Introduction
The first demonstration that tumor cells could
generate ROS at rates that approach the capacity of phagocytic
leukocytes occurred over two decades ago (1,2). At
that time, it was appreciated that oxygen radical generation by
tumor cells might contribute to invasion and metastasis, as well as
the development of ROS-related DNA damage (2–4).
However, a complete understanding of the sources of tumor cell ROS
has only recently begun to be developed, having awaited the
discovery over the past decade of the family of six epithelial
NADPH oxidases (Noxs) that have significant homology with the
membrane oxidase of leukocytes (5), and the development of reagents that
allow evaluation of expression of the members of the Nox gene
family across different tissues and tumors. Recent evidence
suggests that some NADPH oxidases may play a critical role in
enhancing tumor cell proliferation and angiogenesis across a broad
range of histological subtypes of malignancy (6,7).
Dual oxidase 2 (Duox2) is one member of the
epithelial Nox family that generates H2O2 in
the service of several critical physiological functions, including
thyroid hormone biosynthesis and host defense (8,9). It
has two catalytic sites: an NADPH oxidase as well as a heme
peroxidase that function to generate extracellular
H2O2(10).
Duox2 is one of two closely-related Nox isoforms, the other being
Duox1, that share greater than 85% homology at the amino acid level
(11); the membrane-spanning
regions of these proteins are highly homologous to the gp91phox
domain of the phagocytic oxidase (Nox2). The N-terminal heme
peroxidase-like extracellular domain is also related to other
peroxidases that convert O2•− to
H2O2. In addition to the NADPH oxidase and
peroxidase-like domains, two cytosolic, calcium-binding EF-hand
domains have been described which may explain the requirement for
the presence of micromolar calcium concentrations to generate
functional oxidase activity. Finally, it has recently become clear
that reactive oxygen formation in vivo requires the presence
in cells of a dual oxidase maturation factor (DuoxA2), an
ER-resident protein that is necessary for post-translational
processing and translocation of an enzymatically functional Duox2
complex to the plasma membrane (12).
Duox2 has also been implicated in the pathogenesis
of chronic inflammatory, pre-neoplastic conditions, such as
inflammatory bowel disease and chronic pancreatitis (13–15).
In the case of inflammatory bowel disease, the expression of Duox2
is significantly increased in human colon biopsies, and in isolated
intestinal epithelial cells, from patients with both Crohn’s
disease and ulcerative colitis compared to expression levels in
normal adjacent colonic mucosa, suggesting that an unchecked ROS
response to pathogens could contribute to the tissue injury
observed in these chronic inflammatory disorders (13). These results are consistent with
the observation that the expression of Duox2 is upregulated 10-fold
in pre-malignant adenomatous polyps of the colon compared to
adjacent colonic mucosa as determined by expression array analysis
(16), as well as our finding that
Duox2 expression at the mRNA level is dramatically increased in
some surgically-resected colon cancers (7).
Unfortunately, although certain physiological
functions of Duox2 are known in detail, such as its role in thyroid
hormone biosynthesis, immunochemical detection studies of Duox2
that could have important clinical implications remain to be
initiated because of a lack of specific Duox2 antibodies. The
expression of Duox2 at the protein level in human tumors or in
pre-malignant conditions is, therefore, effectively unknown, as
well as its relative intracellular localization in specific tissues
both normal and malignant. Only a small number of studies have been
performed that have attempted to examine Duox2 expression in human
tissues by immunohistochemical techniques; in some of these
studies, antisera were prepared against a short stretch of a Duox2
peptide that might make establishing specificity difficult
(17). Currently-available
polyclonal antibodies used to detect Duox2 have been developed
without always identifying the initiating antigen or establishing
specificity by genetic means, western blot analysis or
immunohistochemistry. Hence, we chose to develop a Duox2 monoclonal
antibody that would be applicable to a variety of investigative
applications in clinical specimens so that a full characterization
of Duox2 expression in normal as well tumor tissues would be
possible.
Herein we report the production and characterization
of a high quality monoclonal antibody that appears to be specific
for the detection of functional Duox protein and that can be used
effectively for many immunochemical applications. We have utilized
this antibody to evaluate the expression of Duox in both normal
tissues and in a variety of human tumors by tissue microarray. Our
results demonstrate for the first time that Duox protein is highly
overexpressed in cancers of the prostate, lung, colon and breast
compared to normal tissues from these organs; and that, in
contrast, Duox protein is not found in abundance in non-Hodgkin
lymphomas or glioblastoma multiforme.
Materials and methods
Materials
Recombinant human IFN-γ (catalog no. 285-IF) was
purchased from R&D Systems. Antibody against human β-actin
(catalog no. A3853) was acquired from Sigma-Aldrich. Human Duox2
primer (catalog no. Hs00204187_m1), human Duox1 (catalog no.
Hs00213694), human β-actin (catalog no. Hs99999903_m1), and TaqMan
Universal PCR mix (catalog no. 4364340) were purchased from Applied
Biosystems.
Cell culture
The human pancreatic cancer cell lines BxPC-3
(catalog no. CRL-1687), MIA PaCa-2 (catalog no. CRL-1420™), and
PANC-1 (catalog no. CRL-1469™) were obtained from the American Type
Culture Collection (Manassas, VA). BxPC-3 cells were cultured in
RPMI-1640 medium (catalog no. SH30255.01; HyClone) with 1% pyruvate
and 10% FBS. MIA PaCa-2 cells were cultured in Dulbecco’s modified
Eagle’s medium with 10% FBS and horse serum to a final
concentration of 2.5%. PANC-1 cells were cultured in Dulbecco’s
modified Eagle’s medium with 10% FBS. Cells were cultured in a
humidified incubator at 37°C in an atmosphere of 5% CO2
in air.
Cloning, expression and purification of a
partial recombinant Duox2 protein
To generate a monoclonal antibody specific for
Duox2, the human Duox2 protein sequence was obtained from the NCBI
data base; it contains 1,548 amino acids of an integral membrane
glycoprotein. Initially, we were unsuccessful in expressing the
full length Duox2 protein in BL21 (DE3) E. coli utilizing
different plasmid vector backbones (data not shown). After a
careful bioinformatics approach to total protein structure, we
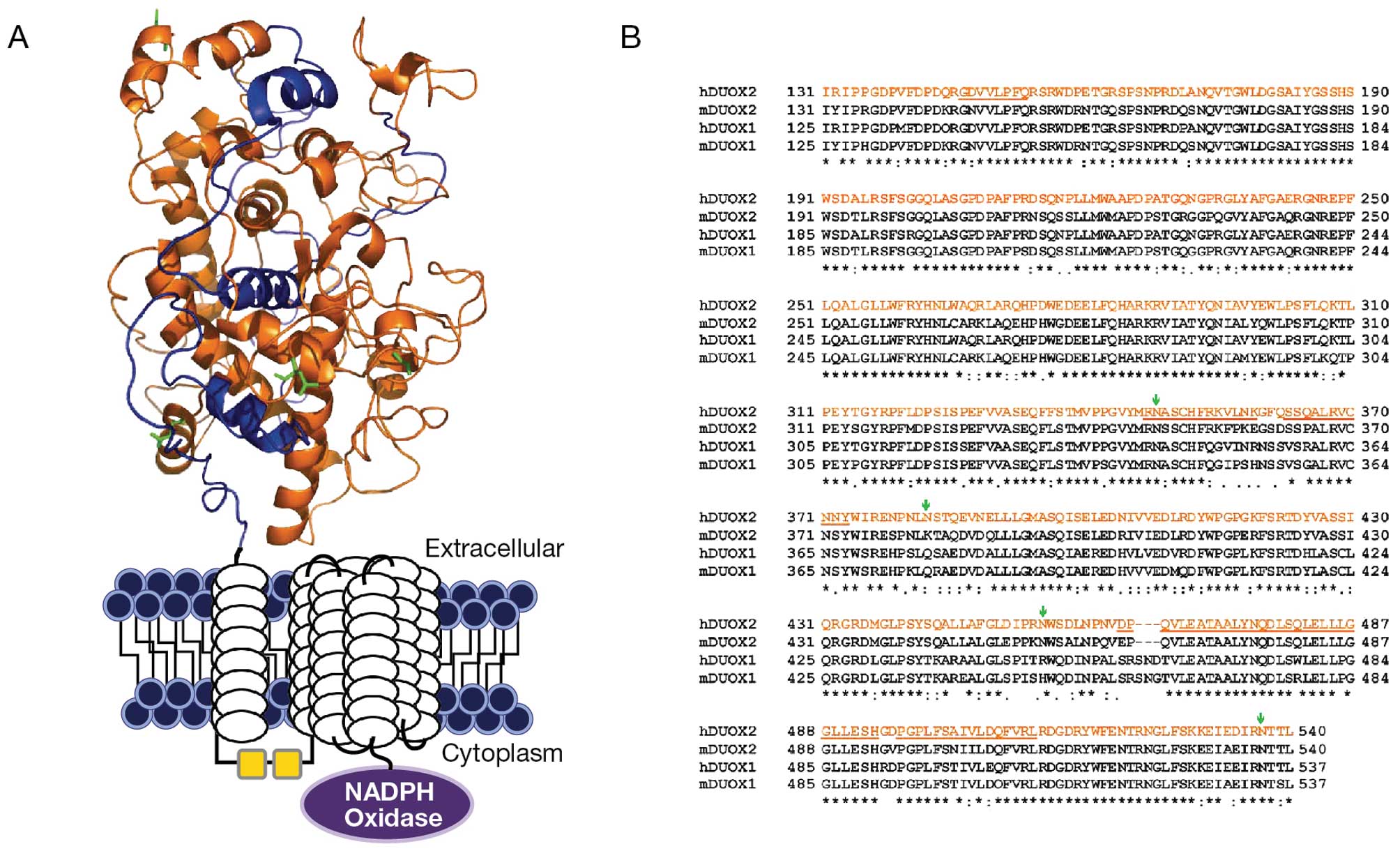
identified the amino terminal end of the Duox2 sequence that
represents 410 amino acids (NH2 terminal 131–540 amino acid
peptides) to be a sequence of high potential immunogenicity for
antibody production (data not shown). Using a human full length
Duox-cDNA plasmid as template, through PCR, a 1,230 BP fragment
corresponding to a 131–540 amino acid sequence was amplified and
sub-cloned into a PET30a(+) vector. The NH2 terminal 131–540 (410)
amino acids represent a unique peroxidase-like domain region of the
Duox2 sequence that we felt would be suitable for antibody
production (Fig. 1). Prior to
expression of the pET30a(+)-DUOX2-410AA, the nucleotide sequence of
the entire gene construct was re-sequenced; we found that our
desired sequence was in the right order. Expression of Duox2-410AA
was detected in the culture after 2 h of induction with IPTG by
analysis of the SDS-PAGE bacterial pellet, where the appearance of
a ∼45-kDa band indicated the synthesis of the recombinant
Duox2-410AA-His-Tag. Soluble cytosolic fractions of induced BL21
lysate demonstrated that most of the induced protein band was found
in the soluble fraction, as confirmed by SDS-PAGE (data not shown).
The truncated Duox2-410AA protein was purified to near homogeneity
using Ni-NTA sepharose resin as evaluated by SDS-PAGE (data not
shown). From 1 liter of E. coli culture, we purified 5.0 mg
protein with an apparent purity of 98% as revealed by Coomassie
Blue staining. The recovery and purification fold were greater than
80% (data not shown).
Production of monoclonal antibodies
To generate monoclonal antibodies, four Balb/c mice
were immunized subcutaneously with a fusion protein containing 50
μg Duox2-410AA-His-Tag antigen in complete Freund’s adjuvant
(Sigma, Gillingham, UK); following this protocol, approximately 200
μg of protein was injected per animal over ten weeks. This
program was followed by three further subcutaneous booster
immunizations of 50 μg Duox2-410AA-His-Tag antigen in
incomplete Freund’s adjuvant (Gibco-BRL, Grand Island, NY) at
15-day intervals. Seven days after the final booster immunization,
test bleeds were taken from each mouse, and the resulting serum
samples (test sera) were screened alongside the corresponding
pre-immune serum sample from each mouse for antibody binding to
Duox2-410AA-His-Tag antigen. Ninety-six-well micro-titer plates
were used for the HRP-conjugate enzyme-linked immunosorbent assay
to screen pre- and post-immune sera (data not shown).
Panel of monoclonal antibodies against
Duox2
According to standard procedures, splenocytes from
the immunized mouse were fused with SP2/0 mouse myeloma cells at a
ratio of 10:1 using a conventional polyethylene glycol (PEG) 1500
(Sigma) fusion protocol, and the resulting hybridomas were selected
in HAT medium. Cell culture supernatants of the hybrid cell
colonies were screened for antibodies by ELISA, and positive cell
lines were subcloned three times by limiting dilution. Finally,
ELISA titer results were calculated as the mean absorbance at 450
nm for each serial dilution of the test and pre-immune serum
samples corrected by subtracting the blank mean absorbance at 450
nm (mean absorbance 450 nm for non-specific binding of the
anti-mouse Ig polyvalent HRP conjugate to Duox2-410AA-His-Tag
coated wells) (data not shown). Hybridoma cell lines producing
anti-Duox2-410AA-His-Tag antibodies were cloned from single cells,
expanded and cryo-preserved according to standard procedures.
Additionally, the hybridomas were further screened to identify
antibodies reacting with both Duox2-410AA-His-Tag antigens. The
experimental screenings with Duox2-410AA-His-Tag coated on ELISA
plates selected several monoclonal antibodies; those that reacted
to the His-tag were discarded. Many hybridoma clones were initially
identified (47 clones) as producing anti-Duox2-410AA-His-Tag
antibodies; 34 clones reacted only with truncated Duox2-410AA.
During further passage in tissue culture, 10 of these 34 clones
either died or stopped producing antibody. We successfully
developed and cryo-preserved 24 stable anti-Duox2-410AA producing
hybridoma cell lines (data not shown).
Development of MIA PaCa-2 cells stably
transfected with Duox2 cDNA
MIA PaCa-2 cells were transfected with an HA-tagged
full length human Duox2 gene in a CMV driven expression vector
(pcDNA3.1) using the Lonza transfection protocol in 100 μl
of transfection buffer (Amaxa Cell line Nucleofector Kit V)
(Program: T-027) utilizing the Amaxa Nucleofector Device (Lonza,
ME). Stable clones of MIA PaCa-2 cells with empty vectors as well
as those expressing the HA-tag-Duox2 were developed by selection in
G418. Because the production of H2O2 by the
Duox2 complex requires the presence of both Duox2 and its
maturation factor (DuoxA2) (12),
the HA-tag-Duox2 stable MIA PaCa-2 clonal cells were further
transiently transfected with the human full length DuoxA2 gene in a
mammalian expression vector (pcDNA3.1) to evaluate functional
enzymatic activity.
Transient transfection of COS-7
cells
Transfection of COS-7 cells was performed according
to the manufacturer’s instructions using the Lonza transfection
protocol and transfection reagent in 100 μl of transfection
buffer (Amaxa Cell line Nucleofector Kit R) (program: A-024)
utilizing the Amaxa Nucleofector Device (Lonza, Rockland, ME). For
each transfection, 2 μg of plasmid DNA (pcDNA3.1/HA-Duox1 or
HA-Duox2 or empty vector) was used. After 48 h of incubation, cells
were lysed and analyzed for RNA and protein content.
RNA extraction, cDNA synthesis and
quantitative real-time RT-PCR assay
Total RNA was extracted with the RNeasy mini kit
(catalog no. 74104; Qiagen) according to the manufacturer’s
instructions. Two micrograms of total RNA was used for cDNA
synthesis, using SuperScript II reverse transcriptase (catalog no.
18080-044) and random primers (catalog no. 48190-011; Invitrogen)
in a 20 μl reaction system, with the following cycles: 25°C
for 5 min, 42°C for 50 min and 75°C for 5 min. After the reaction
was complete, the RT-PCR products were diluted with
diethylpyrocarbonate/H2O to 100 μl for real-time
PCR. Real-time RT-PCR was performed in 384-well plates in a 20
μl reaction system containing 2 μl of diluted cDNA, 1
μl of primer mixture, 7 μl of H2O, and 10
μl of TaqMan 2X reaction mixture. PCR was carried out under
default cycling conditions, and fluorescence was detected with the
ABI 7900HT Sequence Detection System (Applied Biosystems, Foster
City, CA). Triplicate determinations were performed for each sample
that was used for real-time PCR; the mean value was calculated and
the data in the final figures represent the results of three
independent experiments. Relative gene expression was calculated as
the ratio of the target gene to the internal reference gene
(β-actin) multiplied by 103 based on Ct
values.
Western blot analysis
For preparation of whole-cell extracts, cell pellets
from BxPC-3, MIA PaCa-2, and PANC-1 cells, treated with or without
IFN-γ, were lysed with 1X RIPA lysis buffer (catalog no. 20–188;
Millipore, Temecula, CA), with the addition of a phosphatase
inhibitor tablet (catalog no. 04-906-837001; Roche) and a protease
inhibitor tablet (catalog no. 11-836-153001; Roche). The protein
concentrations of whole-cell extracts were measured by using the
BCA Protein Assay Kit (Pierce). Cell extracts were mixed with an
equal volume of 2X SDS protein gel loading buffer (catalog no.
351-082-661; Quality Biological); and when required, the samples
were denatured by heating at 95°C for 5 min. A total of 50
μg of whole-cell extract was loaded onto a 4–20% Tris
glycine gel (catalog no. EC6028; Invitrogen), and the proteins were
separated and electrophoretically transferred to nitro-cellulose
membranes using I Blot gel transfer stacks (catalog no. IB 3010-01;
Invitrogen). The membranes were blocked in 1X TBST buffer with 5%
non-fat milk for 1 h at room temperature and then incubated with
primary antibody overnight in TBST buffer. Membranes were washed
three times in 1X TBST buffer and incubated with HRP-conjugated
secondary antibody for 1 h at room temperature with shaking. The
antigen-antibody complex was visualized with SuperSignal West Pico
Luminol/Enhancer Solution (catalog no. 1856136, Thermo Scientific).
Final characterization and evaluation of Duox2 protein expression
was determined from the whole-cell extract, mixed with an equal
volume of 2X SDS loading buffer but without boiling. For the
analysis of proteins other than Duox2, the mixture of cell extract
with loading buffer was boiled for 5 min. Although the pancreatic
cancer cell lines utilized for these experiments do not contain
measurable Duox1 mRNA, because our antibody cross-reacts with
Duox1, we have referred to the protein it detects as ‘Duox’.
Extracellular H2O2
measurement using Amplex Red®
The Amplex Red® Hydrogen
Peroxide/Peroxidase Assay Kit (catalog no. A22188; Invitrogen) was
used to detect extracellular H2O2 release.
MIA PaCa-2 cells stably expressing Duox2 were transiently
transfected with DuoxA2; 48 h following transient transfection with
DuoxA2, extracellular H2O2 release was
measured. In preparation for determination of
H2O2 release, MIA PaCa-2 cells were washed
twice with 1X PBS, trypsinized and dispersed thoroughly. Cells were
counted to produce a 20-μl cell suspension containing
2×104 live cells in 1X Krebs-Ringer phosphate glucose
(KRPG) buffer. The cells were mixed with 100 μl of Amplex
Red reagent containing 50 μM Amplex Red and 0.1 units of HRP
per ml in KRPG buffer with or without 1 μM ionomycin and
incubated at 37°C for 60 min. The fluorescence of the oxidized
10-acetyl-3,7-dihydroxyphenoxazine was measured at an excitation
wavelength of 530 nm and an emission wavelength of 590 nM, using a
SpectraMax Multiplate reader (Molecular Devices, Sunnyvale, CA).
H2O2 was quantified with an
H2O2 standard curve over a concentration
range from 0 to 2 μM. Each value in the figure represents a
mean of quadruplicate samples from 16 readings.
Immunofluorescence microscopy analysis of
HA-Duox2-expressing MIA PaCa-2 cells
MIA PaCa-2 cells (1×105)
stably-transfected with HA-tagged human full length Duox2 cDNA were
plated in 4-well glass slides (Lab-TekII Chamber slide, Cat no.
154526, Thomas Scientific, Swedesboro, NJ) containing 1.0 ml of
complete growth medium. Cells in the slide chamber were grown
overnight, then were fixed with cold methanol at −20°C for 5 min
and washed once with 1X PBS, pH 7.4. Non-specific binding of
proteins to the section was reduced (blocked) with 0.1% Triton in
1X PBS containing 5% BSA for 60 min at room temperature (RT). After
1 h, the medium was aspirated and the sections incubated for an
additional 60 min with the primary antibody (either an anti-HA
monoclonal or the Duox S-12 monoclonal antibody) reconstituted in
1X PBS + 5% BSA in a dilution of 1:100 and 1:500 respectively.
Next, the slides were washed three times for 3 min with 1X PBS and
then incubated for an additional 60 min with 1X PBS + 5% BSA
containing the secondary antibody conjugated with fluorescein
isothiocyanate (FITC); anti-rat for the HA antibody or anti-mouse
for the Duox S-12 monoclonal antibody (1:200, Jackson Immune
Research Laboratories). The sections were washed again as above
with 1X PBS + 5% BSA and followed by a short (less than 10 sec)
wash with MilliQ water, allowed to air-dry at RT, and mounted in
vectashield mounting medium with 4’,6-diamidino-2-phenylindole
(DAPI) (Vector Laboratories Inc., Burlingame, CA; Cat no. H-1200)
within 1 h. Fluorescence microscopy was performed with a Leica DM
500B fluorescence microscope by selecting the green emission filter
for FITC, with an excitation filter transmitting light with a
wavelength of 480±40 nm and an emission filter transmitting light
with a wavelength of 527±30 nm. The images were viewed with a Leica
oil-immersion objective lens (40×). Nuclear counter staining was
performed with DAPI, and visualized by selecting a blue filter. The
fluorescence excitation maximum for DAPI is 360±40 nm and the
emission maximum is 470±40 nm.
Immunohistochemical staining of
Duox2-expressing MIA PaCa-2 cells
Ectopically- and stably-expressed human Duox2 cDNA
in MIA PaCa-2 cells was evaluated in sections from formalin-fixed
and paraffin-embedded cells using a standardized method. In brief,
the mouse monoclonal antibody to Duox2 (Duox S-12) in a dilution of
1:1,000 was applied for 1 h at room temperature to
paraffin-embedded MIA PaCa-2 cells transfected with either Duox2 or
vector alone. Binding of the primary antibodies to their antigenic
sites in sections was amplified using Vectastain Elite
avidin-biotin-peroxidase complex kits (Vector Laboratories Inc.).
The antigen-antibody reaction sites were visualized using
3,3-diaminobenzidine for 7 min and, subsequently, sections were
counterstained with Mayer’s hematoxylin. Negative controls were
performed using isotype immunoglobulins appropriate to the primary
mouse antibod ies used (Zymed Laboratories, South San Francisco,
CA).
Immunohistochemical analysis of Duox
expression in human tumors and normal tissues
Immunohistochemistry was performed on a National
Cancer Institute TARP multi-tumor tissue microarray [TMA (MTA3)]
with Duox antibody applied at 1:500 dilution, after antigen
retrieval with pH 6.0 buffer (Dako) with a pressure cooker for 20
min. The antigen-antibody complex was detected with Envision+
(Dako) and DAB chromagen. Staining was scored as 0, 1, 2 and 3
corresponding to negative, weak, intermediate and strong
respectively, and interpreted as negative (0 and 1+) or positive
(2+ and 3+).
Statistical analyses
Two tailed Student’s t-tests and χ2
analyses were performed; values of p<0.05 were considered
significant.
Results
Determination of antibody specificity by
western blot analysis
Monoclonal antibodies (denoted S-3, IgG1; S-12,
IgG1; and S-40, IgG2b) were selected according to their ELISA
immunoreactivity for detailed characterization; specificity was
monitored by western blot analysis. The remaining 21 hybridoma cell
lines were cryopreserved. Previously, we demonstrated that IFN-γ
upregulates the mRNA expression of Duox2 in BxPC-3 human pancreatic
cancer cells in a time- and concentration-dependent manner
(14). In the same study, we found
that Duox2 expression in MIA PaCa-2 and PANC-1 human pancreatic
cancer cell lines was unresponsive to IFN-γ exposure. Using
supernatants from our three clones (S-3, S-12 and S-40), we
performed western blot analysis on IFN-γ-induced, as well as
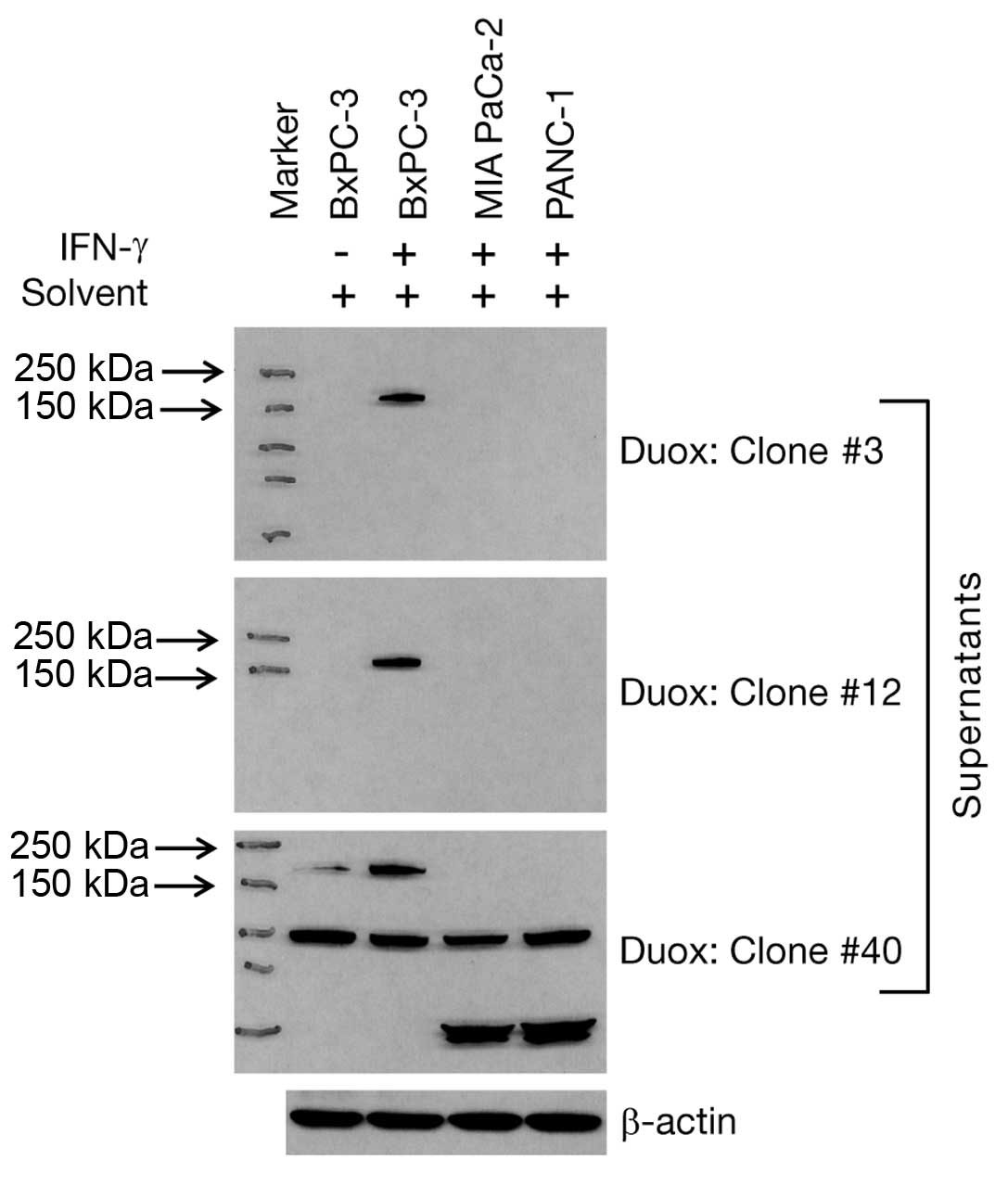
solvent-treated samples of these three cell lines. As shown in
Fig. 2, only IFN-γ-treated BxPC-3
cells responded with upregulated Duox2 protein that was detected by
all three supernatant antibodies. However, the S-40 clone
supernatant demonstrated non-specific protein recognition in all
three cell lines, whether or not treatment with IFN-γ was employed.
Hence, we did not pursue further studies with the S-40 clone.
Because the ELISA affinity for clone S-12 was slightly better than
for the supernatant from clone S-3 (0.78 vs. 0.76), we expanded
cells from the S-12 clone and have affinity purified the S-12 Duox
monoclonal antibody to allow further characterization of its
specificity for western blot analysis, immunofluorescence and
immunohistochemistry, including tissue microarray analysis.
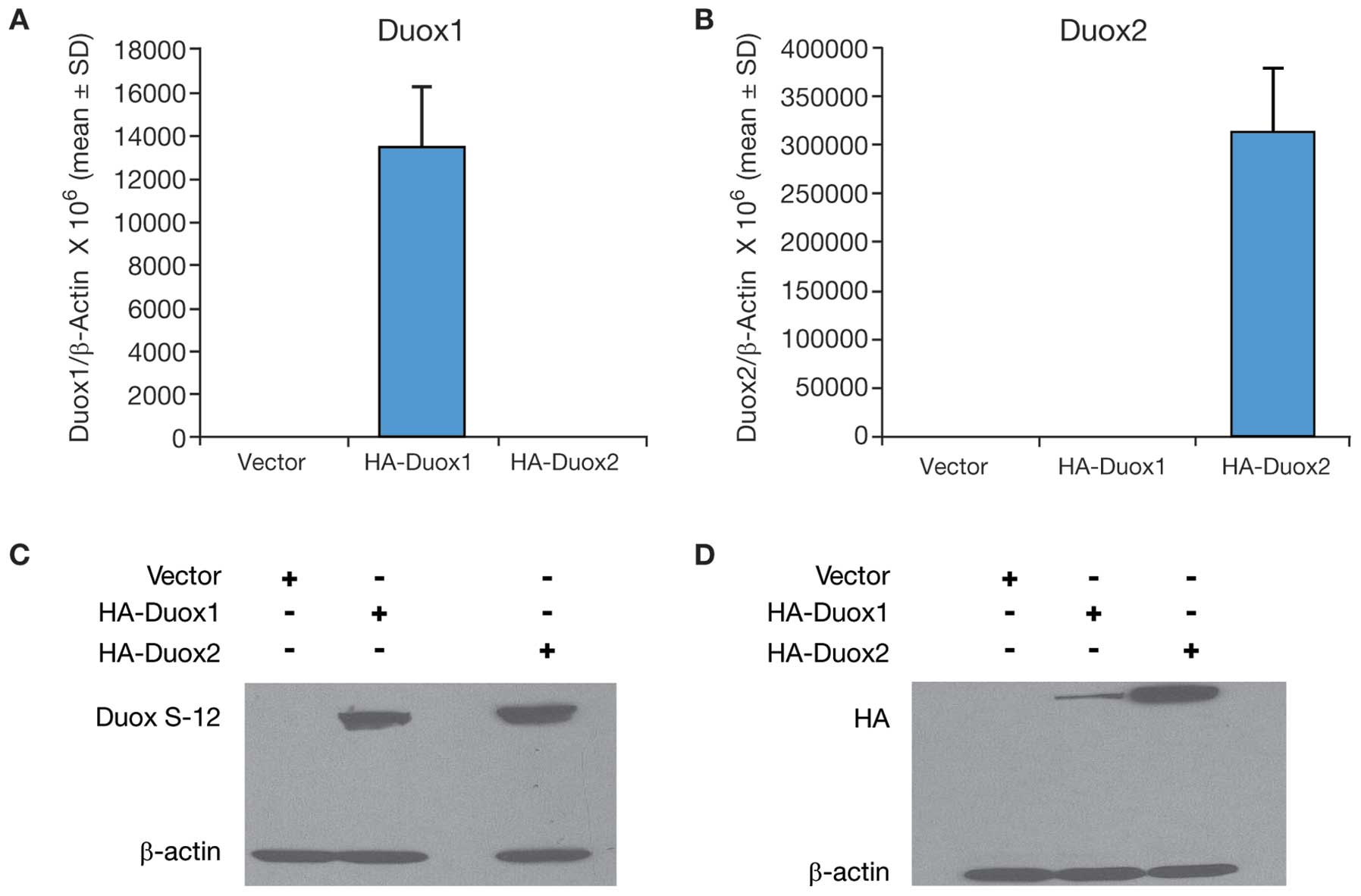
Characterization of Duox2 overexpression
in MIA PaCa-2 cells
To explore Duox2 expression in additional human
tumor cell lines, MIA PaCa-2 pancreatic cancer cells were stably
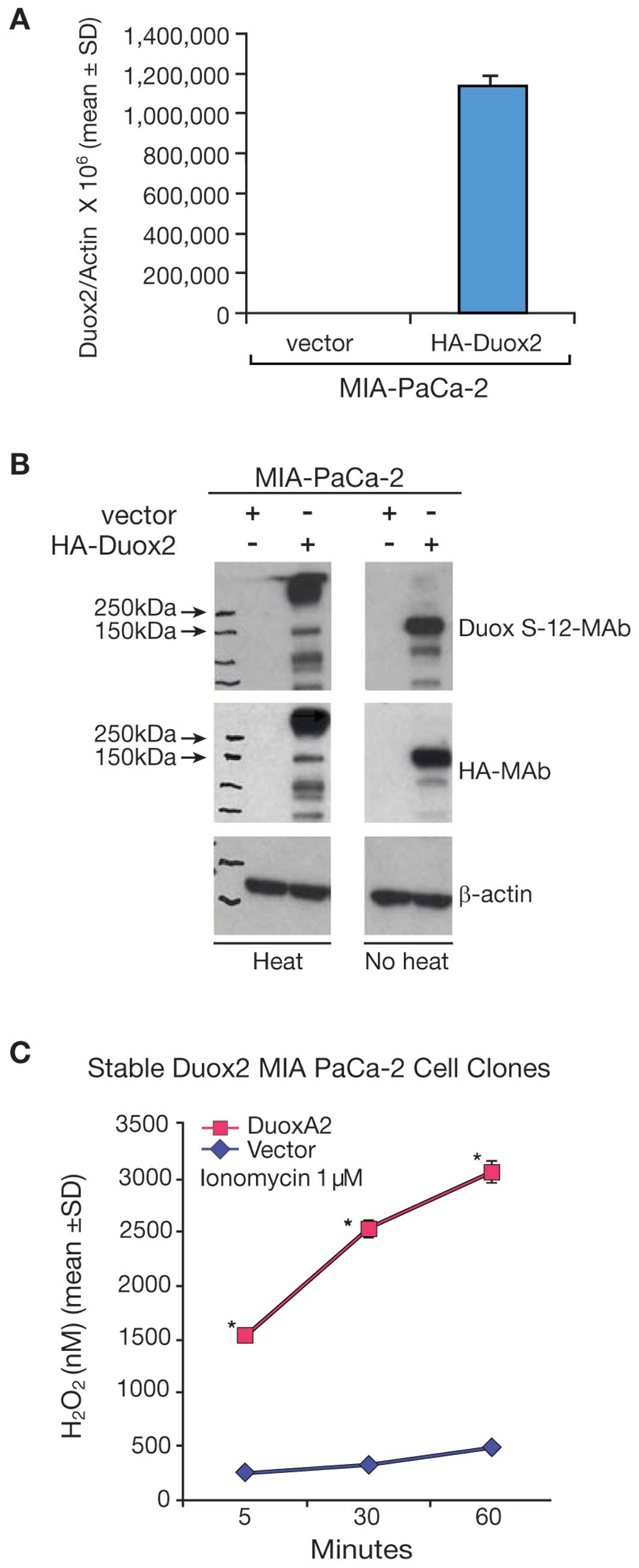
transfected with a full length, human HA-tagged Duox2 cDNA. Duox2
mRNA expression was significantly higher in the Duox2-transfected
clone (Fig. 3A) than in the empty
vector-transfected clone of MIA PaCa-2 cells. We found by western
blot analysis that the Duox S-12 monoclonal antibody selectively
recognized cells that overexpressed Duox2 mRNA (Fig. 3B). We found, furthermore, that
following heat-denaturation of our tumor cell lysates, Duox2
protein appeared to aggregate as a high molecular weight band,
whereas Duox2 protein was recognized at the expected, ∼185 kDa size
in non-heat denatured samples from the overexpressing cell line. We
made the same observation utilizing an antibody directed against HA
(Fig. 3B), where the HA antibody
recognized a protein in the heat-denatured sample with a molecular
weight above ∼250 kDa. Hence, immunoblots of the same cell extracts
demonstrated that the Duox S-12 monoclonal antibody specifically
recognized human Duox2 as a unique protein that was of the same
size (∼185 kDa) as the protein recognized by the HA-tag antibody.
Pre-immune serum exhibited negligible background staining in the
whole transferred blot (data not shown). These results reinforce
the previous immunoblots (Fig. 2)
demonstrating that IFN-γ enhances Duox2 expression in crude cell
extracts from BxPC-3 pancreatic cancer cells; in those experiments,
IFN-γ upregulated the mRNA expression of Duox2, but not Duox1 or
any other member of the NADPH oxidase gene family (data not
shown).
Expression of Duox2 and DuoxA2 leads to
H2O2 production in MIA PaCa-2 cells
To demonstrate the functional activity of Duox2 in
MIA PaCa-2 cells, the stably-transfected, Duox2 MIA PaCa-2 cell
clones were transiently transfected with a DuoxA2 cDNA or an empty
vector; ionomycin-enhanced production of H2O2
was then examined using the Amplex Red® reagent. As
shown in Fig. 3C, MIA PaCa-2 cells
stably expressing Duox2 cDNA alone produced minimal amounts of
H2O2; similarly, MIA PaCa-2 cells expressing
the empty vector exhibited no significant
H2O2 production, even in presence of the
calcium ionophore, ionomycin (data not shown). However, transient
expression of DuoxA2 cDNA (which has virtually no constitutive
expression in this cell line) in the stably expressing Duox2 MIA
PaCa-2 cells resulted in a dramatic increase in
H2O2 production, p<0.05.
Immunohistochemical analysis of MIA
PaCa-2 cells stably transfected with Duox2 cDNA
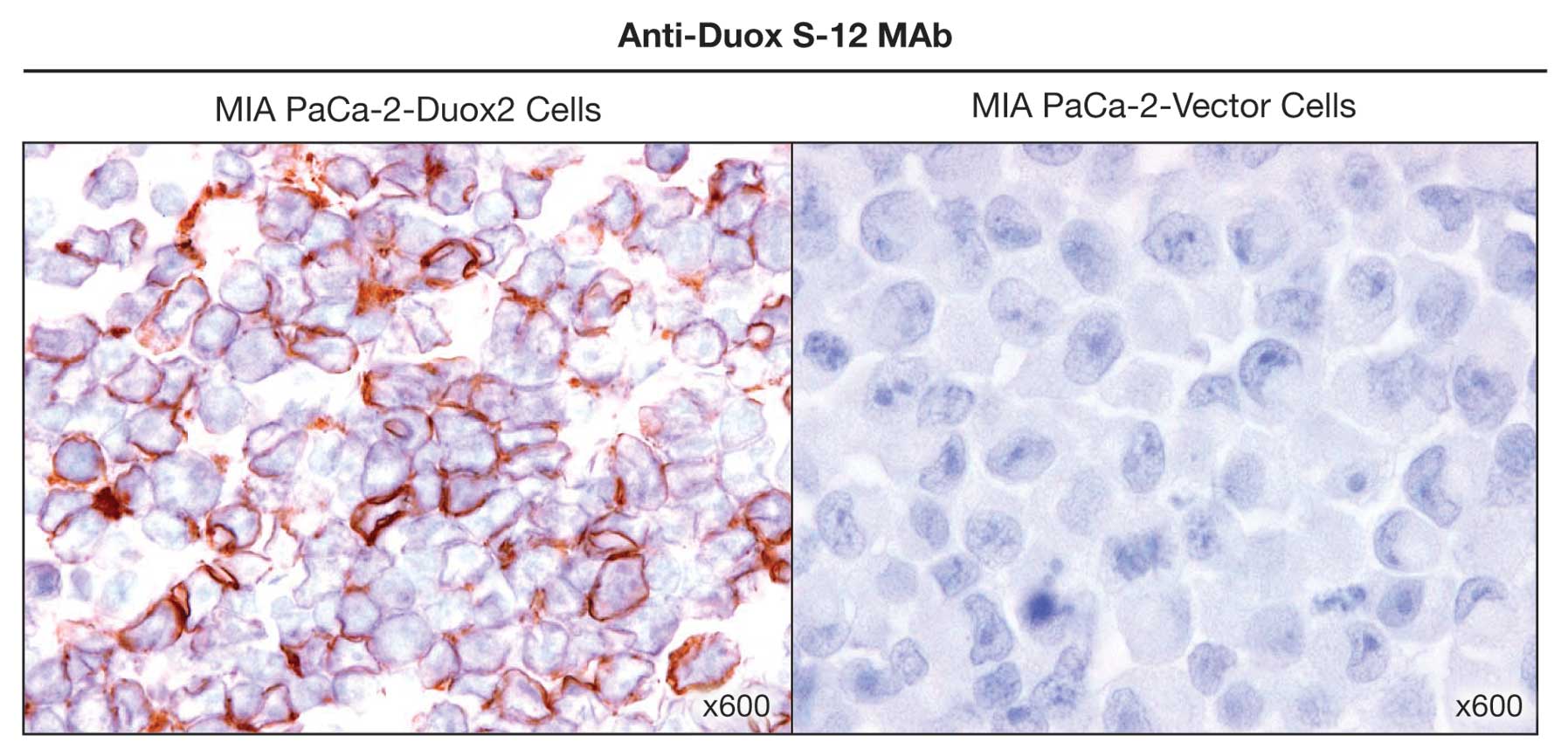
We next investigated whether the anti-Duox S-12
monoclonal antibody would be useful for immunohistochemical
staining. Using fixed sections of MIA PaCa-2 cells that
overexpressed HA-tagged, full length human Duox2, we found
negligible background staining when the sections were reacted with
pre-immune serum (data not shown). When paraffin-embedded,
Duox2-overexpressing MIA PaCa-2 cell pellets were examined with our
Duox S-12 antibody (Fig. 4), we
found the expected, prominent immuno-staining of the tumor cell
plasma membrane. On the other hand, expression of Duox2 protein
could not be detected in vector-transfected MIA PaCa-2 cells
(Fig. 4).
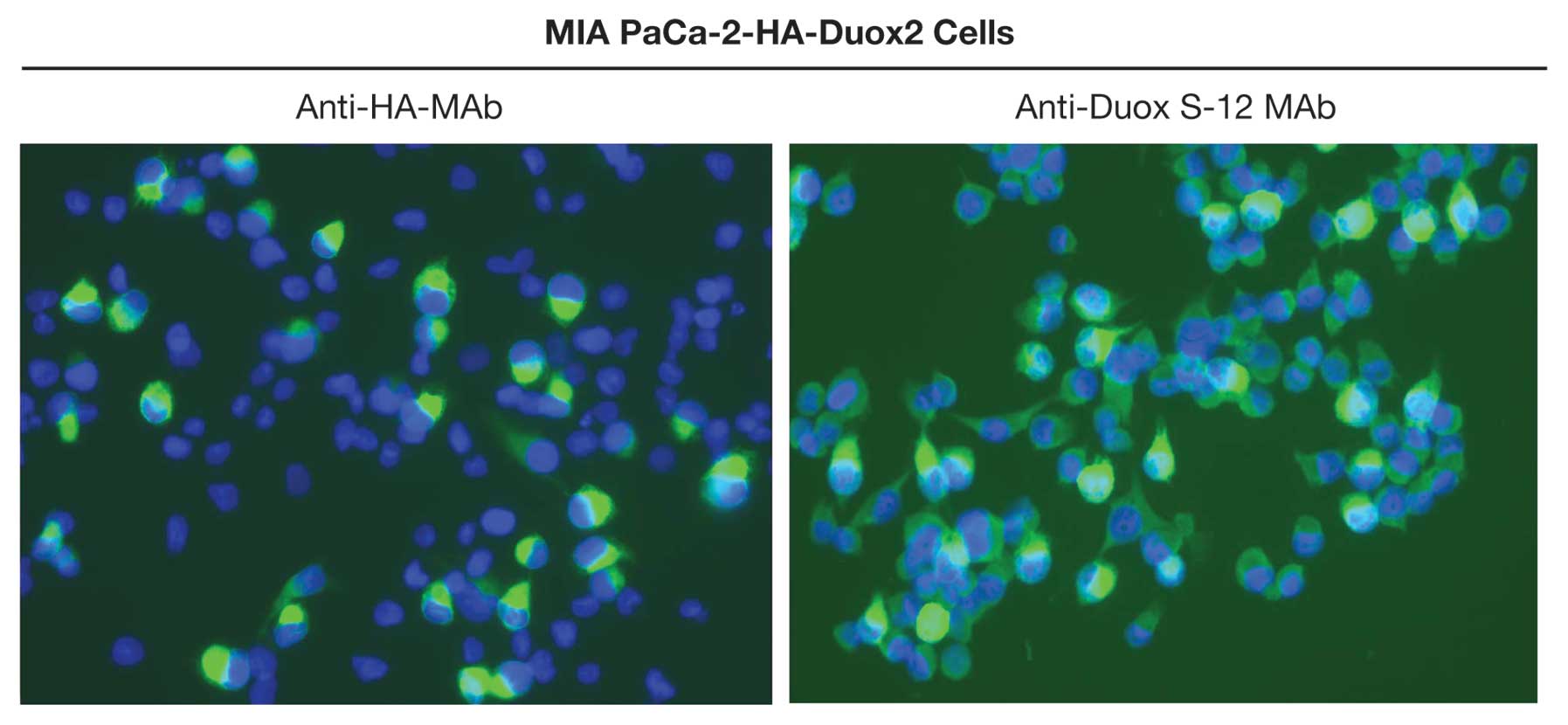
Immunofluorescence analysis of MIA PaCa-2
cells expressing Duox2 cDNA
The Duox S-12 monoclonal antibody was further
characterized by performing immunofluorescent staining of stably
transfected, clonally selected MIA PaCa-2 cells expressing both
Duox2 mRNA and an HA tag. Duox2-transfected MIA PaCa-2 cells
stained positively with either the anti-HA antibody or the
anti-Duox S-12 MAb (Fig. 5). The
immunofluorescence pattern for both Duox2 and HA demonstrated
intense cytoplasmic staining, with an apparent further enhancement
in the plasma and nuclear membranes of some cells. In contrast,
cells expressing a control vector did not demonstrate
immunofluorescence with either the anti-HA antibody or the
anti-Duox S-12 monoclonal antibody (data not shown).
Duox S-12 monoclonal antibody
cross-reacts with human Duox1 protein
As shown in Fig. 1,
there is significant homology (∼85%) between Duox 2 and Duox1 at
the amino terminus of both proteins. This raised the question as to
whether our Duox S-12 monoclonal antibody might cross-react with
the human Duox1 protein. To resolve this issue, we transiently
transfected both HA-tagged human Duox1 and HA-tagged human Duox2
cDNAs into COS-7 cells along with the appropriate vector controls.
Analysis by real-time RT-PCR revealed that expression of both Duox1
and Duox2 was detected in COS-7 cells following transient
transfection compared to their vector controls (Fig. 6A and B). As demonstrated in
Fig. 6C, our Duox S-12 monoclonal
antibody recognized both Duox2 and Duox1 proteins. This observation
is supported by the western blot analysis shown in Fig. 6D, where an antibody against HA
recognized both human Duox1 and Duox2, since both cDNAs were tagged
with HA. Thus, we describe our newly-characterized antibody as Duox
S-12 because it detects both Duox1 and 2 proteins.
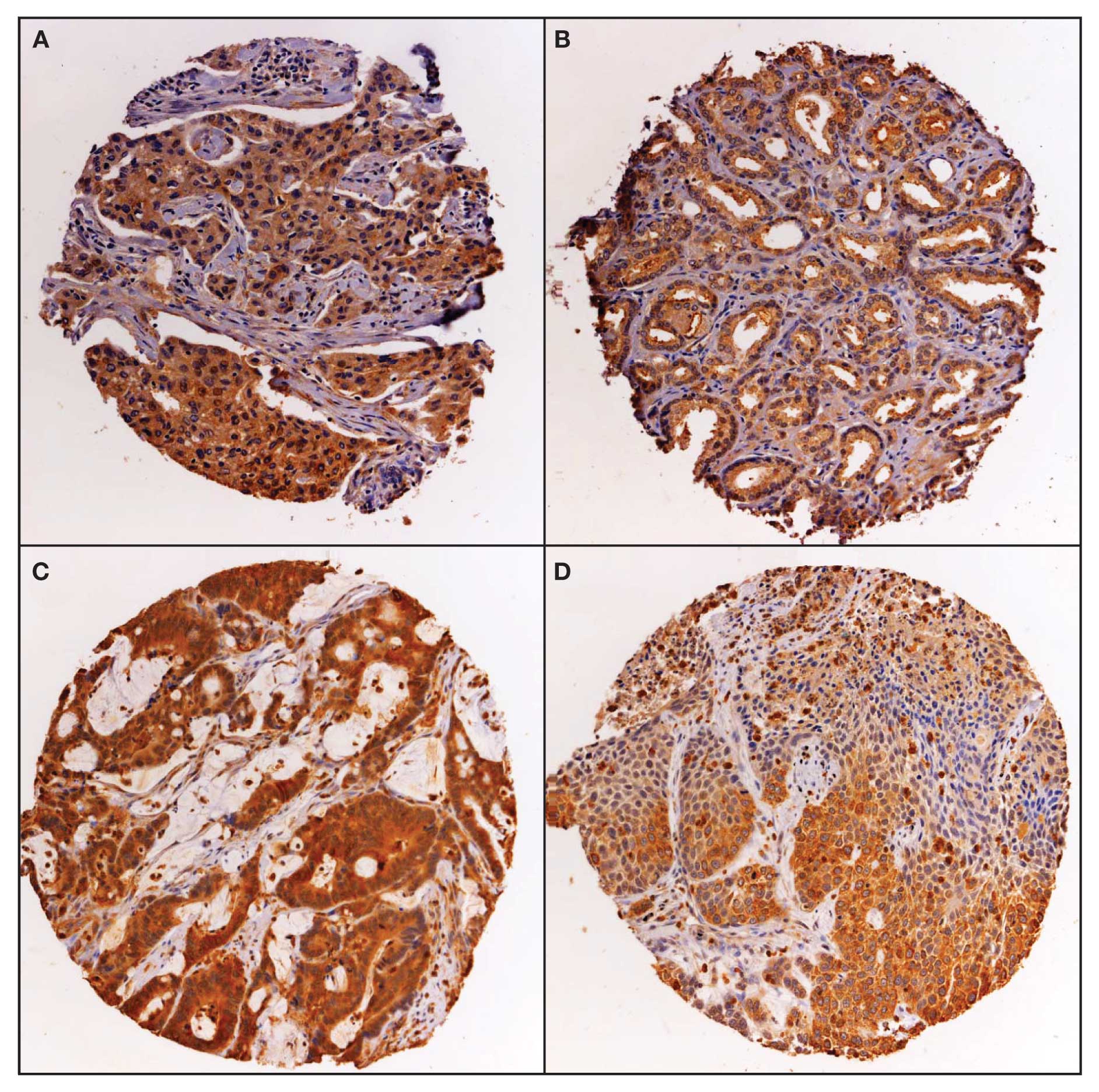
Expression of Duox in human tumors and
normal tissues
Expression of Duox was studied by
immunohistochemistry on a multi-tumor tissue microarray (TARP MTA3)
containing 217 analyzable tumor samples and a sampling of normal
tissue. Duox stains in a cytoplasmic pattern, with some nuclear
expression and was scored as positive/negative (Fig. 7). Duox expression was weakly
positive in normal bone marrow, pancreas and stomach tissues; and
negative in the other normal tissues evaluated, including bladder,
brain, liver, lung, lymph node, small bowel and testis. The
distribution of positive staining by tumor type for the multi-tumor
TMA is presented in Table I. The
distribution of positive staining was statistically different
between tumor types by χ2 statistic (p<0.01). The
brain tumors (glioblastoma multiforme) had the lowest rate of
expression at 14%, while the prostate cancer samples had the
highest frequency of expression at 92%.
 | Table IDistribution of expression levels of
dual oxidase in human malignancies. |
Table I
Distribution of expression levels of
dual oxidase in human malignancies.
| Tumor type
(MTA-3) | Negative | Positive |
|---|
| Glioblastoma
multiforme | 12 (86%) | 2 (14%) |
| Lymphoma | 23 (79%) | 6 (21%) |
| Melanoma | 7 (64%) | 4 (36%) |
| Ovarian Cancer | 16 (55%) | 13 (45%) |
| Breast Cancer | 11 (34%) | 21 (66%) |
| Colon Cancer | 18 (38%) | 29 (62%) |
| Lung Cancer | 4 (14%) | 25 (86%) |
| Prostate
Cancera | 2 (8%) | 24 (92%) |
Discussion
The goal of the present study was to generate
monoclonal antibodies against the human Duox2 protein to provide
reagents that would be useful for investigating the role of Duox2
in cancer, where alterations in oxidant tone play a critical role
in cell growth and proliferation (18). High quality, commercial monoclonal
antibodies against Duox2 are not available; and thus, Duox protein
expression has not been widely examined in cancers of any histology
or chronic inflammatory conditions in comparison to normal tissues.
Available polyclonal antibodies against human Duox2 have not been
convincingly demonstrated to be reliable for most laboratory
research purposes; the lack of widely available antibodies has also
limited biochemical studies of this oxidase. Hence, we successfully
focused our efforts on generating monoclonal antibodies against
human Duox2 protein, which could be used in various immunological
assays, including western blot analysis and immunohistochemistry,
and that would allow a more detailed study of the physiological and
pathophysiological role of Duox2 at the protein level.
Using the Duox S-12 monoclonal antibody, we
confirmed our previous results demonstrating that IFN-γ upregulates
Duox2 at the protein as well as mRNA levels (Fig. 2) in BxPC-3 human pancreatic cancer
cells (14). More importantly, we
found that in the MIA PaCa-2 human pancreatic cancer cell line,
which is not responsive to IFN-γ and does not constitutively
express Duox2, overexpression of Duox2 and its cognate maturation
factor, DuoxA2, produced a functionally active Duox2 protein that
could be quantitated with the Duox S-12 antibody (Fig. 3B). We have also clearly
demonstrated the localization of Duox2 in the plasma membranes of
MIA PaCa-2 cells by immunohistochemistry, and both the cytoplasmic
and peripheral expression of Duox2 in these cells by
immunofluorescence (Figs. 4 and
5). This distribution is similar,
only in part, to that described for normal thyroid tissue (19), and respiratory and gastrointestinal
epithelium (17,20) where Duox2 appears to localize at
the apical surface of thyroid follicles or at the enterocyte brush
border, suggesting that Duox2 is expressed only in the most
differentiated of these normal cells.
As demonstrated in Fig.
6, probably as a result of the extensive amino acid homologies
that exist between the two dual oxidases, we found that the Duox
S-12 antibody cross-reacted with Duox1 in COS-7 cells transfected
with a Duox1 cDNA. However, our recent studies have shown using
real time RT-PCR that Duox1 is only minimally expressed at the mRNA
level, and clearly not upregulated, in many human malignancies,
including cancers of the gastrointestinal tract, breast, lung,
prostate, brain and melanoma (7).
Furthermore, investigators have demonstrated that expression of
Duox1 is epigenetically silenced in non-small cell lung cancer
(21). Thus, we felt that it was
reasonable to examine human tumor tissue microarrays for expression
of Duox protein with our Duox S-12 antibody under the operating
assumption that we would, for the most part, be evaluating the
expression and distribution of Duox2 in such experiments.
Immunohistochemical examination of Duox expression
in normal human tissues and in a range of human tumors suggests
that expression in carcinomas and adenocarcinomas is higher than in
tumors of other histological types (melanoma, lymphoma,
glioblastoma multiforme). Expression was highest in prostate
adenocarcinoma and lung cancers (both adeno-carcinoma and squamous
cell carcinoma); and greater than 60% in breast and colon cancer.
Duox2 was expressed in only a limited number of normal tissues,
none of which demonstrated strong (3+) expression, compared to the
cancers, where 73% of the prostate adenocarcinomas were 3+ in
expression, and 24% of breast, colon and lung cancers were 3+.
Overexpression of Duox in colon cancer is consistent
with the upregulation of Duox2 mRNA that has been demonstrated in
patients with two pre-cancerous conditions, Crohn’s disease and
ulcerative colitis (13). The
expression of Duox2 in the gastrointestinal tract has recently been
demonstrated to be under the control of pro-inflammatory cytokines
that are known to be associated with inflammatory bowel disease
(22). Thus, the
immunohistochemical studies presented here may support the
hypothesis that cytokine-mediated upregulation of Duox2 could
contribute to the cascade of reactive oxygen production known to
accompany pre-malignant, chronic inflammatory disorders of the
gastrointestinal tract (23).
Furthermore, in light of recent studies demonstrating that either
inhibition of Nox-related oxidant stress or other anti-inflammatory
interventions can significantly diminish the late effects of
gastrointestinal inflammation (24,25),
our demonstration of increased Duox expression in gastrointestinal
cancer suggests that pharmacologic inhibition of Nox expression
might be a novel therapeutic intervention capable of interdicting
the development of oxidant-mediated neoplasia.
In summary, we have developed a novel monoclonal
antibody against the dual oxidase members of the Nox gene family
and have used that antibody to demonstrate the over-expression of
Duox2 in several human malignancies. In future studies, we will use
this tool to evaluate the role of Duox2 in the development and
prognosis of a variety of solid tumors, and in mechanistic studies
aimed at discovering small molecule inhibitors that will
specifically block the oxidase function of this protein.
Abbreviations:
|
Duox
|
dual oxidase
|
|
ROS
|
reactive oxygen species
|
|
Nox
|
NADPH oxidase
|
|
DuoxA2
|
dual oxidase maturation factor
|
Acknowledgements
This study was supported by the
Division of Cancer Treatment and Diagnosis and the Center for
Cancer Research of the National Cancer Institute, National
Institutes of Health. The content of this publication does not
necessarily reflect the views of policies of the Department of
Health and Human Services, nor does mention of trade names,
commercial products, or organizations imply endorsement by the
United States Government. In addition, we wish to thank Dr Thomas
Leto of the National Institute of Allergy and Infectious Diseases,
NIH for his kind gift of the HA-Duox1 plasmid and Dr Helmut
Grasberger, of the University of Michigan, for his kind gift of the
HA-Duox2 and Myc-DuoxA2 plasmids.
References
|
1
|
Leroyer V, Werner L, Shaughnessy S,
Goddard GJ and Orr FW: Chemiluminescence and oxygen radical
generation by Walker carcinosarcoma cells following chemotactic
stimulation. Cancer Res. 47:4771–4775. 1987.PubMed/NCBI
|
|
2
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
3
|
Orr FW, Adamson IY, Warner D, Leroyer V,
Werner L, Shaughnessy S and Young L: The effects of oxygen
radical-mediated pulmonary endothelial damage on cancer metastasis.
Mol Cell Biochem. 84:189–198. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shaughnessy SG, Whaley M, Lafrenie RM and
Orr FW: Walker 256 tumor cell degradation of extracellular matrices
involves a latent gelatinase activated by reactive oxygen species.
Arch Biochem Biophys. 304:314–321. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kamata T: Roles of Nox1 and other Nox
isoforms in cancer development. Cancer Sci. 100:1382–1388. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Juhasz A, Ge Y, Markel S, Chiu A,
Matsumoto L, van Balgooy J, Roy K and Doroshow JH: Expression of
NADPH oxidase homologues and accessory genes in human cancer cell
lines, tumours and adjacent normal tissues. Free Radic Res.
43:523–532. 2009. View Article : Google Scholar
|
|
8
|
Caillou B, Dupuy C, Lacroix L, Nocera M,
Talbot M, Ohayon R, Deme D, Bidart JM, Schlumberger M and Virion A:
Expression of reduced nicotinamide adenine dinucleotide phosphate
oxidase (ThoX, LNOX, Duox) genes and proteins in human thyroid
tissues. J Clin Endocrinol Metab. 86:3351–3358. 2001.
|
|
9
|
Bae YS, Choi MK and Lee WJ: Dual oxidase
in mucosal immunity and host-microbe homeostasis. Trends Immunol.
31:278–287. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pachucki J, Wang D, Christophe D and Miot
F: Structural and functional characterization of the two human
ThOX/Duox genes and their 5’-flanking regions. Mol Cell Endocrinol.
214:53–62. 2004.PubMed/NCBI
|
|
11
|
Donko A, Peterfi Z, Sum A, Leto T and
Geiszt M: Dual oxidases. Philos Trans R Soc Lond B Biol Sci.
360:2301–2308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Grasberger H and Refetoff S:
Identification of the maturation factor for dual oxidase. Evolution
of an eukaryotic operon equivalent. J Biol Chem. 281:18269–18272.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lipinski S, Till A, Sina C, Arlt A,
Grasberger H, Schreiber S and Rosenstiel P: DUOX2-derived reactive
oxygen species are effectors of NOD2-mediated antibacterial
responses. J Cell Sci. 122:3522–3530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Y, Antony S, Juhasz A, Lu J, Ge Y,
Jiang G, Roy K and Doroshow JH: Up-regulation and sustained
activation of Stat1 are essential for interferon-gamma
(IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2
(DuoxA2) expression in human pancreatic cancer cell lines. J Biol
Chem. 286:12245–12256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fukushima N, Koopmann J, Sato N, Prasad N,
Carvalho R, Leach SD, Hruban RH and Goggins M: Gene expression
alterations in the non-neoplastic parenchyma adjacent to
infiltrating pancreatic ductal adenocarcinoma. Mod Pathol.
18:779–787. 2005. View Article : Google Scholar
|
|
16
|
Kita H, Hikichi Y, Hikami K, Tsuneyama K,
Cui ZG, Osawa H, Ohnishi H, Mutoh H, Hoshino H, Bowlus CL, Yamamoto
H and Sugano K: Differential gene expression between flat adenoma
and normal mucosa in the colon in a microarray analysis. J
Gastroenterol. 41:1053–1063. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
El Hassani RA, Benfares N, Caillou B,
Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D,
Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M,
Virion A and Dupuy C: Dual oxidase2 is expressed all along the
digestive tract. Am J Physiol Gastrointest Liver Physiol.
288:G933–G942. 2005.PubMed/NCBI
|
|
18
|
Lambeth JD: Nox enzymes, ROS, and chronic
disease: an example of antagonistic pleiotropy. Free Radic Biol
Med. 43:332–347. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lacroix L, Nocera M, Mian C, Caillou B,
Virion A, Dupuy C, Filetti S, Bidart JM and Schlumberger M:
Expression of nicotinamide adenine dinucleotide phosphate oxidase
flavoprotein DUOX genes and proteins in human papillary and
follicular thyroid carcinomas. Thyroid. 11:1017–1023. 2001.
View Article : Google Scholar
|
|
20
|
Forteza R, Salathe M, Miot F, Forteza R
and Conner GE: Regulated hydrogen peroxide production by Duox in
human airway epithelial cells. Am J Respir Cell Mol Biol.
32:462–469. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Luxen S, Belinsky SA and Knaus UG:
Silencing of DUOX NADPH oxidases by promoter hypermethylation in
lung cancer. Cancer Res. 68:1037–1045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hamm CM, Reimers MA, McCullough CK, Gorbe
EB, Lu J, Gu CC, Li E, Dieckgraefe BK, Gong Q, Stappenbeck TS,
Stone CD, Dietz DW and Hunt SR: NOD2 status and human ileal gene
expression. Inflamm Bowel Dis. 16:1649–1657. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Farrow B and Evers BM: Inflammation and
the development of pancreatic cancer. Surg Oncol. 10:153–169. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Masamune A, Watanabe T, Kikuta K, Satoh K
and Shimosegawa T: NADPH oxidase plays a crucial role in the
activation of pancreatic stellate cells. Am J Physiol Gastrointest
Liver Physiol. 294:G99–G108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Guerra C, Collado M, Navas C, Schuhmacher
AJ, Hernandez-Porras I, Canamero M, Rodriguez-Justo M, Serrano M
and Barbacid M: Pancreatitis-induced inflammation contributes to
pancreatic cancer by inhibiting oncogene-induced senescence. Cancer
Cell. 19:728–739. 2011. View Article : Google Scholar : PubMed/NCBI
|