Introduction
Oral squamous cell carcinoma (OSCC), a subtype of
head and neck cancer is the most common neoplasm of the oral
cavity, which accounts for 90% of all diagnosed oral malignancies
(1). OSCC constitutes the 8th most
frequent cancer worldwide (2) and
more than 300,000 new cases are diagnosed annually (3), whereas cancer of the head and neck
accounts for approximately 643,000 new cases and 350,000 deaths per
year (4,5). Over 85% of tumors of this region are
epithelial tumors, especially squamous cell carcinomas (HNSCCs)
(6). Chemoradiation after surgery
is the standard treatment for patients with locally advanced HNSCC,
but long-term survival in HNSCC patients is poor despite the recent
advances in cancer therapy (7).
Conventional chemotherapeutic modalities of OSCC/HNSCC consists of
treating the patients with various drugs; e.g., cisplatin,
fluorouracils, taxans, irinotecan (5) at their maximum tolerated doses (MTD),
interrupted with long break periods between successive cycles.
However, these chemotherapeutic regimens have a myriad of
side-effects including neutropenia with increased rate of
infection, diarrhea, renal failure, nausea, vomiting, dermatitis,
and mucositis (5,8,9).
Among the f luorouracil-based drugs, S-1 (Taiho
Pharmaceutical Co. Ltd., Tokyo, Japan) is potentially more active
and less toxic than of 5-fluorouracil (5-FU) and reported to be
effective against head and neck cancer with 34% response rate
(8). S-1 consists of tegafur
(prodrug of 5-FU), 5-chloro-2,4-dihydroxypyridine (CDHP; augments
the activity of 5-FU by inhibiting DPD) and potassium oxonate (Oxo;
reduces gastrointestinal toxicity by inhibiting 5-FU
phosphorylation) at a molar ratio of 1:0.4:1 (10). There are numerous reports of S-1
clinical trials on HNSCC patients either alone or in combination
with other drugs or radiotherapy (11–14),
but high doses (60–80 mg/m2) of S-1 causes both
hematological and non-hematological toxicities in patients
(8,9,11).
In contrast to the conventional MTD chemotherapy,
metronomic chemotherapy, which refers to low-dose chemotherapy
administered at a close regular intervals with no prolonged breaks
(15) has shown effective
antitumor efficacy by inhibiting tumor angiogenesis with reduced
toxicity (16,17). Metronomic low-dose chemotherapy
with S-1 in combination with other drugs is reported to be
effective in colorectal (10,18),
hepatocellular (19) carcinoma
mouse xenograft models, whereas metronomic tegafur-uracil (UFT) was
effective in breast (20,21), gastrointestinal (22), ovarian cancer (23) and lung adenocarcinoma (24). Ooyama et al (10) have shown the efficacy of metronomic
S-1 and capecitabine against human colon cancer xenografts and also
confirmed the anti-angiogenic properties of S-1, suggesting that
pharmacokinetics of S-1 might be similar to the concept of
metronomic chemotherapy. There are few reports on metronomic
chemotherapy with placitaxel (25), celecoxib and methotrexate (26) in oral/head and neck cancer.
However, no preclinical/clinical reports on S-1 metronomic
chemotherapy against OSCC/HNSCC are available. Therefore, rational
strategies for developing new metronomic protocols and schedules
with conventional drugs are necessary for gaining more favorable
outcome of OSCC treatments.
The molecular and cellular mechanisms involved in
growth, survival and expansion of solid tumors in HNSCC are not
completely understood, but it is established that formation of new
blood vessels (angiogenesis) and the co-option of existing vessels
play a central role (27). Shaked
et al (28) reported that,
metronomic chemotherapy inhibits tumor angiogenesis and tumor
growth by various mechanisms: i) killing endothelial cells through
upregulation of anti-angiogenic factors [e.g. thrombospondin-1
(TSP-1)] and downregulation of pro-angiogenic factors [e.g.
vascular endothelial growth factor (VEGF), platelet-derived growth
factor (PDGF) or hypoxia-inducible factor-1 (HIF-1)]; ii)
decreasing viability and mobilization of bone marrow-derived
circulating endothelial progenitor cells (CEPs); iii) eradication
and disruption of cancer stem cells (CSCs); iv) suppression of T
regulatory cells; and v) killing the tumor cells directly (28–30).
VEGF or VEGF-A is a key stimulator (10,31)
of angiogenesis, whereas anti-angiogenic factors TSP-1 promotes
endothelial cell apoptosis (10,32)
and can suppress the mobilization of CEPs (28). Moreover, early growth response-1
(EGR-1) plays crucial role in angiogenesis by mediating the
expression of TSP-1 (33), VEGF
(34) and fibroblast growth
factor-2 (FGF-2). Several reports on metronomic chemotherapy with
S-1, placitaxel and other drugs in colorectal, hepatocellular,
gastrointestinal and breast cancers have confirmed the association
of downregulated VEGF expression and upregulated TSP-1 expression
with the anti-angiogenic efficacy of metronomic chemotherapy
(10,19,21,22,25,35).
Reports on a wide variety of cancers have
demonstrated that only a distinct subpopulation of tumor cells, the
CSCs, have the ability to undergo self-renewal and differentiation
to initiate tumorigenesis and contribute to the recurrence and
metastasis of cancers in humans (36,37).
CSCs are also present in perivascular niches; release angiogenic
factors in hypoxic conditions, and establish a permissive vascular
niche in tumors (37). The
molecular markers of CSC, which are most commonly used to detect
tumor pathogenicity and angiogenesis in HNSCC are the cluster of
differentiation (CD)34, CD133, CD24, CD44, CD29 and CD31 markers
(38). In HNSCC, it has been shown
that CD44-positive cancer cells can initiate in vivo tumor
formation and have increased resistance to drug and radiation
therapies. CD44 overexpression is also associated with poor
prognosis (39,40). Moreover, Hollemann et al
(27) demonstrated new vessel
formation can be detected by the endothelial marker CD31 in HNSCC.
Therefore, CD31 and CD44 can be used to evaluate the degree of
tumor angiogenesis and can act as prognostic marker of OSCC
(39–42).
In this study, we used OSCC xenograft models to
clarify the suitable administration method of S-1 against oral
squamous cell carcinoma as a metronomic chemotherapy. We also
checked the efficacy of metronomic 5-FU against OSCC in
vitro. In addition, we investigated the mechanisms of the
antitumor and anti-angiogenic effects of metronomic S-1
chemotherapy by evaluating the expression of VEGF, TSP-1 and EGR-1.
The effect of metronomic S-1 on the suppression of CSC in tumors
was also evaluated by detecting the expression pattern of CD44 and
CD31. Moreover, we quantified microvessel density in tumors.
Materials and methods
OSCC cell line and nude mice
Human tongue squamous cell carcinoma (HSC2) cell
line was purchased from Cell Bank, RIKEN BioResource Center
(Ibaraki, Japan) and maintained in Dulbecco’s modified Eagle’s
medium (DMEM) (Sigma-Aldrich, St. Louis, MO, USA) supplemented with
10% fetal bovine serum (FBS) (Invitrogen, Carlsbad, CA, USA), 100
μg/ml streptomycin, 100 U/ml penicillin (Invitrogen) in a
humidified atmosphere containing 5% CO2. Four-week-old
female CAnN. Cg-Foxnlnu/CrlCrlj athymic nude mice (average weight
15.0 g) were purchased from CLEA Japan Co. Ltd. (Tokyo, Japan). The
mice were maintained under specific-pathogen-free conditions and
provided with sterile water and food ad libitum. All
manipulations were carried out aseptically inside a laminar flow
hood. The mice were maintained and handled in accordance with the
Guidelines for Animal Experimentation of Yamaguchi University.
Examination of antitumor activity of
metronomic S-1 in vivo
HSC2 cells (1×106) were suspended in 0.1
ml of serum-free medium and injected into the subcutaneous tissue
of 5-weeks-old mice using a 27-gauge needle. When the estimated
tumor volume (0.5 × length × width2) reached 100–150
mm3, the tumor-bearing mice were randomly divided into
one control and three treatment groups (5 mice/group). HSC2 tumors
were treated with three different regimens of S-1 (6.9 mg/kg)
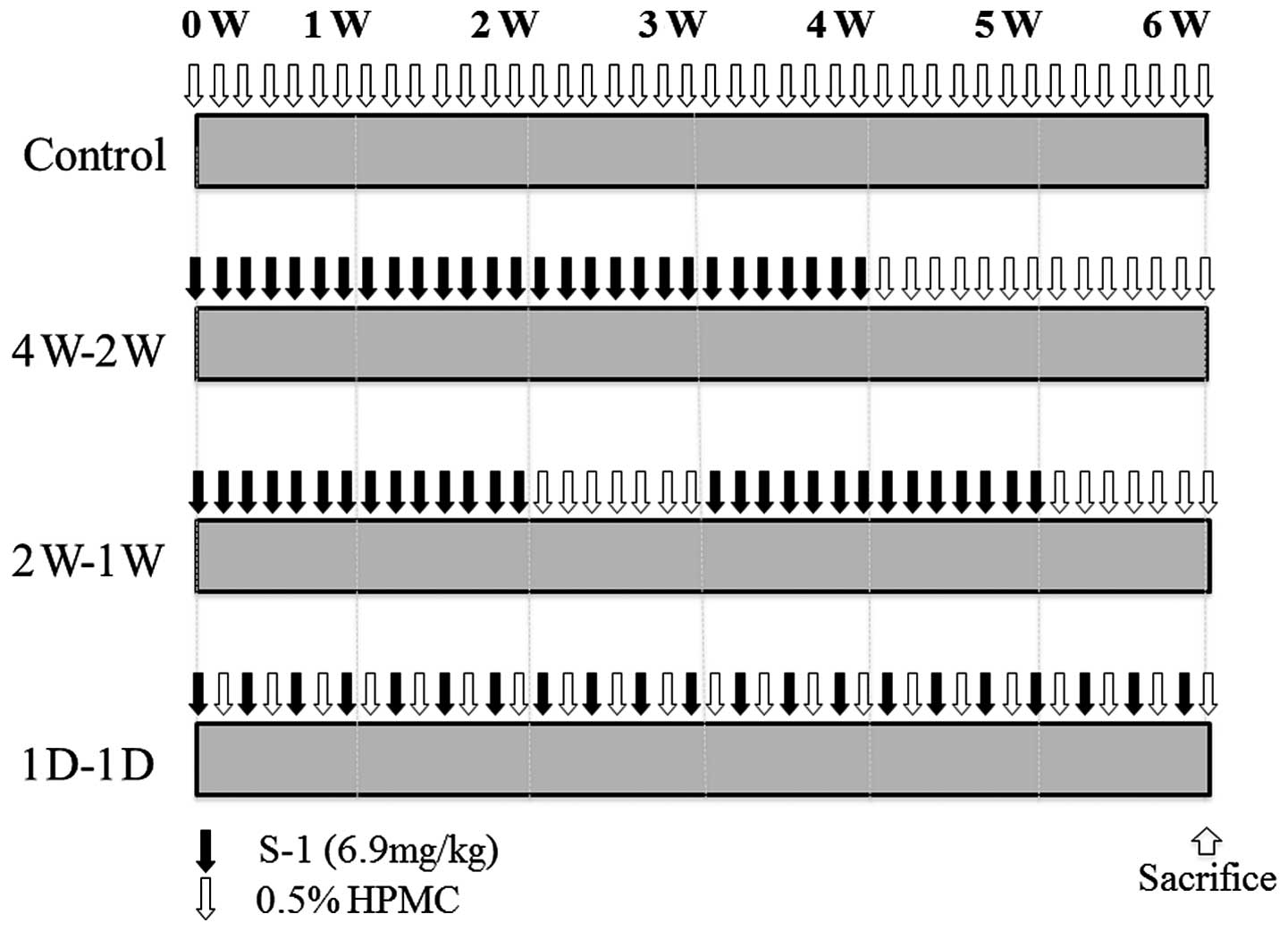
dissolved in 0.5% hydroxypropyl methylcellulose (HPMC). The
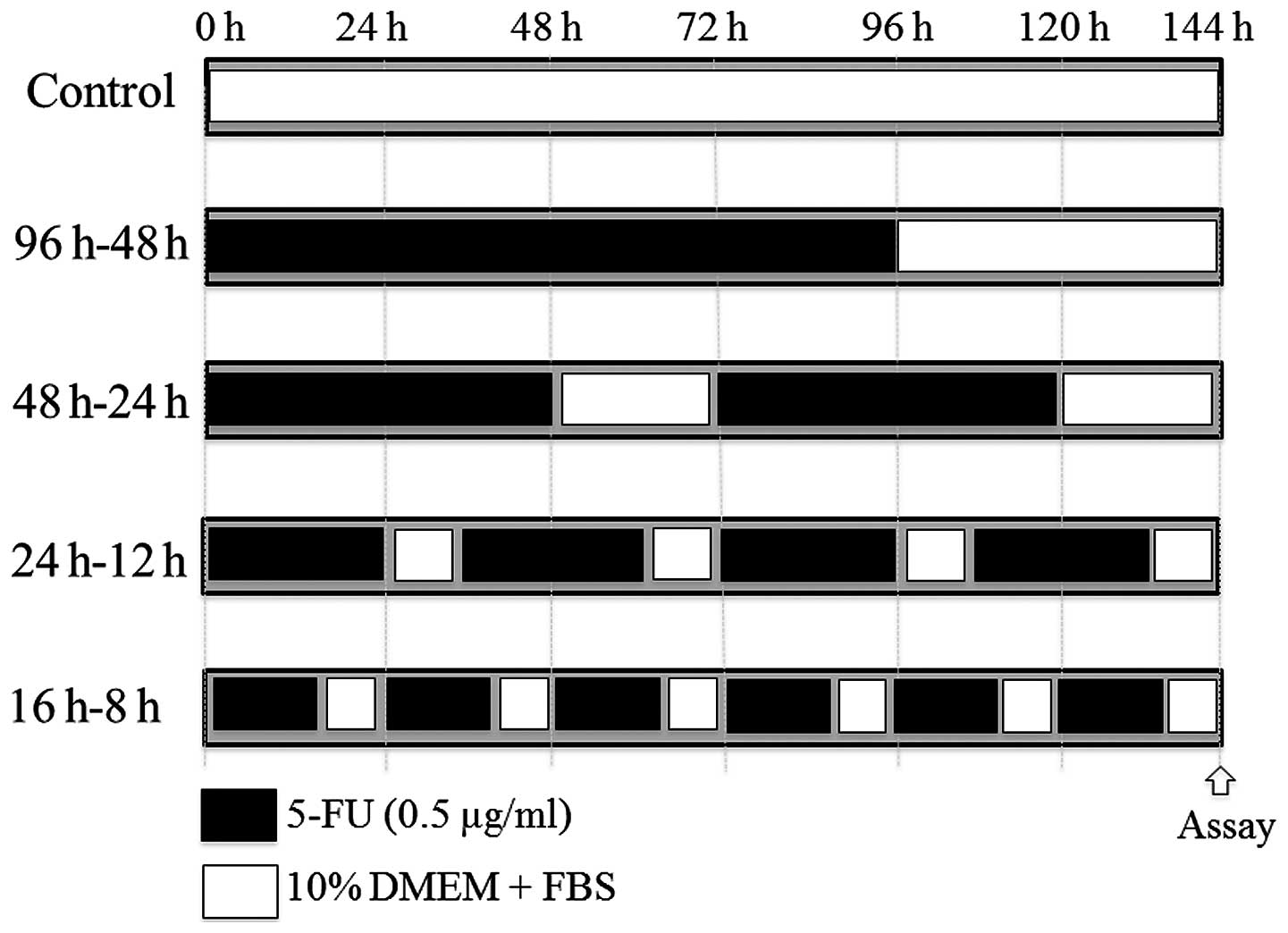
treatment groups were: group 1 (4W-2W; 1 cycle); S-1 administered
for four weeks with 2-week rest, group 2 (2W-1W; 2 cycles); 2-week
treatment with S-1 and 1-week rest, and group 3 (1D-1D; 6 weeks);
treatment in alternate days with S-1. HPMC (0.5%) was administered
to group 4, the control group (Fig.
1). Tumor size and body weight were monitored and measured
twice/week. All mice were sacrificed at the end of each treatment
regimen. Antitumor effects and body weight changes were compared
among the groups.
Immunohistochemical assay for EGR-1,
TSP-1, VEGF, CD44 and CD31
HSC2 tumors harvested at autopsy were embedded in
paraffin blocks. Four-micrometer-thick sections were prepared from
the blocks and mounted on slides. These sections were fixed and
then processed for immunostaining using the anti-EGR-1 mouse
monoclonal antibody (Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA), anti-TSP-1 mouse monoclonal antibody (Santa Cruz), anti-VEGF
mouse monoclonal antibody (Santa Cruz), anti-HCAM (CD44) mouse
monoclonal antibody (Santa Cruz), or anti-PCAM (CD31) rabbit
polyclonal antibody (Santa Cruz), and appropriate peroxidase
conjugated anti-rabbit or mouse IgG second antibody. Negative
controls were done using non-specific IgG. The blocking and
immunostaining were performed using Dako EnVision kit (Dako,
Glostrup, Denmark). All the specimens were counterstained with
hematoxylin. The slides were then examined under a bright-field
microscope. A positive reaction was detected in the cytoplasm or
nucleus as reddish-brown precipitates. For quantification of
microvessel density (MVD), CD31-positive vessels were counted in
randomly selected 10 areas per tumor in each treatment group at
200-fold magnification.
Examination of antitumor activity of
metronomic 5-FU in vitro
HSC2 cells (5×103 cells per well) were
seeded on 96-well plates (Becton-Dickinson Labware, Franklin Lakes,
NJ, USA) in DMEM supplemented with 10% FBS. Twenty-four hours
later, the cells were treated with four different regimens using
0.5 μg/ml 5-FU. The treatment regimens were: 96-h treatment
and 48-h rest (96 h-48 h, 1 cycle); the 48-h treatment and 24-h
rest (48 h-24 h; 2 cycles); the 24-h treatment and 12-h rest (24
h-12 h; 4 cycles); and the 16-h treatment and 8-h rest (16 h-8 h; 6
cycles). A fifth group served as control (Fig. 5). At the end of each treatment,
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide
(MTT) was added to each well (25 μl/well) of the 96-well
plate and incubated for 4 h. The blue dye taken up by cells was
dissolved in dimethyl sulfoxide (100 μl/well), and the
absorbance was measured with a spectrophotometer (Bio-Rad
Laboratories, Hercules, CA, USA) at 490 nm. Growth inhibitory
effects were compared among the groups. All assays were run in
triplicate.
Western blot analysis for TSP-1, EGR-1,
VEGF and CD44 in vitro
Control and 5-FU-treated HSC2 cells were lysed with
RIPA Buffer (Thermo Scientific, Kanagawa, Japan). Whole cell
lysates containing 50 μg protein/sample were subjected to
electrophoresis on 10% SDS-polyacrylamide gels, and then
transferred to a PVDF membrane. The membranes were blocked and
incubated with the anti-EGR-1 mouse monoclonal antibody (Santa
Cruz), anti-TSP-1 mouse monoclonal antibody (Santa Cruz), anti-VEGF
mouse monoclonal antibody (Santa Cruz), or anti-HCAM (CD44) mouse
monoclonal antibody (Santa Cruz). All antibodies were detected
using Western Breeze chromogenic immunodetection system
(Invitrogen) according to the manufacturer’s instructions. Also,
anti-α-tubulin monoclonal antibody (Santa Cruz) was used for
normalization of western blot analysis.
Statistics
The significance of the in vivo and in
vitro results was determined by the Mann-Whitney U test or
one-way ANOVA. The differences were considered statistically
significant at P<0.05.
Results
Evaluation of the antitumor effect of
metronomic S-1 chemo-therapy in vivo
An in vivo experiment was carried out with
HSC2 tumor-bearing nude mice to examine the antitumor activity of
four different treatment regimens of low dose metronomic S-1 (6.9
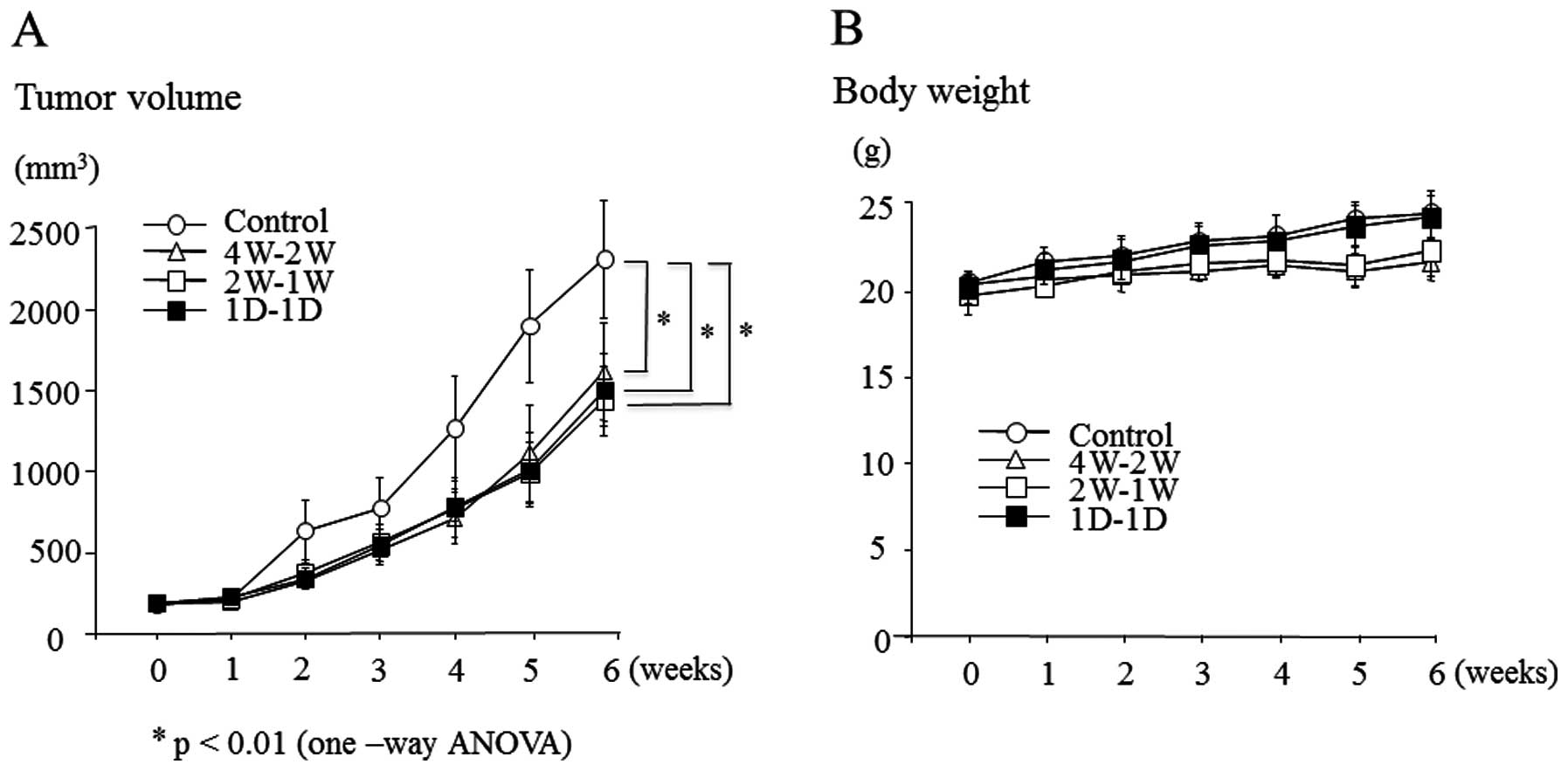
mg/kg). The treatment regimens and schedules are showed in Fig. 1. As shown in Fig. 2A, all treated-groups showed
significant tumor growth inhibition compared to the control group
(P<0.01). However, the relative tumor growth inhibition was not
significantly different between the treated groups. Briefly, each
relative tumor growth inhibition was 32.4% (4W-2W), 39.6% (2W-1W)
and 37.0% (1D-1D). Neither treatment regimen induced a significant
body weight loss during treatment periods. However, body weights
were lower in the mice with 4W-2W or 2W-1W, than 1D-1D or control
group (Fig. 2B).
Evaluation of expression of EGR-1, TSP-1,
VEGF, CD44 and CD31 in tumor tissues in vivo by
immunohistochemistry and quantification of MVD
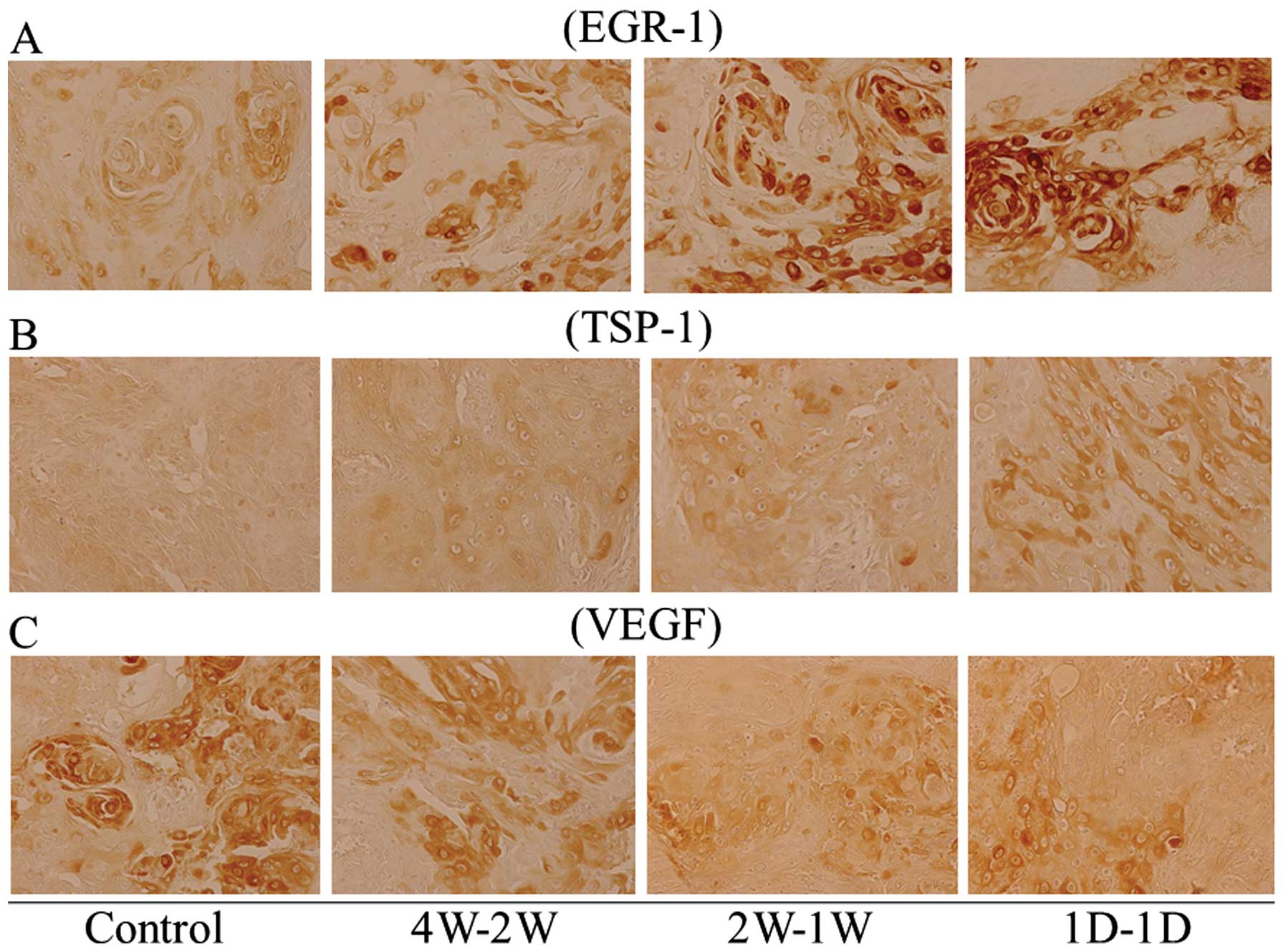
We carried out immunohistochemistry experiments to
evaluate the expression pattern of angiogenic and anti-angiogenic
factors in mouse tumors treated with metronomic S-1. The expression
of anti-angiogenic factors, TSP-1 and EGR-1 was markedly induced in
1D-1D-treated tumors compared to 4W-2W-, 2W-1W-treated tumors or
untreated control tumors (Fig. 3A and
B). The expression level of VEGF, a key angiogenic factor was
also evaluated. A significant reduction of VEGF expression was
observed in 1D-1D-treated mouse tumors than 4W-2W, 2W-1W or
untreated control (Fig. 3C).
Moreover, in order to examine the efficacy of metronomic S-1 in the
suppression of CSCs, the expression of cancer stem cell factor CD44
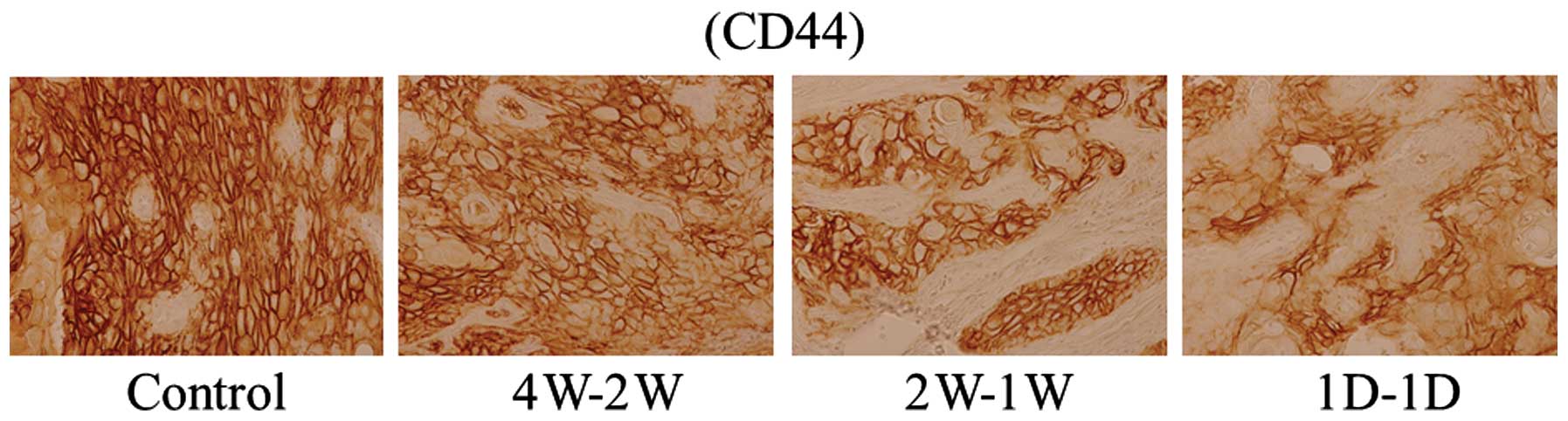
was evaluated. Downregulated expression of CD44 was observed in
treated mouse tumors compared with the untreated control, with
1D-1D-treated tumors showing the most reduced expression of CD44
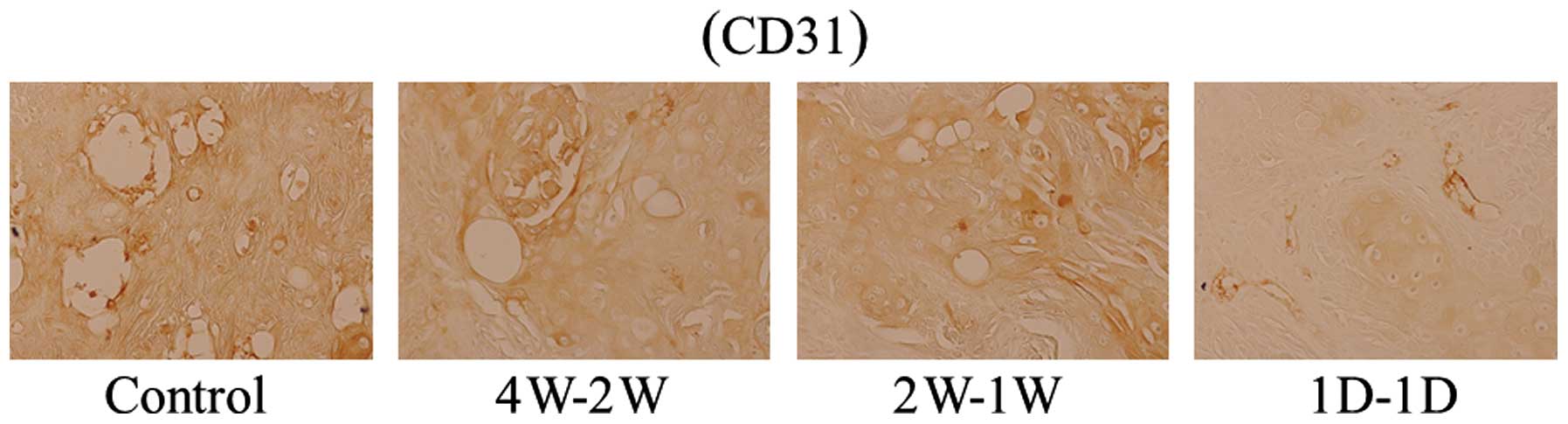
compared with 4W-2W- and 2W-1W-treated tumors (Fig. 4). Expression of endothelial marker
CD31 was also decreased in all treatment groups compared with the
untreated control, with marked reduction of CD31 expression in
1D-1D-treated tumors (Fig. 5).
Accordingly, MVD was significantly reduced in all
three treated groups compared with the control (P<0.01).
However, reduction of MVD was significantly higher in 1D-1D
treatment group compared with the other treatment groups (Table I).
 | Table I.Microvessel density in tumors. |
Table I.
Microvessel density in tumors.
| Treatment | Vessels |
|---|
| Control | 14.6±3.75 |
| 4W-2W | 8.80±1.32a |
| 2W-1W | 6.90±1.45a |
| 1D-1D |
3.40±1.51a,b |
Evaluation of growth inhibitory effect of
metronomic 5-FU in vitro
To further examine the efficacy of metronomic S-1
chemotherapy against HSC2 cells, we investigated the growth
inhibitory effect of low dose metronomic 5-FU (0.5 μg/ml)
in vitro. HSC2 cells were treated with four different
treatment regimens as shown in Fig.
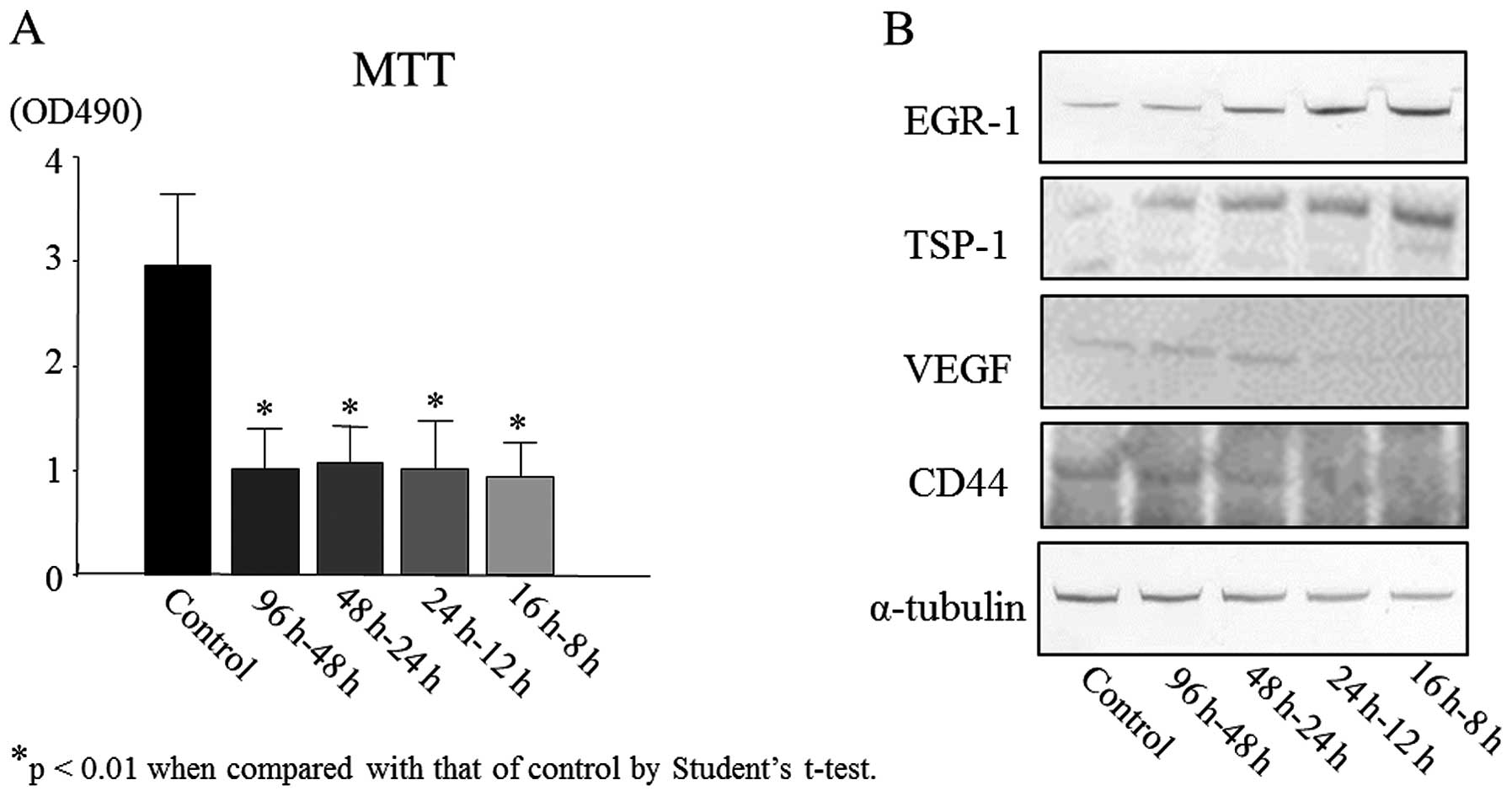
6. As observed by MTT assay, 96 h-48 h, 48 h-24 h, 24 h-12 h
and 16 h-8 h treatment groups showed significant (P<0.01) growth
inhibition of HSC2 cells compared with the untreated control
(Fig. 7A). There was no
significant difference among the treatment groups. However, the
highest inhibition of cell growth was detected in 16 h-8 h
treatment group (Fig. 7A).
Evaluation of expression of EGR-1, TSP-1,
VEGF and CD44 in vitro by western blot analysis
Western blot analysis results in Fig. 7B show the expression of EGR-1,
TSP-1, VEGF and CD44 in treated tumor tissues. The expression level
of EGR-1 was relatively upregulated in 48 h-24 h, 24 h-12 h and 16
h-8 h treatment groups compared to the control. Moreover, compared
with the control, the expression of TSP-1 was increased markedly in
all four treatment groups (96 h-48 h, 48 h-24 h, 24 h-12 h and 16
h-8 h). In 16 h-8 h group, the expression of TSP-1 was highest
which was approximately 3- to 4-fold increased expression to that
of control (Fig. 7B). In contrast
to EGR-1 and TSP-1 expression, VEGF expression was considerably
downregulated in 24 h-12 h and 16 h-8 h treatment groups compared
with 96 h-48 h, 48 h-24 h treatment groups or control. Similarly,
the expression of cancer stem factor CD44 decreased slightly in 96
h-48 h, 48 h-24 h treatment groups compared with the control, while
24 h-12 h and 16 h-8 h treatment groups showed significantly
reduced expression of CD44. Among all the treatment groups, the 16
h-8 h treatment group showed highest expression of EGR-1 and TSP-1,
and lowest expression of VEGF and CD44 compared to control
(Fig. 7B).
Discussion
In our present study, we evaluated the efficacy of
metronomic chemotherapy with low dose S-1 against OSCC both in
vivo and in vitro. Metronomic chemotherapy, which refers
to the administration of comparatively low doses of drugs on a
frequent schedule, with no extended interruptions lacks the acute
toxicity of conventional MTD chemotherapy, but effective even in
low doses against tumor growth and expansion because it inhibits
angiogenesis and decreases nutrition supply in tumors (16,22).
The anti-angiogenic efficacy of metronomic chemotherapy is reported
to increase when it is administered in combination with specific
anti-angiogenic drugs and molecularly targeted therapies (23,24).
Metronomic chemotherapy can be administered orally and is quite
effective as adjuvant chemotherapy (16,20,24).
Being less toxic, it is effective in palliative care (22,24)
and can ensure normal oral functions in OSCC patients. Moreover,
because of its anti-angiogenic property, metronomic chemotherapy
might not induce drug-resistance in tumors (32,43).
Iwamoto et al (19)
reported that hepatocellular tumor cell line that was intrinsically
resistant to high concentration of fluorouracils in vitro,
responds well to metronomic low dose S-1.
There are pre-clinical and clinical reports on the
efficacy and anti-angiogenic property of metronomic S-1 and UFT
against various cancers (11,19–25).
Kato et al (24) reported a
beneficial effect of low dose UFT as adjuvant chemotherapy in 999
lung adenocarcinoma (stage I) patients. In that study, UFT (250
mg/m2/day) was administered twice daily for two years,
which significantly improved overall survival of the patients with
grade II adverse effects/toxicity in only 2% of the patients. Kato
et al (24) also stated
that, as fluorouracil is not a dose-dependent but a time-dependent
drug and acts as anti-angiogenic agent, daily, low-dose, long-term
administration of uracil-tegafur might be effective. Ooyama et
al (10) and Iwamoto et
al (19) have reported
beneficial effects of metronomic S-1 in combination with
anti-angiogenic agents against colon and hepatocellular cancer
xenografts. Placitaxel is also effective as metronomic chemotherapy
(25,44). A study conducted with 722
metastatic breast cancer patients who received 90 mg of
paclitaxel/m2 for 3 days/4 weeks with 10 mg of
bevacizumab reported significantly prolonged progression-free
survival of the patients with minimal toxicity (44).
Our results suggest that, administration of
metronomic 6.9 mg/kg S-1 in alternate days (1D-1D) for 6 weeks is
effective against OSCC tumors than treatment regimens with long
intervals (4W-2W or 2W-1W), as 1D-1D markedly exert anti-angiogenic
effects and reduce the expression of cancer stem cell markers. In
our study, we chose S-1 over UFT because administration of S-1 in
cancer patients often resulted in high concentration of 5-FU in
blood and tumor tissue for long-term periods due to its biochemical
modulation (19). Thus, S-1 might
have stronger antitumor effect than UFT. Moreover, previous reports
on nude mice xenograft experiments have confirmed the efficacy of
S-1 in OSCC tumor growth inhibition, apoptosis and angiogenesis
in vivo (45,46).
According to literature, metronomic chemotherapy
inhibits tumor growth by targeting genetically stable endothelial
cells within the vascular bed in tumors, upregulating
anti-angiogenic factors (i.e. TSP-1, EGR-1) and downregulating
angiogenic factors (i.e. VEGF), killing or disrupting
pro-angiogenic bone marrow-derived cells, CEPs and CSCs (16,28–30).
In our study, metronomic S-1, especially 1D-1D upregulated
anti-angiogenic factors, TSP-1 and EGR-1 in mouse tumors. There are
several reports that describe similar expression pattern of TSP-1
and EGR-1 in colorectal, gastrointestinal, hepatocellular cancer
after administration of S-1, UFT or 5-FU (10,19,22,47).
Allegrini et al (22) and
Caballero et al (25)
reported reduced expression of VEGF in gastrointestinal cancer
treated with metronomic UFT and in HNSCC treated with metronomic
placitaxel, respectively. We also observed downregulated expression
of VEGF in all treatment groups of metronomic S-1, but the
expression was reduced more markedly in tumors treated with 1D-1D.
However, this finding contradicts with the results of Ooyama et
al (10) and Iwamoto et
al (19), as they reported no
significant difference of VEGF expression between control and
metronomic S-1-treated tumors. Bancroft et al (48) have shown that, expression of VEGF
in HNSCC cell lines is associated with co-activation of both
nuclear factor (NF)-κB and activator protein-1 (AP-1). Since 5-FU
and S-1 are reported to suppress the expression of NF-κB (49,50)
and VEGF (50) in OSCC cell lines,
metronomic S-1 doses are expected to deliver similar results.
There are several factors other than TSP-1 and
EGR-1, which act as inhibitors of angiogenesis, i.e. interferons,
TGF-β, angiostatin, and endostatin. Moreover, VEGF is the major
contributor of angiogenesis, and that bFGF, FGF, angiogenin,
hepatocyte growth factor (HGF) and interleukin 8 (IL-8) is also
known as stimulator of angiogenesis. In this study, we examined the
expression of VEGF, TSP-1 and EGR-1 only. Further studies with
other angiogenic and anti-angiogenic factors might help to clarify
the efficacy and anti-angiogenic property of metronomic S-1 against
OSCC.
We also examined the expression of cancer stem cell
marker CD44, endothelial marker CD31, and MVD in tumors. Ooyama
et al (10) and Iwamoto
et al (19) showed
downregulated CD31 expression and reduced number of microvessels in
colon and hepatocellular tumors after treatment with metronomic
S-1. Similarly, in our in vivo study, metronomic S-1 notably
downregualted the expression of CD44 and CD31, and significantly
reduced MVD in 1D-1D compared to the other treatment groups.
Moreover, in order to clarify the efficacy of
metronomic S-1 in vitro, we carried out growth inhibition
assay with metronomic, low dose 5-FU (0.5 μg/ml) and
evaluated the expression of TSP-1, EGR-1, VEGF and CD44 in
5-FU-treated OSCC cells. In our in vitro study, metronomic
5-FU treatment regimens significantly inhibited OSCC tumor growth
compared with the control group. Although, growth inhibition
pattern of tumors were almost same among the 5-FU treatment
regimens, we concluded that 16-h treatment and 8-h rest regimen (16
h-8 h; 6 cycles) is more effective against OSCC since it noticeably
induced the expression of anti-angiogenic factors TSP-1 and EGR,
and reduced the expression of angiogenic factor VEGF, and cancer
stem cell marker CD44. Our experiment results in vitro
regarding the expression of TSP-1. EGR-1, VEGF and CD44 coincided
with our in vivo data with metronomic S-1.
Preclinical and clinical evidence suggests that, a
drug that is administered at a specific point of the circadian
clock of a patient which is designed to target specific cell cycle
events or angiogenesis might be more effective against tumor
progression and can achieve patient’s maximum tolerance towards the
toxicity of the drug (51).
Chronomodulated delivery of fluorouracil at the early part of the
resting period allowed better control of the plasma concentrations
of the drug and showed less toxicity in patients (51). Therefore, close attention on
designing the drug delivery scheduling of metronomic chemotherapy
will accelerate favorable outcome of the patients.
In the present study, although the different
scheduling of metronomic S-1 delivery did not show significant
difference in tumor growth both in vivo and in vitro,
schedules with more frequent drug administration and shorter
resting period (1D-1D, 16 h-8 h treatment regimens) was most
effective against OSCC as it induced the expression of
anti-angiogenic factors, downregulated the expression of angiogenic
factors and suppressed the cancer stem cell markers. In future,
in vivo toxicological study will be carried out to
investigate the possible adverse effects of metronomic S-1 among
the treatment groups.
In conclusion, we have demonstrated the efficacy of
metronomic low dose S-1 and 5-FU against OSCC preclinically. Our
data suggests that, the efficacy of metronomic S-1 chemotherapy not
only lies on the cytotoxic effect of S-1, but also its inhibitory
action on angiogenesis and suppression of cancer stem cells.
Therefore, metronomic S-1 might be a promising strategy for OSCC
treatment. Further studies with metronomic S-1 in combination with
various anti-angiogenic agents (sorafenib, bevacizumab, irinotecan,
semaxinib, sunitinib) might deliver more promising results for the
treatment of oral cancer.
Acknowledgements
This study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Education, Science and
Culture.
References
|
1.
|
Erdem NF, Carlson ER, Gerard DA and Ichiki
AT: Characterization of 3 oral squamous cell carcinoma cell lines
with different invasion and/or metastatic potentials. J Oral
Maxillofac Surg. 65:1725–1733. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Exarchos KP, Goletsis Y and Fotiadis DI: A
multiscale and multiparametric approach for modeling the
progression of oral cancer. BMC Med Inform Decis Mak. 12:1362012.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Tanaka T, Tanaka M and Tanaka T: Oral
carcinogenesis and oral cancer chemoprevention: a review. Patholog
Res Int. 2011:4312462011.PubMed/NCBI
|
|
4.
|
Garg P and Karjodkar F: ‘Catch them before
it becomes too late’ - oral cancer detection. Report of two cases
and review of diagnostic AIDS in cancer detection. Int J Prev Med.
3:737–741. 2012.
|
|
5.
|
Wise-Draper TM, Draper DJ, Gutkind JS,
Molinolo AA, Wikenheiser-Brokamp KA and Wells SI: Future directions
and treatment strategies for head and neck squamous cell
carcinomas. Transl Res. 160:167–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sawicki M, Szudy A, Szczyrek M, Krawczyk P
and Klatka J: Molecularly targeted therapies in head and neck
cancers. Otolaryngol Pol. 66:307–312. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cripps C, Winquist E, Devries MC,
Stys-Norman D and Gilbert R; Head and Neck Cancer Disease Site
Group: Epidermal growth factor receptor targeted therapy in stages
III and IV head and neck cancer. Cur Oncol. 17:37–48.
2010.PubMed/NCBI
|
|
8.
|
Tahara M, Minami H, Kawashima M, Kawada K,
Mukai H, Sakuraba M, Matsuura K, Takashi O, Hayashi R and Ohtsu A:
Phase I trial of chemoradiotherapy with the combination of S-1 plus
cisplatin for patients with unresectable locally advanced squamous
cell carcinoma of the head and neck. Cancer Sci. 102:419–424. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Ogata Y, Sasatomi T, Akagi Y, Ishibashi N,
Mori S and Shirouzu K: Dosage escalation study of S-1 and
irinotecan in metronomic chemotherapy against advanced colorectal
cancer. Kurume Med J. 56:1–7. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ooyama A, Oka T, Zhao H, Yamamoto M,
Akiyama S and Fukushima M: Anti-angiogenic effect of
5-Fluorouracil-based drugs against human colon cancer xenografts.
Cancer Lett. 267:26–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tsukuda M, Kida A, Fujii M, Kono N,
Yoshihara T, Hasegawa Y and Sugita M; Chemotherapy Study Group of
Head and Neck Cancer: Randomized scheduling feasibility study of
S-1 for adjuvant chemotherapy in advanced head and neck cancer. Br
J Cancer. 93:884–889. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Tsukuda M, Ishitoya J, Mikami Y, Matsuda
H, Horiuchi C, Taguchi T, Satake K, Kawano T, Takahashi M,
Nishimura G, Kawakami M, Sakuma Y, Watanabe M, Shiono O, Komatsu M
and Yamashita Y: Analysis of feasibility and toxicity of concurrent
chemoradiotherapy with S-1 for locally advanced squamous cell
carcinoma of the head and neck in elderly cases and/or cases with
comorbidity. Cancer Chemother Pharmacol. 64:945–952. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kuratomi Y, Satoh S, Monji M, Yokogawa K,
Suzuki K, Shimazu R, Tokumaru S and Inokuchi A: A comparative study
of concurrent chemoradiotherapy with S-1 or CDDP for pharyngeal or
laryngeal cancer. Gan To Kagaku Ryoho. 37:1471–1476. 2010.(In
Japanese).
|
|
14.
|
Kubota K, Sato H, Sakaki H, Nakagawa H,
Kon T, Narita N, Kobayashi W and Kimura H: A case of submandibular
gland cancer in elderly patients showed significant effect by S-1
and intravenous docetaxel chemotherapy concurrent with
radiotherapy. Gan To Kagaku Ryoho. 37:1937–1940. 2010.(In
Japanese).
|
|
15.
|
Abu Lila AS, Okadaa T, Doia Y, Ichiharaa
M, Ishidaa T and Kiwadaa H: Combination therapy with metronomic S-1
dosing and oxaliplatin-containing PEG-coated cationic liposomes in
a murine colorectal tumor model: Synergy or antagonism? Int J
Pharmaceutics. 426:263–270. 2012.
|
|
16.
|
Kerbel R and Kamen B: The anti-angiogenic
basis of metronomic chemotherapy. Nat Rev Cancer. 4:423–436. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Man S, Bocci G, Francia G, Green SK, Jothy
S, Hanahan D, Bohlen P, Hicklin DJ, Bergers G and Kerbel RS:
Antitumor effects in mice of low-dose (metronomic) cyclophosphamide
administered continuously through the drinking water. Cancer Res.
62:2731–2735. 2002.PubMed/NCBI
|
|
18.
|
Doi Y, Okada T, Matsumoto H, Ichihara M,
Ishida T and Kiwada H: Combination therapy of metronomic S-1 dosing
with oxaliplatin-containing polyethylene glycol-coated liposome
improves antitumor activity in a murine colorectal tumor model.
Cancer Sci. 101:2470–2475. 2010. View Article : Google Scholar
|
|
19.
|
Iwamoto H, Torimura T, Nakamura T,
Hashimoto O, Inoue K, Kurogi J, Niizeki T, Kuwahara R, Abe M, Koga
H, Yano H, Kerbel RS, Ueno T and Sata M: Metronomic S-1
chemotherapy and vandetanib: an efficacious and nontoxic treatment
for hepatocellular carcinoma. Neoplasia. 13:187–197.
2011.PubMed/NCBI
|
|
20.
|
Munoz R, Shaked Y, Bertolinic F,
Emmenegger U, Man S and Kerbel RS: Anti-angiogenic treatment of
breast cancer using metronomic low-dose chemotherapy. Breast.
14:466–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Munoz R, Man S, Shaked Y, Lee CR, Wong J,
Francia G and Kerbel RS: Highly efficacious nontoxic preclinical
treatment for advanced metastatic breast cancer using combination
oral UFT-cyclophosphamide metronomic chemotherapy. Cancer Res.
66:3386–3391. 2006. View Article : Google Scholar
|
|
22.
|
Allegrini G, Di Desidero T, Barletta MT,
Fioravanti A, Orlandi P, Canu B, Chericoni S, Loupakis F, Di Paolo
A, Masi G, Fontana A, Lucchesi S, Arrighi G, Giusiani M, Ciarlo A,
Brandi G, Danesi R, Kerbel RS, Falcone A and Bocci G: Clinical,
pharmacokinetic and pharmacodynamic evaluations of metronomic UFT
and cyclophosphamide plus celecoxib in patients with advanced
refractory gastrointestinal cancers. Angiogenesis. 15:275–286.
2012. View Article : Google Scholar
|
|
23.
|
Kerbel RS: Improving conventional or low
dose metronomic chemotherapy with targeted antiangiogenic drugs.
Cancer Res Treat. 39:150–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Kato H, Ichinose Y and Ohta M, Hata E,
Tsubota N, Tada H, Watanabe Y, Wada H, Tsuboi M, Hamajima N and
Ohta M; Japan Lung Cancer Research Group on Postsurgical Adjuvant
Chemotherapy: A randomized trial of adjuvant chemotherapy with
uracil-tegafur for adenocarcinoma of the lung. N Engl J Med.
350:1713–1721. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Caballero M, Grau JJ, Blanch JL,
Domingo-Domenech J, Auge JM, Jimenez W and Bernal-Sprekelsen M:
Serum vascular endothelial growth factor as a predictive factor in
metronomic (weekly) paclitaxel treatment for advanced head and neck
cancer. Arch Otolaryngol Head Neck Surg. 133:1143–1148. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Patil V, Noronha V, D’cruz AK, Banavali SD
and Prabhash K: Metronomic chemotherapy in advanced oral cancers. J
Cancer Res Ther. 8:106–110. 2012.
|
|
27.
|
Hollemann D, Yanagida G, Rüger BM,
Neuchrist C and Fischer MB: New vessel formation in peritumoral
area of squamous cell carcinoma of the head and neck. Head Neck.
34:813–820. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shaked Y, Emmenegger U, Man S, Cervi D,
Bertolini F, Ben-David Y and Kerbel RS: Optimal biologic dose of
metronomic chemotherapy regimens is associated with maximum
antiangiogenic activity. Blood. 106:3058–3061. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Bertolini F, Paul S, Mancuso P,
Monestiroli S, Gobbi A, Shaked Y and Kerbel RS: Maximum tolerable
dose and low-dose metronomic chemotherapy have opposite effects on
the mobilization and viability of circulating endothelial
progenitor cells. Cancer Res. 63:4342–4346. 2003.PubMed/NCBI
|
|
30.
|
Loven D, Hasnis E, Bertolini F and Shaked
Y: Low-dose metronomic chemotherapy: from past experience to new
paradigms in the treatment of cancer. Drug Discov Today.
18:193–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Christopoulos A, Ahn SM, Klein JD and Kim
S: Biology of vascular endothelial growth factor and its receptors
in head and neck cancer: Beyond angiogenesis. Head Neck.
33:1220–1229. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Hamano Y, Sugimoto H, Soubasakos MA,
Kieran M, Olsen BR, Lawler J, Sudhakar A and Kalluri R:
Thrombospondin-1 associated with tumor microenvironment contributes
to low-dose cyclophosphamide-mediated endothelial cell apoptosis
and tumor growth suppression. Cancer Res. 64:1570–1574. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kundumani-Sridharan V, Niu J, Wang D, Van
Quyen D, Zhang Q, Singh NK, Subramani J, Karri S and Rao GN:
15(S)-hydroxyeicosatetraenoic acid-induced angiogenesis requires
Src-mediated Egr-1-dependent rapid induction of FGF-2 expression.
Blood. 115:2105–2116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Shimoyamada H, Yazawa T, Sato H, Okudela
K, Ishii J, Sakaeda M, Kashiwagi K, Suzuki T, Mitsui H, Woo T,
Tajiri M, Ohmori T, Ogura T, Masuda M, Oshiro H and Kitamura H:
Early growth response-1 induces and enhances vascular endothelial
growth factor-A expression in lung cancer cells. Am J Pathol.
177:70–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Bocci G, Francia G, Man S, Lawler J and
Kerbel RS: Thrombospondin 1, a mediator of the antiangiogenic
effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci.
100:12917–12922. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Rastogi P: Emergence of cancer stem cells
in head and neck squamous cell carcinoma: a therapeutic insight
with literature review. Dent Res J (Isfahan). 9:239–244.
2012.PubMed/NCBI
|
|
37.
|
Loges S, Schmidt T and Carmeliet P:
Mechanisms of resistance to anti-angiogenic therapy and development
of third-generation anti-angiogenic drug candidates. Genes Cancer.
1:12–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sayed SI, Dwivedi RC, Katna R, Garg A,
Pathak KA, Nutting CM, Rhys-Evans P, Harrington KJ and Kazi R:
Implications of understanding cancer stem cell (CSC) biology in
head and neck squamous cell cancer. Oral Oncol. 47:237–243. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Kokko LL, Hurme S, Maula SM, Alanen K,
Grénman R, Kinnunen I and Ventelä S: Significance of site-specific
prognosis of cancer stem cell marker CD44 in head and neck
squamous-cell carcinoma. Oral Oncol. 47:510–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Prince ME, Sivanandan R, Kaczorowski A,
Wolf GT, Kaplan MJ, Dalerba P, Weissman IL, Clarke MF and Ailles
LE: Identification of a subpopulation of cells with cancer stem
cell properties in head and neck squamous cell carcinoma. Proc Natl
Acad Sci. 104:973–978. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Wang W, Lin P, Han C, Cail W, Zhao X and
Sun B: Vasculogenic mimicry contributes to lymph node metastasis of
laryngeal squamous cell carcinoma. J Exp Clin Cancer Res.
29:602010. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Dales JP, Garcia S, Carpentier S, Andrac
L, Ramuz O, Lavaut MN, Allasia C, Bonnier P and Charpin C:
Long-term prognostic significance of neoangiogenesis in breast
carcinomas: comparison of Tie-2/Tek, CD105, and CD31
immunocytochemical expression. Hum Pathol. 35:176–183. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Kerbel RS: Inhibition of tumor
angiogenesis as a strategy to circumvent acquired resistance to
anti-cancer therapeutic agents. Bioessays. 13:31–36. 1991.
View Article : Google Scholar
|
|
44.
|
Miller K, Wang M, Gralow J, Dickler M,
Cobleigh M, Perez EA, Shenkier T, Cella D and Davidson NE:
Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic
breast cancer. N Engl J Med. 357:2666–2676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Harada K, Kawaguchi S, Supriatno,
Kawashima Y, Yoshida H and Sato M: S-1, an oral fluoropyrimidine
anti-cancer agent, enhanced radiosensitivity in a human oral cancer
cell line in vivo and in vitro: involvement possibility of
inhibition of survival signal, Akt/PKB. Cancer Lett. 226:161–168.
2005. View Article : Google Scholar
|
|
46.
|
Harada K, Kawaguchi S, Supriatno, Onoue T,
Yoshida H and Sato M: Enhancement of apoptosis in salivary gland
cancer cells by the combination of oral fluoropyrimidine anticancer
agent (S-1) and radiation. Int J Oncol. 25:905–911. 2004.PubMed/NCBI
|
|
47.
|
Zhao HY, Ooyama A, Yamamoto M, Ikeda R,
Haraguchi M, Tabata S, Furukawa T, Che XF, Zhang S, Oka T,
Fukushima M, Nakagawa M, Ono M, Kuwano M and Akiyama S: Molecular
basis for the induction of an angiogenesis inhibitor,
thrombospondin-1, by 5-fluorouracil. Cancer Res. 68:7035–7041.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Bancroft CC, Chen Z, Dong G, Sunwoo JB,
Yeh N, Park C and Van Waes C: Coexpression of proangiogenic factors
IL-8 and VEGF by human head and neck squamous cell carcinoma
involves coactivation by MEK-MAPK and IKK-NF-kappaB signal
pathways. Clin Cancer Res. 7:435–442. 2001.PubMed/NCBI
|
|
49.
|
Motegi K, Azuma M, Aota K, Yamashita T,
Tamatani T, Harada K, Yoshida H and Sato M: Effect of a mutant form
of IkappaB-alpha on 5-fluorouracil-induced apoptosis in transformed
human salivary gland cells. Oral Oncol. 37:185–192. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Harada K, Supriatno, Kawashima Y, Yoshida
H and Sato M: S-1 inhibits tumorigenicity and angiogenesis of human
oral squamous cell carcinoma cells by suppressing expression of
phosphorylated Akt, vascular endothelial growth factor and
fibroblast growth factor-2. Int J Oncol. 30:365–374. 2007.
|
|
51.
|
Lévi F: Circadian chronotherapy for human
cancers. Lancet Oncol. 2:307–315. 2001.
|