Introduction
Lung cancer (LC) is the leading cause of cancer
death in men and women, accounting for 28% of all cancer deaths in
2012 (1). Approximately 226,160
new cases (14% of all new cancers) were estimated to be diagnosed
in 2012 (116,470 in men and 109,690 in women). Since only 15% of
lung cancer cases are diagnosed at an early stage, in the majority
of lung cancer patients, metastasis has occurred by the time of
diagnosis. Over half of people with lung cancer die within one year
of being diagnosed. Five-year survival is only 3.5% (2). Malignant mesothelioma (MM), a rare
highly aggressive tumor with no known cure, is associated with
asbestos exposure. MM is characterized by aggressive local growth,
invasion of vital mediastinal structures and death within 4–18
months. Metastasis has been documented in 50% of patients (3).
Metastasis occurs secondary to cancer cell
detachment from the primary tumor, invasion through degraded
basement membrane into the surrounding stroma and entry into and
transport through the vascular or lymphatic system to distal sites
such as the liver, lungs and brain and extravasation, tumor cell
proliferation and angiogenesis at distal sites (4–8).
Tumor cell invasion depends upon degradation of the extracellular
matrix (ECM), which, when intact, acts as a barrier to block cancer
cell invasion. The ECM is composed of collagen, proteoglycans,
fibronectin, laminin and other glycoproteins (9–11).
Two families of proteases, the matrix metalloproteinases (MMPs) and
urokinase plasminogen activators (u-PA) are involved in tumor
invasion and metastasis. Numerous clinical and experimental studies
have demonstrated that elevated levels of u-PA and MMPs are
associated with tumor growth, cancer progression, metastasis and
shortened survival in patients (12–18).
MMPs, especially MMP-2 and -9 play key roles in
tumor cell invasion and metastasis due to their ability to degrade
type IV collagen, a major component of the ECM (11,19,20).
MMP-2 and -9 are secreted in their latent zymogenic form as
inactive pro-enzymes and cleaved by other MMPs or proteases into
activated forms of 68, 58 and 54 kDa for MMP-2 and 84 kDa for
MMP-9. Proteolytic activities of MMP-2 and -9 are inhibited by
specific inhibitors, tissue inhibitors of metalloproteinases
(TIMPs). Thus, a critical determinant of net proteolytic
degradation is the balance between MMP and TIMP levels. Clinical
studies note the association of MMP expression with progression of
lung cancer (13,14) and malignant mesothelioma (15,16).
The serine protease u-PA converts plasminogen to
plasmin, which is capable of promoting tumor growth and
angiogenesis, degrading the ECM and basement membrane and
activating pro-MMPs (21).
Components of the uPA system such as u-PA, plasminogen activator
inhibitor-1 (PAI-1) and urokinase-type plasminogen activator
receptor (uPAR) are overexpressed in a variety of cancer types,
most notably in breast cancer (22), but also in lung cancer (17,23)
and malignant mesothelioma (18,24)
and correlate with cancer progression, metastasis and poor
prognosis. Thus the uPA system represents a potential target for
anticancer strategies.
Rath and Pauling (25) proposed using nutrients such as
lysine and ascorbic acid to target plasmin-mediated connective
tissue degradation as a universal approach to tumor growth and
expansion. Binding to plasminogen active sites, lysine blocks
plasminogen activation into plasmin by tissue plasminogen activator
(t-PA). Thus it modulates the plasmin-induced MMP activation
cascade (26). Subsequent studies
confirmed this approach and resulted in identifying a novel
formulation composed of lysine, ascorbic acid, proline and green
tea extract and other micronutrients (NM), which has shown
significant anticancer activity against a large number (∼40) of
cancer cell lines, blocking cancer growth, tissue invasion and MMP
expression both in vitro and in vivo(27). In this study, we focused on the
modulating effect of NM on the activities of MMP-2 and -9, TIMPs
and u-PA in human lung cancer (A-549 and Calu-3) and malignant
mesothelioma (MSTO-211H) cell lines.
Materials and methods
Materials
Human lung cancer cells A-549 and Calu-3 and
malignant mesothelioma MSTO-211H, along with their culture media
Ham F-12 and RPMI were obtained from ATCC. Antibiotics, penicillin
and fetal bovine serum (FBS), were obtained from Gibco (BRL, Long
Island, NY). Twenty-four well tissue culture plates were obtained
from Costar (Cambrdige, MA). Gelatinase zymography was performed in
10% Novex pre-cast SDS polyacrylamide gel (Invitrogen Inc.) with
0.1% gelatin in non-reducing conditions. The nutrient mixture (NM),
prepared by VitaTech (Hayward, CA) was composed of the following
ingredients in the relative amounts indicated: vitamin C (as
ascorbic acid and as Mg, Ca and palmitate ascorbate) 700 mg;
L-lysine 1000 mg; L-proline 750 mg; L-arginine 500 mg; N-acetyl
cysteine 200 mg; standardized green tea extract (80% polyphenol)
1000 mg; selenium 30 μg; copper 2 mg; manganese 1 mg. All
other reagents used were of high quality and were obtained from
Sigma, unless otherwise indicated.
Cell cultures
The LC (A-549 and Calu-3) and MM (MSTO-211H) cell
lines were grown in Ham F-12 and RPMI media, supplemented with 10%
FBS, penicillin (100 U/ml) and streptomycin (100 μg/ml) in
24-well tissue culture plates. The cells were plated at a density
of 1×105 cells/ml and grown to confluency in a
humidified atmosphere at 5% CO2 at 37°C.
Serum-supplemented media were removed and the cell monolayer was
washed once with PBS with the recommended serum-free media. The
cells were treated with the nutrient mixture, dissolved in media
and tested at 0, 50, 100, 250, 500 and 1000 μg/ml in
triplicate at each dose. Parallel sets of cultures were treated
with PMA (100 ng/ml) for induction of MMP-9. Control and PMA
treatments were done in triplicates. The plates were then returned
to the incubator. The conditioned media were collected separately,
pooled and centrifuged at 4°C for 10 min at 3,000 rpm to remove
cells and cell debris. The supernatant was collected and used to
assess for u-PA activity (by fibrin zymography on 10% SDS-PAGE gels
containing fibrinogen and plasminogen), MMP-2 and -9 (by gelatinase
zymography) and TIMPs (by reverse zymography).
Fibrin zymography
Fibrin zymography was used to analyze u-PA activity
on 10% SDS-PAGE gels containing fibrinogen (5.5 mg/ml) and
plasminogen (50 μg/ml). After electrophoresis, the gels were
washed twice with 2.5% Triton X-100 for 30 min. The gels were then
incubated overnight at 37°C with 0.1% glycine buffer pH 7.5 and
then stained with 0.5% Coomassie Brilliant Blue R250 and destained.
Electrophoresis of u-PA and t-PA were conducted for comparison.
Fibrin zymograms were scanned using CanoScan 9950F Canon
Scanner.
Gelatinase zymography
Gelatinase zymography was performed in 10% NOVEX
Pre-Cast SDS polyacrylamide gel (Invitrogen Corp.) in the presence
of 0.1% gelatin under non-reducing conditions. Culture media (20
μl) were mixed with sample buffer and loaded for SDS-PAGE
with Tris-glycine SDS buffer as suggested by the manufacturer
(Novex). Samples were not boiled before electrophoresis. Following
electrophoresis the gels were washed twice in 2.5% Triton X-100 for
30 min at room temperature to remove SDS. The gels were then
incubated at 37°C overnight in substrate buffer containing 50 mM
Tris-HCl and 10 mM CaCl2 at pH 8.0 and stained with 0.5%
Coomassie Blue R250 in 50% methanol and 10% glacial acetic acid for
30 min and destained. Upon renaturation of the enzyme, the
gelatinases digest the gelatin in the gel and give clear bands
against an intensely stained background. Protein standards were run
concurrently and approximate molecular weights were determined by
plotting the relative mobilities of known proteins.
Reverse zymography
TIMPs were analyzed by reverse zymography on 15% SDS
gels containing serum-free conditioned medium from cells. After
electrophoresis the gels were washed twice with 2.5% Triton-X for
30 min at room temperature to remove SDS. The gels were then
incubated at 37°C overnight in 50 mM Tris-HCl and 10 mM Ca
Cl2 at pH 7.6 and stained with 0.5% Coomassie Blue R25,
destained and scanned.
Scanning of gelatinase and fibrin
zymograms
Gelatinase, reverse and fibrin zymograms were
scanned using CanoScan 9950F Canon scanner at 300 dpi. The
intensity of the bands was evaluated using the pixel-based
densitometer program Un-Scan-It, Version 5.1, 32-bit, by Silk
Scientific Corp. (Orem, UT, USA), at a resolution of 1 Scanner Unit
(1/100 of an inch for an image that was scanned at 100 dpi). The
pixel densitometer calculates the optical density of each pixel
(values 0–255) using the darkly stained background of the gel as a
pixel value of 0. A logarithmic optical density scale was used
since the optical density of films and gels is logarithmically
proportional to the concentration. The pixel densitometer sums the
optical density of each pixel to give a band’s density. In all
graphs, band densities were reported as percentages of the sums of
all pixels in a given lane (treatment) of a gel.
Statistical analysis
Pearson’s correlation coefficient was determined
between mean MMP-2, u-PA and TIMP-2 expressions of lung cancer cell
line A-549, mean u-PA and TIMP-2 expressions of lung cancer Calu-3
and between mean MMP-9 and TIMP-2 expression of malignant
mesothelioma cell line MSTO-211H using MedCalc Software
(Mariakerke, Belgium).
Results
Effect of NM on u-PA activity in human
lung cancer and mesothelioma cell lines
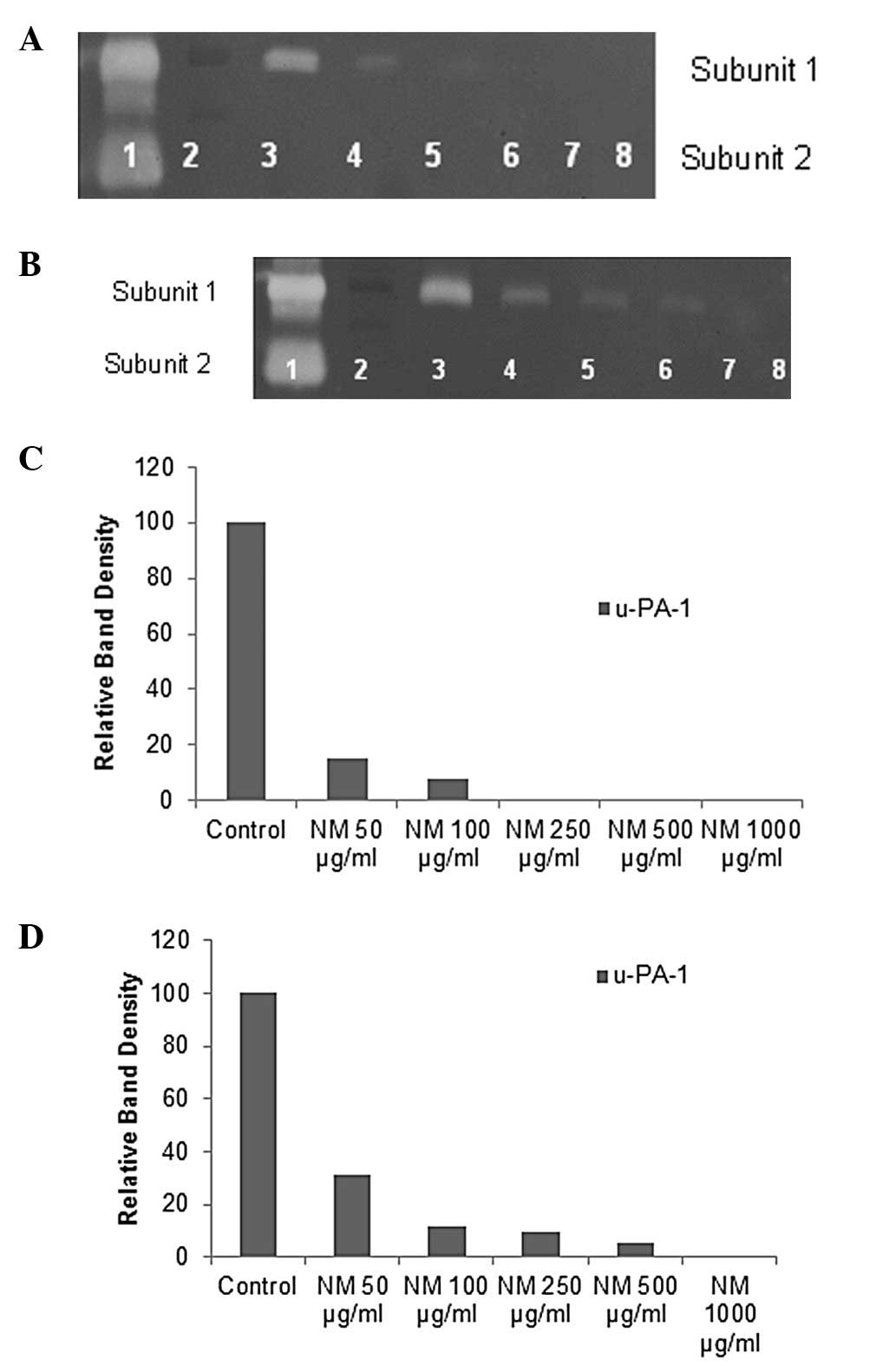
Activity of u-PA was detected in both lung cancer
cell lines showing one band corresponding to subunit 1 (55 kDa).
However, no bands corresponding to u-PA were detected for
mesothelioma cell line. NM exerted dose response inhibition with
virtual block of u-PA activity at 250 μg/ml in A-549 cells
(linear trend R2=0.563) and 1000 μg/ml in Calu-3
cells (linear trend R2=0.680) (see Fig. 1 for respective fibrin zymograms and
densitometry analyses).
Effect of NM on MMP-2 and -9 expression
by human lung cancer and mesothelioma cell lines
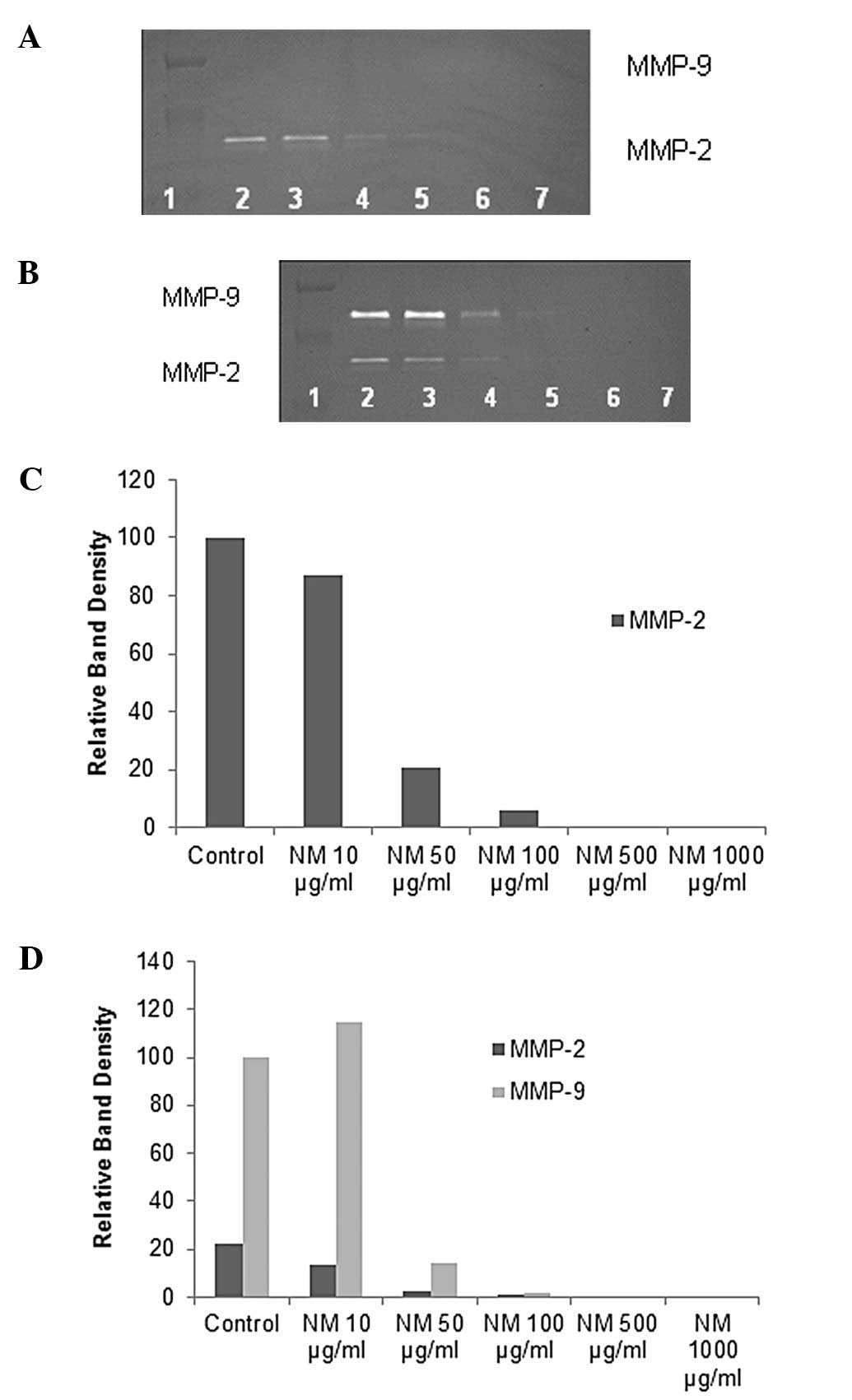
On gelatinase zymography, a band corresponding to
MMP-2 was detected in normal A-549 cells and strong induction of
MMP-9 with PMA (100 ng/ml) treatment. NM inhibited MMP-2 and -9 in
a dose-dependent manner with total block at 500 μg/ml
(linear trend R2=0.781 for MMP-2 and 0.722 for MMP-9).
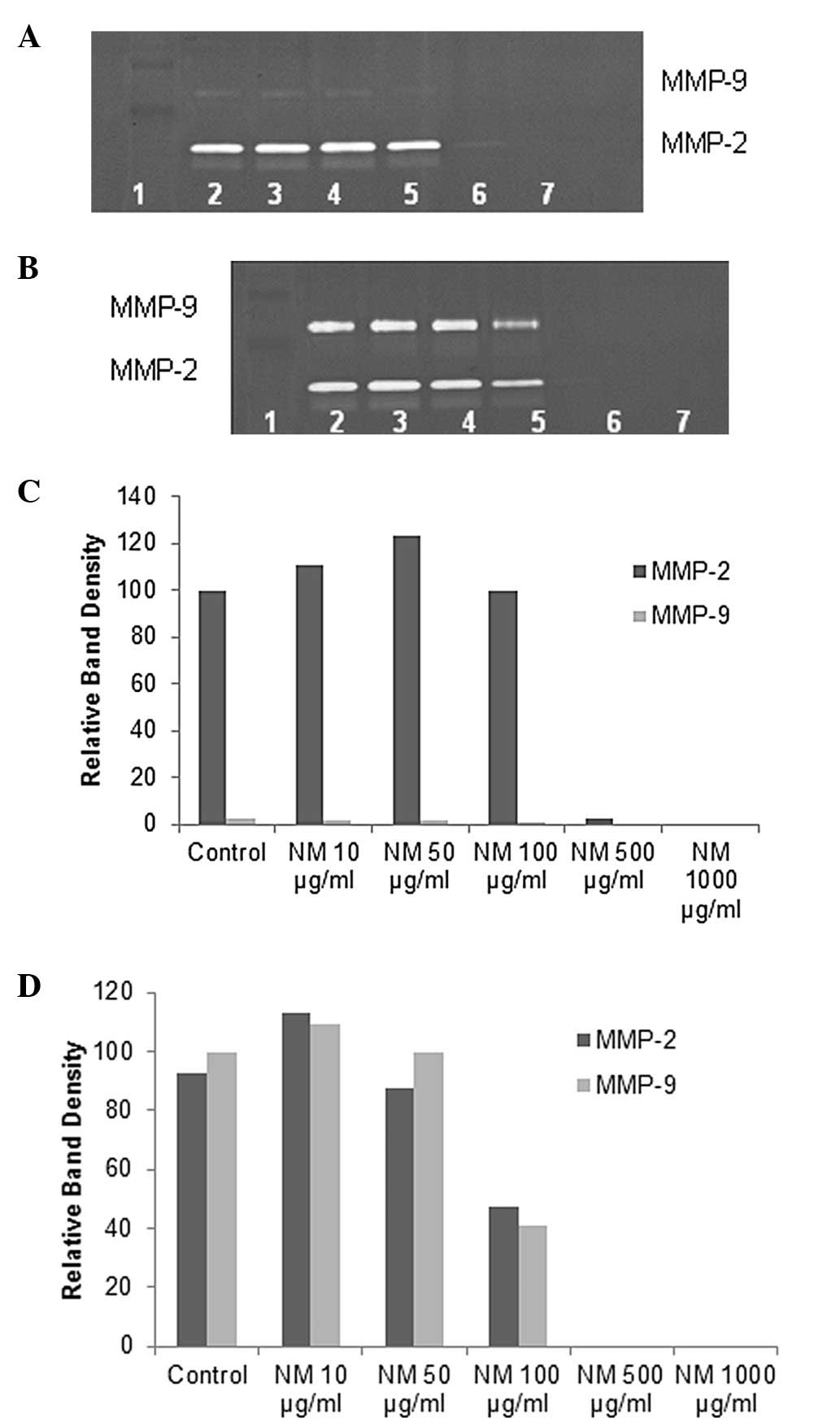
Mesothelioma MSTO-211H cells showed two bands, an intense band
corresponding to MMP-2 and a faint band corresponding to MMP-9,
which was significantly enhanced with PMA treatment. NM inhibited
MMP expression, with complete block of MMP-2 and -9 at 500
μg/ml (linear trend R2 = 0.849 for MMP-2 and
R2=0.853 for MMP-9) (see Figs. 2 and 3 for gelatinase zymograms and
densitometry analyses).
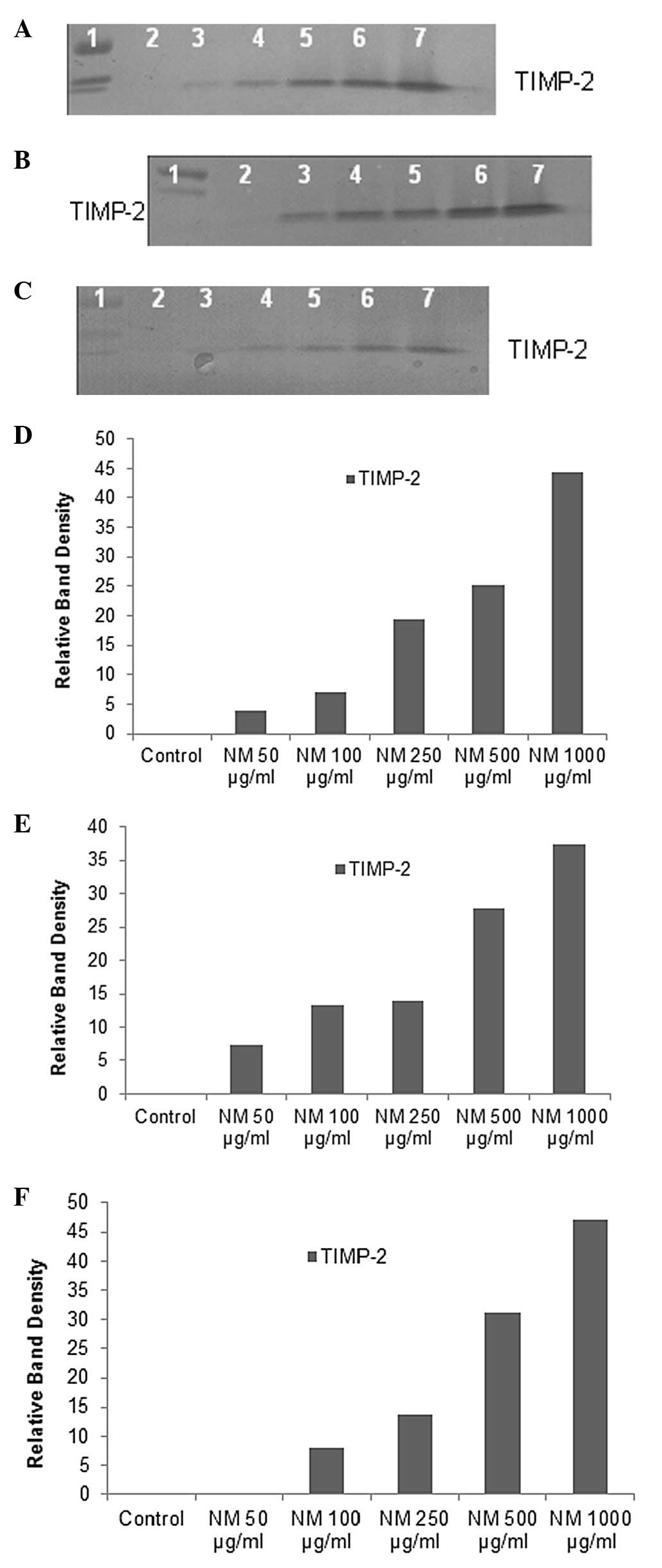
Effect of NM on TIMP activity in human
lung and cancer and mesothelioma cell lines
Reverse zymography revealed upregulation of TIMP-2
activity with NM treatment in all cancer cell lines in a
dose-dependent manner. Minimum activity was expressed at 50 and
maximum at 1,000 μg/ml NM (see Fig. 4 for respective reverse zymograms
and densitometry analyses).
Correlation between lung cancer and
mesothelioma u-PA, TIMP-2 and MMP expressions
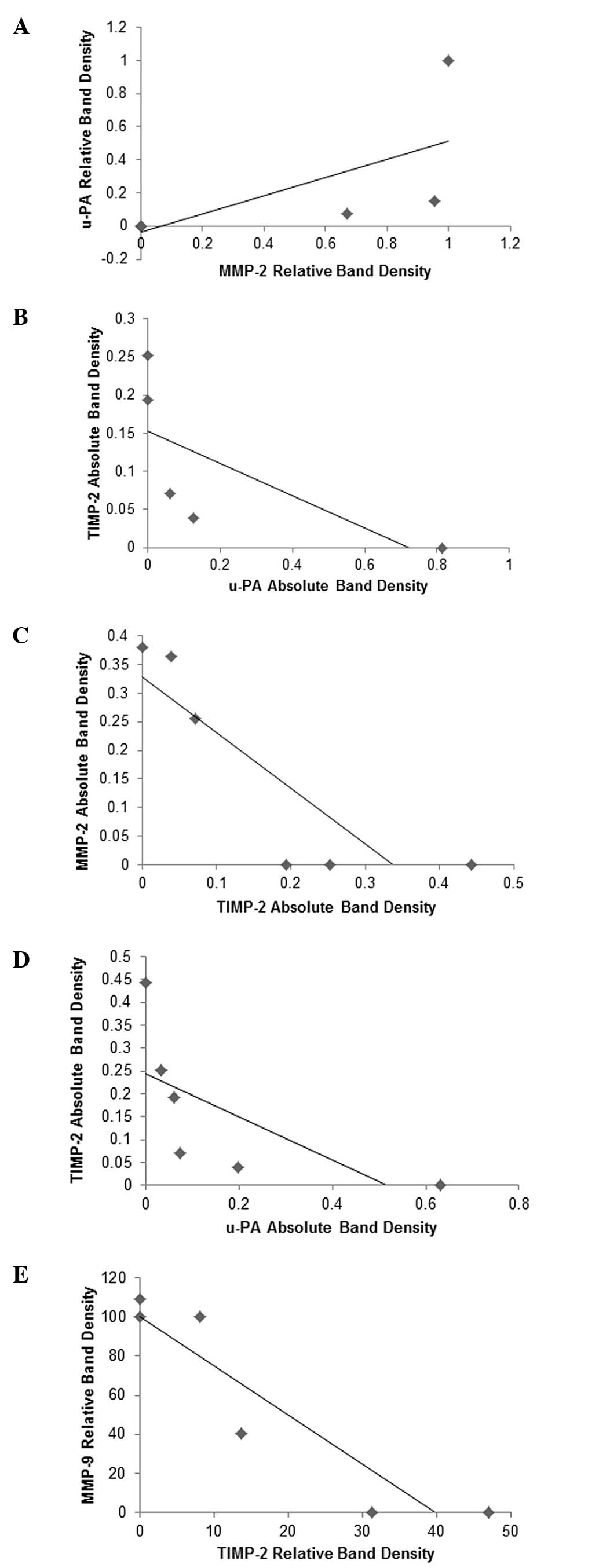
Analysis revealed a positive correlation between
NM-treated lung cancer cell line A-549 u-PA and MMP-2 expressions,
as shown in Fig. 5A, with a
correlation coefficient r= 0.679. Negative correlations were found
between the expressions of A-549 u-PA and TIMP-2 (correlation
coefficient r=−0.685, Fig. 5B) and
MMP-2 and TIMP-2 (correlation coefficient r=−0.862, Fig. 5C). Negative correlations were found
between Calu-3 expression of TIMP-2 and u-PA (correlation
coefficient r=−0.674, Fig. 5D) and
between MSTO-211H expression of TIMP-2 and MMP-9 (correlation
coefficient r=−0.925, Fig.
5E).
Discussion
Tumor cell invasion requires the critical steps of
cell attachment, degradation of the ECM and migration through the
disrupted matrix. The two families of proteases, matrix
metalloproteinases and urokinase plasminogen activators play key
roles in tumor cell invasion. Experimental studies have
demonstrated the role of urokinase plasminogen, especially cell
surface u-PA, as an initiator of ECM proteolysis and associated
tumor cell invasion (26). The
protease u-PA converts plasminogen to plasmin, which is capable of
promoting tumor growth and angiogenesis, degrading the ECM and
basement membrane and activating pro-MMPs (21). Duffy et al first reported
the prognostic value of u-PA in breast cancer patients, showing a
positive correlation between high levels of u-PA and cancer
progression (22). Overexpression
of u-PA in lung cancer has been correlated with cancer progression,
metastasis and poor prognosis (17). Though the pathogenesis of the
aggressive neoplasm malignant mesothelioma remains unclear, the
invasiveness of MM cells has been correlated with disordered fibrin
turnover and pro-coagulant and fibrinolytic activity (17). MM is locally invasive and tumor
cells are often surrounded by a dense collagenous stroma;
neoplastic spread is thought to be facilitated by fibrin and
plasminogen activators, which control inflammatory cell traffic
through the tumor matrix, as well as by promoting
neovascularization (24). Idell
et al found that fibrinolytic activity of human pleural
mesothelioma cell line MS-1 was mainly due to urokinase and was
responsive to cytokine stimulation (24). Matrix metalloproteinases,
especially MMP-2 and -9 play pivotal roles in tumor cell invasion
and metastasis due to their ability to degrade type IV collagen, a
major component of the ECM. Overproduction of MMPs, especially
MMP-2 and -9 has been associated with a more aggressive behavior of
lung cancer and mesothelioma (13–16).
Our study demonstrated that the specific mixture of
nutrients tested significantly inhibited u-PA secretion in lung
cancer cell A-549 and Calu-3 (malignant mesothelioma cell line
MSTO-211H was not found to secrete u-PA in this study).
Furthermore, the NM demonstrated dose-dependent decrease in MMP
secretion and increase in TIMP-2 secretion by both lung cancer
A-549 and mesothelioma cell lines. As expected, a significant
positive correlation was found between the secretion of u-PA and
MMP-2 and a significant negative correlation between u-PA and
TIMP-2 secretion by NM treatment of lung cancer A-549 cells. As
anticipated, a significant negative correlation was found between
MMP-9 and TIMP-2 secretion by MSTO-211H cell line. Furthermore, a
previous study demonstrated significant correlation between NM
inhibition of Matrigel invasion and NM modulation of the MMP-2 and
-9 activity of the lung cancer and MM cell lines studied (28). A significant negative correlation
was found between inhibition of NM modulation of Matrigel invasion
and MMP-2 secretion with lung cancer A-549 (r=−0.905). For
malignant mesothelioma MSTO-211H cells, a significant negative
correlation (r=−0.955 was found between inhibition of NM modulation
of Matrigel invasion and MMP-9 secretion. Previous in vivo
studies of the effects of NM on lung cancer growth and metastasis
support these results in that it demonstrated significant (47%,
p<0.0001) inhibition of A-549 xenograft tumor growth in nude
mice (29).
In contrast to the associated toxicity and limited
efficacy of standard cancer chemotherapy and radiation therapy, the
efficacy and safety of dietary and botanical natural compounds in
cancer prevention has been extensively documented (30). The nutrient mixture was formulated
by selecting nutrients that act on critical physiological targets
in cancer progression and metastasis, as documented in both
clinical and experimental studies. Combining these micronutrients
expands metabolic targets, maximizing biological impact with lower
doses of components. A previous study of the comparative effects of
NM, green tea extract and EGCG on inhibition of MMP-2 and -9
secretion of different cancer cell lines with varying MMP secretion
patterns, revealed the superior potency of NM over GTE and EGCG at
equivalent doses (31). These
results can be understood from the more comprehensive treatment
offered by the combination of nutrients in NM over individual
components of NM since MMP-2 and -9 are mediated by differential
pathways.
Optimal ECM structure depends upon adequate supplies
of ascorbic acid and the amino acids lysine and proline to ensure
proper synthesis and hydroxylation of collagen fibers. In addition,
lysine contributes to ECM stability as a natural inhibitor of
plasmin-induced proteolysis (25,32).
Manganese and copper are also essential for collagen formation.
There is considerable documentation of the potency of green tea
extract in modulating cancer cell growth, metastasis, angiogenesis
and other aspects of cancer progression (33–39).
N-acetyl cysteine and selenium have demonstrated inhibition of
tumor cell MMP-9 and invasive activities, as well as migration of
endothelial cells through ECM (40–42).
Ascorbic acid demonstrates cytotoxic and antimetastatic actions on
malignant cell lines (43–47) and cancer patients have been found
to have low levels of ascorbic acid (48,49).
Low levels of arginine, a precursor of nitric oxide (NO), can limit
the production of NO, which has been shown to predominantly act as
an inducer of apoptosis (50).
In conclusion, the NM demonstrated potent anticancer
activity by targeting primary mechanisms responsible for the
aggressive spread of lung cancer and malignant mesothelioma. In
this in vitro study, the NM significantly inhibited
secretion of u-PA and increased secretion of TIMP-2 in lung cancer
cell lines A-549 and Calu-3, as well as decreased MMP-2 secretion
in A-549 cells, suggesting its potential in modulating lung cancer
invasion and metastasis. Malignant mesothelioma MSTO-211H cells did
not secrete u-PA; however, MMP-9 secretion by MSTO-211H was
inhibited by NM and secretion of TIMP-2 was enhanced by NM. NM
inhibition of MMP secretion was found to be correlated
significantly with Matrigel invasion of lung cancer A-549 and
MSTO-211H cells. Furthermore, use of the nutrient mixture would not
pose any toxic effect clinically, especially in the relevant doses,
as in vivo safety studies demonstrate. An in vivo
toxicology study showed that NM had no adverse effects on vital
organs (heart, liver and kidney), or on the associated functional
serum enzymes (51).
Acknowledgements
Mr. Monterrey provided assistance in
scanning the gels. This study was funded by Dr Rath Health
Foundation (Santa Clara, CA, USA) a non-profit organization.
References
|
1
|
American Cancer Society: Lung cancer: What
are the key statistics about lung cancer. http://www.cancer.org/cancer/lungcancer-non-small-cell/detailedguide/non-smallcell-lung-cancer-key-statistics
(Accessed 12/3/2012) Last revised: 10/12/2012.
|
|
2
|
American Lung Association: Lung Cancer
Fact Sheet. http://www.lung.org/lung-disease/lung-cancer/resources/facts-figures/lung-cancer-fact-sheet.html
(Accessed 12/3/2012) Last revised 2012.
|
|
3
|
American Cancer Society: Malignant
Mesothelioma. http://www.cancer.org/cancer/malignantmesothelioma/detailedguide/malignant-mesothelioma-survival-statistics
(Accessed 12/3/2012) Last revised 9/20/2012.
|
|
4
|
Fidler IJ: Molecular biology of cancer:
invasion and metastasis. Cancer: Principles and Practice of
Oncology. 5th edition. De Vita VT, Hellman S and Rosenberg SA:
Lippincott-Raven; Philadelphia, PA: pp. 135–152. 1997
|
|
5
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folkman J: Role of angiogenesis in tumor
growth and metastasis. Semin Oncol. 29(Suppl 16): 15–18. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chambers AF and Matrisian LM: Changing
views on the role of matrix metalloproteinases in metastasis. J
Natl Cancer Inst. 89:1260–1270. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kleiner DL and Stetler-Stevenson WG:
Matrix metalloproteinases and metastasis. Cancer Chemother
Pharmacol. 43(Suppl): S42–S51. 1999. View Article : Google Scholar
|
|
9
|
Yurchenko PD and Schitny JC: Molecular
architecture of basement membranes. FASEB J. 4:1577–1590.
1990.PubMed/NCBI
|
|
10
|
Barsky SH, Siegel GP, Jannotta F and
Liotta LA: Loss of basement membrane components by invasive tumors
but not by their benign counterparts. Lab Investig. 49:140–147.
1983.PubMed/NCBI
|
|
11
|
Liotta LA, Tryggvason K, Garbisa A, Hart
I, Foltz CM and Shafie S: Metastatic potential correlates with
enzymatic degradation of basement membrane collagen. Nature.
284:67–68. 1980. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nelson AR, Fingleton B, Rothenberg ML and
Matrisian LM: Matrix metalloproteinases: biologic activity and
clinical implications. J Clin Oncol. 18:1135–1149. 2000.PubMed/NCBI
|
|
13
|
Schültz A, Schneidenbach D, Aust G,
Tannpfel A, Steinert M and Wittekind C: Differential expression and
activity status of MMP-1, MMP-2 and MMP-9 in tumor and stromal
cells of squamous cell carcinomas of the lung. Tumor Biol.
23:179–184. 2002.PubMed/NCBI
|
|
14
|
Takashi M, Takashi M, Kazumitsu O, Kazuo
K, Yoshio N, Fujio N, Mamoru K, Motohiko F, Nanao N and Yukiyasu N:
Analysis of circulating plasma matrix metalloproteinases (MMP) and
tissue inhibitors of MMP in lung cancer patients. J Nihon Univ Med
Assoc. 59:97–102. 2000.
|
|
15
|
Hirano H, Tsugu M, Kizaki T, Sashikata T,
Yosgi Y, Okada Y and Mori H: Expression of matrix
metalloproteinases, tissue inhibitors of metalloproteinase,
collagens and ki67 antigen in pleural malignant mesothelioma: an
immunohistochemical and electron microscopic study. Med Electron
Microsc. 35:16–23. 2002. View Article : Google Scholar
|
|
16
|
Edwards JC, McLaren J, Jones JL, Walker DA
and O’Byrne KJ: Matrix metalloproteinases 2 and 9 (gelatinases A
and B) expression in malignant mesothelioma and benign pleura. Br J
Cancer. 88:1553–1559. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Werle B, Kotzsch M, Lah TT, Kos J,
Gabrielcic-Geiger D, Spiess E, Schirren J, Ebert W, Fiehn W, Luther
T, Magdolen V, Schmitt N and Harbeck N: Cathepsin B, plasminogen
activator-inihbitor (PAI-1) and plasminogen activator-receptor
(uPAR) are prognostic factors for patients with non-small cell lung
cancer. Anticancer Res. 24:4147–4161. 2004.
|
|
18
|
Shetty S, Kumar A, Johnson A, Pueblitz S
and Idell S: Urokinase receptor in human malignant mesothelioma
cells: role in tumor cell mitogenesis and proteolysis. Am J Physiol
Lung Cell Mol Physiol. 268:L972–L982. 1995.PubMed/NCBI
|
|
19
|
Stetler-Stevenson WG: The role of matrix
metalloproteinases in tumor invasion, metastasis and angiogenesis.
Surg Oncol Clin North Am. 10:383–392. 2001.PubMed/NCBI
|
|
20
|
Stetler-Stevenson WG: Type IV collagenases
in tumor invasion and metastasis. Cancer Metastasis Rev. 9:289–303.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dano K, Andreasen PA, Grondahl-Hansen J,
Kristensen P, Nielsen LS and Skriver L: Plasminogen activators,
tissue degradation and cancer. Adv Cancer Res. 44:139–266. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Duffy MJ, Duggan C, Mulcahy HE, McDermott
EW and O’Higgins NJ: Urokinase plasminogen activator: a prognostic
marker in breast cancer including patients with axillary
node-negative disease. Clin Chem. 44:1177–1183. 1998.
|
|
23
|
Henneke I, Greschus S, Saval R, Korfel M,
Markart P, Mahavadi P, Schermuly RT, Wygrecka M, Strüzebecher J,
Seeger W, Günther A and Ruppert C: Inhibition of urokinase activity
reduces primary tumor growth and metastasis formation in a murine
lung carcinoma model. Am J Respir Crit Care Med. 181:612–619. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Idell S, Pueblitz S, Emri S, Gungen Y,
Gray L, Kumar A, Holiday D, Koenig KB and Johnson AR: Regulation of
fibrin deposition by malignant mesothelioma. Am J Pathol.
147:1318–1329. 1995.PubMed/NCBI
|
|
25
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomolecular Med. 7:17–23. 1992.
|
|
26
|
Andreasen PA, Kjøller L, Christensen L and
Duffy MJ: The urokinase-type plasminogen activator system in cancer
metastasis: a review. Int J Cancer. 72:1–22. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Roomi MW, Monterrey JC, Kalinovsky T,
Niedzwiecki A and Rath M: Inhibition of invasion and MMPs by a
nutrient mixture in human cancer cell lines: a correlation study.
Exp Oncol. 32:243–248. 2010.PubMed/NCBI
|
|
29
|
Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: In vivo and in vitro anti-tumor effect of
a unique nutrient mixture on lung cancer cell line A-549. Exp Lung
Res. 32:441–453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Amin ARMR, Kucek O, Khuri FR and Shin DM:
Perspectives for cancer prevention with natural compounds. J Clin
Oncol. 27:2712–2725. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Comparative effects of EGCG, green tea and a
nutrient mixture on the patterns of MMP-2 and MMP-9 expression in
cancer cell lines. Oncol Rep. 24:747–757. 2010.PubMed/NCBI
|
|
32
|
Sun Z, Chen YH, Wang P, Zhang J, Gurewich
V, Zhang P and Liu JN: The blockage of high-affinity lysine binding
sites of plasminogen by EACA significantly inhibits
prourokinase-induced plasminogen activation. Biochem Biophys Acta.
1596:182–192. 2002.PubMed/NCBI
|
|
33
|
Kemberling JK, Hampton JA, Keck RW, Gomez
MA and Selman SH: Inhibition of bladder tumor growth by the green
tea derivative epigallocatechin-3-gallate. J Urol. 170:773–776.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sato D and Matsushima M: Preventive
effects of urinary bladder tumors induced by
N-butyl-N-(4-hydroxybutyl)-nitrosamine in rat by green tea leaves.
Int J Urol. 10:160–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valcic S, Timmermann BN, Alberts DS,
Wachter GA, Krutzsch M, Wymer J and Guillen JM: Inhibitory effect
of six green tea catechins and caffeine on the growth of four
selected human tumor cell lines. Anticancer Drugs. 7:461–468. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mukhtar H and Ahmed N: Tea polyphenols:
prevention of cancer and optimizing health. Am J Clin Nutr.
71:S1698–S1702. 2000.PubMed/NCBI
|
|
37
|
Yang GY, Liao J, Kim K, Yurtow EJ and Yang
CS: Inhibition of growth and induction of apoptosis in human cancer
cell lines by tea polyphenols. Carcinogenesis. 19:611–616. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taniguchi S, Fujiki H, Kobayashi H, Go H,
Miyado K, Sadano H and Shimikawa R: Effect of (-) epigallocatechin
gallate, the main constituent of green tea, on lung metastasis with
mouse B16 melanoma cell lines. Cancer Lett. 65:51–54. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hara Y: Green tea: Health Benefits and
Applications. Marcel Dekker; New York, NY, Basel: 2001, View Article : Google Scholar
|
|
40
|
Kawakami S, Kageyama Y, Fujii Y, Kihara K
and Oshima H: Inhibitory effects of N-acetyl cysteine on invasion
and MMP 9 production of T24 human bladder cancer cells. Anticancer
Res. 21:213–219. 2001.PubMed/NCBI
|
|
41
|
Morini M, Cai T, Aluigi MG, Noonan DM,
Masiello L, De Floro S, D’Agostinin F, Albini A and Fassima G: The
role of the thiol N-acetyl cysteine in the prevention of tumor
invasion and angiogenesis. Int J Biol Markers. 14:268–271.
1999.PubMed/NCBI
|
|
42
|
Yoon SO, Kim MM and Chung AS: Inhibitory
effects of selenite on invasion of HT 1080 tumor cells. J Biol
Chem. 276:20085–20092. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Naidu KA, Karl RC and Coppola D:
Antiproliferative and proapoptotic effect of ascorbyl stearate in
human pancreatic cancer cells: association with decreased
expression of insulin-like growth factor 1 receptor. Dig Dis Sci.
48:230–237. 2003. View Article : Google Scholar
|
|
44
|
Anthony HM and Schorah CJ: Severe
hypovitaminosis C in lung-cancer patients: the utilization of
vitamin C in surgical repair and lymphocyte-related host
resistance. Br J Cancer. 46:354–367. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Maramag C, Menon M, Balaji KC, Reddy PG
and Laxmanan S: Effect of vitamin C on prostate cancer cells in
vitro: effect on cell number, viability and DNA synthesis.
Prostate. 32:188–195. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Koh WS, Lee SJ, Lee H, Park C, Park MH,
Kim WS, Yoon SS, Park K, Hong SI, Chung MH and Park CH:
Differential effects and transport kinetics of ascorbate
derivatives in leukemic cell lines. Anticancer Res. 8:2487–2493.
1998.PubMed/NCBI
|
|
47
|
Chen Q, Espey MG, Krishna MC, Mitchell JB,
Corpe CP, Buettner GR, Shacter E and Levine M: Pharmacologic
ascorbic acid concentrations selectively kill cancer cells: Action
as a pro-drug to deliver hydrogen peroxide to tissues. Proc Natl
Acad Sci USA. 102:13604–13609. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nunez C, Ortiz de Apodaca Y and Ruiz A:
Ascorbic acid in the plasma and blood cells of women with breast
cancer. The effect of consumption of food with an elevated content
of this vitamin. Nutr Hosp. 10:368–372. 1995.PubMed/NCBI
|
|
49
|
Kurbacher CM, Wagner U, Kolster B,
Andreotti PE, Krebs D and Bruckner HW: Ascorbic acid (vitamin C)
improves the antineoplastic activity of doxorubicin, cisplatin and
paclitaxel in human breast carcinoma cells in vitro. Cancer Lett.
103:183–189. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cooke JP and Dzau VJ: Nitric oxide
synthase: role in the genesis of vascular disease. Annu Rev Med.
48:489–509. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Roomi MW, Ivanov V, Netke SP, Niedzwiecki
A and Rath M: Serum markers of the liver, heart and kidney and
lipid profile and histopathology in ODS rats treated with nutrient
synergy. J Am Coll Nutr. 22:77abs. 86. 2003.
|