Introduction
Chemotherapy has been documented to be efficacious
in treating men and women with stage III colon cancer (1–5) and
the clinical guidelines are clear about recommending adjuvant
chemotherapy for these patients after resection (1,5). For
patients with stages II–III rectal cancer, the combined-modality of
adjuvant chemotherapy and radiation therapy is currently the
standard of care (1,5). Although chemotherapy is not well
documented in randomized trials for those with stage I or II colon
cancer (6–11), a substantial number of patients
with stage II colon cancer received chemotherapy (12,13).
What has not been well studied is the potential association between
chemotherapy utilization and the risk of cognitive impairments in
patients with cancer treated with chemotherapy. There have been
some anecdotal reports since the 1980s, indicating that patients
treated with chemotherapy complained of changes in their memory,
attention, concentration, and language skills primarily in those
with cancer (14–23). The first study that examined this
relationship within the context of a randomized trial was reported
by van Dam and colleagues in 1998 for patients with breast cancer
(24). In the treatment group,
they administered four cycles of combination chemotherapy agents
(fluorouracil, doxorubicin and cyclophosphamide); and a fifth
course of high-dose combination chemotherapy (cyclophosphamide and
carboplatin) (24). The subsequent
small-scale studies seemed to support the original findings
(25–32). However, minor cognitive impairments
(such as minor decrease in memory, attention, concentration and
language skills) may be reversible after a short time use of
chemotherapy, even if there were true causal relationships. No
clinical trials have been conducted to determine the association
between chemotherapy and long-term follow-up of outcomes such as
dementia, which are unlikely to be reversed.
There have been four population-based studies
examining a long-term relationship between chemotherapy and
dementia (33–36) and one of these studies examined the
5 types of cognitive impairments (36), all of which were conducted in
breast cancer patients. No nationwide large population-based study
has been conducted on this research question in patients with
colorectal cancer. Because similar chemotherapy agents such as
fluorouracil are commonly used in patients with colorectal cancer
as well as in patients with breast cancer, it is reasonable to
posit that, if chemotherapy use increased the risk of cognitive
impairments in patients with breast cancer, this relationship will
likely be observed in those with colorectal cancer who received
similar chemotherapy agents. Therefore, this study aimed to
primarily determine the long-term risk of drug-induced dementia
associated with chemotherapy use and also explore if the use of
chemotherapy was associated with the risks of other cognitive
impairments, including Alzheimer’s disease, in a large
population-based cohort of patients with colorectal cancer in the
US with up to 17 years of follow-up. In this study, only those
patients who were free of any cognitive impairment at baseline were
included. Moreover, additional matched-cohort analysis according to
the propensity of receiving chemotherapy was conducted to verify
the study findings and to assess the potential impact of selection
bias that might have affected the study findings. Our hypothesis
was that there were significant differences in the occurrence of
developing cognitive impairments in men and women diagnosed with
colorectal cancer who received chemotherapy compared to those who
did not use chemotherapy.
Patients and methods
Data sources and study population
The National Cancer Institute’s Surveillance,
Epidemiology and End Results (SEER) 16 cancer registries and
Medicare linked databases were used for this analysis (37,38).
The Committee for the Protection of Human Subjects at the
University of Texas Health Science Center at Houston approved this
study.
The study population consisted of 120,111 patients
who were diagnosed with colorectal cancer as the only primary tumor
at age ≥65 years from 1991 through 2002. Cases from Atlanta and
rural Georgia were combined due to small numbers in the same state.
We excluded 35,475 subjects who did not have full coverage of
Medicare Parts A and B or were enrolled with Health Maintenance
Organizations from the year of diagnosis to the last follow-up
(December 2006 or date of death). For the purpose of this study in
determining the relationship between chemotherapy and cognitive
impairments, we included only patients who were free of any
cognitive impairment at the time of cancer diagnosis. By doing so,
we excluded 4,078 cases with preexisting cognitive impairments,
5,129 patients aged ≥90 and 3,073 cases who received first
chemotherapy after 12 months of diagnosis, leaving 72,374 subjects
for the final analysis.
Matched cohort
In order to minimize selection bias due to factors
that may have influenced physicians or patients to choose
chemotherapy, we first calculated the propensity (or conditional
probability) of receiving chemotherapy for all patients, and then
matched patients who actually received chemotherapy with those who
had the same or similar propensity but did not receive
chemotherapy. The propensity of receiving chemotherapy was created
through the logistic regression model based on the following
patient and tumor characteristics: age, sex, ethnicity, marital
status, tumor stage, tumor grade, tumor size, number of positive
lymph nodes, comorbidity, surgery, radiotherapy, socioeconomic
status, cancer type, year of diagnosis and SEER areas. The matching
through the 5-1 digit propensity of receiving chemotherapy was
performed using the greedy matching algorithm by Parsons (39). A total of 15,921 patients receiving
chemotherapy were matched with 15,921 patients who did not receive
chemotherapy.
Chemotherapy
The methods of identifying chemotherapy use through
the Medicare claims was discussed elsewhere (40) and the validity of Medicare claims
for chemotherapy have been reasonably well confirmed (41–48).
Cognitive impairments and mood
disorders
Cognitive impairment was defined if there were at
least two claims in all Medicare claim files (including inpatient,
outpatient and physician claims) that were 30 days apart for each
of the following diagnoses (with ICD-9-CM codes) (46) after chemotherapy use: unspecified
cognitive disorder (294.9), amnestic disorder (294.0), Alzheimer’s
disease (331.0), vascular dementia (290.x), unspecified dementia
(294.8) or drug-induced dementia and psychoses (292.x).
Mood disorder was defined if there were at least two
claims that were 30 days apart for each of the following diagnoses
(46) before or after chemotherapy
use: anxiety state, unspecified (300.0); anxiety depression
(300.4); unspecified depressive disorder, (311.x;); alteration of
consciousness (780.9); and other depressions (296.2, 296.3,
296.5–296.7, 298.0, 301.10, 301.12, 301.13, 309.0, 309.1).
Other variables
Comorbidity was ascertained from Medicare claims
data through diagnoses or procedures that were made between 1 year
prior to and 1 month after the diagnosis of colorectal cancer using
the previously validated comorbidity index (49–51)
and SAS macro program (52). The
percent of persons living below the poverty line at the census
tract level from the 1990 census for cases in 1991–1999 and from
the 2000 census for cases in 2000–2002 was used to define the
socioeconomic status (SES). These percentages were then classified
into quartiles.
Analyses
The χ2 statistic (at a significance level
of 0.05) was used to compare baseline characteristics between
patients who received chemotherapy and those who did not in the
entire cohort and in the matched cohort. Incidence rate (density)
was defined as the ratio of the number of new cognitive impairments
over the total number of person-years. Person-years were calculated
as the number of patients multiplied by the number of years from
diagnosis to the date of the first cognitive impairment or date of
death or date of last follow-up, whichever occurred first. The
cumulative incidence (probability) of cognitive impairments was
calculated using the statistical program by Penman and Johnson
(53). The time to event
(cognitive impairment) analysis was conducted using the Cox
proportional hazard regression model available in SAS (54).
Results
Table I presents
the distribution of baseline characteristics among the entire
cohort of patients with colorectal cancer according to chemotherapy
status and also presents the comparisons of the matched cohort
based on propensity score of receiving chemotherapy. In the entire
cohort, a significantly higher proportion of younger patients and
married people received chemotherapy. A slightly higher percentage
of women and Caucasians received chemotherapy. The higher
percentage of not receiving chemotherapy was related to lower
socioeconomic status, earlier tumor stage, smaller tumor size,
fewer positive lymph nodes, low grade tumors and higher comorbidity
scores. A higher proportion of patients who had resection and
radiation therapy also received chemotherapy. The distribution of
these characteristics was significantly different between patients
receiving chemotherapy and those who did not. However, in the
matched cohort, there were no significant differences between these
two groups in terms of all baseline characteristics.
 | Table IComparisons of characteristics among
women with colon cancer according to the receipt of chemotherapy
(chemo) in both entire cohort and propensity-matched cohort. |
Table I
Comparisons of characteristics among
women with colon cancer according to the receipt of chemotherapy
(chemo) in both entire cohort and propensity-matched cohort.
| Column % of the
entire cohort | Column % of the
matched cohort |
|---|
|
|
|---|
|
Characteristics | Chemo
(n=23,484) | No chemo
(n=48,890) | P-value | Chemo
(n=15,921) | No chemo
(n=15,921) | P-value |
|---|
| Median age
(range) | 73 (65–89) | 78 (65–89) | | 75 (65–89) | 75 (65–89) | |
| Age (years) | | | | | | |
| 65–69 | 28.1 | 14.4 | <0.001 | 21.4 | 21.4 | 0.817 |
| 70–74 | 30.7 | 19.8 | | 27.5 | 27.4 | |
| 75–79 | 24.7 | 24.2 | | 27.9 | 27.8 | |
| 80–84 | 12.8 | 24.1 | | 17.7 | 17.6 | |
| 85–89 | 3.7 | 17.4 | | 5.4 | 5.8 | |
| Gender | | | | | | |
| Male | 48.6 | 43.9 | <0.001 | 47.2 | 47.1 | 0.893 |
| Female | 51.4 | 56.1 | | 52.8 | 52.9 | |
| Race/ethnicity | | | | | | |
| Caucasians | 85.1 | 84.3 | <0.001 | 84.4 | 84.1 | 0.725 |
| African
Americans | 6.7 | 8.1 | | 7.5 | 7.8 | |
| Others | 8.2 | 7.6 | | 8.1 | 8.1 | |
| Marital status | | | | | | |
| Married | 59.3 | 45.2 | <0.001 | 53.7 | 53.3 | 0.712 |
| Unmarried | 37.9 | 50.5 | | 42.9 | 43.1 | |
| Unknown | 2.8 | 4.3 | | 3.4 | 3.5 | |
| Socioeconomic
status (SES) | | | | | | |
| First quartile
(high) | 26.8 | 23.6 | <0.001 | 24.7 | 24.5 | 0.789 |
| Second
quartile | 25.2 | 24.3 | | 24.8 | 24.6 | |
| Third
quartile | 24 | 24.9 | | 24.9 | 24.8 | |
| Fourth quartile
(low) | 22.5 | 25.5 | | 24.1 | 24.5 | |
| Missing SES | 1.4 | 1.7 | | 1.5 | 1.6 | |
| Tumor stage | | | | | | |
| I | 5.5 | 33.0 | <0.001 | 8.2 | 7.8 | 0.676 |
| II | 22.9 | 29.4 | | 29.7 | 29.8 | |
| III | 41.0 | 12.9 | | 29.2 | 28.9 | |
| IV | 25.7 | 15.8 | | 26.7 | 27.1 | |
| Unstaged | 4.9 | 8.8 | | 6.2 | 6.4 | |
| Tumor size
(cm) | | | | | | |
| <1 | 0.5 | 2.9 | <0.001 | 0.7 | 0.7 | 0.959 |
| 1–<2 | 2.1 | 4.1 | | 2.3 | 2.2 | |
| 2–<3 | 7.6 | 8.6 | | 7.5 | 7.4 | |
| 3–<4 | 14.6 | 12.9 | | 13.7 | 13.8 | |
| ≥4 | 56.1 | 41.0 | | 53.7 | 53.9 | |
| Missing | 19.0 | 30.6 | | 22.1 | 22.0 | |
| N of positive lymph
nodes | | | | | | |
| 0 (negative) | 27.1 | 50.6 | <0.001 | 35.6 | 35.1 | 0.889 |
| 1 | 14.9 | 5.4 | | 12.1 | 12 | |
| 2–3 | 16.7 | 5.4 | | 12.2 | 12.1 | |
| 4–9 | 15.8 | 5.0 | | 11.4 | 11.5 | |
| 10–51 | 6.7 | 2.6 | | 5.5 | 5.7 | |
| Missing | 18.8 | 31.1 | | 23.2 | 23.5 | |
| Tumor grade | | | | | | |
|
Well-differentiated | 5.5 | 9.9 | <0.001 | 6.2 | 6.0 | 0.815 |
|
Moderately-differentiated | 61.5 | 56.4 | | 60.1 | 60.1 | |
|
Poorly-differentiated | 24.6 | 16.2 | | 22.8 | 23.0 | |
|
Unknown/missing | 8.4 | 17.5 | | 10.8 | 10.9 | |
| Comorbidity
scores | | | | | | |
| 0 | 61.5 | 51.0 | <0.001 | 57.6 | 57.2 | 0.799 |
| 1 | 25.4 | 26.7 | | 26.3 | 26.6 | |
| ≥2 | 12.1 | 22.3 | | 16.1 | 16.2 | |
| Primary surgery
(resection) | | | | | | |
| No | 11.3 | 17.6 | <0.001 | 14.5 | 15.0 | 0.200 |
| Yes | 88.7 | 82.4 | | 85.5 | 85.0 | |
| Radiotherapy | | | | | | |
| No | 71.7 | 88.6 | <0.001 | 79.6 | 79.5 | 0.911 |
| Yes | 28.3 | 11.4 | | 20.4 | 20.5 | |
| Year of
diagnosis | | | | | | |
| 1991 | 7.2 | 7.2 | <0.001 | 7.4 | 7.6 | 0.945 |
| 1992 | 7.3 | 7.1 | | 7.2 | 7.4 | |
| 1993 | 6.9 | 6.8 | | 6.6 | 6.9 | |
| 1994 | 6.6 | 6.7 | | 6.6 | 6.6 | |
| 1995 | 7.0 | 6.5 | | 6.7 | 6.8 | |
| 1996 | 6.6 | 6.3 | | 6.6 | 6.2 | |
| 1997 | 6.8 | 6.3 | | 6.8 | 6.8 | |
| 1998 | 7.0 | 6.4 | | 6.5 | 6.6 | |
| 1999 | 6.0 | 6.4 | | 6.1 | 5.9 | |
| 2000 | 10.2 | 14.2 | | 12.0 | 11.9 | |
| 2001 | 14.0 | 12.8 | | 13.5 | 13.8 | |
| 2002 | 14.4 | 13.3 | | 13.8 | 13.5 | |
| SEER areas | | | | | | |
| Connecticut | 12.2 | 11.8 | <0.001 | 11.5 | 12.1 | 0.899 |
| Detroit | 14.0 | 12.8 | | 13.6 | 13.2 | |
| Hawaii | 2.1 | 2.0 | | 2.0 | 1.9 | |
| Iowa | 13.0 | 13.6 | | 12.9 | 12.7 | |
| New Mexico | 2.9 | 3.4 | | 3.2 | 3.2 | |
| Seattle | 7.4 | 7.5 | | 7.2 | 7 | |
| Utah | 3.1 | 3.5 | | 3.3 | 3.2 | |
| Atlanta/rural
Georgia | 4.7 | 4.4 | | 4.6 | 4.8 | |
| Kentucky | 3.6 | 3.8 | | 3.8 | 3.9 | |
| Louisiana | 2.8 | 2.9 | | 2.8 | 2.9 | |
| New Jersey | 7.5 | 7.1 | | 7.4 | 7.2 | |
| California | 26.7 | 27.2 | | 27.7 | 27.8 | |
| Cancer type | | | | | | |
| Colon cancer | 69.9 | 77.1 | <0.001 | 74.6 | 74.3 | 0.550 |
| Rectal
cancer | 30.1 | 22.9 | | 25.4 | 25.7 | |
| Total | 100.0 | 100.0 | | 100.0 | 100.0 | |
Table II presents
the incidence density of cognitive impairments by chemotherapy
status, age, gender, tumor stage and comorbidity. The incidence
density of drug-induced dementia was higher in patients receiving
chemotherapy than those who did not receive chemotherapy in all
strata of age, sex, stage and comorbidity score. For example, the
incidence of drug-induced dementia in patients aged 65–69 was 1.84
times higher in patients receiving chemotherapy than in those not
receiving chemotherapy (3.33 versus 1.81 per 10,000 person-years),
whereas the relative risk was 1.42 in patients aged 80–84 years
(5.15 versus 3.63 per 10,000 person-years) between the two groups.
However, the incidence of other types of cognitive impairments such
as Alzheimer’s disease, vascular disorder, cognitive disorder or
other dementia appeared to be higher in patients who did not
receive chemotherapy. Overall, the incidence rate of various
cognitive impairments increased with advanced age and higher
comorbidity scores but was relatively similar across gender and
tumor stage.
 | Table IIIncidence density of cognitive
impairments by chemotherapy status and other factors in the entire
cohort of patients with colon cancer. |
Table II
Incidence density of cognitive
impairments by chemotherapy status and other factors in the entire
cohort of patients with colon cancer.
| Incidence density
of cognitive impairments (number of cases per 1,000 person-years),
by chemotherapy status
|
|---|
| Patient and tumor
characteristics | Drug-induced
dementia | Alzheimer’s
disease | Vascular
dementia | Cognitive disorder,
NOSa | Other dementias or
dementia, NOSa | Any dementia (any
of these 5) |
|---|
| Chemotherapy | | | | | | |
| Age (years) | | | | | | |
| 65–69 | 3.33 | 3.24 | 3.92 | 0.84 | 4.76 | 11.16 |
| 70–74 | 2.87 | 6.09 | 7.06 | 0.71 | 8.06 | 16.89 |
| 75–79 | 3.74 | 10.06 | 12.58 | 0.49 | 13.75 | 26.89 |
| 80–84 | 5.15 | 15.02 | 19.25 | 1.44 | 20.67 | 41.83 |
| 85–89 | 3.91 | 19.95 | 29.36 | 0.43 | 26.44 | 59.63 |
| Gender | | | | | | |
| Male | 3.52 | 6.49 | 8.33 | 0.81 | 9.7 | 20.09 |
| Female | 3.45 | 8.17 | 9.86 | 0.73 | 10.45 | 21.64 |
| Tumor stage | | | | | | |
| I | 2.70 | 9.78 | 11.47 | 0.56 | 11.82 | 22.1 |
| II | 2.80 | 7.32 | 9.32 | 1.07 | 10.13 | 20.54 |
| III | 3.16 | 7.49 | 9.37 | 0.57 | 10.18 | 20.56 |
| IV | 7.82 | 3.75 | 5.89 | 0.71 | 6.60 | 20.5 |
| Unstaged | 3.42 | 11.4 | 9.12 | 1.21 | 14.48 | 26.81 |
| Comorbidity
scores | | | | | | |
| 0 | 2.96 | 6.68 | 7.49 | 0.7 | 8.67 | 17.74 |
| 1 | 3.83 | 8.44 | 11.04 | 1.04 | 11.56 | 23.98 |
| ≥2 | 6.04 | 9.45 | 15.43 | 0.59 | 15.95 | 34.55 |
| Total | 3.48 | 7.38 | 9.14 | 0.77 | 10.10 | 20.91 |
| No
chemotherapy | | | | | | |
| Age (years) | | | | | | |
| 65–69 | 1.81 | 4.13 | 6.43 | 0.71 | 5.95 | 12.82 |
| 70–74 | 2.65 | 8.90 | 12.74 | 0.82 | 12.29 | 24.37 |
| 75–79 | 2.70 | 14.98 | 22.22 | 1.02 | 20.49 | 40.59 |
| 80–84 | 3.63 | 22.43 | 35.01 | 1.57 | 31.87 | 63.85 |
| ≥85 | 3.77 | 25.84 | 48.15 | 1.41 | 43.44 | 89.29 |
| Gender | | | | | | |
| Male | 2.76 | 12.08 | 18.86 | 1.09 | 17.61 | 35.84 |
| Female | 2.86 | 15.46 | 24.43 | 1.05 | 22.39 | 44.08 |
| Tumor stage | | | | | | |
| I | 2.40 | 11.67 | 16.91 | 0.89 | 16.21 | 31.17 |
| II | 2.88 | 14.75 | 23.62 | 1.01 | 21.66 | 42.59 |
| III | 3.74 | 20.45 | 33.01 | 1.81 | 31.11 | 60.89 |
| IV | 8.65 | 18.72 | 38.06 | 0.96 | 31.58 | 84.29 |
| Unstaged | 2.12 | 18.13 | 35.3 | 1.88 | 27.28 | 64.14 |
| Comorbidity
scores | | | | | | |
| 0 | 2.22 | 12.30 | 16.8 | 0.85 | 16.28 | 31.53 |
| 1 | 3.19 | 16.14 | 27.03 | 1.22 | 22.96 | 47.51 |
| ≥2 | 4.73 | 17.74 | 36.15 | 1.70 | 33.30 | 68.01 |
| Total | 2.82 | 14.03 | 22.06 | 1.07 | 20.36 | 40.56 |
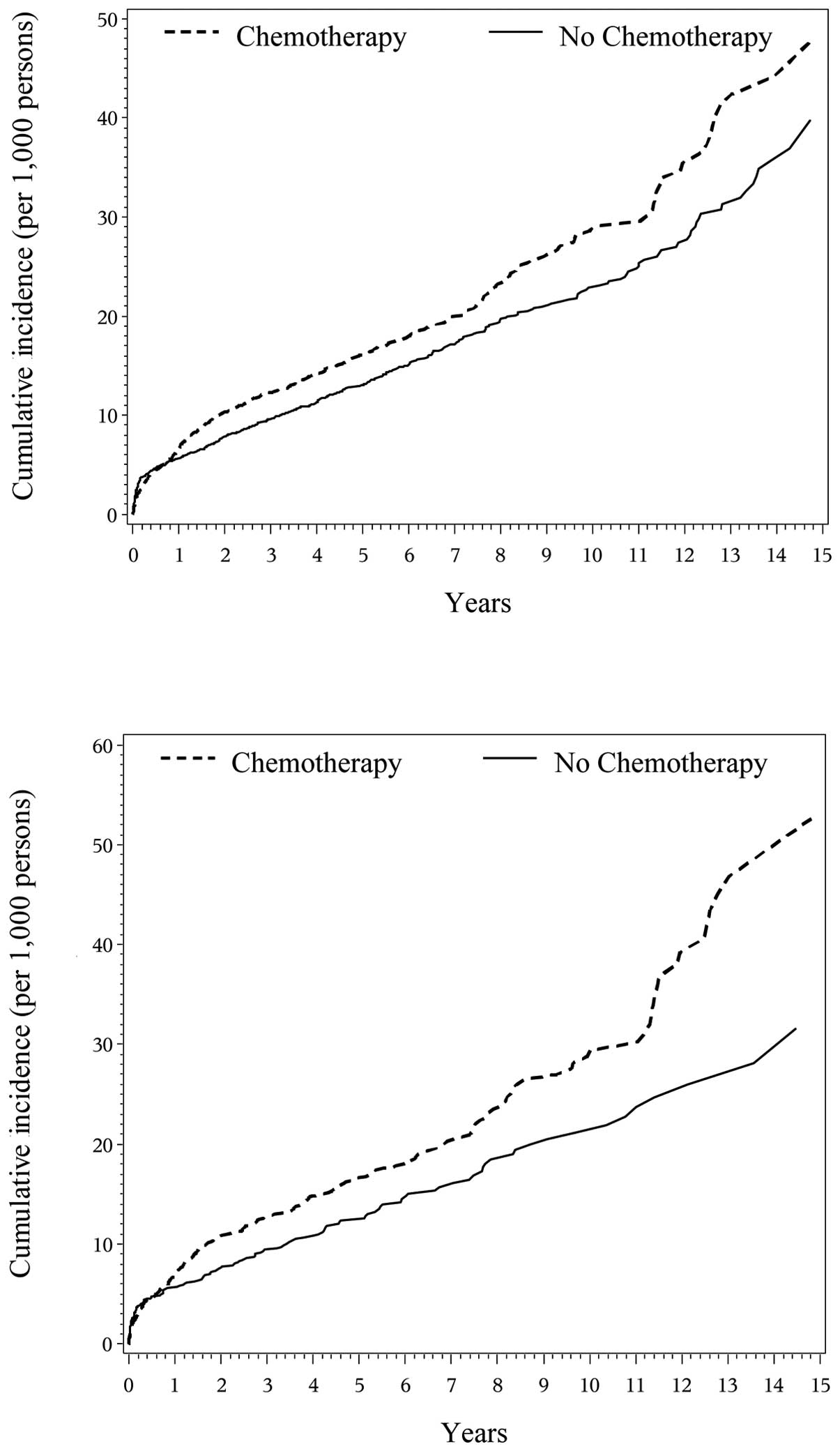
Fig. 1 presents the
cumulative incidence curve of drug-induced dementia over the
15-year period by chemotherapy status. The probability of
drug-induced dementia was similar for chemotherapy group compared
to no chemotherapy group in the first year. After the first year,
the incidence of drug-induced dementia became higher in the
chemotherapy group than in the no chemotherapy group and the gap
between the two groups appeared to widen over time (Fig. 1, top). The cumulative incidence of
drug-induced dementia at 3 years was 12.3 cases per 1,000 persons
for the chemotherapy group and 9.7 cases per 1,000 persons for no
chemotherapy group, while the cumulative incidence was 16.2 and
13.0 per 1,000 persons at 5 years and 29.0 and 22.8 per 1,000
persons at 10 years for both groups. Similar curves were observed
among the matched cohorts (Fig. 1,
bottom). However, the patterns of cumulative incidence were
different for four other types of cognitive impairments, in which
the incidence was lower in patients receiving chemotherapy than
those without chemotherapy except for cognitive disorder in which
incidence probability overlapped in years 7–12 in both the entire
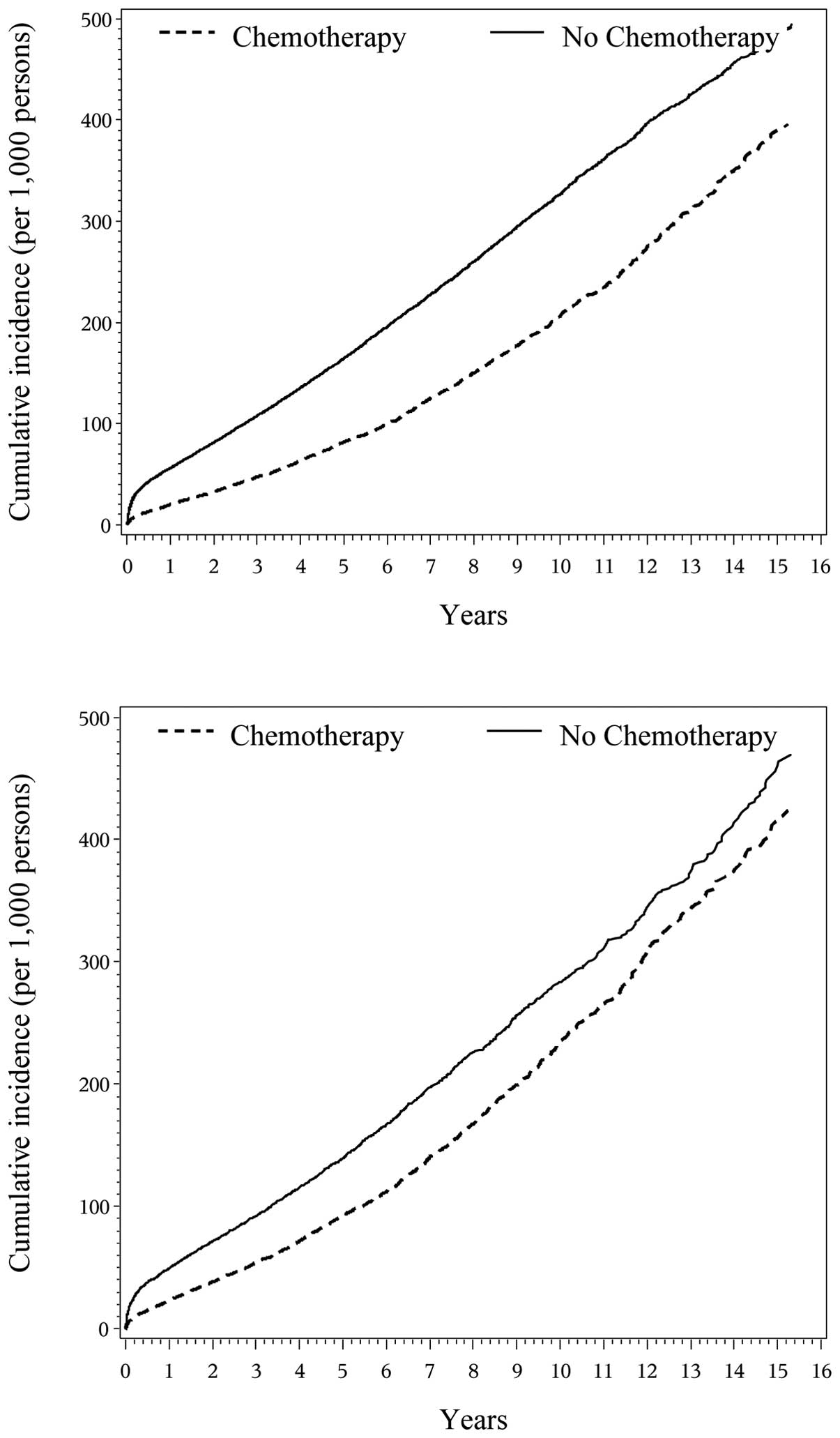
and matched cohorts (data not shown). Fig. 2 presents the cumulative incidence
curve of any dementia (all above dementias combined) over the
15-year period by chemotherapy status. The results were similar to
those from the four types of cognitive impairments other than
drug-induced dementia.
Table III presents
the time-to-event analysis for the hazard ratio of having various
types of cognitive impairments. Patients who received chemotherapy
were 24% significantly more likely to develop drug-induced dementia
compared to those without chemotherapy after adjusting for patient
and tumor characteristics (hazard ratio 1.24, 95% CI 1.05–1.47). On
the contrast, the risk of developing Alzheimer’s disease, vascular
dementia, or other dementias was significantly lower in patients
receiving chemotherapy than that in those who did not receive
chemotherapy, except for cognitive disorder which was not
significantly different between the two groups. As expected, the
risk of all types of cognitive impairments increased significantly
with age and comorbidity scores. There were no significant
differences in the risk of developing drug-induced dementia,
Alzheimer’s disease and vascular dementia between men and women,
but women appeared to have slightly lower risks of cognitive
disorders and other non-specified dementias. The risk of all types
of cognitive impairments was not significantly associated with the
receipt of radiation therapy.
 | Table IIIRelative risk (hazard ratio) of
cognitive impairments in the entire cohort of patients receiving
chemotherapy compared to those not, controlling for other
factors. |
Table III
Relative risk (hazard ratio) of
cognitive impairments in the entire cohort of patients receiving
chemotherapy compared to those not, controlling for other
factors.
| Hazard
ratioa (95% CI) of
having cognitive impairments
|
|---|
|
Characteristics | Drug-induced
dementia | Alzheimer’s
disease | Vascular
dementia | Cognitive disorder,
NOS | Other dementias or
dementia, NOSa | Any dementia (any
of the previous 5) |
|---|
| Chemotherapy | | | | | | |
| No
chemotherapy | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) |
| Chemotherapy | 1.24
(1.05–1.47) | 0.67
(0.61–0.74) | 0.55
(0.50–0.60) | 0.78
(0.57–1.07) | 0.63
(0.58–0.69) | 0.66
(0.62–0.70) |
| Age (years) | | | | | | |
| 65–69 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 70–74 | 1.08
(0.88–1.32) | 2.10
(1.82–2.43) | 1.89
(1.67–2.14) | 0.98
(0.68–1.42) | 1.94
(1.71–2.19) | 1.73
(1.59–1.88) |
| 75–79 | 1.18
(0.97–1.45) | 3.64
(3.17–4.18) | 3.32
(2.95–3.72) | 1.05
(0.73–1.52) | 3.38
(3.01–3.80) | 2.85
(2.63–3.09) |
| 80–85 | 1.49
(1.20–1.84) | 5.70
(4.96–6.56) | 5.11
(4.55–5.74) | 1.83
(1.27–2.63) | 5.37
(4.78–6.03) | 4.41
(4.07–4.78) |
| 85–89 | 1.37
(1.04–1.79) | 6.92
(5.94–8.06) | 6.90
(6.09–7.81) | 1.46
(0.92–2.33) | 7.61
(6.72–8.63) | 6.02
(5.52–6.57) |
| Gender | | | | | | |
| Male | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 1.02
(0.89–1.18) | 1.03
(0.96–1.12) | 0.98
(0.92–1.04) | 0.76
(0.59–0.98) | 0.92
(0.87–0.98) | 0.95
(0.91–0.99) |
| Comorbidity
scores | | | | | | |
| 0 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 1 | 1.32
(1.13–1.54) | 1.26
(1.16–1.36) | 1.51
(1.42–1.62) | 1.50
(1.14–1.96) | 1.33
(1.24–1.42) | 1.38
(1.32–1.45) |
| ≥2 | 1.85
(1.55–2.20) | 1.40
(1.28–1.54) | 2.04
(1.90–2.19) | 1.81
(1.31–2.49) | 1.96
(1.82–2.11) | 1.92
(1.82–2.03) |
| Radiotherapy | | | | | | |
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.06
(0.88–1.27) | 0.95
(0.86–1.05) | 0.99
(0.91–1.08) | 1.12
(0.80–1.56) | 0.94
(0.86–1.02) | 0.96
(0.90–1.02) |
Table IV presents
the risks of developing cognitive impairments in patients who were
treated with chemotherapy compared to those who were not,
stratified by the status of mood disorder. The risk of developing
drug-induced dementia seemed to be only significantly elevated in
those without mood disorder who received chemotherapy compared to
those who did not receive chemotherapy in both the entire cohort
(hazard ratio 1.26, 95% CI 1.06–1.50) and the matched cohort (1.29,
1.04–1.59). In those who had a history of mood disorder, there was
no longer a significant difference in the risk of developing
drug-induced dementia between the chemotherapy and no chemotherapy
groups (1.13, 0.58–2.18 for the entire cohort; 1.29, 0.52–3.20 for
the matched cohort). On the other hand, the risk of developing
Alzheimer’s disease, vascular dementia or other unspecified
dementias was significantly lower in patients receiving
chemotherapy compared to those without chemotherapy while the risk
of cognitive disorder was not significantly different between the
two groups. Also, there was no evidence of effect modification by
status of mood disorder. The findings from any dementia (all above
dementias combined) were similar to those from the four types of
cognitive impairments other than drug-induced dementia.
 | Table IVHazard ratio of developing cognitive
impairments in entire cohort and matched cohort of patients with
colon cancer who received chemotherapy compared to those without
receiving chemotherapy, stratified by the status of mood
disorder. |
Table IV
Hazard ratio of developing cognitive
impairments in entire cohort and matched cohort of patients with
colon cancer who received chemotherapy compared to those without
receiving chemotherapy, stratified by the status of mood
disorder.
| Hazard ratio (95%
CI)a of having
cognitive impairments in patients receiving chemotherapy compared
to those who did not, by status of mood disorder prior to cancer
diagnosis
|
|---|
| Type of cognitive
impairments | Had mood
disorder | No mood
disorder |
|---|
| Entire cohort | (n=2,908) | (n=69,466) |
| Drug-induced
dementia | 1.13
(0.58–2.18) | 1.26
(1.06–1.50) |
| Alzheimer’s
disease | 0.66
(0.44–1.00) | 0.68
(0.61–0.75) |
| Vascular
dementia | 0.49
(0.35–0.68) | 0.56
(0.51–0.61) |
| Cognitive
disorder- NOS | 0.91
(0.22–3.74) | 0.78
(0.56–1.09) |
| Other dementias
or dementia NOS | 0.66
(0.48–0.91) | 0.64
(0.59–0.69) |
| Any dementia (any
of above) | 0.61
(0.48–0.77) | 0.67
(0.63–0.71) |
| Matched cohort | (n=1,193) | (n=30,649) |
| Drug-induced
dementia | 1.29
(0.52–3.20) | 1.29
(1.04–1.59) |
| Alzheimer’s
disease | 0.58
(0.35–0.98) | 0.65
(0.58–0.73) |
| Vascular
dementia | 0.44
(0.29–0.67) | 0.53
(0.48–0.58) |
| Cognitive
disorder, NOS | - | 0.77
(0.53–1.13) |
| Other dementias
or dementia NOS | 0.60
(0.41–0.90) | 0.61
(0.55–0.67) |
| Any dementia (any
of above) | 0.51
(0.41–0.74) | 0.64
(0.60–0.69) |
Discussion
This study found that patients who received
chemotherapy were 24% significantly more likely to develop
drug-induced dementia compared to those without chemotherapy after
adjusting for patient and tumor characteristics. The significantly
increased risk was limited to those without a history of mood
disorder. The risk of developing Alzheimer’s disease, vascular
dementia or other dementias was significantly lower in patients
receiving chemotherapy than those who did not receive chemotherapy,
except for cognitive disorder that was not significantly different
between the two groups, which all were not affected by the history
of mood disorder. These findings should have important clinical and
public health implications, including key messages about potential
chemotherapy-induced dementia but no evidence about chemotherapy
associated with the increased risk of other dementias.
Possible relationships between cognitive impairments
and chemotherapy have been examined in clinical or community
settings involving patients treated with chemotherapy for breast
cancer (24–36) and none was conducted in men or
women with colorectal cancer. It is important to note that previous
pioneering small-scale clinical trials in the 1990s showed a
significant association between chemotherapy use and cognitive
impairments (24–31). Those small trials with short
follow-up times mostly tested the changes in memory and attention
which could be reversed after stopping chemotherapy. Our study
followed patients up to 17 years after cancer diagnosis, therefore
making it possible to examine long-term cognitive impairments such
as dementia at the late clinical stages.
The link between chemotherapy and cognitive
impairments and its potential mechanisms have been examined in
animal models (55–60). For example, it was suggested that
deficits in DNA-repair mechanisms and/or a deregulated immune
response, coupled with the effect of chemotherapy on these systems,
might have contributed to cognitive decline in rats following
chemotherapy (56). In another
study (59), cyclophosphamide- and
doxorubicin-treated rats showed significantly impaired performance
on the novel place recognition task compared with untreated
controls, suggesting a significant decline in neurogenesis in
chemotherapy-treated animals. However, a study by Fremouw et
al(57) showed that despite
significant toxic effects, chemotherapy-treated mice performed as
well as control mice on all tasks, concluding that as are some
humans, these mice may be resistant to at least some aspects of
chemotherapy-induced cognitive decline. Moreover, the study by
Fardell et al(58) showed
that exercising rats had improved cognition relative to
non-exercising rats after fluorouracil and oxaliplatin, suggesting
that physical activity may help ameliorate the cognitive
impairments induced by chemotherapy.
Our study has a number of strengths. First, the
study population covered a large cohort of community-based Medicare
beneficiaries, leading to much greater generalization of the study
findings to the elderly population aged ≥65 across the country.
Second, a unique feature of our study was that we included only
those patients free of any cognitive impairment at the time of
cancer diagnosis, thus leading to clearer cause-effect temporal
relationships. Third, because of potential selection bias in which
some patients with certain characteristics were given chemotherapy
while other patients were not given this therapy according to
preferences by patients or providers, or because of potential
confounding by indication in which whether or not chemotherapy was
given was influenced by other conditions such as cognitive decline,
mood disorder or other comorbid conditions, the matched cohort
analyses based on the conditional probability of receiving
chemotherapy would minimize selection bias and potential
confounding. In addition, the analysis was further stratified by
the status of mood disorder which demonstrated important
differences in the associations between chemotherapy use and
cognitive impairments. Finally, a large population-based cohort of
patients with colorectal cancer were followed-up from 4 to 17
years, allowing for more time to capture chronic conditions such as
dementia that would be otherwise missed in short follow-up
studies.
It is important to note several important
limitations of this study. First, study outcomes only included more
serious and late-stage cognitive impairments (such as Alzheimer’s
disease), which might not be comparable to early stage of cognitive
impairments identified in previous clinical trials (19–21,25,26).
The claims data did not allow assessment of the early-stage disease
process (such as decline in memory or attention) of cognitive
impairments. Due to this limitation, our study population who were
free of dementia at the baseline might have included those who
already had early stage cognitive impairments, which could have
affected the association between chemotherapy and outcomes. Second,
even though we adjusted for some measured confounding factors and
used the matched cohort analysis based on the probability of
receiving chemotherapy, there could have been unmeasured or unknown
factors which could have influenced physicians to prescribe
chemotherapy or not to do so. In particular, if physicians were
aware of some studies on potential link between chemotherapy and
cognitive impairments, they might hesitate to prescribe
chemotherapy to those with suspected cognitive problems. Hence, the
potential for selection bias could not be ruled out in this study.
Third, the finding that the risk of drug-induced dementia was
significantly increased only in those patients without mood
disorder may be vulnerable to surveillance bias because of the link
between mood disorder and dementia (61). In other words, patients without a
history of mood disorder could be more likely labeled by the
treating clinicians or coders as having ‘drug-induced dementia’,
whereas those with a history of mood disorder might be more likely
labeled as having dementias other than drug-induced. Similarly,
because of difficulty in confirming if a dementia was induced by
chemotherapy, clinical or coding staff might likely code a dementia
as drug-induced for patients receiving chemotherapy but code
differently for patients without receiving chemotherapy, leading to
certain degrees of differential misclassification of study
outcomes. One way to see if this was the case is to analyze all
dementias combined regardless of drug-induced one. We performed
additional analyses and found that the receipt of chemotherapy was
associated with the decreased risk of all cognitive impairments
combined (Tables II–IV and Fig.
2). Fourth, Medicare claims had limited information on the
dosage and intensity of chemotherapy that could have affected the
occurrence and severity of cognitive impairments. We relied on the
common procedure codes that specified the standard dose for each
chemotherapy agent but in practice the treating physician might
have modified the chemotherapy doses according to each patient’s
characteristics. Furthermore, this study did not examine the types
of chemotherapy utilized, the number of cycles administered, and
their potential effects on the outcomes.
In conclusion, there was a significant association
between chemotherapy and the risk of developing drug-induced
dementia in patients with colorectal cancer who received
chemotherapy but did not have a history of mood disorder. This
study with long-term follow-up found that the risks of Alzheimer’s
disease, vascular dementia, or other non-specified dementias were
even lower in patients with colorectal cancer with chemotherapy use
than those without this therapy.
Acknowledgements
We acknowledge the efforts of the
National Cancer Institute; Center for Medicare and Medicaid
Services; Information Management Services, Inc.; and the SEER
Program tumor registries in the creation of this database. The
interpretation and reporting of these data are the sole
responsibilities of the authors. This study was supported in part
by a grant from the Agency for Healthcare Research and Quality
(R01-HS018956) and in part by a grant from the Cancer Prevention
and Research Institute of Texas (RP101207).
References
|
1
|
NIH Consensus Conference: Adjuvant therapy
for patients with colon and rectal cancer. JAMA. 264:1444–1450.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moertel CG, Fleming TR, Macdonald JS, et
al: Fluorouracil plus levamisole as effective adjuvant therapy
after resection of stage III colon carcinoma: a final report. Ann
Intern Med. 122:321–326. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sargent DJ, Goldberg RM, Jacobson SD, et
al: A pooled analysis of adjuvant chemotherapy for resected colon
cancer in elderly patients. N Engl J Med. 345:1091–1097. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gill S, Loprinzi CL, Sargent DJ, et al:
Pooled analysis of fluorouracil-based adjuvant therapy for stage II
and III colon cancer: who benefits and by how much? J Clin Oncol.
22:1797–1806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
National Cancer Institute: Colon and
Rectal cancer Treatment. http://www.cancer.gov/cancertopics/treatment/colon-and-rectal.
Accessed September 16, 2012.
|
|
6
|
O’Connell MJ, Martenson JA, Wieand HS, et
al: Improving adjuvant therapy for rectal cancer by combining
protracted-infusion fluorouracil with radiation therapy after
curative surgery. N Engl J Med. 331:502–507. 1994.PubMed/NCBI
|
|
7
|
Figueredo A, Charette ML, Maroun J,
Brouwers MC and Zuraw L: Adjuvant therapy for stage II colon
cancer: a systematic review from the Cancer Care Ontario Program in
evidence-based care’s gastrointestinal cancer disease site group. J
Clin Oncol. 22:3395–3407. 2004.
|
|
8
|
Benson AB III, Schrag D, Somerfield MR, et
al: American Society of Clinical Oncology recommendations on
adjuvant chemotherapy for stage II colon cancer. J Clin Oncol.
22:3408–3419. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sargent DJ, Wieand HS, Haller DG, et al:
Disease-free survival versus overall survival as a primary end
point for adjuvant colon cancer studies: individual patient data
from 20,898 patients on 18 randomized trials. J Clin Oncol.
23:8664–8670. 2005. View Article : Google Scholar
|
|
10
|
Goldberg RM, Tabah-Fisch I, Bleiberg H, et
al: Pooled analysis of safety and efficacy of oxaliplatin plus
fluorouracil/leucovorin administered bimonthly in elderly patients
with colorectal cancer. J Clin Oncol. 24:4085–4091. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
André T, Boni C, Navarro M, et al:
Improved overall survival with oxaliplatin, fluorouracil, and
leucovorin as adjuvant treatment in stage II or III colon cancer in
the MOSAIC trial. J Clin Oncol. 27:3109–3116. 2009.PubMed/NCBI
|
|
12
|
Schrag D, Rifas-Shiman S, Saltz L, Bach PB
and Begg CB: Adjuvant chemotherapy use for Medicare beneficiaries
with stage II colon cancer. J Clin Oncol. 20:3999–4005. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O’Connor ES, Greenblatt DY, LoConte NK, et
al: Adjuvant chemotherapy for stage II colon cancer with poor
prognostic features. J Clin Oncol. 29:3381–3388. 2011.PubMed/NCBI
|
|
14
|
Silberfarb PM, Philibert D and Levine PM:
Psychosocial aspects of neoplastic disease: II. Affective and
cognitive effects of chemotherapy in cancer patients. Am J
Psychiatry. 137:597–601. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oxman TE and Silberfarb PM: Serial
cognitive testing in cancer patients receiving chemotherapy. Am J
Psychiatry. 137:1263–1265. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Devlen J, Maguire P, Phillips P and
Crowther D: Psychological problems associated with diagnosis and
treatment of lymphomas. II: Prospective study. Br Med J.
295:955–957. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parth P, Dunlap WP, Kennedy RS, et al:
Motor and cognitive testing of bone marrow transplant patients
after chemoradiotherapy. Percept Mot Skills. 68:1227–1241. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Copeland DR, Moore BD III, Francis DJ, et
al: Neuropsy-chologic effects of chemotherapy on children with
cancer: a longitudinal study. J Clin Oncol. 14:2826–2835.
1996.PubMed/NCBI
|
|
19
|
Waber DP, Tarbell NJ, Fairclough D, et al:
Cognitive sequelae of treatment in childhood acute lymphoblastic
leukemia: cranial radiation requires an accomplice. J Clin Oncol.
13:2490–2496. 1995.PubMed/NCBI
|
|
20
|
Ahles TA, Silberfarb PM, Rundle AC, et al:
Quality of life in patients with limited small-cell carcinoma of
the lung receiving chemotherapy with or without radiation therapy,
for cancer and Leukemia Group B. Psychother Psychosom. 62:193–199.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wieneke MH and Dienst ER:
Neuropsychological assessment of cognitive functioning following
chemotherapy for breast cancer. Psychooncology. 4:61–66. 1995.
View Article : Google Scholar
|
|
22
|
Meyers CA, Byrne KS and Komaki R:
Cognitive deficits in patients with small cell lung cancer before
and after chemotherapy. Lung Cancer. 12:231–235. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schwartz CL: Late effects of treatment in
long-term survivors of cancer. Cancer Treat Rev. 21:355–366. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
van Dam FS, Schagen SB, Muller MJ, et al:
Impairment of cognitive function in women receiving adjuvant
treatment for high-risk breast cancer: high-dose versus
standard-dose chemotherapy. J Natl Cancer Inst. 90:210–218.
1998.PubMed/NCBI
|
|
25
|
Schagen SB, van Dam FS, Muller MJ, et al:
Cognitive deficits after postoperative adjuvant chemotherapy for
breast carcinoma. Cancer. 85:640–650. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schagen SB, Hamburger HL, Muller MJ, et
al: Neurophysiological evaluation of late effects of adjuvant
high-dose chemotherapy on cognitive function. J Neurooncol.
51:159–165. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schagen SB, Muller MJ, Boogerd W, et al:
Change in cognitive function after chemotherapy: a prospective
longitudinal study in breast cancer patients. J Natl Cancer Inst.
98:1742–1745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kreukels BP, van Dam FS, Ridderinkhof KR,
et al: Persistent neurocognitive problems after adjuvant
chemotherapy for breast cancer. Clin Breast Cancer. 8:80–87. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brezden CB, Phillips KA, Abdolell M, et
al: Cognitive function in breast cancer patients receiving adjuvant
chemotherapy. J Clin Oncol. 18:2695–2701. 2000.PubMed/NCBI
|
|
30
|
Wefel JS, Lenzi R, Theriault RL, Davis RN
and Meyers CA: The cognitive sequelae of standard-dose adjuvant
chemotherapy in women with breast carcinoma: results of a
prospective, randomized, longitudinal trial. Cancer. 100:2292–2299.
2004. View Article : Google Scholar
|
|
31
|
Hurria A, Rosen C, Hudis C, et al:
Cognitive function of older patients receiving adjuvant
chemotherapy for breast cancer: a pilot prospective longitudinal
study. J Am Geriatr Soc. 54:925–931. 2006. View Article : Google Scholar
|
|
32
|
Deprez S, Amant F, Smeets A, et al:
Longitudinal assessment of chemotherapy-induced structural changes
in cerebral white matter and its correlation with impaired
cognitive functioning. J Clin Oncol. 30:274–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Heck JE, Albert SM, Franco R and Gorin SS:
Patterns of dementia diagnosis in surveillance, epidemiology, and
end results breast cancer survivors who use chemotherapy. J Am
Geriatr Soc. 56:1687–1692. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Baxter NN, Durham SB, Phillips KA,
Habermann EB and Virning BA: Risk of dementia in older breast
cancer survivors: a population-based cohort study of the
association with adjuvant chemotherapy. J Am Geriatr Soc.
57:403–411. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raji MA, Tamborello LP, Kuo YF, Ju H,
Freeman JL, Zhang DD, Giordano SH and Goodwin JS: Risk of
subsequent dementia diagnoses does not vary by types of adjuvant
chemotherapy in older women with breast cancer. Med Oncol.
26:452–459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du XL, Xia R and Hardy D: Relationship
between chemotherapy use and cognitive impairments in older women
with breast cancer: findings from a large population-based cohort.
Am J Clin Oncol. 33:533–543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
National Cancer Institute: About the SEER
(Surveillance Epidemiology and End Results) Registries. http://seer.cancer.gov/registries/.
Accessed September 15, 2012.
|
|
38
|
Warren JL, Klabunde CN, Schrag D, Bach PB
and Riley GF: Overview of the SEER-Medicare data: content, research
applications, and generalizability to the United States elderly
population. Med Care. 40(Suppl): 3–18. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Parsons LS: Reducing Bias in a Propensity
Score Matching-Pair Sample Using GreedyMatching Techniques.
available at http://www2.sas.com/proceedings/sugi26/p214-26.pdf.
Accessed July 2, 2012.
|
|
40
|
Du XL and Goodwin JS: Patterns of use of
chemotherapy for breast cancer in older women: findings from
Medicare claims data. J Clin Oncol. 19:1455–1461. 2001.PubMed/NCBI
|
|
41
|
Warren JL, Harlan LC, Fahey A, et al:
Utility of the SEER-Medicare data to identify chemotherapy use. Med
Care. 40:IV-55–IV-61. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Du XL, Key CR, Dickie L, et al: External
validation of medicare claims for breast cancer chemotherapy
compared with medical chart reviews. Med Care. 44:124–131. 2006.
View Article : Google Scholar
|
|
43
|
Lamont EB, Herndon JE II, Weeks JC, et al:
Criterion validity of Medicare chemotherapy claims in cancer and
leukemia Group B breast and lung cancer trial participants. J Natl
Cancer Inst. 97:1080–1083. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liang SY, Phillips KA, Wang G, et al:
Tradeoffs of using administrative claims and medical records to
identify the use of personalized medicine for patients with breast
cancer. Med Care. 49:e1–e8. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lund JL, Stürmer T, Harlan LC, et al:
Identifying specific chemotherapeutic agents in medicare data: a
validation study. Med Care. Nov 10–2011, (Epub ahead of print).
|
|
46
|
US Public Health Services: International
Classification of Diseases, 9th Revision, Clinical Modification.
5th edition. Practice Management Information Corp.; Los Angeles,
CA: 1996
|
|
47
|
American Medical Association: Physicians’
Current Procedural Terminology-CPT 2000. American Medical
Association; Chicago, IL: 2000
|
|
48
|
Health Care Financing Administration: HCFA
Common Procedure Coding System: National Level II Medicare Codes.
Practice Management Information Corp.; Los Angeles, CA: 2000
|
|
49
|
Charlson ME, Pompei P, Ales KL and
MacKenzie CR: A new method of classifying prognostic comorbidity in
longitudinal studies: development and validation. J Chronic Dis.
40:373–383. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Romano PS, Roos LL and Jollis JG: Adapting
a clinical comorbidity index for use with ICD-9-CM administrative
data: differing perspectives. J Clin Epidemiol. 46:1075–1079. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Klabunde CN, Potosky AL, Legler JM and
Warren JL: Development of a comorbidity index using physician
claims data. J Clin Epidemiol. 53:1258–1267. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
National Cancer Institute: SEER-Medicare:
Calculation of Comorbidity Weights. http://healthservices.cancer.gov/seermedicare/program/comorbidity.html.
Accessed July 18, 2012.
|
|
53
|
Penman AD and Johnson WD: A SAS program
for calculating cumulative incidence of events (with confidence
limits) and number at risk at specified time intervals with
partially censored data. Comput Methods Programs Biomed. 89:50–55.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Allison PD: Survival Analysis Using the
SAS System: A Practical Guide. SAS Institute Inc; Cary, NC:
1995
|
|
55
|
Ahles TA and Saykin AJ: Candidate
mechanisms for chemotherapy-induced cognitive changes. Nat Rev
Cancer. 7:192–201. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
56
|
Long JM, Lee GD, Kelley-Bell B, Spangler
EL, Perez EJ, Longo DL, de Cabo R, Zou S and Rapp PR: Preserved
learning and memory following 5-fluorouracil and cyclophosphamide
treatment in rats. Pharmacol Biochem Behav. 100:205–211. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fremouw T, Fessler CL, Ferguson RJ and
Burguete Y: Preserved learning and memory in mice following
chemotherapy: 5-fluorouracil and doxorubicin single agent
treatment, doxorubicin-cyclophosphamide combination treatment.
Behav Brain Res. 226:154–162. 2012. View Article : Google Scholar
|
|
58
|
Fardell JE, Vardy J, Shah JD and Johnston
IN: Cognitive impairments caused by oxaliplatin and 5-fluorouracil
chemotherapy are ameliorated by physical activity.
Psychopharmacology. 220:183–193. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Christie LA, Acharya MM, Parihar VK,
Nguyen A, Martirosian V and Limoli CL: Impaired cognitive function
and hippocampal neurogenesis following cancer chemotherapy. Clin
Cancer Res. 18:1954–1965. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Winocur G, Henkelman M, Wojtowicz JM,
Zhang H, Binns MA and Tannock IF: The effects of chemotherapy on
cognitive function in a mouse model: a prospective study. Clin
Cancer Res. 18:3112–3121. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Byers AL and Yaffe K: Depression and risk
of developing dementia. Nat Rev Neurol. 7:323–331. 2011. View Article : Google Scholar : PubMed/NCBI
|