Introduction
Better understanding of the molecular mechanisms
underlying pulmonary oncogeneisis is critical for the development
of optimally therapeutic modalities (1–3).
Thus far, numerous molecules have been indentified to be implicated
in pulmonary oncogenesis. Among these molecules, the abnormalities
in cell cycle regulatory proteins are common in lung cancer
(4). The cell cycle is governed by
cyclin-dependent kinases (CDKs) whose activities are regulated
positively by cyclins, but negatively by CDK-inhibitors (CKIs)
(5,6).
Cyclin-dependent kinase 2-associated protein 1
(CDK2AP1), also named as deleted in oral cancer-1 (DOC-1), is a
growth suppressor originally isolated from normal hamster oral
keratino cytes by suppression subtractive hybridization (6–8).
Human CDK2AP1 is a highly conserved cellular gene and has been
mapped to chromosome 12q24 (8).
The human CDK2AP1 cDNA is 1.6-kilobase pairs (kb) in length,
encoding 115 amino acids with a molecular weight of 12.4 kDa (pI of
9.62) (8). CDK2 activity is
thought to play a key role in late G1 to S phase progression by
phosphorylating and inactivating the retinoblastoma (Rb) protein.
Phosphorylated and inactive Rb allows the transcription of genes
under the control of E2F, which are required for DNA replication
(5,6). Therefore, the downregulation of
CDK2AP1, an inhibitor of CDK2 and hence G1/S transition, is
expected to result in an unregulated cell cycle progression. There
is evidence to suggest that CDK2AP1 is implicated in negative
regulation of CDK2 activity by sequestering monomeric CDK2 and/or
targeting CDK2 for proteolysis (7). CDK2AP1 was shown to interact with DNA
polymerase α/primase and/or CDK2 and to mediate phosphorylation of
the large p180 subunit, suggesting a regulatory role in DNA
replication during S phase of the cell cycle. In addition, recent
studies have shown that CDK2AP1 mediates the growth suppressing
signal from TGF-β (9).
Studies have shown that CDK2AP1 gene is usually
expressed in normal human tissues. However, emerging evidence
suggests that loss or reduced expression of CDK2AP1 might
contribute to the multi-step nature of oral carcinogenesis in many
types of malignancies including oral cancer, prostate cancer,
esophageal carcinoma, gastric cancer and colorectal cancer
(9–15) and that its loss may be an event
that is associated with tumor progression. Given the significant
association of CDK2AP1 expression with tumorigenesis (7), CDK2AP1 may play a role in oncogenesis
and serves as a molecular target for cancer therapy. It is perhaps
no surprise that CDK2AP1 also plays a functional role in human
pulmonary tumorigenesis. However, to date, the specific roles of
CDK2AP1 in lung cancers have not yet been reported.
A recently developed technique that was used to
specifically down- or upregulate gene expression opens a new avenue
for cancer study. The RNA interference (RNAi) technique, a powerful
tool for carrying out loss-of-function assay, represents a novel
alternative to gene inhibition and provides a new approach for
studying cancer gene therapy (1,2).
Furthermore, another recently developed technique of ectopic
overexpression of a gene adds another indispensible dimension to
study of cancer gene therapy. Figueiredo and associates have shown
that liposome-based ectopic overexpression of CDK2AP1 in squamous
cell carcinoma VII/SF (SCC-VII/SF) significantly induced antitumor
responses in an in vivo mouse model of head and neck cancer
(16). Ectopic expression of
CDK2AP1 in hamster oral carcinoma HCPC-1 cells, which were induced
by 7,12-dimethylbenz(a)anthracene, has been associated with growth
suppression and a significant antiproliferative effects.
Additionally, transfection of CDK2AP1 into HCPC-1 cells
significantly increased apoptosis compared with untransfected
controls (17).
To study whether and how CDK2AP1 is involved in
tumorigenesis of the lung, we adopted the lentiviral
vector-mediated RNAi and upregulation system to achieve highly
stable silence and ectopic overexpression of CDK2AP1 in A549 cells.
The impacts of CDK2AP1 suppression or overexpression on
proliferation, cell cycling and chemosensitivity of A549 cells were
investigated.
Materials and methods
Animals
Male Balb/c nude mice (Shanghai SLAC Laboratory
Animal Co. Ltd., Shanghai, China) between 4- and 6-week of age were
used for the study. These mice were kept under specific pathogenic
free (SPF) conditions and 12-h light-dark cycles, with free access
to water and standard laboratory diet. Animal manipulation was
approved by the Animal Care and Use Ethics Committee of the Second
Hospital of Jilin University, China.
Lentiviral vector construction and
transfection
For CDK2AP1 expression vector construction, the
fragment of CDK2AP1 CDS was amplified and inserted into MCS of pCDH
cDNA cloning vector. Blank pCDH vector was used as a control. For
CDK2AP1 RNAi, shRNA cassette against human CDK2AP1 gene was
designed based on the CDK2AP1-specific targeting sequence
5′-CATGGCAACGTCTTCACAGTA-3′; Scrambled sequence
5′-AATGTACTGCGCGTGGAGA-3′ was used as negative control. The shRNA
sequences were synthesized, annealed and ligated into pLKO
vector.
For lentivirus packing, the re-constructed
pCDH-CDK2AP1, pCDH-Control, pLKO-siCDK2AP1 and pLKO-siControl were
co-transfected into 293T cells together with packing helper
plasmids. The lentiviral particles were then harvested from the
culture medium 96 h post-transfection and then subjected to
ultracentrifugation. The constructed lentivirus was referred as
Lv-CDK2AP1 for overexpression of CDK2AP1, or Lv-si-CDK2AP1 for
specific interfering of CDK2AP1.
Human lung adenocarcinoma cell line A549 (Cell Bank
of Chinese Academy of Sciences, Shanghai, China) was maintained in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5%
CO2. For cell infection, 40% confluent A549 cells were
incubated with si-CDK2AP1, si-control, CDK2AP1 and control
lentivirus for 96 h, respectively, with a replacement of medium 24
h after treatment. Lentivirus transduction efficiency was
determined by fluorescence microscopy.
Western blot analysis
Cells were harvested 72 h after lenti-virus
transduction and Western blot analysis was subsequently performed.
Cells were lysed by pre-cooled lysis buffer (10 mM Tris-HCl, pH
7.4, 1 mM EDTA, 0.1% Triton X-100, 0.1% SDS). The protein
concentration was analyzed by Bradford assay kit (Pierce, Rockford,
IL, USA). Thirty microgram protein extracted from cells was loaded
on 10% polyacrylamide gel and electrophorised at 30 mA for 2 h. The
resulting membrane was blocked in 5% non-fat dry milk blocking
buffer and then probed with polyclonal antibodies against the
CDK2AP1 (Santa Cruz Biotechnology, Santa Cruz, CA), GAPDH (Sigma
Chemical Co., St. Louis, MO, no. G8795), CDK4 (Cell Signaling
Technology, no. 2906), CDK7 (Cell Signaling Technology, no. 2090)
and anti-Rb (phospho S780) antibody (Abcam, ab47763) respectively
overnight at 4°C. The membrane was washed three times with
Tris-buffered saline Tween-20 (TBST), followed by incubation for 2
h with anti-mouse IgG at a 1:5000 dilution (Santa Cruz). The
membrane was developed using enhanced chemiluminescence (Amersham
Life Science, Arlington Hts, IL).
Real-time PCR analysis
Total RNAs were extracted using TRIzol reagent
(Invitrogen, Carlsbad, CA). The reverse transcription reactions
were carried out following the protocol of the M-MLV Reverse
Transcriptase (Promega Corp., Madison, WI). The primers used were
as follows: for CDK2AP1, 5′-AAG AGCAACCCACCAAACC-3′ and
5′-ATCAACTTACAATAA ACGCAGAAC-3′; for actin, 5′-GGCGGCACCACCATGTA
CCCT-3′ and 5′-AGGGGCCGGACTCGTCATACT-3′. The relative mRNA
expression of CDK2AP1 was calculated by the 2−ΔΔCt
method, using actin mRNA level for normalization. All samples were
analyzed in triplicates.
Cell proliferation assay by
methylthiazoletetrazolium
Cells were trypsinized 96 h post-lentivirus
treatment, resuspended, seeded into 96-well plate and incubated at
37°C. The number of viable cells in all groups was counted at
indicated times. At each time point, 10 μl
3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT)
(Dingguo Biotechnology) at 5 mg/ml was added into each well. The
plate was incubated for 4 h, washed and added with 100 μl of
dimethyl sulfoxide. The absorbance was measured at 490 nm. Each
experiment was repeated three times.
BrdU cell proliferation assay
The BrdU Cell Proliferation Assay kit was obtained
from Chemicon International (Temecula, CA). Cells were re-plated in
a 96-well plate at 2×104 cell/ml 96 h after lentivirus
treatment. BrdU was added in each well of the plates and incubated
for 2 h, followed by fixation and wash. The anti-BrdU monoclonal
and the goat anti-mouse IgG, peroxidase conjugate antibodies were
used successively. The plate was added with TMB peroxidase
substrate for reaction and read at dual wavelength of 490 nm.
Colony formation assay
After infection, cells in all four groups were
seeded into a 6-well plate at a concentration of 200 cells per well
and maintained at 37°C for 14 days. The culture medium was changed
every 2–3 days. At the end of incubation, the plate was washed with
PBS, fixed with paraformaldehyde, stained with Giemsa stain (Sigma)
for 10 min and washed with ddH2O, sequentially. The
stained cells were photographed with a digital camera. The number
of colonies in each well was counted and analyzed.
Flow cytometric analysis
Cells in all four groups were harvested by
centrifugation at 1,200 rpm for 5 min 48 h after infection. The
cell pellets were washed with cold PBS, fixed with 70% ethanol and
harvested after centrifugation. Then the pellets were resuspended
with PBS and filtrated through 400-mesh membrane. The cell samples
were stained with propidium iodide (PI) (Sigma)/RNase/PBS (100
μg/ml PI and 10 μg/ml RNase A) solution at 4°C for 30
min in dark. Stained samples were analyzed by a FACsCalibur II
sorter and CellQuest FACS system (BD Biosciences, San Diego, CA,
USA). The percentage of cells in each cell cycle phase was
determined. All samples were measured in triplicates.
Assessment of the effects on the
chemosensitivity to cisplatin and paclitaxel
A549 cells were seeded in a 6-well plate at a
concentration of 5×104 cells/well for 24 h and then
infected with CDK2AP1 overexpression lentivirus. After 96-h
incubation, cells were harvested and re-seeded in a 96-well plate
and cultured for 24 h. For chemosensitivity assessment, parental
cells, control or CDK2AP1-overexpressing lentivirus infected cells
were treated with cisplatin (10 μg/ml) or paclitaxel (100
μmol/ml) for 24 h, respectively and subjected to cell
viability analysis by MTT assay.
Tumorigenesis
Tumor cell inoculation into the nude mice was
performed using the modified technique previously described by us
(1,2). A549 cells (5×106) infected
with lentiviruses containing si-CDK2AP1, si-control, CDK2AP1 and
control sequences were injected subcutaneously into the back of
male nude mice (n=8 per group). The development and growth of solid
tumors were monitored by measuring tumor size at 0, 10, 17 and 24
days after inoculation. Tumor volume was calculated by the formula:
V = 0.5 × L × W2 (mm3), where V, L and W represent the
volume, length and width of tumors, respectively. The tumor volume
in each group was analyzed at 0, 10, 17 and 24 days after
inoculation. On day 24, mice were euthanized and tumors were
excised for measurement of tumor weight.
Statistical analysis
Data are expressed as the mean ± SD. Student’s
t-test was performed to evaluate inter-group differences. P<0.05
was taken as statistical significance threshold. All statistical
analyses were performed using Prism 5 (GraphPad Software Inc.).
Results
Efficacy of lentivirus-mediated RNAi and
ectopic overexpression of CDK2AP1
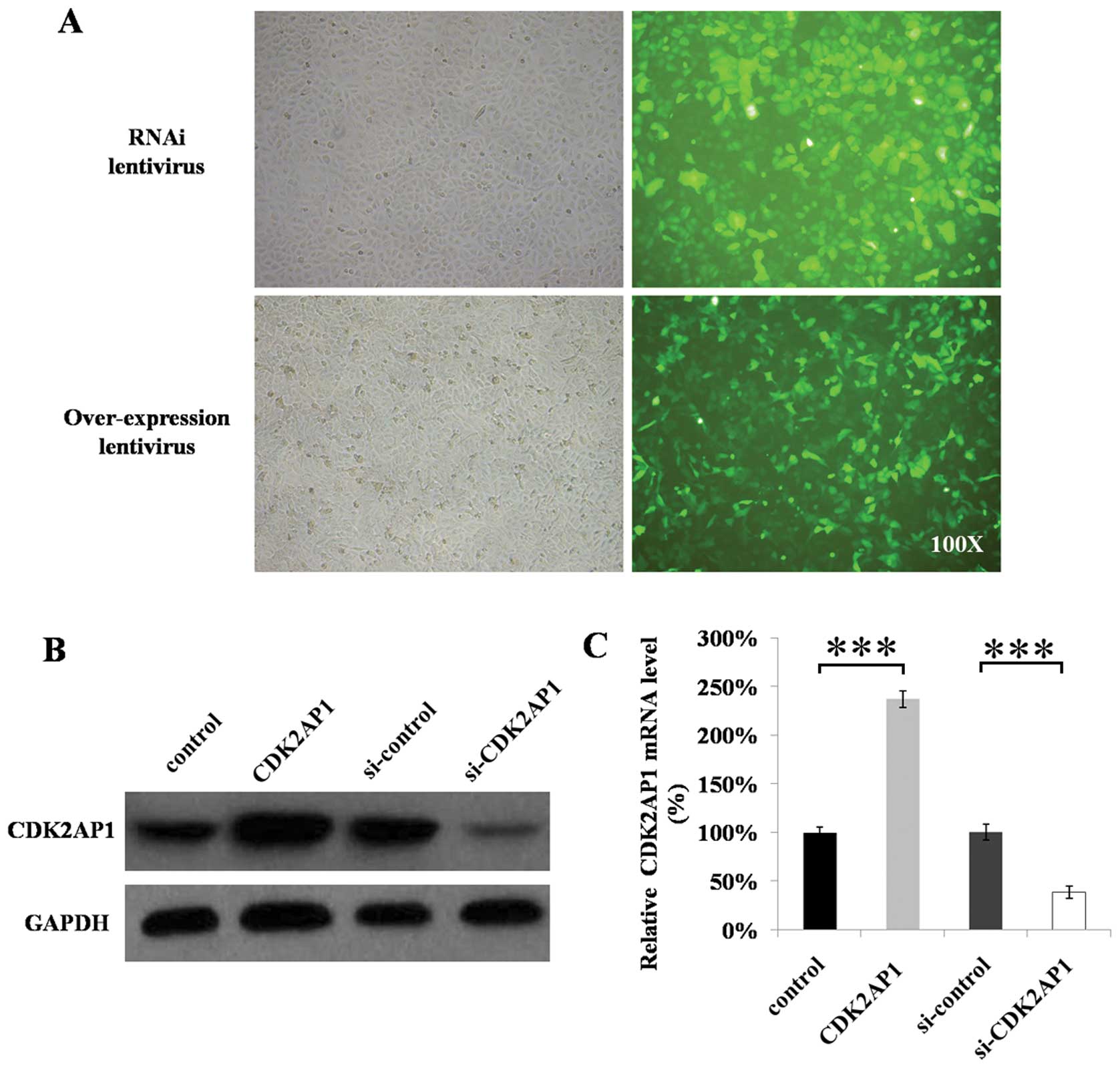
Fluorescence microscopy examination showed that
>90% cells were successfully infected as visualized by the GFP
tag in lentiviruses (Fig. 1A). The
overexpression or shRNA-containing constructs have successfully
down- or upregulated the expression levels of CDK2AP1 gene
(Fig. 1B and C). The levels of
CDK2AP1 mRNA transcripts in mock control did not significantly
differ from si-control. The levels of CDK2AP1 mRNA transcripts in
A549 cells transduced with si-CDK2AP1 lentivirus were significantly
lower than that transduced with si-control (Fig. 1B, P<0.01), whereas the levels in
A549 cells transduced with overexpression lentiviral construct were
significantly higher than that transduced with mock control
(Fig. 1B, P<0.01). Western
blotting showed that the expression levels of CDK2AP1 protein in
A549 cells transduced with si-CDK2AP1 lentivirus were significantly
lower, but significantly higher in that ransduced with
overexpression lentiviral construct than those in the controls
(Fig. 1C, P<0.01), suggesting
that the si-CDK2AP1 lentiviral contruct can effectively
downregulate CDK2AP1 gene expression at both mRNA and protein
levels in A549 cells, whereas the CDK2AP1 lentiviral construct can
effectively upregulate CDK2AP1 gene expression at both mRNA and
protein levels in A549 cells.
Impact of down- or upregulation of
CDK2AP1 on cell growth in vitro
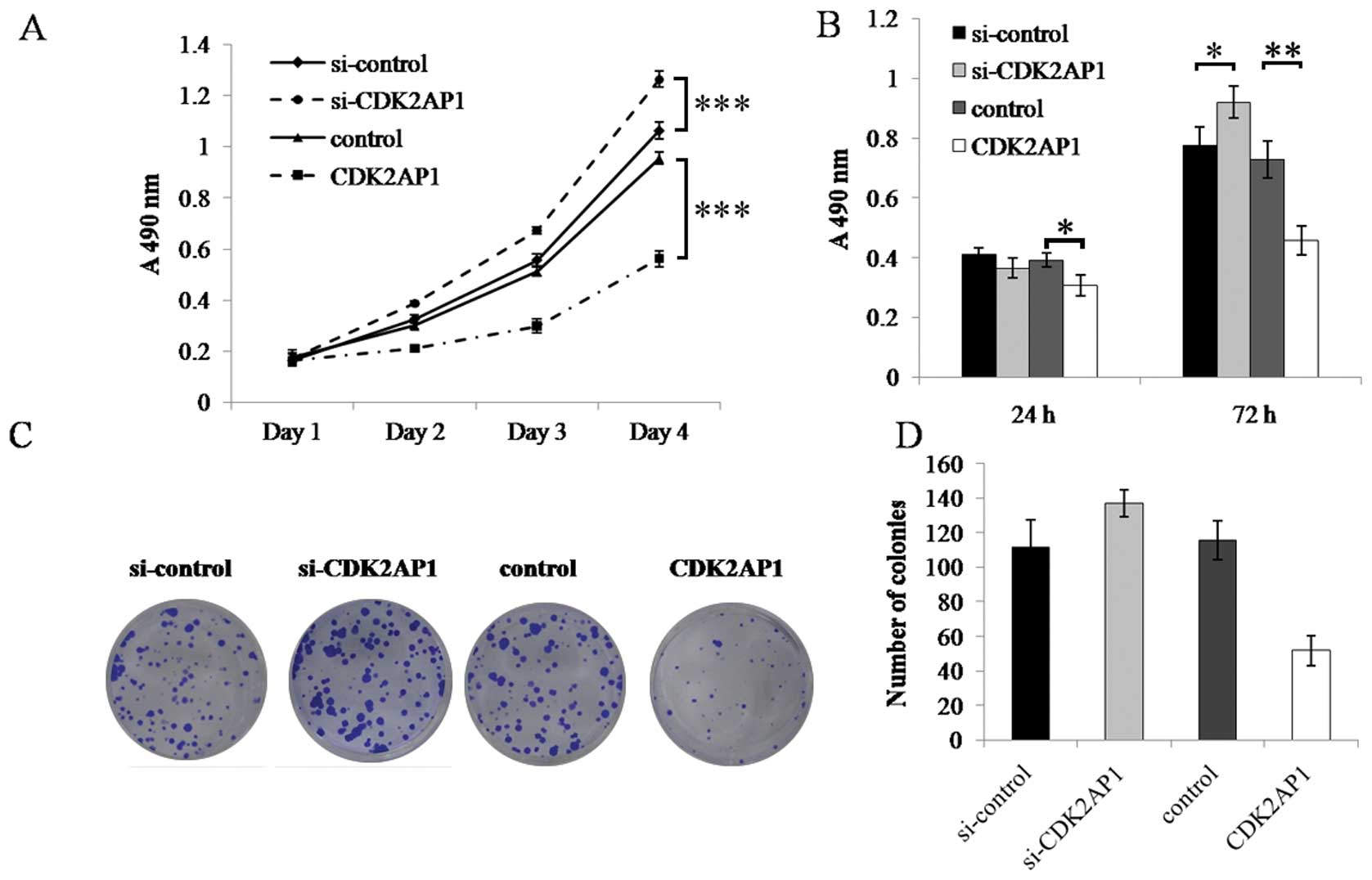
To explore the role of CDK2AP1 in the growth of A549
cells, the growth dynamics of A549 cells transduced with si-CDK2AP1
and CDK2AP1 lentiviral construct was determined by MTT and BrdU
cell proliferation assay. MTT assay showed that, during the 4-day
incubation period, the growth of control A549 cells did not
significantly differ from si-control A549 cells and both had strong
proliferation activity. However, the growth of CDK2AP1 knockdown
cells was significantly faster than that of si-control cells,
whereas that of CDK2AP1 overexpression cells was significantly
slower than that of mock control at day 4 (P<0.001, Fig. 2A). BrdU incorporation assay
revealed that after 72 h of incubation, knockdown of CDK2AP1
resulted in increased DNA synthesis (P<0.05), as compared with
si-control. In contrast, CDK2AP1 over-expression significantly
inhibited DNA synthesis (P<0.001).
To evaluate the relative long-term effect of CDK2AP1
knockdown or overexpression, the colony formation ability of A549
cells transduced with different lentivirus was studied as well.
Quantitative analysis of colony counts showed that following
incubation for 14 days, the colony number of control A549 cells was
indistinguishable from that of si-control A549 cells. The colony
number of Lv-si-CDK2AP1 infected A549 cells was significantly
higher than that of control, whereas the colony count of Lv-CDK2AP1
infected A549 cells was significantly slower than that of
si-control (Fig. 2C and D).
Therefore, these results suggest that downregulation of CDK2AP1
gene expression enhances the growth of A549 cells, whereas ectopic
overexpression of CDK2AP1 gene inhibits A549 cell growth in
vitro.
Effects of down- or upregulation of
CDK2AP1 on cell cycle distribution
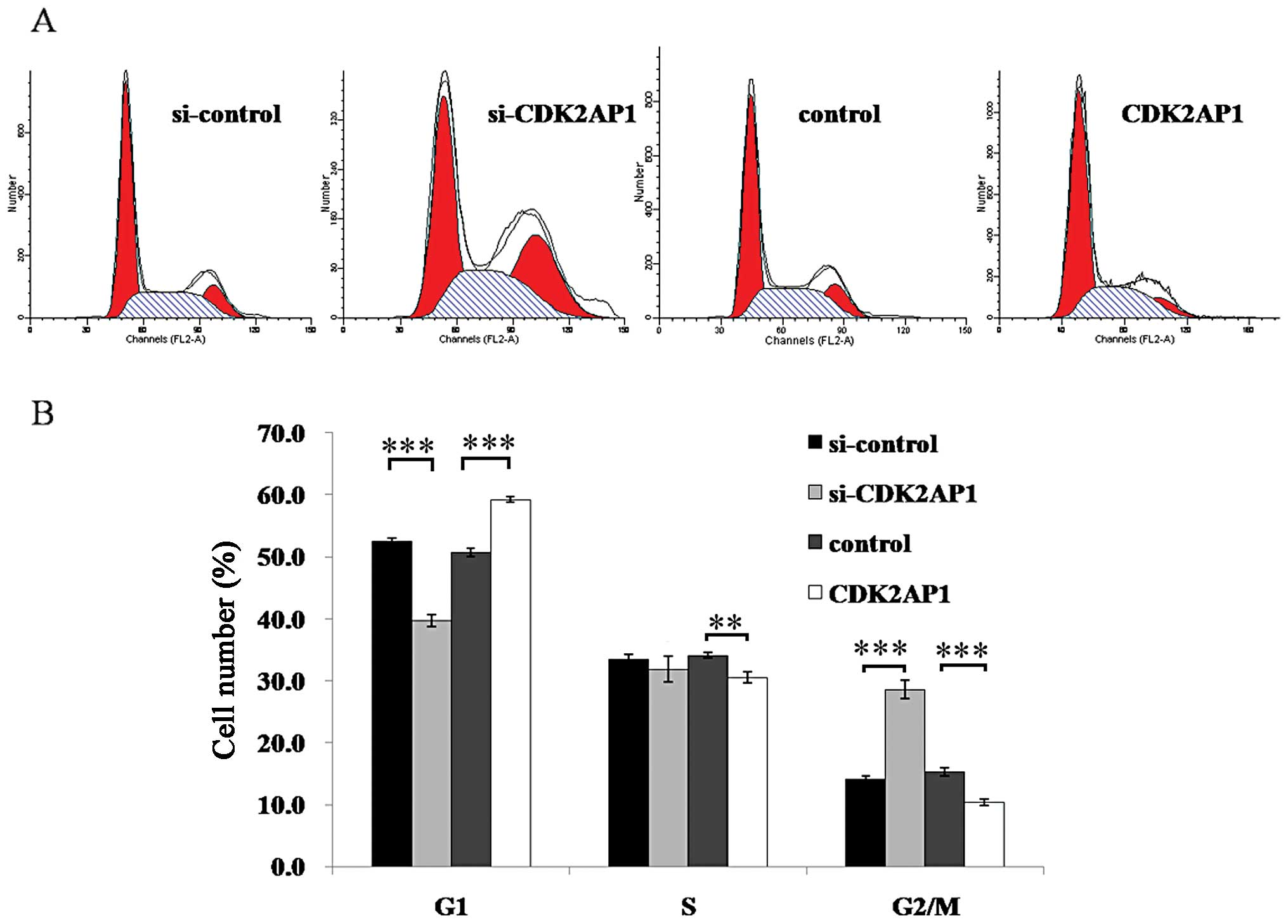
To explore the potential mechanism underlying the
action of CDK2AP1 on the growth of A549 cells, the cell cycling
patterns of CDK2AP1 knockdown and overexpression of A549 cells were
determined by flow cytometric analysis. As shown in Fig. 3, the CDK2AP1 RNAi construct
resulted in a substantial decrease in G1-phase and an increase in
G2/M phase populations (P<0.001). In contrast, CDK2AP1
over-expression construct caused an elevated G1-phase proportion
and a reduced G2/M phase proportion (P<0.01). Moreover, there
was an obvious impairment in the frequency at S phase of Lv-CDA2AP1
transduced cells in comparison to mock control. Overall, these
results suggest that CDK2AP1 plays an important role in cell cycle
control for A549 cells.
Effects of CDK2AP1 overexpression in A549
cells on chemo-sensitivity
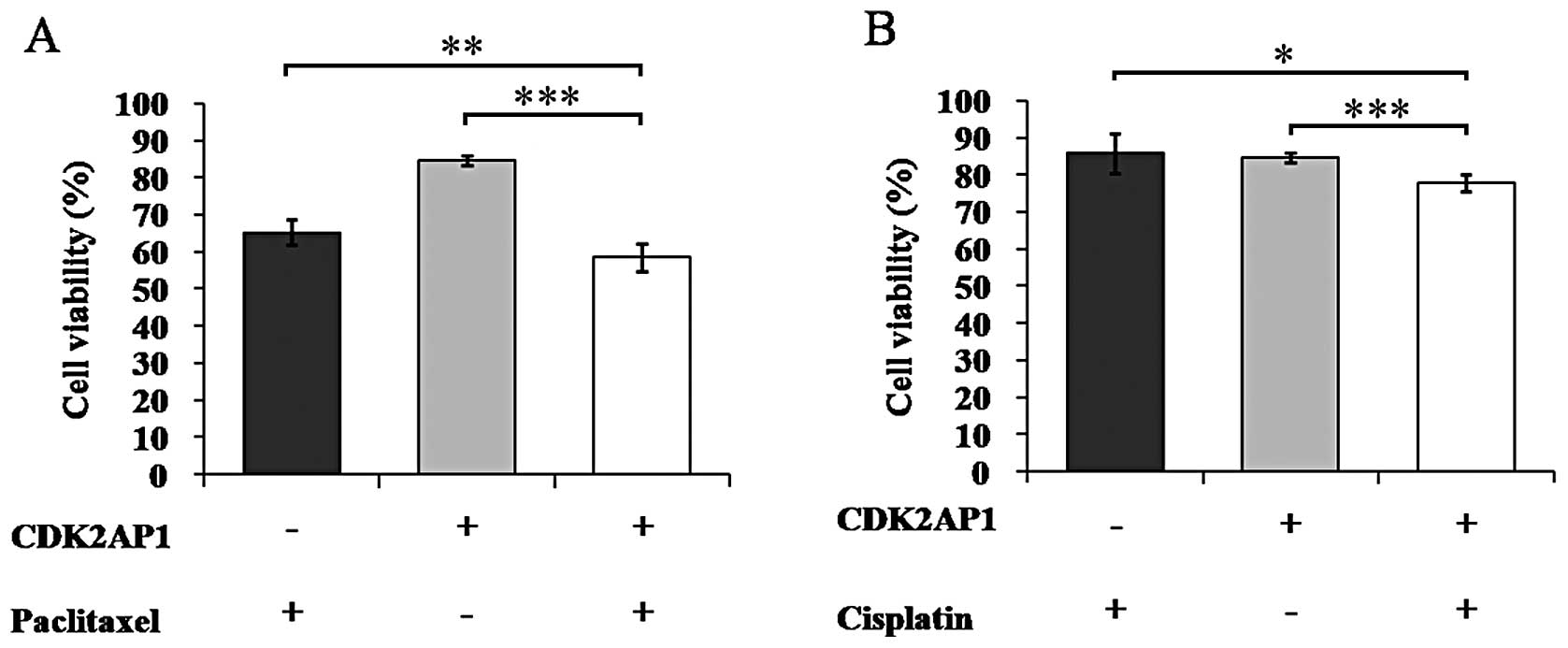
Cisplatin and paclitaxel are commonly used in lung
cancer treatment and thus improvement of their chemo-sensitivity is
critical (18). Here we combined
the treatment of CDK2AP1 overexpression lentivirus with cisplatin
or paclitaxel in A549 cells to evaluate its effects on
chemosensitivity (Fig. 4).
Notably, the viability of A549 cells treated with the combination
of Lv-CDK2AP1 and paclitaxel was significantly decreased than that
with Lv-CDK2AP1 (P<0.001) or paclitaxel alone (P<0.01), which
was 58.5, 84.6 and 65.2%, respectively (P<0.01, Fig. 4A). Similar results were observed in
the combination treatment of Lv-CDK2AP1 and cisplatin (Fig. 4B). Therefore, CDK2AP1
overexpression can drastically enhance the chemosensitivity of A549
cells to paclitaxel and cisplatin.
Effects of down- or upregulation of
CDK2AP1 on tumorigenesis
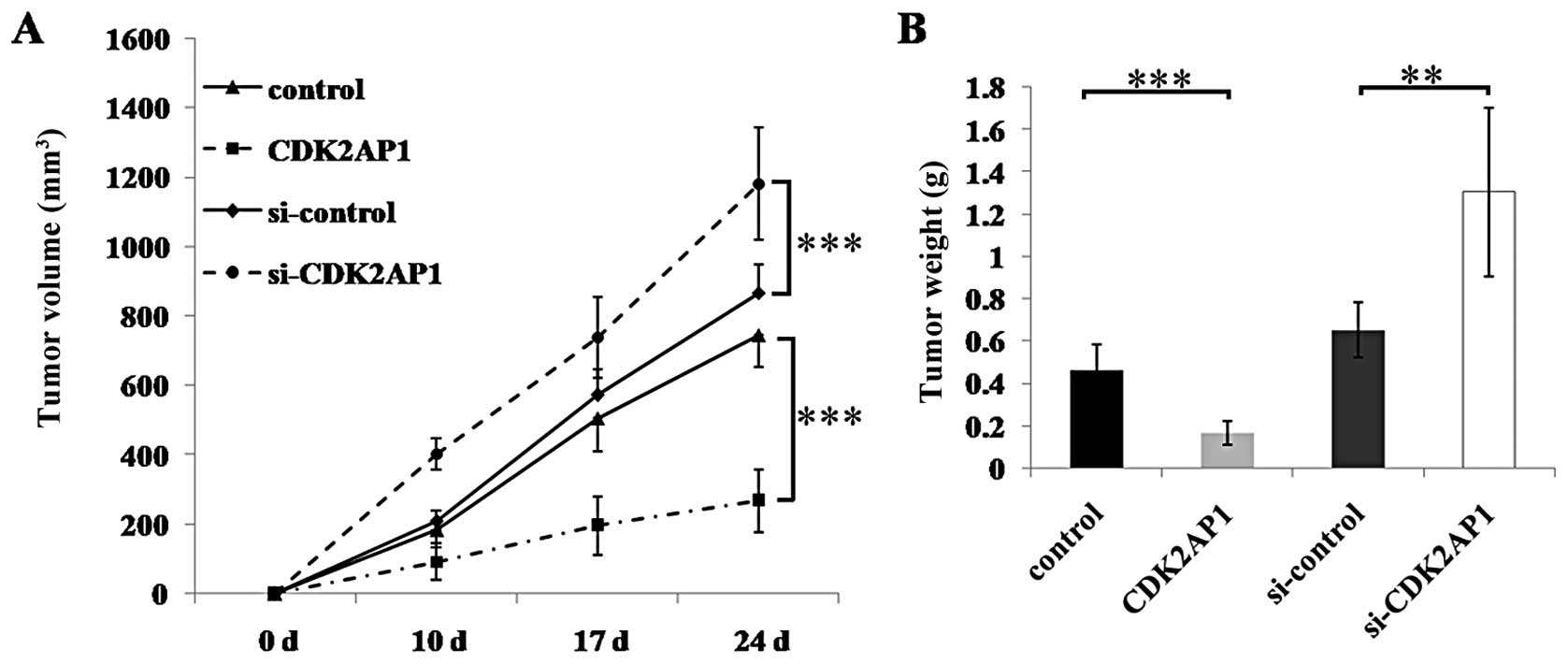
To determine the role of CDK2AP1 in tumorigenicity,
CDK2AP1 knockdown or overexpressing A549 cells were injected
subcutaneously into athymic nude mice (Fig. 5). The tumor growth curves and tumor
weight results showed that the developed solid tumor in CDK2AP1
overexpression group grew significantly slower than the controls
after 24 days of inoculation, whereas the tumors in CDK2AP1
knockdown group grew significantly faster than the controls, being
well in agreement with the results of cell proliferation and colony
formation in vitro. These results demonstrate that
downregulation of CDK2AP1 expression enhanced tumorigenicity of
A549 cells, whereas ectopic overexpression of CDK2AP1 gene
decreased tumorigenicity of A549 cells in vivo.
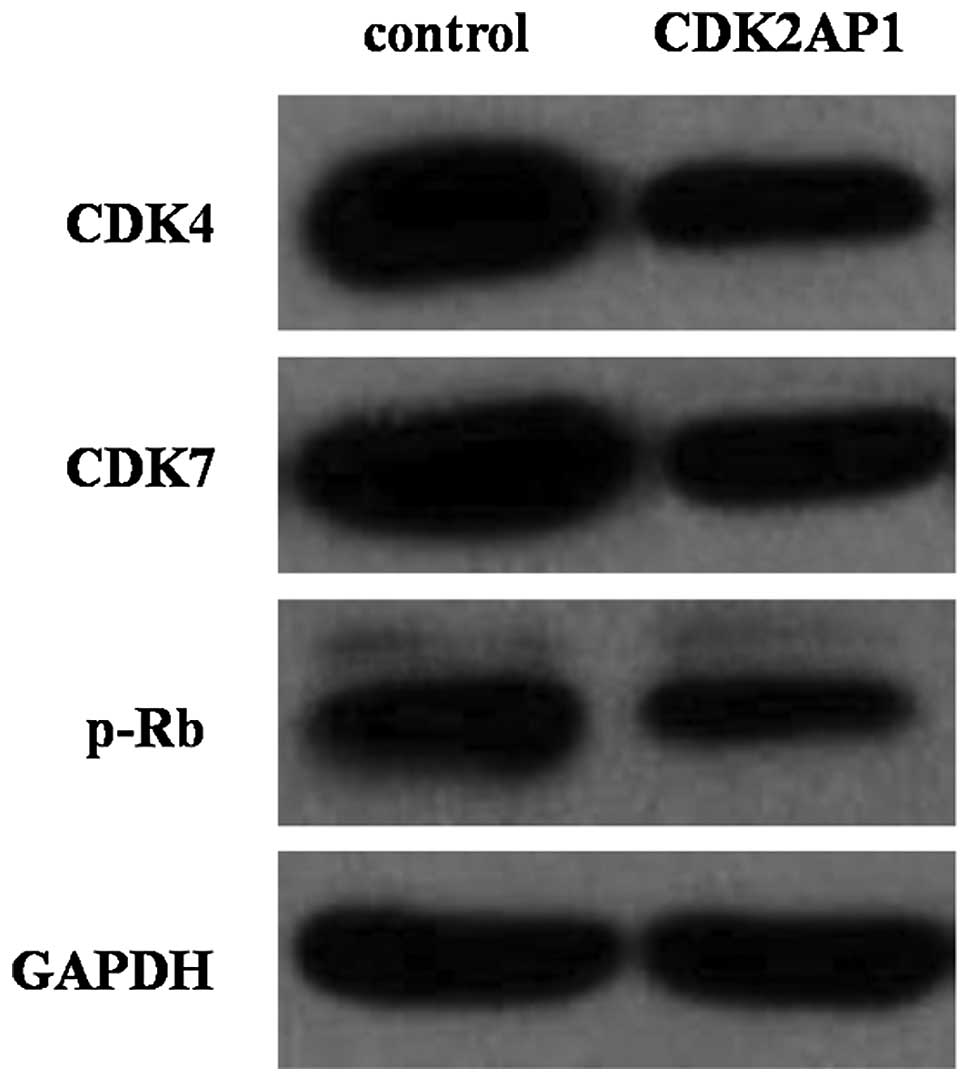
Effects of down- or upregulation of
CDK2AP1 on cell cycle regulators
CDK2AP1 is known to be associated with CDK2 activity
during G1-S phase. To explore the regulator mechanism of CDK2AP1 on
A549 cell cycle progression, western blotting was performed on
Lv-CDK2AP1 transduced cells. Fig.
6 showed that overexpression of CDK2AP1 resulted in significant
decrease in the expression of CDK4, CDK7 as well as the
retinoblastoma tumor suppressor gene product (p-Rb) that plays a
pivotal role in G1- to S-phase cell.
Discussion
In the present study, we determined the role of
CDK2AP1 in the growth of A549 cells, a cell line that was initially
derived from a highly malignant human lung adenocarcinoma. Using
the constructed lentiviral vectors containing CDK2AP1 shRNA or
full-length CDK2AP1 cDNA, we infected A549 cells to silence
endogenous CDK2AP1 or overexpress CDK2AP1 and investigated
systemically the impacts of CDK2AP1 down- or upregulation on lung
cancer growth and chemosensitivity in vitro and in
vivo. Our results revealed that ectopic overexpression of
CDK2AP1 in A549 cells can greatly impair the proliferation and
colony-forming ability and enhance the chemosensitivity to
cisplatin and paclitaxel in vitro. Notably, we found that
CDK2AP1 overexpression caused cell cycle arrest at G1/S transition
of A549 cells, as evidenced by the accumulation of G1 phase.
Injection of the ectopic overexpressing A549 cells into nude mice
resulted in inhibited growth of solid lung tumors in vivo.
Consistently with the aforementioned results, knockdown of CDK2AP1
in A549 cells gave rise to the opposite effect on
proliferation/growth, cell cycling and tumorigenesis in
vivo. Collectively, these findings not only lend weight to the
growing body of evidence that CDK2AP1 is involved in cancer
development and progression, but represent the first report that
CDK2AP1 is a novel proliferation regulator in lung cancer.
Our data are consistent with previous studies, which
have demonstrated the antitumor activity of CDK2AP1 in various
malignancies (11,15,16,19–21).
In addition, accumulated evidence has indicated that the expression
levels of CDK2AP1 may be used as an indicator in diagnostics for
the invasiveness and prognosis of oral squamous cell carcinoma,
esophageal squamous cell carcinomas and gastric cancer tissues in
humans (10,14). Moreover, a recent study revealed
that targeted disruption of murine Cdk2ap1 resulted in embryonic
lethality with a high penetration rate, which highlights the
essential role of CDK2AP1 in the mammal (22).
Cancer research has recently devoted the collective
efforts toward an understanding of the molecular regulation and
functional significance of cell cycle regulators in the
pathogenesis and development of cancers (4,11,13).
It has been demonstrated that CDK2 is an important candidate target
for therapeutic intervention (23,24).
In this study, attention is also paid to the issues regarding
association between the antitumor activity of CDK2AP1 and
expression of other cell cycle regulators and our finding shows
that ectopic over-expression of CDK2AP1 reduces the expression of
CDK4, CDK7 and p-Rb in A549 cells. Therefore, we would like to put
forward a tentative hypothesis that the antitumor activity of
CDK2AP1 in lung cancer may act via a mechanism impacting on other
cell cycle regulators in addition to CDK2.
In this study, to evaluate the potential value of
CDK2AP1 in lung cancer treatment, we examined the chemosensitivity
of A549 cells to cisplatin and paclitaxel, which are commonly used
in clinical study (18). Many
studies have focused on increasing the chemosensitivity of lung
cancer cells to chemotherapeutics. Kim and associates have shown
that p12CDK2 mediates DNA damage responses induced by cisplatin in
mouse oral/head and neck cancer model (25). Our finding represents the first
report that CDK2AP1 overexpression can enhance the chemosensitivity
to cisplatin and paclitaxel, suggesting that CDK2AP1 may be a
potential molecular target in lung cancer therapy.
Several growth factors or growth suppressors have
been implicated in cell proliferation and growth as well as
invasion-metastasis cascade of malignant tumors (1,2,13).
CDK2AP1 has been selected as a functional target of gene therapy
for human colorectal cancer and mouse model of head and neck cancer
(12,15,16).
Our results show that modulation of CDK2AP1 gene expression in A549
cells can affect cell growth and chemosensitivity of lung cancer
in vitro and/or in vivo, which is consistent with
previous reports (16,21).
Emerging evidence suggests that several factors are
implicated in genesis of lung cancer, such as new fusion genes,
changing expression level of p53, growth factors, MED19 (a subunit
of mediator complex), cytokines and chemokine receptors and STAT3
(signal transducer and activator of tran s cription 3) (1,2). It
has been suggested that CDK2AP1 suppresses malignant biological
interactions between prostate cancer and bone cells and modifies
the androgen-responsive pathway function (11,13).
However, to date, the issue as to whether and how CDK2AP1
interplays with other regulators is poorly understood and further
investigation is warranted to elucidate the mechanism underlying
the action of CDK2AP1.
In conclusion, our findings strongly suggest that
CDK2AP1 facilitates important regulatory roles in lung cancer cell
proliferation and cell cycle progression of lung cancer and also
has significant effects on the chemosensitivity of pulmonary
malignancies. Hence, this study extends our current knowledge on
oncogenesis of lung cancer and indicates that CDK2AP1 may serve as
a new molecular target for lung cancer therapy.
Acknowledgements
This study was supported by the grant
from the Natural Science Foundation of China (#81272472).
References
|
1
|
Sun M, Jiang R, Li JD, et al: MED19
promotes proliferation and tumorigenesis of lung cancer. Mol Cell
Biochem. 355:27–33. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang CG, Wang RY, Sun M, Li JD, Gao N and
Zhang XY: In vivo antitumor effect of siRNA against STAT3 on
transplanted Lewis lung cancer in mice. Chem Res Chin Univ.
24:322–329. 2008.
|
|
3
|
Luo SL, Sun M, Jiamg R, Wang G and Zhang
XY: Establishment of primary mouse lung adenocarcinoma cell
culture. Oncol Lett. 2:629–632. 2011.PubMed/NCBI
|
|
4
|
Eymin B and Gazzeri S: Role of cell cycle
regulators in lung carcinogenesis. Cell Adh Migr. 4:114–123. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shintani S, Mihara M, Terakado N, et al:
Reduction of p12DOC-1 expression is a negative prognostic indicator
in patients with surgically resected oral squamous cell carcinoma.
Clin Cancer Res. 7:2776–2782. 2001.PubMed/NCBI
|
|
6
|
Shintani S, Ohyama H, Zhang X, et al:
p12(DOC-1) is a novel cyclin-dependent kinase 2-associated protein.
Mol Cell Biol. 20:6300–6307. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Todd R, McBride J, Tsuji T, et al: Deleted
in oral cancer-1 (doc-1), a novel oral tumor suppressor gene. FASEB
J. 9:1362–1370. 1995.PubMed/NCBI
|
|
8
|
Daigo Y, Suzuki K, Maruyama O, et al:
Isolation, mapping and mutation analysis of a human cDNA homologous
to the doc-1 gene of the Chinese hamster, a candidate tumor
suppressor for oral cancer. Genes Chromosomes Cancer. 20:204–207.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng H, Shintani S, Kim Y and Wong DT:
Loss of p12CDK2-AP1 expression in human oral squamous cell
carcinoma with disrupted transforming growth factor-beta-Smad
signaling pathway. Neoplasia. 8:1028–1036. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hiyoshi Y, Watanabe M, Hirashima K, et al:
p12CDK2-AP1 is associated with tumor progression and a poor
prognosis in esophageal squamous cell carcinoma. Oncol Rep.
22:35–39. 2009.PubMed/NCBI
|
|
11
|
Zolochevska O and Figueiredo ML: Cell
cycle regulator cdk2ap1 inhibits prostate cancer cell growth and
modifies androgen-responsive pathway function. Prostate.
69:1586–1597. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zolochevska O and Figueiredo ML:
Expression of cell cycle regulator cdk2ap1 suppresses tumor cell
phenotype by non-cell-autonomous mechanisms. Oral Oncol.
45:e106–112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zolochevska O and Figueiredo ML:
Cell-cycle regulators cdk2ap1 and bicalutamide suppress malignant
biological interactions between prostate cancer and bone cells.
Prostate. 71:353–367. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Choi MG, Sohn TS, Park SB, et al:
Decreased expression of p12 is associated with more advanced tumor
invasion in human gastric cancer tissues. Eur Surg Res. 42:223–229.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Z, Gaba AG, Kent TS, Bennett A,
Miller A and Weber TK: Modulation of CDK2-AP1 [p12(DOC-1)]
expression in human colorectal cancer. Oncogene. 24:3657–3668.
2005.PubMed/NCBI
|
|
16
|
Figueiredo ML, Kim Y, St John MA and Wong
DT: p12CDK2-AP1 gene therapy strategy inhibits tumor growth in an
in vivo mouse model of head and neck cancer. Clin Cancer Res.
11:3939–3948. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cwikla SJ, Tsuji T, McBride J, Wong DT and
Todd R: doc-1-mediated apoptosis in malignant hamster oral
keratinocytes. J Oral Maxillofac Surg. 58:406–414. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Galetta D, Pisconti S, Cinieri S, et al:
Induction pemetrexed and cisplatin followed by maintenance
pemetrexed versus carboplatin plus paclitaxel plus bevacizumab
followed by maintenance bevacizumab: a quality of life-oriented
randomized phase III study in patients with advanced non-squamous
non-small-cell lung cancer (ERACLE). Clin Lung Cancer. 12:402–406.
2011.
|
|
19
|
Zolochevska O and Figueiredo ML: Novel
tumor growth inhibition mechanism by cell cycle regulator cdk2ap1
involves antiangiogenesis modulation. Microvasc Res. 80:324–331.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wong DT, Kim JJ, Khalid O, Sun HH and Kim
Y: Double edge: CDK2AP1 in cell-cycle regulation and epigenetic
regulation. J Dent Res. 91:235–241. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zheng J, Xue H, Wang T, et al: miR-21
downregulates the tumor suppressor P12 CDK2AP1 and stimulates cell
proliferation and invasion. J Cell Biochem. 112:872–880. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim Y, McBride J, Kimlin L, Pae EK,
Deshpande A and Wong DT: Targeted inactivation of p12, CDK2
associating protein 1, leads to early embryonic lethality. PLoS
One. 4:e45182009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wadler S: Perspectives for cancer
therapies with cdk2 inhibitors. Drug Resist Updat. 4:347–367. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang TY, Chang GC, Chen KC, et al:
Sustained activation of ERK and Cdk2/cyclin-A signaling pathway by
pemetrexed leading to S-phase arrest and apoptosis in human
non-small cell lung cancer A549 cells. Eur J Pharmacol. 663:17–26.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim Y, McBride J, Zhang R, Zhou X and Wong
DT: p12(CDK2-AP1) mediates DNA damage responses induced by
cisplatin. Oncogene. 24:407–418. 2005. View Article : Google Scholar : PubMed/NCBI
|