Introduction
Colorectal cancer (CRC) is one of the most common
cancers in the world and ∼90% of CRC deaths are caused by
metastasis, not by primary solid tumors (1). Despite recent advances, systemic
chemotherapy for metastatic disease is considered palliative and
long-term survivors are rarely seen treated only by chemotherapy
(2).
Overexpression of fatty acid synthase (FASN) is
common in many human cancers and blocking FASN inhibits growth and
leads to apoptosis in these cancer cells (3). Cerulenin is a small molecular FASN
inhibitor, which has been isolated from Cephalosporium caerulens.
Cerulenin contains an epoxy group that reacts with the ketoacyl
synthase domain. It has revealed significant antitumor activity in
various cancer cells by inducing apoptosis and growth inhibition
(4).
Oxaliplatin is a platinum-based drug considered to
be the most promising chemotherapy for CRC (5). Oxaliplatin treatment produces high
levels of single- and double-strand breaks in DNA due to
replication fork collapse and nuclease attack at the site of
platinated cross-links and finally causes cell cycle arrest
(6). Several clinical trials have
shown, however, that oxaliplatin by itself is less effective
compared to oxaliplatin combination therapy (7).
Our previous study showed the ability of cerulenin
to cause cytotoxicity and induce apoptosis in murine CRC cells and
in a murine xenograft model (8).
In this study we reveal that cerulenin causes cytotoxicity of human
CRC cell line HCT116 in vitro and in vivo. Next, we
hypothesized that cerulenin can potentiate the cytotoxicity of
oxaliplatin. In this study we report cerulenin and oxaliplatin have
synergistic cytotoxicity and cerulenin can reduce the dosage of
oxaliplatin in the treatment of human CRC.
Materials and methods
Reagents
Cerulenin and oxaliplatin were obtained from Sigma
(St. Louis, MO, USA). For cell culture and i.p. injections,
cerulenin was dissolved in acetone at a concentration of 20 mg/ml
and stored at −20°C. Oxaliplatin was dissolved in sterile water. In
in vitro experiments, 12.5–100 μM of cerulenin and
0.5–2.5 μM of oxaliplatin were added to the medium. Cell
viability assay and western blot experiments were performed 24 h
later after adding cerulenin and oxaliplatin. In in vivo
experiments, treatment with cerulenin at 15 and 30 mg/kg were given
i.p. at days 7, 10, 14 and 17 after tumor inoculation. In in
vivo treatment with oxaliplatin, 2.5 and 5 mg/kg of oxaliplatin
were given i.p. at the same schedule as cerulenin.
Cell culture
The human CRC cell lines HCT116 and RKO were used
and tested for mycoplasm-free cell lines. These cancer cells were
subdivided in multiple tubes for stock in liquid nitrogen
immediately after possession. All cell lines were subjected to the
present experiment within 6 months of resuscitation. Stock cultures
were grown in high-glucose DMEM containing 10% FBS and 1%
antibiotics. The cells were grown in growth medium at 37°C in a 95%
air, 5% CO2-humidified incubator.
Cell viability assay
To measure the cytotoxicity of cerulenin against
HCT116 and RKO cells, 3×103 cells were plated per well
onto 96-well plates. Following overnight culture, cerulenin and
oxaliplatin were added at specified concentrations. After 24 h of
incubation, cell viability was measured by the mitochondrial
activity in reducing
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium
monosodium salt (WST-8) to formazan using a Cell Counting kit-8
(Dojindo Laboratories, Kumamoto, Japan). Cells were incubated with
a reagent according to the manufacturer’s instructions. Plates were
read at A450 on a spectrometer.
Cell proliferation assay
To measure the cell proliferation activity of
cerulenin and oxaliplatin against HCT116 and RKO cancer cells,
3×103 cells were plated per well onto 96-well plates.
Following overnight culture, cerulenin and oxaliplatin were added
at specified concentrations. After 24 h of incubation, cell
proliferation was measured with a BrdU assay kit (Roche
Diagnostics, Penzberg, Germany). Cells were incubated with a
reagent as per the manufacturer’s instructions. Plates were read at
A450 on a spectrometer.
Apoptosis assay
The In situ Cell Death Detection kit (Roche
Diagnostics, Basel, Switzerland) was used for the demonstration of
apoptotic cell death of cell culture. Cells (3×104) were
plated per well onto Lab-Tek II Chamber Slides (Nalge Nunc
International, Tokyo, Japan) and were incubated with the terminal
deoxynucleotidyl transferase-mediated dUTP nick-end labeling
(TUNEL) reaction mixture according to the manufacturer’s
recommendations.
Western blot analysis
For western blot analysis, total protein extracts of
HCT116 cells were obtained 24 h after cerulenin and oxaliplatin
treatment and separated by 10% SDS-PAGE and transferred to
nitrocellulose membrane (Millipore, Bedford, MA, USA). The
following antibodies were used as primary antibodies: total Akt
(9272), phosphoserine 473 Akt (9271), cleaved caspase-3 (9661),
phospho-p38 (4511p), phosphoserine 15 p53 (9284p), p21waf1 (2947p)
and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (2118) (Cell
Signaling Technology, Beverly, MA, USA). Purified mouse anti-FAS
antibody (610962) was purchased from BD Biosciences (San Jose, CA,
USA). Secondary goat anti-rabbit and goat anti-mouse antibodies
conjugated with horseradish peroxidase were purchased from Cell
Signaling Technology. Immunoblots were analyzed by enhanced
chemiluminescence.
Animals
Eight-week-old male severe combined immunodeficiency
(SCID) mice (Clea, Tokyo, Japan), weighing 24–28 g were utilized.
The mice were kept in a temperature-controlled room on a 12-h
light-dark cycle. They had free access to water and standard chow
throughout the experiment. After an acclimation period of ≥7 days,
the mice were separated into four groups as follows: control group,
mice without any treatment (n=12); cerulenin group, mice with
cerulenin treatment 15 (n=5) and 30 mg/kg (n=5); oxaliplatin group,
mice with oxaliplatin treatment 2.5 (n=8) and 5 mg/kg (n=5); and
combination group, mice with 15 mg/kg of cerulenin and 2.5 mg/kg of
oxaliplatin treatment (n=10). All animal experiments were carried
out in a humane manner after receiving approval from the
Institutional Animal Committee of Teikyo University and in
accordance with the Regulation for Animal Experiments of the
University and Fundamental Guidelines for Proper Conduct of Animal
Experiments and Related Activities in Academic Research
Institutions under the jurisdiction of the Ministry of Education,
Culture, Sports, Science and Technology of Japan.
Xenograft
Cells (2×106) of HCT116 were injected
subcutaneously into the right flank of each mouse with a 27-gauge
needle. Tumors were detected by palpation and measured periodically
with calipers. Seven days after tumor injection, cerulenin and
oxaliplatin were injected intraperitoneally every 3 days.
Twenty-one days after inoculation, the mice were sacrificed and
tumors were removed for examination. Tumor tissue, fixed in 10%
buffered formalin, was used for histological analyses.
Statistical analysis
All data are expressed as the mean ± SD of samples.
Comparisons between various points were made using one-way ANOVA.
Significant data were examined by the Bonferroni-Dunn multiple
comparisons post hoc test. In all cases, P<0.05 was
considered significant.
Results
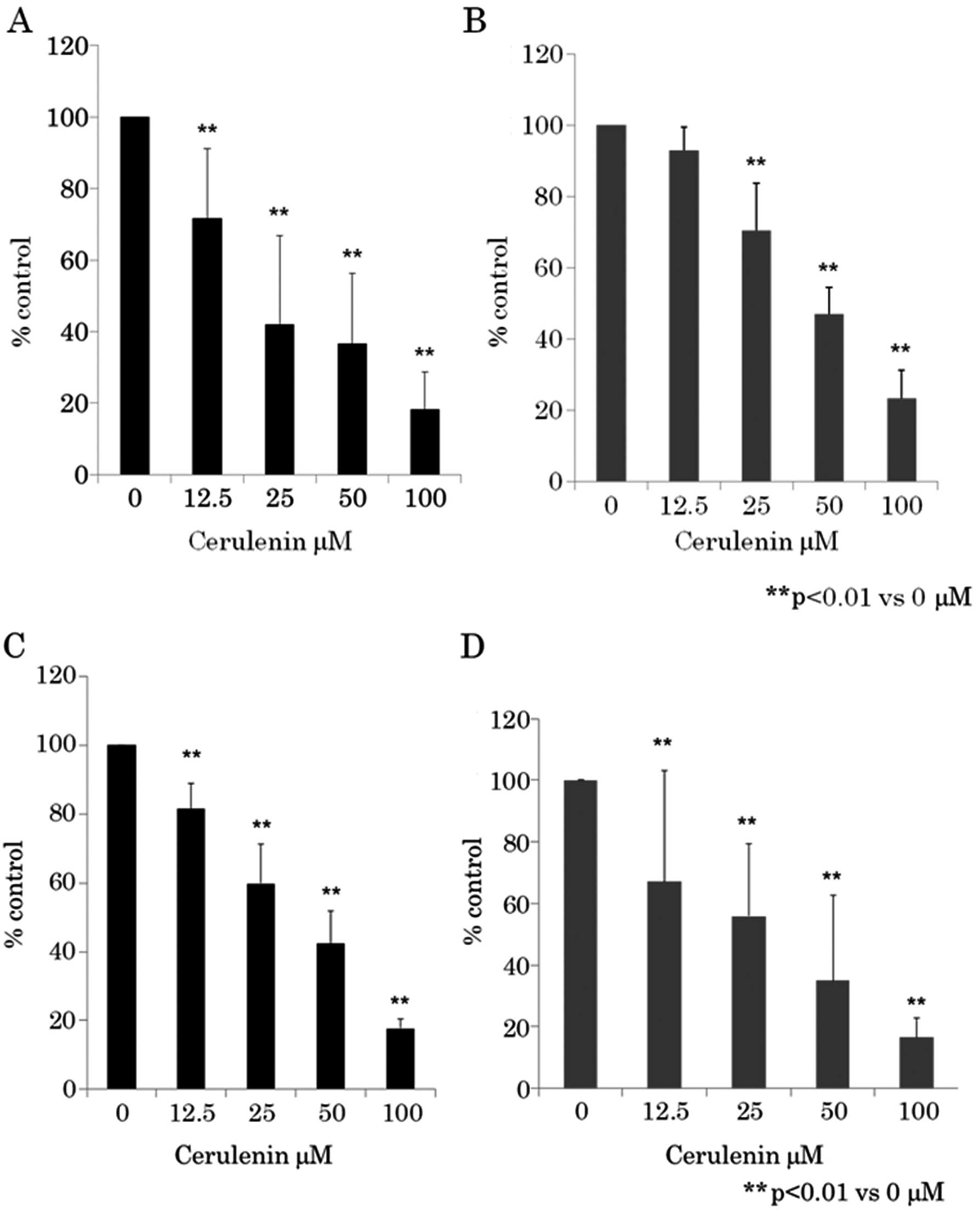
Dose-dependent inhibition of
proliferation of human CRC cell lines by cerulenin
We initially determined whether cerulenin treatment
led to the inhibition of human CRC cell proliferation. CRC cells
were treated with various doses of cerulenin for 24 h and cell
viability was assayed using WST-8 assay (Fig. 1A and B) and BrdU assay (Fig. 1C and D). Fig. 1 shows that as the dose of cerulenin
increased from 12.5 to 100 μM, cell growth inhibition
increased in a dose-dependent manner in CRC cell lines HCT116 and
RKO. Cerulenin-induced growth inhibition was found to be
statistically significant (p<0.01) (one-way ANOVA) in 12.5–100
μM of cerulenin compared to 0 μM.
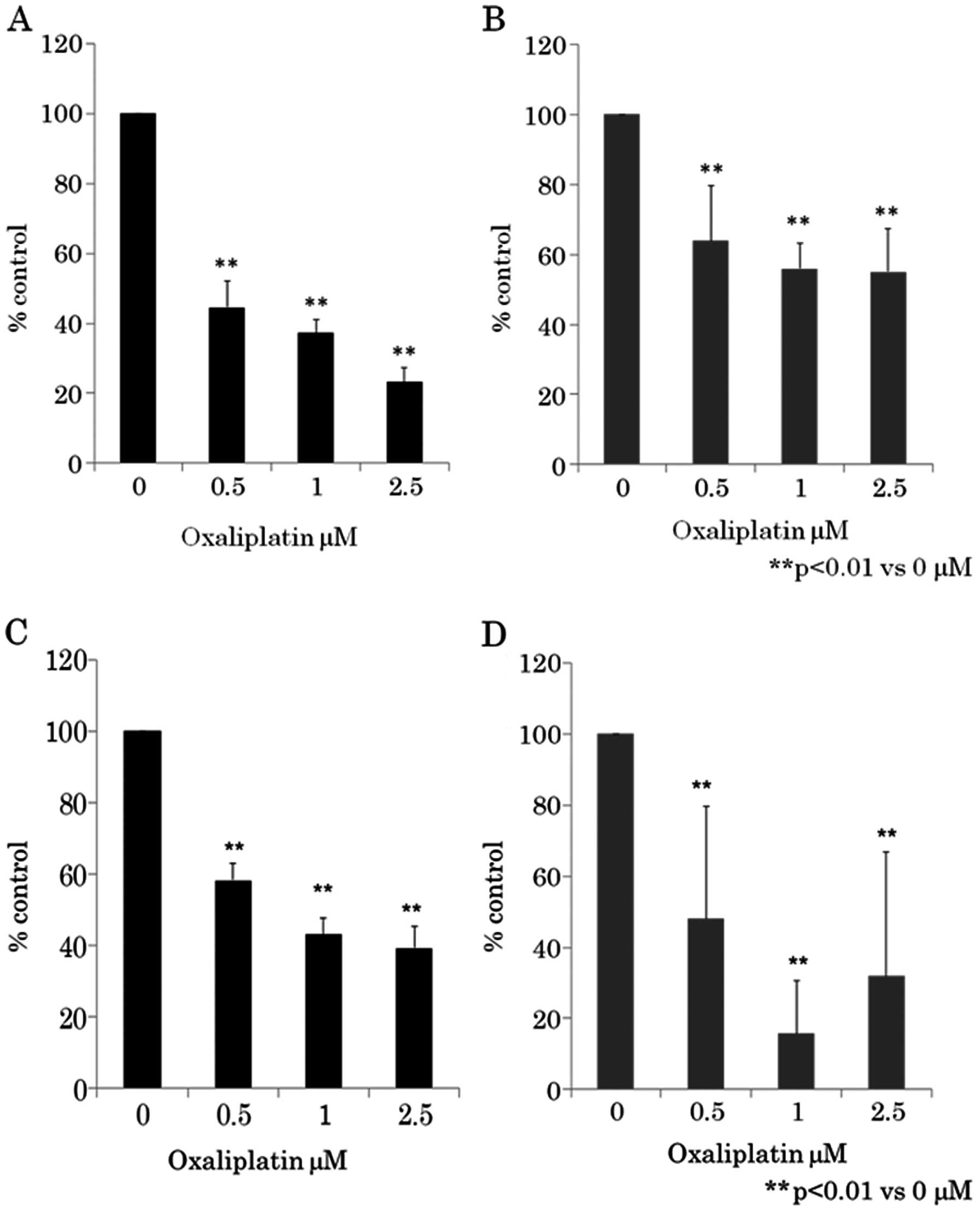
Dose-dependent inhibition of
proliferation of human CRC cell lines by oxaliplatin
Next, we determined whether oxaliplatin treatment
led to the inhibition of human CRC cell proliferation. CRC cells
were treated with various doses of oxaliplatin for 24 h and cell
viability was assayed using WST-8 assay (Fig. 2A and B) and BrdU assay (Fig. 2C and D). Fig. 2 shows that as the dose of
oxaliplatin increased from 0.5 to 2.5 μM, cell growth
inhibition increased in a dose-dependent manner in CRC cell lines
HCT116 and RKO. Oxaliplatin-induced growth inhibition was found to
be statistically significant (p<0.01) (one-way ANOVA) in 0.5–2.5
μM of oxaliplatin compared to 0 μM.
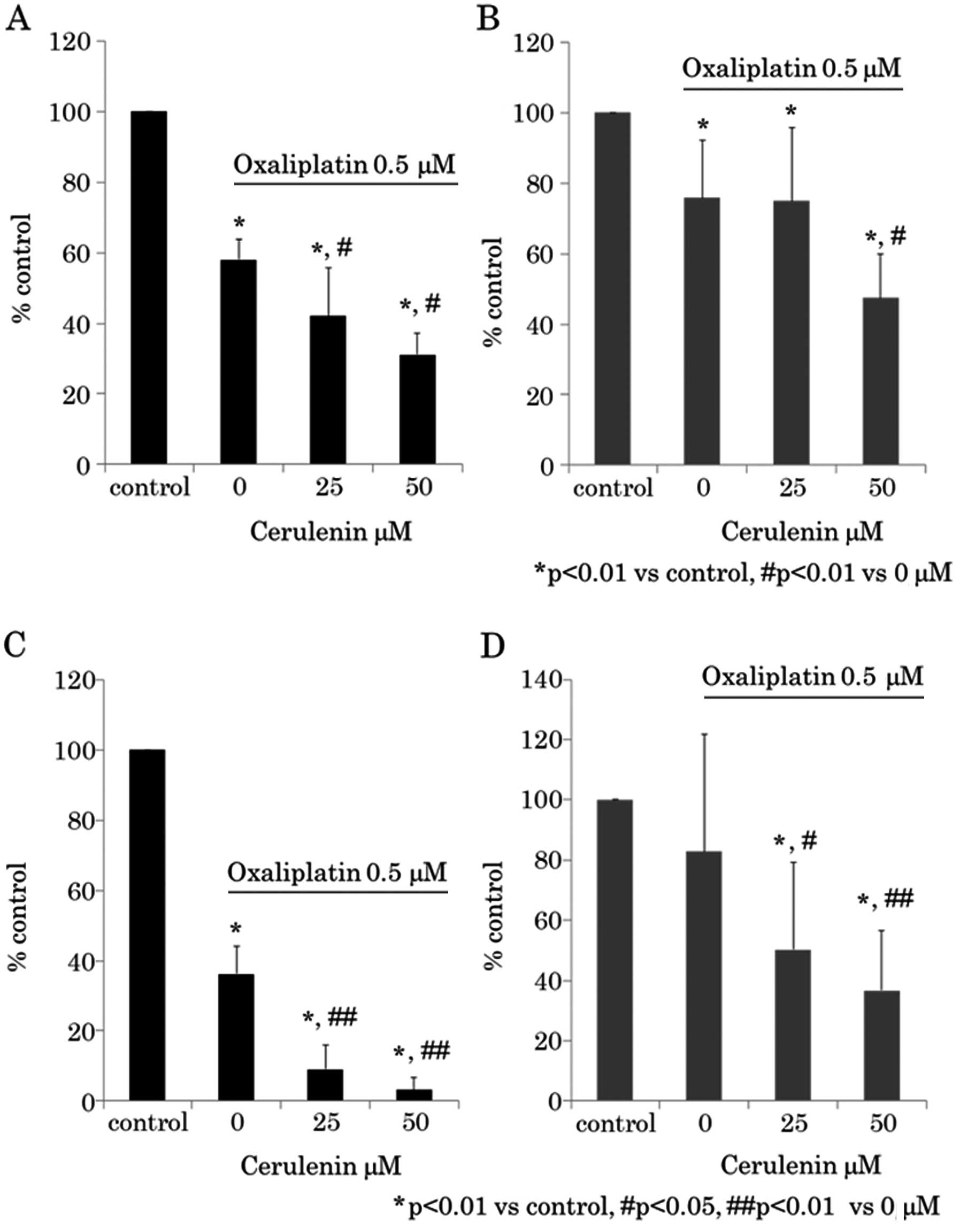
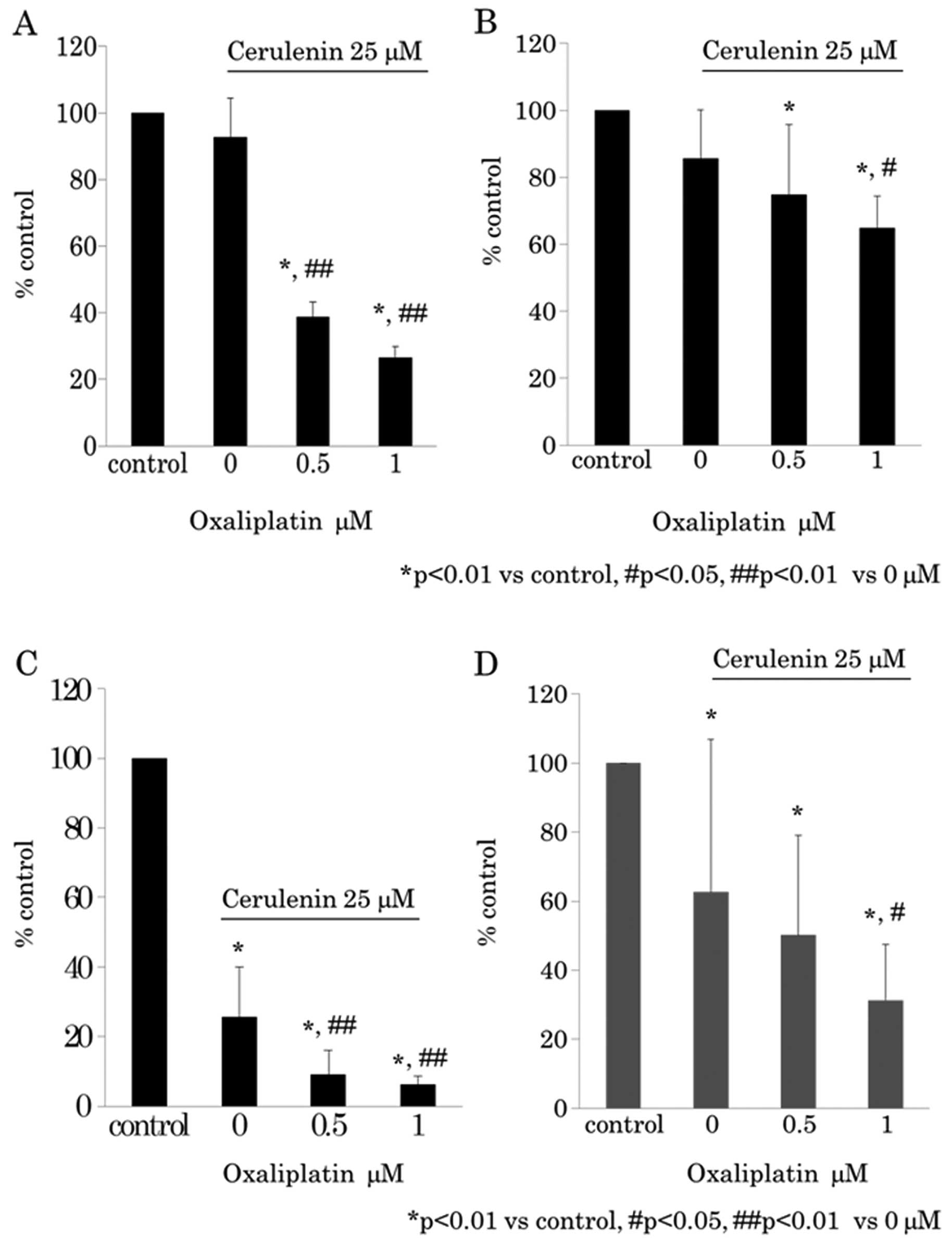
Synergistic antitumor effect between
cerulenin and oxaliplatin
Next we determined whether a synergistic antitumor
effect exists between cerulenin and oxaliplatin. The cerulenin
effect under 0.5 μM of oxaliplatin was evaluated using WST-8
assay (Fig. 3A and B) and BrdU
assay (Fig. 3C and D). The
oxaliplatin effect under 25 μM of cerulenin was evaluated
using WST-8 assay (Fig. 4A and B)
and BrdU assay (Fig. 4C and D).
The results indicated that cerulenin and oxaliplatin have synergic
antitumor effects.
Induction of apoptosis via activation of
caspase-dependent pathway by cerulenin combined with
oxaliplatin
In subsequent experiments, we determined the
mechanism of the observed suppressive effect of combination therapy
by WST-8 and BrdU assays. The overexpression of FASN has been
observed to cooperate with survival pathways, including the
phosphatidylinositol-3-kinase (PI3K)/Akt pathway. HCT116 cells
expressed FASN and p-Akt constitutively and treatment of cerulenin
suppressed FAS expression in 100 μM of cerulenin as
previously reported by us (data not shown). Dephosphorylated
constitutive activated Akt, activation of p38 and increased cleaved
caspase-3 in cerulenin treatment (Fig.
5A). Oxaliplatin induced p53–p21 pathway and p38, but did not
increase cleaved caspase-3 (Fig.
5B). In combination therapy, p53–p21 pathway and p38 activation
occurred in a lower dose and induced caspase-3 cleavage (Fig. 5C).
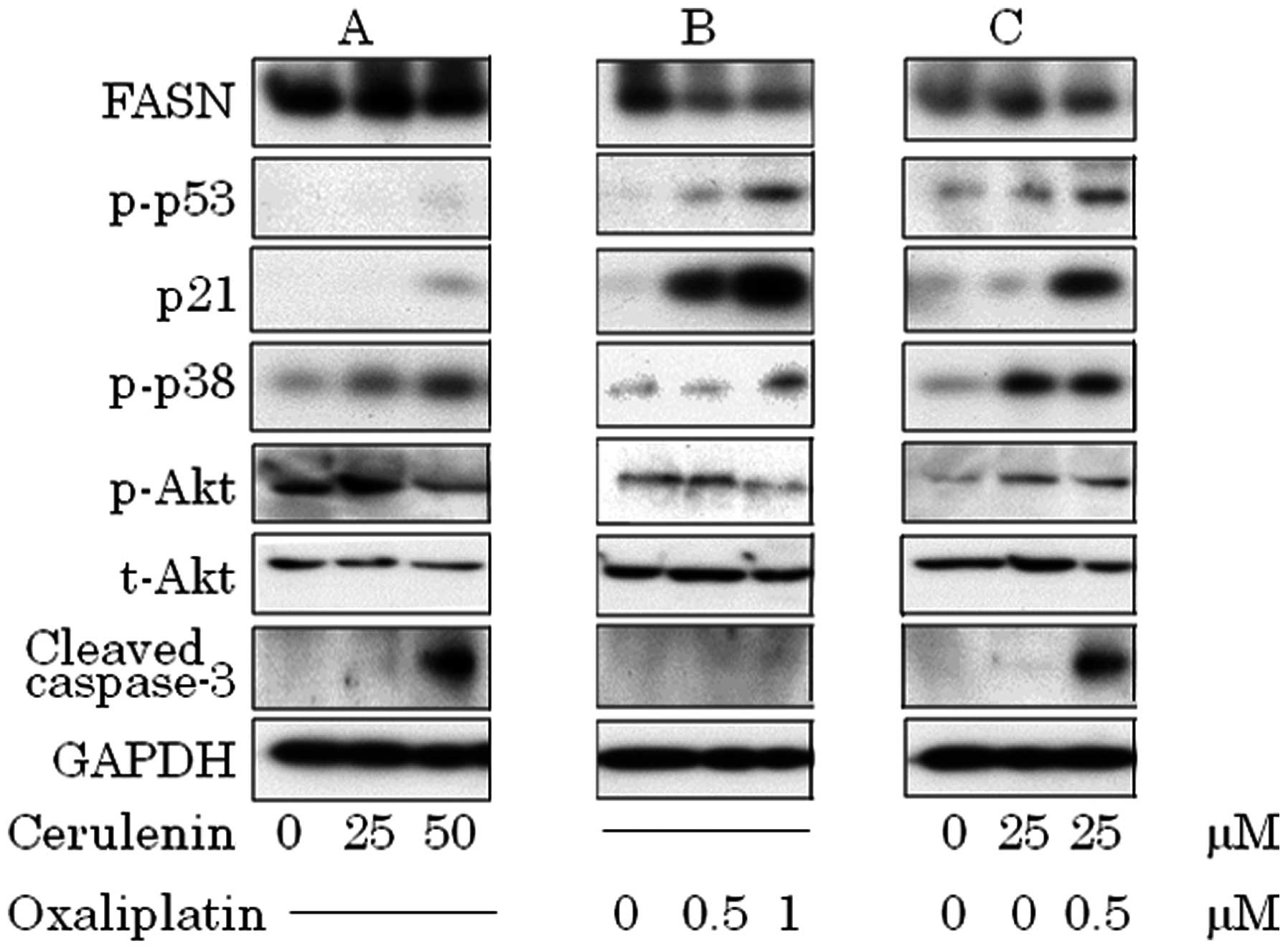 | Figure 5.(A) Cerulenin treatment causes p38
phosphorylation, dephosphorylation of constitutive phosphorylation
of Akt and accumulation of cleaved caspase-3. (B) Oxaliplatin
treatment causes p53 phosphorylation, and p21 activation. (C) In
combination, activation of p53–p21 pathway, p38 and caspase-3
cleavage. (A) HCT116 was treated with 0, 25 and 50 μM of
cerulenin for 24 h. (B) HCT116 was treated with 0, 0.5 and 1.0
μM of oxaliplatin for 24 h. (C) HCT116 was treated with 25
μM of cerulenin combined with 0.5 μM of oxaliplatin.
After cell lysis, equal amounts of proteins were separated by
SDS-PAGE, transferred to Immobilon membrane and immunoblotted with
antibodies against FASN, p-p53, p21, p-p38, p-Akt, t-Akt, cleaved
caspase-3 and GAPDH as indicated. |
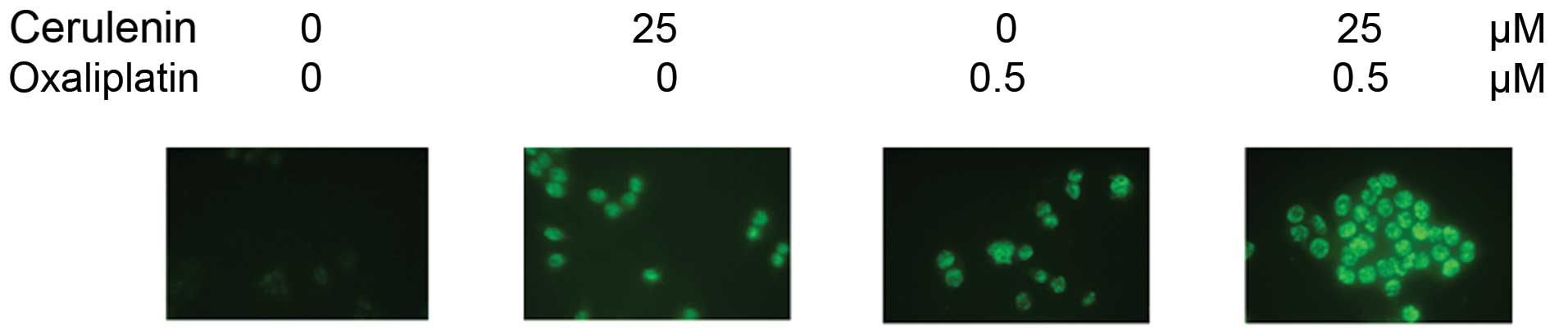
Apoptotic effect of HCT116 by cerulenin
and oxaliplatin combination therapy
TUNEL staining of HCT116 cells shows apoptotic cells
in 25 μM of cerulenin and 0.5 μM of oxaliplatin.
Combination with cerulenin and oxaliplatin induced apoptosis
significantly (Fig. 6).
Cerulenin combined with oxaliplatin
inhibits tumor growth of HCT116 xenografts
We evaluated the potential effectiveness of
cerulenin and oxaliplatin combination for a xenograft model of
HCT116, subcutaneously injected into the right flank of each mouse.
Fig. 7A shows the weight of
animals in control, cerulenin 15 mg/kg (cer 15) and 30 mg/kg (cer
30), oxaliplatin 2.5 mg/kg (ox 2.5) and 5 mg/kg (ox 5) and
combination group, with 15 mg/kg of cerulenin and 2.5 mg/kg of
oxaliplatin (cer 15 ox 2.5). In control, cer 15, ox 2.5 and ox 5
groups, significant body weight loss was observed during treatment.
However, in cer 15 ox 2.5 group, significant weight loss was not
observed. Fig. 7B shows tumors
removed from the representative control, cer 15, cer 30, ox 2.5, ox
5 and cer 15 ox 2.5 groups. Tumor growth was significantly
inhibited in the cer 30 and ox 5 group compared to the control
group. In cer 15 ox 2.5 group, the tumor growth was inhibited at
the same level compared to cer 30 and ox 5. Fig. 7C indicates tumor weight in the 6
groups. Tumor growth was significantly reduced in cer 30, ox 5 and
cer 15 ox 2.5 groups compared to control group.
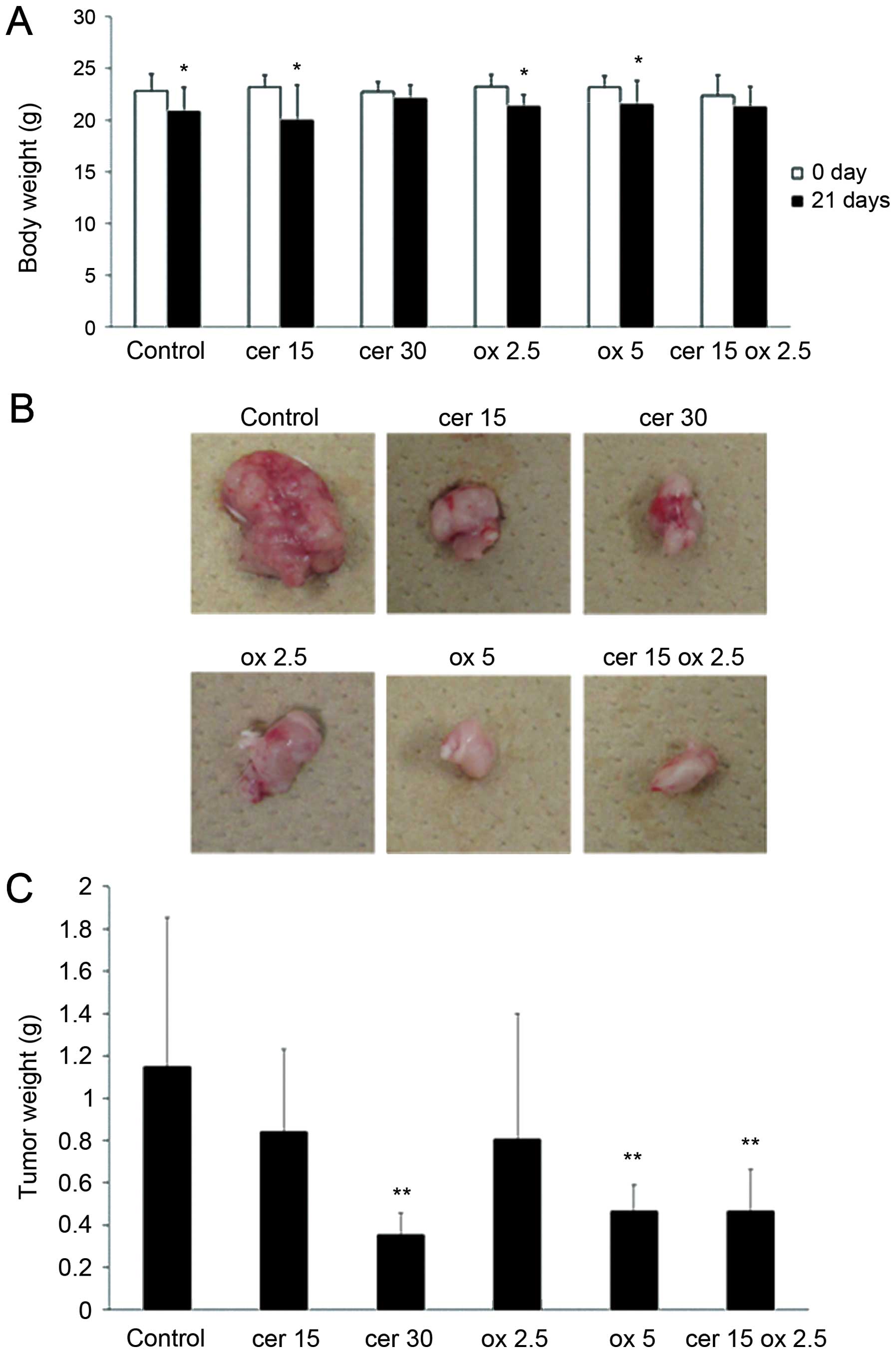 | Figure 7.Cerulenin and oxaliplatin combination
therapy significantly inhibits tumor growth of the xenograft HCT116
tumors in SCID mice. HCT116 was injected to the right flank
subcutaneously. Seven days later, cerulenin 15 and 30 mg/kg,
oxaliplatin 2.5 and 5 mg/kg and cerulenin 15 mg/kg and oxaliplatin
2.5 mg/kg were treated 4 times. cer 15, cerulenin 15 mg/kg; cer 30,
cerulenin 30 mg/kg; ox 2.5, oxaliplatin 2.5 mg/kg; ox 5,
oxaliplatin 5 mg/kg; cer 15 ox 2.5, cerulenin 15 mg/kg and
oxaliplatin 2.5 mg/kg. (A) Weight of animals in 6 groups. Columns,
mean; bars, SD. White bar, on the day of tumor injection. Black
bar, 21 days after tumor inoculation. *p<0.05 versus
0 day of control group, t-test. (B) Representative xenograft tumors
of the 6 groups. (C) Tumor weight of the 6 groups.
**p<0.01 versus control group. |
Discussion
The role of increased FASN in cancer cells and the
mechanisms of cell killing by inhibitors of FASN are still not
fully understood (9). The effect
of an intermediate metabolite of fatty acid synthesis on cancer
cells is likely mediated through cell signaling pathways,
especially Akt (10). The
overexpression of FASN has been observed to cooperate with survival
pathways including the phosphatidylinositol-3-kinase (PI3K)/Akt
pathway. HCT116 expressed FASN and p-Akt constitutively and
treatment with cerulenin suppressed FASN expression,
dephosphorylated constitutive activated Akt, activated p38 and
increased cleaved caspase-3, finally causing apoptosis.
p38 MAP kinase is a member of the MAP kinase family
and is activated by a variety of cellular stresses including
osmotic shock, inflammatory cytokines, lipopolysaccharide, UV light
and growth factors (11–15). Activated p38 MAP kinase appears to
have multiple targets in the apoptotic pathway. In nitric oxide
(NO)-induced neuronal apoptosis, p38 MAP kinase activates caspase
and induces apoptosis (16). C75
is one of the FASN inhibitors reported to induce p38 activation
(17). Oxaliplatin activates p38
MAP kinase phosphorylation in human colon carcinoma cells (18).
The p53 protein, a well-characterized tumor
suppressor, plays a pivotal role in the maintenance of genomic
stability (19,20). Activation of p53 can lead to either
cell cycle arrest and DNA repair or apoptosis (21). DNA damage induces phosphorylation
of p53 at Ser15 and phosphorylation promotes both the accumulation
and activation of p53 in response to DNA damage (22). Activated p53 up-regulates p21
transcription (23). HCT116 cell
line, which harbors a wild-type p53 protein, is sensitive to
oxaliplatin treatment (24). The
p53–p21 pathway is a major determinant of sensitivity to
oxaliplatin of the p53 wild-type HCT116 cell line (25). The p53–p21 pathway leads to cell
cycle arrest without apoptosis (26). Based on our results, oxaliplatin
induces the p53–p21 pathway, causing cell cycle arrest without
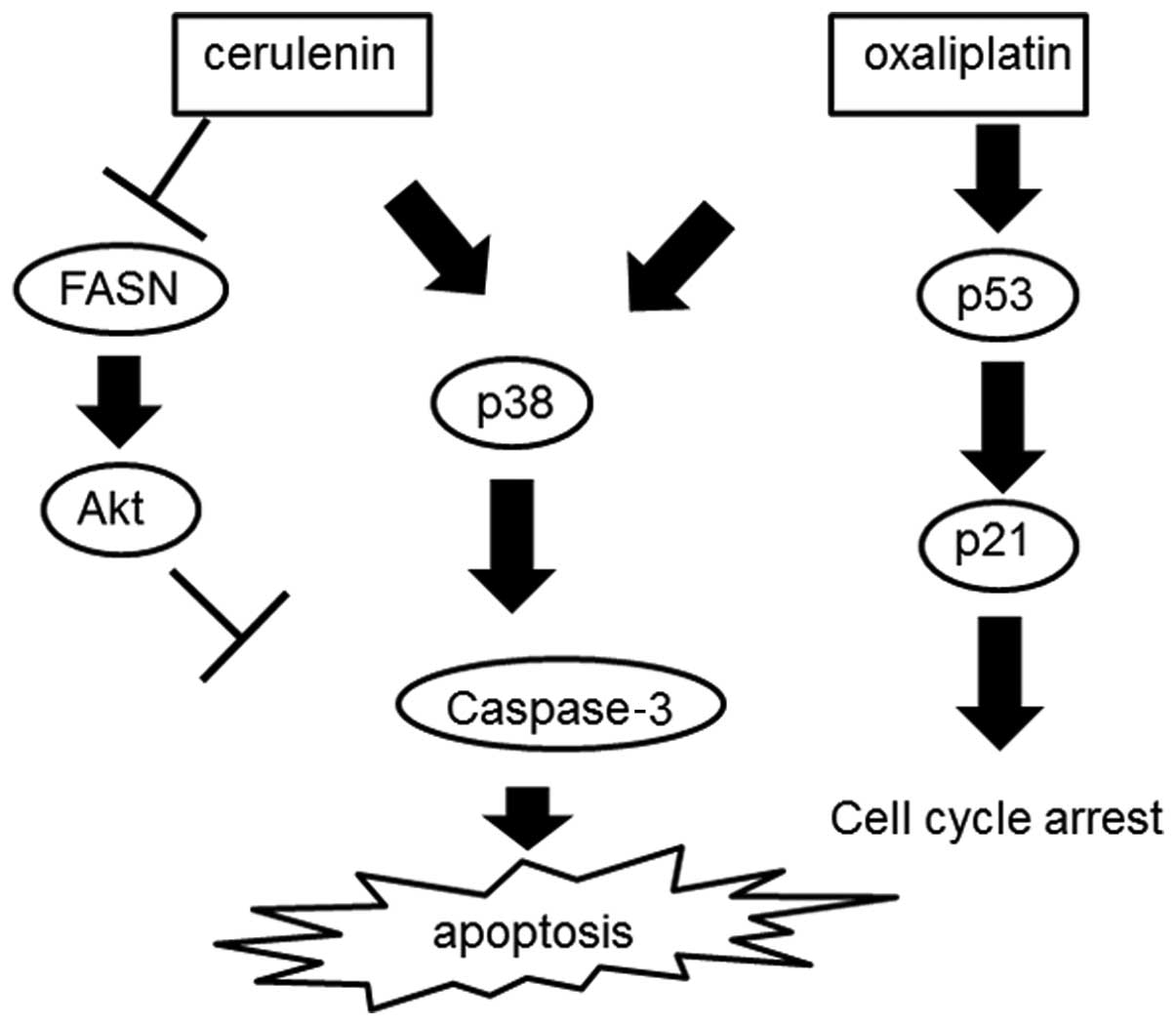
activating apoptotic pathways. In combination with cerulenin and
oxaliplatin, p53–p21 pathway and p38 were activated, which causes
cell cycle arrest and apoptosis. In a xenotransplant mouse model,
the combination therapy, which consists of 2.5 mg/kg of oxaliplatin
and 15 mg/kg of cerulenin, had the same tumor shrinkage effect
compared to the oxaliplatin 5 mg/kg group, which means that by
adding cerulenin oxaliplatin dose could be reduced. In the
combination therapy, mitotic figures of the tumor were
significantly decreased and TUNEL-positive cancer cells were
increased. Fig. 8 shows the scheme
of the combination therapy consisting of cerulenin and
oxaliplatin.
Oxaliplatin is a most promising chemotherapeutic
agent, which consists of FOLFOX for unresectable CRC (5). Neurotoxicity is a severe and
treatment-limiting side-effect of several chemotherapeutic agents
(27). Sensory neurotoxicity is a
potentially limiting factor in many patients who might otherwise
achieve good results with oxaliplatin therapy (28). In patients with severe
neurotoxicity, reduction or discontinuation of oxaliplatin is often
required. This study revealed that cerulenin can potentiate
oxaliplatin in in vitro and in vivo. Cerulenin is one
of the better combinations with oxaliplatin, which achieves
reduction of oxaliplatin and long-term tolerated chemotherapy for
unresectable CRC.
In conclusion, cerulenin has a cytotoxic effect on
human CRC cell line HCT116. Moreover, cerulenin potentiated
cytotoxicity of oxaliplatin. Cerulenin would be effective in
treatment of unresectable CRC in combination with oxaliplatin,
which reduces the dose of oxaliplatin and would make it possible to
endure the chemotherapy over a longer period.
Acknowledgements
The authors thank Satoko Nakabayashi
for technical assistance. This study was supported in part by
grants-in-aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan (MEXT).
References
|
1.
|
Gupta GP and Massague J: Cancer
metastasis: building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Tachimori A, Yamada N, Amano R, et al:
Combination therapy of S-1 with selective cyclooxygenase-2
inhibitor for liver metastasis of colorectal carcinoma. Anticancer
Res. 28:629–638. 2008.PubMed/NCBI
|
|
3.
|
Kuhajda FP: Fatty acid synthase and
cancer: new application of an old pathway. Cancer Res.
66:5977–5980. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Elbaz A, Wu X, Rivas D, et al: Inhibition
of fatty acid biosynthesis prevents adipocyte lipotoxicity on human
osteoblasts in vitro. J Cell Mol Med. 14:982–991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Wils J: Adjuvant treatment of colon
cancer: past, present and future. J Cemother. 19:115–122. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Pires IM, Ward TH and Dive C: Oxaliplatin
responses in colorectal cancer cells are modulated by CHK2 kinase
inhibitors. Br J Pharmacol. 159:1326–1338. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Ramanathan RK, Clark JW, Kemeny NE, et al:
Safety and toxicity analysis of oxaliplatin combined with
fluorouracil or as a single agent in patients with previously
treated advanced colorectal cancer. J Clin Oncol. 21:2904–2911.
2003. View Article : Google Scholar
|
|
8.
|
Murata S, Yanagisawa K, Fukunaga K, Oda T,
Kobayashi A, Sasaki R and Ohkohchi N: Fatty acid synthase inhibitor
cerulenin suppresses liver metastasis of colon cancer in mice.
Cancer Sci. 101:1861–1865. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Orita H, Coulter J, Lemmon C, et al:
Selective inhibition of fatty acid synthase for lung cancer
treatment. Clin Cancer Res. 13:7139–7145. 2007.PubMed/NCBI
|
|
10.
|
Wang HQ, Altomare DA, Skele KL, et al:
Positive feedback regulation between AKT activation and fatty acid
synthase expression in ovarian carcinoma cells. Oncogene.
24:3574–3582. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Rouse J, Cohen P, Trigon S, et al: A novel
kinase cascade triggered by stress and heat shock that stimulates
MAPKAP kinase-2 and phosphorylation of the small heat shock
proteins. Cell. 78:1027–1037. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Han J, Lee JD, Bibbs L and Ulevitch RJ: A
MAP kinase targeted by endotoxin and hyperosmolarity in mammalian
cells. Science. 265:808–811. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Lee JC, Laydon JT, McDonnell PC, et al: A
protein kinase involved in the regulation of inflammatory cytokine
biosynthesis. Nature. 372:739–746. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Freshney NW, Rawlinson L, Guesdon F, et
al: Interleukin-1 activates a novel protein kinase cascade that
results in the phosphorylation of Hsp27. Cell. 78:1039–1049. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Raingeaud J, Gupta S, Rogers JS, et al:
Pro-inflammatory cytokines and environmental stress cause p38
mitogen-activated protein kinase activation by dual phosphorylation
on tyrosine and threonine. J Biol Chem. 270:7420–7426. 1995.
View Article : Google Scholar
|
|
16.
|
Ghatan S, Larner S, Kinoshita Y, et al:
p38 MAP kinase mediates Bax translocation in nitric oxide-induced
apoptosis in neurons. J Cell Biol. 150:335–347. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Gao Y, Lin LP, Zhu CH, Chen Y, Hou YT and
Ding J: Growth arrest induced by C75, A fatty acid synthase
inhibitor, was partially modulated by p38 MAPK but not by p53 in
human hepatocellular carcinoma. Cancer Biol Ther. 8:978–985. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Liu HF, Hu HC and Chao JI: Oxaliplatin
down-regulates survivin by p38 MAP kinase and proteasome in human
colon cancer cells. Chem Biol Interact. 188:535–545. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Schwartz D and Rotter V: p53-dependent
cell cycle control: response to genotoxic stress. Semin Cancer
Biol. 8:325–336. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shieh SY, Ikeda M, Taya Y and Prives C:
DNA damage-induced phosphorylation of p53 alleviates inhibition by
MDM2. Cell. 91:325–334. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Wang Y and Prives C: Increased and altered
DNA binding of human p53 by S and G2/M but not G1 cyclin-dependent
kinases. Nature. 376:88–91. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Gourdier I, Del Rio M, Crabbe L, et al:
Drug specific resistance to oxaliplatin is associated with
apoptosis defect in a cellular model of colon carcinoma. FEBS Lett.
529:232–236. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Toscano F, Parmentier B, Fajoui ZE, et al:
p53 dependent and independent sensitivity to oxaliplatin of colon
cancer cells. Biochem Pharmacol. 74:392–406. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Schwartz GK: Development of cell cycle
active drugs for the treatment of gastrointestinal cancers: a new
approach to cancer therapy. J Clin Oncol. 23:4499–4508. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Benett BK, Park SB, Lin CSY, et al: Impact
of oxaliplatin-induced neuropathy: a patient perspective. Support
Care Cancer. 20:2959–2967. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Gœbel FM, Tournigand C, André T, et al:
Oxaliplatin reintroduction in patients previously treated with
leucovorin, fluorouracil and oxaliplatin for metastatic colorectal
cancer. Ann Oncol. 15:1210–1214. 2004.PubMed/NCBI
|