Introduction
The expression of miR-205, transcribed from the
MIR205HG gene on chromosome 1q, is specific for epithelial tissues
(reviewed in ref. 1). Loss of
expression of this microRNA in cancer has been found to be involved
in the epithelial to mesenchymal transition (EMT), a reversible
series of molecular changes that lead to the conversion of
polarised immobile epithelial cells to more motile mesenchymal
cells. EMT is required for embryonic development, but also occurs
in epithelial tumours, leading to metastasis. Other microRNAs that
are repressed during the EMT are the members of the miR-200 family,
namely miR-200a, miR-200b, miR-200c, miR-141 and miR-429. The
decreased expression of these miRNAs indirectly downregulates
E-cadherin expression through the increased expression of ZEB1 and
ZEB2, which repress the CDH1 gene (2), reviewed in (1). MiR-205 has been found to be
downregulated in different tumour types, including breast cancer
(3), head and neck cancer
(4) and invasive bladder cancer,
in contrast to non-invasive tumours (5). Microarray based screens of prostate
cancer tissue also indicated a consistent downregulation of miR-205
(6–8). Nevertheless, upregulation of miR-205
in cancer has also been observed, e.g., in endometrial cancer
(9,10), esophageal cancer (11) and non-small cell lung cancer
(12). In the tumour types where
increased expression of miR-205 occurs, it appears not to decrease
cell growth, but it does still inhibit the EMT through repression
of ZEB2 (11) and inhibits
metastasis (13).
Another confirmed target of this microRNA is the
transcription factor E2F1 (14,15),
which is involved in cell division and apoptosis. E2F1, in a
negative feedback loop, appears to upregulate miR205 expression
(16). In melanoma cell lines,
miR-205 was also found to be positively regulated by full length
p73 and repressed by the N-terminally truncated p73 isoform DNp73.
Although p53 binds to the same sites as p73, it did not
significantly regulate miR-205 in this system (16). In contrast, in breast cancer cell
lines, a robust upregulation by p53 was seen. These authors did not
test for the effects of p73, though (15). Positive regulation by DNp63, which
also binds to the same sites at the promoter as p53 and p73, has
also been described (17,18). In breast cancer it has recently
been shown that overexpression of ErbB2 represses miR-205 leading
to increased growth in the soft-agar assay (19), but whether this is a direct
regulation remains elusive. Another possibility is silencing by
methylation of the miR-205 promoter. This hypothesis is further
supported by the fact that the CpG island located upstream of the
first exon of miR205HG was found to be methylated in multiple
prostate cancer cell lines (20)
and 5′Aza treatment was able to increase miR-205 expression in
breast cancer cell lines (15).
In the present study, we investigated the
downregulation of miR-205 in prostate cancer samples. The levels of
miR-205 correlated significantly inversely with tumour size and
decreased from Gleason score 7a=3+4 to 8=4+4. Additionally, we
identified the anti-apoptotic gene BCL2 as a target of miR-205 in
prostate cancer. We also correlated BCL2 expression to miR-205
levels in prostate cancer tissues. BCL2 is a potentially relevant
miR-205 target specifically in prostate cancer, due to its known
association to prognosis and disease progression (21,22).
Materials and methods
Patient collective
A series of 111 formalin-fixed paraffin-embedded
(FFPE) prostatectomy specimens with primary prostate adenocarcinoma
(PCa) from the Department of Pathology, Ruhr University Bochum,
diagnosed between 2009 and 2011 were collected for the study. The
composition of the cohort concerning Gleason grade, age, PSA levels
and stage is shown in Table I. The
PCa specimens were subjected to histological examination by an
expert pathologist for confirmation of the Gleason grading, WHO
classification of the tumour and staging according to the
tumour-node-metastasis system. No patients had distant metastases
at the time of diagnosis. The study was approved by the local
Ethics Committee (Votum no. 3991-11).
 | Table I.Characteristics of the patient
collective. |
Table I.
Characteristics of the patient
collective.
| Cohort feature | Median | Range | N |
|
| Age (years) | 63.5 | 47.00–75.5 | 111 |
| Tumour size
(cm3) | 2.30 | 0.16–18.00 | 111 |
| PSA (ng/ml) | 7.70 | 2.3–38 | 104 |
|
| Tumour
characteristics | No. of samples
(%) |
|
| Node-positive (N1)
cases | 6 (5.41) |
| Distant metastasis
(M1) | 0 (0) |
| Lymphatic vessel
invasion (L1) | 8 (7.21) |
| Vascular invasion
(V1) | 1 (0.90) |
| Perineural invasion
(Pn1) | 65 (78.38) |
| Gleason sum | |
| 3+3 | 14 (12.61) |
| 3+4 | 47 (42.34) |
| 4+3 | 37 (33.33) |
| 4+4 | 13 (11.71) |
| Stage | |
| pT2a | 10 (8.93) |
| pT2b | 1 (0.89) |
| pT2c | 72 (96.29) |
| pT3a | 14 (12.50) |
| pT3b | 13 (11.61) |
| pT4 | 1 (0.89) |
Determination of miR-205 and BCL2 levels
in patient samples
The histopathological tumour regions of interest
were micro-dissected with the corresponding HE slide as a guide
(tumour was marked with a pen on the HE slide) and used for RNA
extraction using the miRNA FFPE kit (Qiagen, Hilden, Germany),
following the manufacturer’s instructions.
For the detection of miRNA levels, 500 ng total RNA
was transcribed using the miScript reverse transcription kit
(Qiagen); qRT-PCR was carried out with 25 ng cDNA per reaction
using the miScript SYBR green PCR system (Qiagen) with the miR-205
primer (#MS00003780) on an Eppendorf Realplex4 cycler (Eppendorf,
Hamburg, Germany). RNU6B (U6 small nuclear RNA 2,
#MS00014000) was used for the normalization to total RNA (23). For the detection of BCL2 in
FFPE samples and cell culture, RNA was transcribed to cDNA using
the High Capacity cDNA system (Life Technologies, Darmstadt,
Germany). qRT-PCR was carried out with the TaqMan system (primer
set #Hs99999018_m1) with 2X Universal Master Mix (Life
Technologies). Template cDNA (25 ng) was used per PCR. 18S rRNA
(#Hs99999901_s1) was used as internal control.
Cell culture
The prostate cancer cell lines PC3 and LnCap and the
osteosarcoma cell line U2-OS were obtained from ATCC (Manassas, VA,
USA). PC3 and LnCap cells were cultivated in RPMI-1640 (Pan
Biotech, Aidenach, Germany) and U2-OS was cultivated in DMEM high
glucose (Pan Biotech), both supplemented with 10% fetal calf serum
(Hyclone-Thermo Scientific, Bonn, Germany), 100 U/ml penicillin and
100 μg/ml streptomycin (PAN Biotech); cisplatin and
doxorubicin were obtained from Sigma-Aldrich (Taufkirchen,
Germany).
Transfection of prostate cell lines with
miRNA mimics
PC3 cells were seeded at 5×105 per well
and LnCap were seeded at 8×105 cells per well in 6-well
plates in their normal medium and transfected in suspension with 10
nM miRNA mimics (Qiagen) or AllStars negative control siRNA,
labelled with AlexaFluor-647 (Qiagen), using 6 μl HiPerFect
(Qiagen). The transfection efficiency was determined by measuring
the fluorescence of the AllStars oligos by flow cytometry and was
between 90 and 96%. Whenever the assay required, the cells were
treated with cisplatin or doxorubicin 72 h after transfection.
Reporter assay
Fragments of the 3′ untranslated region (UTR) of
E2F1 (262 bp), E2F5 (389 bp) and BCL2 (438 bp) containing the
potential miR-205 binding sites were cloned into pGL3 as 3′UTR of
the firefly luciferase gene by PCR. U2-OS cells were transiently
transfected in triplicates with 40 ng of the pGL3 construct, 10 ng
of the expression vector for renilla luciferase pRL-CMV (Promega,
Mannheim, Germany) and 10 nmol of miRNA mimics (Qiagen), using the
transfection reagent Attractene according to the manufacturer’s
instructions (Qiagen). Cells were harvested 30 h after transfection
and activities of firefly and renilla luciferase were measured
using the Dual-Luciferase reporter assay system (Promega). The
resulting luminescence was measured with a Tecan M200 microplate
reader (Tecan, Crailsheim, Germany).
MTT assay
Cells were plated at 10,000 (PC3) or 5,000 (LnCap)
per well in 24-well plates. One, three, five and seven days after
plating, respectively, 100 μl of 5 mg/ml MTT (thiazolyl blue
tetrazolium bromide, Carl Roth, Karlsruhe, Germany) in PBS was
added per well; cells were lysed after 4 h by addition of 250
μl triplex solution (10% SDS; 5% isobutanol, 0.012 M HCl).
Absorbance was measured at 562 nm.
Apoptosis assays
Caspase activities were measured using the Caspase
Glo 3/7 assay (Promega), following the manufacturer’s instructions.
Values were corrected for differences in cell numbers by doing an
MTT assay in parallel.
The mitochondrial membrane potential was determined
in LnCap by JC-1 (Axxora, Lörrach, Germany) staining. JC-1 was
added to the cells at a final concentration of 5 μg/ml,
incubated for 10 min at 37°C, after which the cells were
trypsinized and washed extensively with PBS. In PC3, the
mitochondrial membrane potential was instead measured using the
FlowCellect™ MitoPotential Red kit (Millipore, Schwalbach,
Germany), following the manufacturer’s instructions. In both cases,
treatments were performed in triplicates; fluorescence was measured
on a Guava easyCyte 8HT flow cytometer (Millipore).
For the determination of the sub-G1 fraction,
prostate carcinoma cells were transfected with miR-205 mimics or
AllStars control oligos, trypsinized, plated at 5×104
cells per well in 6-well plates in triplicates the next day and
treated with doxorubicin or cisplatin 48 h after transfection.
Forty-eight hours after treatment with doxorubicin and cisplatin,
respectively, cells were harvested by trypsinisation and combined
with the floating cells. Cells were fixed in 70% ice-cold ethanol
and stained in 0.1% Triton X-100 (Carl Roth), 0.5 mg/ml RNaseA
(Sigma-Aldrich) and 60 μg/ml propidium iodide (MP
Biochemicals, Illkirch, France) in PBS. The sub-G1 fraction of
propidium iodide stained cells were measured on a Guava easyCyte
8HT flow cytometer (Millipore).
Western blotting
Cells were harvested by scraping into ice-cold RIPA
buffer (1% NP40, 0.5% sodium deoxycholate, 0.1% SDS, 150 mM NaCl,
50 mM Tris-HCl pH 8.0) containing EDTA-free complete protease
inhibitors (Roche, Mannheim, Germany). Protein concentration was
measured using the BCA assay (Thermo Scientific) according to the
manufacturer’s instructions. Incubation with primary antibodies
anti-BCL2 (Cell Signaling, Frankfurt, Germany) or anti-α-tubulin
(clone DM1A, Thermo Fisher, Bonn, Germany) was done overnight at
4°C. Blots were incubated with horseradish peroxidase labelled
secondary antibodies (Cell Signaling) and the signal was detected
using ECL (Thermo Scientific). Changes in protein expression were
quantified using ImageJ Software (V.1.43u; NIH; USA). By
normalizing integrated density values (IDV) of the protein bands of
interest were normalized to the corresponding tubulin IDVs.
Statistics
Comparisons between pairs of values were done using
the t-test; the Welch t-test was applied if the F-test indicated a
significant difference between the variances. Statistical
inferences were derived from single experiments. For statistical
testing on expression levels of miR-205 and BCL2, data were
log2 transformed, to allow the use of normal distribution-based
tests and Pearson’s correlation coefficient. For post hoc
testing between individual pairs of conditions, the Scheffé test
was applied. Statistical analysis was carried out by the Statistica
10 program (StatSoft, Tulsa, OK, USA).
Results
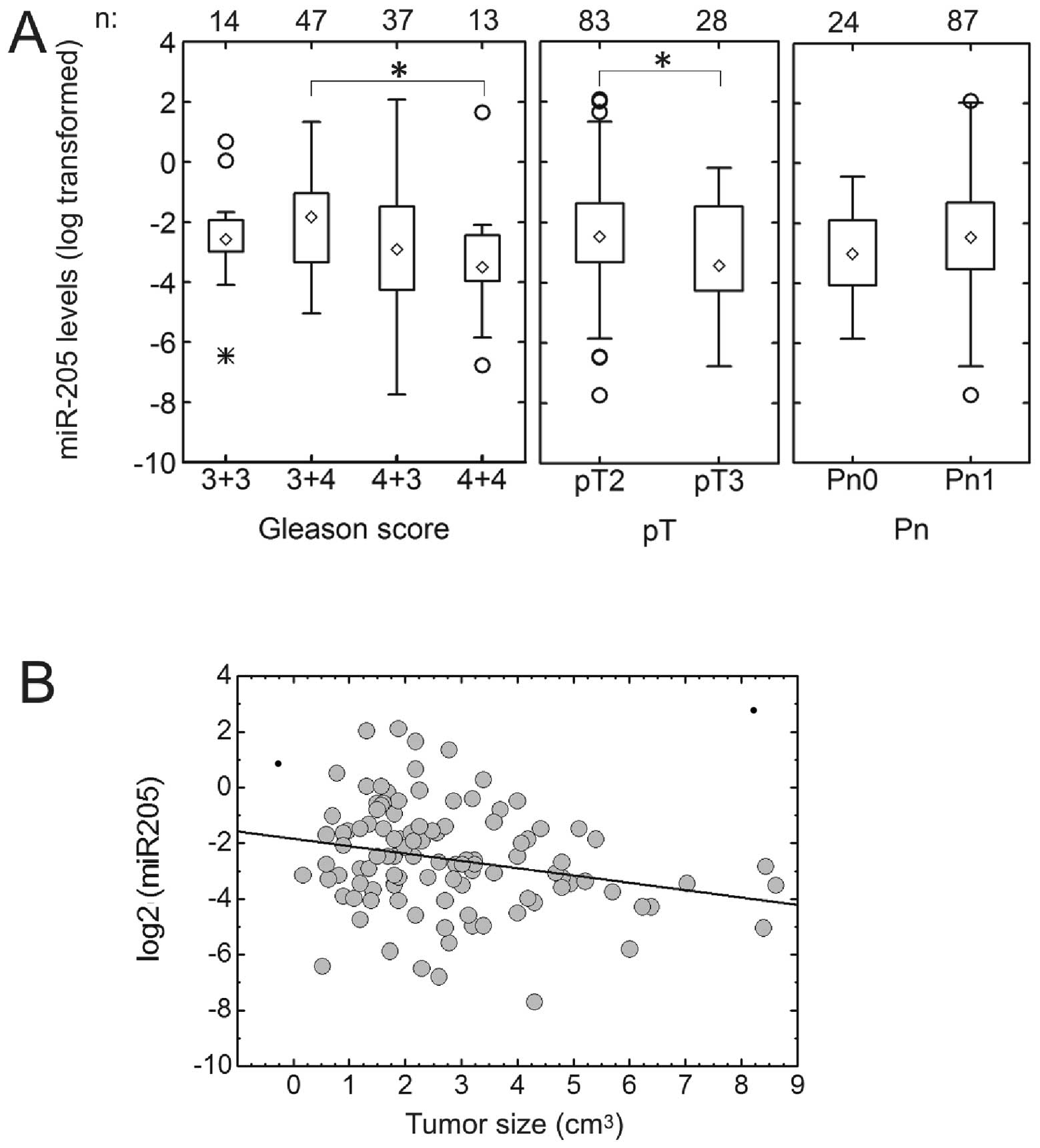
MiR-205 is downregulated in prostate
cancer
We determined the expression of miR-205 by qRT-PCR
in 111 FFPE specimens of prostate carcinoma. The miR-205 expression
was found to be strongly reduced in 102 of 111 analyzed prostate
cancer samples, with a median relative expression level of 0.160 in
comparison to benign tissue from the same patient (interquartile
range, IQR, 0.085–0.350). MiR-205 expression levels decreased with
increasing Gleason score from 7a=3+4 to 8=4+4, but Gleason score
6=3+3 samples showed lower expression than Gleason score 7a samples
(median expression levels: Gleason 3+6, 0.168; Gleason 7a, 0.303;
Gleason 7b, 0.150; Gleason 8, 0.087; p=0.050 by ANOVA on log
transformed data) (Fig. 1A). Its
expression was also higher in tumours confined to the prostate
(pT2) than in those that extend beyond the prostate (pT3)
(p=0.0425). However, differences in miR-205 expression between
perineural invasion categories were not significant (Fig. 1A). Only 9 of 111 samples showed an
increased expression of miRNA 205 in tumour tissue in comparison to
the benign controls; these were all relatively small tumours (<4
cm3). The inverse correlation between miR-205 expression
and tumour size was significant (r=−0.234, p=0.0087) (Fig. 1B).
The anti-apoptotic gene BCL2 is a target
of miR-205
The reduced expression of miR-205 in prostate cancer
indicates that its targets may aid tumour progression. From the
targets suggested by the miRGen program (www.diana.pcbi.upenn.edu/miRGen/html), we selected
BCL2 as a novel candidate. BCL2 appears interesting in this
context, as its overexpression in prostate cancer is a known marker
for poor prognosis (24).
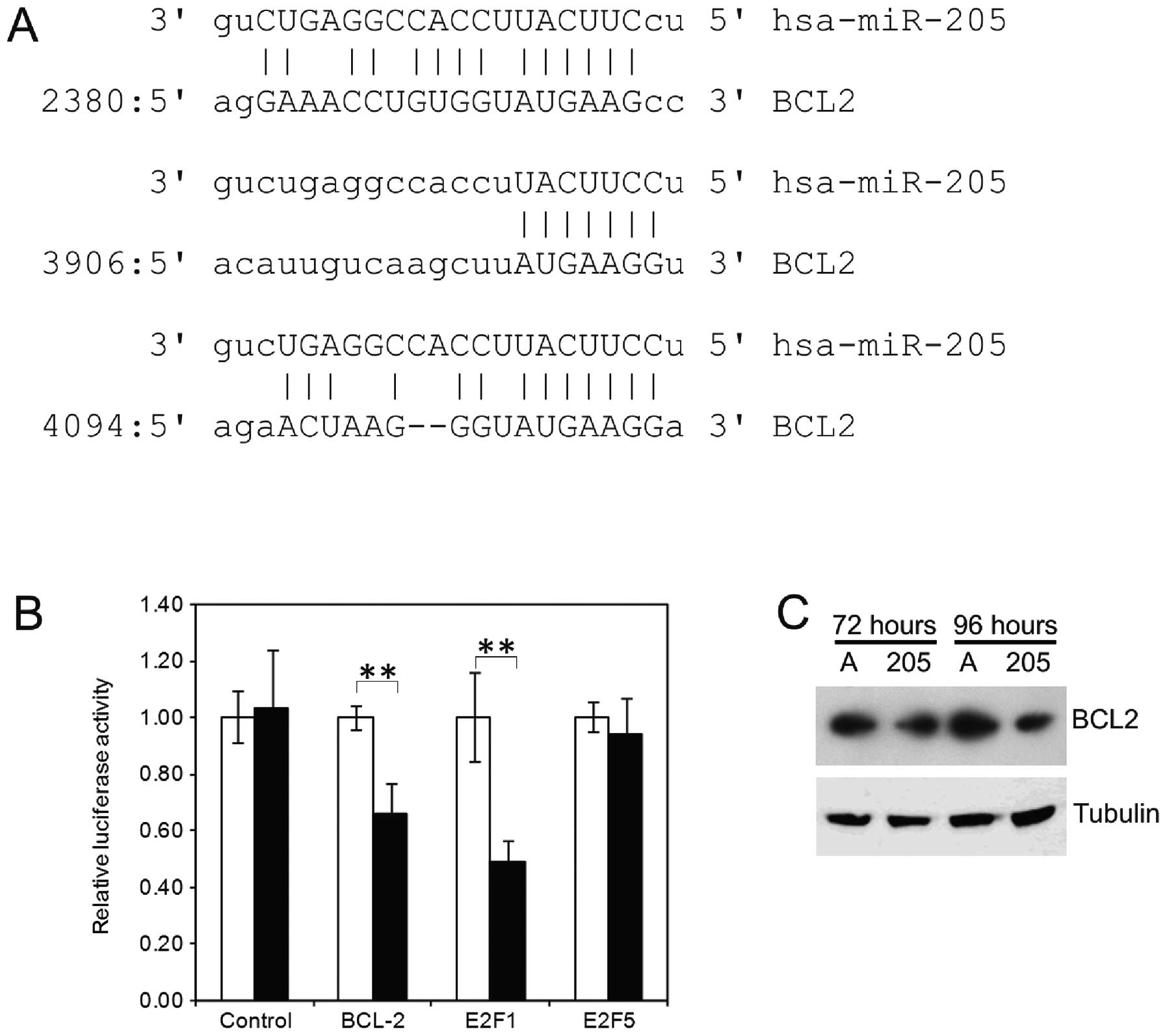
In the ∼5-kb long 3′UTR, 3 potential miR-205 binding
sites are found, one weak (6-mer + a) centrally located at position
2380 and two potentially stronger sites near the 3′ end of the
3′UTR at positions 3906 (7-mer-m8) and 4094 (7-mer-1a) (Fig. 2A), both with a ΔG above −13.40
kCal/mol (25). For a random
sequence of this length, a ΔG of −13.40 kCal/mol would be expected
(26). Additionally, these sites
are located relatively close together and near the 3′ end of a long
3′UTR, which are also typical characteristics of functional miRNA
binding sites (27).
The fragment containing these two most 3′ terminal
sites was cloned into the pGL3 vector as 3′UTR for the luciferase
gene (28). A reporter assay was
done in U2-OS cells with miR-205 or control oligos to test for
reduced luciferase activity after binding the microRNA. The
osteosarcoma cell line U2-OS was used for this assay due to its
ease of transfection and the stability of the assay in this cell
line. As positive controls, equivalent constructs containing
miR-205 binding sites in E2F1 and E2F5 were used. In
comparison to the control oligos, miR-205 caused a 34% reduction in
the luciferase signal when the BCL2 UTR was tested. In comparison,
the E2F1 UTR caused a 51% percent reduction in signal
intensity and the E2F5 UTR only 6%. The decrease in
luminescence was significant for both BCL2 and E2F1
(p<0.01, t-test). The control vector did not show significantly
reduced luciferase activity in the presence of miR-205 (Fig. 2B).
Next, we analyzed if overexpression of miR-205 leads
to a decrease in the BCL2 protein levels of the prostate cancer
cell line PC3. We observed a decrease in the BCL2 level by western
blotting at 72 and 96 h after transfection with miR-205 mimics, in
comparison those transfected with control oligos (Fig. 2C).
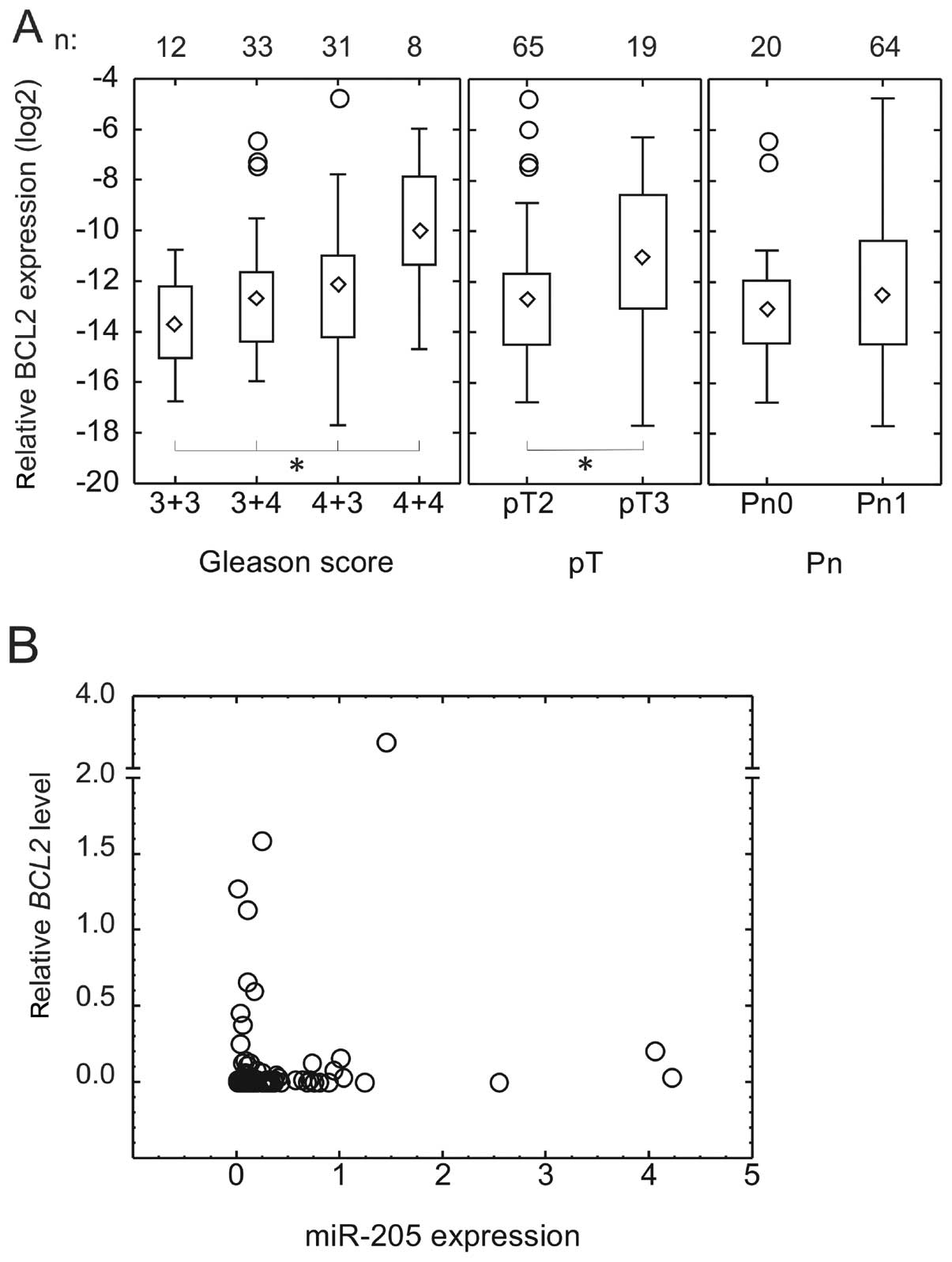
BCL2 expression in comparison to miR-205
levels in patient samples
Having confirmed BCL2 as a miR-205 target, we
determined the expression of BCL2 at the mRNA level by TaqMan assay
in the series of archival samples for which miR-205 levels were
measured by qRT-PCR. The quality of the RNA was sufficient for
reliable PCR results in 84 of 111 samples. The mRNA levels of
BCL2 have been found to correlate well with its protein
level (29), allowing for the use
of qRT-PCR to measure BCL2 expression. BCL2 is
strongly expressed in the basal cells of benign glands (24,30),
making the comparison to benign tissue difficult for this gene.
Therefore, we calculated BCL2 expression levels as ΔCt
relative to 18S levels in tumour tissue. BCL2 mRNA
expression increased significantly with increasing Gleason grade
(median expression relative to 18S: Gleason 3+3, 0.0074% (n=12);
Gleason 3+4, 0.0148% (n=33); Gleason 4+3, 0.022% (n=31); Gleason
4+4, 0.100% (n=8); p=0.173. Expression was also stronger in tumours
with extension beyond the prostate (pT3, median, 0.0488%) in
comparison to those confined to the prostate (pT2, median, 0.0148%)
(p=0.0399). There was no significant difference between those with
(Pn1, median, 0.0172%) and without perineural invasion (Pn0,
median, 0.0118%) (Fig. 3A).
BCL2 levels did not correlate to tumour size either.
Although there was no linear correlation between
miR-205 and BCL2 expression, all the samples except one with high
BCL2 expression, had low (<30% of benign) miR-205 expression
(Fig. 3B).
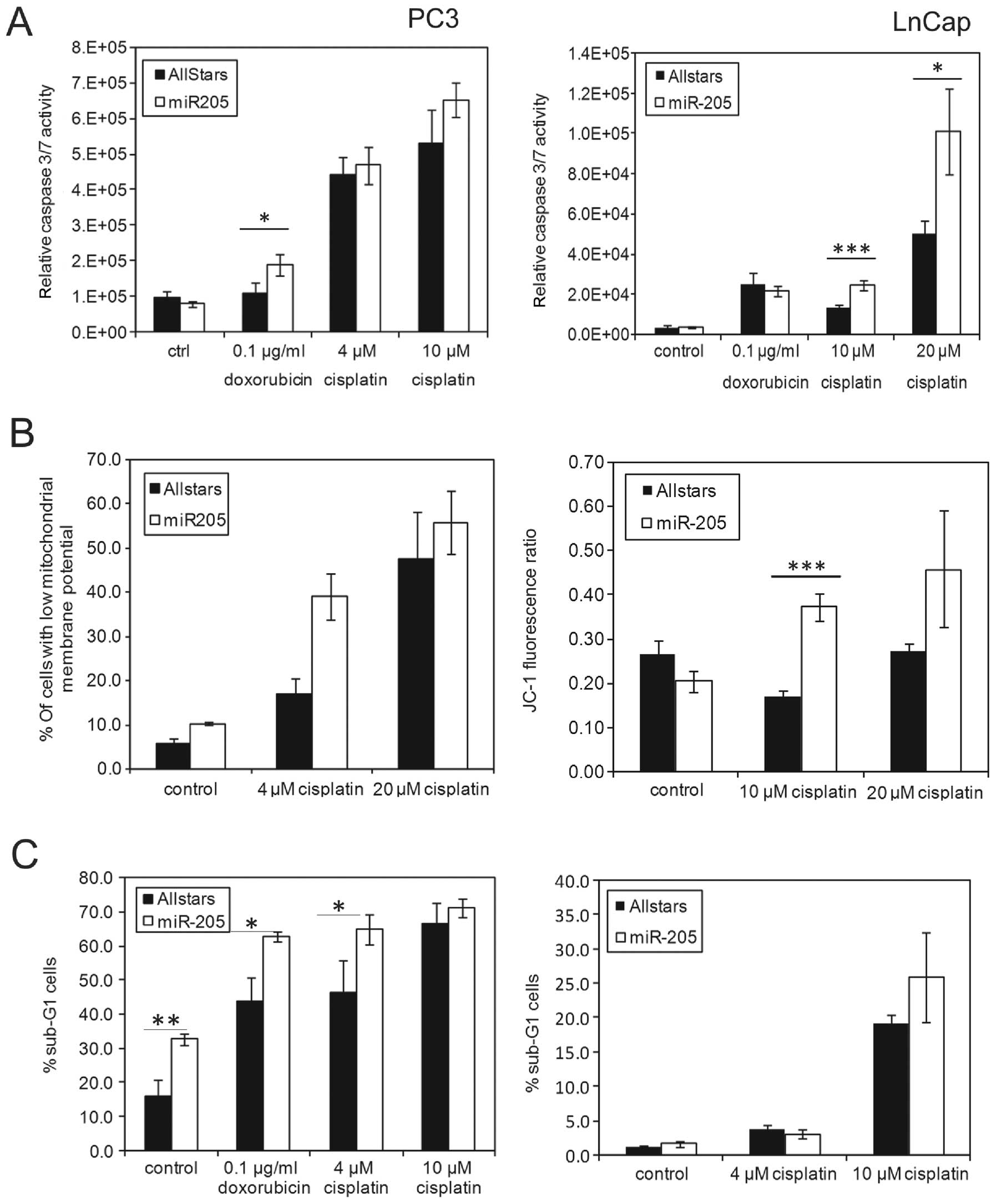
MiR-205 promotes apoptosis in prostate
cancer cells
Having confirmed anti-apoptotic gene BCL2 as
a miR-205 target, we investigated the effect of miR-205
overexpression on the induction of cell death in prostate cancer
cells. We measured caspase 3/7 activity in cells transfected with
miR-205 mimics after treatment for 24 h with 0.1 μg/ml
doxorubicin (0.1724 μM), 4 and 10 μM cisplatin (PC3)
or 10 and 20 μM (LnCap) cisplatin. A consistent increase in
caspase activity was observed in cells transfected with miR-205 in
comparison to control oligos in both cell lines in combination with
cisplatin treatment (Fig. 4A).
Cisplatin (4 μM) does not induce significant cell death in
LnCap cells, therefore higher doses were used in this cell line
(Fig. 4B and data not shown).
Doxorubicin only caused a significant difference in PC3 cells in
this assay.
As a marker for apoptosis, we determined the sub-G1
fraction by flow cytometry 48 h after genotoxic treatment. We
observed a significant increase in the fraction of apoptotic cells
both in control cells and cells treated with doxorubicin or 4
μM cisplatin (Fig. 4C) in
PC3. LnCap shows the strongest increase in apoptosis at 10
μM cisplatin concentration in combination with miRNA205
mimics. In these cells there was no increase in the spontaneous
apoptosis rate after miR-205 transfection, in contrast to PC3.
Apoptosis is also characterized by loss of mitochondrial membrane
potential (ΔΨM), which can be measured through a change in the
fluorescence spectrum of the JC-1 dye. Loss of ΔΨM was
significantly increased in LnCap cells transfected with miR-205
after treatment with 10 μM cisplatin, as compared to control
transfected cells, confirming the results for caspase 3/7
activation (Fig. 4B).
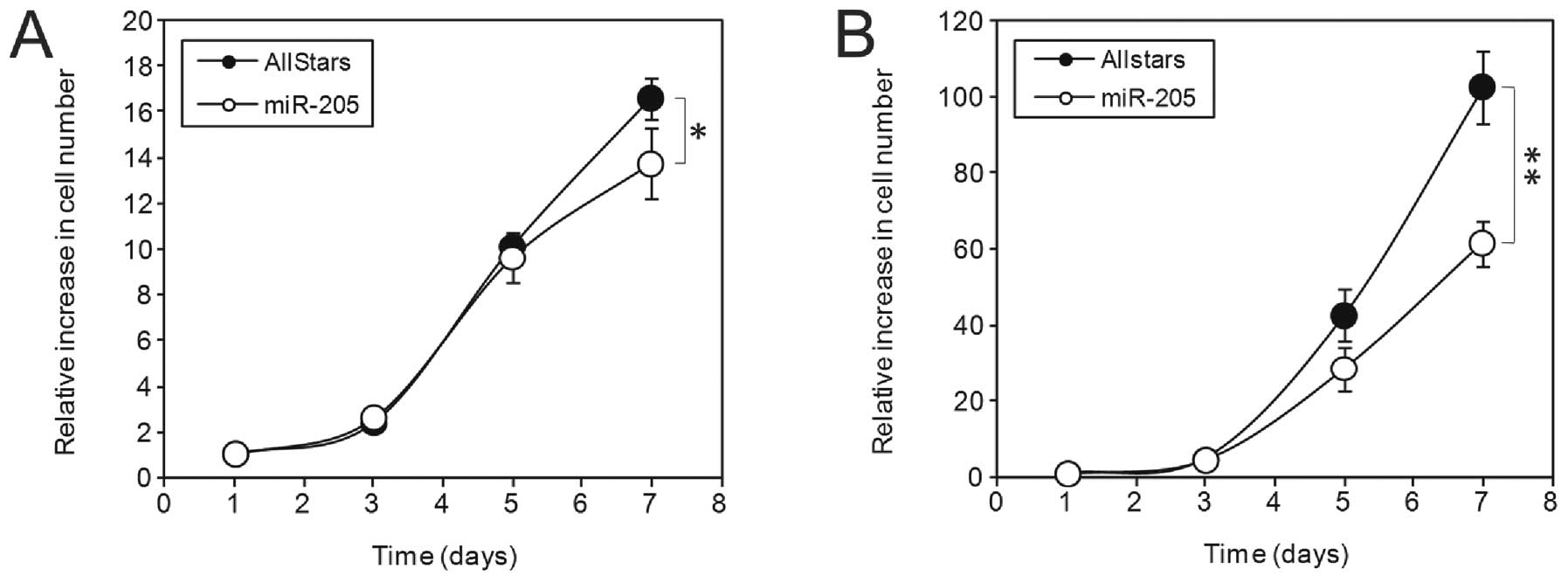
MiR-205 inhibits cell division in
prostate cancer cells
We next determined the effect of miR-205
overexpression on cell growth. For this, PC3 and LnCap cells were
transfected with either AllStars negative control siRNA or miR-205
mimics and then plated out at low densities. An MTT assay was
performed at the starting point and at 3, 5 and 7 days thereafter.
We observed a significantly lower cell number in the miR-205
transfected cells (p<0.05), in comparison to control transfected
cells at 7 days (Fig. 5) in both
cell lines.
Discussion
In this study, we describe the downregulation of the
microRNA miR-205 in prostate cancer, which is known to be involved
in the epithelial to mesenchymal transition (1,20).
In our series of samples, expression of miR-205 was reduced in 76
of 84 prostate cancer specimens, with a median relative expression
level of 18.0% in tumour tissue compared to benign tissue of the
same patient. This finding is in accordance with earlier data from
microarray experiments (7,8) and from qRT-PCR in a smaller series of
patients (31). The level of
miR-205 correlated inversely with tumour size, which fits with the
growth inhibitory effects we observed for this microRNA. Moreover,
there was a trend for miR-205 expression to decrease with
increasing Gleason grade from 7a (3+4) to 8 (4+4). This is
noteworthy as it corresponds to the worse prognosis of patients
with primarily Gleason grade 4 as opposed to primarily Gleason
grade 3 (32).
The role of miR-205 in cancer development depends on
the tissue type, as it has been found to be either over-expressed
or downregulated, depending on the tumour origin. Its functions
include the prevention of epithelial to mesenchymal transition,
which is associated with metastasis, through repression of ZEB1 and
ZEB2 and consequent high E-cadherin levels (reviewed in ref.
1). On the other hand, several of
its described targets, such as E2F1 (14), PTEN (33), BCL2L2 (BCL-W) (20) and PKCε (31) are involved in cell growth and
survival, whereas VEGFA (13) is an important factor in
angiogenesis.
In the present study, we describe the anti-apoptotic
protein BCL2 as a novel target of miR-205. This protein is likely
an important target in prostate cancer, as high BCL2 expression in
primary prostate cancer is a marker for poor prognosis, with an
increased risk for recurrence (21,22,24,34).
Biochemical recurrence during anti-androgen therapy is frequently
associated with increased BCL2 expression (35,36).
Loss of miR-205 may play a role in androgen independence, as one
study reported reduced expression only in androgen-independent
tumours (7), which would then
correspond to higher BCL2 levels.
The regulation of BCL2 expression in prostate cancer
is complex. It is a known target of several other microRNAs, such
as miR-15, miR-16 (37) and the
miR-34-family (38,39). A different and independent
mechanism for low BCL2 expression in prostate cancer is methylation
of the BCL2 promoter (30,40), which would explain why many cases
do not express BCL2 at all, whereas those that express it, are
strongly positive. Gene rearrangements of BCL2 appear to be rare in
prostate cancer (21). Taken
together, these factors may explain the poor correlation between
BCL2 levels in vivo in our samples, in contrast to
the in vitro results in PC3 and LnCap cells. Further
research will be needed to clarify this issue.
With BCL2 as a target, in combination with the
expected effects of previously published targets like E2F1, we
found that overexpression of miR-205 in prostate cancer cell lines
decreases cell division and increases apoptosis in response to
chemotherapeutical agents. Moreover, these data are in accordance
with results in different prostate cancer cell lines after
treatment with cisplatin and docetaxel. The authors attribute their
results to repression of BCL-W (encoded by BCL2L2), which is
another anti-apoptotic member of the BH3 family (20). As we confirmed the repression of
the anti-apoptotic BCL2 by miR-205, the observed effects may also
in part be caused by this protein.
In conclusion, we identified the downregulation of
miR-205 in prostate cancer and describe BCL2 as a novel
target of this microRNA, with potential prognostic and therapeutic
implications. Increased expression of miR-205 was found to inhibit
cell growth and to sensitize cells to apoptosis, caused by
chemotherapeutical agents in a BCL2-dependent manner.
Acknowledgements
This study was funded by the Ministry
for Innovation, Science and Research of Nordrhein-Westfalen and by
the Protein Research Unit Ruhr within Europe, PURE.
References
|
1.
|
Gregory PA, Bracken CP, Bert AG and
Goodall GJ: MicroRNAs as regulators of epithelial-mesenchymal
transition. Cell Cycle. 7:3112–3118. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Puhr M, Hoefer J, Schafer G, et al:
Epithelial-to-mesenchymal transition leads to docetaxel resistance
in prostate cancer and is mediated by reduced expression of
miR-200c and miR-205. Am J Pathol. 181:2188–2201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sempere LF, Christensen M, Silahtaroglu A,
et al: Altered MicroRNA expression confined to specific epithelial
cell subpopulations in breast cancer. Cancer Res. 67:11612–11620.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Childs G, Fazzari M, Kung G, et al:
Low-level expression of microRNAs let-7d and miR-205 are prognostic
markers of head and neck squamous cell carcinoma. Am J Pathol.
174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wiklund ED, Bramsen JB, Hulf T, et al:
Coordinated epigenetic repression of the miR-200 family and miR-205
in invasive bladder cancer. Int J Cancer. 128:1327–1334. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Coppola V, De Maria R and Bonci D:
MicroRNAs and prostate cancer. Endocr Relat Cancer. 17:F1–F17.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Schaefer A, Jung M, Mollenkopf HJ, et al:
Diagnostic and prognostic implications of microRNA profiling in
prostate carcinoma. Int J Cancer. 126:1166–1176. 2010.PubMed/NCBI
|
|
9.
|
Chung TK, Cheung TH, Huen NY, et al:
Dysregulated microRNAs and their predicted targets associated with
endometrioid endometrial adenocarcinoma in Hong Kong women. Int J
Cancer. 124:1358–1365. 2009. View Article : Google Scholar
|
|
10.
|
Karaayvaz M, Zhang C, Liang S, Shroyer KR
and Ju J: Prognostic significance of miR-205 in endometrial cancer.
PLoS One. 7:e351582012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Matsushima K, Isomoto H, Yamaguchi N, et
al: MiRNA-205 modulates cellular invasion and migration via
regulating zinc finger E-box binding homeobox 2 expression in
esophageal squamous cell carcinoma cells. J Transl Med. 9:302011.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Markou A, Tsaroucha EG, Kaklamanis L,
Fotinou M, Georgoulias V and Lianidou ES: Prognostic value of
mature microRNA-21 and microRNA-205 overexpression in non-small
cell lung cancer by quantitative real-time RT-PCR. Clin Chem.
54:1696–1704. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wu H, Zhu S and Mo YY: Suppression of cell
growth and invasion by miR-205 in breast cancer. Cell Res.
19:439–448. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Dar AA, Majid S, de Semir D, Nosrati M,
Bezrookove V and Kashani-Sabet M: miRNA-205 suppresses melanoma
cell proliferation and induces senescence via regulation of E2F1
protein. J Biol Chem. 286:16606–16614. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Piovan C, Palmieri D, Di Leva G, et al:
Oncosuppressive role of p53-induced miR-205 in triple negative
breast cancer. Mol Oncol. 6:458–472. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Alla V, Kowtharapu BS, Engelmann D, et al:
E2F1 confers anticancer drug resistance by targeting ABC
transporter family members and Bcl-2 via the p73/DNp73-miR-205
circuitry. Cell Cycle. 11:3067–3078. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gandellini P, Profumo V, Casamichele A, et
al: miR-205 regulates basement membrane deposition in human
prostate: implications for cancer development. Cell Death Differ.
19:1750–1760. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tucci P, Agostini M, Grespi F, et al: Loss
of p63 and its microRNA-205 target results in enhanced cell
migration and metastasis in prostate cancer. Proc Natl Acad Sci
USA. 109:15312–15317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Adachi R, Horiuchi S, Sakurazawa Y,
Hasegawa T, Sato K and Sakamaki T: ErbB2 down-regulates
microRNA-205 in breast cancer. Biochem Biophys Res Commun.
411:804–808. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Bhatnagar N, Li X, Padi SK, Zhang Q, Tang
MS and Guo B: Downregulation of miR-205 and miR-31 confers
resistance to chemotherapy-induced apoptosis in prostate cancer
cells. Cell Death Dis. 1:e1052010. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Fleischmann A, Huland H, Mirlacher M, et
al: Prognostic relevance of Bcl-2 overexpression in surgically
treated prostate cancer is not caused by increased copy number or
translocation of the gene. Prostate. 72:991–997. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Cho IC, Chung HS, Cho KS, et al: Bcl-2 as
a predictive factor for biochemical recurrence after radical
prostatectomy: an interim analysis. Cancer Res Treat. 42:157–162.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Schaefer A, Jung M, Miller K, et al:
Suitable reference genes for relative quantification of miRNA
expression in prostate cancer. Exp Mol Med. 42:749–758. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Revelos K, Petraki C, Gregorakis A,
Scorilas A, Papanastasiou P and Koutsilieris M: Immunohistochemical
expression of Bcl2 is an independent predictor of
time-to-biochemical failure in patients with clinically localized
prostate cancer following radical prostatectomy. Anticancer Res.
25:3123–3133. 2005.
|
|
25.
|
Zuker M: Mfold web server for nucleic acid
folding and hybridization prediction. Nucleic Acids Res.
31:3406–3415. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Martin MM, Buckenberger JA, Jiang J, et
al: The human angiotensin II type 1 receptor +1166 A/C polymorphism
attenuates microrna-155 binding. J Biol Chem. 282:24262–24269.
2007.
|
|
27.
|
Grimson A, Farh KK, Johnston WK,
Garrett-Engele P, Lim LP and Bartel DP: MicroRNA targeting
specificity in mammals: determinants beyond seed pairing. Mol Cell.
27:91–105. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Tarasov V, Jung P, Verdoodt B, et al:
Differential regulation of microRNAs by p53 revealed by massively
parallel sequencing: miR-34a is a p53 target that induces apoptosis
and G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shen Y, Iqbal J, Huang JZ, Zhou G and Chan
WC: BCL2 protein expression parallels its mRNA level in normal and
malignant B cells. Blood. 104:2936–2939. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Carvalho JR, Filipe L, Costa VL, et al:
Detailed analysis of expression and promoter methylation status of
apoptosis-related genes in prostate cancer. Apoptosis. 15:956–965.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gandellini P, Folini M, Longoni N, et al:
miR-205 Exerts tumor-suppressive functions in human prostate
through down-regulation of protein kinase C epsilon. Cancer Res.
69:2287–2295. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wright JL SC, Lin DW, Kolb S, et al:
Prostate cancer specific mortality and Gleason 7 disease
differences in prostate cancer outcomes between cases with Gleason
4+3 and Gleason 3+4 tumors in a population based cohort. J Urol.
182:2702–2707. 2009.
|
|
33.
|
Greene SB, Gunaratne PH, Hammond SM and
Rosen JM: A putative role for microRNA-205 in mammary epithelial
cell progenitors. J Cell Sci. 123:606–618. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Yoshino T, Shiina H, Urakami S, et al:
Bcl-2 expression as a predictive marker of hormone-refractory
prostate cancer treated with taxane-based chemotherapy. Clin Cancer
Res. 12:6116–6124. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Colombel M, Symmans F, Gil S, et al:
Detection of the apoptosis-suppressing oncoprotein bc1-2 in
hormone-refractory human prostate cancers. Am J Pathol.
143:390–400. 1993.PubMed/NCBI
|
|
36.
|
McDonnell TJ, Troncoso P, Brisbay SM, et
al: Expression of the protooncogene bcl-2 in the prostate and its
association with emergence of androgen-independent prostate cancer.
Cancer Res. 52:6940–6944. 1992.PubMed/NCBI
|
|
37.
|
Cimmino A, Calin GA, Fabbri M, et al:
miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl
Acad Sci USA. 102:13944–13949. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Hagman Z, Larne O, Edsjo A, et al: miR-34c
is down regulated in prostate cancer and exerts tumor suppressive
functions. Int J Cancer. 127:2768–2776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Cole KA, Attiyeh EF, Mosse YP, et al: A
functional screen identifies miR-34a as a candidate neuroblastoma
tumor suppressor gene. Mol Cancer Res. 6:735–742. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Cho NY, Kim BH, Choi M, et al:
Hypermethylation of CpG island loci and hypomethylation of LINE-1
and Alu repeats in prostate adenocarcinoma and their relationship
to clinicopathological features. J Pathol. 211:269–277. 2007.
View Article : Google Scholar : PubMed/NCBI
|