Introduction
Oesophageal cancer is the seventh most common cancer
worldwide (1) with its 5-year
survival rate being dismally low at ≤15% (2). Oesophageal squamous cell carcinoma
(OESCC) is an exceptionally drug-resistant tumour. Despite recent
advances in the detection of OESCC and the development of
multimodal therapy (3,4), its incidence is on the rise and
outcome for patients remains poor (5,6).
Thus, a greater understanding of the initiation and progression of
OESCC is required in order to be able to identify predictive and
prognostic factors that may in the future lead to novel therapeutic
strategies.
The mitogen-activated protein kinases (MAPKs) are
serine/threonine kinases and include the extracellular-regulated
kinase (ERK), c-jun NH2-terminal kinase (JNK) and p38
MAPK families. The p38 MAPK family consists of four members; p38α
(MAPK14) of which there are two splice variants (7), p38β (MAPK11), p38γ (MAPK12) and p38δ
(MAPK13) (8). Although these
isoforms are 60–70% identical in amino acid sequence they differ
greatly in their tissue distribution (9), substrate specificity (10) and sensitivity to chemical
inhibitors (11). In recent years,
we have gained an increased appreciation of the importance of p38
isoforms for a variety of cellular functions including
proliferation, differentiation, transformation and programmed cell
death (12). Their roles, however,
are more complex than previously thought, with distinct members
appearing to have different functions. In addition, the roles of
p38 in various pathologic conditions remain to be elucidated
(13).
To-date most of the published literature refers to
the p38 family as a whole or indeed have focused on the first
discovered isoform p38α (10,13).
There is an obvious dearth of research pertaining to the latter two
isoforms, p38γ and -δ, due partly to the lack of commercially
available specific chemical activators or inhibitors for each of
these isoforms (14). In the
present study we have overcome this obstacle using an
enzyme-substrate fusion approach for the generation of
constitutively active p38δ. We now provide new information
regarding the role(s) of p38δ and active (phosphorylated) p38δ
(p-p38δ) in OESCC. We identified differential p38δ expression in
OESCC. Lack of p38δ expression in OESCC allows for a more
aggressive phenotype including increased proliferation, increased
migration and increased capacity for anchorage-independent growth.
Restoration of p38δ expression, however, reverses these effects.
Together, our results provide evidence for a novel role for
p38δ-induced suppressive effects in OESCC. With survival rates
being poor for patients with OESCC, there is an urgent need to find
novel strategies to improve current therapy. Our study suggests
isoform specific activation of p38δ as a possible potential
approach for treatment of patients with OESCC.
Materials and methods
Reagents
All chemicals and cell culture reagents were
purchased from Sigma-Aldrich (Wicklow, Ireland), enzymes from New
England BioLabs (Hertfordshire, UK) and primary antibodies from
Cell Signaling Technologies (Hertfordshire, UK), unless otherwise
stated.
Specimens
The patient cohort consisted of ten patients with
OESCC of both genders ranging in age from 44 to 81 years.
Formalin-fixed, paraffin-embedded (FFPE) oesophagectomy specimens
from ten patients consisted of ten paired samples of primary tumour
and metastatic lymph nodes with 10 samples of non-tumour adjacent
tissues (NAT). Patient features are summarized in Table IA.
 | Table IPatient characteristics, and cell
lines used. |
Table I
Patient characteristics, and cell
lines used.
| A, OESCC patient
features |
|---|
|
|---|
| Patient
features | No. of
patients |
|---|
| Gender | |
| Male | 4 |
| Female | 6 |
| Age, median
(years) | 63 (44–81) |
| TNM7 stage | |
| T stage | |
| T3 | 10 |
| N stage | |
| N1 | 3 |
| N2 | 7 |
| Histological
grade | |
| Well
differentiated | 1 |
| Moderately
differentiated | 6 |
| Poorly
differentiated | 3 |
| B, KE (OESCC) cell
line features |
|---|
|
|---|
| KE features | |
|---|
| Gender | |
| Male | KE-3, -4, -5,
-6 |
| Female | KE-8, -10 |
| Age, median
(years) | 67 (50–71) |
| TNM7 stage | |
| T stage | |
| T1 | KE-10 |
| T3 | KE-3, -5, -6,
-8 |
| T4 | KE-4 |
| N stage | |
| N0 | KE-5 |
| N1 | KE-3, -4, -6, -8,
-10 |
| Histological
grade | |
| Well
differentiated | KE-5, -6 |
| Moderately
differentiated | KE-3, -10 |
| Poorly
differentiated | KE-4, -8 |
| C, p38δ MAPK
expression in patient specimens |
|---|
|
|---|
| Diagnosis | p38δ MAPK
expression
|
|---|
| Positive | Negative |
|---|
| NAT (n=10) | 9 | 1 |
| OESCC primary
(n=10) | 6a | 4 |
| OESCC nodes
(n=10) | 2 | 8 |
Cell culture
The KE oesophageal cancer cell lines (kind gifts
from Professor T. Fujii, Kurume University School of Medicine,
Japan) (15–17) as well as KYSE-70, OE-19, OE-21 and
OE-33 (ATCC, Rockville, MD, USA) were cultured in RPMI-1640
supplemented with 10% FCS, 100 μg/ml streptomycin and 100
U/ml penicillin. KE cell line features are summarized in Table IB. The metastatic oesophageal
cancer cell line, OC-3 [a kind gift from Cork Cancer Research
Centre, (Biosciences Institute, National University of Ireland,
Cork, Ireland] (18) was cultured
in DMEM supplemented with 10% FCS, 100 μg/ml streptomycin
and 100 U/ml penicillin. KYSE-450 cells (ATCC, Rockville, MD, USA)
were maintained in 45% RPMI-1640/45% Ham’s F-12 nutrient mixture
supplemented with 10% FCS, 100 μg/ml streptomycin and 100
U/ml penicillin.
Proliferation assay
KE cells were plated at a density of
3×104 cells/well in a 6-well tissue culture plate. Cell
viability was assessed by trypan blue (0.4% w/v) exclusion assay at
the indicated times (18).
Nuclear and cytosolic extraction
Nuclear and cytosolic fractions were isolated from
2×106 cells using the NE-PER Isolation kit (Pierce
Biotechnology, Rockford, IL, USA) according to the manufacturer’s
instructions.
Generation of MKK6b-p38δ MAPK,
MKK6b(E)-p38δ MAPK and MKK6b(E)-p38δDN MAPK fusion
proteins
p38δ (pcDNA3-FLAG-p38δ) and constitutively active
MKK6b [pcDNA3-MKK6b(E)] plasmids were a kind gift from Professor J.
Han (Scripps Research Institute, La Jolla, CA, USA) and have
previously been described (19).
To construct the pcDNA3-MKK6b(E)-FLAG-p38δ (p-p38δ) fusion plasmid
the TAA stop codon of MKK6b(E) was replaced with a unique
SwaI restriction sequence using a QuikChange Lightening
Site-Directed Mutagenesis kit (Agilent Technologies) (5′-CAT
CTTTTGTAAAACTGATTCTTGGAGAATTTAAATCAG TGGACTTAATCGGTTGACCCTACTG-3′;
5′-CAGTAG GGTCAACCGATTAAGTCCACTGATTTAAATTCTCCAA
GAATCAGTTTTACAAAAGATG-3′). A PCR generated DraI-DraI
fragment encoding FLAG-p38δ with a 5′ (Gly-Glu)5 linker
(5′-CCGCGCTTTAAAGGCGAGGGCGAGGG CGAGGGCGAGGGCGAGATGGACTACAAGGACGAC
GAT-3′; 5′-TTGATCTTTAAATTATTACAGCTTCATG CCACTTCGT-3′) to facilitate
folding as previously described (20) was ligated to SwaI linearised
pcDNA3-MKK6b(E) with T4 DNA ligase. The pcDNA3-MKK6b-FLAG-p38δ
plasmid (inactive MKK6b) was created by the substitution of
Glu151 and Glu155 with Ser and Thr
respectively by site-directed muta-genesis
(5′-TGGAATCAGTGGCTATTTGGTGGACTCTGT
TGCTAAAACAATTGATGCAGGTTGCAAACCATAC-3′;
5′-GTATGGTTTGCAACCTGCATCAATTGTTTTAGCAA
CAGAGTCCACCAAATAGCCACTGATTCCA-3′). The
pcDNA3-MKK6b(E)-FLAG-p38δDN (dominant negative)
(p-p38δDN) plasmid was created by substituting
Thr180 and Tyr182 of p38δ with Ala and Phe
respectively by site-directed mutagenesis
(5′-GACGCCGAGATGGCTGGCTTCGTGG TGACCCG-3′;
5′-CGGGTCACCACGAAGCCAGCCAT CTCGGCGTC-3′). DNA sequence analysis
confirmed the integrity of all plasmids.
Stable transfection
KE-3 cells were transfected using Lipofectamine™
2000 reagent (Life Technologies™) and a total of 4 μg of
plasmid DNA according to the manufacturer’s instructions.
Twenty-four hours following transfection cells were transferred to
100-mm diameter dishes and transfected cells were selected in
growth medium containing 800 μg/ml Geneticin. After 4–8
weeks, individual cell colonies were transferred for clone
expansion.
Immunoblot analysis
Supernatants used for immunoblotting with specific
antibodies, p38α and -δ, phospho-p38 MAPK and MKK6 antibodies (New
England Biolabs), p38γ (Upstate) and p38β2 antibody
(Zymed Laboratories Inc.) have previously been described by us
(18,21). Chemiluminescent detection was
performed using SuperSignal® WestDura Extended Duration
Substrate (Pierce Biotechnology) and bands were visualized using a
Syngene G:Box ChemiXR5 Gel Documentation System.
Immunohistochemistry
This was performed as previously described by us
(21). Briefly, FFPE OESCC and NAT
sections were de-parrafinized in xylene and re-hydrated prior to
analysis. Antigen retrieval was performed by microwave irradiation
in 0.01 M citrate buffer, pH 6.0. In addition cultured cells grown
on coverslips were fixed in 2-4% paraformaldehyde and permeabilised
with 0.5% Triton-X-100. Samples were blocked with 5% NGS in TS/SAP.
Slides were incubated with primary antibody overnight at 4°C.
Antibody binding was localized using a biotinylated secondary
antibody, avidin-conjugated HRP and DAB substrate, contained within
the Vectastain ABC detection kit (Vector Laboratories, Burlingame,
CA, USA). Slides were counterstained with hematoxylin.
ELISA
Cell lysates were analysed for p38δ phosphorylation
at T180/Y182 using the R&D Systems DuoSet® IC Human
phospho-p38δ (T180/Y182) sandwich ELISA (DYC2124-5) according to
the manufacturer’s instructions. Absorbance was read at 450 nm on a
Tecan Sunrise spectrophotometric plate reader and analysed using
the XRead software program.
Boyden chamber cell migration assay
Cells were plated in starvation medium at a density
of 3×104 cells/well into a 96-well plate of the upper
chamber. The bottom chamber contained 10% FCS as the
chemoattractant. Cells were left migrate for 24 h through the
matrigel filter (8 mm). Migrated cells were treated with MTT
(3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5
mg/ml) and absorbance read at 540 nm to calculate viable cell
numbers as previously described (21).
Wound-healing assay
Cell migration was assessed by in vitro
wound-healing assay as previously described (22). A linear wound track was made by use
of a sterile tip through confluent cells. Cells migrating into the
wound were captured under a phase-contrast microscope 24 and 48 h
after wounding. Migration was determined using the ImageJ program
as an average closed area of the wound relative to the initial
wound area at 24 and 48 h after wounding.
Colony forming assay
The role of p38δ in anchorage-independent growth was
assayed using a soft agar colony-forming assay as previously
described (21). Cells were plated
at a density of 3×105 cells/100-mm dish in medium
containing 0.4% (w/v) agar on an underlay of 0.8% (w/v) agar. After
a 21-day incubation colonies were stained with MTT (5 mg/ml)
overnight and counted.
siRNA
KE-6 cells at 75% confluency in antibiotic-free
media were transfected with 100 nM p38δ MAPK siRNA or control
siRNA-A (Santa Cruz Biotechnology, Santa Cruz, CA, USA) according
to the manufacturer’s instructions and as recently described
(23).
RT-PCR
First-strand cDNA was synthesised using
SuperScript® VILO™ cDNA Synthesis kit (Life
Technologies) from total RNA isolated from cells using an Illustra
RNASpin Mini kit (GE Healthcare, Buckinghamshire, UK) according to
the manufacturer’s instructions. p38δ mRNA was amplified from
cellular cDNA under the following conditions: ddH2O, 1X
DreamTaq buffer, 0.2 mM dNTPs, 0.25 μM p38δ forward primer:
5′-CCACGTTAAACTGCCCATCT-3′, 0.25 μM p38δ reverse primer:
5′-CCGCCACAAGCTAAAAAGAG-3′, 1 μl cDNA and 1 U DreamTaq DNA
polymerase (Thermo Fisher Scientific; Waltham, MA, USA). RT-PCR
products were analysed by agarose gel electrophoresis.
Proteome Profiler™ antibody array
The relative levels of phosphorylation of 26 kinases
was examined in cell lysates using a Proteome Profiler Human
Phospho-MAPK array (R&D Systems, Abingdon, UK) according to the
manufacturer’s instructions. Following chemiluminescent detection,
pixel density of each spot was analysed using Scion image
software.
Ethics
The research was approved by the Clinical Research
Ethics Committee of the Cork Teaching Hospitals.
Statistical analysis
Results are expressed as mean ± SE. Statistical
comparisons were made by using analysis of variance with subsequent
application of Student’s t-test, as appropriate. GraphPad InStat 3
software was used also for statistical analysis.
Results
p38α, -β, -γ and -δ isoforms and MKK3,
-4, -6 and 7 are differentially expressed in oesophageal
cancer
The expression of p38 as a family has previously
been outlined in oesophageal cancer as well as other cancer types
(10,13,24,25).
While these reports refer to the p38 family, analysis of individual
p38 isoform expression in oesophageal cancer has to date never been
reported. A previous study by us outlining differential p38 isoform
expression in renal cancer prompted us to investigate further the
effects of individual p38 family members in cancer in general
(26). Using western blot analysis
we examined p38 MAPK isoform expression in nine OESCC cell lines
(KE-3, -4, -5, -6, -8, -10, KYSE-70, KYSE-450 and OE-21) and three
oesophageal adenocarcinoma cell lines (OC-3, OE-19 and OE-33). We
used antibodies specific for each isoform p38α, -β2, -γ
and -δ as previously described by us (26). All twelve oesophageal cancer cell
lines (squamous and adenocarcinoma) expressed p38α, -β and -γ
(albeit at different levels) (Fig.
1A). In contrast p38δ expression was present in the three
adenocarcinoma cell lines but absent in four of the OESCC cell
lines KE-3, -8, KYSE-70 and OE-21 (Fig. 1A). The specific loss of p38δ
isoform expression only has previously been reported by us in renal
carcinoma (786-0) (26) and also
observed by us in liver (Huh-7), lung (A-549) prostate (PC-3 and
DU-145) and skin (MeWo) cancer cell lines (Barry et al,
unpublished data). Upstream MKK3 and -6 are thought to be the major
protein kinases responsible for p38 activation (24) but the selectivity of p38 isoform
activation is stimulus type and strength dependent (27). We observed strong MKK3 and -4
expression for all cell lines except KE-3 and -8 OESCC which were
MKK3 negative. In contrast levels of MKK6 and -7 expression were
considerably lower (Fig. 1B).
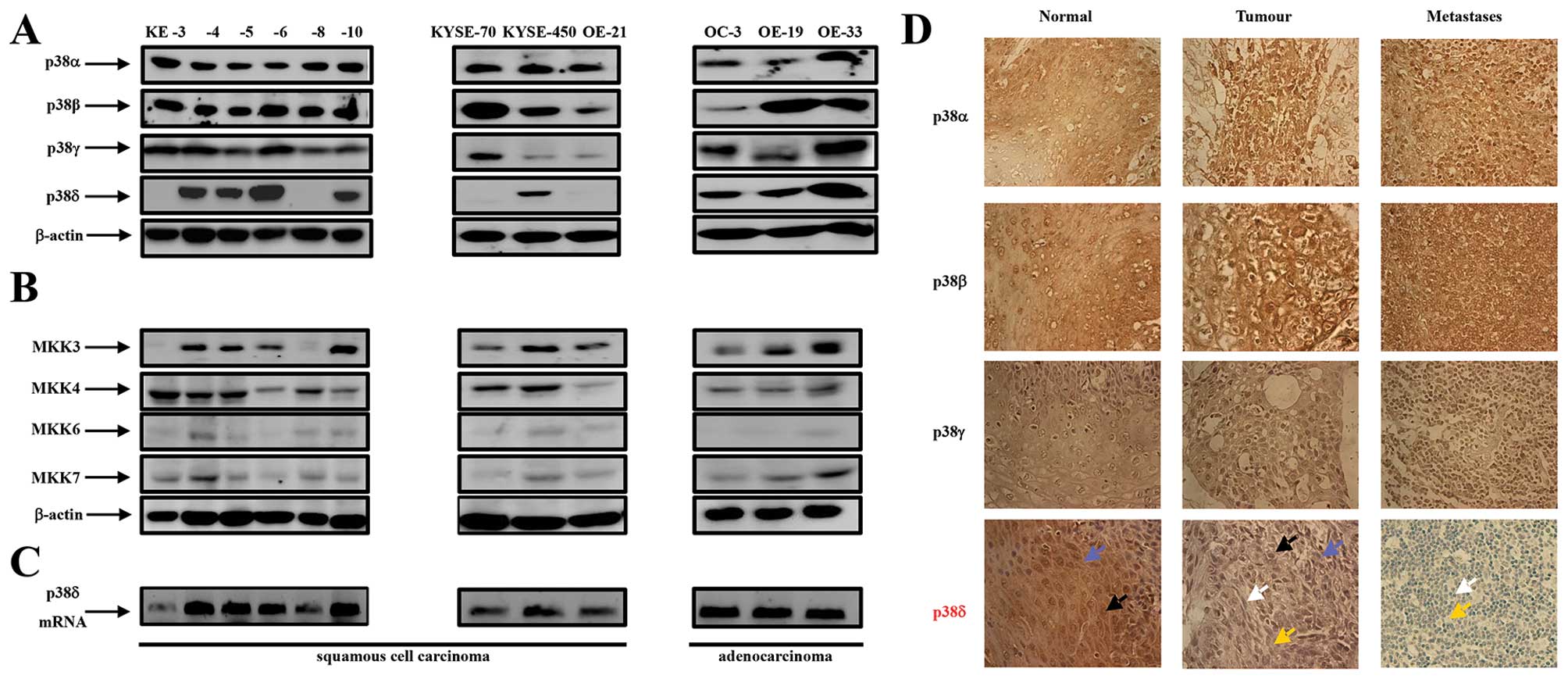 | Figure 1Expression of p38 MAPK isoforms,
MKK3, -4, -6 and -7 in oesophageal cancer. (A) Western blot
analysis of p38 isoform expression in KE-3, -4, -5, -6, -8 and -10,
KYSE-70, -450 and OE-21 (oesophageal squamous cell carcinoma cell
lines) as well as OC-3, OE-19 and OE-33 (oesophageal adenocarcinoma
cell lines). (B) Western blot analysis of MKK3, -4, -6 and -7 in
the same twelve cell lines. Aliquots of 30 μg of protein
lysate were loaded on a 10% SDS-PAGE gel and analyzed by
immunoblotting using antibodies specific for p38α, -β2,
-γ and -δ. β-actin analysis served as a loading control. The
results shown are representative of four independent experiments.
(C) Agarose gel electrophoresis analysis of DNA fragments produced
by PCR amplification of p38δ mRNA from oesophageal squamous (KE3,
-4, -5, -6, -8, 10, KYSE70, -450 and OE21) and adenocarcinoma (OC3,
OE19 and -33) cell lines. (D) Immunohistochemical staining of p38α,
-β2, -γ and -δ isoforms in normal, tumourigenic and
metastatic (lymph node) oesophageal human tissue.
Immunohistochemical staining was performed as outlined in Materials
and methods. Blue arrow indicates cytoplasmic staining; black arrow
indicates nuclear staining; white arrow indicates blue unstained
nuclei and yellow arrow indicates blue unstained cytoplasm.
Magnification, ×400. The results shown are representative of ten
patients. |
Finally, analysis of p38δ at the mRNA level
surprisingly proved positive for all cell lines examined including
the four OESCC cell lines that were negative for p38δ protein
expression (Fig. 1C). Primers
specific for a 292-bp fragment of the 3′-untranslated region of
p38δ mRNA amplified cDNA from all twelve cell lines. Other primer
sets within the coding sequence yielded similar results (data not
shown). In addition DNA sequence analysis of PCR products did not
identify any mutations such as a stop codon or a missense mutation
which could possibly explain loss of p38δ protein expression (data
not shown).
To investigate whether the p38 isoform expression
pattern we observed in vitro with the OESCC cell lines could
be translatable to the in vivo situation we analyzed the
expression profile and localization of all four p38 isoforms (α,
-β, -γ and -δ) in FFPE oesophagectomy specimens from ten patients
with squamous cell carcinoma. Samples consisted of ten paired
primary tumour and metastatic (lymph nodes) as well as
corresponding non-tumour adjacent tissues (NAT) as outlined in
Table IA. Samples were staged
according to the new TNM7 categorization for oesophageal cancer
(Table IA) (28). Consistent levels of p38α and -β
expression was evident in all ten normal, primary and metastatic
OESCC samples (Fig. 1D).
Similarly, we did not observe a change in p38γ expression between
normal, primary tumour and metastatic samples albeit the intensity
of brown staining was less than that observed for p38α and -β
(Fig. 1D). p38δ expression,
however, was considerably different in normal vs primary tumour vs
metastatic disease (Fig. 1D and
Table IC). p38δ expression was
observed in both the nuclei and cytoplasm of nine of the ten
oesophageal NAT tissue samples. However, a significant decrease in
expression was observed in both the nuclei and cytoplasm in the ten
primary tumour specimens as evidenced from the lighter brown
staining compared to NAT samples in six patient samples and
complete loss of expression in four of the samples (Fig. 1D and Table IC). Furthermore, eight out of the
ten metastatic tissue specimens demonstrated complete loss of p38δ
expression with both the nuclei and cytoplasm appearing blue in
colour (Fig. 1D). This is an
important finding considering identification of lymph node
metastasis is the single most important prognostic factor in
oesophageal cancer (1).
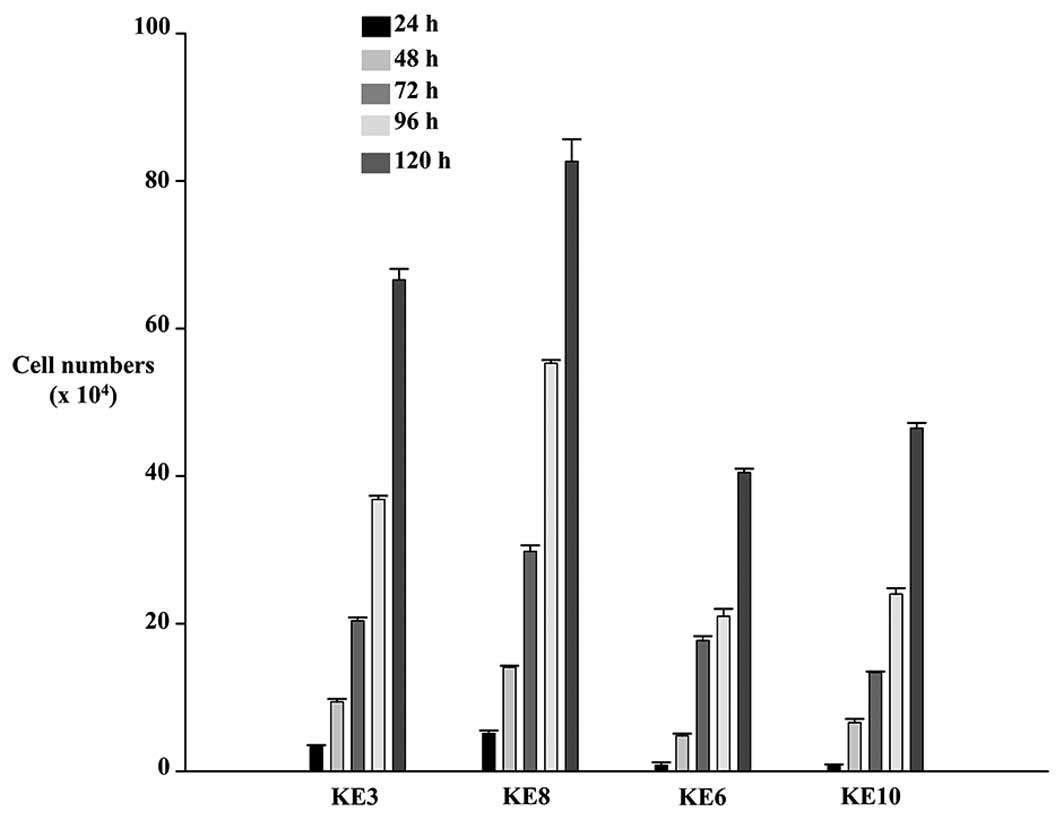
OESCC cell lines lacking endogenous p38δ
MAPK expression proliferate faster than those which express this
isoform
The results obtained for differential p38δ
expression in both the oesophageal cell lines and the human samples
prompted us to investigate further the effect(s) if any this
particular isoform may have on the tumourigenicity of OESCC.
Firstly, we examined whether the absence or presence of endogenous
p38δ expression could have an effect on the proliferation rate of
our OESCC cell lines. Using the trypan blue exclusion assay we
compared the proliferation rate of KE-3 and -8 cell lines (which do
not express p38δ) versus KE-6 and -10 (which express p38δ). We
observed that at all time-points studied (24–120 h) both cell lines
KE-3 and -8 proliferated faster than KE-6 and -10 cells (Fig. 2).
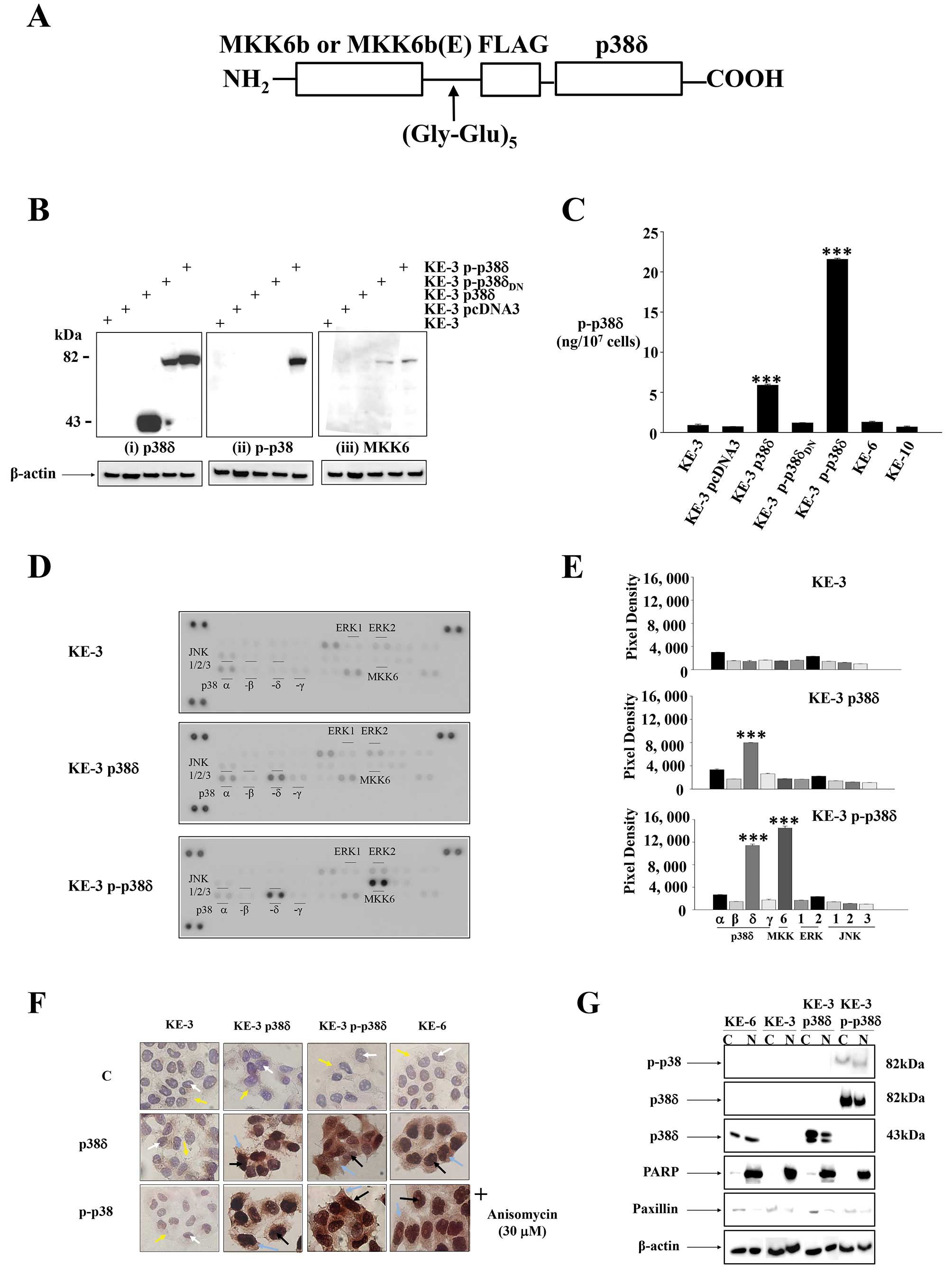
Generation of active (phosphorylated)
p38δ (p-p38δ) MAPK fusion proteins
To investigate whether p38δ or active
(phosphorylated) p38δ (p-p38δ) drives the observed
anti-proliferative phenotype (Fig.
2) we re-introduced wild-type p38δ into KE-3 cells which have
lost its expression. In the absence of a specific commercially
available p38δ activator [and to investigate the effect(s) of
active (p-p38δ)] we generated a constitutively active p38δ through
enzyme substrate fusion as previously described for JNK (Fig. 3A) (20). Western blot analysis of stable
transfections of KE-3 cells demonstrated that
pcDNA3-MKK6b-(Gly-Glu)5-FLAG-p38δ (data not shown) as
well as pcDNA3-MKK6b(E)-(Gly-Glu)5-FLAG-p38δ both
produced a single polypeptide with a molecular mass of 82 kDa as
expected when using p38δ, p-p38 and MKK6 antibodies, respectively
(Fig. 3B). As both MKK6b and
MKK6b(E) fused in frame to p38δ produced the same desired result
only one plasmid (MKK6b(E)-p38δ) was used for subsequent
experiments. Western blot analysis of KE-3 cells stably transfected
with pcDNA3-MKK6b(E)-(Gly-Glu)5-FLAG-p38δDN
also produced a single polypeptide with a molecular mass of 82 kDa
upon incubation with p38δ and MKK6 antibodies (Fig. 3Bi and iii) but did not demonstrate
p38 activation (phosphorylation) (Fig.
3Bii). Of note the antibody used in Fig. 3Bii is a pan phospho-p38 antibody.
To our knowledge there is no commercially available antibody to
test for active (phosphorylated) p-p38δ specifically by western
blot analysis. Therefore, to confirm p38δ activation we performed a
sandwich ELISA which measures p38δ isoform phosphorylation
specifically. Transfection of KE-3 cells with wild-type p38δ alone
revealed activation (Fig. 3C).
This is in strong agreement with previous reports where
adenovirally expressed wild-type p38δ was activated in head and
neck squamous cell carcinoma (29)
and human keratinocytes (30). A
4-fold (p<0.001) increase in activation of p38δ was observed
following stable transfection of KE-3 cells with p-p38δ (Fig. 3C). This level of activation is
similar to KE-3 p38δ transfected cells upon activation with
anisomycin (30 μM) (data not shown). As expected we did not
observe phosphorylation of p38δ in cells transfected with
p-p38δDN (Fig. 3C). We
also analysed KE-6 and KE-10 cell lines (which express endogenous
p38δ expression) but did not observe p38δ phosphorylation in either
cell line (Fig. 3C).
To ensure specific phosphorylation of p38δ only and
not the other three p38 isoforms (α, -β and -γ) we performed a
human phospho-MAPK antibody array (R&D Systems). We did not
observe phosphorylation of p38α, -β or -γ in nontransfected KE-3
cells or cells stably transfected with p38δ or p-p38δ (Fig. 3D and E). We did, however, observe
an increase (p<0.001) in phosphorylation in KE-3 p38δ wild-type
transfected cells which was amplified in KE-3 p-p38δ transfected
cells (Fig. 3D and E) in agreement
with our ELISA results (Fig. 3C).
These results confirm phosphorylation of p38δ only in our studies.
We also observed MKK6 phosphorylation in KE-3 p-p38δ as expected
(Fig. 3D and E). A previous report
outlined p38δ induced inactivation of ERK1/2 (31) however, we did not find any change
in ERK1/2 or indeed JNK1/2/3 (Fig. 3D
and E).
Finally, the physical location of a protein either
in the nucleus or the cytoplasm directly influences its biological
function. Members of the p38 family do not contain either a nuclear
localisation signal (NLS) or a nuclear export signal (NES) but
their subcellular localisation can be regulated in part by their
interacting proteins (32). We
compared the subcellular localization of p38δ and p-p38 in KE-3
transfected cells with endogenous p38δ expression in KE-6 cells. As
expected p38δ and p-p38 were absent from both compartments in
non-transfected KE-3 cells (Fig.
3F). p38δ and p-p38 were detected in both the cytoplasm and the
nucleus of KE-3 stably transfected cells (Fig. 3F). This pattern of expression
correlated with the subcellular localization of p38δ and p-p38 in
KE-6 cells in the presence and absence of anisomycin (30
μM). To confirm our immunohistochemical findings cytosolic
and nuclear extracts were prepared from transfected and
non-transfected KE-3 and KE-6 cells and examined by western blot
analysis. The use of PARP as a nuclear-restricted marker and
Paxillin as a cytosolic marker ensured that there was no cross
contamination between the subcellular fractions (21). Similar results were observed
demonstrating the presence of p38δ and p-p38 in both the cytoplasm
and nucleus of KE-3 and -6 cells (Fig.
3G).
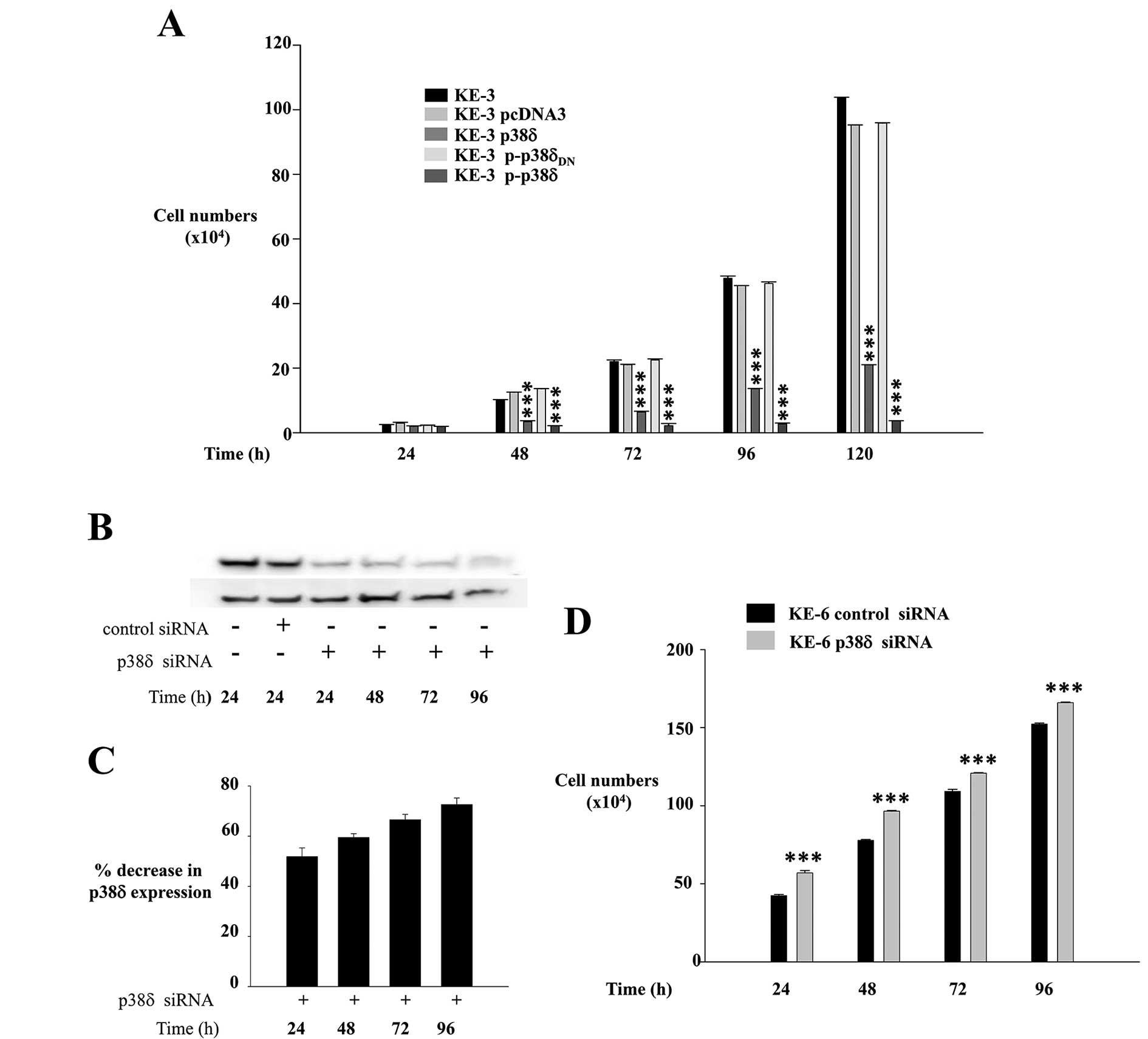
KE-3 cells transfected with p38δ and
p-p38δ MAPK show reduced proliferation
Uncontrolled cellular proliferation is a hallmark of
cancer. To investigate if loss of p38δ expression specifically
drives the higher growth kinetics observed in Fig. 2 we compared the growth rates of
KE-3 non-transfected and transfected cells. We observed a
significant (p<0.001) time-dependent decrease in the
proliferation rate of KE-3 cells when transfected with wild-type
p38δ compared with nontransfected cells and cells transfected with
empty pcDNA3 vector (Fig. 4A).
This anti-proliferative effect was amplified further in KE-3 cells
transfected with active p-p38δ (Fig.
4A). KE-3 cells transfected with p-p38δDN
demonstrated the same proliferation rate as non-transfected cells
or cells transfected with pcDNA3 only (Fig. 4A).
To further examine the hypothesis that p38δ is
anti-proliferative in OESCC we employed a siRNA approach using the
KE-6 cell line which expresses endogenous p38δ (Figs. 1, 3F
and G). KE-6 cells were transiently transfected with p38δ siRNA
or control siRNA as previously described (23). We observed a 51.9±6.5% reduction in
KE-6 p38δ expression at 24 h following p38δ siRNA transfection
which increased to 72.6±2.6% by 96 h when compared to control siRNA
transfected KE-6 cells (Fig. 4B and
C). No change in p38δ expression was observed when KE-6 cells
were transfected with control siRNA for all time-points studied
(24–96 h) (only 24 h is shown in Fig.
4B). A significant (p<0.001) increase in cell proliferation
was observed for KE-6 cells transfected with p38δ siRNA compared to
cells transfected with control siRNA for all time-points studied
(Fig. 4D). The anti-proliferative
effect was observed even in the absence of active p38δ in KE-6
cells (Fig. 3C). This effect on
proliferation may be independent of its kinase activity as has
previously been reported for p38α in regulating HeLa cell
proliferation (33) and p38γ in
rat intestinal epithelial cells (34).
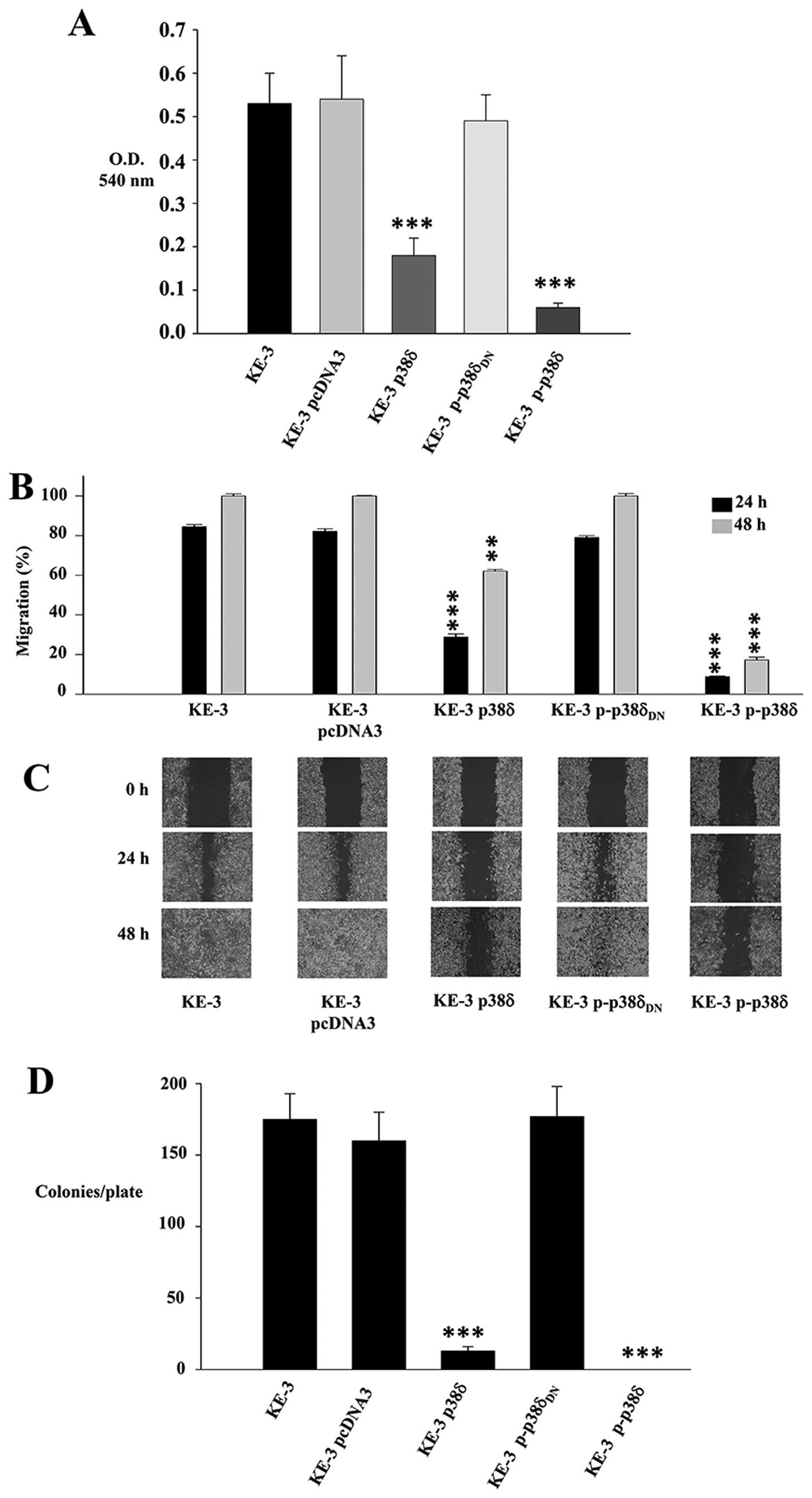
p38δ and p-p38δ MAPK play a role in
migration and anchorage-independent growth of KE-3 cells
A key characteristic of cancer cells is their
ability to migrate and progress from primary tumours to metastases
in distant organs. A recent report summarizes the roles of p38
MAPKs in cancer invasion and metastasis (35). This review, however, as in previous
reports documents the roles of p38 family as a whole or p38α
(10,13). We examined the role of p38δ in
OESCC cell migration using both a Boyden chamber assay and a wound
healing assay. We observed a 66±7.5 and 88.7±1.9% decrease in
migration after 24 h for KE-3 p38δ and p-p38δ cells respectively
compared to non-transfected cells (Fig. 5A). In addition p38δ and p-p38δ
induced a significant decrease in KE-3 migration at 24 h [55.65±1.5
and 75.65±0.3% (p<0.001), respectively] and 48 h [37.9±0.8,
(p<0.01) and 82.7±1.4%, (p<0.001) respectively] compared with
non-transfected KE-3 cells using a wound healing assay (Fig. 5B and C). Finally, to further
examine the influence of p38δ and p-p38δ on the growth
characteristics of KE-3 cells, we measured their ability to grow in
an anchorage-independent manner. Non-transfected KE-3 cells growing
in soft agar for 21 days gave rise to 175±18 colonies/plate
(Fig. 5D). This was similar to the
number of colonies/plate that grew for cells transfected with empty
vector (160±20) or p-p38δDN (177±21) (Fig. 5D). In contrast, however, p38δ and
p-p38δ transfected cells produced a significant (p<0.001)
decrease in colony numbers in p38δ transfected cells (13±3) with no
colonies observable for p-p38δ transfected cells (Fig. 5D).
Discussion
Oesophageal cancer is a highly aggressive
treatment-refractory disease with a high mortality rate (2,5,6). As
conventional therapy is ineffective, targeting specific potential
molecular tumour markers may prove to be the future of oesophageal
cancer treatment. Despite current studies of molecular targets in
oesophageal cancer (36), we are
still somewhat hindered by limited knowledge of the genes and
pathways involved in the tumourigenesis of the oesophagus when it
comes to treatment.
Emerging role(s) for p38 MAPKs in different aspects
of cancer has recently been outlined. To-date the best studied and
reviewed isoform in cancer is p38α. It has been characterized as
both a potential tumour suppressor (25,37–39)
and tumour promoter (29,35). In comparison the role(s) of p38δ in
cancer is largely uncharacterised. The limited current knowledge
pertaining to p38δ, however, also alludes to disparate role(s) for
this kinase in tumour development. An oncogenic role for p38δ has
been suggested in p38δ-deficient mice that have reduced
susceptibility to skin carcinogenesis (40) as well as promoting head and neck
squamous carcinoma cell growth (29). In contrast a very recent study
outlined a role for p38δ as a tumour suppressor in mouse
fibroblasts (41). In our study
outlined here we show for the first time the differential
expression of p38δ in OESCC cell lines and in vivo. The loss
of p38δ expression provides a survival advantage for OESCC which
demonstrates increased cell proliferation, migration and contact
inhibition. Re-introduction of p38δ, however, leads to reversal of
these tumourigenic effects. Thus, recent evidence (41) as well as our present study suggests
that targeting p38δ may offer a powerful protection against
carcinogenesis. Targeting p38 MAPK isoforms or pathways for
therapeutic purposes, however, should perhaps be strictly dependent
on cell context, tumour cell type and tumour stage.
The fusion of p38δ to its upstream kinase MKK6b or
active MKK6b [MKK6b(E)] generated a constitutively active p38δ
which was used as a tool to study its specific effect(s) in OESCC.
Re-introduction of p38δ (with subsequent activation) or active
p-p38δ into KE-3 OESCC attenuated cell proliferation, migration and
anchorage-independent growth. The strength and duration of p38
activation has been shown to play a crucial role in determining
cell fate. Strong activation has been shown to induce apoptosis
whereas lower levels results in cell survival (27,39).
In our study we observed strong anti-proliferative, anti-migratory
effects as well as effects on anchorage-independent growth upon
re-introduction of p38δ into KE-3 cells which subsequently became
active. These antitumourigenic effects were amplified further in
KE-3 cells transfected with constitutively active p-p38δ. It is
possible that owing to the localization of both p38δ and p-p38δ in
the nucleus and the cytoplasm of OESCC that this kinase may modify
its target(s) either structurally or subcellularly. We are
presently researching whether they are in free form or docked with
specific cytoplasmic or nuclear partners (24). Furthermore, p38δ and p-p38δ induced
antitumourigenic effects in OESCC may arise by a combination of
both phosphorylation-dependent and independent effects as
previously described (33,34).
There are many paradigms in the literature of
cross-talk between different MAPK pathways. In this instance,
however, when KE-3 cells were stably transfected with p38δ or
p-p38δ we did not observe changes in either p38 isoform (α, -β and
-γ), ERK1/2 or JNK1/2/3 expression (data not shown) or activation
levels. This is in agreement with a recent bio-informatics analysis
of MAPK pathways which specifically identified that persistent
activation of p38δ is resistant to interaction with other MAPKs
(42). This lack of interference
from other MAPKs permits us to specifically study the effects of
p38δ on cell cycle control, pathway components and regulatory
mechanisms in OESCC which is currently ongoing in our laboratory.
In addition negative feedback mechanisms have been shown to
contribute to fine-tuning p38 MAPK activity levels. One such report
outlines an increase in MKK6 expression and stability in
p38α−/− cardiomyocytes from transgenic mice (43). We did not observe a correlation
between the presence or absence of p38δ expression in OESCC cells
and MKK expression. Of notable exception is MKK3 whose expression
is absent from KE-3 and -8 cells (both negative for p38δ) but
present in KE-4, -5, -6 and -10 cells (all positive for p38δ).
However, this pattern of expression does not hold for KYSE-70 and
OE-21 OESCC cell lines which express MKK3 but are also negative for
p38δ protein expression.
Reports of the involvement of p38 MAPKs in a variety
of different pathological conditions is continuing to increase
fuelling interest in the development of potent and specific drugs
for modulating the activity of these kinases. Presently there are a
number of p38 inhibitors undergoing clinical trials for the
treatment of inflammatory diseases (44,45).
Results arising from our study demonstrate that loss of p38δ
expression in OESCC provides a more sinister phenotype with
increased proliferation, migration and anchorage-independent
growth. Thus, it is possible that isoform specific activation
(rather than inhibition) of p38δ may provide a therapeutic benefit
for patients with OESCC which express this isoform. In addition,
how p38δ activators may interact and enhance the effectiveness of
traditional therapeutics in combination therapy warrants
attention.
In conclusion, our results reveal previously
undocumented p38δ differential expression and function in OESCC. We
identified a subset of OESCC cell lines as well as human primary
and metastatic tumour samples that exhibit downregulation of p38δ
protein expression. We now provide evidence that loss of expression
of this particular isoform may be a mechanism by which OESCC cells
promote carcinogenesis. Re-introduction of p38δ into OESCC negative
cell lines suppressed different aspects of tumourigenesis. Our data
warrant further investigation to understand the important
physiological and pathophysiological effects of p38δ in OESCC and
is currently in progress. This knowledge should identify which
pathways, substrates or regulators are affected specifically by
p38δ in providing an antitumourigenic effect in OESCC. Armed with
this information uncovering novel targets and the development of
new therapeutics may be possible for this common cancer that
continues to demonstrate a generally poor clinical outcome.
Acknowledgements
This study was supported by the Health
Research Board, Ireland (grant HRA/2009/17).
References
|
1.
|
Kayani B, Zacharakis E, Ahmed K and Hanna
GB: Lymph node metastases and prognosis in oesophageal carcinoma -
a systematic review. Eur J Surg Oncol. 37:747–753. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Almhanna K and Strosberg JR: Multimodality
approach for locally advanced esophageal cancer. World J
Gastroenterol. 18:5679–5687. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Thallinger CM, Kiesewetter B, Raderer M
and Hejna M: Preand postoperative treatment modalities for
esophageal squamous cell carcinoma. Anticancer Res. 32:4609–4627.
2012.PubMed/NCBI
|
|
5.
|
Klein CA and Stoecklein NH: Lessons from
an aggressive cancer: evolutionary dynamics in esophageal cancer.
Cancer Res. 69:5285–5288. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bird-Lieberman EL and Fitzgerald RC: Early
diagnosis of oesophageal cancer. Br J Cancer. 101:1–6. 2009.
View Article : Google Scholar
|
|
7.
|
Sanz V, Arozarena I and Crespo P: Distinct
carboxy-termini confer divergent characteristics to the
mitogen-activated protein kinase p38α and its splice isoform Mxi2.
FEBS Lett. 474:169–174. 2000.PubMed/NCBI
|
|
8.
|
Raman M, Chen W and Cobb MH: Differential
regulation and properties of mapks. Oncogene. 26:3100–3112. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hui L, Bakiri L, Mairhorfer A, Schweifer
N, Haslinger C, Kenner L, Komnenovic V, Scheuch H, Beug H and
Wagner EF: P38alpha suppresses normal and cancer cell proliferation
by antagonizing the jnk-c-jun pathway. Nat Genet. 39:741–749. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ono K and Han J: The p38 signal
transduction pathway: activation and function. Cell Signal.
12:1–13. 2000. View Article : Google Scholar
|
|
11.
|
Zarubin T and Han J: Activation and
signaling of the p38 MAP kinase pathway. Cell Res. 15:11–18. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kyriakis JM and Ayruch J: Mammalian MAPK
signal transduction pathways activated by stress and inflammation:
a 10-year update. Physiol Rev. 92:689–737. 2012.PubMed/NCBI
|
|
13.
|
Cuenda A and Rousseau S: p38 MAP-kinases
pathway regulation, function and role in human diseases. Biochim
Biophys Acta. 1773:1358–1375. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Eyers PA, Craxton M, Morrice N, Cohen P
and Goedert M: Conversion of SB 203580-insensitive MAP kinase
family members to drug-sensitive forms by a single amino-acid
substitution. Chem Biol. 5:321–328. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Yamana H, Kakegawa T, Tanaka T, Higaki K,
Fujii T and Tou U: Experimental studies on immunotargeting therapy
for esophageal carcinoma. Gan To Kagaku Ryoho. 21:755–760.
1994.PubMed/NCBI
|
|
16.
|
Tsukahara T, Nabeta Y, Kawaguchi S, Ikeda
H, Sato Y, Shimozawa K, Ida K, Asanuma H, Hirohashi Y, Torigoe T,
Hiraga H, Nagoya S, Wada T, Yamashita T and Sato N: Identification
of human autologous cytotoxic T-lymphcyte-defined osteosarcoma gene
that encodes a transcriptional regulator, papillomavirus binding
factor. Cancer Res. 64:5442–5448. 2004. View Article : Google Scholar
|
|
17.
|
Nakao M, Yamann H, Imai Y, Toh Y, Toh U,
Kimura A, Yanoma S, Kakegawa T and Hoir K: HLA A2601-restricted
CTLs recognize a peptide antigen expressed on squamous cell
carcinoma. Cancer Res. 55:4248–4252. 1995.PubMed/NCBI
|
|
18.
|
Barry OP, Mullan B, Sheehan D, Kazanietz
MG, Shanahan F, Collins JK and O’Sullivan GC: Constitutive ERK1/2
activation in esophagogastric rib bone marrow micrometastatic cells
is MEK-independent. J Biol Chem. 276:15537–15546. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Pramanik R, Qi X, Borowicz S, Choubey D,
Schultz RM, Han J and Chen G: p38 isoforms have opposite effects on
AP-1-dependent transcription through regulation of c-Jun. The
determinant roles of the isoforms in the p38 MAPK signal
specificity. J Biol Chem. 278:4831–4839. 2003. View Article : Google Scholar
|
|
20.
|
Zheng C, Xiang J, Hunter T and Lin A: The
JNKK2-JNK1 fusion protein acts as a constitutively active c-Jun
Kinase that stimulates c-Jun transcription activity. J Biol Chem.
274:28966–28971. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
O’Sullivan GC, Tangney M, Casey G, Ambrose
M, Houston A and Barry OP: Modulation of p21-activated kinase 1
alters the behavior of renal cell carcinoma. Int J Cancer.
121:1930–1940. 2007.PubMed/NCBI
|
|
22.
|
Chen L, Zhang JJ and Huang XY: cAMP
inhibits cell migration by interfering with Rac-induced
lamellipodium formation. J Biol Chem. 283:13799–13805. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Adhikary G, Chew YC, Reece EA and Eckert
RL: PKC-delta and -eta, MEKK-1, MEK-6, MEK-3 and p38-delta are
essential mediators of the response of normal human epidermal
keratinocytes to differentiating agents. J Invest Dermatol.
130:2017–2030. 2010. View Article : Google Scholar
|
|
24.
|
Cargnello M and Roux P: Activation and
function of the MAPKs and their substrates, the MAPK-activated
protein kinases. Microbiol Mol Biol Rev. 75:50–83. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wagner EF and Nebreda ÁR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ambrose M, Ryan A, O’Sullivan GC, Dunne C
and Barry OP: Induction of apoptosis in renal cell carcinoma by
reactive oxygen species: involvement of extracellular
signal-regulated kinase 1/2, p38delta/gamma, cyclooxygenase-2
down-regulation and translocation of apoptosis-inducing factor. Mol
Pharmacol. 69:1879–1890. 2006. View Article : Google Scholar
|
|
27.
|
Remy G, Risco AM, Iñesta-Vaquera FA,
González-Terán B, Sabio G, Davis RJ and Cuenda A: Differential
activation of p38MAPK isoforms by MKK6 and MKK3. Cell Signal.
22:660–667. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Reid TD, Sanyaolu LN, Chan D, Williams GT
and Lewis WG: Relative prognostic value of TNM7 vs TNM6 in staging
oesophageal cancer. Br J Cancer. 105:842–846. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Junttila MR, Ala-aho R, Jokilehto T,
Peltonen J, Kallajoki M, Grenman R, Jaakkola P, Westermarck J and
Kähäri V-M: p38α and p38δ mitogen-activated protein kinase isoforms
regulate invasion and growth of head and neck squamous carcinoma
cells. Oncogene. 26:5267–5279. 2007.
|
|
30.
|
Efimova T, Broome A-M and Eckert RL:
Protein kinase Cδ regulates keratinocyte death and survival by
regulating activity and subcellular localization of a
p38δ-extracellular signal-regulated kinase 1/2 complex. Mol Cell
Biol. 24:8167–8183. 2004.
|
|
31.
|
Efimova T, Broome AM and Eckert RL: A
regulatory role for p38δ MAPK in keratinocyte differentiation.
Evidence for p38δ-ERK1/2 complex formation. J Biol Chem.
278:34277–34285. 2003.
|
|
32.
|
Li Q, Zhang N, Zhang D, Wang Y, Lin T,
Wang Y, Zhou H, Ye Z, Zhang F, Lin S-C and Han J: Determinants that
control the distinct subcellular localization of p38α-PRAK and
p38β-PRAK complexes. J Biol Chem. 283:11014–11023. 2008.PubMed/NCBI
|
|
33.
|
Fan L, Yang X, Du J, Marshall M, Blanchard
K and Ye X: A novel role of p38α MAPK in mitotic progression
independent of its kinase activity. Cell Cycle. 4:1616–1624.
2005.
|
|
34.
|
Tang J, Qi X, Mercola D, Han J and Chen G:
Essential role of p38γ in K-Ras transformation independent of
phosphorylation. J Biol Chem. 280:23910–23917. 2005.
|
|
35.
|
Del Barco Barrantes I and Nebreda AR:
Roles of p38 MAPKs in invasion and metastasis. Biochem Soc Trans.
40:79–84. 2012.PubMed/NCBI
|
|
36.
|
Izzo JG, Luthra R, Sims-Mourtada J, Chao
KSC, Lee JH, Wu T-T, Correa AM, Luthra M, Aggarwal B, Hung M-C and
Ajani JA: Emerging molecular targets in esophageal cancers.
Gastrointest Cancer Res. 1:S3–S6. 2007.PubMed/NCBI
|
|
37.
|
Bulavin DV and Fornace AJ: p38 MAP
kinases’s emerging role as a tumour suppressor. Adv Cancer Res.
92:95–118. 2004.
|
|
38.
|
Hui L, Bakiri L, Stepniak E and Wagber EF:
p38α a supressor of cell proliferation and tumorigenesis. Cell
Cycle. 6:2429–2433. 2007.
|
|
39.
|
Ventura JJ, Tenbaum S, Perdiguero E, Huth
M, Guerra C, Barbacid M, Pasparakis M and Nebreda AR: p38α MAP
kinase is essential in lung stem and progenitor cell proliferation
and differentiation. Nat Genet. 39:750–758. 2007.
|
|
40.
|
Schindler EM, Hindes A, Gribben EL, Burns
CJ, Yin Y, Lin M-H, Owen RJ, Longmore GD, Kissling GE, Arthur JSC
and Efimova T: p38δ mitogen-activated protein kinase is essential
for skin tumor development in mice. Cancer Res. 69:4648–4655.
2009.
|
|
41.
|
Cerezo-Guisado MI, Del Reino P, Remy G,
Kuma Y, Arthur JSC, Gallego-Ortega D and Cuenda A: Evidence of p38γ
and p38δ involvement in cell transformation processes.
Carcinogenesis. 32:1093–1099. 2011.
|
|
42.
|
Sundaramurthy P, Gakkhar S and Sowdhamini
R: Analysis of the impact of ERK5, JNK and p38 kinase cascades on
each other: a systems approach. Bioinformation. 3:244–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Ambrosino C, Mace G, Galban S, Fritsch C,
Vintersten k, Black E, Gorospe M and Nebreda AR: Negative feedback
regulation of MKK6 mRNA stability by p38α mitogen-activated protein
kinase. Mol Cell Biol. 23:370–381. 2003.PubMed/NCBI
|
|
44.
|
Coulthars LR, White DE, Jones DL,
McDermott MF and Burchill SA: p38MAPK: stress responses
from molecular mechanisms to therapeutics. Trends Mol Med.
15:369–379. 2009.
|
|
45.
|
Gaestel M and Kracht M: Peptides as
signalling inhibitors for mammalian MAP kinase cascades. Curr Pharm
Des. 15:2471–2480. 2009. View Article : Google Scholar : PubMed/NCBI
|