Introduction
The non-Hodgkin’s lymphomas (NHLs) are a group of
lymphoproliferative malignancies with divergent clinical courses.
These disorders, which may originate from transformed B or T cells,
can be grouped into aggressive and indolent categories (1). The aggressive NHLs have a relatively
short natural history if untreated, but a proportion of patients
can be cured with intensive chemotherapy (2). In contrast, the indolent lymphomas
have a relatively long natural history, with median survival times
as long as 10 years (3). During
the early course of disease, the indolent lymphomas are generally
responsive to radiation therapy, chemotherapy and monoclonal
antibody (mAb)-based therapy and can be re-treated upon relapse. As
the disease progresses, it can become refractory to therapy, with
subsequent disease-free intervals becoming shorter (4). Although the NHLs have historically
been treated with radiation therapy and/or chemotherapy, the
standard of care has evolved to incorporate the use of rituximab, a
mAb, which is directed against the CD20 antigen expressed on the
surface of transformed B-lymphocytes (5).
CD80 (B7-1) is a cell-surface receptor of the
immunoglobulin superfamily that is best known for its role in the
costimulation of T-cell function causing T-cell proliferation,
cytokine production and generation of cytotoxic T lymphocytes
(6,7). The ligands for CD80 are CD28 and
cytotoxic T-lymphocyte antigen 4 (CTLA-4, CD152) (8). CD80 is transiently expressed on
antigen-presenting cells, T cells and activated B cells, but is
constitutively expressed on the surface of many B-cell lymphomas
(9–12). When cell-surface CD80 is
cross-linked with anti-CD80 antibodies, cell proliferation is
inhibited, pro-apoptotic molecules are upregulated and
antibody-dependent cell cytotoxicity (ADCC) is induced (13). These studies provide the rationale
for using an anti-CD80 mAb to treat lymphoma.
Galiximab is a primatized anti-CD80 mAb that has
been tested as monotherapy in phase I/II clinical trials involving
patients with relapsed/refractory follicular lymphoma (FL),
producing an overall response rate of 11% and tumor reductions in
46% of patients (14). In a recent
phase I/II clinical trial involving patients with relapsed or
refractory FL, combined therapy with galiximab and rituximab
yielded an overall response rate of 66% and a median
progression-free survival of 12.1 months at the recommended phase
II dose of galiximab (500 mg/m2) (15). A phase II CALGB trial of galiximab
and rituximab was undertaken in patients with previously untreated
FL. The regimen was well tolerated and efficacious in patients with
low risk FLIPI scores (16).
Recently, we have reported that treatment of
CD80+ B-NHL cells with galiximab resulted in cell
signaling-induced inhibition of the constitutively activated NF-κB
pathway. Inhibition of this pathway resulted downstream in the
inhibition of both the metastasis-inducer and resistant
transcription factor, SNAIL and the transcription and resistant
factor Ying Yang 1 (YY1) and these inhibitory effects led to
sensitization of tumor cells to apoptosis by drugs (e.g. CCDP and
TRAIL) (17).
The present study sought to examine the in
vivo efficacy of galiximab treatment on the growth of lymphoma
tumor xenografts. In addition, since galiximab sensitizes tumor
cells in vitro to apoptosis by drugs (17), we have also examined in vivo
the antitumor effect of galiximab used in combination with
fludarabine or doxorubicin. We show here that galiximab exhibits
antitumor activity as a single agent in solid and disseminated
human lymphoma xenografts in SCID mice. Further, the antitumor
activity of galiximab was enhanced when used in combination with
fludarabine.
Materials and methods
Cell lines
The human Epstein-Barr virus-transformed
B-lymphocyte cell line, SKW6.4 (TIB-215) and the Burkitt lymphoma
cell line, Raji (CCL-86), were obtained from ATCC (Manassas, VA,
USA). Cells were cultured in RPMI-1640 medium (ATCC, 30-2001)
supplemented with 10% fetal bovine serum (FBS; SH30071.03; HyClone,
Logan, UT), L-glutamine 2 mmol/l, sodium pyruvate 1 mmol/l, and 1%
penicillin-streptomycin at 37°C in an atmosphere of 5%
CO2. All cells used in this study were within 15
passages after resuscitation. The cells were checked routinely by
morphology and tested for mycoplasma contamination with the
CELLshipper Mycoplasma Detection kit (Bionique Testing
Laboratories).
Animals
Female CB17 mice at 6–8 weeks of age with severe
combined immunodeficiency (SCID) were used for in vivo tumor
modeling studies (Charles River Laboratories Inc., Holister, CA)
and were housed in polycarbonate cages using a HEPA-filtered,
ventilated rack system (Allentown Inc., Allentown, NJ). All animal
studies and procedures were performed under an institutionally
approved protocol for animal care and use (IACUC #SD12-04; Biogen
Idec, Cambridge, MA). The Biogen Idec animal facility is accredited
by the Association for Assessment and Accreditation of Laboratory
Animal Care International.
Drugs/antibodies
Galiximab (IDEC-114) is a high-affinity,
PRIMATIZED®, anti-CD80 immunoglobulin (Ig) G1, λ mAb.
This antibody was obtained by immunizing cynomolgus monkeys with
recombinant CD80 antigen, followed by cell fusion and cloning of
the antibody-secreting heterohybridoma. The variable regions of the
light and heavy chains were cloned and incorporated into a cassette
vector containing human constant region genes. The primatized
antibody, therefore, contains variable regions of cynomolgus
macaque origin and constant regions of human origin. The N5LG1
vector, which encodes the antibody, is expressed in the Chinese
hamster ovary transfectoma cell line DG44. The secreted antibody is
subsequently purified from the medium using chromatography and
filtration. Galiximab is formulated for human intravenous
administration as a sterile product in a buffer containing sodium
acetate 25 mmol/l, glycine 220 mmol/l, and 0.05% polysorbate 80 v/v
at pH 6.0. CE9.1 (Biogen Idec), a primatized, anti-CD4 IgG1 mAb,
served as an isotype-matched negative control. Fludarabine
(NDC#0703-4852-11; Teva Parenteral Medicines Inc., Irvine, CA) and
doxorubicin (NDC#55390-237-01; Bedford Labs, Bedford, OH) were the
chemotherapeutic agents used.
In vitro sensitization of Raji cells by
galiximab to apoptosis by fludarabine or doxorubicin
Raji cells were treated with different
concentrations of galiximab for 18 h and then treated with various
concentrations of fludarabine or doxorubicin for an additional 18
h. The cells were harvested and examined by flow for apoptosis for
the activation of caspase 3 as described previously (17).
The human lymphoma subcutaneous tumor
model
Mice with SCID were subcutaneously (s.c.) injected
in the flank with Raji cells (2×106) in 50% Matrigel
basement membrane (BD Biosciences, Bedford, MA, USA) on day 0.
After the tumors reached >100 mm3 in size, the mice
were randomized into groups (n=10) and intraperitoneally injected
with vehicle, control antibody (CE9.1), or various concentrations
of galiximab (0.1, 1, 3 and 10 mg/kg) as a single agent to
determine the optimum doses. Because the pharmacokinetic estimation
indicated that galiximab has a half-life of 8.6 days (data not
shown), galiximab was dosed once weekly. The mice received a total
of 3 treatments. The tumors were measured biweekly with calipers
and tumor volume was calculated using the formula: (length ×
width2)/2. The day-34 treatment effect was analyzed for
statistical significance using an unpaired Student’s t-test with a
95% confidence interval.
In addition, to determine combination effects, when
tumors reached 100–150 mm3 (early) or 200–400
mm3 (late), the mice were randomized into groups (n=10)
and intraperitoneally injected with vehicle, control antibody
(CE9.1), or galiximab (3 mg/kg per week). Some mice were then
treated with intraperitoneal fludarabine (100 mg/kg per day) 3 days
following the first antibody injection. These mice were treated
with fludarabine for 5 consecutive days. Treatment effects were
analyzed using an unpaired Student’s t-test with a 95% confidence
interval.
Disseminated human lymphoma model
SCID mice were intravenously injected in the tail
vein with SKW6.4 lymphoma cells (4×106 cells) on day 0.
On day 3, the mice were randomized into groups (n=10) and
intraperitoneally treated with vehicle, isotype-matched control
antibody (CE9.1), or varying concentrations of galiximab (5, 10
mg/kg). A total of 4 injections was administered on days 3, 10, 17
and 24; the mice were evaluated daily for disease-free survival
(DFS) and disease-related events. For the doxorubicin experiments,
the tumors were implanted as mentioned above. On day 3, the mice
were randomized into groups (n=10) and intraperitoneally injected
with vehicle, control antibody (CE9.1), or galiximab (10 mg/kg per
week). Re-treatment with antibody occurred on days 7, 10 and ≥24.
Two days after the first antibody injection, a group of mice was
intravenously injected with doxorubicin (2.5 mg/kg per week).
Re-treatment with doxorubicin occurred on days 12 and 19. DFS and
disease-related events were recorded using Kaplan-Meier survival
analysis. Because the progression of disseminated disease
subsequent to intravenous administration of malignant cells rapidly
leads to death and is preceded by hind-limb paralysis, a
disease-related event was defined as <20% weight loss (with
muscle weakness) and neurologic motor deficit. The anti-tumor
response was analyzed using a log-rank test with a 95% confidence
interval, and data were analyzed using GraphPad Prism (GraphPad
Software Inc., San Diego, CA).
Results
Galiximab demonstrates in vivo antitumor
activity in a human lymphoma tumor xenograft model
The antitumor activity of galiximab was tested in
vivo in mice bearing the Raji human tumor xenograft. SCID mice
were s.c. injected in vivo in the flank with Raji cells
(2×106) in 50% Matrigel on day 0. After the tumor
reached >100 mm3, the mice were randomized into
groups (n=10) and injected intraperitoneally with vehicle, control
IgG isotype (CE9.1), or various concentrations of galiximab (0.1,
1.0, 3.0, 10 and 30 mg/kg) as single agent. The mice received a
total of three treatments with galiximab (d14, d21 and d28). The
mice were measured bi-weekly with calipers and tumor volume was
calculated as described in methods. The day 34 treatment effect was
analyzed for statistical significance. The findings summarized in
Fig. 1A demonstrate a
dose-dependent decrease in the mean tumor volume. Tumor reduction
reached statistical significance with all galiximab concentrations
used (see table below Fig. 1A).
Analysis of the survival of the treated mice revealed that there
was a significant prolongation of survival in all mice treated with
concentrations of galiximab ranging from 5–30 mg/kg (Fig. 1B, and table below).
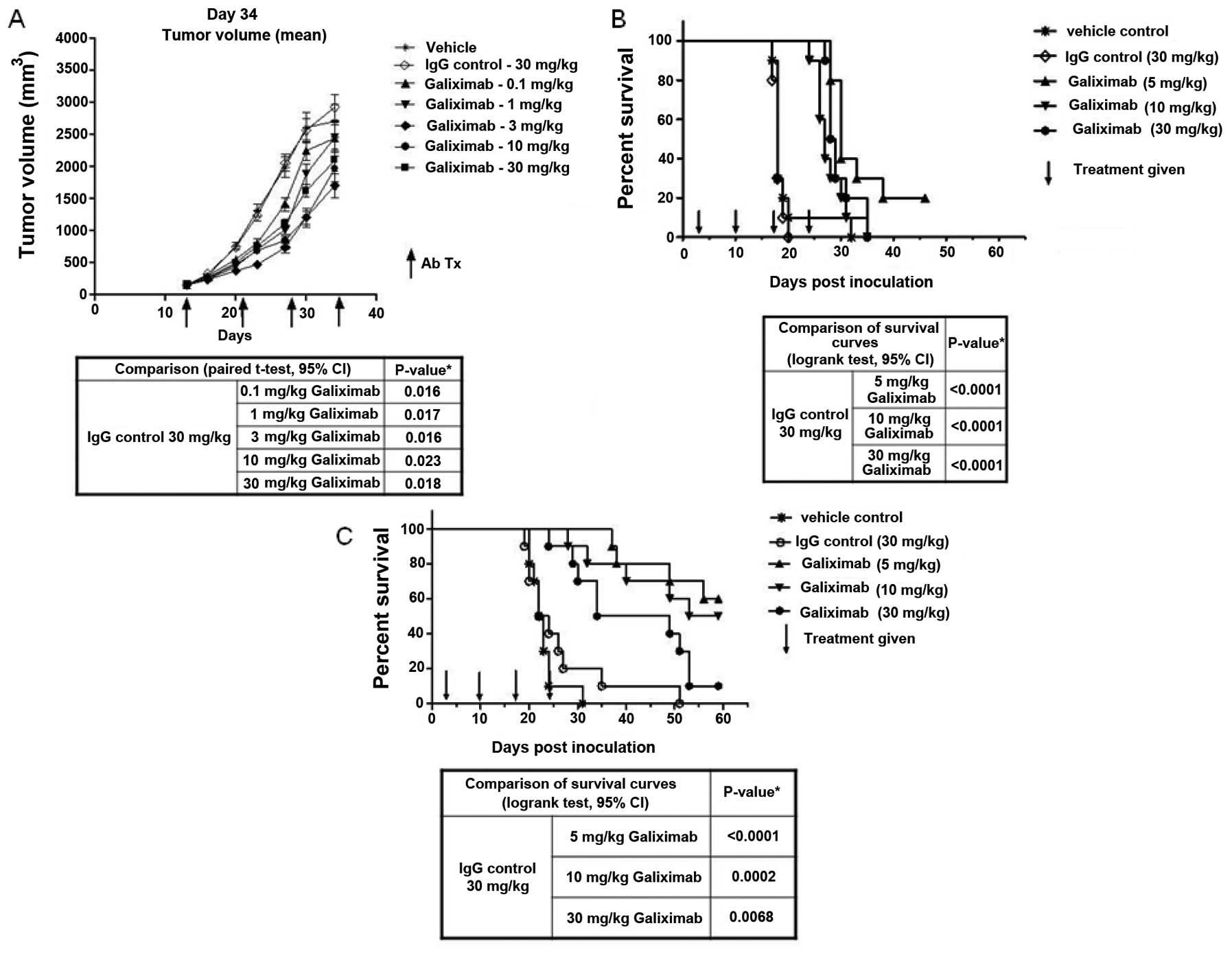 | Figure 1.Galiximab demonstrates antitumor
response and survival in solid tumor and disseminated lymphoma
tumor models. (A and B) On day 0, SCID mice were inoculated
subcutaneously in the flank with Raji lymphoma cells
(2×106) in 50% Matrigel. When the solid tumors reached
150 mm3, the mice were randomized into groups (n=10) and
treated weekly with intraperitoneal injections of vehicle, control
antibody or varying concentrations of galiximab (0.1–30 mg/kg) or
isotype-matched control antibody (CE9.1) (30 mg/kg) for a total of
4 injections. Tumor volume was calculated twice weekly, from
bidirectional caliper measurements using the formula: (length ×
width2)/2. On day 34, antitumor response was analyzed
for statistical significance using an unpaired Student’s t-test
with a 95% confidence interval (A). The mice were evaluated daily
and disease events were recorded using Kaplan-Meier survival
analysis (B). (C) On day 0, SKW lymphoma cells (4×106)
were intravenously inoculated into the tail vein of SCID mice.
Three days after inoculation, the mice were randomized into groups
(n=10) and intraperitoneally injected with vehicle, isotype-matched
control (30 mg/kg), or varying concentrations of galiximab (5, 10
or 30 mg/kg) for a total of 4 weekly injections. The mice were
evaluated daily, and disease events were recorded using
Kaplan-Meier survival analysis. A disease event was categorized as
<20% weight loss (with muscle weakness) and neurological motor
deficit. The antitumor response was analyzed with a log-rank test
with a 95% confidence interval. |
The disseminated lymphoma tumor model SKW was
examined. SCID mice were treated with various concentrations of
galiximab (5, 10 and 30 mg/kg) and examined at 60 days for
survival. There was significant prolongation of survival with all
concentrations of galiximab used (Fig.
1C). The statistical analyses for significance are summarized
in a table below Fig. 1C.
Potentiation of tumor growth inhibition
by the combination treatment of galiximab and fludarabine
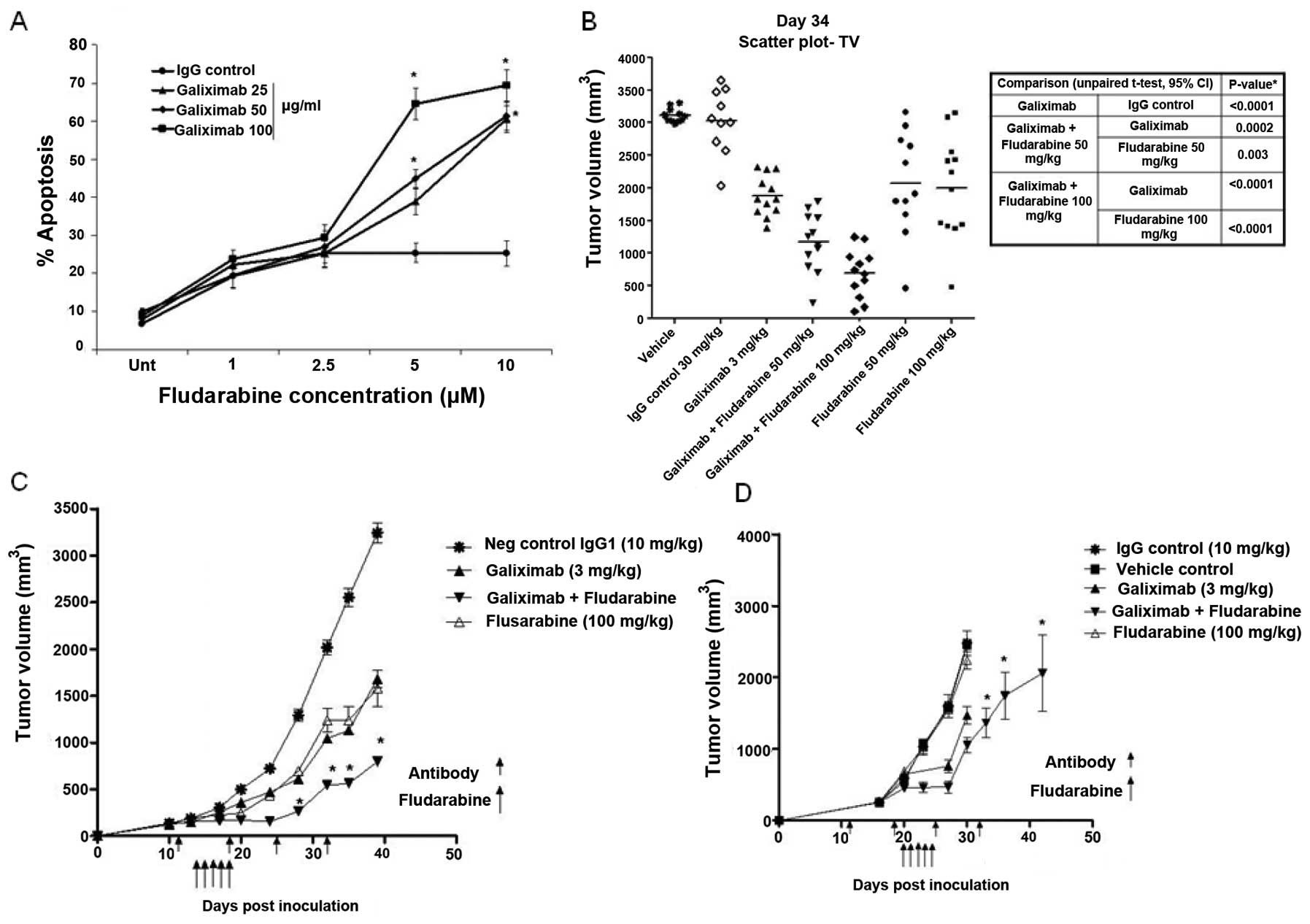
We examined whether the combination treatment of
galiximab and fludarabine may potentiate the antitumor response
in vivo. In vitro, Raji cells were treated with various
concentrations of galiximab (25, 50 and 100 μg/ml) and with
various concentrations of fludarabine (1–10 μM) and the
cells were analyzed for apoptosis as described in methods. The
findings shown in Fig. 2A
demonstrate that whereas treatment with either single agent alone
resulted in modest apoptosis, however, the combination treatment
resulted in significant potentiation of apoptosis.
These above in vitro findings prompted us to
examine in vivo the effect of the combination treatment of
galiximab and fludarabine on the growth of Raji tumor. Mice were
treated with a single dose of galiximab (3 mg/kg) and two doses of
fludarabine (50 and 100 mg/kg) and tumor volumes were measured. The
findings in Fig. 2B demonstrate
that whereas galiximab used alone or fludarabine used alone showed
reduction in tumor volume, however, the combination treatment was
more potent in reducing tumor weight. The statistical analyses are
summarized in the table adjacent to Fig. 2B.
We then examined the efficacy of the above
treatments in two different models with preexisting different tumor
sizes. Two protocols were used, namely, treatment was initiated in
mice with a tumor xenograft reaching either 100–180 or 200–400
mm3 prior to drug treatment for early or late advanced
tumor models, respectively. In the early model, treatment with
galiximab (3 mg/kg) or fludarabine (100 mg/kg) resulted in a
significant decrease in tumor volume (p<0.001). However,
treatment with the combination of galiximab and fludarabine
improved the antitumor effect with a significant reduction of tumor
volume when compared with treatment of galiximab alone (p<0.001)
or fludarabine alone (p<0.001) as shown in Fig. 2C. In the late advanced tumor model,
treatment with fludarabine alone had no effect. Treatment with
galiximab retarded tumor growth. However, a specific tumor
reduction was observed when galiximab was used in combination with
fludarabine and the effect was statistically significant
(p<0.001) (Fig. 2D).
Tumor growth inhibition and survival in
mice treated with galiximab and doxorubicin
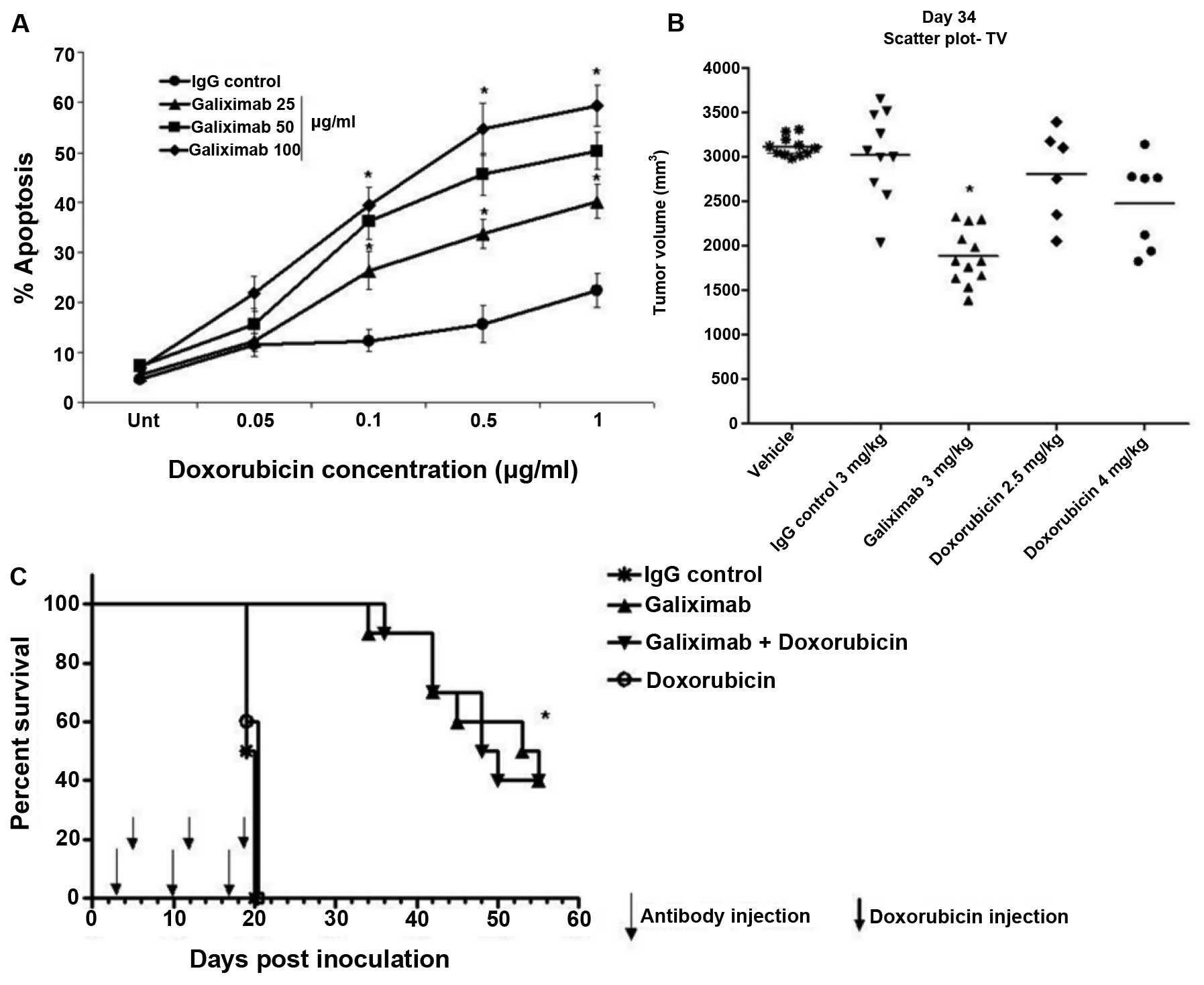
We first examined the cytotoxic effect observed
following treatment of Raji cells with galiximab, doxorubicin, or
combination. Raji cells were treated with various concentrations of
galiximab (25, 50, 100 μg/ml) for 18 h and treated for an
additional 18 h with various concentrations of doxorubicin
(0.05–1.0 μg/ml) and apoptosis was determined as described
in methods. While treatment with galiximab resulted in no effect,
treatment with doxorubicin showed modest apoptosis (about 10%) at
high concentrations. However, the combination treatment resulted in
significant potentiation of apoptosis (Fig. 3A).
In vivo, the Raji tumor-bearing mice were
treated with galiximab (3 mg/kg) or doxorubicin (2.5 and 4 mg/kg)
and tumor growth was monitored. As expected, treatment with
galiximab resulted in significant inhibition of tumor growth.
However, there was modest antitumor effect by the treatment with
doxorubicin alone (4 mg/kg) (Fig.
3B). Mice treated with the combination of galiximab and
doxorubicin were also monitored for survival. Doxorubicin alone was
not effective. However, galiximab alone or the combination of
galiximab and doxorubicin revealed significant prologation of
survival, though, doxorubicin did not augment the survival of the
mice treated with galiximab alone (Fig. 3C).
Discussion
Galiximab is a primatized mAb that is directed
against CD80, an antigen that is expressed on the surface of many B
cell lymphomas (9–12,18).
Treatment of B cell tumors with galiximab induces cell death via
ADCC (13). Recently, we have
reported that galiximab triggers B-NHL cell lines and inhibits the
constitutively activated survival/anti-apoptotic NF-κB pathway and
downstream transcription factors that regulate drug resistance such
as Snail and YYI. The inhibition of either Snail or YY1 expression
and activity by siRNAs sensitized the tumor cells to CCDP and
TRAIL-induced apoptosis (17). The
present study examined in vivo in mice the antitumor
activity of galiximab used alone or in combination with drugs such
as fludarabine or doxorubicin. The findings clearly demonstrate
that treatment with galiximab inhibited B-NHL tumor xenografts
growth and prolonged survival and its antitumor activity was
potentiated when combined with fludarabine. The antitumor activity
in vivo by galiximab was shown both in a solid and
disseminated B-NHL tumor xenografts.
In vitro findings demonstrated that treatment
of B-NHL cells with galiximab inhibited cell proliferation
(13). This in vitro
finding was validated preclinically in vivo in SCID mice
bearing B-NHL tumor xenografts. The extent of inhibition of tumor
growth was the function of the galiximab dose used. Further, there
was a significant prolongation of survival with galiximab treatment
correlating with the inhibition of tumor growth. A comparison
between the antitumor effect of galiximab alone and its
administration post-tumor inoculation was examined in an early
tumor model (100–180 mm3) and a late tumor model
(>200 mm3) and the findings demonstrated significant
inhibition of tumor growth in both models.
In vitro findings demonstrated that galiximab
sensitized Raji cells to apoptosis by fludarabine. Accordingly,
analysis in vivo was performed to validate the in
vitro findings. Treatment with fludarabine had a modest
antitumor activity, whereas, the combination of galiximab and
fludarabine demonstrated a significant inhibition of tumor growth
and which was more significant than tumor growth inhibition by
galiximab monotherapy. The mechanism by which galiximab potentiates
the activity of fludarabine in vivo may be due, in part, to
the findings showing the galiximab signals B-NHL cells and inhibits
the constitutively activated survival/anti-apoptotic NF-κB pathway
and downstream inhibition of the resistant factors such as Bcl-2,
BCL-xL, Snail and YY1 as recently reported by us (17).
The in vitro findings obtained with the
sensitization to apoptosis by the combination of galiximab and
fludarabine were confirmed with the combination of galiximab and
doxorubicin. In vitro analysis revealed that treatment of
Raji cells with galiximab sensitized the cells to apoptosis to
doxorubicin. In vivo treatment with doxorubicin showed a
modest inhibition of tumor growth; however, there was no
significant survival of mice treated with doxorubicin. In contrast,
whereas treatment by galiximab alone resulted in significant
prolongation of survival, there was no potentiation by the
combination with doxorubicin. These findings in vivo did not
concur with the in vitro findings as expected. Further
studies with different doses and schedules are necessary to show if
additive or synergistic effects are achieved by the combination or
galiximab and doxorubicin.
The in vivo findings observed in the
immuno-compromised SCID mice ruled out a contribution by the host
immune system. It is not clear whether a more significant antitumor
response by galiximab alone or its combination with drugs might
have been achieved. We have reported that treatment of B-NHL cells
with rituximab sensitized the tumor cells to both FasL and TRAIL,
ligands that are expressed on host effector cells such as T, NK and
macrophages (19,20). Thus, it is likely that the
antitumor response in immuno-competent mice might have been
augmented by the additional contribution of host effector antitumor
activity to the direct effect of the combination treatment with
galiximab alone or with drugs.
Clinically, galiximab has been tested in a clinical
trial of phase I and II studies in patients with indolent
follicular lymphoma (FL). A dose escalation trial of galiximab
treatment as single agent was found to be well tolerated, with
modest antitumor activity in patients with relapsed FL (14). In another phase I/II trial of
galiximab plus rituximab in a relapsed/refractory FL population
demonstrated a 66% overall response rate (ORR); 33% partial
response (PR) with a median PFS of 12.1 month (15). Based on these findings, the CALGB
50402, phase II trial of galiximab plus rituximab was undertaken in
previously untreated FL patients (16). The findings showed that the
administration of galiximab plus rituximab was well tolerated and
appears efficacious in patients with low risk FLIPI scores. The
clinical findings observed with the combination of galiximab and
rituximab in vivo may be the result of several, although
unclear, underlying mechanisms of action. Clearly,
antibody-dependent cellular cytotoxicity (ADCC) and
complement-dependent cellular cytotoxicity (CDC) play a role in the
inhibition of cell proliferation and in direct cytotoxicity. In
addition, we have reported that both galiximab (17) and rituximab (19–21)
inhibit survival pathways in B-NHL cells leading to susceptibility
of tumor cells to direct cytotoxicity by both chemotherapeutic
drugs and by host immune effector cells bearing FasL or TRAIL. For
CD80, the role of the tumor microenvironment cannot be ruled out
since CD80 is expressed on the surface of tumor-associated
macrophages (TAM) and the inhibition of CD80-CD28 interaction by
galiximab interferes with TAM-mediated cytokine responses and
proangiogenic signals, all of which induce antitumor activity
(8).
The present findings suggest the potential clinical
application of the combination of galiximab and fludarabine in the
treatment of B-NHL. This treatment strategy may be a complement to
the current rituximab/CHOP therapy. The present findings and those
of others also suggest the potential use of the triple combination
of galiximab, rituximab and chemotherapy in the treatment of
patients who are poor responders.
Abbreviations:
|
ADCC
|
antibody-dependent cell-mediated
cytotoxicity;
|
|
B-NHL
|
B-non-Hodgkin’s lymphoma;
|
|
CDC
|
complement-dependent cytotoxicity;
|
|
CDDP
|
cis-diamminedichloroplatinum(II);
|
|
CTLA-4
|
cytotoxic T-lymphocyte antigen 4;
|
|
DLBCL
|
diffuse large B cell lymphoma;
|
|
TRAIL
|
tumor necrosis factor (TNF)-related
apoptosis-inducing ligand;
|
|
siRNA
|
small interfering RNA;
|
|
FL
|
follicular lymphoma;
|
|
galiximab
|
anti-CD80 mAb;
|
|
mAb
|
monoclonal antibody;
|
|
SCID
|
severe combined immunodeficiency;
|
|
DFS
|
disease free survival;
|
|
NF-κB
|
nuclear factor κ-light-chain-enhancer
of activated B cells;
|
|
rituximab
|
anti-CD20 mAb;
|
|
YY1
|
Yin Yang 1
|
Acknowledgements
This study was supported, in part by
Jonsson Comprehensive Cancer Center (B.B. and M.I.V.); UCLA AIDS
Institute (M.I.V.); Fogarty International Center Fellowship (D43
TW00013-14, M.I.V.); and FIS, IMSS (grant FIS/IMSS/PROT/556,
M.I.V.).
References
|
1.
|
Armitage JO: Treatment of non-Hodgkin’s
lymphoma. N Engl J Med. 328:1023–1030. 1993.
|
|
2.
|
Sawas A, Diefenbach C and O’Connor OA: New
therapeutic targets and drugs in non-Hodgkin’s lymphoma. Curr Opin
Hematol. 18:280–287. 2011.
|
|
3.
|
Ferrario A, Pulsoni A, Olivero B, et al:
Fludarabine, cyclophosphamide, and rituximab in patients with
advanced, untreated, indolent B-cell nonfollicular lymphomas: phase
2 study of the Italian Lymphoma Foundation. Cancer. 118:3954–3961.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Montoto S and Fitzgibbon J: Transformation
of indolent B-cell lymphomas. J Clin Oncol. 29:1827–1834. 2011.
View Article : Google Scholar
|
|
5.
|
Molina A: A decade of rituximab: improving
survival outcomes in non-Hodgkin’s lymphoma. Annu Rev Med.
59:237–250. 2008.PubMed/NCBI
|
|
6.
|
Coyle AJ and Gutierrez-Ramos JC: The
expanding B7 superfamily: increasing complexity in costimulatory
signals regulating T cell function. Nat Immunol. 2:203–209. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Lanier LL, O’Fallon S, Somoza C, et al:
CD80 (B7) and CD86 (B70) provide similar costimulatory signals for
T cell proliferation, cytokine production, and generation of CTL. J
Immunol. 154:97–105. 1995.PubMed/NCBI
|
|
8.
|
Pardoll DM: The blockade of immune
checkpoints in cancer immunotherapy. Nat Rev Cancer. 12:252–264.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Dorfman DM, Schultze JL, Shahsafaei A, et
al: In vivo expression of B7-1 and B7-2 by follicular lymphoma
cells can prevent induction of T-cell anergy but is insufficient to
induce significant T-cell proliferation. Blood. 90:4297–4306.
1997.PubMed/NCBI
|
|
10.
|
Munro JM, Freedman AS, Aster JC, et al: In
vivo expression of the B7 costimulatory molecule by subsets of
antigen-presenting cells and the malignant cells of Hodgkin’s
disease. Blood. 83:793–798. 1994.PubMed/NCBI
|
|
11.
|
Vyth-Dreese FA, Dellemijn TA, Majoor D and
de Jong D: Localization in situ of the co-stimulatory molecules
B7.1, B7.2, CD40 and their ligands in normal human lymphoid tissue.
Eur J Immunol. 25:3023–3029. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Delabie J, Ceuppens JL, Vandenberghe P, de
Boer M, Coorevits L and De Wolf-Peeters C: The B7/BB1 antigen is
expressed by Reed-Sternberg cells of Hodgkin’s disease and
contributes to the stimulating capacity of Hodgkin’s
disease-derived cell lines. Blood. 82:2845–2852. 1993.PubMed/NCBI
|
|
13.
|
Suvas S, Singh V, Sahdev S, Vohra H and
Agrewala JN: Distinct role of CD80 and CD86 in the regulation of
the activation of B cell and B cell lymphoma. J Biol Chem.
277:7766–7775. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Czuczman MS, Thall A, Witzig TE, et al:
Phase I/II study of galiximab, an anti-CD80 antibody, for relapsed
or refractory follicular lymphoma. J Clin Oncol. 23:4390–4398.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Leonard JP, Friedberg JW, Younes A, et al:
A phase I/II study of galiximab (an anti-CD80 monoclonal antibody)
in combination with rituximab for relapsed or refractory,
follicular lymphoma. Ann Oncol. 18:1216–1223. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Czuczman MS, Leonard JP, Jung S, et al:
Phase II trial of galiximab (anti-CD80 monoclonal antibody) plus
rituximab (CALGB 50402): Follicular Lymphoma International
Prognostic Index (FLIPI) score is predictive of upfront
immunotherapy responsiveness. Ann Oncol. 23:2356–2362. 2012.
View Article : Google Scholar
|
|
17.
|
Martinez-Paniagua MA, Vega MI,
Huerta-Yepez S, et al: Galiximab signals B-NHL cells and inhibits
the activities of NF-kappaB-induced YY1- and snail-resistant
factors: mechanism of sensitization to apoptosis by
chemoimmuno-therapeutic drugs. Mol Cancer Ther. 11:572–581. 2012.
View Article : Google Scholar
|
|
18.
|
Younes A, Hariharan K, Allen RS and Leigh
BR: Initial trials of anti-CD80 monoclonal antibody (Galiximab)
therapy for patients with relapsed or refractory follicular
lymphoma. Clin Lymphoma. 3:257–259. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Vega MI, Huerta-Yepez S, Jazirehi AR,
Garban H and Bonavida B: Rituximab (chimeric anti-CD20) sensitizes
B-NHL cell lines to Fas-induced apoptosis. Oncogene. 24:8114–8127.
2005.PubMed/NCBI
|
|
20.
|
Jazirehi AR and Bonavida B: Cellular and
molecular signal transduction pathways modulated by rituximab
(rituxan, anti-CD20 mAb) in non-Hodgkin’s lymphoma: implications in
chemosensitization and therapeutic intervention. Oncogene.
24:2121–2143. 2005.PubMed/NCBI
|
|
21.
|
Vega MI, Baritaki S, Huerta-Yepez S,
Martinez-Paniagua MA and Bonavida B: A potential mechanism of
rituximab-induced inhibition of tumor growth through its
sensitization to tumor necrosis factor-related apoptosis-inducing
ligand-expressing host cytotoxic cells. Leuk Lymphoma. 52:108–121.
2011. View Article : Google Scholar
|