Introduction
Colorectal cancer (CRC) presents a problematic issue
in the developed world as it is the major cause of cancer-related
death. Over 1.2 million new cases were diagnosed in 2008 and
600,000 deaths were reported (1).
There has been considerable interest in understanding
carcinogenesis of CRC throughout the last decades. Hereditary and
sporadic forms of colon cancer have been reported (2). Germline mutations in the APC gene and
the DNA mismatch repair complex are described as the genetic
explanations for inherited familial adenomatous polyposis syndrome
and hereditary non-polyposis colon cancer (HNPCC) respectively
(3–6). Sporadic cancers of the colon are
associated with K-ras, TGF-β receptor, p53 and somatic APC
mutations (3,6–8).
Oxidative stress has been implicated in a number of
colon pathologies including inflammatory bowel disease,
diverticulitis and cancer (9–11).
Tumor progression is greatly regulated by hypoxia, the bulky nature
of a tumor distances the cells from oxygen-rich blood vessels,
which implicates cellular response (12). Intratumoral hypoxia has been
associated with increased risk of mortality (13). Oxidative stress allows for the
activation of several gene products that would promote metabolic
adaptation to hypoxia, such as those responsible for angiogenesis
and glycolytic enzymes (14–16).
Hypoxia inducible factors (HIFs) function physiologically in
adaptation to hypoxic conditions, they present the most notable
group assciated with hypoxia-induced reactions (17).
The phosphoglycerate kinase 1 (PGK1) is an
ATP-generating enzyme of the glycolytic pathway catalyzing the
conversion of 1,3-diphosphoglycerate to 3-phosphoglycerate and
regulated by hypoxia-induced factor-1α (HIF-1α), which is the most
important factor involved in the cellular response to hypoxia
(18,19). Solid tumor cells employ glycolytic
enzymes such as PGK1, to generate ATP when their supply of oxygen
is limited (20). Several
malignancies including prostate cancer, breast cancer, pancreatic
ductal adenocarcinoma, multidrug-resistant ovarian cancer and as we
have demonstrated, metastatic gastric cancer, have all been shown
to exhibit an increased expression of PGK1 (21–25).
An increased interest in PGK1 has arisen due to its possible role
in invasion and metastasis; several possible pathways have been
described, including the chemokine axis (26), allowing for an indirect impact of
PGK1 on migration, angiogenesis and tumor growth (27). Furthermore, β-catenin, a molecule
involved in cell proliferation, invasion, metastasis, angiogenesis
and drug resistance, seems to be a downstream target of PGK1
(18,28,29).
The expression of PGK1 has not yet been investigated in metastatic
colon cancer. In this study, we examined whether the PGK1
expression varies between metastatic and non-metastatic colon
cancer to find potential pathways accounting for such
observations.
Patients and methods
Patients, tissue specimens and RNA
extraction
Tissue samples were obtained from patients who
underwent hemi- or total colectomy at the Department of General,
Viszeral- and Transplant Surgery of the University of Tübingen,
Germany. Manual micro-dissection and harvesting was performed
immediately after tumor resection to ensure high tumor cell content
of the samples, specimens were then snap-frozen in liquid nitrogen
and stored at −80°C until utilization. Only specimens from patients
with histologically confirmed diagnosis of adenocarcinoma were
considered. A total of 27 samples (17 males and 10 females) were
utilized for RNA extraction. Thirteen from patients without
metastasis (mean age, 68 years; range, 52–80; pT1-category, 2;
pT2-category, 2; pT3-category, 9) and 14 from patients with
histologically confirmed metastasis of at least N1 category
according to UICC (30) (mean age,
69 years; range, 51–92; pT2-category, 1; pT3-category, 9;
pT4-category, 4; N1, 4; pN1-category, 4; pN2-category, 6;
pN3-category, 4; M0-category, 8; M1-category, 6). All patients
signed an informed written consent to participate in the study.
RNA extraction was performed using the
RNAeasy® Mini kit (Qiagen, Hilden, Germany) according to
the manufacturer’s instructions. The quality and quantity of the
mRNA yield was monitored using the NanoDrop ND-1000
spectrophotometer (NanoDrop Technologies, Wilmington, DE, USA).
Specimens used for immunhistochemical staining were
retrieved from a tissue bank of the comprehensive cancer center of
the University of Tübingen, Germany. Twenty-seven paraffin-embedded
tissue blocks of adenocarcinoma samples were randomly chosen with
the help of a corresponding database, 17 from non-metastatic colon
cancer patients (pT2-category, 3; pT3-category, 14; localization: 9
ascending colon, 2 transverse colon, 6 descending colon) and 10
from metastatic colon cancer patients (pT2-category, 2;
pT3-category, 8; pN1-category, 7; pN2-category, 3; M1-category, 2;
tumor localization: 5 ascending colon, 2 transverse colon, 2
descending colon, 1 recto-sigmoid region).
Real-time LightCycler®
RT-PCR
cDNA was prepared using the first-strand cDNA
synthesis kit (Roche Applied Science, Mannheim, Germany) according
to the manufacturer’s instructions. Real-time PCR was then
performed with the SYBR Green Jump Start TAQ ReadyMix (Sigma,
Taufkirchen, Germany) and LightCycler (Roche Applied Science) as
described previously (26). The
number of specific transcripts was normalized to β-actin as a
housekeeping gene. For analysis of relative expression, the
2−ΔΔT method was utilized according to Livak and
Schmittinger (31). The
Mann-Whitney U test was used for comparison of expression data,
p<0.05 was considered statistically significant. Sequences of
the primers used in this study are listed in Table I.
 | Table I.Genes and corresponding primers used
for real-time PCR. |
Table I.
Genes and corresponding primers used
for real-time PCR.
| Gene product | Sense primer | Antisense primer |
|---|
| Phosphoglycerate
kinase 1 (PGK1) |
CATACCTGCTGGCTGGATGG |
CCCACAGGACCATTCCACAC |
| Early growth response
1 (EGR1) |
CTGACCGCAGAGTCTTTTC |
AAGGTGTTGCCACTGTTG |
| Jun oncogene
(JUN) |
TGACGGACTGTTCTATGACT |
AAGGTGTTGCCACTGTTG |
| FBJ murine
osteosarcoma viral oncogene homolog (FOS) |
GCAAGGTGGAACAGTTATCT |
TTCAGCAGGTTGGCAATC |
| Cysteine-rich,
angiogenic inducer, 61 |
GAAGAGTGTCAGAATCAGAATCA |
TACCTTAATGCTCCTCAAGAATG |
Immunohistochemistry
For immunhistochemistry of colon cancer samples,
serial paraffin sections (2 μm) of the selected tumor blocks
were cut and deparaffinized. After protease antigen retrieval (6
min), PGK1 staining with PGK1/2 primary antibody (dilution 1:200;
clone: sc-48342; Santa Cruz Biotechnology Inc., Santa Cruz, CA,
USA) was carried out on an automated immunostainer (Benchmark,
Ventana Medical Systems, USA) with an incubation time of 32 min.
Counterstaining was performed using a biotin-free ultraView
Universal DAB Detection kit (Ventana Medical Systems).
Semi-quantative analysis was performed by 2 pathologists based on
the intensity of tumor cell staining.
Cell culture
The HCT116 (ATCC Manassas, VA, USA) cell line was
utilized for PGK1 knockdown and microarray investigations as
described below. Cells were cultured in Dulbecco’s modified Eagle’s
medium (DMEM) (Lonza, Basel, Switzerland) + 10% fetal calf serum
(FCS) (PAA, Pasching, Austria) and maintained in a humid chamber at
a 37°C and 5% CO2 atmospheric condition.
Small hairpin RNA knockdown of PGK1
HCT cells were incubated with an adenovirus (Sirion
Biotech, Martinsried, Germany) containing shPGK1 for 48 h. RNA was
then isolated using the RNAeasy Mini kit (Qiagen) and quantified
with the NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies).
Microarray analysis
To generate gene expression profiles, both steady
state and actively translated RNA was isolated from wild-type
HCT116 and PGK1-silenced HCT116 cell lines. Three aliquots
containing 10 μg RNA each were harvested from both cell
lines and quantified using the NanoDrop ND-1000 spectrophotometer
(NanoDrop Technologies). The GeneChip 3′ IVT Express kit was used
to synthesize biotinylated amplified RNA (aRNA) that was hybridized
to the Affymetrix GeneChip® human genome U133 Plus 2.0
array (Affymetrix Inc., Santa Clara, CA, USA). In brief, first
strand cDNA was synthesized via reverse transcription, then
converted into a double stranded template for transcription. In
vitro transcription allowed for synthesis of aRNA incorporating
a biotin-conjugated nucleotide. aRNA was then fragmented and
hybridized onto the Chip Arrays according to the manufacturer’s
instructions. Chips were washed and stained using a Fluidics
Station and scanned with a GeneArray Scanner 3000, both provided by
the GeneChip® System 3000Dx v.2 by Affymetrix. Scanned
images were inspected visually to check for hybridization artifacts
and proper grid alignment. Images were quantifed to produce
transcript level data using the Affymetrix Microarray Analysis
Suite (MAS) 5.0, which were then loaded to GeneSpring Software 7.2
(Agilent, Palo Alto, CA, USA) and normalized across arrays.
Principal component analysis was performed to test dissociation
between the groups. Fold change was derived as the ratio of average
differences from the three experimental arrays as compared to the
three control arrays. Multiple testing correction was performed and
a 2-fold cutoff was set. Expression profiles were compared using
one-way analyses of variance (ANOVA), p<0.05 was considered
statistically significant. Web-based Ingenuity Pathway software
(Ingenuity® Systems, www.ingenuity.com) was employed to examine the
function and pathway of the different genes.
Results
mRNA expression levels of PGK1 by means
of QRT-PCR
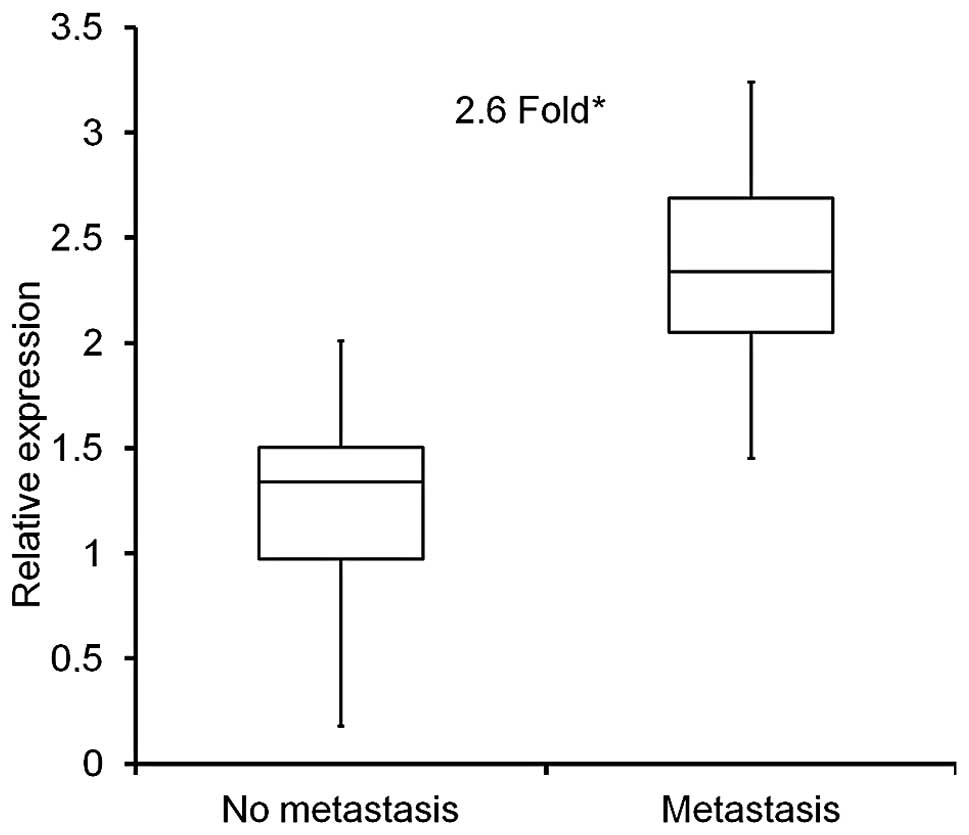
Quantative real-time PCR (qRT-PCR) was used to
assess mRNA expression of PGK1 in colon cancer tissue extracts,
either from patients without metastasis (n=13) or with
histologically confirmed metastasis (n=14) as mentioned above. PGK1
expression levels were significantly higher in metastatic samples
by 2.6-fold (p<0.001), when compared to non-metastatic colon
cancer samples (Fig. 1).
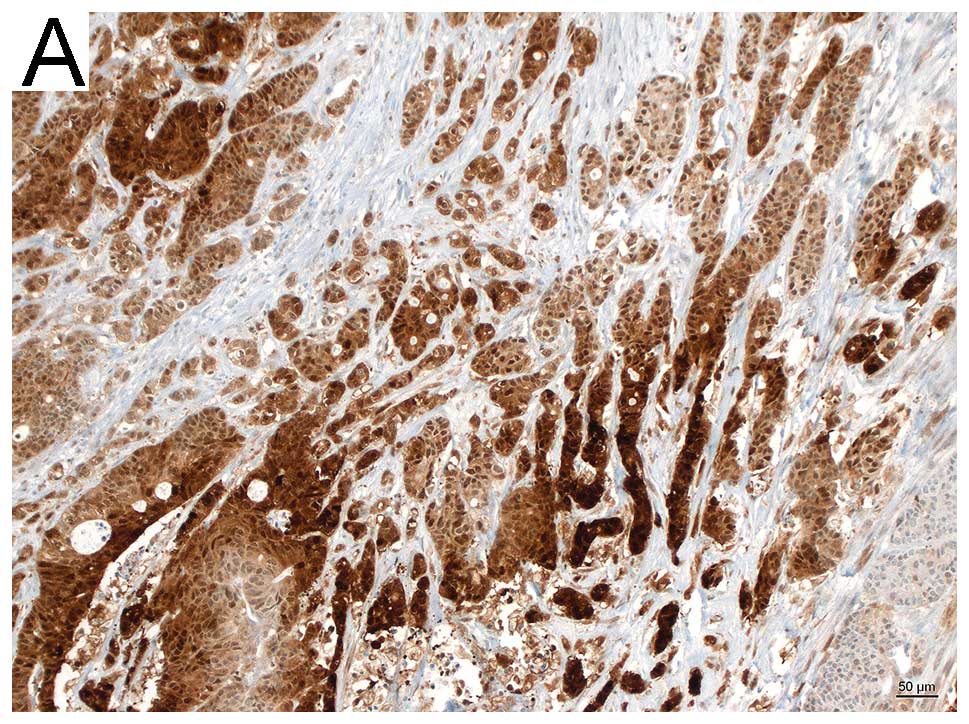
Semi-quantative analysis by means of
immunohistochemistry
PGK1 stained sections of 27 colon tumor specimens,
either without metastasis (n=17), or with metastasis (n=10) were
evaluated by two pathologists. Semi-quantative analysis was
performed by assessing the stained tumor area (>50%, strong;
<50%, moderate; <10%, weak). Sections of the metastasis group
(9/10) showed strong to moderate staining (6 strong, 3 moderate), 1
section was negative. Whereas 13/17 of the non-metastatic sections
showed weak to moderate staining (1 negative, 6 weak, 6 moderate)
and only 4/17 strong. Fig. 2 shows
representative histological images.
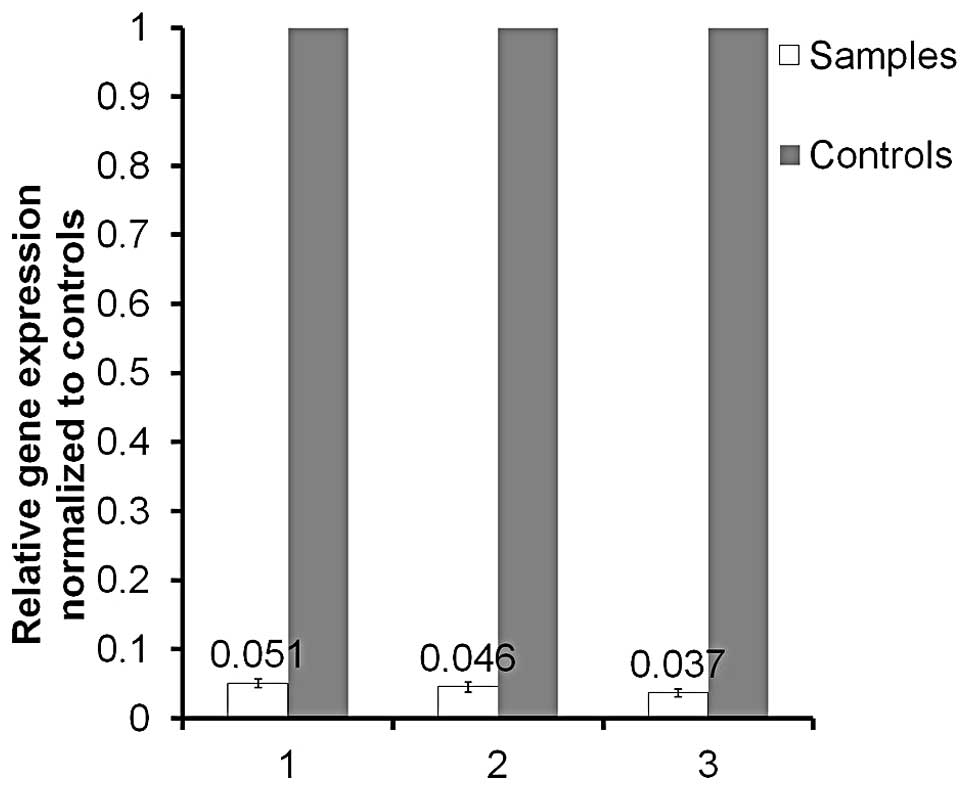
Microarray analysis and validation
Successful PGK1 knockdown was achieved in HCT116
cells (Fig. 3), from which 3 RNA
extracts from 3 individual experiments were utilized for microarray
analysis. Comparison was made to corresponding native HCT116 RNA
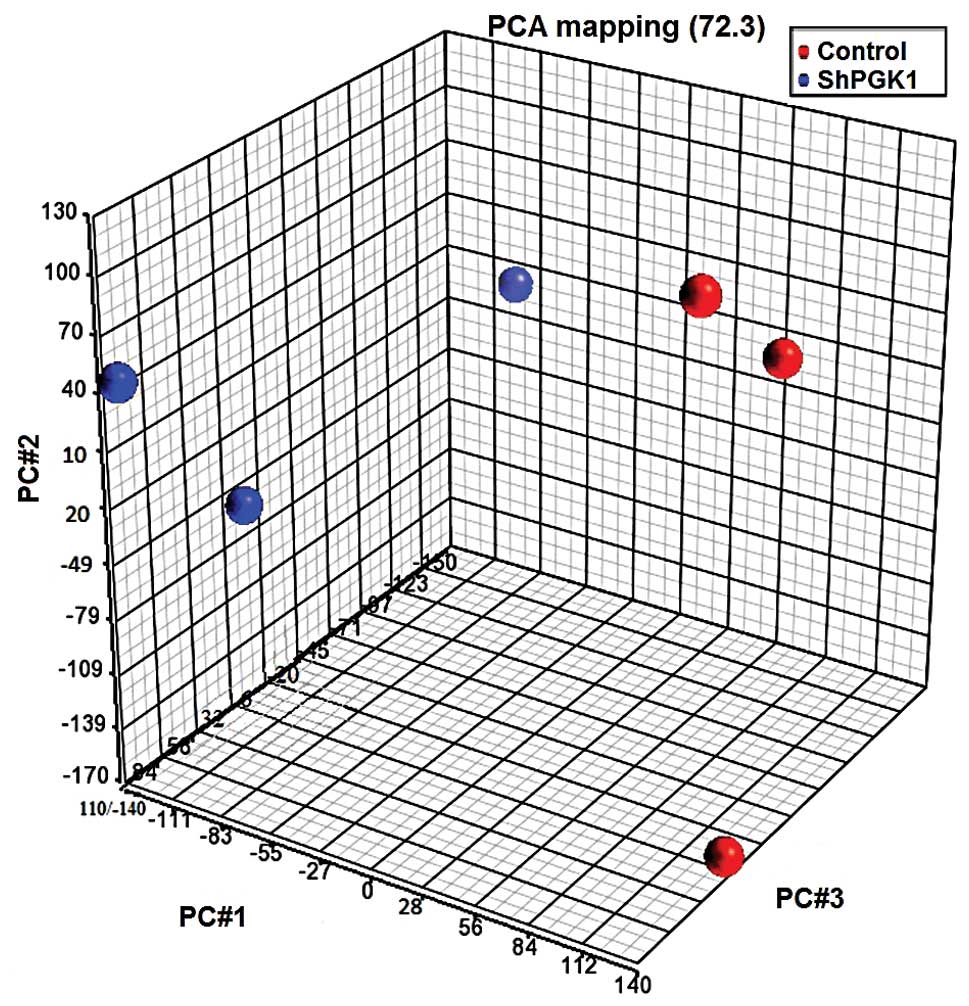
extracts of the same passage. Fig.
4 shows a 3D visualization of the relationships between the
samples using PCA, which is based on the expression levels of the
probe sets: PGK1 knockdown and native groups clustered in separate
areas of the 3-dimentional visualization, indicating a clear
difference in the molecular composition between them. PCA captured
72.3% of the variation observed in the experiment in the first
three principal components, which are plotted on x-, y- and z-axes,
respectively, representing the largest fraction of the overall
variability in samples. At a significance level of <0.05 and a
cutoff fold change of ≥2, 92 genes were upregulated and 180
downregulated. After uploading data sets containing gene
identifiers into Ingenuity Pathway software (Ingenuity Systems,
www.ingenuity.com), genes of interest were further
tested by means of RT-PCR for microarray validation showing
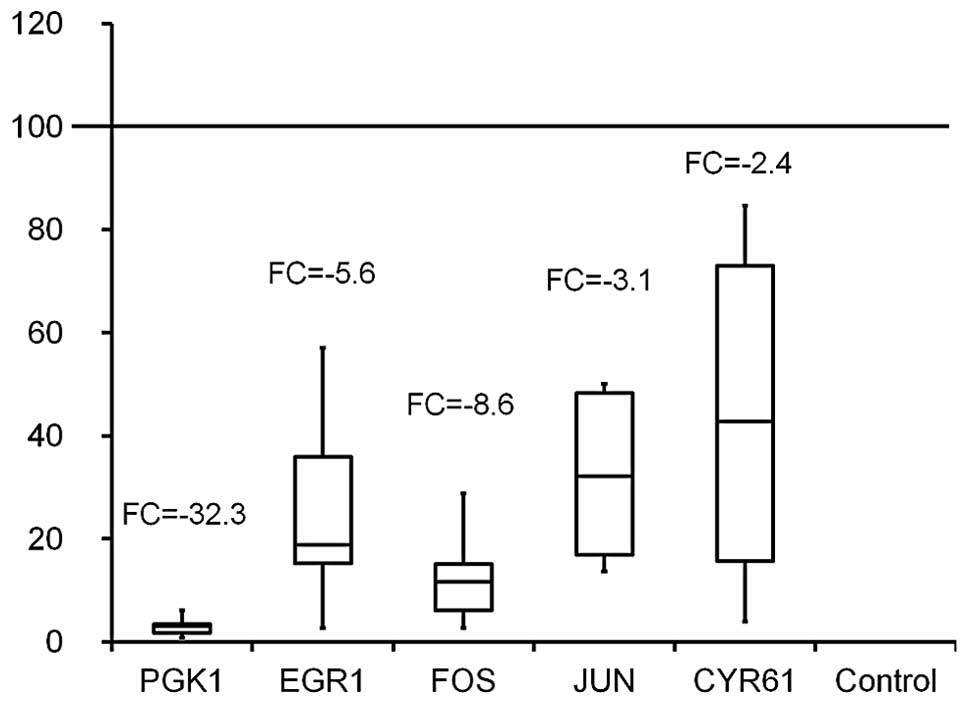
downregulation of PGK1 fold change (FC) -32.3; early growth
response 1 (EGR1) F -5.6; cysteine-rich 61 (CYR61) FC -2.4; FBJ
murine osteosarcoma viral oncogene homolog (FOS) FC -8.6; Jun
oncogene (JUN) FC -3.2 (Fig.
5).
Discussion
The data presented in this study indicate that colon
cancer cells that tend to metastasize possess an increased
expression of PGK1. This has been shown both by PCR on RNA level
and immunhistochemical staining. Previous studies described an
increased expression of PGK1 in prostate cancer, breast cancer,
pancreatic ductal adenocarcinoma, multidrug-resistant ovarian
cancer and as we have previously demonstrated, metastatic gastric
cancer (21–25). PGK1 is known to be a downstream
target of HIF-1α which is the major factor regulating cellular
response to hypoxia (19).
The bulky nature of tumor masses and thus the
impaired conditions for oxygen diffusion is believed to trigger the
induction of PGK1, this allows for ATP production and
supplementation of cells under hypoxic conditions (24). Furthermore, PGK1 has been shown to
be associated with the induction of several pathways, one of which
has been described by Wang and his group demonstrating the
relationship between PGK1 and the CXCR4/CXCL12 axis in prostate
cancer cell lines (18). These
findings were reproduced by our group in metastatic gastric cancer
(26) as described earlier, but
not colon cancer. Knockdown of PGK1 in HCT116 cancer cells was not
seen to cause alteration in CXCR4 or CXCL12 expression according to
our microarray results.
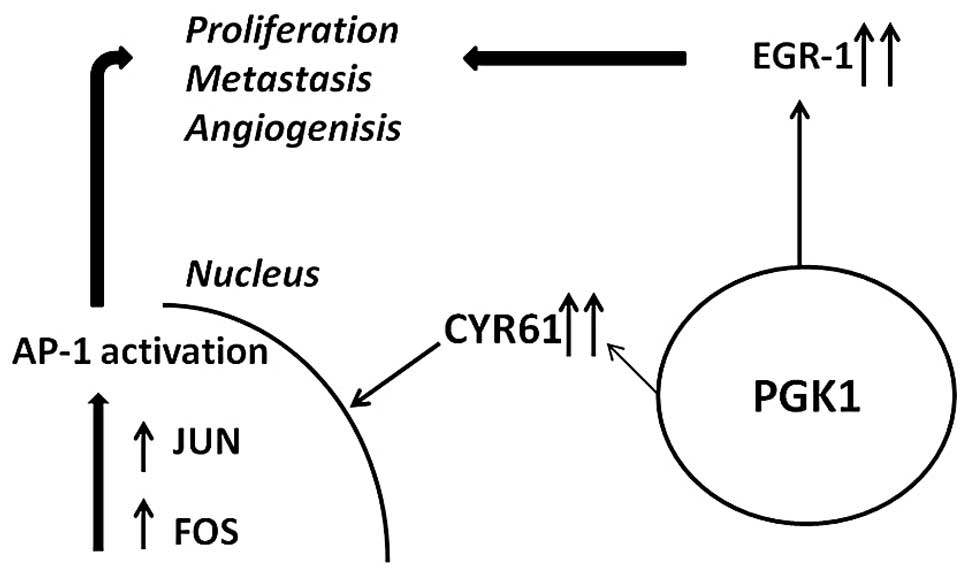
Several genes, however, appeared to be linked to
PGK1 based on the current data. FOS and JUN form a heterodimeric
complex which regulates gene transcription by binding to
transcriptional control elements containing activator protein-l
(AP-1) binding sites (32). This
interaction is known to be induced by CYR61 resulting in invasive
growth, migration and angiogenesis (Fig. 6) (33,34).
EGR1, a member of the early growth family has also
shown an association with PGK1. Interestingly, it has been
indicated that EGR1 plays a role in angiogenesis, growth and
metastasis (35). These findings
were reproduced by Zheng and his group demonstrating that the
majority of gastric cancer tissues expressed EGR1 and that the
increased expression was associated with metastasis and growth
(36).
In conclusion, the data of this study indicate that
an increased expression of PGK1 in colon cancer tissue is
associated with metastasis. Furthermore, we propose several genes
induced by PGK1 that could account for cell migration, mainly EGR1
and CYR61 together with its downstream transcription factors FOS
and JUN (33,36).
References
|
1.
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2.
|
Markowitz SD, Dawson DM, Willis J and
Willson JK: Focus on colon cancer. Cancer Cell. 1:233–236. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Goss KH and Groden J: Biology of the
adenomatous polyposis coli tumor suppressor. J Clin Oncol.
18:1967–1979. 2000.PubMed/NCBI
|
|
4.
|
Kinzler KW and Vogelstein B: Lessons from
hereditary colorectal cancer. Cell. 87:159–170. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kolodner R: Biochemistry and genetics of
eukaryotic mismatch repair. Genes Dev. 10:1433–1442. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Markowitz S: TGF-beta receptors and DNA
repair genes, coupled targets in a pathway of human colon
carcinogenesis. Biochim Biophys Acta. 1470:M13–M20. 2000.PubMed/NCBI
|
|
7.
|
Fearon ER: K-ras gene mutation as a
pathogenetic and diagnostic marker in human cancer. J Natl Cancer
Inst. 85:1978–1980. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Markowitz S, Wang J, Myeroff L, et al:
Inactivation of the type II TGF-beta receptor in colon cancer cells
with microsatellite instability. Science. 268:1336–1338. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Borek C: Dietary antioxidants and human
cancer. Integr Cancer Ther. 3:333–341. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Seril DN, Liao J, Yang GY and Yang CS:
Oxidative stress and ulcerative colitis-associated carcinogenesis:
studies in humans and animal models. Carcinogenesis. 24:353–362.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rhodes JM and Campbell BJ: Inflammation
and colorectal cancer: IBD-associated and sporadic cancer compared.
Trends Mol Med. 8:10–16. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Mazure NM, Brahimi-Horn MC, Berta MA, et
al: HIF-1: master and commander of the hypoxic world. A
pharmacological approach to its regulation by siRNAs. Biochem
Pharmacol. 68:971–980. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Semenza GL: Hypoxia-inducible factors:
mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Carmeliet P, Dor Y, Herbert JM, et al:
Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ryan HE, Lo J and Johnson RS: HIF-1 alpha
is required for solid tumor formation and embryonic
vascularization. EMBO J. 17:3005–3015. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Seagroves TN, Ryan HE, Lu H, et al:
Transcription factor HIF-1 is a necessary mediator of the pasteur
effect in mammalian cells. Mol Cell Biol. 21:3436–3444. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Semenza GL: Oxygen sensing, homeostasis
and disease. N Engl J Med. 365:537–547. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Wang J, Wang J, Dai J, et al: A glycolytic
mechanism regulating an angiogenic switch in prostate cancer.
Cancer Res. 67:149–159. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Dayan F, Roux D, Brahimi-Horn MC,
Pouyssegur J and Mazure NM: The oxygen sensor factor-inhibiting
hypoxiainducible factor-1 controls expression of distinct genes
through the bifunctional transcriptional character of
hypoxia-inducible factor-1alpha. Cancer Res. 66:3688–3698. 2006.
View Article : Google Scholar
|
|
20.
|
Daly EB, Wind T, Jiang XM, Sun L and Hogg
PJ: Secretion of phosphoglycerate kinase from tumour cells is
controlled by oxygen-sensing hydroxylases. Biochim Biophys Acta.
1691:17–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Migita T, Oda Y, Naito S, Morikawa W,
Kuwano M and Tsuneyoshi M: The accumulation of angiostatin-like
fragments in human prostate carcinoma. Clin Cancer Res.
7:2750–2756. 2001.PubMed/NCBI
|
|
22.
|
Duan Z, Lamendola DE, Yusuf RZ, Penson RT,
Preffer FI and Seiden MV: Overexpression of human phosphoglycerate
kinase 1 (PGK1) induces a multidrug resistance phenotype.
Anticancer Res. 22:1933–1941. 2002.PubMed/NCBI
|
|
23.
|
Hwang TL, Liang Y, Chien KY and Yu JS:
Overexpression and elevated serum levels of phosphoglycerate kinase
1 in pancreatic ductal adenocarcinoma. Proteomics. 6:2259–2272.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhang D, Tai LK, Wong LL, Chiu LL, Sethi
SK and Koay ES: Proteomic study reveals that proteins involved in
metabolic and detoxification pathways are highly expressed in
HER-2/neu-positive breast cancer. Mol Cell Proteomics. 4:1686–1696.
2005. View Article : Google Scholar
|
|
25.
|
Zieker D, Konigsrainer I, Tritschler I, et
al: Phosphoglycerate kinase 1 a promoting enzyme for peritoneal
dissemination in gastric cancer. Int J Cancer. 126:1513–1520.
2010.PubMed/NCBI
|
|
26.
|
Zieker D, Konigsrainer I, Traub F, et al:
PGK1 a potential marker for peritoneal dissemination in gastric
cancer. Cell Physiol Biochem. 21:429–436. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Gerard C and Rollins BJ: Chemokines and
disease. Nat Immunol. 2:108–115. 2001. View
Article : Google Scholar
|
|
28.
|
Lowy AM, Clements WM, Bishop J, et al:
beta-catenin/Wnt signaling regulates expression of the membrane
type 3 matrix metalloproteinase in gastric cancer. Cancer Res.
66:4734–4741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Yamada T, Takaoka AS, Naishiro Y, et al:
Transactivation of the multidrug resistance 1 gene by T-cell factor
4/beta-catenin complex in early colorectal carcinogenesis. Cancer
Res. 60:4761–4766. 2000.PubMed/NCBI
|
|
30.
|
Sobin LH: TNM, sixth edition: new
developments in general concepts and rules. Semin Surg Oncol.
21:19–22. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Curran T and Franza BR Jr: Fos and Jun:
the AP-1 connection. Cell. 55:395–397. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Tan TW, Yang WH, Lin YT, et al: Cyr61
increases migration and MMP-13 expression via alphavbeta3 integrin,
FAK, ERK and AP-1-dependent pathway in human chondrosarcoma cells.
Carcinogenesis. 30:258–268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Eferl R and Wagner EF: AP-1: a
double-edged sword in tumorigenesis. Nat Rev Cancer. 3:859–868.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
35.
|
de Mestre AM, Rao S, Hornby JR, Soe-Htwe
T, Khachigian LM and Hulett MD: Early growth response gene 1 (EGR1)
regulates heparanase gene transcription in tumor cells. J Biol
Chem. 280:35136–35147. 2005.
|
|
36.
|
Zheng L, Pu J, Jiang G, et al: Abnormal
expression of early growth response 1 in gastric cancer:
association with tumor invasion, metastasis and heparanase
transcription. Pathol Int. 60:268–277. 2010. View Article : Google Scholar
|