Introduction
Ovarian cancer is the most common cause of cancer
death from gynecologic tumours and currently causes ∼100,000 deaths
per year world-wide (1). While
treatment of ovarian cancer with platinum-based agents has been
established for decades, these still remain the most active
substances for this entity (2).
Accordingly, primary resistance to platinum-based therapy is
associated with a worse disease-free and overall survival (3). However, virtually all patients
eventually develop secondary resistance to platinum based agents
and compounds used for second- or third-line treatment. Other
substances display substantially less anticancer activity as
compared to platinum (4). There is
a definite clinical need to develop new treatment strategies to
overcome platinum resistance. As survival is strongly influenced by
immunological parameters, immunotherapeutic strategies appear
promising, therefore a better understanding of the interaction
between ovarian tumour cells and cells of the immune system is
necessary.
Natural-killer (NK)-cells play an important role in
immune surveillance and co-ordinating responses of other immune
cells. Most tumour cells express surface molecules that can be
recognized by activating receptors on NK-cells (5). The expression of these receptors make
such cells susceptible to endogenous NK-cells, but malignant cells
have developed mechanisms to evade innate immune surveillance
(6–8). In patients with cancer, it is
presumed that tumour cells have developed mechanisms to suppress
NK-cell activation and resist lysis by endogenous NK-cells, but the
molecular basis for tumour cell resistance against this lysis is
not well understood.
Alterations of the serine/threonine kinase AKT/PKB
pathway have been detected in several human malignancies including
ovarian cancer (9). AKT has a
broad range of downstream effectors that regulate cell processes
such as cell growth, cell cycle progression, survival, migration,
and angiogenesis (10). The AKT
pathway is a promising target for cancer therapy, as it is a main
nodal point where extracellular and intracellular oncogenic signals
are integrated. Due to the key role of AKT in malignant
transformation numerous inhibitors of the AKT-pathway have been
developed, and are currently in various stages of clinical
development (11).
In human specimens of ovarian cancer AKT was found
to be activated in 68% (9) and
PI3K, an upstream component of the AKT-pathway, was found to be
mutated in 12% of the cases (12).
Recent evidence by our group and others has shown that
overactivation of the AKT-pathway may be associated with platinum
resistance (13–17). It was demonstrated that parental
A2780 cells become platinum resistant by overexpression of AKT and
that platinum resistance in A2780cis cells can be overcome by
transfection with siRNA downregulating AKT (17).
In the present study we enlightened the interaction
between ovarian tumour cells with different AKT expression levels
and NK-cells.
Materials and methods
Cell culture
A2780 and A2780cis cell lines (both are p53 and KRAS
wild-type cell lines) were obtained from ECACC (Salisbury, UK). The
cis-platinum-resistant A2780cis cell line has been developed by
chronic exposure of the parental cis-platinum-sensitive A2780 cell
line to increasing concentrations of cis-platinum (18).
Preparation of cell lysates and western
blotting
Preparation of cell lysates was performed as
previously described (17).
Membranes were probed overnight with anti-phospho-Akt antibody from
Epitomics (Burlingame, CA, USA), anti-B7-H1 antibody from
eBioscience (Frankfurt, Germany) or anti-β-actin antibody from
Abcam (Cambridge, UK), respectively. Secondary horseradish
peroxidase (HRP)-conjugated antibodies were from Cell Signaling
(Frankfurt, Germany). The chemiluminescent HRP substrate solution
(Millipore, Schwalbach, Germany) was used for detection.
NK-cell preparation and lysis assay
PBMC were obtained from healthy volunteers by
density gradient centrifugation (Biocoll; Biochrom AG, Berlin,
Germany). Monocytes were depleted by adherence and the remaining
non-adherent PBL were further cultured on irradiated (30 Gy)
RPMI-8866 feeder cells to obtain polyclonal NK-cell populations
(19). After 6 days of co-culture
500 U IL-2 (Peprotech, Hamburg, Germany) were added per ml and 48 h
later the polyclonal NK-cell population (effector cells) was used
in different killing assays. Therefore, the NK-cells were labeled
with eFluor 670 (eBioscience) and lytic activity against
CFSE-stained (eBioscience) tumour cells (targets; 105
cells/well) was assessed in a modified 5 h FATAL assay using
various effector:target ratios (20). Cells were detached by
trypsinisation and target cell lysis was determined by flow
cytometric analysis of 30,000 target cells in a FACScan flow
cytometer (Calibur, BD Biosciences, Heidelberg, Germany). eFluor
670-negative target cells were selected by gating and the
percentage of CFSE cells within this population was determined.
Spontaneous leakage of CFSE was determined by incubating the target
cells with medium alone.
Flow cytometry analysis
Cells/sample (106) were detached with
Accutase (PAA, Cölbe, Germany), blocked and stained with Alexa
Fluor® 488 conjugated anti-human MICA/B antibody
(eBioscience) or with the relevant isotype control according to the
manufacturer’s instructions.
RT-PCR
Total cellular RNA was extracted from cells by
RNeasy kit (Qiagen, Hilden, Germany). The quality and quantity of
RNA preparations was assessed with NanoDrop ND-1000 (PEQLAB
Biotechnologie GmBH, Erlangen, Germany). Generation of cDNAs by
reverse transcription and RT-PCR reaction was performed in one step
(Qiagen OneStep RT-PCR kit; Qiagen) according to the manufacturer’s
instructions. The gene for the constitutively expressed ribosomal
protein L13A (rpL13A) was used as housekeeping gene (21) in order to monitor RNA quality and
cDNA synthesis, and to ensure that equivalent amounts of cDNA were
used in all PCR amplifications. Oligonucleotides were synthesized
by Sigma-Aldrich (Taufkirchen, Germany): rpL13A f,
5′-TACGCTGTGAAGGCATCAAC-3′; rpL13A r, 5′-CACCATCCGCTTT
TTCTTGT-3′; bcl-2 f, 5′-ATGGCGCACGCTGGGAGAAC-3′;
bcl-2 r, 5′-GCATGCTGGGGCCGTACAGT-3′; bcl-xL f,
5′-CGGTGAATGGAGCCACTGCG-3′; bcl-xL r,
5′-GTCACTGAATGCCCGCCGGT-3′; bcl-w f,
5′-CCCAGGCTCAGCCCAGCAAC-3′; bcl-w r,
5′-CCCAGTTCCCCTCCCGCAGA-3′; ciap-1 f,
5′-AGCCTGCTTTGCCTGTGGTGG-3′; ciap-1 r,
5′-GCCGCAGCATTTCCTTTAACCCA-3′; ciap-2 f,
5′-GCCTTGATGAGAAGTTCCTACCCCT-3′; ciap-2 r,
5′-AGCCCATTTCCACGGCAGCA-3′; bim f,
5′-CAGCCACCCTGCGAACCCTG-3′; bim r,
5′-GGGCAGCTGTCCCCTTCACC-3′; bak f, 5′-ACGG
CAGCTCGCCATCATCG-3′; bak r, 5′-GAAGAGCCACCACACGGCCC-3′;
bax f, 5′-TGATGGACGGGTCCGGGGAG-3′; bax r,
5′-GGGGAGAGGGCACCACTGTGA-3′; PVRL2 (coding for CD112)l; f,
5′-GGACCCTGGCCGGAACTGTC-3′; PVRL2 r,
5′-AATGATGGCGGCGATGATGCCC-3′; PVR (coding for CD155) f,
5′-CTGATCCTGCTGGGGATCGGG-3′; PVR r,
5′-CCCCTCTCAGTCCCGACGCT-3′; ulbp1 f,
5′-CCCCGCGTTCCTTCTGTGCC-3′; ulbp1 r,
5′-TAGACAGGCGGCCTCCCTGAA-3′; ulbp2 f,
5′-CCACGGTGGTGTGCGGTTCA-3′; ulbp2 r,
5′-TGGCCAGACAGAAGGGCGAGT-3′; ulbp3 f,
5′-CTTCCGCGCCTCGCGATTCT-3′; ulbp3 r,
5′-AGTCTGAGCCTCTGCCCCACC-3′; MICA f,
5′-CTGCCTGCAGGAACTACGGCG-3′; MICA r,
5′-AGCAGCCAGCAGCAACAGCAGAA-3′; MICB f,
5′-GAGCGGGGCGCAGGTGACTAA-3′; MICB r,
5′-AGCGCAGGAAGGGCTGACCA-3′; mmp-9 f,
5′-TTGACAGCGACAAGAAGTGG-3′; mmp-9 r,
5′-GCCATTCACGTCGTCCTTAT-3′; adam10 f,
5′-GGCGGGGATGGGAGGTCAGT-3′; adam10 r,
5′-AGGTGCTCCAACCCAAGCCA-3′; adam17 f,
5′-GCTGCAACAGCGACTGCACG-3′; adam17 r,
5′-GCGCCGAAGGGATCACAGGG-3′; timp-3 f,
5′-AAAGGAGGGGCCCTTCGGCA-3′; timp-3 r,
5′-CTTCTGCCGGATGCAGGCGT-3′. All PCR products were analyzed by
separation on a 2% agarose gel stained with GelRed (Biotium,
Hayward, CA, USA).
Results
This study focused on natural-killer (NK)-cells and
their interaction with a platinum-sensitive parental human ovarian
cancer cell line A2780 and the corresponding platinum-resistant
cell line A2780cis. These two cell lines were used because they are
well characterized and furthermore they have the same genetic
background but differ with regard to platinum resistance. First of
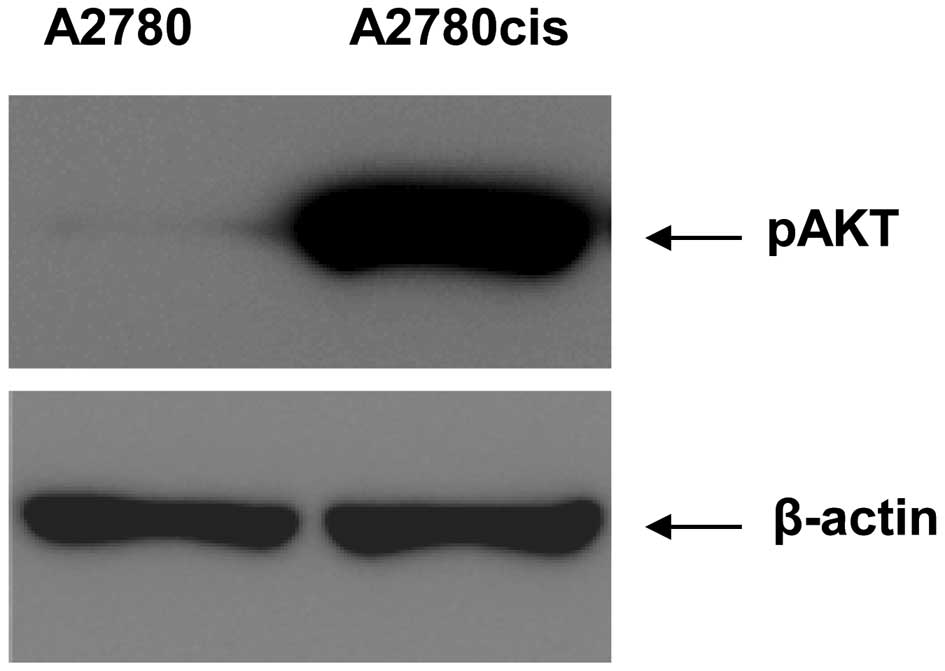
all the different pAKT expression levels were analyzed in the two
cell lines by western blotting (Fig.
1). The expression of pAKT is strongly induced in A2780cis
cells in comparison to the expression in the parental A2780
cells.
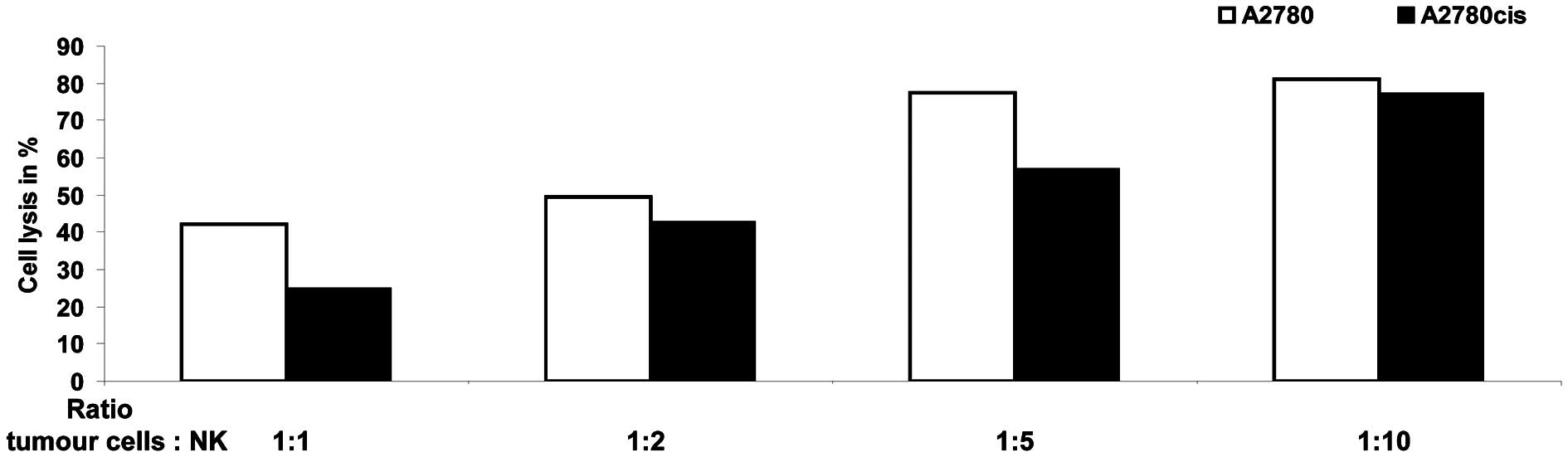
Next the killing efficiency of NK-cells, prepared as
described in the Material and methods, were confirmed with a
luciferase-based assay in the reporter cell line K562 (data not
shown). After their killing capacity was established, the NK-cells
were used in the modified FATAL assay with A2780 and A2780cis
cells. As shown in Fig. 2 the
killing rates of tumour cells differ significantly between the two
cell lines. Parental A2780 cells seem to be better targets for
NK-cell mediated killing than the A2780cis cells at all ratios
between NK-cells and tumour cells used in this experiment. Only if
10 times more NK-cells than tumour cells are used the killing rate
of A2780cis cells reached nearly that of wild-type A2780 cells.
Similar results were obtained with NK-cell preparations derived
from other healthy donors. Therefore the observed differences in
the killing rate between A2780 and A2780cis cells are neither
dependent on the healthy donor nor the NK-cell preparation.
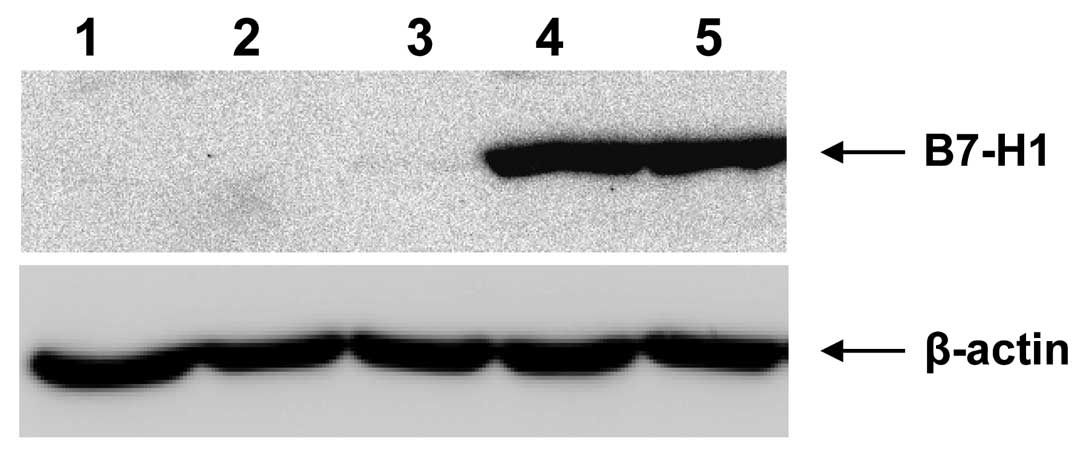
Next we addressed the question why the interaction
and killing between NK-cells and A2780 cells is much better. B7-H1
(programmed cell death 1 ligand 1) ligand for the receptor PD1
(programmed death 1) protects cells against NK-cell mediated lysis,
because the interaction of B7-H1 with PD1 results in inhibition of
T-cell-receptor-mediated proliferation and cytokine production.
However, as shown in Fig. 3
neither A2780 (Fig. 3, lane 1) nor
A2780cis cells (Fig. 3, lane 2)
express the B7-H1 ligand. Likewise B7-H1 was not expressed in the
ovarian cancer cell line OAW42 (Fig.
3, lane 3). In contrast the B7-H1 protein can be detected with
the specific antibody in other ovarian cancer cell lines, e.g.,
SKOV-3 (Fig. 3, lane 4) and
OVCAR-3 (Fig. 3, lane 5), which
were used as positive controls in this experiment. Therefore, B7-H1
ligand seems not to be involved in the observed different NK-cell
mediated lysis rates.
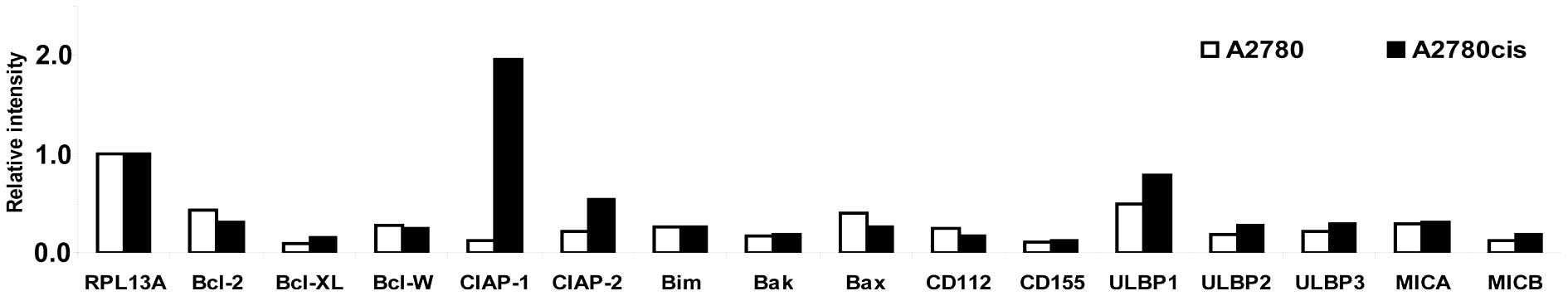
In the next step the expression rates of different
anti- and pro-apoptotic genes were studied by RT-PCR (Fig. 4). The expression rate of the
anti-apoptotic gene bcl-2 is decreased in A2780cis cells
compared to parental A2780 cells. The anti-apoptotic genes
bcl-xL and bcl-w were expressed nearly at the same
rate in the two cell lines. Obviously the expression of the
anti-apoptotic genes cellular inhibitor of apoptosis (ciap)
-1 and -2 is strongly induced in A2780cis cells
compared to A2780 cells. The pro-apoptotic genes such as bim
and bak showed no expression differences in the two cell
lines but bax expression is decreased in the
platinum-resistant cell line. Also the expression rate of CD112 and
CD155, both ligands for the NK-cell activating receptor DNAM-1,
were analyzed. There was no difference in CD155 expression in the
two cell lines and CD112 expression was slightly increased in A2780
cells. The expression rate for NKG2D ligands, e.g., MICB, ULBP1,
ULBP2 and ULBP3, were increased in A2780cis cells compared to the
parental A2780 cells. Even if the expression rate of MICA was found
to be nearly the same in both cell lines we decided to analyse the
expression differences of MICA and MICB between A2780 and A2780cis
cells, because these two proteins are highly related and both
activate cytotoxicity by NK-cells through the NKG2D receptor as a
mechanism of immunological defence. Furthermore, shedding of MICA
and MICB seems to be one of the mechanisms by which tumours evade
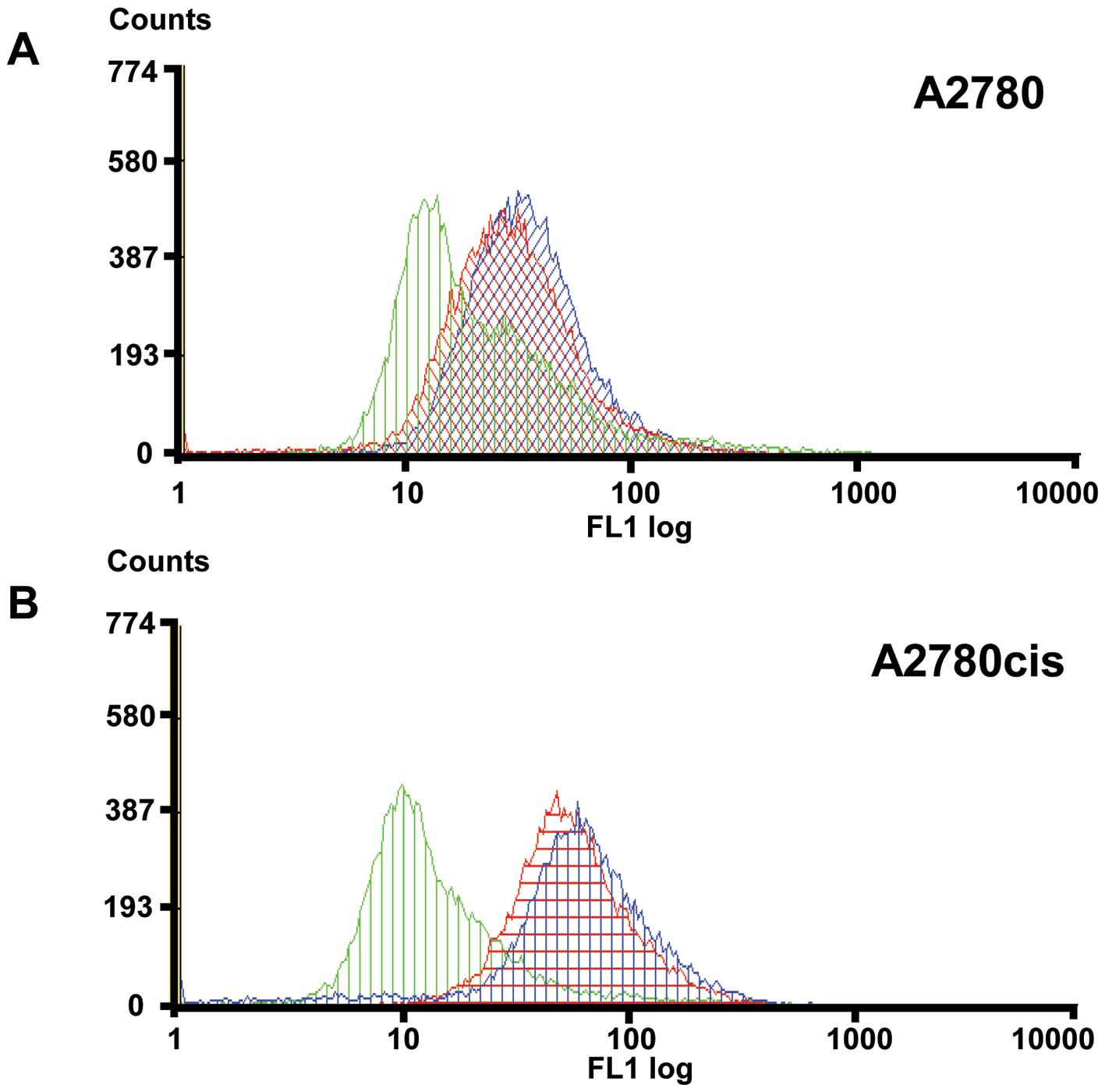
host immune surveillance. Expression of MICA/B was analyzed by FACS
with and without addition of GM6001 a broad-spectrum inhibitor of
proteinases (Fig. 5).
Pre-incubation of A2780cis cells with GM6001 results in 5.3% higher
amount of MICA/B protein on the cell surface compared to untreated
A2780cis cells. Therefore it is very likely that A2780cis cells
could be protected against NK-cell mediated lysis by shed soluble
MICA/B. In contrast the amount of MICA/B protein on the surface of
parental A2780 cells is not significantly influenced (increased
<0.1%) by pre-incubation with the proteinase inhibitor. This
observation led us to have a look on the expression pattern of
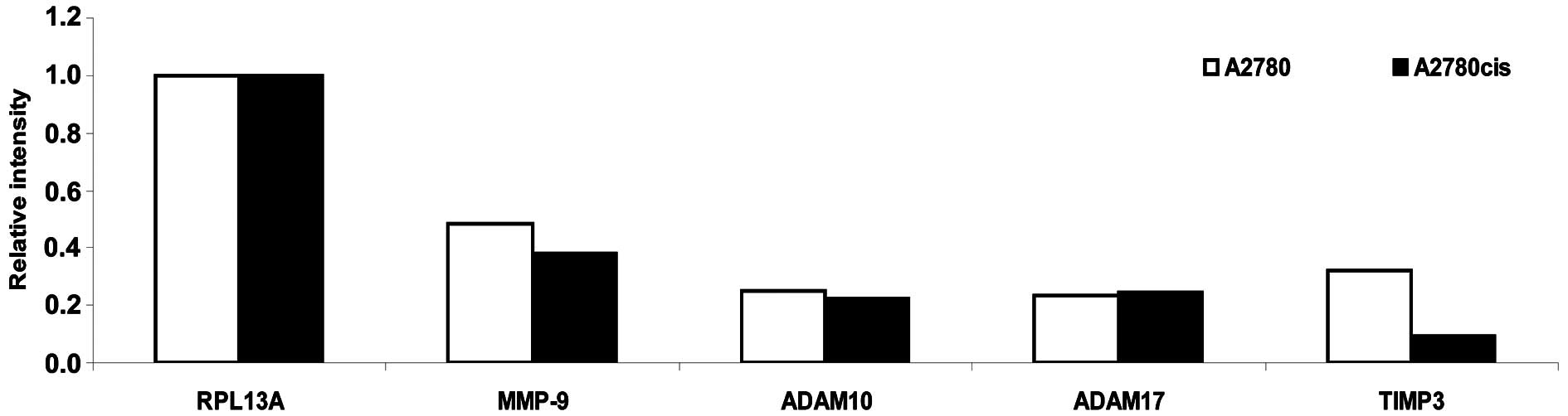
different proteinases especially MMP-9, ADAM-10 and ARAM-17 known
to be involved in cleavage of MICA/B as well as TIMP3 a known
inhibitor of the proteolysis shedding process (Fig. 6). As illustrated in Fig. 6 the expression rate of MMP-9 is
slightly decreased in A2780cis cells compared to the parental A2780
cells whereas the expression of ADAM-10 and ADAM-17 is the same in
both cell lines. Expression of TIMP3 is significantly reduced in
A2780cis cells compared to A2780 cells and therefore the
proteolytic shedding of MICA/B is probably more pronounced in the
A2780cis cell line.
Discussion
Natural-killer (NK)-cells are a critical component
of the innate immune response against infectious pathogens and
malignant transformation (22,23).
NK-cells mediate this activity through the elaboration of various
cytokines as well as through direct cytolytic activity. However,
unlike adaptive immune cells, which utilize specific clonal
recognition receptors, NK-cell activation depends on a complex
balance between activating and inhibitory signals (24,25).
Nevertheless, NK-cells play an important role in immune
surveillance and coordinating responses of other immune cells. Most
tumour cells express surface molecules that can be recognized by
activating receptors on NK-cells (5). The expression of these receptors make
such cells susceptible to endogenous NK-cells, but malignant cells
have developed mechanisms to evade these mechanisms of innate
immune surveillance (6–8). In patients with cancer, it is
presumed that tumour cells have developed mechanisms to suppress
NK-cell activation and resist lysis by endogenous NK-cells, but the
molecular basis for target resistance is not well understood. The
goal of our studies was to characterize the molecular basis for
these resistance mechanisms in ovarian cancer with different AKT
expression levels. Therefore the interactions of NK-cells with a
platinum-sensitive parental human ovarian cancer cell line and the
corresponding platinum-resistant cell line was analyzed. These cell
lines have the same genetic background but differ with regard to
the AKT expression level. We found great differences in the
efficiency of NK mediated cell lysis between the cell lines. The
cis-platinum-resistant A2780cis cells are less accessible for
NK-cell mediated killing. This finding is in agreement with a
recent report by Bellucci et al(26). Using a lentiviral shRNA library
targeting >1,000 human genes they identified 83 genes that
promote target cell resistance to human NK-cell-mediated killing
(26). Many of the genes
identified in this genetic screen belong to common signalling
pathways including members of the AKT/PI3K-pathway such as PIK3CA
and PIK3CB (26).
The comparison of cancer cell lines A2780 and
A2780cis revealed that the observed differences with regard to
NK-cell mediated killing are most probably based on two mechanisms.
First of all the observed increased expression of anti-apoptotic
genes (especially ciap-1 and -2) in A2780cis cells
compared to A2780 cells most probably renders A2780cis cells more
resistant against apoptosis. Second the CD112 ligand for NK-cell
receptor DNAM-1 was expressed at a reduced level in A2780cis cells
but ligands for the NK-cell receptor NKG2D were expressed more
strongly in the platinum-resistant cells compared to parental A2780
cells.
A2780cis cells express lower levels of TIMP-3 the
inhibitor of MICA/B shedding. At the same time the proteases for
shedding are expressed which result in a net increase of soluble
MICA/B in A2780cis cell cultures as shown by FACS analysis in this
study. It is well known that cleaved MICA/B protect cells against
NK mediated cell killing (27,28).
Therefore, we conclude that most probably the increased amount of
soluble MICA/B is responsible for the lower killing rate of
platinum-resistant A2780cis cells compared to their parental A2780
cells. Previously it was demonstrated that PI3K/AKT pathway is
involved in inducing MICA/B expression in breast cancer cells
(29). Our results revealed that
the cells with an increase in phosphorylated AKT/activated PI3K/AKT
pathway have a higher MICA/B expression. Recently Bellucci et
al demonstrated that treatment of tumour cells with JAK
inhibitors increased their susceptibility to NK-cell mediated
killing (26). The authors
concluded that common signalling pathways can regulate
susceptibility of human tumour cells to killing by immunologic
effector cells and that small molecule inhibitors of these kinases
may have important immunologic effects in vivo(26). Whether in analogy inhibition of
PI3K/AKT pathway renders the platinum-resistant A2780cis cells
accessible for NK-cell mediated killing must be evaluated in
further studies. Our study presented here characterizes the
molecular basis for resistance mechanisms in ovarian cancer with
different AKT expression levels regarding NK-cell mediated killing.
In conclusion, the cis-platinum-resistant A2780cis cells are less
accessible for NK-cell mediated killing in comparison to parental
A2780 cells. Different mechanisms seem to be relevant; first an
increased expression of anti-apoptotic genes (especially
ciap-1 and -2) and second an increase of soluble
MICA/B seems to be present in cis-platinum-resistant cells.
Acknowledgements
We appreciate the permission to use
the INTAS ChemoStar Imager (Department of Microbiology, University
of Würzburg), and we thank especially Professor T. Rudel and Dr B.
Bergmann. This study was supported by IZKF Würzburg.
References
|
1.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2.
|
Pignata S, Cannella L, Leopardo D, Pisano
C, Bruni GS and Facchini G: Chemotherapy in epithelial ovarian
cancer. Cancer Lett. 303:73–83. 2011. View Article : Google Scholar
|
|
3.
|
Gonzalez-Martin AJ: Medical treatment of
epithelial ovarian cancer. Expert Rev Anticancer Ther. 4:1125–1143.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Matsuo K, Lin YG, Roman LD and Sood AK:
Overcoming platinum resistance in ovarian carcinoma. Expert Opin
Investig Drugs. 19:1339–1354. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Lanier LL: NK cell recognition. Annu Rev
Immunol. 23:225–274. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Dunn GP, Bruce AT, Ikeda H, Old LJ and
Schreiber RD: Cancer immunoediting: from immunosurveillance to
tumor escape. Nat Immunol. 3:991–998. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Orr MT and Lanier LL: Natural killer cell
education and tolerance. Cell. 142:847–856. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Smyth MJ, Dunn GP and Schreiber RD: Cancer
immunosurveillance and immunoediting: the roles of immunity in
suppressing tumor development and shaping tumor immunogenicity. Adv
Immunol. 90:1–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Altomare DA, Wang HQ, Skele KL, et al: AKT
and mTOR phosphorylation is frequently detected in ovarian cancer
and can be targeted to disrupt ovarian tumor cell growth. Oncogene.
23:5853–5857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cheng JQ, Lindsley CW, Cheng GZ, Yang H
and Nicosia SV: The Akt/PKB pathway: molecular target for cancer
drug discovery. Oncogene. 24:7482–7492. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Steelman LS, Chappell WH, Abrams SL, et
al: Roles of the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways in
controlling growth and sensitivity to therapy-implications for
cancer and aging. Aging. 3:192–222. 2011.PubMed/NCBI
|
|
12.
|
Levine DA, Bogomolniy F, Yee CJ, et al:
Frequent mutation of the PIK3CA gene in ovarian and breast cancers.
Clin Cancer Res. 11:2875–2878. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Benedetti V, Perego P, Luca Beretta G, et
al: Modulation of survival pathways in ovarian carcinoma cell lines
resistant to platinum compounds. Mol Cancer Ther. 7:679–687. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Engel JB, Schonhals T, Hausler S, et al:
Induction of programmed cell death by inhibition of AKT with the
alkylphosphocholine perifosine in in vitro models of platinum
sensitive and resistant ovarian cancers. Arch Gynecol Obstet.
283:603–610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Santiskulvong C, Konecny GE, Fekete M, et
al: Dual targeting of phosphoinositide 3-kinase and mammalian
target of rapamycin using NVP-BEZ235 as a novel therapeutic
approach in human ovarian carcinoma. Clin Cancer Res. 17:2373–2384.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Westfall SD and Skinner MK: Inhibition of
phosphatidylinositol 3-kinase sensitizes ovarian cancer cells to
carboplatin and allows adjunct chemotherapy treatment. Mol Cancer
Ther. 4:1764–1771. 2005. View Article : Google Scholar
|
|
17.
|
Hahne JC, Honig A, Meyer SR, et al:
Downregulation of AKT reverses platinum resistance of human ovarian
cancers in vitro. Oncol Rep. 28:2023–2028. 2012.PubMed/NCBI
|
|
18.
|
Behrens BC, Hamilton TC, Masuda H, et al:
Characterization of a cis-diamminedichloroplatinum(II)-resistant
human ovarian cancer cell line and its use in evaluation of
platinum analogues. Cancer Res. 47:414–418. 1987.PubMed/NCBI
|
|
19.
|
Valiante NM, Rengaraju M and Trinchieri G:
Role of the production of natural killer cell stimulatory factor
(NKSF/IL-12) in the ability of B cell lines to stimulate T and NK
cell proliferation. Cell Immunol. 145:187–198. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Sheehy ME, McDermott AB, Furlan SN,
Klenerman P and Nixon DF: A novel technique for the fluorometric
assessment of T lymphocytic antigen specific lysis. J Immunol
Methods. 249:99–110. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Jesnowski R, Backhaus C, Ringel J and Lohr
M: Ribosomal highly basic 23-kDa protein as a reliable standard for
gene expression analysis. Pancreatology. 2:421–424. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Caligiuri MA: Human natural killer cells.
Blood. 112:461–469. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Vivier E, Raulet DH, Moretta A, et al:
Innate or adaptive immunity? The example of natural killer cells.
Science. 331:44–49. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Lanier LL: Up on the tightrope: natural
killer cell activation and inhibition. Nat Immunol. 9:495–502.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Moretta L, Biassoni R, Bottino C, Mingari
MC and Moretta A: Human NK-cell receptors. Immunol Today.
21:420–422. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bellucci R, Nguyen HN, Martin A, et al:
Tyrosine kinase pathways modulate tumor susceptibility to natural
killer cells. J Clin Invest. 122:2369–2383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Boutet P, Agüera-Gonza’lez S, Atkinson S,
et al: Cutting edge: the metalloproteinase ADAM17/TNF-α-converting
enzyme regulates proteolytic shedding of the MHC class I-related
chain B protein. J Immunol. 182:49–53. 2009.PubMed/NCBI
|
|
28.
|
Waldhauer I, Goehlsdorf D, Gieseke F, et
al: Tumor-associated MICA is shed by ADAM proteases. Cancer Res.
68:6368–6376. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Okita R, Mougiakakos D, Ando T, et al:
HER2/HER3 signaling regulates NK cell-mediated cytotoxicity via MHC
class I chain-related molecule A and B expression in human breast
cancer cell lines. J Immunol. 188:2136–2145. 2012. View Article : Google Scholar : PubMed/NCBI
|