Introduction
Ovarian cancer is one of the most common gynecologic
cancers, preceded only by cervical carcinoma. But epithelial
ovarian carcinoma (or EOC) still ranks first as the leading cause
of death among gynecological malignancies (1,2).
Because of the broad transfer of abdominopelvic cavity, limitations
of surgery and resistance or relapse after chemotherapy, the 5-year
survival rate is only approximately 30% in 70% of patients with
advanced disease (3).
Although significant progress has been made in
understanding the biology of EOC, due to the asymptomatic nature of
early ovarian cancer, patients are frequently diagnosed at advanced
stages (III–IV) when intraperitoneal carcinomatosis is already
apparent and the disease is disseminated (4). It is almost impossible to make an
early detection (5), due to the
lack of specificity of clinical symptoms and the absence of
effective screening programs. Therefore, revealing the mechanisms
of tumor invasion and metastasis and developing efficient targeted
therapies may benefit the management of ovarian cancer.
During proliferation, invasion and migration of
various tumors, cells may encounter hypoxia conditions, due to poor
or altered vascularization (6). A
number of studies have shown that hypoxia is an independent
predictor of poor prognosis (7). A
key regulator of the cellular response to oxygen deprivation is
hypoxia-inducible factor-1 (or HIF-1), which comprises a
constitutively expressed β-subunit and an oxygen-labile α-subunit
(8). Hypoxic conditions stimulate
HIF-1α to accumulate, which activates transcription of target genes
involved in angiogenesis, energy metabolism, adaptive survival or
apoptosis (9,10).
Lysyl oxidase (LOX) is a copper-dependent amine
oxidase that is thought to function only in the extracellular
milieu by cross-linking collagens or elastin to increase
extracellular matrix tensile strength (11). This protein is essential for
embryonic development, wound healing and adult tissue remodeling
(12). The findings that LOX
activity is modulated by oxygen levels, and also that LOX is able
to regulate cell migration and adhesion (13), have generated considerable interest
in the role of LOX during tumor progression. Both up- and
downregulation of LOX have been observed in different cancer cell
lines and primary tumors (12).
However, in breast cancer, elevated LOX expression has been
positively-correlated with migration and invasion (14). There is no report on the role of
LOX in migration and invasion in ovarian carcinoma.
Hypoxia has been shown to upregulate LOX expression,
via HIF-1α binding to hypoxia responsive elements in LOX promoter,
leading to enhanced invasion in an invasive or meta-static breast
cancer cell line. Conversely, inhibition of LOX expression and
catalytic activity in this cell line significantly reduced the
number of distant metastases to the lung and liver in vivo
(15). Furthermore, overexpression
of LOX in poorly invasive breast cancer cell lines results in an
increase in in vitro migration and invasion (16). However, there is no research on the
role of LOX in hypoxia of ovarian cancer. The aim of the present
study was to investigate the expressions of LOX in ovarian cancer
and relationships between expressions of LOX in hypoxia and
clinical parameters or prognosis, and to explore the role of
constitutive activation of LOX-HIF-1α signaling pathway in the
invasion and metastasis of ovarian cancer. We hypothesized that
hypoxia-induced LOX upregulates the expression of HIF-1α which
promotes ovarian cancer cell invasion and metastasis.
We report the relationship between
hypoxia/reoxygenation, LOX catalytic activity, and LOX-induced
migration in the ovarian cancer cells HO8910 and HO8910-PM. We
demonstrate that LOX expression correlates with HIF-1α in 61 cases
ovarian tumor tissues and that hypoxia upregulates LOX and HIF-1α
expression and migration/invasion of HO8910/HO8910-PM cells via
HIF-1α and HIF-2α. Furthermore, the activation of AKT and MMPs/FAK
is involved in LOX/HIF-1α-induced invasion of EOC cells. The
identification of hypoxia-HIF-1α-LOX pathway provides novel
insights into the mechanisms that control cancer cell migration in
hypoxia and reoxygenation regions. Manipulation of the tumor
microenvironment serves as a potential therapeutic approach for
ovarian cancer.
Materials and methods
Sample preparation
Consecutive patients between February 2005 and
August 2010 to Renji Hospital affiliated School of Medicine,
Shanghai Jiao Tong University, who had histologically proven
epithelial ovarian carcinoma (PEOC, n=41), borderline ovarian tumor
(n=20), innocent ovarian tumor (n=27) and normal ovarian tissue
(n=28) were studied. None of 116 patients had received radiation
therapy or chemotherapy before surgery, and had no diabetes and
other metabolic diseases. Their mean age was 56 and median age 60
years (range 28–76 years). A total of 41 PEOC patients had serious
cystadenocarcinoma (n=25), mucinous cystadenocarcinoma (n=6), clear
cell carcinoma (n=5) and endometrial carcinoma (n=5) by
histological type. All the immunoreactions were separately
evaluated by two senior pathologists.
Immunohistochemical staining
Sections (4-μm thick) were prepared from the
paraffin-embedded tissues. Immunostaining was performed by the
streptavidin-peroxidase (S-P) method (MaiXin, China). The specimens
were incubated with primary antibody or negative control antibody,
followed by biotinylated linking antibody and streptavidin
peroxidase. The primary antibodies were anti-LOX (rabbit polyclonal
antibody, 1:200, Novus, Littleton, CO, USA) and anti-HIF-1α (mouse
monoclonal antibody, 1:400, BD Transduction Laboratories, San Jose,
CA, USA). Breast cancer tissue sections with strong LOX and HIF-1α
expression were used as positive controls. The primary anti-IgG was
used as negative control. The peroxidase reaction was developed
with DAB staining. LOX positive staining was found mainly in the
cytoplasm, staining pale yellow-brown, small pieces or diffuse
distribution. Staining intensity scoring criteria were: no color,
0; light yellow, 1; yellow, 2; and brown, 3. For the percentage
tumor positivity, the following scoring was used: negative, 0;
1–25%, 1; 25–50%, 2; and >50%, 3. Both the staining intensity
and percentage positivity scores were summed and tumors with scores
ranging from 0 to 9 were assigned to: negative (0–1), + (2), ++ (3–4), +++
(≥5).
HIF-1α is present in the cytoplasm and nucleus. The
appearance of brown particles in the nucleus or cytoplasm is
considered as a positive immunohistochemistry score: no staining,
0; nucleus positive cells <10% and/or weak cytoplasmic staining,
1; the nucleus of 10–50% positive cells and/or moderate staining in
the cytoplasm, 2; >50% positive cells in the nucleus and/or
strong cytoplasmic staining, 3.
Cells and culture conditions
Human ovarian carcinoma cell line HO8910-PM, HO8910
and SKOV3 were purchased from China Academy of Science Cell Bank
(Shanghai, China). Human ovarian cancer line COC1 was purchased
from China Typical Culture Collection Center (Wuhan, China). All
cells were cultured in RPMI-1640 medium (Gibco-BRL, Grand Island,
NY, USA) supplemented with 10% heat-inactivated fetal calf serum, 2
mM L-glutamine and 100 U/ml penicillin-streptomycin mixture
(Gibco-BRL, Grand Island, NY, USA) in 5% CO2 and 95% air
humidified atmosphere at 37°C. For experiments, cells were treated
with the indicated concentrations of β-aminopropionitrile (βAPN;
100, 200 or 300 mM; Sigma Aldrich, St. Louis, MO, USA). For
hypoxia, cells were cultured in a modular incubator chamber (Thermo
Electron, Forma, MA, USA) that was infused with a mixture of 1%
O2, 5% CO2 and 94% N2 at 37°C.
Cells were incubated in normoxic or hypoxic condition for 4, 12 and
24 h, respectively.
Quantitative PCR
Total RNA was isolated from cells in the logarithmic
growth phase by TRIzol Reagent (Invitrogen, Carlsbad, CA, USA).
First-strand cDNA synthesis was performed using the Superscript
reverse transcription kit (Invitrogen, Germany). Q-PCR was carried
out on an ABI Prism 7300 PCR Detection System (Applied Biosystems,
Foster City, CA, USA) with fluorescence dye SYBR-Green (SYBR-Green
Real-Time PCR Master mix, Toyobo, Japan). The sequences of the
primers were as follows: LOX-F: 5′-TTGAGTCCTGGCTG TTATGATACC-3′,
LOX-R: 5′-TGATGTCCTGTGTAGCGA ATGTC-3′. HIF-1α-F:
5′-ACTCAGGACACAGATTTAGA CTTG-3′, HIF-1α-R:
5′-TGGCATTAGCAGTAGGTTCTTG-3′. GAPDH-F:
5′-TGATGTCCTGTGTAGCGAATGTC-3′; GAPDH-R:
5′-TGATGTCCTGTGTAGCGAATGTC-3′. The thermal cycling conditions were:
95°C 60 sec, 40 cycles of 95°C for 15 sec, 60°C for 45 sec,
Melting/Dissociation Curve Analysis. Data analysis was carried out
by ABI sequence detection software using relative quantification.
Relative expression levels were calculated using the
2−ΔΔCt method. For quantification, the target sequence
was normalized to the GAPDH mRNA levels.
siRNA transfection
RNA interference and siRNA preparation was performed
in 24 h, the following sequences for LOX and HIF-1α were used at
100 nM. HIF_1F: GCAUUGUAUGUG UGAAUUAdTdT; HIF_1R:
UAAUUCACACAUACAAUGCdTdT; HIF_2F: CGAUGGAAGCACUAGACAAdTdT; HIF_2R:
UUGUCUAGUGCUUCCAUCGdTdT; HIF_3F: CGAUCAGUUGUCACCAUUAdTdT; HIF_3R:
UAAUGGUGACAACUGAUCGdTdT; LOX_1F: GGGCAGAUGUCAGAGAUUAdTdT; LOX_1R:
UAAUCUCUGACAUCUGCCCdTdT; LOX_2F: GCACAGUUGUCAUCAACAUdTdT; LOX_2R:
AUGUUGAUGACAACUGUGCdTdT; LOX_3F: GAAUCUGACUAUACCAACAdTdT; LOX_3R:
UGUUGGUAUAGUCAGAUUCdTdT. Effects of siRNA for LOX (siLOX) or HIF-1α
(siHIF-1α) were compared with those of a random siRNA sequence.
Cells were plated onto 6-well plate and grown to 30–50% confluence
before transfection. Transfection methods were: mixing 250
μl serum-free Opti-MEM medium and 5 μl liposomes, and
mixing 250 μl serum-free Opti-MEM medium and 5 μl
HIF-1α or LOX siRNA solution. Then all components were mixed for 5
min and incubated for 20 more min at room temperature. Twenty-four
hours later, the cells were subjected to hypoxia treatment.
Western blot analysis
The cells exposed to the various treatments were
harvested, lysed and subjected to SDS-polyacrylamide gel
electrophoresis and blotting, transferred to Immun-Blot membrane
(polyvinylidene difluoride, Bio-Rad, Hercules, CA, USA), and
immunoreactivity levels were evaluated by hybridization using the
following antibodies: rabbit polyclonal antibody anti-LOX (1:1,000,
Novus, Littleton, CO, USA); mouse monoclonal antibody (mAb)
anti-HIF-1α (1:800, BD, USA); rabbit monoclonal antibody (mAb)
β-actin antibody (1:2,000, Cell Signaling Technology, USA); FAK and
Phospho-FAK (Tyr576) polyclonal antibody (1:1,000, Cell Signaling
Technology, USA); AKT and Phospho-AKT (Ser473) polyclonal antibody
(1:1,000, Cell Signaling Technology, USA); MMP-2 and MMP-9
polyclonal antibody (1:1,000, Cell Signaling Technology, USA). The
signal was then detected by chemiluminescence with SuperSignal kit
(Pierce, Rockford, IL, USA).
Lysyl oxidase activity assay
Ovarian cancer cells were plated onto 96-well tissue
culture plates (100 μl/well) under different conditions, and
stimulated as desired. Cell culture media were collected. LOX
activity was measured using Lysyl Oxidase Activity Assay Kit
(Fluorometric) (Abcam, UK). The assay reaction mixture consisted of
HRP substrate, assay buffer, horseradish peroxidase and DMSO. Prior
to assay, stock solutions and assay reaction mixture were prepared
according to the protocols. A total of 50 μl of assay
reaction mixture was added to each well of the cell media, and
those of lysyl oxidase standards. The mixture was incubated at 37°C
for 10 to 30 min, protected from light. The fluorescence increase
was measured with a fluorescence plate reader at Ex/Em = 530 to
570/590 to 600 nm (maximum Ex/Em = 540/590 nm).
Matrigel cell invasion and migration
assays
Ovarian cancer cells (5×104) (48 h
post-transfection or increasing concentrations of βAPN treated)
were placed into the upper wells of a Matrigel-coated 24-well
Transwell chamber (8-μm pore size, 12-mm diameter, Costar,
USA) in the 24-well plate and cultured in hypoxic or normoxic
environment. The medium containing 20% FBS was added to the lower
chamber. After 24 h of incubation, the non-invasive cells on the
upper membrane surface were removed with a Q tip, and the invasive
cells that invaded through the Matrigel and 8-mm pore size membrane
were fixed with 4% paraformaldehyde and stained with crystal
violet. The number of invasive cells was counted under inverted
microscope (×200). Data presented are representative of four
individual wells. Cell migration was assayed using a similar
approach without Matrigel coating, and the treated cells in
migration chamber were incubated under normoxic (N) or hypoxic (H)
conditions for 16 h.
Statistical analysis
Each experiment comparing the effects of different
treatments used the same endometrial sample, and each experiment
was repeated at least three times on different specimens. Data are
presented as mean ± SD and analyzed by SPSS software using
non-parametric statistical analysis (Mann-Whitney U test for
independent comparisons and Wilcoxon signed-ranks test for paired
comparisons). P≤0.05 was defined as statistically significant.
Results
Correlation between LOX/HIF-1α expression
and clinico-pathological parameters in epithelial ovarian
cancer
To determine whether LOX and HIF-1α expression
increases in ovarian cancer tissues, we examined the expression of
LOX and HIF-1α in 44 cases of human epithelial ovarian carcinoma,
20 cases of borderline ovarian tumor, 27 cases of benign ovarian
tumor and 28 cases of normal ovarian tissues. Immunohistochemical
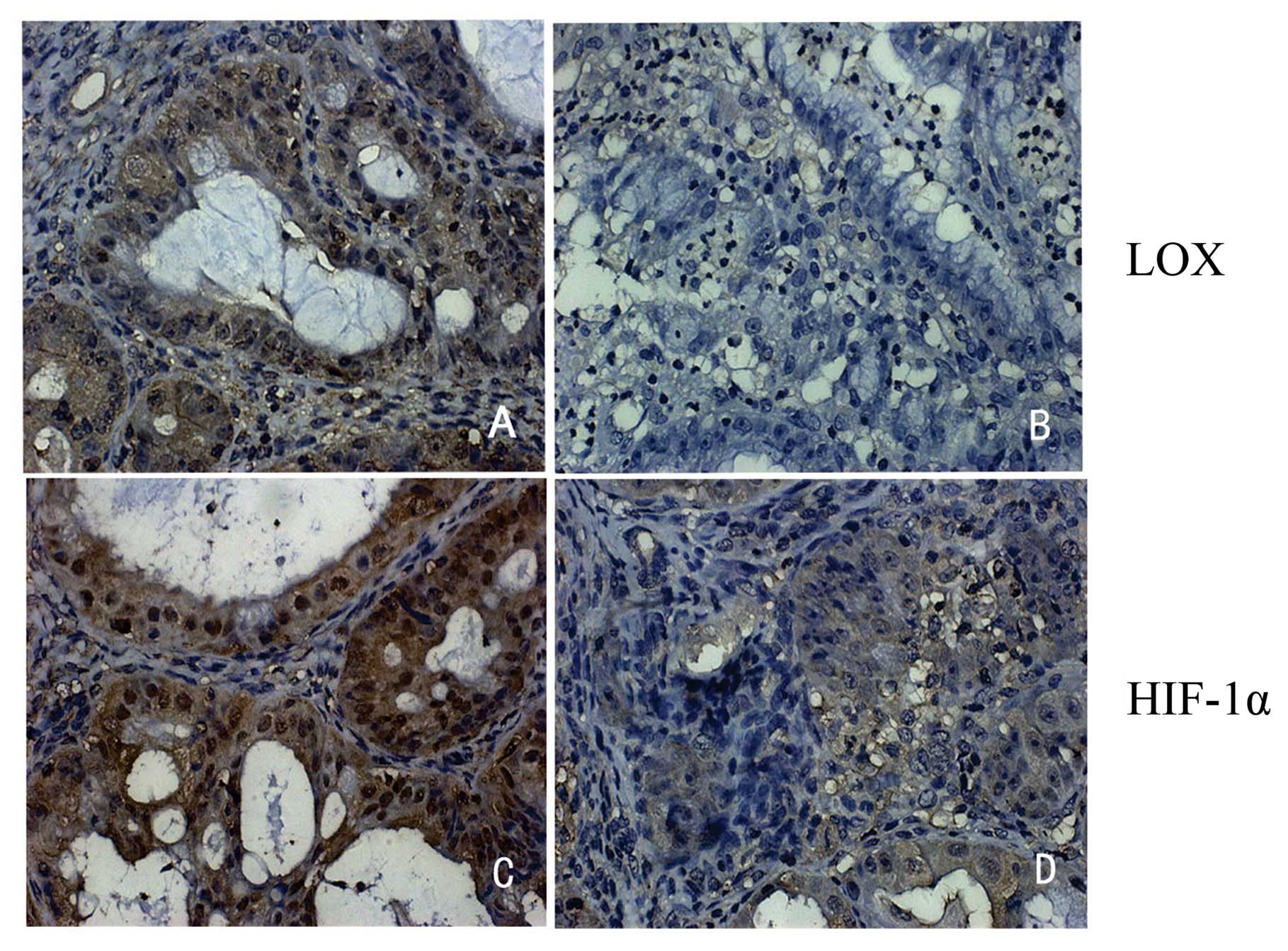
data revealed that the predominant staining pattern of LOX and
HIF-1α is cytoplasmic and/or nuclear (Fig. 1). LOX is positively expressed in
97.6% (40/41) epithelial ovarian cancer, 80% (16/20) borderline
ovarian cancer, 48.1% (13/27) benign ovarian cancer and 7.1% (2/28)
normal ovarian tissues (Table I).
HIF-1α is positively expressed in 87.8% (36/41) epithelial cancer,
90% (18/20) borderline cancer, 40.7% (11/27) benign tumors and
17.9% (5/28) normal tissues (Table
I). The data indicate that LOX and HIF-1α expression is related
to ovarian cancer malignancy. The association of LOX and HIF-1α
expression with clinical pathological parameters was then analyzed.
Clinicopathological analysis showed that both LOX and HIF-1α
expression are significantly associated with tumor FIGO
classification (p=0.035 and p=0.032), tumor size (p=0.033 and
p=0.032) and lymph node metastasis (p=0.016 and p=0.028,
respectively) (Table II).
Statistical analysis showed that LOX expression is correlated
positively with the expression of HIF-1α (r=0.423, p=0.005)
(Table III).
 | Table IExpression of LOX and HIF-1α in PEOC
tissue sections. |
Table I
Expression of LOX and HIF-1α in PEOC
tissue sections.
| No. | LOX | HIF-1α | P-valuea |
|---|
| POEC | 41 | 97.6% (40/41) | 87.8% (36/41) | |
| Borderline ovarian
tumor | 20 | 80% (16/20) | 90% (18/20) | P<0.001 |
| Benign ovarian
tumor | 27 | 48.1% (13/27) | 40.7% (11/27) | |
| Normal ovarian
tissue | 28 | 7.1% (2/28) | 17.9% (5/28) | |
 | Table IICorrelation between the
clinicopathological features and expression of LOX and HIF-1α in
PEOC. |
Table II
Correlation between the
clinicopathological features and expression of LOX and HIF-1α in
PEOC.
| LOX | HIF-1α |
|---|
|
|
|---|
| Clinicopathological
variable | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| Age (years) | | | | | | | | | | |
| <60 | 0 | 4 | 8 | 8 | | 3 | 6 | 4 | 7 | |
| ≥60 | 1 | 6 | 6 | 8 | 0.645 | 2 | 5 | 4 | 10 | 0.850 |
| FIGO disease
stage | | | | | | | | | | |
| I–II | 1 | 8 | 7 | 4 | | 4 | 8 | 4 | 4 | |
| III–IV | 0 | 2 | 7 | 12 | 0.035a | 1 | 3 | 4 | 13 | 0.032a |
| Tumor size
(cm) | | | | | | | | | | |
| ≤2 | 1 | 8 | 6 | 4 | | 4 | 8 | 2 | 5 | |
| >2 | 0 | 2 | 8 | 12 | 0.033a | 1 | 3 | 6 | 12 | 0.032a |
| Metastasis | | | | | | | | | | |
| Positive | 0 | 3 | 11 | 13 | | 1 | 6 | 5 | 15 | |
| Negative | 1 | 7 | 3 | 3 | 0.016a | 4 | 5 | 3 | 2 | 0.028a |
| Ascites | | | | | | | | | | |
| Present | 0 | 6 | 11 | 14 | | 2 | 8 | 7 | 15 | |
| Absent | 1 | 4 | 3 | 2 | 0.126 | 3 | 3 | 1 | 3 | 0.191 |
| CA125 | | | | | | | | | | |
| Abnormal | 0 | 5 | 10 | 13 | | 3 | 5 | 5 | 15 | |
| Normal | 1 | 5 | 4 | 3 | 0.172 | 2 | 6 | 3 | 2 | 0.109 |
 | Table IIIRelationship between the expression
of HIF-1α and LOX in PEOC. |
Table III
Relationship between the expression
of HIF-1α and LOX in PEOC.
| | LOX
|
|---|
| HIF-1α | No. | - | + | ++ | +++ |
|---|
| − | 5 | 1 | 3 | 1 | 0 |
| + | 11 | 0 | 4 | 4 | 3 |
| ++ | 8 | 0 | 2 | 3 | 3 |
| +++ | 17 | 0 | 1 | 7 | 9 |
| Total | 41 | 1 | 10 | 15 | 15 |
Hypoxia promotes LOX and HIF-1α
expression in epithelial ovarian cancer
Ovarian cancer is a kind of multivessel solid tumor
and hypoxia is accompanied with tumor growth. Under hypoxia
condition, HIF acts as a considerable regulator which helps tumor
cells to endure hypoxia and promote tumor infiltration and
metastasis. Oxygen concentration <6% induces HIF-1α expression.
To study how hypoxia affects LOX and HIF-1α expression, we exposed
the ovarian cancer cell lines HO8910-PM, HO8910, COC1 and SKOV3 in
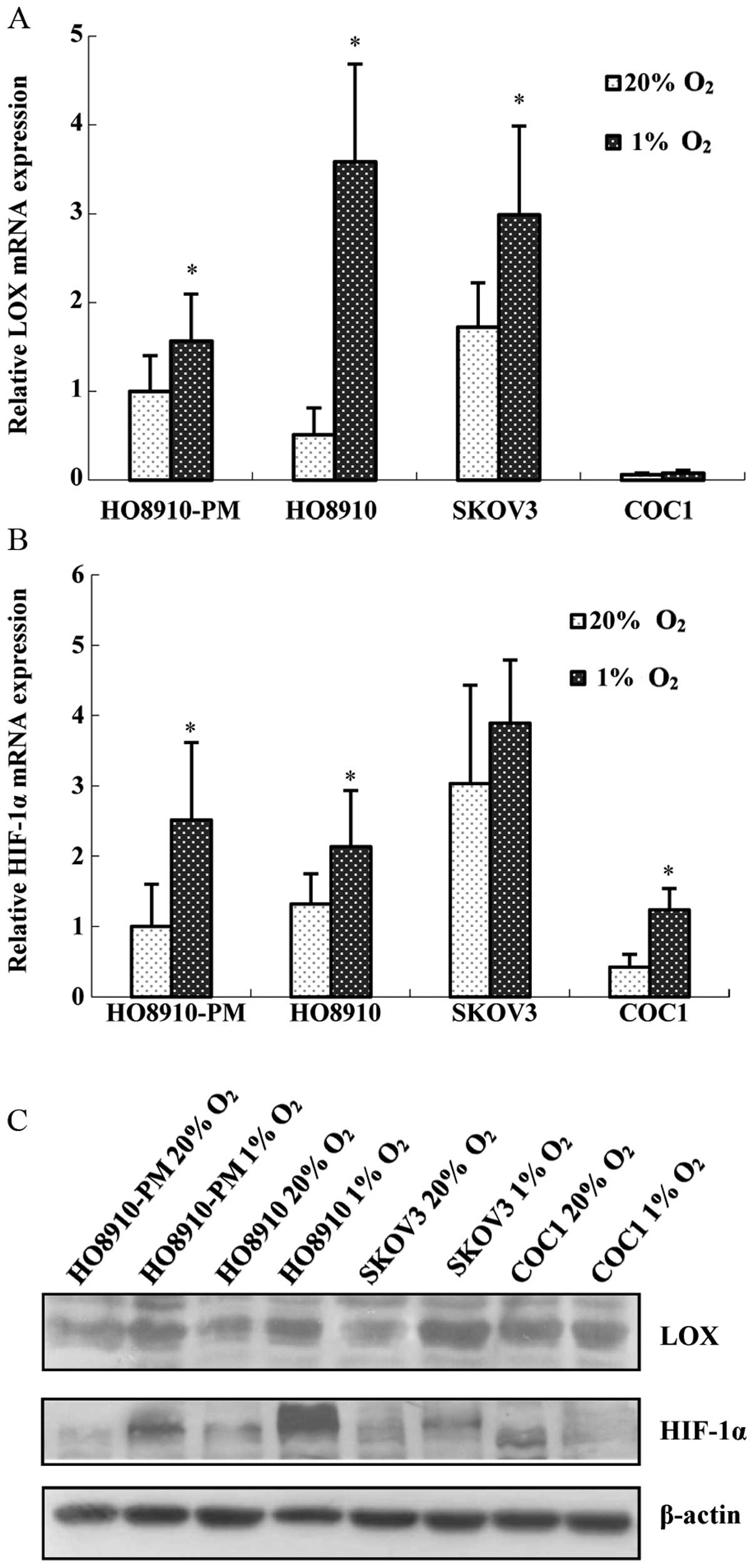
1% oxygen to mimic hypoxia condition. The results showed that all
four cell lines are positive for LOX and HIF-1α mRNA. After being
stimulated with 1% oxygen for 24 h, LOX and HIF-1α mRNA expression
(Fig. 2A and B), as well as
protein expression (Fig. 2C)
increased significantly in HO8910-PM and HO8910 cells. However, LOX
mRNA and protein expression increased marginally in COC1, SKOV3
cells (Fig. 2A and C). Therefore,
we carried out hypoxia treatment in HO8910-PM (high invasion) and
HO8910 (low invasion) cells. HO8910-PM, HO8910 cell lines were
treated with 1% oxygen for different duration, and then given 20%
oxygen for 6 or 24 h. Expression of LOX and HIF-1α was measured.
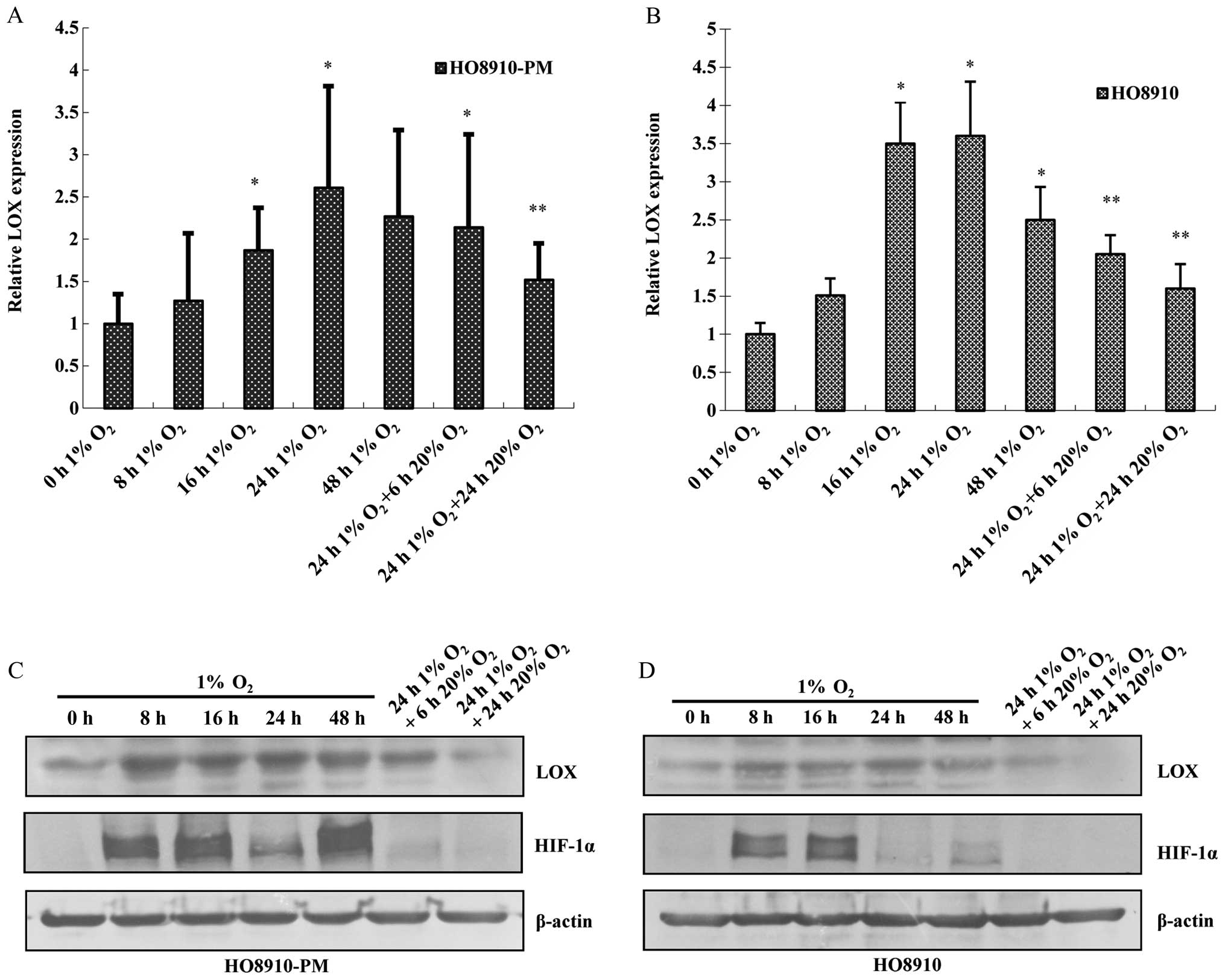
The data showed that LOX mRNA and protein expression increases in a
time-dependent manner under hypoxia condition with the peak at 24 h
(Fig. 3A and B). HIF-1α protein
expression also increases at 8 h after hypoxia treatment, peaks at
16 h, remains elevated at 24 h and returns to the basal level at 48
h. LOX along with HIF-1α mRNA and protein expression are
downregulated due to reoxygenation administration for 6 h,
reverting to the level before hypoxia treatment (Fig. 3C and D).
Inter-regulation between LOX and HIF-1α
in ovarian cancer cell lines under hypoxia condition
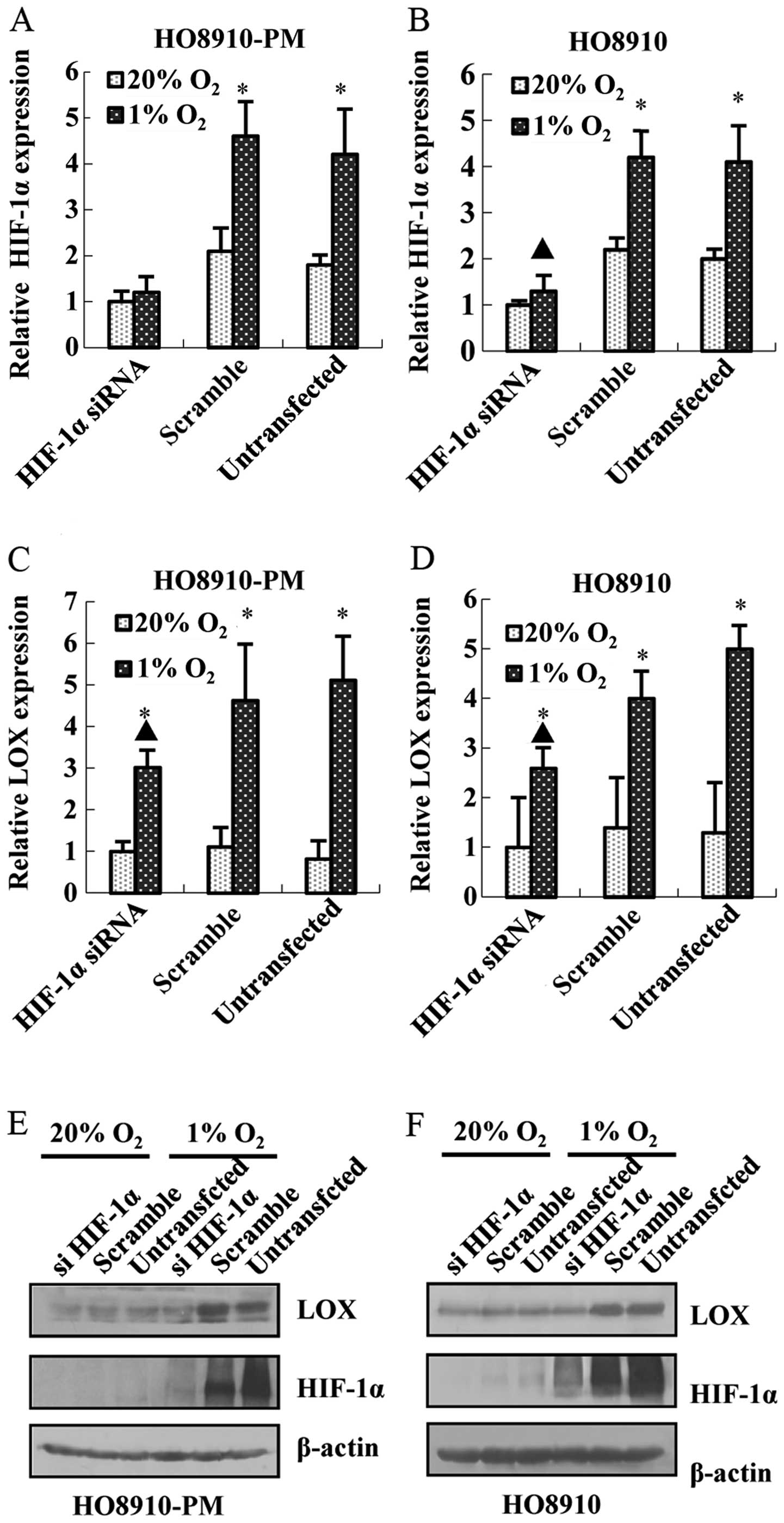
To study the relationship between LOX and HIF1α
under hypoxia condition, HIF-1α siRNA was prepared and transfected
in HO8910-PM and HO8910 cell lines. The results showed that HIF-1α
siRNA transfection leads to the decrease of HIF-1α mRNA and protein
expression in HO8910-PM (Fig. 4A and
E) and HO8910 (Fig. 4B and F).
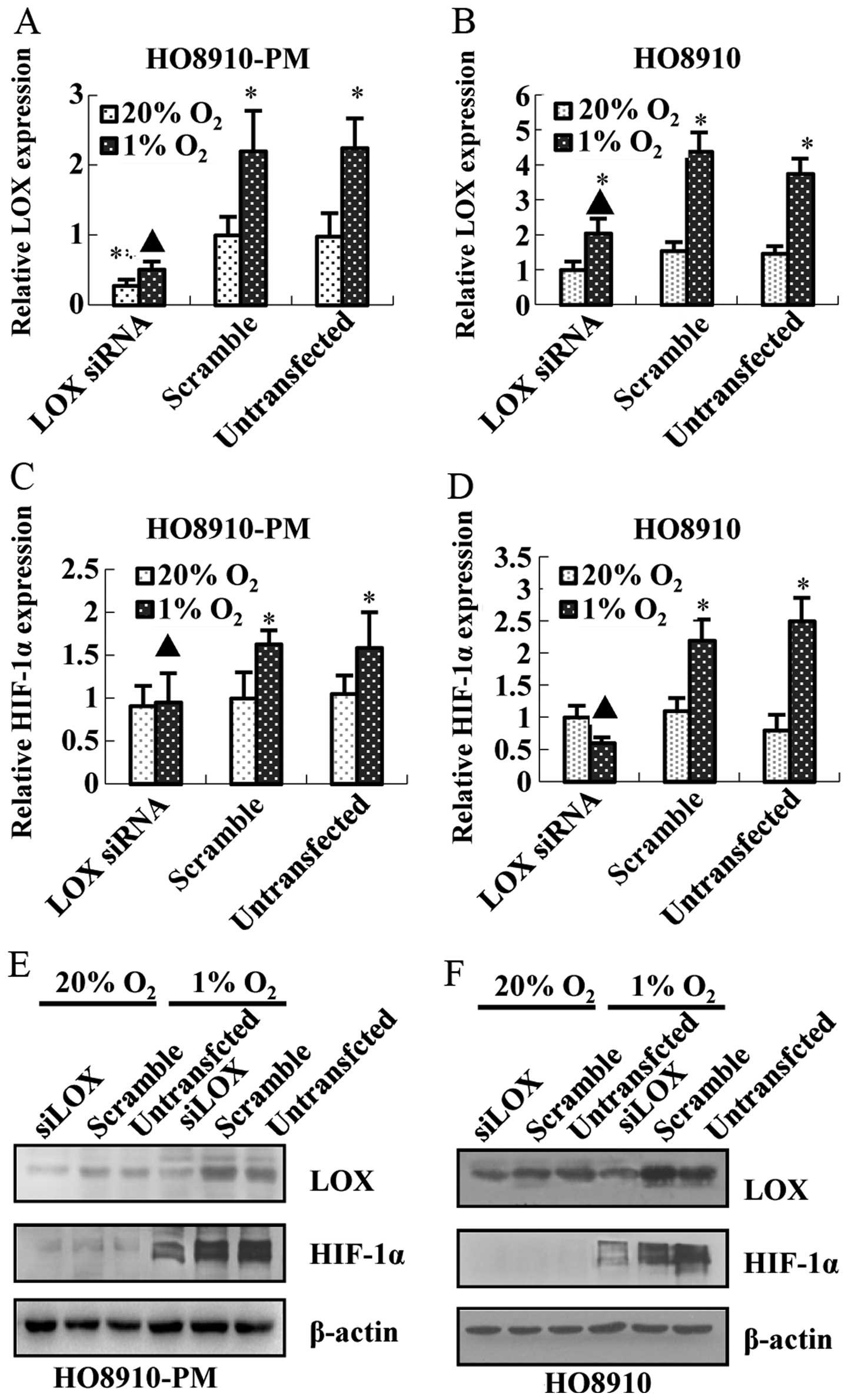
Meanwhile, knockdown of HIF-1α down-regulates LOX mRNA (Fig. 4C and D) and protein expression
(Fig. 4E and F). Furthermore,
knockdown of LOX represses LOX mRNA and protein expression
(Fig. 5A, B, E and F) as expected,
and downregulates HIF-1α mRNA and protein expression (Fig. 5C–F) regardless of hypoxia. The data
above suggest that LOX positively regulates HIF-1α expression and
support the notion that LOX possibly acts as a pivotal upregulator
on HIF-1α expression after hypoxia.
LOX catalytic activity is necessary for
regulating HIF-1α expression
Recent studies have indicated that the biological
functions of LOX proteins are dependent on its catalytic domain
(11,17–19).
We used the specific inhibitor of LOX catalytic activity,
β-aminoproprionitrile (βAPN), to address the role of active LOX.
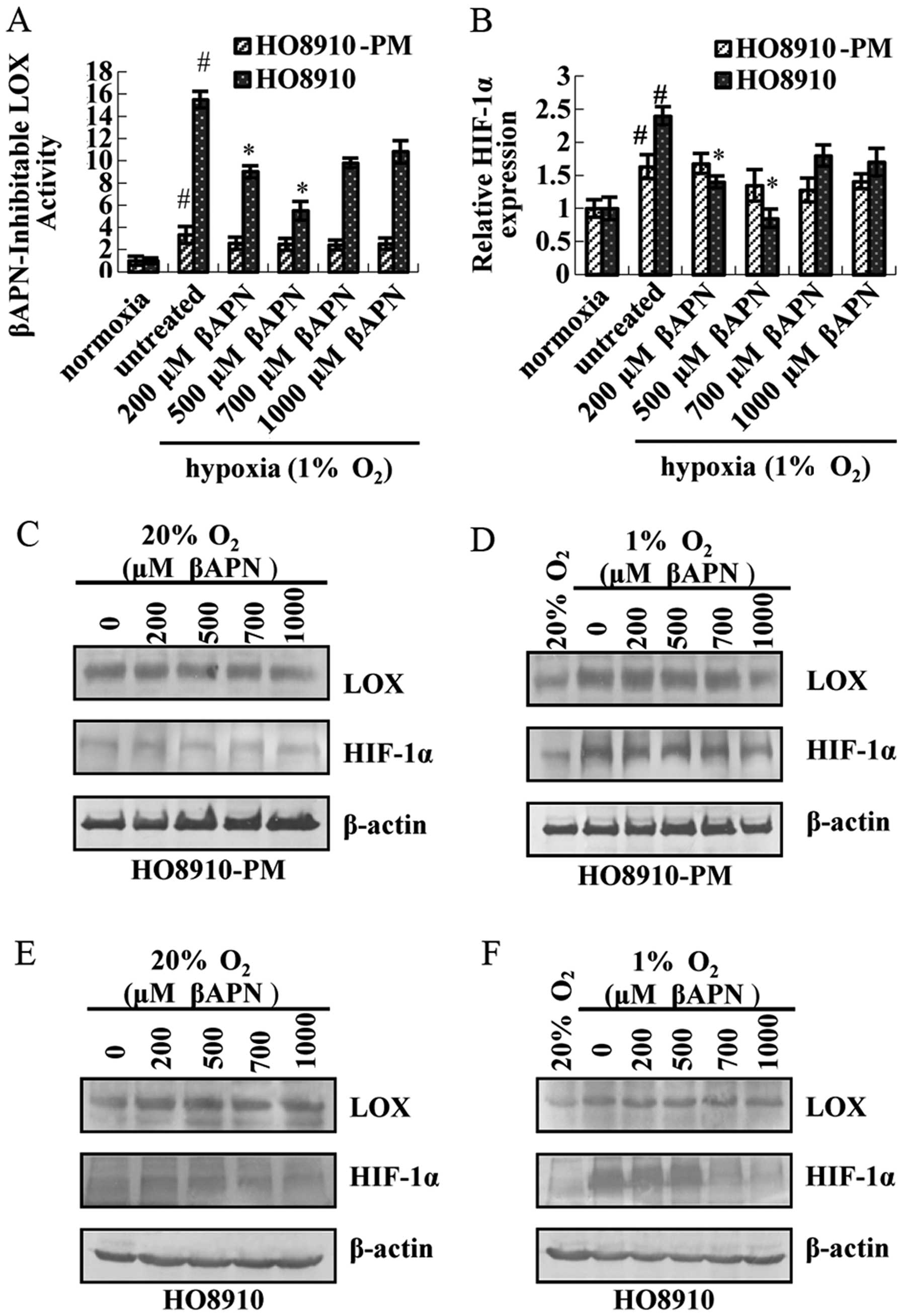
The results showed that LOX catalytic activity (Fig. 6A), HIF-1α mRNA expression (Fig. 6B), LOX and HIF-1α protein
expression (Fig. 6D) do not change
dramatically in HO8910-PM cells under hypoxia condition after
treatment by βAPN. LOX catalytic activity is inhibited by βAPN,
especially at 500 μM (Fig.
6A). LOX and HIF-1α mRNA (Fig.
6B), HIF-1α protein expression (Fig. 6E) is downregulated. In contrast,
LOX protein expression remains unaltered. LOX and HIF-1α expression
in both cells under normoxia condition are lower than that under
hypoxia condition. Thus, no noticeable change exists in either LOX
or HIF-1α at mRNA and protein level (Fig. 6C and E).
LOX facilitated ovarian cancer cell
migration and invasion depends on its catalytic activity
Transwell migration assay and cell invasion assay,
together with LOX siRNA transfection method were performed to study
the effect of LOX on ovarian cancer cell migration and invasion
capability. The results demonstrated that migration and invading
capability in ovarian cancer cells increase under hypoxia condition
as compared with those under normoxia condition without LOX siRNA
transfection (Fig. 7A and B).
Ovarian cancer cell migration and invasion capability decrease
markedly after LOX siRNA transfection under both normoxia and
hypoxia conditions (Fig. 7A and
B). These data suggest that LOX is likely to be involved in
ovarian cancer cell migration and invasion capability regulation.
Ovarian cancer cell migration and invasion capability are blocked
by βAPN in a dose-dependent manner under normoxia condition
(Fig. 7C and D). βAPN impacts
ovarian cancer cell migration and invasion capability to a lesser
extent under hypoxia condition than that under normoxia condition
(Fig. 7C and D). Reoxygenation
followed by hypoxia rescues inhibitory activity of βAPN on ovarian
cancer cell migration and invasion capability to the level under
normoxia condition (Fig. 7C and
D).
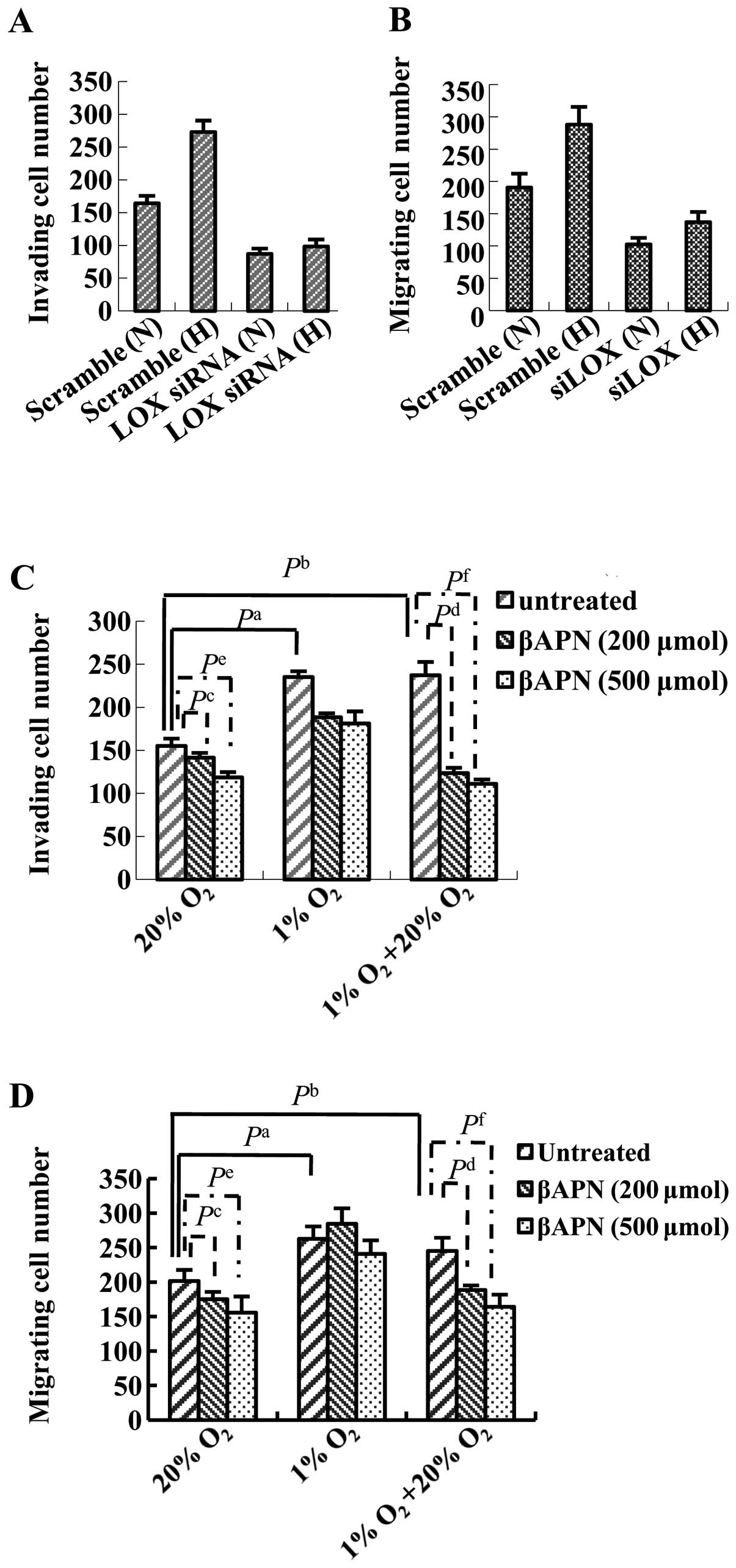 | Figure 7Effect of LOX on the invasion and
migration of HO8910 cells under normoxic and hypoxic conditions. (A
and B) HO8910 cells were transfected with scrambled siRNA or a
siRNA against LOX and then subjected to the (A) Transwell invasion
assay or (B) Transwell Matrigel migration assay under normoxic (N)
or hypoxic (H) conditions for 24 h. Hypoxia increased cell invasion
and migration; knockdown of LOX reduced cell invasion in
both normoxic and hypoxic conditions. (C and D) HO8910 cells were
cultured in 20% O2, 1% O2 or reoxygenation
conditions (24 h at 1% O2 followed by 6 h at 20%
O2). Where indicated, the cells were treated with
increasing concentrations of βAPN for 24 h prior to and during the
assays. (C) Transwell invasion assay. (D) Transwell Matrigel
migration assay. A significant increase in cell invasion and
migration were observed when HO8910 cells were cultured under
reoxygenation or hypoxic conditions, compared to 20% O2.
Inhibition of LOX catalytic activity using βAPN decreased cell
invasion and migration in cells exposed to hypoxia; this effect was
more obvious when the cells were cultured in 1% O2
followed by 6 h reoxygenation in 20% O2 (compared to 1%
O2 or 20% O2 alone). Each experiment was
performed at least three times and representative data are shown;
data are the mean ± SD of five ×200 fields of view. N, normoxia; H,
hypoxia. (A and B) Pa, Scramble (N) vs. Scramble (H); Pb, LOX siRNA
(N) vs. Scramble (N); Pc, LOX siRNA (H) vs. Scramble (H). (C and D)
Pa <0.05, untreated 1% O2 vs. untreated 20%
O2; Pb <0.05, untreated 1% O2 + 20%
O2 vs. untreated 20% O2; Pc <0.05, βAPN
(200 μmol) 20% O2 vs. untreated 20%
O2; Pd <0.05, βAPN (200 μmol) 1% O2
+ 20% O2 vs. untreated 1% O2 + 20%
O2; Pe <0.05, βAPN (500 μmol) 20%
O2 vs. untreated 20% O2; Pf <0.05, βAPN
(500 μmol) 1% O2 + 20% O2 vs.
untreated 1% O2 + 20% O2. |
LOX regulated ovarian cell migration and
invasion via migration-related molecules and protein kinases
To further study the mechanism through which LOX
regulates ovarian cell migration and invasion, migration related
molecules including MMP2, MMP9 and protein kinases which are
involved in tumor migration such as FAK, AKT were examined after
LOX siRNA transfection in HO8910-PM, HO8910 cells under normoxia
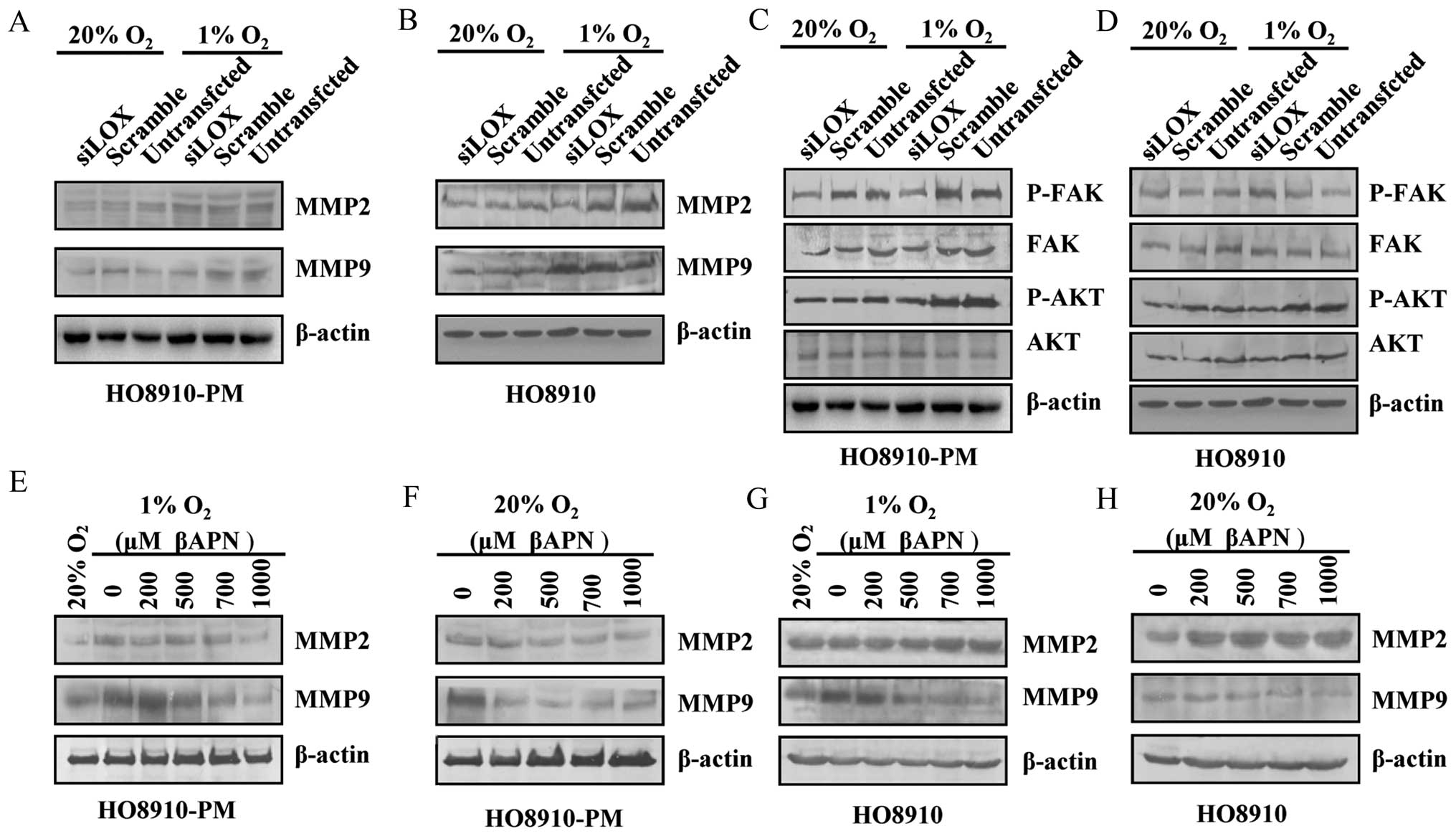
and hypoxia conditions. The results showed that MMP9, FAK, AKT
protein expression decreases in HO8910-PM cells under normoxia and
hypoxia conditions (Fig. 8A and
C). MMP2, P-FAK, P-AKT protein expression decreases in HO8910
cells under hypoxia condition (Fig. 8B
and D). P-FAK, P-AKT protein expression decreases in HO8910
cells under normoxia condition (Fig.
8B and D). MMP2, MMP9 protein expression is downregulated by
βAPN in HO8910-PM cells under reoxygenation condition followed by
hypoxia similar to the level under normoxia condition (Fig. 8E and F). MMP9 expression is
downregulated by βAPN in HO8910 cells under both hypoxia plus
reoxygenation, and normoxia (Fig. 8G
and H). Our data suggest that LOX likely regulates various
types of ovarian cancer cells through different signaling
pathways.
Discussion
Epithelial ovarian cancer (EOC) is the leading cause
of death from gynecologic cancer and the fifth most common cause of
cancer mortality in women. EOC ranks first in gynecological cancer
mortality and is prone to invasion and distant metastasis. In 2011,
there was an estimated 21,990 new diagnoses and an estimated 15,460
deaths from this neoplasm in the United States, less than 40% of
women with ovarian cancer are cured (20,21).
The distant metastasis of EOC is one of the main reasons for their
poor 5-year survival rate. Therefore, the current challenge is to
understand the molecular mechanisms underlying epithelial ovarian
cancer metastasis in order to design novel therapeutics.
Hypoxia is a characteristic of many malignancies
arising from various sites (22).
Ovarian cancer is a multi-blood vessel solid tumor. As tumor volume
increases, there become a considerable number of hypoxic tumor
cells. In hypoxic cancer cells, HIF-1α binds to the hypoxia
responsive element (HRE) in the promoter region (13) of many target genes including LOX
(15). LOX has been intensively
studied in cancers as its importance in tumor progression becomes
more thoroughly realized (16).
Although LOX has been shown to play roles in tumor metastasis, the
mechanism by which LOX expres sion is upregulated in ovarian cancer
cells and the correlation with hypoxia are still poorly understood.
Here, we present data that LOX is overexpressed in EOC tumor
tissues compared with non-cancer tissues. Additionally, LOX is
expressed intracellularily within ovarian cancer cells and
facilitates cell migration through the regulation of HIF-1α.
Furthermore, EOC cells display a marked increase in LOX-dependent
FAK/AKT activation and cell migration following
hypoxia/reoxygenation.
In this study, first, the association between
hypoxia and increased LOX expression was validated in clinically
relevant ovarian cancer paraffin-embedded tissues by
immunohistochemical method. Using HIF-1α as a surrogate marker of
hypoxia in malignant tissue, we observed a significant correlation
between hypoxia and LOX expression. LOX and HIF-1α proteins are
both overexpressed in ovarian cancer cells and the expression is
significantly associated with advanced clinical stages and
metastasis in EOC. Moreover, statistical analysis showed that high
level of LOX correlates positively with the high expression of
HIF-1α (r=0.423, p=0.005). Our data suggest that HIF-1α promotes
EOC progression and metastasis via upregulation of LOX expression
in EOC.
In order to further investigate whether hypoxia
regulates LOX, we examined the effect of hypoxia on LOX expression
in EOC cells. The expression of LOX mRNA and protein is strongly
elevated in hypoxia-exposed HO8910 and HO8910-PM cells compared
with the normoxic control cells. Furthermore, we demonstrated that
the poorly invasive cancer cells HO8910, which express little to no
endogenous LOX, aberrantly express high levels of LOX when exposed
to hypoxic conditions. These findings are in agreement with those
previously described in other cancer cells (15). Therefore, in addition to making
invasive/metastatic cancer cells more invasive, hypoxia may induce
LOX expression in poorly invasive cancer cells, thereby enabling
them to acquire invasive competence.
It has been demonstrated that HIF-1α is a hypoxia
responsive factor (8). We
investigated whether hypoxia upregulates LOX via HIF-1α. Our data
showed that the expressions of LOX mRNA and protein are greatly
reduced when HIF-1α is downregulated by siRNA, suggesting that
hypoxia may upregulate LOX via HIF-1α pathway in HO8910 and
HO8910-PM cells. Studies have demonstrated that HIFs are involved
in tumor invasion and metastasis (23). We investigated whether hypoxia
could promote tumor invasion and migration via HIF-1α and LOX. In
our study, we observed that the migration and invasion capability
decreases markedly after LOX siRNA transfection in HO8910 cells
under both normoxia and hypoxia conditions. Our data suggest that
LOX is involved in ovarian cancer cell migration and invasion
capability regulation and is related to HIFs. We further showed
that LOX regulates cell motility/migration via LOX activity,
supported by the data on βAPN, an inhibitor of LOX.
Multiple mechanisms may be involved in
hypoxia-induced metastasis (13).
Since hypoxia followed by reoxygenation may also provide a
physiological pressure in tumors selecting for metastatic cell
phenotypes (24,25), it is essential to determine whether
hypoxia-induced LOX in ovarian cancer cells is active under hypoxic
conditions. Our results show that under hypoxic conditions LOX is
not catalytically active. This finding is in agreement with the LOX
mechanism of action in that LOX requires oxygen in order to
regenerate catalytic activity (11). As a consequence of its dependency
on oxygen, hypoxia induced LOX is unable to induce cell migration
until the microenvironment is reoxygenated. Moreover, this increase
in migration is also correlated with the oxygen level and
activation of MMPs and FAK. These novel findings validate the role
of LOX in ovarian cancer metastasis and indicate the importance of
reoxygenation when studying hypoxia induced phenomena.
While the invasive mechanism mediated by LOX-HIF-1α
has been poorly understood in EOCs, accumulating data in other
cancer cells have indicated that multiple signaling mechanisms
exist to regulate cell migration. We showed that the LOX-HIF-1α
mutual regulation mechanism activates the AKT pathway and thus
promotes tumor cells migration. This result agrees with those
published earlier showing that PI3K/AKT pathway increases the rate
of HIF-1α protein synthesis (26).
Such activation of AKT pathway by LOX is consistent with recently
published results, which have shown that LOX modulates ovarian
tumor progression by stimulating MMPs/FAK/AKT signaling. It has
been shown that HIF-1α-inducible lysyl oxidase activates HIF-1α via
PI3K/AKT pathway in colorectal cancer (26). PI3K and AKT pathways appear to
operate independently (27), but
sometimes the two pathways are intermingled (28). Further studies are needed to
clarify the relationship between PI3K/AKT and other pathways in LOX
signaling of ovarian cancer cells.
In conclusion, our study demonstrates that
hypoxia-HIF-1α-LOX-AKT pathway regulates the invasion and migration
of ovarian cancer cells under hypoxic/reoxygenation
microenvironments. HIF-1α and LOX activation might play a role in
promoting a more malignant phenotype of EOC and presents targets
for novel therapeutic strategies.
Acknowledgements
This study was supported by grants
from National Natural Science Foundation of China (nos. 81001160,
30772306 and 81072138), Science Foundation of Shanghai Municipal
Health Bureau (no. 2009060), Shanghai Health Bureau Key Disciplines
and Specialties Foundation, Shanghai Education Commission Key
Disciplines Foundation, Key Discipline Project of Renji Hospital,
Shanghai Jiatong University School of Medicine and NIH (P20
RR016457 from INBRE Program of the National Center for Research
Resources, Y.W.).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar
|
|
2.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3.
|
Rustin G, van der Burg M, Griffin C, Qian
W and Swart AM: Early versus delayed treatment of relapsed ovarian
cancer. Lancet. 377:380–381. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Vergara D, Merlot B, Lucot JP, Collinet P,
Vinatier D, Fournier I and Salzet M: Epithelial-mesenchymal
transition in ovarian cancer. Cancer Lett. 291:59–66. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Ricciardelli C and Oehler MK: Diverse
molecular pathways in ovarian cancer and their clinical
significance. Maturitas. 62:270–275. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Choi JY, Jang YS, Min SY and Song JY:
Overexpression of MMP-9 and HIF-1α in breast cancer cells under
hypoxic conditions. J Breast Cancer. 14:88–95. 2011.
|
|
7.
|
Dayan F, Mazure NM, Brahimi-Horn MC and
Pouyssegur J: A dialogue between the hypoxia-inducible factor and
the tumor microenvironment. Cancer Microenviron. 1:53–68. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Allen M and Louise Jones J: Jekyll and
Hyde: the role of the microenvironment on the progression of
cancer. J Pathol. 223:162–176. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Semenza GL: Defining the role of
hypoxia-inducible factor 1 in cancer biology and therapeutics.
Oncogene. 29:625–634. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chen CL, Chu JS, Su WC, Huang SC and Lee
WY: Hypoxia and metabolic phenotypes during breast carcinogenesis:
expression of HIF-1alpha, GLUT1, and CAIX. Virchows Arch.
457:53–61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Lucero HA and Kagan HM: Lysyl oxidase: an
oxidative enzyme and effector of cell function. Cell Mol Life Sci.
63:2304–2316. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Payne SL, Hendrix MJ and Kirschmann DA:
Paradoxical roles for lysyl oxidases in cancer - a prospect. J Cell
Biochem. 101:1338–1354. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Postovit LM, Abbott DE, Payne SL, Wheaton
WW, Margaryan NV, Sullivan R, Jansen MK, Csiszar K, Hendrix MJ and
Kirschmann DA: Hypoxia/reoxygenation: a dynamic regulator of lysyl
oxidase-facilitated breast cancer migration. J Cell Biochem.
103:1369–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Nagaraja GM, Othman M, Fox BP, Alsaber R,
Pellegrino CM, Zeng Y, Khanna R, Tamburini P, Swaroop A and Kandpal
RP: Gene expression signatures and biomarkers of noninvasive and
invasive breast cancer cells: comprehensive profiles by
representational difference analysis, microarrays and proteomics.
Oncogene. 25:2328–2338. 2006. View Article : Google Scholar
|
|
15.
|
Erler JT, Bennewith KL, Nicolau M,
Dornhofer N, Kong C, Le QT, Chi JT, Jeffrey SS and Giaccia AJ:
Lysyl oxidase is essential for hypoxia-induced metastasis. Nature.
440:1222–1226. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Payne SL, Fogelgren B, Hess AR, Seftor EA,
Wiley EL, Fong SF, Csiszar K, Hendrix MJ and Kirschmann DA: Lysyl
oxidase regulates breast cancer cell migration and adhesion through
a hydrogen peroxide-mediated mechanism. Cancer Res. 65:11429–11436.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Polgar N, Fogelgren B, Shipley JM and
Csiszar K: Lysyl oxidase interacts with hormone placental lactogen
and synergistically promotes breast epithelial cell proliferation
and migration. J Biol Chem. 282:3262–3272. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Fogelgren B, Polgar N, Szauter KM,
Ujfaludi Z, Laczko R, Fong KS and Csiszar K: Cellular fibronectin
binds to lysyl oxidase with high affinity and is critical for its
proteolytic activation. J Biol Chem. 280:24690–24697. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Bouez C, Reynaud C, Noblesse E, Thepot A,
Gleyzal C, Kanitakis J, Perrier E, Damour O and Sommer P: The lysyl
oxidase LOX is absent in basal and squamous cell carcinomas and its
knockdown induces an invading phenotype in a skin equivalent model.
Clin Cancer Res. 12:1463–1469. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
21.
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
22.
|
Cassavaugh J and Lounsbury KM:
Hypoxia-mediated biological control. J Cell Biochem. 112:735–744.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Gort EH, Groot AJ, van der Wall E, van
Diest PJ and Vooijs MA: Hypoxic regulation of metastasis via
hypoxia-inducible factors. Curr Mol Med. 8:60–67. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rofstad EK: Microenvironment-induced
cancer metastasis. Int J Radiat Biol. 76:589–605. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Ruan K, Song G and Ouyang G: Role of
hypoxia in the hallmarks of human cancer. J Cell Biochem.
107:1053–1062. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Pez F, Dayan F, Durivault J, Kaniewski B,
Aimond G, Le Provost GS, Deux B, Clezardin P, Sommer P, Pouyssegur
J and Reynaud C: The HIF-1-inducible lysyl oxidase activates HIF-1
via the Akt pathway in a positive regulation loop and synergizes
with HIF-1 in promoting tumor cell growth. Cancer Res.
71:1647–1657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Denko NC, Fontana LA, Hudson KM, Sutphin
PD, Raychaudhuri S, Altman R and Giaccia AJ: Investigating hypoxic
tumor physiology through gene expression patterns. Oncogene.
22:5907–5914. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Choi YK, Kim CK, Lee H, Jeoung D, Ha KS,
Kwon YG, Kim KW and Kim YM: Carbon monoxide promotes VEGF
expression by increasing HIF-1alpha protein level via two distinct
mechanisms, translational activation and stabilization of
HIF-1alpha protein. J Biol Chem. 285:32116–32125. 2010. View Article : Google Scholar
|