Introduction
Breast cancer is the most frequently diagnosed
cancer, second only to lung cancer, and is the leading cause of
cancer death in women worldwide, accounting for 23% (1.38 million)
of the total new cancer cases and 14% (458,400) of the total cancer
deaths in 2008 (1,2). Breast cancer continues to have a 20%
rate for advanced or metastatic disease with a palliative
prognosis, although it has achieved higher cure rates because of
earlier detection and the likelihood that better treatments have
led to lower mortality rates (1).
However, the optimization of treatment procedures in metastatic
disease remains an unmet need.
A number of cytotoxic drugs have substantial
antitumor activity against breast cancer. Combinations of cytotoxic
regimens are associated with higher response rates (RR) and longer
durations of response than single-agent regimens (3). However, the survival rate is very low
for the patients who ultimately develop metastatic disease
(4). Thus, improved therapies for
patients with advanced stages of the disease are necessary. Many
compounds have shown activity in metastatic breast cancer; however,
there is a need for well-tolerated active combinations,
particularly in high-risk groups, e.g., patients with visceral
metastasis having a fast progression rate (5,6).
Pemetrexed, as a single agent, in anthracycline- and
taxane-pretreated breast cancer patients has shown a RR of 9–28%
(7–9). Platinum compounds are being
increasingly incorporated in the treatment of metastatic breast
cancer (10–13) as they have a synergistic action
with pemetrexed and gemcitabine (14). Carboplatin, with a similar activity
to other platinum compounds, is a possible combination partner with
pemetrexed, due to its better tolerability and safety profile
(13,15). Moreover, this combination has shown
an overall response rate (ORR) of 54% and a manageable toxicity
profile as a first-line therapy in patients with locally advanced
and metastatic breast cancer (13).
Gemcitabine, as a single agent, has been studied in
phase II trials (first-, second- and third-line) and a registration
trial in combination with paclitaxel after anthracycline
pretreatment received approval, reflecting its increasing
acceptance as a standard treatment in first- and second-line
metastatic breast cancer (16–22).
After anthracycline and taxane pretreatment there has been an
increasing list of trials with gemcitabine, both as a single agent
but also in combinations (14,17,21,23).
It is known to have a better toxicity profile and non-overlapping
toxicity with other chemotherapeutic agents and is thus
advantageous for combination therapies (24,25).
The most promising of these was the phase III trial with
gemcitabine and vinorelbine (26,27).
Vinorelbine has been studied in phase II trials and
has shown efficacy as a single agent in first- and second-line
treatment after anthracycline pretreatment (25,28,29).
In other phase II studies, vinorelbine in combination with
gemcitabine showed efficacy and tolerability as first-line therapy
as well as after pretreatment either with anthracycline alone or
with anthracycline/taxane-based regimens (30–33).
A phase III trial with a vinorelbine/gemcitabine doublet in
pretreated patients has also shown activity with an acceptable
safety profile (27).
The pemetrexed-carboplatin combination has been
tested (13,15) and needs additional evaluation as a
possible efficacious combination treatment after anthracycline and
taxane pretreatment. The potential synergism between these
compounds makes them an attractive combination to be compared to
another well-tolerated combination in metastatic breast cancer
disease such as gemcitabine-vinorelbine. The present study was
conducted to elucidate the activity of two chemotherapy regimens in
advanced breast cancer in a randomized phase II study. The primary
objective was to determine the antitumor activity of
pemetrexed-carboplatin and gemcitabinevinorelbine in anthracycline-
and taxane-pretreated patients with advanced breast cancer, by
measuring the RR [complete response (CR) and partial response
(PR)]. The secondary objective was to estimate time-to-event
efficacy variables, safety and quality of life with both of those
combination regimens.
Materials and methods
Patients
Adult females with a histologic or cytologic
diagnosis of advanced breast cancer, who had received at least 1
prior chemotherapy containing anthracycline and taxanes, had at
least 1 unidimensionally measurable lesion meeting the Response
Evaluation Criteria in Solid Tumors (RECIST) (34), and had an Eastern Cooperative
Oncology Group performance status (ECOG PS) of 0–2 with an
estimated life expectancy of ≥3 months were included in the study.
Previous radiation therapy to less than 25% of the bone marrow was
allowed, provided that the therapy was completed 30 days prior to
study entry. Patients were not eligible if they had previously
received pemetrexed, gemcitabine, carboplatin or vinorelbine,
whether in clinical practice or in another clinical trial. Other
exclusion criteria included the following: active infection;
history of malignant conditions (except non-melanotic skin cancer
or carcinoma in situ of the cervix); untreated cerebral
metastases; inability or unwillingness to take folic acid, vitamin
B12 supplementation or dexamethasone; or having received any
investigational drug within 30 days of study entry.
The study was conducted according to the principles
of good clinical practice, applicable laws and regulations, and the
Declaration of Helsinki. Each institution’s review board approved
the study and all patients signed an informed consent document
before study participation.
Study design
This was a multicenter, randomized (1:1), two-stage,
open-label, non-comparative, parallel-group phase II study
(NCT00325234) conducted between June 2006 and April 2010. Eligible
patients with advanced breast cancer previously treated with
anthracycline and taxanes were randomized either to Arm A
(pemetrexed and carboplatin) or to Arm B (vinorelbine and
gemcitabine). In Arm A, patients were administered with pemetrexed
600 mg/m2 (intravenously for 10 min on day 1) based on
results from phase I study (35)
and phase II study (13) and
carboplatin [given over approximately 30 min beginning after the
end of the pemetrexed infusion for target area under the curve
(AUC) 5.0] on day 1, after pretreatment with folic acid, vitamin
B12 and dexamethasone. In Arm B, vinorelbine 30 mg/m2
(given over approximately 6–10 min) and gemcitabine 1,200
mg/m2 (given over approximately 30 min) were
administered on day 1 and day 8. For treatment, a cycle was defined
as an interval of 21 days. Patients were treated until unacceptable
toxicity or progressive disease. Dose adjustments were based on the
National Cancer Institute Common Toxicity Criteria (NCI-CTC)
version 3.0 (36). Patients were
stratified by line of treatment (first/second line), visceral
disease (yes/no), and ECOG PS (0–1/2).
Efficacy and health outcome measures
The primary objective of this study was to assess
antitumor activity independently for each of the arms, as measured
by tumor RR (proportion of patients with CR or PR) according to
RECIST (1.0). Radiological assessments were routinely performed
before drug administration at every other cycle throughout the
treatment. The secondary objectives included the assessment of
time-to-event efficacy variables along with characterization of the
quantitative and qualitative toxicities in each treatment arm in
this patient population and assessment of quality of life. The
time-to-event efficacy variables were duration of response (DoR)
defined as the time from the date when the measurement criteria are
met for complete response or partial response (whichever status is
recorded first) until the date of first observation of disease
progression or death from study disease, time-to-response (TTR)
defined as the time from the date of study enrollment to the first
date when the measurement criteria are met for complete response or
partial response (whichever status is recorded first),
time-to-progressive disease (TTPD) defined as the time from the
date of study enrollment to the first documented date of
progressive disease or death from study disease, and
time-to-treatment failure (TTTF), defined as the time from date of
study enrollment to the first documented date of death from any
cause, progressive disease, or study treatment discontinuation due
to adverse event. Quality of life (QoL) was assessed using the
European Organization for Research and Treatment of Cancer (EORTC)
questionnaires QLQ-C30 and QLQ-BR23 (37).
Safety measures
Patients who received at least 1 dose of study
medication were evaluated for safety according to the following
variables: extent of exposure; treatment-emergent adverse events
[(TEAEs), graded using the Common Terminology Criteria for Adverse
Events (CTCAE) scale, Version 3.0]; discontinuations due to adverse
events (AEs); deaths; and serious adverse events (SAEs). All
patients continued clinical follow-up visits for approximately 30
days after the last day of study-drug administration and every
three months thereafter until disease progression, or for up to one
year.
Statistical analysis
A two-stage design was employed independently for
each of the arms, with the possibility of stopping each treatment
early due to lack of response (38). For the first stage, 28 patients
were to be evaluated per treatment arm. If fewer than or equal to 7
out of the 28 patients showed response to the investigational
regimen, the accrual for this regimen was to be stopped and the
conclusion was to be drawn that this regimen is not worthy of
further study in this tumor type. If more than 7 patients showed
response, accrual was to be continued until 68 qualified patients
had been enrolled. If, at the end of stage 2, fewer than or equal
to 20 out of 68 patients had responded, this regimen was to be
deemed not worthy of any further investigation in this patient
population, unless clinical considerations suggest otherwise.
This sample size gives no less than a 0.738 chance
of terminating enrollment early at the end of the first stage if
the true RR is less than or equal to 22% (H0). This procedure
provided a Type I error of 0.045 for testing of the null hypothesis
that the RR is no greater than 22% and the statistical power is 90%
when the RR is 40% (H1). All statistical tests were conducted at a
two-sided α level of 0.05, unless stated otherwise.
The primary analysis was completed using a
‘qualified for clinical tumor response population’, defined as
females with histologic or cytologic diagnosis of advanced breast
cancer previously treated with anthracyclines and taxanes,
receiving no concurrent antitumor therapy, having presence of
measurable disease as defined by RECIST, and receiving treatment
with at least 1 dose of the study-drug of the assigned study
regimen.
RR was defined as the sum of the number of patients
with CR plus PR divided by the total number of qualified patients.
Exact 95% Pearson-Clopper confidence intervals (CIs) for the RR of
each arm were calculated (39). RR
was also analyzed by line of treatment (first-line vs. second-line)
and visceral disease (yes vs. no).
Evaluation of time-to-event variables (secondary
efficacy analyses) included quartiles estimated using the product
limit method (40) and the
corresponding 95% CI based on the sign test (41). Safety variables were summarized
descriptively.
For QoL assessment using the EORTC questionnaires
QLQ-C30 and QLQ-BR23, observed values and absolute changes from
baseline were summarized for patients who completed at least 1
questionnaire at baseline and at least 1 questionnaire after the
first study-drug administration (a completed questionnaire was
defined as one with at least 50% of the questions answered).
Analysis was performed according to the EORTC guidelines
(http://www.eortc.be/home/qol).
Results
Patient disposition, baseline
demographics, and disease characteristics
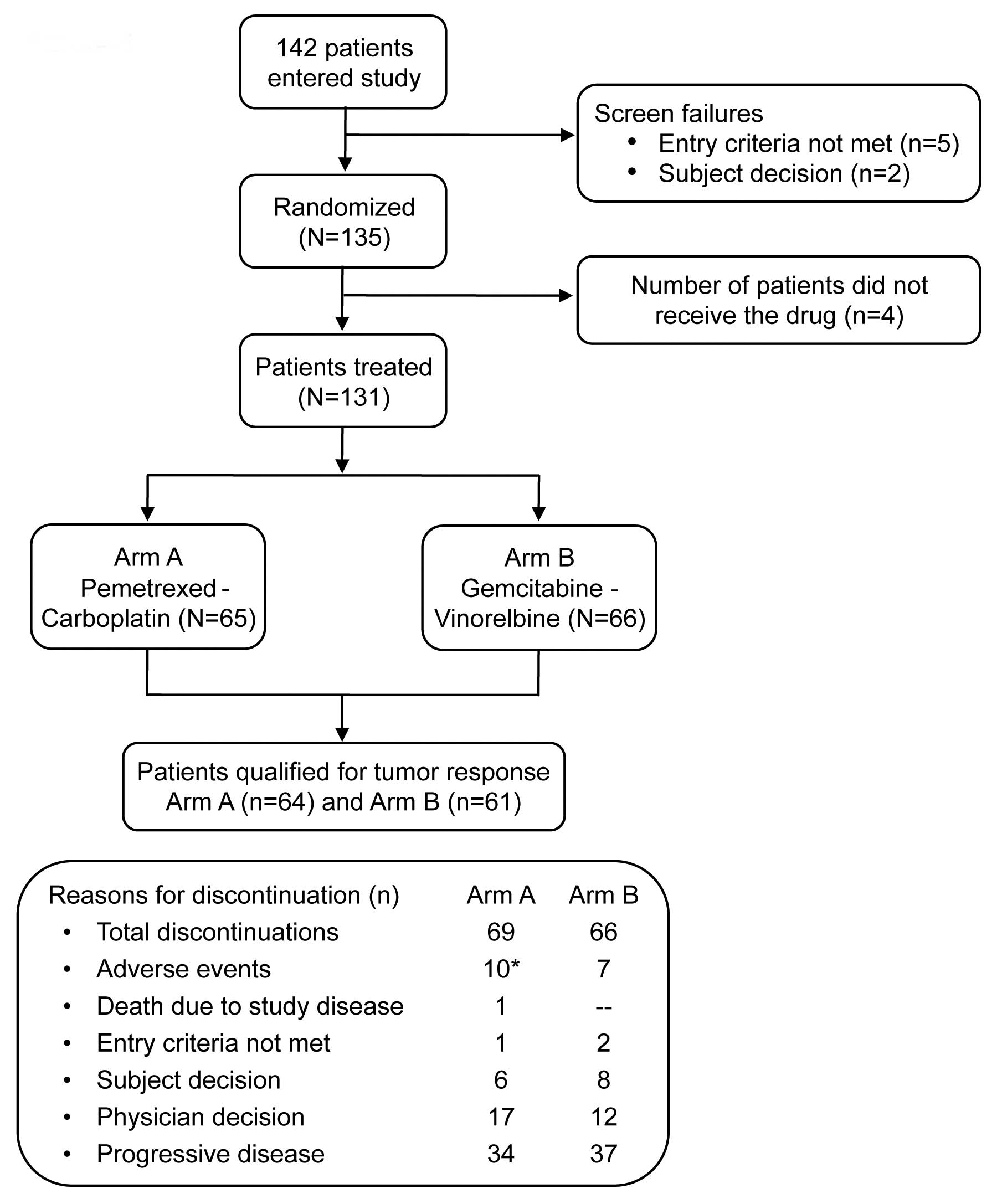
A total of 135 patients, enrolled from June 2006 to
April 2010 across 7 countries at 30 study centers, were randomly
assigned with 69 patients in Arm A and 66 patients in Arm B. A
total of 131 patients were treated (received at least 1 dose of
study treatment), comprising 65 patients in Arm A and 66 patients
in Arm B. The population qualifying for tumor response included 64
patients (92.8%) in Arm A and 61 patients (92.4%) in Arm B. Patient
disposition is represented in Fig.
1.
The baseline demographics and disease
characteristics of the study population are summarized in Table I. A majority of the patients were
Caucasian (94.8% overall) with mean age of 52 years in both
treatment arms. Most of the patients had an ECOG PS of 0 (57.8%
overall), while 2 patients (2.9%), both in Arm A, had an ECOG PS of
2. Most patients were receiving second-line treatment (70.4%
overall) and more than 80% had visceral disease. The most common
target lesion disease sites for the overall enrolled population
were liver (45.5%), lung (24.2%) and lymph node (19.7%).
 | Table IBaseline demographics and clinical
characteristics of all enrolled patients. |
Table I
Baseline demographics and clinical
characteristics of all enrolled patients.
| Variables |
Pemetrexed-carboplatin N=69, n (%) |
Vinorelbine-gemcitabine N=66, n (%) |
|---|
| Age, years | | |
| Mean (SD) | 51.9 (11.4) | 52.3 (10.4) |
| Median (min,
max) | 52.0 (29, 75) | 51.5 (30, 77) |
| Origin | | |
| Caucasian | 67 (97.1) | 61 (92.4) |
| African | 1 (1.4) | 3 (4.5) |
| Hispanic | 0 (0.0) | 1 (1.5) |
| Asian | 1 (1.4) | 1 (1.5) |
| ECOG PS | | |
| 0 | 39 (56.5) | 39 (59.1) |
| 1 | 28 (40.6) | 27 (40.9) |
| 2 | 2 (2.9) | 0 (0.0) |
| Hormonal receptor
status | | |
| E and P
negative | 19 (27.5) | 21 (31.8) |
| E and/or P
positive | 49 (71.0) | 44 (66.7) |
| Unknown | 1 (1.4) | 1 (1.5) |
| HER-2/neutral
assay | | |
| Positive | 12 (17.4) | 13 (19.7) |
| Negative | 53 (76.8) | 49 (74.2) |
| Not
done/unknown | 4 (5.8) | 4 (6.1) |
| Pathological
diagnosis | | |
| Carcinoma,
ductal | 64 (92.8) | 54 (81.8) |
| Carcinoma,
lobular | 4 (5.8) | 5 (7.6) |
| Carcinoma,
inflammatory | 1 (1.4) | 3 (4.5) |
| Other | 0 (0.0) | 4 (6.1) |
| Differentiation
grade | n=63 | n=60 |
| Grade I | 6 (9.5) | 3 (5.0) |
| Grade II | 25 (39.7) | 27 (45.0) |
| Grade III | 32 (50.8) | 30 (50.0) |
| Line of
treatment | | |
| 1st line | 21 (30.4) | 19 (28.8) |
| 2nd line | 48 (69.6) | 47 (71.2) |
| Visceral
disease | | |
| Yes | 55 (79.7) | 56 (84.8) |
| No | 14 (20.3) | 10 (15.2) |
Efficacy
The overall tumor response for the treatment arms is
summarized in Table II. The
population qualifying for tumor response included 64 patients
(92.8%) in Arm A and 61 patients (92.4%) in Arm B. A RR of 26.6%
(95% CI: 16.3, 39.1) was observed in Arm A and 29.5% (95% CI: 18.5,
42.6) was observed in Arm B at the end of stage 2 of the trial,
which was lower than required to meet the primary endpoint (RR of
more than 22%). The PRs were similar in both treatment arms (26.6%,
95% CI: 16.3, 39.1 in Arm A and 26.2%, 95% CI: 15.8, 39.1 in Arm
B). There were 2 CRs and both were in Arm B (3.3%, 95% CI: 0.4,
11.3).
 | Table IIBest overall tumor response in
patients who qualified for tumor response analyses. |
Table II
Best overall tumor response in
patients who qualified for tumor response analyses.
| Parameters |
Pemetrexed-carboplatin N=64, n (%), [95%
CI] |
Vinorelbine-gemcitabine N=61, n (%), [95%
CI] |
|---|
| Response rate (CR +
PR, per RECIST criteria) | 17 (26.6), [16.3,
39.1] | 18 (29.5), [18.5,
42.6] |
| Best overall
response rate | | |
| Complete
response | 0 (0.0), [0.0,
5.6] | 2 (3.3), [0.4,
11.3] |
| Partial
response | 17 (26.6), [16.3,
39.1] | 16 (26.2), [15.8,
39.1] |
| Stable
disease | 23 (35.9), [24.3,
48.9] | 21 (34.4), [22.7,
47.7] |
| Disease
progression | 17 (26.6), [16.3,
39.1] | 17 (27.9), [17.1,
40.8] |
| Unknown/not
done | 7 (10.9), [4.5,
21.2] | 5 (8.2), [2.7,
18.1] |
Among patients who qualified for tumor response, the
first-line treatment RR ws approximately 30% for both treatment
arms [31.6%, 95% CI: 12.6, 56.6 in Arm A (n=19) and 29.4%, 95% CI:
10.3, 56.0 in Arm B (n=17)]. Among patients receiving second-line
treatment who qualified for tumor response, RR (95% CI) was 24.4%
(12.9, 39.5) in Arm A (n= 45) and 29.5% (16.8, 45.2) in Arm B (n=
44). In patients with visceral disease who qualified for tumor
response, RR (95% CI) was 23.5% [12.8, 37.5 in Arm A (n=51)] and
32.1% [19.9, 46.3 in Arm B (n=53)]. In patients with no visceral
disease, RR was 38.5%, 95% CI: 13.9, 68.4 in Arm A (n=13) and
12.5%, 95% CI: 0.3, 52.7 in Arm B (n=8). Analysis of tumor response
using data for the enrolled population yielded results similar to
the findings with the qualified population. The RR in this
population was 17 [24.6%, (95% CI: 15.1, 36.5)] in Arm A and 19
[28.8%, (95% CI: 18.3, 41.3)] in Arm B.
All patients who showed a tumor response were
included in analyses of DoR and TTR. TTPD and TTTF were presented
for all treated patients. Secondary efficacy endpoints are
summarized in Table III. The
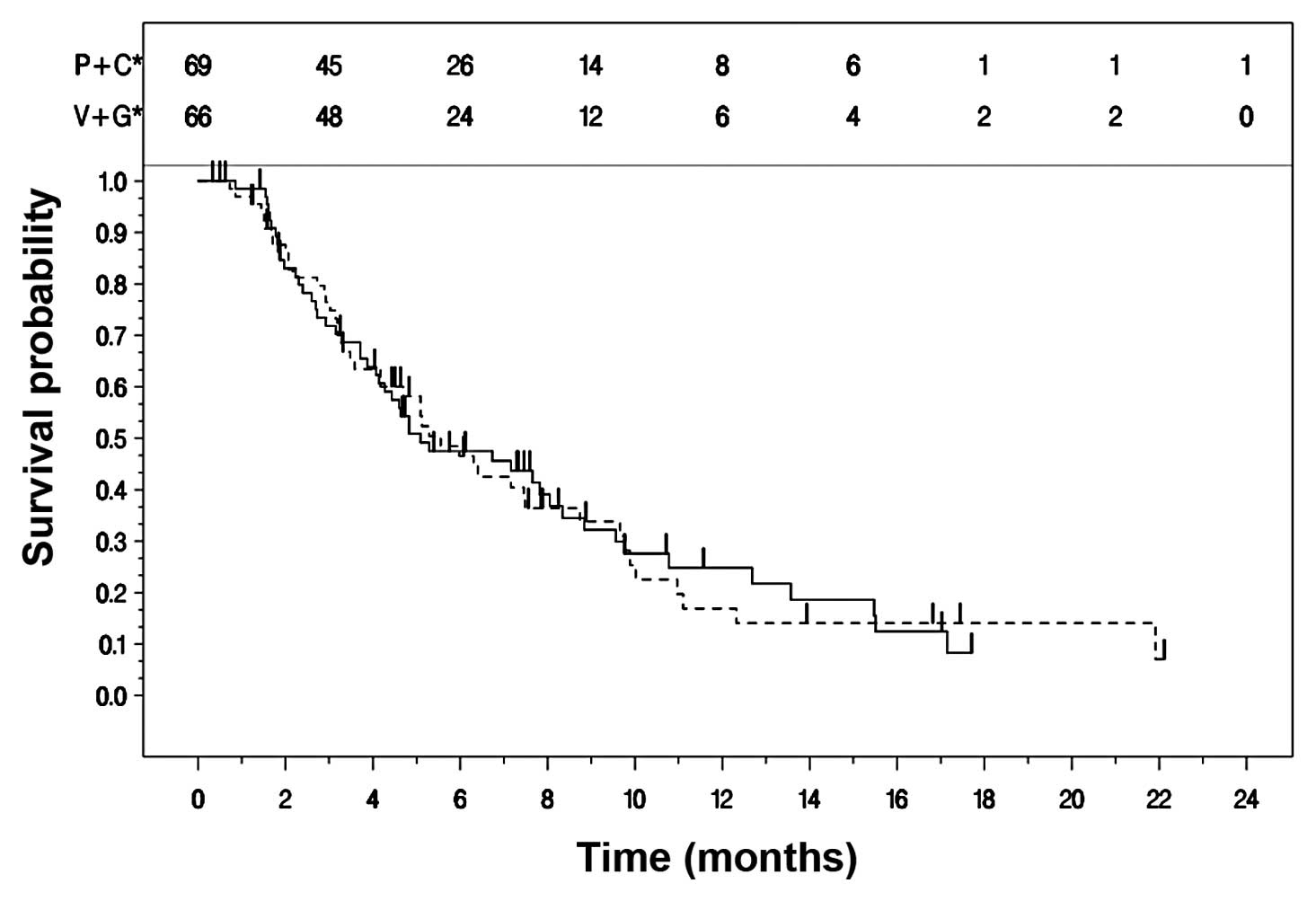
Kaplan-Meier curves for TTPD in the two treatment arms are
presented in Fig. 2. The median
TTPD was 5.1 (95% CI: 4.1, 8.0) months for Arm A and 5.6 (95% CI:
4.2, 7.5) months for Arm B (Table
III).
 | Table IIISecondary efficacy endpoints,
Kaplan-Meier estimates per treatment arm. |
Table III
Secondary efficacy endpoints,
Kaplan-Meier estimates per treatment arm.
|
Pemetrexed-carboplatin
|
Vinorelbine-gemcitabine
|
|---|
| Measure | Patients with
events n/N (%) | Months, median [95%
CI] | Patients with
events n/N (%) | Months, median [95%
CI] |
|---|
| Duration of
responsea | 12/17 (70.6) | 7.7 [4.2,
12.2] | 16/19 (84.2) | 7.5 [4.9, 8.3] |
| Time to
responsea | 17/17 (100) | 1.8 [1.6, 3.3] | 19/19 (100) | 1.8 [1.6, 3.1] |
| Time to progressive
diseaseb | 48/69 (69.6) | 5.1 [4.1, 8.0] | 46/66 (69.7) | 5.6 [4.2, 7.5] |
| Time to treatment
failureb | 60/69 (87.0) | 4.8 [3.3, 7.0] | 58/66 (87.9) | 5.1 [3.5, 6.3] |
Health outcomes
The population who qualified for QLQ-C30 and
QLQ-BR23 analysis included 41 patients in Arm A and 39 patients in
Arm B for QLQ-C30, and 36 patients in Arm A and 38 patients in Arm
B for QLQ-BR23 (Table IV).
Baseline and change-from-baseline values at cycles 3 and 5 and at
follow-up are presented in Table
IV.
 | Table IVHealth outcomes (EORTC QLQ-C30 global
health status; QLQ-BR23 body image) in patients qualified for
QLQ-C30 and QLQ-BR23 analyses. |
Table IV
Health outcomes (EORTC QLQ-C30 global
health status; QLQ-BR23 body image) in patients qualified for
QLQ-C30 and QLQ-BR23 analyses.
|
Pemetrexed-carboplatin
|
Vinorelbine-gemcitabine
|
|---|
| Time point | n | Mean (SD) | n | Mean (SD) |
|---|
| Baseline
QLQ-C30 | 41 | 67.3 (24.2) | 39 | 61.5 (28.5) |
| Cycle 3, change
from baseline | 35 | −9 (26.6) | 31 | −4 (21.8) |
| Cycle 5, change
from baseline | 20 | −10.4 (28.3) | 18 | −2.3 (27.9) |
| Follow-up, change
from baseline | 26 | −21.5 (32.5) | 22 | −4.9 (16.2) |
| Baseline QLQ-BR
23 | 36 | 77.5 (30.5) | 38 | 75.4 (24.1) |
| Cycle 3, change
from baseline | 31 | −3.6 (23.5) | 31 | −2.4 (18.1) |
| Cycle 5, change
from baseline | 18 | −5.4 (29.3) | 19 | −3.9 (13.1) |
| Follow-up, change
from baseline | 24 | −4.7 (29.0) | 22 | −3.7 (20.4) |
Safety
The median (range) number of cycles administered was
similar for both treatment arms [6.0 (1,21) in
Arm A and 6.0 (1,20) in Arm B]. Exposure in the treatment
arms as measured by relative dose intensity were: Arm A [mean%
(SD)] = 88.5% (12.7%) for pemetrexed and 86.1% (13.8%) for
carboplatin, and Arm B = 68.1% (14.4%) for vinorelbine and 68.4%
(14.4%) for gemcitabine. Over all cycles, day 8 dose reductions and
omissions ranged from approximately 30 to 40% in Arm B compared
with no omissions and approximately 20% of dose reductions in Arm
A.
During study period or within the 30-day
post-therapy period, 1 death in Arm A occurred due to the study
disease and 1 death in Arm B occurred due to hepatic failure during
follow-up period after 214 days of first dose. Nine patients
(13.8%) in Arm A and 7 patients (10.6%) in Arm B (safety
population) discontinued due to AEs. One additional patient who did
not receive the drug in Arm A was also discontinued. The most
common TEAE leading to discontinuation was neutropenia, reported in
1 patient (1.5%) in Arm A and 3 patients (4.5%) in Arm B. Two
patients (3.1%) in Arm A discontinued due to drug hypersensitivity
and both were considered to be related to the study drug. Grade 3
congestive cardiac failure (SAE) leading to hospitalization was
seen in 1 patient in Arm B, causing discontinuation.
At least 1 SAE was reported in 18 patients (27.7%)
in Arm A and 22 patients (33.3%) in Arm B; anemia, neutropenia and
thrombocytopenia were the most commonly occurring in Arm A and
occurred in >5% of the safety population. There were no
drug-related SAEs that occurred in >5% of patients in Arm B.
A summary of grades 3 and 4 TEAEs is provided in
Table V. Potentially drug-related
CTCAE grade 3 or 4 TEAEs were seen in 36.9% of patients in Arm A
and 60.6% of patients in Arm B, the most frequent being
neutropenia.
 | Table VGrades 3 and 4 adverse events
possibly related to study-drug that occurred in ≥10% of patients in
each treatment group. |
Table V
Grades 3 and 4 adverse events
possibly related to study-drug that occurred in ≥10% of patients in
each treatment group.
| Adverse events
(laboratory and non-laboratory) | Grade 3 n (%) | Grade 4 n (%) |
|---|
|
Pemetrexed-carboplatin, N=65 | | |
| Neutropenia | 15 (23.1) | 9 (13.8) |
|
Thrombocytopenia | 9 (13.8) | 6 (9.2) |
| Anemia | 10 (15.4) | 2 (3.1) |
| Leukopenia | 9 (13.8) | 1 (1.5) |
|
Vinorelbine-gemcitabine, N=66 | | |
| Neutropenia | 22 (33.3) | 18 (27.3) |
| Leukopenia | 9 (13.6) | 2 (3.0) |
| Fatigue | 8 (12.1) | 1 (1.5) |
Discussion
The present study was conducted to assess the
antitumor activity and safety profiles of two chemotherapy
regimens, pemetrexed-carboplatin and gemcitabine-vinorelbine in
anthracycline- and taxane-pretreated patients with advanced breast
cancer. The gemcitabine-vinorelbine combination has been studied
extensively in various phase II trials and more recently in a phase
III trial in pretreated advanced breast cancer patients, but the
current study is the first to investigate the combination of
pemetrexed-carboplatin in patients with advanced breast cancer
pretreated with anthracycline and taxanes.
The RRs (95% CI), the primary objective of this
study, shown in both arms were moderate [26.6% (16.3, 39.1) in Arm
A and 29.5% (18.5, 42.6) in Arm B], since none met the predefined
response at endpoint.
The RR shown by the gemcitabine-vinorelbine
combination was comparable to previous studies (26,30–33,42–45).
The more recent randomized phase III trial comparing the
gemcitabinevinorelbine combination with capecetabine demonstrated
an RR of 28.4% (27), which was
comparable to the current study. The median TTPD and DoR in the
present study were comparable with other phase II (TTPD, 5.7, 6.0
months; DoR, 6.9, 6.0 months) (33,34)
and phase III studies (TTPD, 5.4 months) (27). Upon safety analysis, the rate of
grades 3–4 neutropenia in this arm was found to be much higher
(60.6%) than that found in the previous studies (16.7–48.0%)
(30–33,42–45).
However, no drug-related SAEs were reported in >5% patients in
this treatment arm. In spite of the fact that there were higher
rate of grade 3–4 neutropenia in this study, only two patients in
Arm B had any infectious complications [1 patient (1.5%) had
staphylococcal sepsis and 1 patient (1.5%) had streptococcal
infection] and none were reported in Arm A.
In an earlier study, where pemetrexed-carboplatin
was given as first-line therapy, the RR was much higher (54%)
(13) compared to the present
study. However, the RR of the combination in the current study was
comparable to an earlier study (21%) with pemetrexed alone in
anthracycline-pretreated patients (8). Also, none of the patients achieved CR
with the pemetrexed-carboplatin combination, either as a first-line
or a second-line therapy. Both median TTPD (10.3 months) and DoR
(11.1 months) were higher when the combination was used as a
first-line treatment (13) than in
the present study (TTPD, 5.1 months; DoR, 7.7 months). The rate of
grade 3 and 4 neutropenia with pemetrexed-carboplatin was lower
compared to first-line treatment studies with similar toxicity
profiles (13). This could be
attributed to the difference in the total durations of drug
exposure in the earlier study compared to the present study (24 vs.
19 weeks). The high incidence of neutropenia despite full vitamin
supplementation could be due to the additive myelosuppressive
activity of the combination of carboplatin and pemetrexed (13).
The mean number of cycles administered was similar
for both arms (Arm A, 6.3; Arm B, 6.2) but the exposure levels were
lower in Arm B. The low dose intensity in Arm B appeared to be
related to missed or reduced doses on day 8; over all cycles, day 8
dose reductions and omissions ranged from approximately 30 to 40%
in Arm B, which might explain the relatively low exposure in Arm
B.
One study limitation is that the majority (70%) of
patients enrolled in the present study had received two lines of
previous treatment, which may have caused some study bias. Such
heterogeneous population from prognosis point of view (1st and 2nd
line) would explain the observed moderate RR. In addition, the
non-comparative design of the 2 arms of treatment prevented
additional valuable conclusions.
From the above results it can be concluded that both
combination therapies showed moderate efficacy and were well
tolerated but further studies are still warranted. The results with
pemetrexed and carboplatin were moderate but promising; results
with gemcitabine and vinorelbine showed potential as in the
previous studies. However, further research with newer drugs and
newer combinations continues to be needed in order to deal with
treatment failures as well as to arrest disease progression and
palliate symptoms in patients with advanced breast cancer.
Clinical practice points
Screening and probably better treatments in early
breast cancer decrease the numbers of metastatic breast cancer
incidence. However, there is a selection of hard to treat
population in this setting with the need to have non
cross-resistant drugs available. Most patients had anthracyclines
and taxanes in the adjuvant setting. Therefore, well-tolerated
active compounds are needed. Pemetrexed and carboplatin are both
well-tolerated apart from the hematologic toxicities, which can
easily be handled. The efficacy parameters are comparable to other
well-tolerated combinations such as gemcitabine and vinorelbine and
can be clinically accepted as an alternative treatment procedure.
However, it must also be considered that pemetrexed is off-label in
breast cancer treatment and the data are of limited value because
these are phase II results. If platinum compounds will be further
developed, particularly in basal or triple-negative breast cancer,
and combination partners are needed, pemetrexed can be accepted as
a promising compound.
Acknowledgements
This study was sponsored by Eli Lilly
and Company, Indianapolis, IN, USA. The authors wish to thank the
investigators, coordinators and patients for their participation in
this study. V. Soldatenkova, V. Moreau and D. Desaiah and T.
Bauknecht are employees of Eli Lilly and Company; M. Martin served
on Lilly advisory boards and received remuneration; D. Amadori, E.
Carrasco, S. Roesel, R. Labianca, B. Uziely and M. Martin served as
investigators on this trial.
References
|
1.
|
Ferlay J, Shin HR, Bray F, et al:
Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int
J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Bray F, Center MM, et al: Global
Cancer Statistics. CA Cancer J Clin. 61:69–90. 2011. View Article : Google Scholar
|
|
3.
|
Henderson IC, Berry DA, Demetri GD, et al:
Improved outcomes from adding sequential Paclitaxel but not from
escalating Doxorubicin dose in an adjuvant chemotherapy regimen for
patients with node-positive primary breast cancer. J Clin Oncol.
21:976–983. 2003. View Article : Google Scholar
|
|
4.
|
Hortobagyi GN: Treatment of breast cancer.
N Engl J Med. 339:974–984. 1998. View Article : Google Scholar
|
|
5.
|
Dean-Colomb W and Esteva FJ: Emerging
agents in the treatment of anthracycline- and taxane-refractory
metastatic breast cancer. Semin Oncol. 35:S31–S38. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Overmoyer B: Options for the treatment of
patients with taxane-refractory metastatic breast cancer. Clin
Breast Cancer. 8:S61–S70. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Llombart-Cussac A, Theodoulou M, Rowland
K, et al: Pemetrexed in patients with locally advanced or
metastatic breast cancer who had received previous anthracycline
and taxane treatment: phase II study. Clin Breast Cancer.
7:380–385. 2006. View Article : Google Scholar
|
|
8.
|
Martin M, Spielmann M, Namer M, et al:
Phase II study of pemetrexed in breast cancer patients pretreated
with anthracyclines. Ann Oncol. 14:1246–1252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Miles DW, Smith IE, Coleman RE, et al: A
phase II study of pemetrexed disodium (LY231514) in patients with
locally recurrent or metastatic breast cancer. Eur J Cancer.
37:1366–1371. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Crown J and Pegram M: Platinum-taxane
combinations in metastatic breast cancer: an evolving role in the
era of molecularly targeted therapy. Breast Cancer Res Treat.
79:S11–S18. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Martín M: Platinum compounds in the
treatment of advanced breast cancer. Clin Breast Cancer. 2:190–208.
2001.PubMed/NCBI
|
|
12.
|
Perez EA, Hillman DW, Stella PJ, et al: A
phase II study of paclitaxel plus carboplatin as first-line
chemotherapy for women with metastatic breast carcinoma. Cancer.
88:124–131. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Garin A, Manikhas A, Biakhov M, et al: A
phase II study of pemetrexed and carboplatin in patients with
locally advanced or metastatic breast cancer. Breast Cancer Res
Treat. 110:309–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Heinemann V, Stemmler HJ, Wohlrab A, et
al: High efficacy of gemcitabine and cisplatin in patients with
predominantly anthracycline- and taxane-pretreated metastatic
breast cancer. Cancer Chemother Pharmacol. 57:640–646. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Perez EA: Carboplatin in combination
therapy for metastatic breast cancer. Oncologist. 9:518–527. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Brodowicz T, Kostler WJ, Möslinger R, et
al: Single-agent gemcitabine as second- and third-line treatment in
metastatic breast cancer. Breast. 9:338–342. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Spielmann M, Llombart-Cussac A, Kalla S,
et al: Single-agent gemcitabine is active in previously treated
metastatic breast cancer. Oncology. 60:303–307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Blackstein M, Vogel CL, Ambinder R, et al:
Gemcitabine as first-line therapy in patients with metastatic
breast cancer: a phase II trial. Oncology. 62:2–8. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Heinemann V: Role of gemcitabine in the
treatment of advanced and metastatic breast cancer. Oncology.
64:191–206. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Heinemann V: Gemcitabine in metastatic
breast cancer. Expert Rev Anticancer Ther. 5:429–443. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Modi S, Currie VE, Seidman AD, et al: A
phase II trial of gemcitabine in patients with metastatic breast
cancer previously treated with an anthracycline and taxane. Clin
Breast Cancer. 6:55–60. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Albain KS, Nag SM, Calderillo-Ruiz G, et
al: Gemcitabine plus Paclitaxel versus Paclitaxel monotherapy in
patients with meta-static breast cancer and prior anthracycline
treatment. J Clin Oncol. 26:3950–3957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Seo JH, Oh SC, Choi CW, et al: Phase II
study of a gemcitabine and cisplatin combination regimen in taxane
resistant meta-static breast cancer. Cancer Chemother Pharmacol.
59:269–274. 2007.PubMed/NCBI
|
|
24.
|
Silvestris N, D’Aprile M, Andreola G, et
al: Rationale for the use of gemcitabine in breast cancer (Review).
Int J Oncol. 24:389–398. 2004.PubMed/NCBI
|
|
25.
|
Jones S, Winer E, Vogel C, et al:
Randomized comparison of vinorelbine and melphalan in
anthracycline-refractory advanced breast cancer. J Clin Oncol.
13:2567–2574. 1995.PubMed/NCBI
|
|
26.
|
Martín M, Ruiz A, Muñoz M, et al:
Gemcitabine plus vinorelbine monotherapy in patients with
metastatic breast cancer previously treated with anthracyclines and
taxanes: final results of the phase III Spanish Breast Cancer
Research Group (GEICAM) trial. Lancet Oncol. 8:219–225. 2007.
|
|
27.
|
Pallis AG, Boukovinas I, Ardavanis A, et
al: A multicenter randomized phase III trial of
vinorelbine/gemcitabine doublet versus capecitabine monotherapy in
anthracycline- and taxanepretreated women with metastatic breast
cancer. Ann Oncol. 23:1164–1169. 2012. View Article : Google Scholar
|
|
28.
|
Degardin M, Bonneterre J, Hecquet B, et
al: Vinorelbine (navelbine) as a salvage treatment for advanced
breast cancer. Ann Oncol. 5:423–426. 1994.PubMed/NCBI
|
|
29.
|
Gregory RK and Smith IE: Vinorelbine - a
clinical review. Br J Cancer. 82:1907–1913. 2000.PubMed/NCBI
|
|
30.
|
Haider K, Kornek GV, Kwasny W, et al:
Treatment of advanced breast cancer with gemcitabine and
vinorelbine plus human granulocyte colony-stimulating factor.
Breast Cancer Res Treat. 55:203–211. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Valenza R, Leonardi V, Gebbia V, et al:
Gemcitabine and vinorelbine in pretreated advanced breast cancer: a
pilot study. Ann Oncol. 11:495–496. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Stathopoulos GP, Rigatos SK, Pergantas N,
et al: Phase II trial of biweekly administration of vinorelbine and
gemcitabine in pretreated advanced breast cancer. J Clin Oncol.
20:37–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Stemmler HJ, diGioia D, Freier W, Tessen,
et al: Randomised phase II trial of gemcitabine plus vinorelbine vs
gemcitabine plus cisplatin vs gemcitabine plus capecitabine in
patients with pretreated metastatic breast cancer. Br J Cancer.
104:1071–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Therasse P, Arbuck SG, Eisenhauer EA, et
al: New guidelines to evaluate the response to treatment in solid
tumors. European Organization for Research and Treatment of Cancer,
National Cancer Institute of the United States, National Cancer
Institute of Canada. J Natl Cancer Inst. 92:205–216. 2000.
View Article : Google Scholar
|
|
35.
|
Hughes A, Calvert P, Azzabi A, et al:
Phase I clinical and pharmacokinetic Study of pemetrexed and
carboplatin in patients with malignant pleural mesothelioma. J Clin
Oncol. 20:3533–3544. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Cancer Therapy Evaluation Program: Common
Terminology Criteria for Adverse Events, Version 3.0, 2003.
National Cancer Institute. Available, Published online August 9,
2006; available at: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcev3.pdf.
Accessed November 14, 2011.
|
|
37.
|
Nagel GC, Schmidt S, Strauss BM, et al:
Quality of life in breast cancer patients: a cluster analytic
approach. Empirically derived subgroups of the EORTC-QLQ BR 23 - a
clinically oriented assessment. Breast Cancer Res Treat. 68:75–87.
2001. View Article : Google Scholar
|
|
38.
|
Simon R: Optimal two-stage designs for
phase II clinical trials. Control Clin Trials. 10:1–10. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Clopper CJ and Pearson ES: The use of
confidence or fiducial limits illustrated in the case of the
binomial. Biometrika. 26:404–413. 1934. View Article : Google Scholar
|
|
40.
|
Kaplan EL and Meier P: Nonparametric
estimation from incomplete observations. J Am Stat Assoc.
53:457–481. 1958. View Article : Google Scholar
|
|
41.
|
Brookmeyer R and Crowley J: A confidence
interval for the median survival time. Biometrics. 38:29–41. 1982.
View Article : Google Scholar
|
|
42.
|
Nicolaides C, Dimopoulos MA, Samantas E,
et al: Gemcitabine and vinorelbine as second-line treatment in
patients with meta-static breast cancer progressing after
first-line taxane-based chemotherapy: a phase II study conducted by
the Hellenic Cooperative Oncology Group. Ann Oncol. 11:873–875.
2000. View Article : Google Scholar
|
|
43.
|
Park IH, Ro J, Lee KS, et al: Phase II
study of gemcitabine in combination with vinorelbine versus
gemcitabine followed by vinorelbine for metastatic breast cancer.
Invest New Drugs. 28:659–669. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Shehata S, Saad E, Goda Y, et al: A phase
II study of gemcitabine combined with vinorelbine as first-line
chemotherapy for metastatic breast cancer. Hematol Oncol Stem Cell
Ther. 3:1–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Morabito A, Filippelli G, Palmeri S, et
al: The combination of gemcitabine and vinorelbine is an active
regimen as second-line therapy in patients with metastatic breast
cancer pretreated with taxanes and/or anthracyclines: a phase I–II
study. Breast Cancer Res Treat. 78:29–36. 2003.
|