these limitations. The reason for the poor transduction
of adenoviral vectors is that tumor cells exhibit limited surface
expression of the adenovirus 5 (Ad5) receptors, specifically the
coxsackievirus and adenovirus receptor (CAR),
αvβ3 and αvβ5
integrins. Therefore, numerous studies have focused on the
modification of the adenoviral fiber region that binds to the
receptors of tumor cells, to facilitate an eventual efficient
infection. The structure of the fiber can be divided into three
domains: an N-terminal tail, that attaches to the penton base, a
central shaft with repeating motifs of ∼15 residues and a
C-terminal globular ‘knob’ domain, that functions as the cellular
attachment site (6). The fiber
knob has a central depression and three symmetry-related valleys,
initially considered to be the binding sites for CAR (7). The first step involving the binding
of the virus to the CAR receptor of target cells occurs via the
C-terminal knob domain of the fiber protein. The second step is the
interaction of Arg-Gly-Asp (RGD) motifs in the penton base with
αvβ3 and αvβ5
integrins, which are the secondary host cell receptors,
facilitating the internalization of virus via receptor-mediated
endocytosis. However, Ad5-mediated gene transfer is inefficient in
a number of tissues, such as endothelial (8,9),
smooth muscle (8), differentiated
airway epithelial (10) and brain
tissue (11), as well as
peripheral blood cells (12), due
to the lack of corresponding primary and/or secondary
receptors.
Several studies have been published, regarding the
direct genetic modification of adenoviral particles as a strategy
to increase the infection efficiency in specific target cells
(13–16). These studies have demonstrated that
the fiber region of adenovirus serotype 35 (Ad35), which possesses
shorter fiber proteins compared to Ad5, exhibits a different
pattern of tropism. Therefore, it has been utilized to construct a
chimeric form with the fiber region of Ad5, with the expectation of
higher transduction efficiency in cancer cells (13,17).
Since CD46 is a receptor for Ad35 that is ubiquitously expressed in
human cells (18), chimeric
Ad5/F35 fiber proteins may be expected to transduce in various
human cancer cell lines more effectively, including CAR-negative
cell lines. Several tumor cell lines, including melanoma, require
high adenovirus doses for sufficient gene expression, due to the
lower expression of CAR (19).
Therefore, the Ad5/F35 chimeric vector may be a promising candidate
for efficient gene transfer into human cancer cell lines. Toyoda
et al(20) reported that
the adenovirus with chimeric Ad5/F35 fiber proteins exhibited
enhanced transfection efficiency in human pancreatic cancer cells.
However, Yu et al(21,22)
demonstrated that the transduction efficiency of Ad5/F35 did not
directly correlate with the expression level of CD46. In addition
to the distinct cell entry pathways of Ad35, another advantage is
the ability of the vector to induce autophagy through the
CD46-Cyt-1/GOPC pathway, via the autophagosome formation complex,
Vps34/Beclin-1 (18), which
ultimately leads to viral replication and oncolysis (23).
Autophagy is a regulated process for the degradation
of cellular components, that has been well-conserved in eukaryotic
cells. Autophagy can be activated in response to various
physiological and pathological stimuli to promote cell survival, or
to act as a mode of cell death (24). Among the genes/proteins that
regulate autophagy, mTOR, which belongs to the phosphatidylinositol
kinase-related protein kinase family and has been identified as the
cellular target of rapamycin, appears to play an important role
(25). mTOR is not only involved
in a nutrient-sensitive signaling pathway and autophagy, but also
modulates apoptotic pathways (26). The modulation of mTOR activity,
however, may have pro- as well as anti-apoptotic effects, since
rapamycin, depending on the context, may exert both pro- and
anti-apoptotic effects (27,28).
To overcome the non-specific transcription of
adenoviral vectors, conditional replication of the adenovirus and
specific targeting of tumor cells have been used (2). Briefly, these efforts can be
categorized into two general approaches. The first one is deleting
the adenoviral genes that are essential for viral replication in
normal cells, but are dispensable in tumor cells (29). The second approach is selectively
limiting the expression of the adenoviral genes that are essential
for viral replication in specific types of tumors or tissues, by
using tumor- or tissue-specific promoters (29–31).
In detail, among various tumor-specific promoters, the survivin
promoter has been proven to be a promising candidate for its
application in transcriptional targeting of adenoviral vector-based
cancer gene therapy or oncolysis for melanoma (32,33).
In the present study, we evaluated the effect of
cytomegalovirus (CMV) promoter-driven oncolytic Ad5/F35 vector on
various cancer cells. Our results suggest that the oncolytic
activity induced by Ad5/F35 specifically correlates with the
survival potential of the cell types, irrespective of their
doubling time, which renders these cancer cells more sensitive to
the autophagy inducer.
Materials and methods
Cell culture
The human cancer cell lines DU145 (prostate
adenocarcinoma), MDA-MB-231 (breast adenocarcinoma), A549 (lung
adenocarcinoma), HCT116 (colon carcinoma) and A375 (skin melanoma),
were cultured in Dulbecco’s modified Eagle’s medium (DMEM) with 10%
fetal bovine serum (FBS) (HyClone, Logan, UT, USA). U373-MG,
U87-MG, U251N and U343 (human malignant glioma), Jurkat (human T
cell leukemia), SK-MEL-2 and SK-MEL-3 (human skin melanoma) cells
were cultured in RPMI-1640 with 10% FBS. HeLa (human cervical
adenocarcinoma) cells were cultured in minimal essential medium
(MEM) with 10% FBS. HEM (human epidermal melanocyte) cells were
cultured in melanocyte growth medium containing low levels of serum
and fully supplemented with growth factors and antibiotics. 293A
cells (a subclone of the 293 human embryonic kidney cell line) were
cultured in DMEM with 10% FBS (HyClone). Cells were maintained in a
37°C humidified atmosphere containing 5% CO2.
Reagents and antibodies
Anti-mTOR, anti-phospho-Akt, anti-Bcl-2,
anti-Bcl-xL, anti-LC3B antibodies and wortmannin were purchased
from Cell Signaling Technology (Beverly, MA, USA). Fluorescein
isothiocyanate (FITC)-conjugated anti-CD46, allophycocyanin
(APC)-conjugated anti-mouse IgG1 and mouse IgG fluorescence control
isotype antibodies were purchased from BD Biosciences (San Jose,
CA, USA). Anti-CAR and anti-actin antibodies, 3-methyladenine
(3-MA) and rapamycin were purchased from Santa Cruz Biotechnology
(Santa Cruz, CA, USA). All other chemicals, including pepstatin A,
E-64d and curcumin were purchased from Sigma-Aldrich (St. Louis,
MO, USA).
Construction of chimeric adenoviral
vectors
For the construction of chimeric sequences of Ad5
and Ad35 fiber regions, a synthetic gene (2374 bp) encoding Ad5 and
Ad35 fibers was cloned into XbaI/KpnI-digested
pBluescript II SK(-) and named pBSK[2374]. DNA synthesis was
carried out by Epoch Biolabs (Sugar Land, TX, USA). The synthesized
DNA sequences spanned a region from the 30470th (XbaI) to
the 33599th (KpnI) nucleotide of the human Ad5 genome
sequence, with substitution of the Ad35 fiber shaft and knob
domain. To remove the KpnI site present within the Ad35
shaft, the nucleotide sequence ggtacc was replaced with the
sequence ggcacc, without altering the amino acid sequence.
The resultant sequences were a mixture of the Ad5 fiber tail domain
and the Ad35 fiber shaft and knob domain. Following the digestion
of pBSK[2374] with XbaI/KpnI, the insert was
subcloned into XbaI/KpnI-digested pSK[5543], a
structure that has been previously described in detail (34). The resultant product was used as a
shuttle vector. This newly constructed adenovirus shuttle vector
with chimeric fibers, pSK[5543 with 5/35 fiber], was digested with
XmnI and the adenoviral vector vmdl324Bst containing the Ad5
genome lacking the E1 region (340–4640 in the nucleotide
sequence of Ad5) and the E3 region (28592–30470 in the
nucleotide sequence of Ad5) and the IX gene was linearized with
SpeI for homologous DNA recombination in the E. coli
strain, BJ5183. To generate Ad5/F35 chimeric fiber-incorporated
green fluorescent protein (GFP)-expressing adenovirus, further
homologous recombination was performed after subcloning the GFP
gene from pEGFP-N1 (AflII-blunt-BamHI) into the
pSP72-ΔE3-adenovirus death protein (ADP) Ad shuttle vector
(35), after digestion with
KpnI-blunt-BamHI for substitution of ADP with GFP.
Subsequently, the second homologous recombination was performed in
BJ5183 following digestion of the shuttle vector with XmnI
and of the adenoviral vector with Srf I. Finally, pCA14
containing the IX gene, which plays a role in efficient viral
proliferation, was linearized with XmnI for homologous DNA
recombination with the previous recombinant adenoviral vectors
following digestion with BstBI. To verify the respective
homologous recombinants, plasmid DNA was purified from an E.
coli culture grown overnight, digested with HindIII and
the digestion pattern was analyzed. The appropriate homologous
recombinant adenoviral plasmid DNA was digested with PacI
and transfected into 293A cells to generate GFP-expressing chimeric
adenoviruses.
These chimeric adenoviruses, with or without the GFP
gene, were propagated in 293A cells and amplified for purification,
according to standard methods. Titration was performed by
estimating the infectious viral particles with a standard plaque
assay kit developed by Qbiogene (Carlsbad, CA, USA) in 293A cells.
The Adeno-X Rapid Titer kit (Clontech; Mountain View, CA, USA) was
used for the titration of GFP-expressing adenovirus.
Construction of oncolytic adenoviral
vectors
For the construction of adenoviral vectors, a
synthetic gene (3484 bp) was used as a backbone construct for the
shuttle vector and the DNA was named pBSK[3484]. It consisted of
inverted terminal repeats (ITR) - packaging signal - mouse survivin
promoter-E1A-BGH polyA-E1B and 55-kDa gene cassette-Ad E1 right
region (in the order listed). The mouse survivin promoter may be
replaced with other promoter types (CMV promoter, human survivin
promoter), through digestion with KpnI and XhoI,
respectively, for the examination of promoter activity.
pcDNA3.1/Hygro was used as a template for PCR amplification of the
CMV promoter, with the sense primer,
5′-CGGGGTACCGATGTACGGGCCAGAT-3′, and the anti-sense primer,
5′-CCGCTCGAGAATTTCGATAAGCCAG-3′. Following digestion of the PCR
product of the CMV promoter with KpnI/XhoI, it was
inserted into KpnI/XhoI-digested pBSK[3484]. Human
survivin promoter was synthesized from +1 to −268 nucleotides of
the survivin promoter and then inserted into
KpnI/XhoI-digested pBSK[3484]. For the replacement of
the CMV promoter or the human survivin promoter, the E1B 55-kDa
gene cassette was deleted by EcoRI and SalI
digestion, followed by blunting, so as to provide oncolytic
activity. These various types of pSK[3484] constructs were again
digested with FspI and BamHI and ligated into
SspI and BglII-digested pCA14 for the formation of
the final E1 shuttle vector. The pCA14-[3484] shuttle vector was
linearized by XmnI digestion. The linearized pCA14-[3484]
was co-transformed into E. coli BJ5183, together with
BstBI-digested vmdl324Bst, for homologous recombination. To
verify the respective homologous recombinants, the plasmid DNA,
purified from an overnight-grown E. coli culture, was
digested with HindIII and the digestion pattern was
analyzed. The homologous recombinant adenoviral plasmid DNA was
digested with PacI and transfected into 293A cells to
generate tumor-selective, replication-competent
Ad-3484-CMV-ΔE1B-derived ΔE1B19/55 or Ad-3484-human survivin
promoter-derived ΔE1B19.
Flow cytometric analysis
Cancer cell surface receptors (CAR and CD46) were
quantified by flow cytometric analysis. After various cancer cells
were trypsinized and washed twice with ice-cold PBS, they were
separately incubated with mouse anti-CAR antibody (Santa Cruz
Biotechnology) for 1 h at 4°C. After washing twice with ice-cold
PBS, cells were incubated with APC-conjugated anti-mouse IgG
antibody for 45 min at 4°C in the dark. Subsequently, the cells
were washed twice with ice-cold PBS, incubated again with
FITC-conjugated anti-CD46 for 1 h at 4°C in the dark, then washed
twice with ice-cold PBS. Mouse IgG fluorescence control antibody
was used as the negative control. Finally, the cells were suspended
again in PBS and analyzed using a FACS Calibur flow cytometer (BD
Biosciences, Lincoln Park, NJ, USA).
Cytopathic effect (CPE) assay
To evaluate the CPE of replication-competent
adenoviruses, cells were first plated to ∼80% confluency into 24-
or 48-well plates. They were pre-treated with 3-MA (Santa Cruz
Biotechnology), wortmannin (Cell Signaling Technology), or
rapamycin (Santa Cruz Biotechnology) for 1 h and were then infected
with replication-competent adenoviruses of various multiplicities
of infection (MOI). After 8 h of infection, cells were monitored
daily under a microscope. When the infected cells exhibited cell
lysis at the lowest MOI, the remaining cells on the plate were
fixed with 4% paraformaldehyde and stained with 0.5% crystal
violet.
Protein extracts and polyacrylamide gel
electrophoresis
Cells were lysed with 1X Laemmli lysis buffer [62.5
mM Tris (pH 6.8), 2% sodium dodecyl sulfate (SDS), 10% glycerol,
0.002% bromophenol blue] and boiled for 10 min. Protein content was
measured using BCA Protein Assay reagent (Pierce, Rockford, IL,
USA). The samples were diluted with 1X lysis buffer and
β-mercaptoethanol was added to a concentration of 350 mM. Equal
amounts of protein were loaded onto 10% SDS polyacrylamide gels.
SDS-polyacrylamide gel electrophoresis (PAGE) analysis was
performed according to the procedure of Laemmli using a Hoefer gel
apparatus.
Immunoblot analysis
Proteins were separated by SDS-PAGE and
electrophoretically transferred onto polyvinylidene fluoride (PVDF)
membranes. Each PVDF membrane was blocked with 5% non-fat dry milk
in PBS-Tween-20 (0.1%, v/v) at room temperature for 1 h. The
membrane was then incubated with the primary antibody (diluted
according to the manufacturer’s instructions) for 2 h. Horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG was used as a
secondary antibody. Immunoreactive proteins were visualized by
chemiluminescence (ECL; Amersham, Arlington Heights, IL, USA).
Non-radioactive cytotoxicity assay
The oncolytic effect of adenoviruses was assessed
using the CytoTox 96® Non-Radioactive Cytotoxicity Assay
kit (Promega, Madison, WI, USA). This assay can quantitatively
measure lactate dehydrogenase (LDH) levels, a stable cytosolic
enzyme that is released upon cell lysis. After the various cancer
cells were treated with 3-MA or rapamycin for 1 h, they were
infected with two types of oncolytic adenovirus of various MOIs and
incubated for 3–7 days in 48-wells. A total of 50 μl of
supernatant from each well was transferred onto a new 96-well
flat-bottom (enzymatic assay) plate and 50 μl of
Reconstituted Substrate Mix were added to each well, followed by
incubation for 30 min at room temperature in the dark. After 30
min, 50 μl of stop solution were added to each well and the
absorbance was examined at 490 nm within 1 h, using an ELISA plate
reader. For LDH positive control, lysis solution (10X) was added to
the control cells and the lysed cells were incubated for 45 min,
after which time the plate was centrifuged at 250 x g for 4 min.
Following centrifugation, 50 μl of supernatant were
transferred to an enzymatic assay plate for LDH assay, as described
above.
Statistical analysis
Data are presented as the means ± SEM of replicate
samples, as described in the figure legends. The Student’s
t-test was used to compare two different groups. A value of
p<0.05 was considered to indicate a statistically significant
difference.
Results
Transduction efficiency of Ad5/F35 in
human cancer cell lines
The infectivity of Ad5/F35 was expected to be higher
than that of Ad5 in the cancer cell lines expressing CAR or CD46.
We first examined the transduction efficiency of GFP-expressing
Ad5/F35 in a variety of human cancer cell lines to identify the
types of cancer cells that may be suitable targets for chimeric
Ad5/F35. Two types of adenoviruses that were replication-defective
or tumor-selective and replication-competent were produced and each
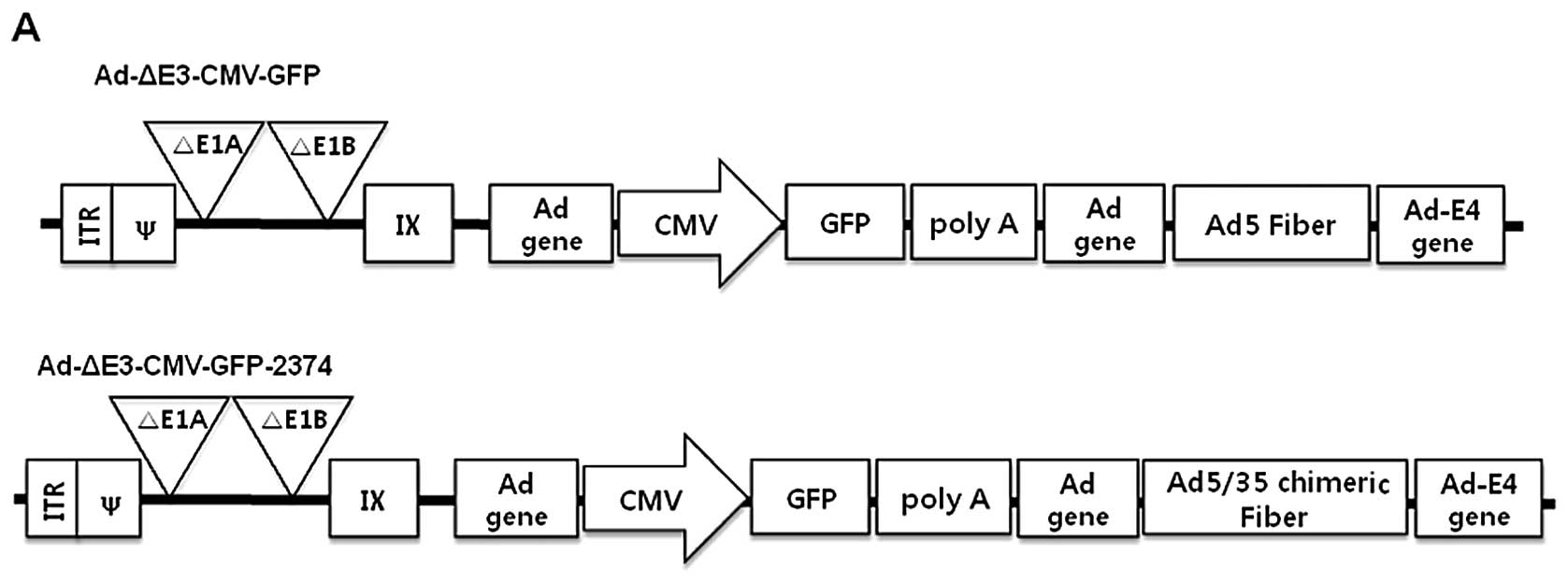
type exhibited an alteration in the fiber region. Their detailed
structures are shown in Fig. 1. In
general, melanoma cells are known to require high doses of
adenovirus for sufficient gene transfer, due to the the paucity of
CAR expression (19). However, it
is unlikely that transduction efficiency correlates with a specific
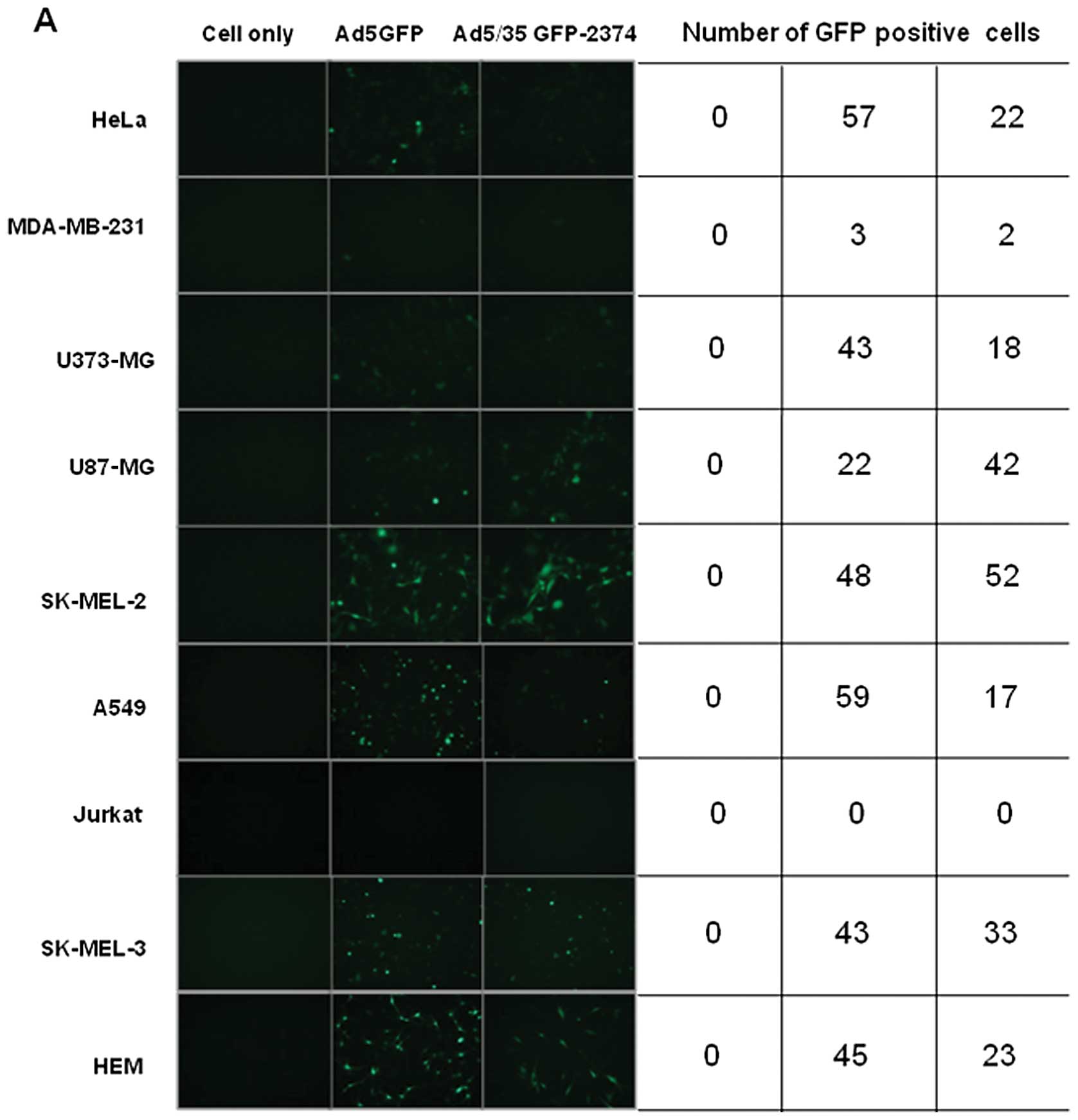
type of cancer cell. Compared to Ad5, chimeric Ad5/F35 did not
exhibit higher transduction efficiency in SK-MEL-2, SK-MEL-3 and
other cancer cell lines. To further investigate the effect of
chimeric Ad5/F35 on gene transfer efficiency in melanoma cells
(SK-MEL-2), chimeric Ad5/F35 adenovirus was infected at various
MOIs. As shown in Fig. 2B, the
transduction efficiency of Ad5/F35 at different MOIs was very
similar to that of Ad5, which was consistent with the findings of
Yu et al(21,22).
Expression levels of CAR and CD46 in
human cancer cell lines
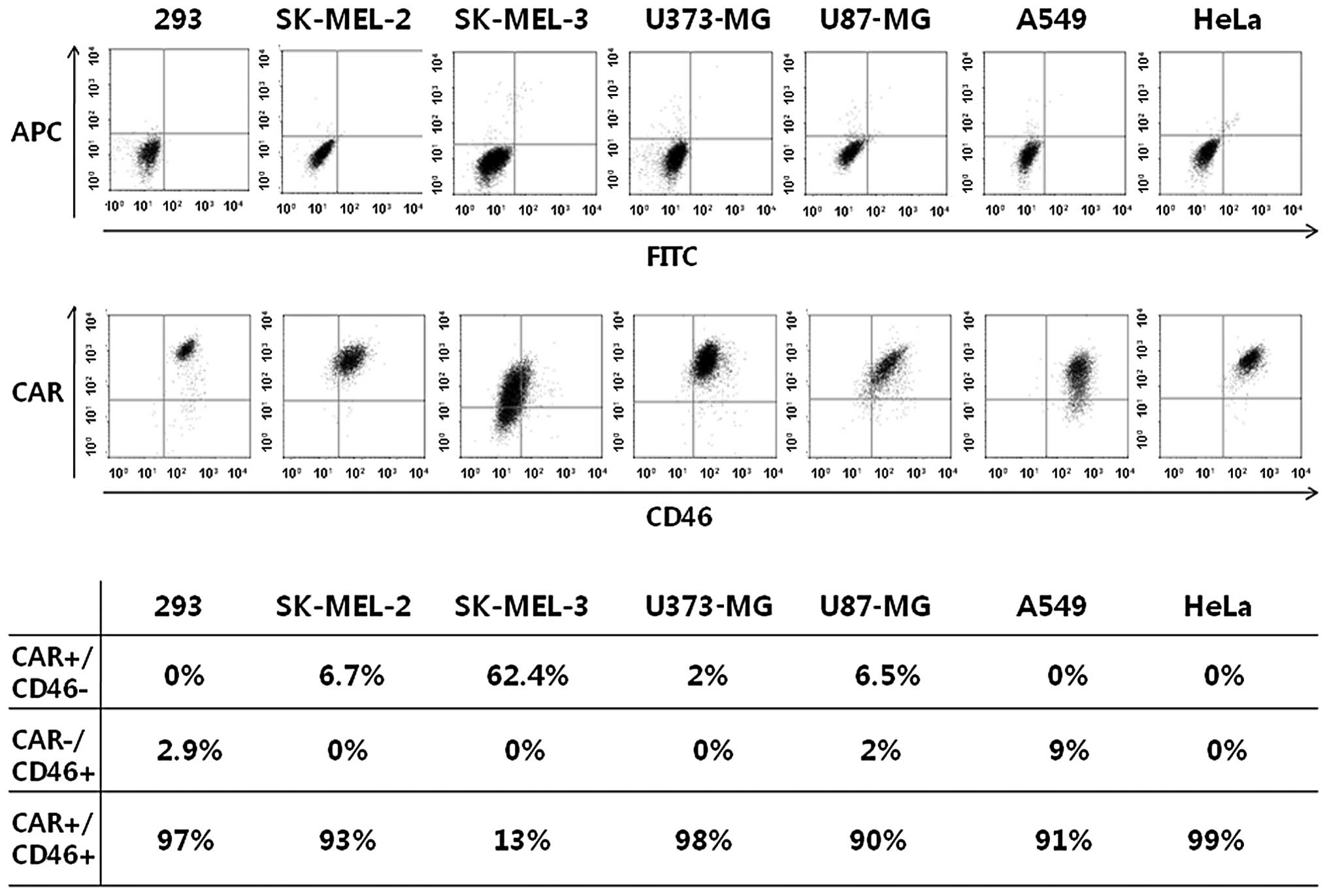
Despite our expectations, the transduction
efficiency of chimeric Ad5/F35 in most cancer cells was not
enhanced compared to that of Ad5. Therefore, we investigated
whether a correlation exists between the transduction efficiency of
Ad5 or Ad5/F35 and CAR or CD46 expression levels in the plasma
membrane of the cancer cells. We did not discover any direct
correlation between the transduction efficiency and CD46 expression
following the transfection of cancer cells with Ad5/F35. CAR and
CD46 were highly expressed in the majority of the cancer cell
lines, apart from the SK-MEL-3 cells (Fig. 3). This indicated that the
infectivity of Ad5/F35 is not modified in relation to cell surface
receptors. To confirm that the transduction efficiency of Ad5/F35
and the levels of CD46 were not the main factors responsible for
optimal gene transfer, we investigated the possibility that
autophagy facilitates a faster induction of Ad5/F35 viral particle
production compared to Ad5. According to recent studies, adenovirus
has the ability to induce autophagy (36–39);
therefore, autophagy may be used to facilitate the release of viral
progeny, promote adenovirus replication and improve oncolysis
(23,39).
Oncolytic activity induced by
tumor-selective, replication-competent adenoviruses in vitro
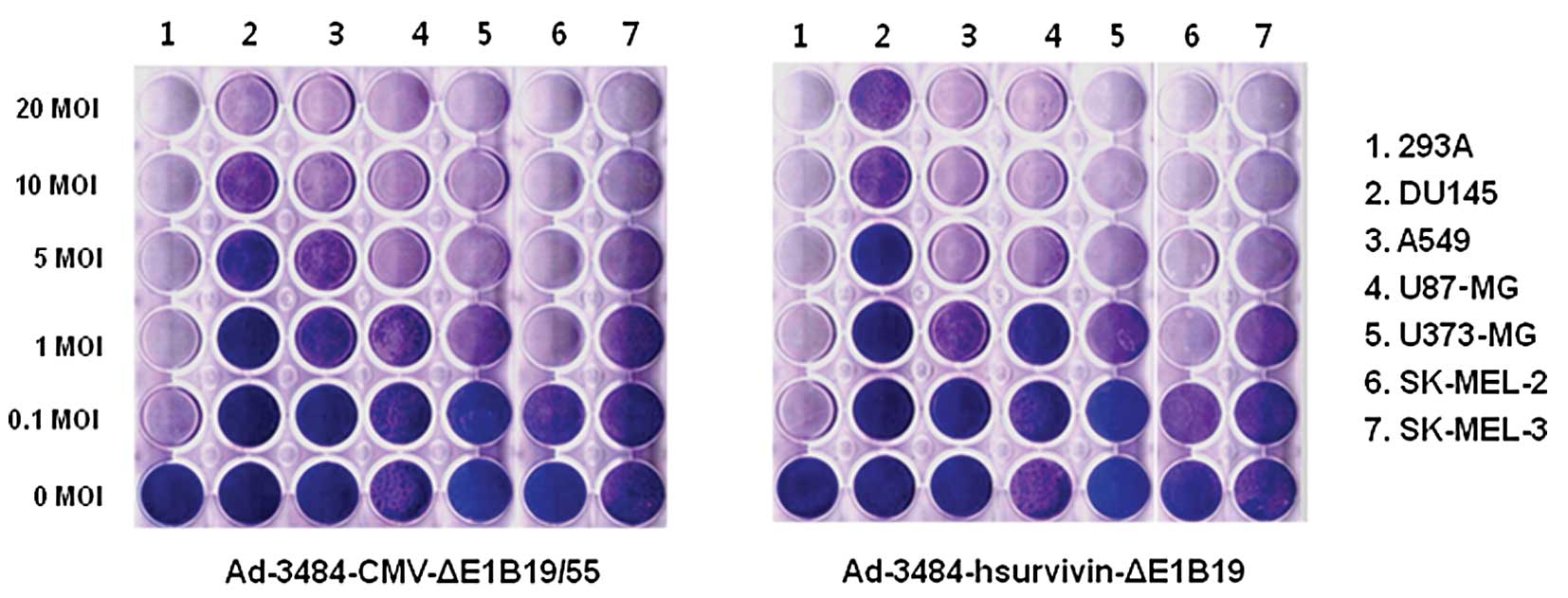
To estimate the oncolytic activity induced by
adenoviruses, various tumor cells were infected with two types of
replication-competent adenovirus (Ad-3484-CMV-ΔE1B and
Ad-3484-hsurvivin-ΔE1B19KD). For Ad-3484-CMV-ΔE1B, E1A gene
expression was regulated by the CMV promoter and the entire E1B
gene was deleted, whereas in the case of
Ad-3484-hsurvivin-ΔE1B19KD, E1A expression was regulated by the
human survivin promoter and only E1B55KD was expressed. The
oncolytic activity induced by these viruses was very similar in all
the cancer cells examined (Fig.
4), whereas no oncolytic activity was observed in normal cells
(data not shown). In this study, we also compared the oncolytic
activity induced by Ad-3484-CMV-ΔE1B with Ad5 fiber (control) with
that of Ad-3484-CMV-ΔE1B-2374 with chimeric Ad5/F35 in various
cancer cell lines (Fig. 5A). As
previously mentioned, we also examined whether the autophagic
condition is essential for oncolytic activity. For this experiment,
rapamycin was used as an autophagy inducer and 3-MA, as well as
wortmannin [phosphoinositide 3-kinase (PI3K) inhibitors], were used
as autophagy inhibitors, based on their inhibitory effect on class
III PI3K activity, which is essential for the induction of
autophagy (40). As shown in
Fig. 5A, the oncolytic activity
induced by chimeric Ad5/F35 was higher in DU145 and A549 cells
following rapamycin treatment, but not in SK-MEL-2, SK-MEL-3, A375
and HeLa cells. To verify this comparative potency of the
treatments, LDH cytotoxicity assay was performed. We discovered
that the oncolytic activity induced by chimeric Ad5/F35 was higher
in the DU145 and A549 cells following pre-treatment with rapamycin,
by comparing the survival rates of the cells treated with rapamycin
to those of the cells transfected with chimeric Ad5/F35 without
rapamycin pre-treatment (DU145, 96→61% and A549, 76→59%), at an MOI
of 10 (Fig. 5B). We then aimed to
determine the factor(s) that mainly control(s) the
autophagy-enhanced oncolytic effect. We first examined the
endogenous levels of several key molecules related to survival or
anti-apoptosis, such as mTOR, phosphorylated Akt and Bcl-2 or
Bcl-xL (Fig. 5C). As shown in
Fig. 5C, DU145, A375 and A549
cells have higher survival potentials, whereas SK-MEL-2, SK-MEL-3
and HeLa cells have lower survival potentials. We also calculated
the doubling time of each cell line in search of a correlation
between oncolytic activity and proliferation rate (Table I). Based on our overall results, we
discovered a correlation between oncolytic activity following
rapamycin pre-treatment and survival potential. Accordingly,
rapamycin-pre-treated DU145 cells exhibited the highest oncolytic
activity. To confirm this finding, several other cancer cells with
a higher survival potential were also examined using rapamycin,
3-MA and wortmannin, after narrowing down the range of MOIs. Our
data indicated that Ad-3484-CMV-Δ E1B-2374 with chimeric Ad5/F35
increased its oncolytic activity in the presence of rapamycin in
DU145, A549, SK-MEL-28 and A375 cells, whereas no increase in
oncolytic activity in the presence of rapamycin was observed in
SK-MEL-2, U251N and HCT116 cells. SK-BR3 and SK-OV3 cells exhibited
some oncolytic activity induced by these adenoviruses, irrespective
of chemical treatment; however, the oncolytic effect of the
Ad-3484-CMV-ΔE1B-2374 virus was less significant than that of the
control virus on HeLa cells (Fig.
6A). As shown in Fig. 6A, the
selective improvement in the oncolytic effect induced by
Ad-3484-CMV-ΔE1B-2374 + rapamycin was also observed in DU145, A549,
SK-MEL-28, SK-BR3 and SK-OV3 cells, as indicated by the survival
rates (DU145, 69→32%; A549, 56→36%; SK-MEL-28, 52→37%; SK-BR3,
67→45% and SK-OV3, 83→50%), at 50 MOI. As regards the latter two
cell lines (SK-BR3 and SK-OV3), the selectivity was only observed
with Ad-3484-CMV-ΔE1B-2374, irrespective of the presence of
inhibitors (Fig. 6A). Similar
patterns of oncolytic activity were also observed by LDH
cytotoxicity assay (Fig. 6B). The
survival potential of each cell line was investigated (Fig. 6C) and the doubling time was
estimated (Table II). However, as
shown in Fig. 6D, the reduced
oncolytic activity induced by Ad5/F35 in HeLa cells may be
explained by the possibility that the cellular autophagic condition
in HeLa cells is already established without an autophagy inducer,
which results in less oncolytic activity induced by Ad5/F35 than by
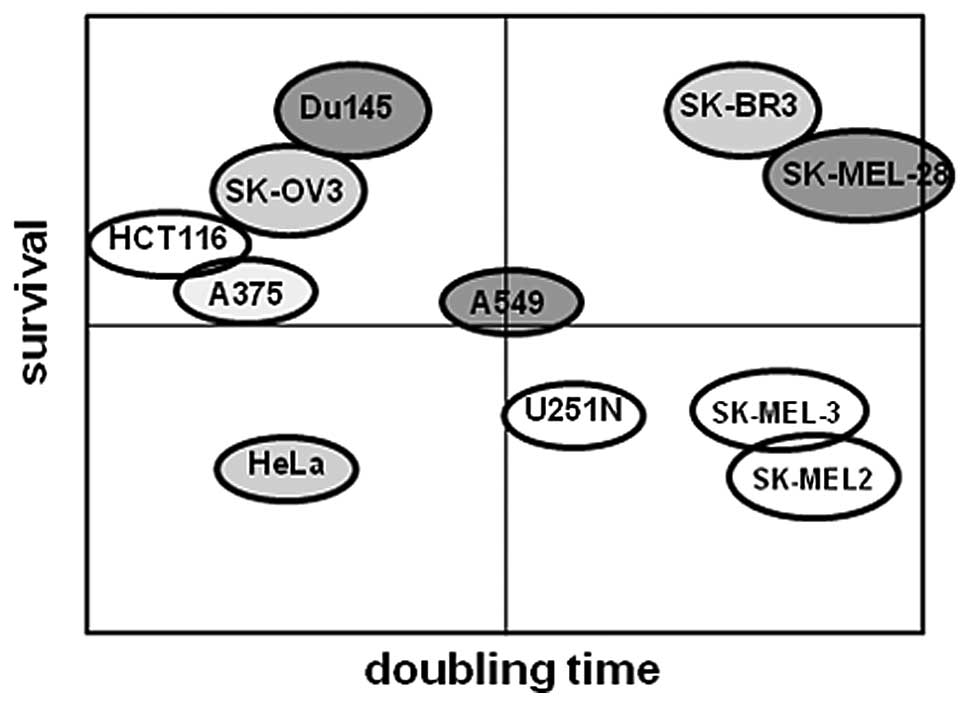
Ad5. Based on these results (Figs.
5 and 6, Tables I and II), we created a frequency diagram to
demonstrate the correlation between survival or cell proliferation
rate and oncolytic activity induced by Ad5/F35 + autophagy inducer.
As shown in Fig. 7, apart from the
HCT116 cells, the majority of cancer cells exhibited higher
survival potentials and increased oncolytic activities induced by
Ad5/F35 + autophagy inducer. However, we discovered that the cell
proliferation rate was not related to oncolytic activity induced by
Ad5/F35 + autophagy inducer. Taken together, our results suggest
that rapamycin, being an autophagy inducer, exerts a pronounced
effect on cancer cells with a higher survival potential.
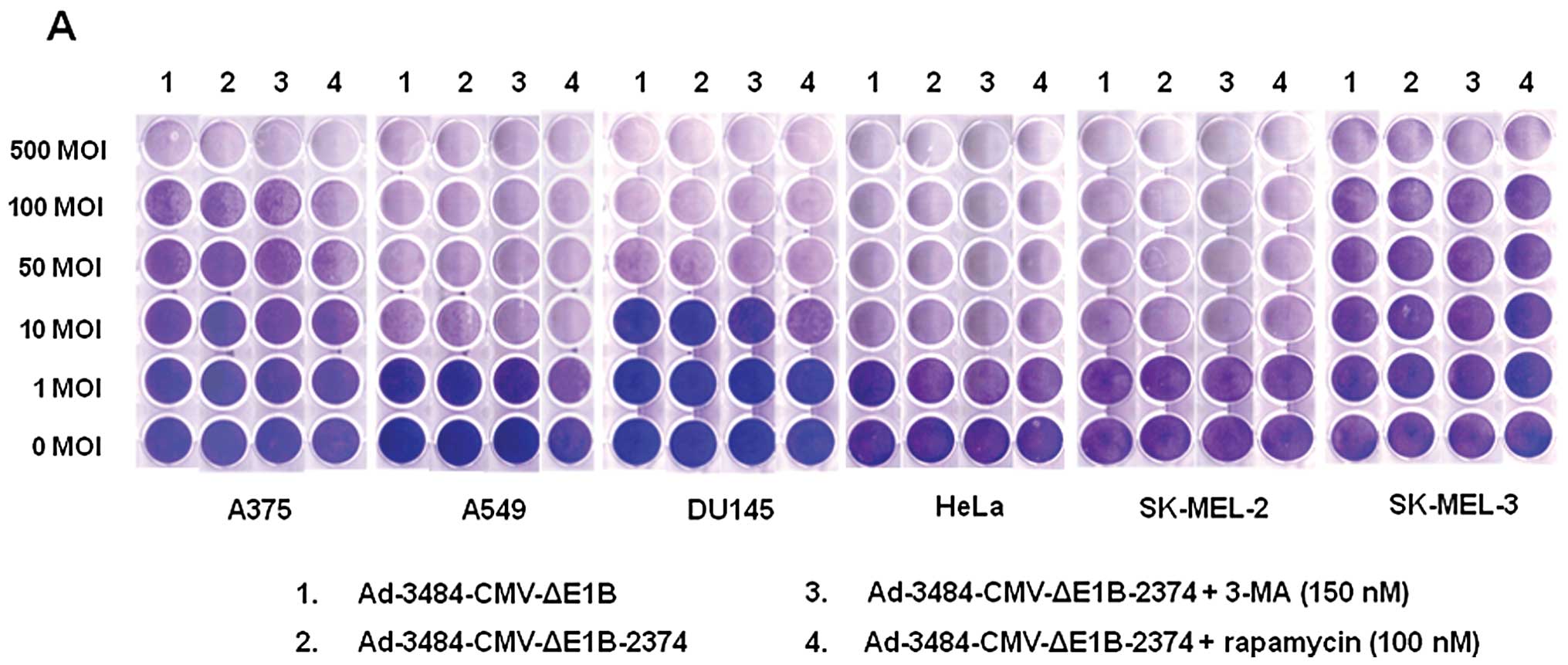 | Figure 5Comparison of the oncolytic activity
induced by Ad-3484-CMV-ΔE1B to that induced by
Ad-3484-CMV-ΔE1B-2374 and survival signals/doubling time of
examined cancer cells. (A) To compare the oncolytic activities of
Ad-3484-CMV-ΔE1B and Ad-3484-CMV-ΔE1B-2374, various cancer cells
were infected with each virus at an MOI between 1 and 500,
following pre-treatment with 3-MA (150 nM) or rapamycin (100 nM)
for 1 h. After seven days of infection (five days for HeLa and
SK-MEL-2 cells), all the cells on the plate were fixed with 4%
paraformaldehyde and stained with 0.5% crystal violet. (B) Various
cancer cells were infected with each virus mentioned in (A) at an
MOI between 1 and 1000, following pre-treatment with 3-MA (150 nM)
or rapamycin (100 nM) for 1 h. After seven days of infection (five
days for HeLa and SK-MEL-2 cells), LDH cytotoxic assay was
performed to determine the comparative potency of each treatment.
3484, Ad-3484-CMV-ΔE1B; 2374, Ad-3484-CMV-ΔE1B-2374; 2374 + 3-MA,
Ad-3484-CMV-ΔE1B-2374 + 3-MA; 2374 + rapamycin,
Ad-3484-CMV-ΔE1B-2374 + rapamycin. Error bars represent standard
errors from three separate experiments. The asterisks (*) indicate
a significant difference of 2374 + rapamycin compared to 3484
(p<0.05). (C) Survival-related signals, such as mTOR,
phosphorylated Akt, Bcl-2 and Bcl-xL, were examined in the cancer
cells shown in (A). |
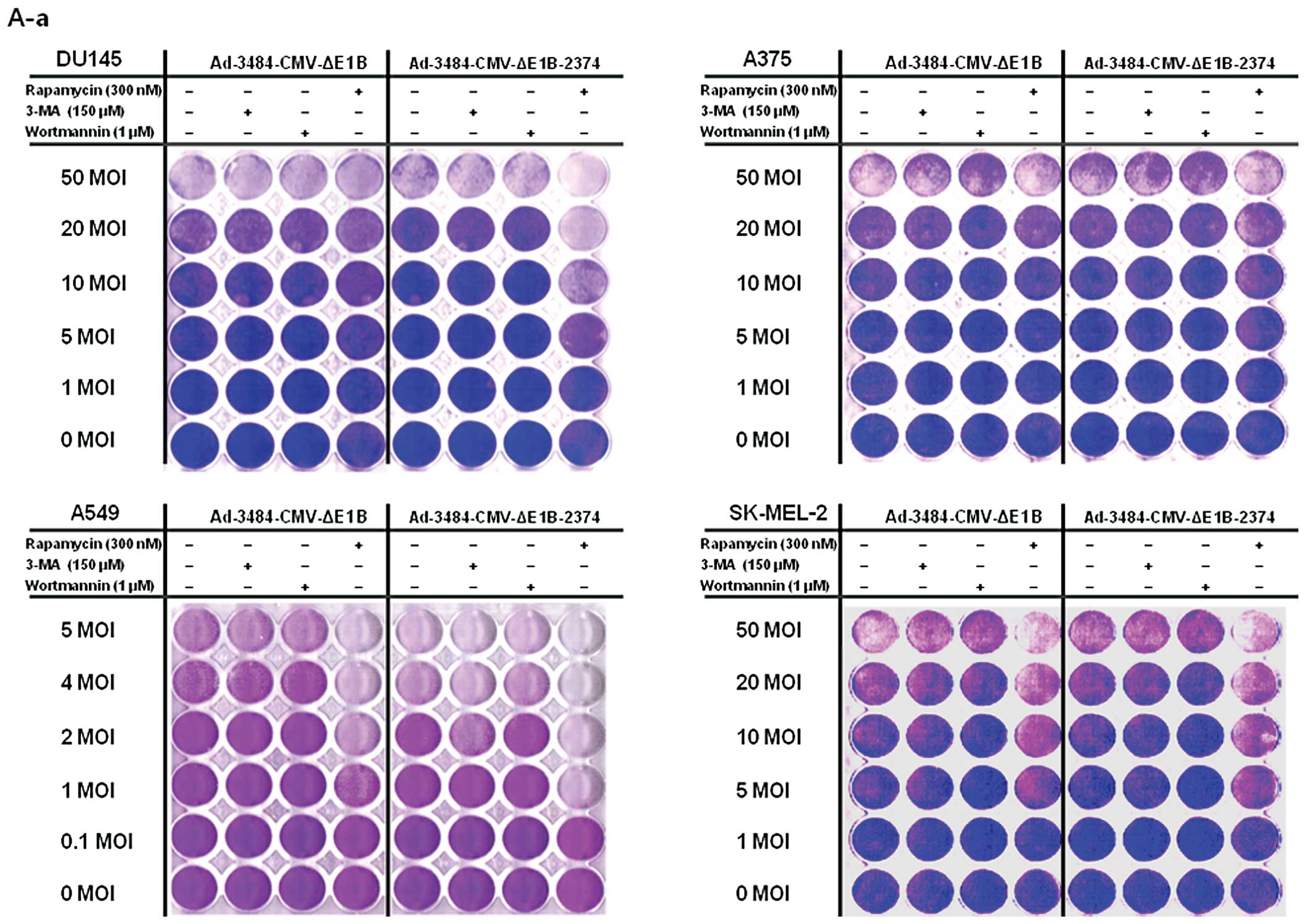 | Figure 6Comparison of oncolytic activity
induced by Ad-3484-CMV-ΔE1B with that induced by
Ad-3484-CMV-ΔE1B-2374 and survival signals/doubling time of
examined cancer cells. (A) To compare the oncolytic activity
induced by Ad-3484-CMV-ΔE1B with that of Ad-3484-CMV-ΔE1B-2374,
cancer cells were infected with each virus at an MOI between 0.1
and 50, following pre-treatment with 3-MA (150 nM) or rapamycin
(300 nM) or wortmannin (1 μM) for 1 h. After seven days of
infection (five days for HeLa and SK-MEL-2 cells), all the cells on
the plate were fixed with 4% paraformaldehyde and stained with 0.5%
crystal violet. Left column: Lane 1, Ad-3484-CMV-ΔE1B; Lane 2,
Ad-3484-CMV-ΔE1B + 3-MA (150 nM), Lane 3, Ad-3484-CMV-ΔE1B +
wortmannin (1 μM), Lane 4, Ad-3484-CMV-ΔE1B + rapamycin (300
nM). Right column: Lane 1, Ad-3484-CMV-ΔE1B-2374; Lane 2,
Ad-3484-CMV-ΔE1B-2374 + 3-MA (150 nM), Lane 3,
Ad-3484-CMV-ΔE1B-2374 + wortmannin (1 μM), Lane 4,
Ad-3484-CMV-ΔE1B-2374 + rapamycin (300 nM). (a) DU145, A375, A549
and SK-MEL-2 cells. (b) SK-BR3, SK-OV3, HeLa and SK-MEL-28 cells.
(c) HCT116 and U251N cells. (B) Various cancer cells were infected
with Ad-3484-CMV-ΔE1B or Ad-3484-CMV-ΔE1B-2374 at an MOI between
0.1 and 50, following pre-treatment with rapamycin (300 nM) for 1
h. After seven days of infection (five days for HeLa and SK-MEL-2
cells), LDH cytotoxic assay was performed to determine the
comparative potency of each treatment. 3484 + rapamycin,
Ad-3484-CMV-ΔE1B + rapamycin (300 nM); 2374 + rapamycin,
Ad-3484-CMV-ΔE1B-2374 + rapamycin (300 nM). Error bars represent
standard errors from three separate experiments. The asterisks (*)
indicate a significant difference between 2374 + rapamycin and 3484
+ rapamycin (p<0.05). (a) DU145, A375, A549, SK-MEL-2, SK-BR3
and SK-OV3 cells. (b) HeLa, SK-MEL-28, HCT116 and U251N cells. (C)
Survival-related signals, such as mTOR, phosphorylated Akt, Bcl-2
and Bcl-xL, were examined in the cancer cells shown in (A). (D)
LC3II conversion as a marker of autophagic activity was detected in
the resting state of HeLa cells by pepstatin A (20 μg/ml) +
E-64d (20 μg/ml) for 4 h. |
 | Table IDoubling times of various cancer cell
lines. |
Table I
Doubling times of various cancer cell
lines.
| Cell line | Doubling time
(h) |
|---|
| DU145 | 16.2±6.2 |
| A549 | 21.7±6.1 |
| HeLa | 12.3±1.4 |
| A375 | 10.8±2.4 |
| SK-MEL-3 | 32.0±10 |
| SK-MEL-2 | 43.6±10 |
 | Table IIDoubling times of various cancer cell
lines. |
Table II
Doubling times of various cancer cell
lines.
| Cell line | Doubling time
(h) |
|---|
| SK-BR3 | 48.9±12 |
| SK-OV3 | 11.7±0.2 |
| HCT116 | 9.85±1.6 |
| U251N | 23.8±1.1 |
| SK-MEL-28 | 58.6±4.7 |
Discussion
The aim of this study was to create a recombinant
adenovirus with a modified fiber, in order to facilitate gene
transfer in cancer cells and to overcome the poor tumor
transduction efficiency of adenovirus serotype 5 (Ad5). Therefore,
a chimeric adenovirus with an Ad5 fiber tail domain and a Ad35
fiber shaft and knob domain (Ad5/F35) was constructed, which is
known to exhibit expanded tropism (17). However, as shown in Fig. 3, the exclusive expression of CD46
was not observed in all the cancer cell lines investigated; these
results are consistent with the results shown in Fig. 2, where GFP-expressing Ad5/F35 did
not show any increase in transduction efficiency. Another possible
explanation of the relatively low transduction efficiency of
Ad5/F35 may be due to the longer stay at endosomal/lysosomal
compartments without endosomal escape for nuclear import, resulting
in less efficient Ad5/F35-mediated gene transfer compared to that
of Ad5 (41). We therefore
investigated other means of enhancing Ad5/F35 transduction
efficiency, on the basis that the CD46 receptor-promoted autophagy
would result in the promotion of viral replication and oncolysis
(23). The chimeric Ad5/F35
adenoviral vector exerted a more significant oncolytic effect on
cancer cells with a higher survival potential under autophagic
conditions generated by pre-treatment with rapamycin (an mTOR
inhibitor) as an autophagy inducer (Figs. 5 and 6). Therefore, chimeric Ad5/F35 may be
applicable in the treatment of cancer cells with a high survival
potential. However, it is possible that rapamycin may increase the
endosomal release of Ad5/F35, resulting in enhanced viral
replication. This possibility is currently under investigation.
Taken together, Ad5/F35-mediated higher gene transfer may be
achieved by the combination of an oncolytic adenovirus with an
autophagy inducer, such as rapamycin, or by incorporating genes
that induce autophagy.
Abbreviations:
|
CAR
|
coxsackievirus and adenovirus
receptor;
|
|
ITR
|
inverted terminal repeats;
|
|
GFP
|
green fluorescent protein;
|
|
FITC
|
fluorescein isothiocyanate;
|
|
APC
|
allophycocyanin;
|
|
ADP
|
adenovirus death protein;
|
|
GOPC
|
Golgi-associated PDZ and coiled-coil
motif-containing protein;
|
|
LDH
|
lactate dehydrogenase
|
Acknowledgements
This study was supported by the
Industrial Strategic Technology Development program (10035562:
Development of nucleic acid-based anticancer drugs overcoming
immunotherapy resistance) funded by the Ministry of Knowledge
Economy (MKE, Korea). This study was also supported by the Basic
Science Research Program through the National Research Foundation
of Korea (NRF) and funded by the Ministry of Education, Science and
Technology (2012-0002108; grant to J.J.S.). S.Y.K. and S.K. were
funded by the Brain Korea 21 Project for Medical Science, Yonsei
University, College of Medicine, Seoul, Republic of Korea.
References
|
1
|
Fukazawa T, Matsuoka J, Yamatsuji T, Maeda
Y, Durbin ML and Naomoto Y: Adenovirus-mediated cancer gene therapy
and virotherapy (Review). Int J Mol Med. 25:3–10. 2010.PubMed/NCBI
|
|
2
|
Sharma A, Tandon M, Bangari DS and Mittal
SK: Adenoviral vector-based strategies for cancer therapy. Curr
Drug Ther. 4:117–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douglas JT: Adenoviral vectors for gene
therapy. Mol Biotechnol. 36:71–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Russell SJ, Peng KW and Bell JC: Oncolytic
virotherapy. Nature Biotech. 30:658–670. 2012. View Article : Google Scholar
|
|
5
|
Edukulla R, Woller N, Mundt B, et al:
Antitumoral immune response by recruitment and expansion of
dendritic cells in tumors infected with telomerase-dependent
oncolytic viruses. Cancer Res. 69:1448–1458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rux JJ and Burnett RM: Adenovirus
structure. Hum Gene Ther. 15:1167–1176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burnett RM: The structure of the
adenovirus capsid. II The packing symmetry of hexon and its
implications for viral architecture. J Mol Biol. 185:125–143.
1985.PubMed/NCBI
|
|
8
|
Wickham TJ, Segal DM, Roelvink PW, et al:
Targeted adenovirus gene transfer to endothelial and smooth muscle
cells by using bispecific antibodies. J Virol. 70:6831–6838.
1996.PubMed/NCBI
|
|
9
|
Stevenson SC, Rollence M, Marshall-Neff J
and McClelland A: Selective targeting of human cells by a chimeric
adenovirus vector containing a modified fiber protein. J Virol.
71:4782–4790. 1997.PubMed/NCBI
|
|
10
|
Zabner J, Freimuth P, Puga A, Fabrega A
and Welsh MJ: Lack of high affinity fiber receptor activity
explains the resistance of ciliated airway epithelia to adenovirus
infection. J Clin Invest. 100:1144–1149. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chillon M, Bosch A, Zabner J, et al: Group
D adenoviruses infect primary central nervous system cells more
efficiently than those from group C. J Virol. 73:2537–2540.
1999.PubMed/NCBI
|
|
12
|
Tomko RP, Xu R and Philipson L: HCAR and
MCAR: the human and mouse cellular receptors for subgroup C
adenoviruses and group B coxsackieviruses. Proc Natl Acad Sci USA.
94:3352–3356. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shayakhmetov DM, Papayannopoulou T,
Stamatoyannopoulos G and Lieber A: Efficient gene transfer into
human CD34(+) cells by a retargeted adenovirus vector. J Virol.
74:2567–2583. 2000.
|
|
14
|
Wickham TJ, Tzeng E, Shears LL II, et al:
Increased in vitro and in vivo gene transfer by adenovirus vectors
containing chimeric fiber proteins. J Virol. 71:8221–8229.
1997.PubMed/NCBI
|
|
15
|
Wickham TJ, Lee GM, Titus JA, et al:
Targeted adenovirus-mediated gene delivery to T cells via CD3. J
Virol. 71:7663–7669. 1997.PubMed/NCBI
|
|
16
|
Okada Y, Okada N, Nakagawa S, et al:
Fiber-mutant technique can augment gene transduction efficacy and
anti-tumor effects against established murine melanoma by
cytokine-gene therapy using adenovirus vectors. Cancer Lett.
177:57–63. 2002. View Article : Google Scholar
|
|
17
|
Mizuguchi H and Hayakawa T: Adenovirus
vectors containing chimeric type 5 and type 35 fiber proteins
exhibit altered and expanded tropism and increase the size limit of
foreign genes. Gene. 285:69–77. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Joubert PE, Meiffren G, Grégoire IP, et
al: Autophagy induction by the pathogen receptor CD46. Cell Host
Microbe. 6:354–366. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hemmi S, Geertsen R, Mezzacasa A, Peter I
and Dummer R: The presence of human coxsackievirus and adenovirus
receptor is associated with efficient adenovirus-mediated transgene
expression in human melanoma cell cultures. Hum Gene Ther.
9:2363–2373. 1998. View Article : Google Scholar
|
|
20
|
Toyoda E, Doi R, Kami K, et al: Adenovirus
vectors with chimeric type 5 and 35 fiber proteins exhibit enhanced
transfection of human pancreatic cancer cells. Int J Oncol.
33:1141–1147. 2008.PubMed/NCBI
|
|
21
|
Yu L, Shimozato O, Li Q, et al: Adenovirus
type 5 substituted with type 11 or 35 fiber structure increases its
infectivity to human cells enabling dual gene transfer in
CD46-dependent and -independent manners. Anticancer Res.
27:2311–2316. 2007.PubMed/NCBI
|
|
22
|
Yu L, Takenobu H, Shimozato O, et al:
Increased infectivity of adenovirus type 5 bearing type 11 or type
35 fibers to human esophageal and oral carcinoma cells. Oncol Rep.
14:831–835. 2005.PubMed/NCBI
|
|
23
|
Rodriguez-Rocha H, Gomez-Gutierrez JG,
Garcia-Garcia A, et al: Adenoviruses induce autophagy to promote
virus replication and oncolysis. Virology. 416:9–15. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abounit K, Scarabelli TM and McCauley RB:
Autophagy in mammalian cells. World J Biol Chem. 3:1–6. 2012.
|
|
25
|
Noda T and Ohsumi Y: Tor, a
phosphatidylinositol kinase homologue, controls autophagy in yeast.
J Biol Chem. 273:3963–3966. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Asnaghi L, Bruno P, Priulla M and Nicolin
A: mTOR: a protein kinase switching between life and death.
Pharmacol Res. 50:545–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Calastretti A, Rancati F, Ceriani MC,
Asnaghi L, Canti G and Nicolin A: Rapamycin increases the cellular
concentration of the BCL-2 protein and exerts an anti-apoptotic
effect. Eur J Cancer. 37:2121–2128. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ravikumar B, Berger Z, Vacher C, O’Kane CJ
and Rubinsztein DC: Rapamycin pre-treatment protects against
apoptosis. Hum Mol Genet. 15:1209–1216. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Everts B and van der Poel HG:
Replication-selective oncolytic viruses in the treatment of cancer.
Cancer Gene Ther. 12:141–161. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo ZS, Thorne SH and Bartlett DL:
Oncolytic virotherapy: molecular targets in tumor-selective
replication and carrier cell-mediated delivery of oncolytic
viruses. Biochim Biophys Acta. 1785:217–231. 2008.PubMed/NCBI
|
|
31
|
Wu L, Johnson M and Sato M:
Transcriptionally targeted gene therapy to detect and treat cancer.
Trends Mol Med. 9:421–429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Diepgen TL and Mahler V: The epidemiology
of skin cancer. Br J Dermatol. 146(Suppl 61): 1–6. 2002. View Article : Google Scholar
|
|
33
|
Lu B, Makhija SK, Nettelbeck DM, et al:
Evaluation of tumor-specific promoter activities in melanoma. Gene
Ther. 12:330–338. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yun CO, Cho EA, Song JJ, et al:
dl-VSVG-LacZ, a vesicular stomatitis virus glycoprotein
epitope-incorporated adenovirus, exhibits marked enhancement in
gene transduction efficiency. Hum Gene Ther. 14:1643–1652. 2003.
View Article : Google Scholar
|
|
35
|
Yun CO, Kim E, Koo T, Kim H, Lee YS and
Kim JH: ADP-overexpressing adenovirus elicits enhanced cytopathic
effect by induction of apoptosis. Cancer Gene Ther. 12:61–71. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Baird SK, Aerts JL, Eddaoudi A, Lockley M,
Lemoine NR and McNeish IA: Oncolytic adenoviral mutants induce a
novel mode of programmed cell death in ovarian cancer. Oncogene.
27:3081–3090. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ito H, Aoki H, Kühnel F, et al: Autophagic
cell death of malignant glioma cells induced by a conditionally
replicating adenovirus. J Natl Cancer Inst. 98:625–636. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang H, Gomez-Manzano C, Aoki H, et al:
Examination of the therapeutic potential of Delta-24-RGD in brain
tumor stem cells: role of autophagic cell death. J Natl Cancer
Inst. 99:1410–1414. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang H, White EJ, Gomez-Manzano C and
Fueyo J: Adenovirus’s last trick: you say lysis, we say autophagy.
Autophagy. 4:118–120. 2008.
|
|
40
|
Wu YT, Tan HL, Shui G, et al: Dual role of
3-methyladenine in modulation of autophagy via different temporal
patterns of inhibition on class I and III phosphoinositide
3-kinase. J Biol Chem. 285:10850–10861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shayakhmetov DM, Li ZY, Ternovoi V, Gaggar
A, Gharwan H and Lieber A: The interaction between the fiber knob
domain and the cellular attachment receptor determines the
intracellular trafficking route of adenoviruses. J Virol.
77:3712–3723. 2003. View Article : Google Scholar
|