Introduction
Hepatocellular carcinoma (HCC) is one of the most
common and lethal cancers in the world and is the second leading
cause of cancer-related death in China (1). Despite remarkable progress in HCC
diagnosis and treatment, the prognosis of patients with HCC remains
very poor due to the high rate of intra-hepatic and distant
metastasis after resection or transplantation (1). The 5-year survival rate is limited to
25–39% after surgery and systemic therapy with cytotoxic agents
provides marginal benefit (2).
Therefore, the discovery of molecules and/or signal transduction
pathways essential to the carcinogenesis and malignant behaviour of
HCC cells, especially their invasion and metastasis, is important
for improving the prognosis of HCC patients.
B cell-specific Moloney murine leukaemia virus
insertion site 1 (Bmi-1), a member of the Polycomb family (PcG) of
proteins, which repress the transcription of their target genes via
an epigenetic mechanism (3–5), was
originally identified as an oncogene cooperating with c-Myc in a
murine lymphomagenesis model (6).
Subsequent studies identified the essential role of Bmi-1 in
embryonic development and the maintenance of self-renewal of both
normal and malignant human mammary stem cells (7). Bmi-1 also regulates cellular
processes including cell cycle progression, apoptosis and
senescence as well as immortalisation by repressing the INK4A
locus, which encodes two tumour repressor proteins,
p16Ink4a and p19Arf (mouse homologue of human
p14ARF) (8) and
inducing telomerase activity (9).
In addition, there is accumulating evidence that Bmi-1 is
overexpressed in a variety of human malignant neoplasms, such as
melanoma (10), breast cancer
(11), bladder cancer (12), pancreatic cancer (13) and HCC (14–16).
Furthermore, Bmi-1 is involved in tumour development and
progression and is associated with a poor prognosis (17). For example, Bmi-1 expression is
significantly correlated with nodal involvement, distant metastasis
and clinical stage of colon and gastric cancers (18,19).
Overexpression of Bmi-1 was associated with the invasion of
nasopharyngeal carcinomas and predicted poor survival (20). Inhibition of Bmi-1 leads to
decreased invasion of cervical cancer cells (21). Taken together, these data strongly
indicate that Bmi-1 contributes to more aggressive behaviour of
cancer cells, particularly with respect to invasion and metastasis.
However, the exact mechanisms by which Bmi-1 mediates tumour cell
invasion and metastasis, especially in HCC, remain largely
unknown.
In the present study, we examined the expression
profile of Bmi-1 in patients with HCC and compared Bmi-1 expression
with clinicopathological parameters by immunohistochemical
analysis. We also determined the survivals and prognostic value of
Bmi-1 expression for HCC patients by Kaplan-Meier method and Cox
proportional hazards model. Finally, we evaluated the effects of
Bmi-1 depletion on the invasive behaviour of HCC cell lines in
vitro and investigated potentially related mechanisms.
Materials and methods
Tissue specimens
Sixty-two HCCs and corresponding non-cancer liver
tissues were obtained from patients of the Department of
Hepatobiliary Surgery, Xijing Hospital of the Fourth Military
Medical University (Xi’an, China), between March 2004 and September
2006. Informed consent for research use of the specimens was
obtained for all cases and all study protocols were approved by the
Ethics Committee for Clinical Research of the Fourth Military
Medical University. None of the patients received radiotherapy or
chemotherapy before routine surgery. All of the specimens were
fixed in 10% buffered formalin solution and embedded in paraffin
and consecutive 4-μm-thick sections were cut.
Immunohistochemistry
Paraffin-embedded sections were deparaffinised with
xylene, rehydrated and then immersed in 3% hydrogen peroxide
solution for 10 min to inhibit endogenous peroxidase activity. For
antigen retrieval, slides were boiled in 0.01 mol/l sodium citrate
buffer (pH 7.0) for 10 min in a microwave oven. After being blocked
with 1% bovine serum albumin (BSA), the sections were incubated
with mouse monoclonal anti-Bmi-1 antibody (1:50, Abcam, Hong Kong,
China) at 4°C overnight. Following incubation with biotinylated
secondary antibody, a streptavidin-biotin
complex/horseradish-peroxidase was applied. Finally, antibody
binding was visualised with 3, 3′-diaminobenzidine (DAB) and
counterstained with hematoxylin. The primary antibody was replaced
by PBS in negative controls. Two pathologists who were blinded to
the clinical and histopathologic outcomes evaluated the results of
the staining independently. The Bmi-1 expression was scored for
staining intensity and extent of involved tissue. The staining
intensity was scored as 0 (no staining), 1 (weakly stained), 2
(moderately stained), or 3 (strongly stained). The extent of
staining was scored as 0 (<5%), 1 (5–25%), 2 (26–50%), or 3
(>50%), according to the percentage of positively stained cells.
The sum of the intensity and extent scores was used as the final
staining score ranging from 0 to 9. We defined Bmi-1 expression
according to the final scores as follows: 0–1, negative; 2–9,
positive.
Cell culture
Three human hepatocellular carcinoma cell lines,
HepG2, SMMC-7721 and MHCC97-H and a normal hepatocyte cell line,
HL-7702, were obtained from American Type Culture Collection
(Manassas, VA, USA) and were maintained in DMEM medium (Gibco,
Gaithersburg, MD, USA) supplemented with 10% fetal bovine serum
(Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified chamber
with 95% air and 5% CO2.
Construction of lentiviral vectors and
transfection
Lentivirus vectors for human Bmi-1 small hairpin RNA
(shRNA) encoding a green fluorescent protein (GFP) and a puromycin
resistance gene were constructed, packed and purified by GeneChem
Corp. (Shanghai, China). Bmi-1 shRNA was designed according to the
human Bmi-1 mRNA sequence (GenBank accession no. NM_005180). The
shRNA target sequence was 5′-CGGAAAGTAAACAAAGACAAA-3′ and a
negative control shRNA was provided by GeneChem. Cells were seeded
in 24-well plates overnight before transfection for a target
confluence of 30–50%. For transfection, according to the MOI value
(number of lentiviruses:number of cells), the appropriate amounts
of lentiviruses mixed with medium containing polybrene were added
to the cells. After 24 h of transfection at 37°C, the medium was
replaced by fresh DMEM medium containing 10% FBS. Three days after
transfection, cells were selected with 2 μg/ml puromycin for 3 days
and harvested for subsequent studies.
RNA extraction and quantitative real-time
PCR
Total RNA was extracted with TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. Total
RNA (1 μg) was reverse-transcribed into cDNA using the Primescript
RT reagent kit (Takara, Japan) in accordance with the
manufacturer’s instructions. Bmi-1 expression levels were
quantified by real-time quantitative polymerase chain reaction
(PCR). Bmi-1 mRNA levels were standardised to glyceraldehyde
3-phosphate dehydrogenase (GAPDH) as a reference housekeeping gene.
The forward primer for Bmi-1 was 5′-GCTTCAAGATGGCCGC TTG-3′; the
reverse primer was 5′-TTCTCGTTGTTCGATGC ATTTC-3′. The forward
primer for GAPDH was 5′-GCACCGT CAAGGCTGAGAAC-3′; the reverse
primer was 5′-TGGTGA AGACGCCAGTGGA-3′. Quantitative real-time PCR
was performed in a Bio-Rad iCycler IQ™ 5 (Bio-Rad, Hercules, CA,
USA) with SYBR Master Mix (Takara) according to the manufacturer′s
instructions. Each reaction was performed in a final volume of 20
μl containing 2.0 μl of appropriately diluted cDNA, 1.0 μl (10 μM)
of forward and reverse primers specific for human Bmi-1 or GAPDH,
10 μl of SYBR Premix Ex Taq and 6.0 μl of water. The cycling
conditions were as follows: a denaturation step at 95°C for 3 min;
40 cycles of denaturation at 95°C for 10 sec, specific annealing at
59°C for 30 sec and elongation at 72°C for 30 sec. At the end of
the cycles, the temperature was raised to 95°C for 1 min. The
melting curve was achieved by first cooling samples to 55°C for 1
min, followed by 81 cycles (30 sec/cycle) in which the temperature
was raised by 0.5°C per cycle to a maximum temperature of 95°C.
Protein extraction and western blot
analysis
Cells were lysed in ice-cold RIPA lysis buffer
containing 50 mM Tris-HCl (pH 7.4), 1% Triton X-100, 5 mM EDTA, 1
mM leupeptin, 1 mM phenylmethylsulfonyl fluoride, 10 mM NaF and 1
mM Na3VO4 and then centrifuged at 20,000 g
for 30 min at 4°C to remove debris. Protein concentrations were
determined by a BCA assay (Pierce, Rockford, IL, USA). Equal
amounts of cell lysate protein were subjected to SDS-polyacrylamide
gel electrophoresis (PAGE) and transferred to polyvinyl difluoride
(PVDF) membranes. Membranes were blocked with 5% non-fat dry milk
in Tris-buffered saline with Tween-20 for 1 h, then incubated
overnight at 4°C with specific primary antibodies. Primary
antibodies against Bmi-1 were purchased from Abcam and primary
antibodies against MMP-2, MMP-9, VEGF, PTEN, Akt, p-Akt and GAPDH
were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
The membranes were next incubated with horseradish
peroxidase-conjugated secondary antibodies and then developed with
an enhanced chemiluminescence detection system (Amersham Life
Science, Piscataway, NJ, USA) according to the manufacturer’s
instructions.
Invasion assay in vitro
Transwell cell culture chambers (8-μm pore size;
Millipore, Billerica, MA, USA) were used for in vitro
invasion assays. The upper side of the filter was covered with
Matrigel (Collaborative Research Inc., Boston, BD, USA) (1:3
dilution with DMEM free of serum) before the assays. Cells
(5×105) were serum-starved for 24 h and then transferred
in 350 μl serum-free DMEM to the upper chamber and DMEM with 15%
fetal bovine serum was added to the lower chamber as a
chemoattractant. The cells were incubated under normoxic conditions
for 24 h. Cells on the upper side of the filter were removed and
cells that remained adherent to the underside of the membrane were
fixed in 4% formaldehyde and stained with 0.5% crystal violet for
10 min. For pharmacological inhibition assays with LY294002, cells
were pre-treated for 2–4 h and the treatment continued during the
invasion experiment. Finally, the number of invasive cells was
counted in ten contiguous fields of each sample and the average was
determined.
ELISA assay
An enzyme-linked immunosorbent assay (ELISA)
(Amersham, Buckinghamshire, UK) was used to quantify the individual
activities of MMP-2, MMP-9 and VEGF. The samples were thawed on ice
and all reagents were equilibrated to room temperature; assays were
carried out according to the manufacturer’s instructions.
Statistical analysis
The data are expressed as the means ± SD.
Correlations between clinicopathological variables and Bmi-1
expression were analysed with Pearson’s χ2 tests.
Survival curves were calculated using the Kaplan-Meier method and
compared using the log-rank test. The Cox proportional hazard model
was carried out to explore the value of clinicopathological factors
and Bmi-1 expression on survival. Variance analysis between groups
was performed by one-way ANOVA and the significance of differences
between control and treatment groups was tested using Dunnett’s
multiple comparisons test. All statistical analyses were performed
using the SPSS software package (SPSS, Chicago, IL, USA). P<0.05
was considered statistically significant.
Results
Overexpression of BMI-1 in HCC
tissues
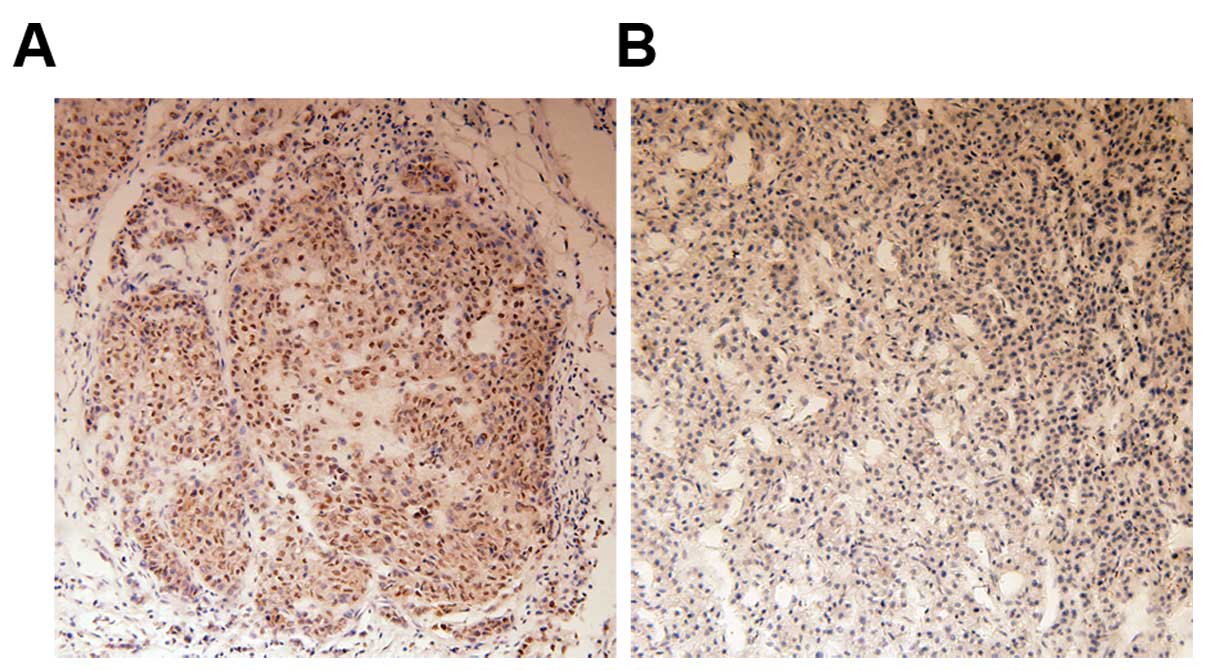
We evaluated 62 tissue specimens from HCC patients
by immunohistochemistry for Bmi-1 expression. Consistent with
previous reports (14), Bmi-1
protein was mainly observed in neoplastic epithelial cell nuclei.
Positive staining for Bmi-1 protein was observed in 46.8% (29/62)
of HCC tissues. By contrast, no staining or only weak staining was
observed in normal liver tissues. Staining of representative
samples is presented in Fig.
1.
Overexpression of Bmi-1 was associated
with the progression of HCC
We compared Bmi-1 expression with the
clinicopathological parameters of 62 patients to investigate the
clinical significance of Bmi-1 expression during hepatocyte
carcinogenesis. As shown in Table
I, there was no correlation between the expression of Bmi-1 and
certain clinical features, such as age, gender, tumour location,
histological grade, satellite lesions, tumour number and AFP level.
However, Bmi-1 expression was strongly associated with tumour size,
metastasis, venous invasion and AJCC TNM stage. This result
indicated a correlation between Bmi-1 expression and HCC invasion
and metastasis.
 | Table IRelationship between Bmi-1 expression
and clinicopathological varibles of patients with HCC. |
Table I
Relationship between Bmi-1 expression
and clinicopathological varibles of patients with HCC.
| | Bmi-1
expression | | |
|---|
| |
| | |
|---|
| Variables | All patients
(n=62) | Positive
(n=29) | Negative
(n=33) | χ2 | P-value |
|---|
| Age (years) |
| <50 | 30 | 13 | 17 | 0.276 | 0.599 |
| ≥50 | 32 | 16 | 16 | | |
| Gender |
| Male | 42 | 20 | 22 | 0.037 | 0.847 |
| Female | 20 | 9 | 11 | | |
| Tumour
location |
| Left | 26 | 14 | 12 | 0.900 | 0.343 |
| Right | 36 | 15 | 21 | | |
| Tumour size
(cm) |
| <5 | 32 | 10 | 22 | 6.402 | 0.011 |
| ≥5 | 30 | 19 | 11 | | |
| Histological
grade |
| Well | 18 | 11 | 7 | 2.094 | 0.148 |
| Moderated or
poorly | 44 | 18 | 26 | | |
| Metastasis |
| Negative | 39 | 14 | 25 | 4.996 | 0.025 |
| Positive | 23 | 15 | 8 | | |
| Satellite
lesions |
| Negative | 40 | 16 | 24 | 2.078 | 0.149 |
| Positive | 22 | 13 | 9 | | |
| Venous
invasion |
| Negative | 46 | 17 | 29 | 6.901 | 0.009 |
| Positive | 16 | 12 | 4 | | |
| Tumour number |
| Single | 48 | 23 | 25 | 0.111 | 0.739 |
| Multiple | 14 | 6 | 8 | | |
| AJCC TNM stage |
| I–II | 17 | 1 | 16 | 15.732 | <0.001 |
| III–IV | 45 | 28 | 17 | | |
| AFP (ng/ml) |
| ≤400 | 23 | 12 | 11 | 0.428 | 0.513 |
| >400 | 39 | 17 | 22 | | |
High Bmi-1 expression is associated with
the adverse prognosis of HCC and is an independent prognostic
factor
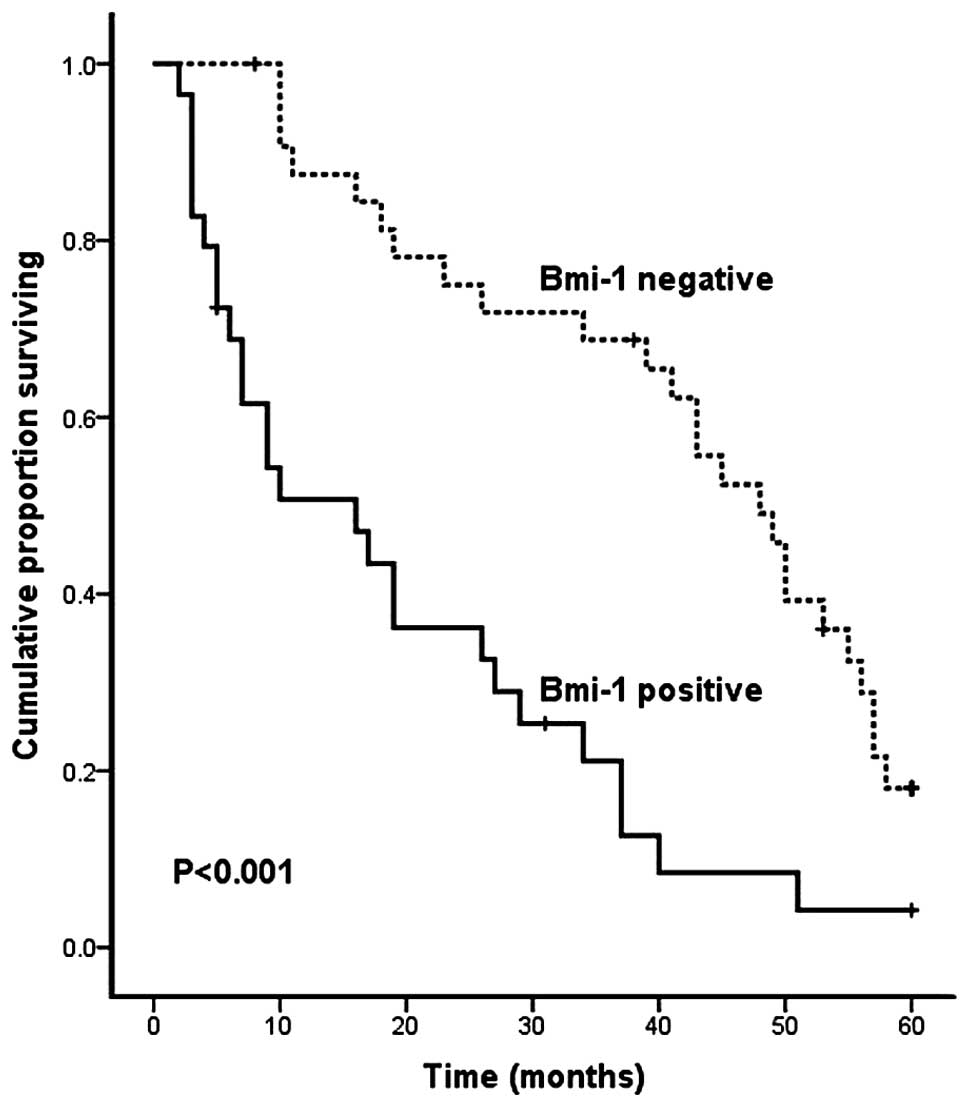
To evaluate the overall survival rate of HCC
patients in relation to Bmi-1 expression, we carried out
Kaplan-Meier survival analysis and log-rank test. The result
demonstrated that patients with positive Bmi-1 expression had a
significantly shorter 5-year survival rate than patients with
negative levels of Bmi-1 expression (P<0.001, log-rank test;
Fig. 2).
A univariate Cox regression analysis showed that the
overall survival was directly influenced by metastasis, venous
invasion, satellite lesions, AJCC TNM stage and Bmi-1 protein
expression (Table II). To
determine the relative importance of each variable, multivariate
Cox regression analyses were performed. Multivariate analysis
revealed that expression of Bmi-1 (P<0.001, HR = 5.095; 95% CI,
2.169–11.969), metastasis (P<0.001, HR = 18.163; 95% CI,
4.854–67.968) and venous invasion (P=0.034, HR = 3.083; 95% CI,
1.091–8.711) were independent prognostic factors for overall
survival in patients who have undergone curative resection for HCC
(Table II).
 | Table IIUnivariate and multivariate analyses
of overall survival for 62 HCC patients. |
Table II
Univariate and multivariate analyses
of overall survival for 62 HCC patients.
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 1.182
(0.678–2.060) | 0.556 | | |
| Gender | 1.202
(0.657–2.197) | 0.551 | | |
| Tumour
location | 0.916
(0.518–1.621) | 0.764 | | |
| Tumour size
(cm) | 1.248
(0.710–2.195) | 0.442 | | |
| Histological | 0.793
(0.427–1.473) | 0.463 | | |
| Metastasis | 18.028
(7.192–45.190) | <0.001 | 18.163
(4.854–67.968) | <0.001 |
| Satellite
lesions | 2.469
(1.380–4.416) | 0.002 | 1.103
(0.566–2.150) | 0.773 |
| Venous
invasion | 14.699
(6.231–34.674) | <0.001 | 3.083
(1.091–8.711) | 0.034 |
| Tumour number | 1.230
(0.636–2.377) | 0.538 | | |
| AJCC TNM stage | 3.948
(1.998–7.801) | <0.001 | 0.993
(0.367–2.687) | 0.989 |
| AFP (ng/ml) | 0.759
(0.429–1.344) | 0.344 | | |
| Bmi-1 | 3.325
(1.855–5.958) | <0.001 | 5.095
(2.169–11.969) | <0.001 |
Bmi-1 shRNA silenced Bmi-1 expression on
the mRNA and protein levels
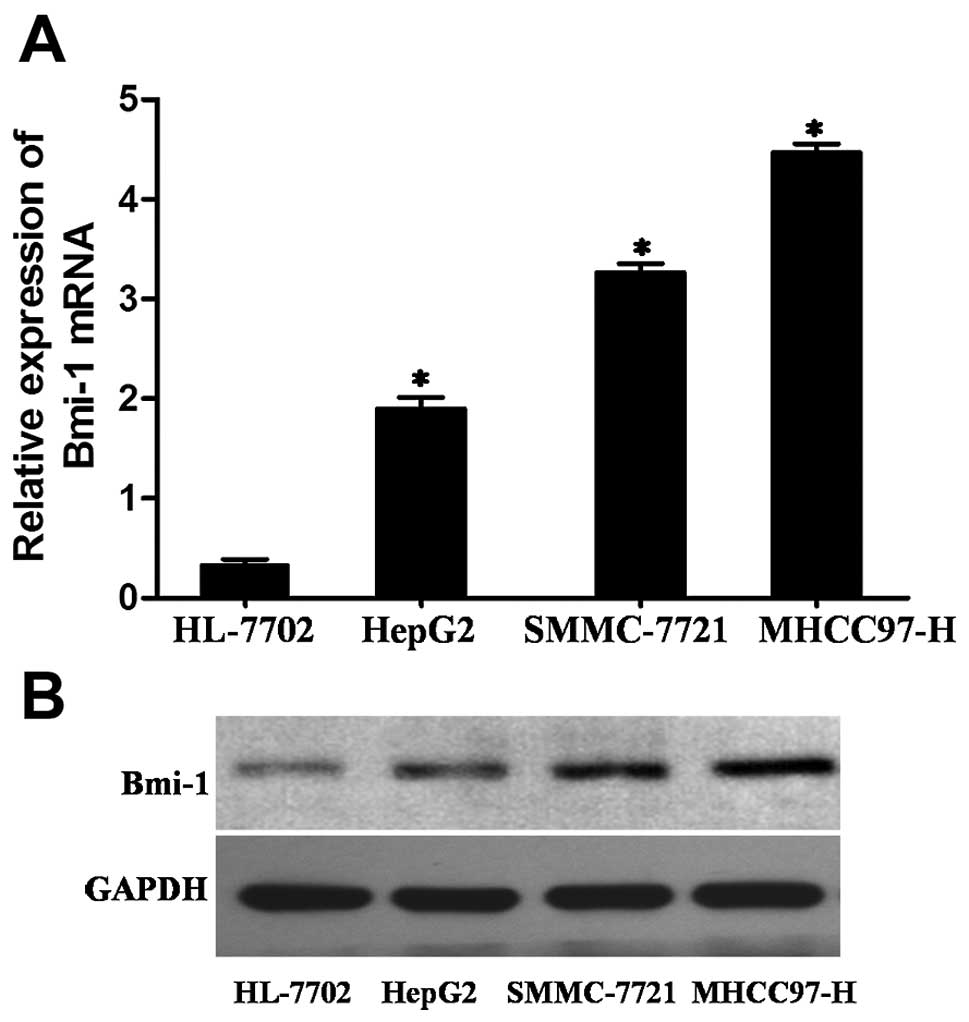
To further describe the role of Bmi-1 in the
progression of HCC, Bmi-1 expression was first compared among three
HCC cell lines (HepG2, SMMC-7721 and MHCC97-H) and an immortal
hepatocyte cell line (HL-7702) that was used as a reference for
Bmi-1 expression by real-time PCR and western blotting. The levels
of Bmi-1 expression were significantly higher in all three HCC cell
lines compared with that of the HL-7702 cells (Fig. 3).
Among these 3 HCC cell lines, HepG2 cells are the
least invasive, SMCC-7721 is moderately invasive and MHCC97H cells
are the most invasive (22). Our
results showed that the invasive abilities of these cells were
consistent with their Bmi-1 expression (Fig. 3). This finding indicated that the
upregulated levels of Bmi-1 may play a role in invasive
behaviour.
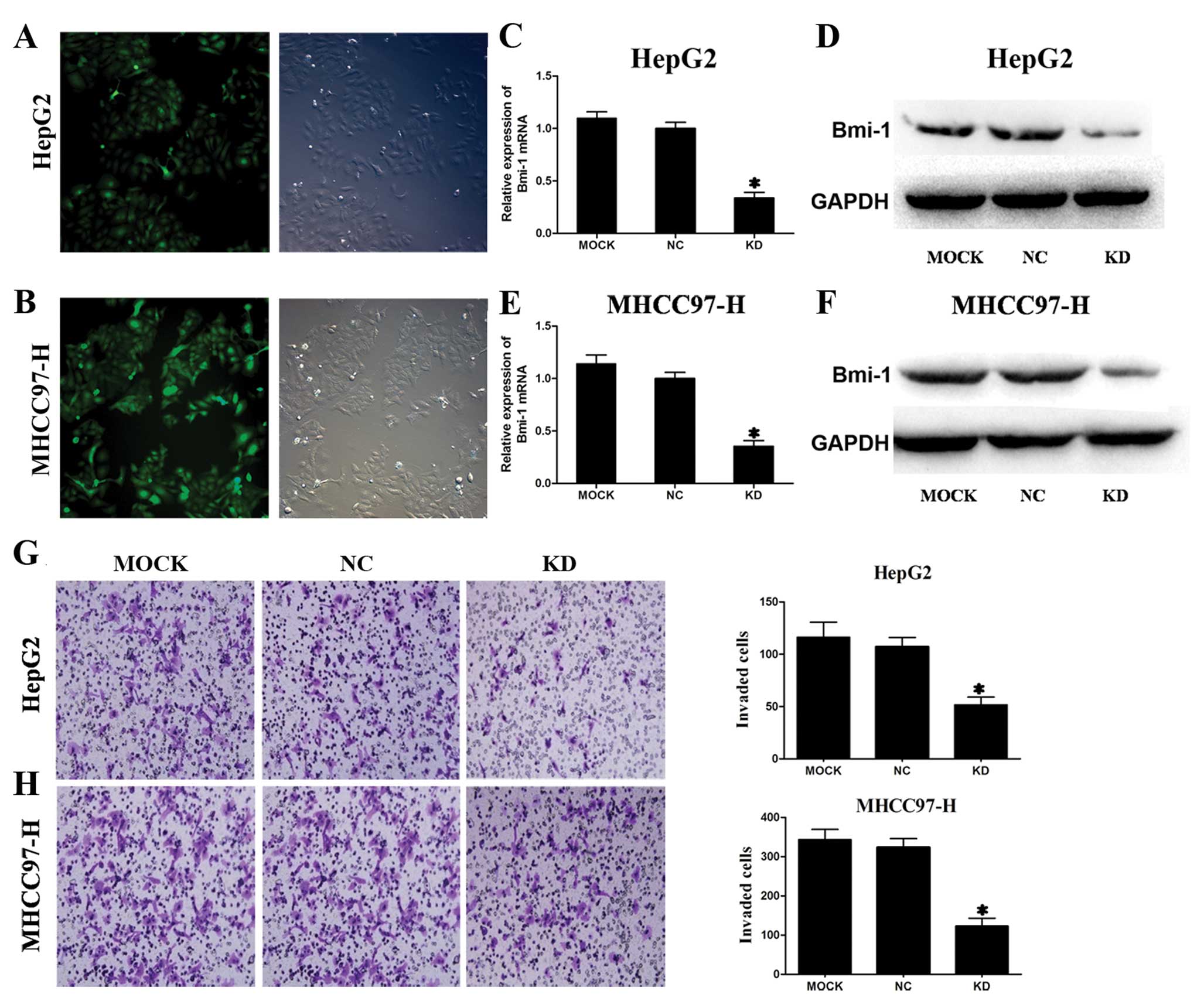
A shRNA vector that co-expresses GFP was generated
for stable and efficient Bmi-1 reduction in HCC cells and the
transfection efficiency was assessed by fluorescence microscopy.
Almost all HepG2 and MHCC97-H cells were successfully transduced
with lentivirus shRNA vector (Fig. 4A
and B). These results confirmed that Bmi-1 shRNA was
successfully introduced into the HepG2 and MHCC97-H cells.
As shown in Fig.
4C–F, endogenous Bmi-1 mRNA and protein levels were
significantly reduced in HepG2 and MHCC97-H cells transfected with
Bmi-1 shRNA vectors compared with the negative control shRNA vector
transfected cells and untransfected cells examined by real-time PCR
and western blotting, respectively. Thus, Bmi-1 expression was
effectively downregulated by Bmi-1 shRNA vectors in two HCC cell
lines in vitro.
Suppression of Bmi-1 repressed invasion
of HCC cells in vitro
Because high Bmi-1 expression was positively
associated with venous invasion (P=0.009) and metastasis (P=0.025),
we further determined whether Bmi-1 was involved in the invasion
and metastasis of HCC. To examine whether suppression of Bmi-1 in
HCC cell lines affected their invasive properties, we conducted
transwell invasion assays in vitro. The numbers of HepG2 and
MHCC97-H cells transfected with Bmi-1 shRNA vectors invading
through the filter were markedly lower than the number of the
negative control groups and mock groups (Fig. 4G and H). Bmi-1 knockdown
dramatically inhibited the invasiveness of HepG2 and MHCC97-H
cells.
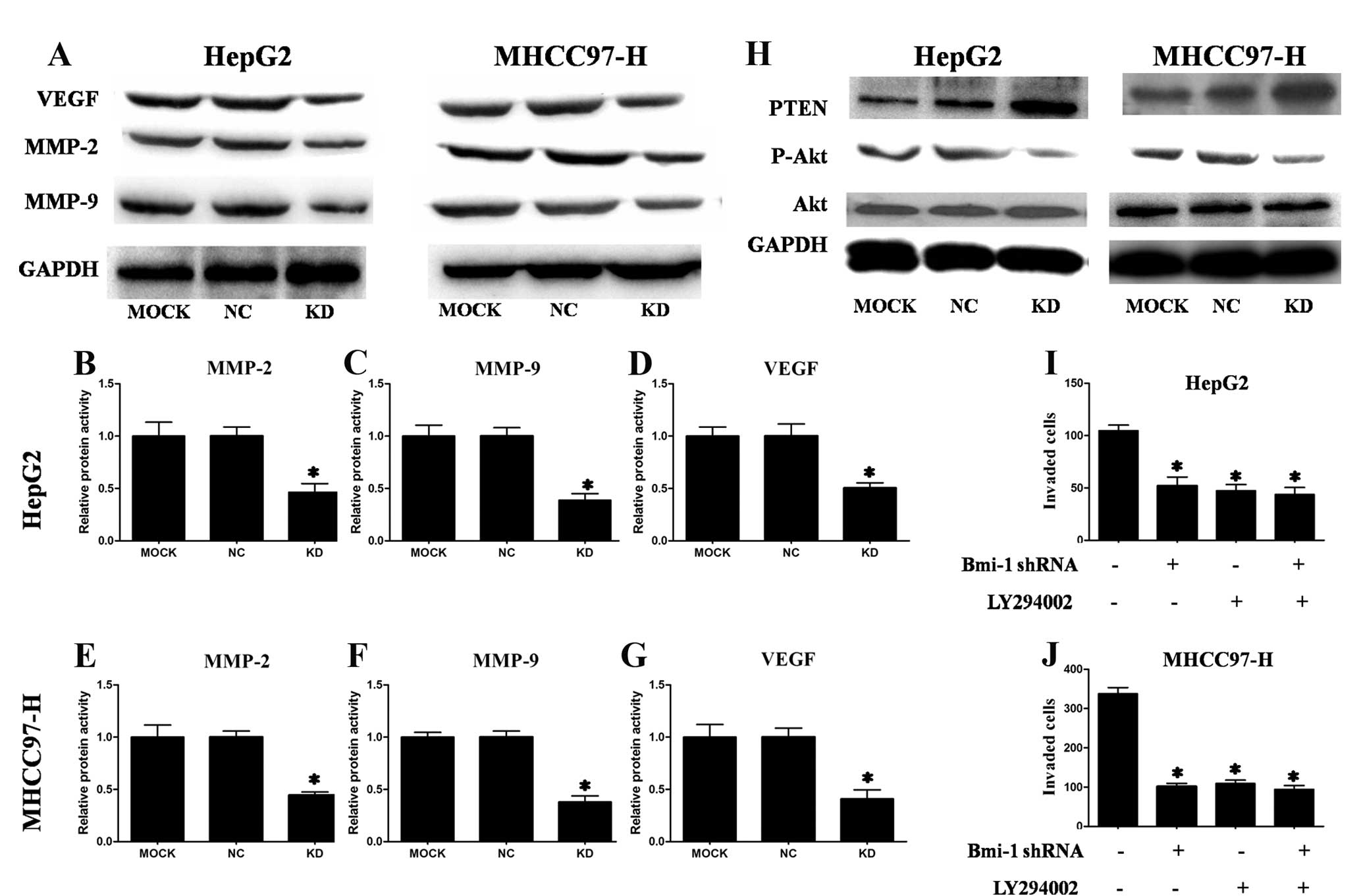
Suppression of Bmi-1 decreased the
expression of MMP-2, MMP-9 and VEGF
Because Bmi-1 knockdown inhibited HCC cell invasion,
we also investigated its effect on metastasis-related genes. MMP-2,
MMP-9 and VEGF play important roles in cancer invasion and
metastasis (23), including HCC
(22). We determined the protein
levels of these three genes by western blotting after transfection.
As shown in Fig. 5A, transfection
of HepG2 and MHCC97-H cells with Bmi-1-shRNA vectors reduced MMP-2,
MMP-9 and VEGF protein levels. We confirmed the effect of
Bmi-1-shRNA on MMP-2, MMP-9 and VEGF levels by ELISA. As shown in
Fig. 5B–G, Bmi-1 knockdown in HCC
cells significantly decreased MMP-2, MMP-9 and VEGF levels. These
data indicate that the effects of Bmi-1 on invasion may be mediated
by MMP-2, MMP-9 and VEGF.
Suppression of Bmi-1 increased PTEN
expression and decreased p-Akt expression
One previous report indicated that Bmi-1 can
downregulate the transcription of PTEN (24). Therefore, we investigated whether
PTEN was upregulated in HCC cells with Bmi-1 knocked down. As shown
in Fig. 5H, PTEN levels were
increased in HCC cells with Bmi-1 knockdown compared to the mock
groups and the control groups. These results demonstrated that PTEN
was upregulated by Bmi-1 silencing.
PTEN is a tumour suppressor with phosphatase
activity that can inhibit tumour metastasis via negative regulation
of the PI3K/Akt pathway (25).
Moreover, the PI3K/Akt signalling pathway is known to play a major
role in signalling pathways responsible for the invasion and
migration of various cancers (26). Furthermore, PTEN regulates the
expression of MMPs and VEGF in HCC (27). Upregulation of Bmi-1 can activate
the PI3K/Akt pathway (24).
Therefore, we considered that Bmi-1 participates in the invasion
and metastasis of HCC by activation of the PI3K/Akt pathway. To
test this hypothesis, we examined the levels of phosphorylated Akt
and total Akt. Western blot analyses showed less phosphorylated Akt
in HCC cells with Bmi-1 knockdown compared to the negative control
groups and mock groups but no change in the total amount of Akt.
This experiment demonstrated that knockdown of Bmi-1 inhibited the
Akt pathway (Fig. 5H).
To further study whether Bmi-1 participates in the
invasion and metastasis of HCC cells via PI3k/Akt pathway, HepG2
and MHCC97-H cells were treated with the highly specific PI3K/Akt
pathway inhibitor LY294002. LY294002 (10 μM) alone reduced HCC cell
invasion. However, treatment with LY294002 in HCC cells with Bmi-1
knockdown did not further reduce the invasion ability compared to
HCC cells treated with LY294002 alone or HCC cells with Bmi-1
knockdown alone (Fig. 5I and J).
These results suggested that Bmi-1 may promote HCC cell invasion
through the activation of the PI3K/Akt pathway with subsequent
regulation of MMP-2, MMP-9 and VEGF expression.
Discussion
HCC is the fifth most common malignancy in the world
and the third most common cause of cancer-related death (28) and the high recurrence rate of
intra-hepatic and distant metastasis is a major obstacle to
improving the survival of patients with HCC (1). Therefore, it is vital to clarify the
mechanisms and identify key factors underlying invasion and
metastasis to develop novel treatments and cures. In this study, we
identified and functionally characterised Bmi-1 as an important
player in HCC progression. Our study demonstrates that Bmi-1 is
overexpressed in HCC tissue and cells and its overexpression
contributes to invasion and metastasis by increasing the expression
of MMP-2, MMP-9 and VEGF via the PTEN/PI3K/Akt pathway.
Recently, many studies have revealed that Bmi-1 is
upregulated in a variety of human malignancies and is involved in
tumour invasion and metastasis. In breast cancer, overexpression of
Bmi-1 is associated with lymph node involvement and distant
metastasis (29). In addition, in
colon cancer, Bmi-1 expression is significantly correlated with
nodal involvement, distant metastasis and clinical stage (18). In this study, we examined the Bmi-1
expression in HCC samples and corresponding non-cancer liver
tissues. We found that Bmi-1 was significantly overexpressed in HCC
tissues compared with matched normal liver tissues, which is
consistent with previous reports (14,15).
Of note, a previous study reported that Bmi-1 was also positively
expressed in surrounding non-cancer liver tissues and cirrhotic
liver but not in distant normal liver tissue (16), which suggested that Bmi-1 might
play a role in the early stages of HCC. We determined that
overexpression of Bmi-1 was strongly associated with tumour size,
metastasis, venous invasion and AJCC TNM stage, while it was not
correlated with other clinicopathological parameters, such as age,
gender, tumour location, histological grade, satellite lesions,
tumour number and AFP level. Our study suggests that Bmi-1 may
participate in late progression and aggressive biological behaviour
of HCC. Our results were consistent with those of Sasaki et
al(15), which indicated that
the expression of Bmi-1 and EZH2 was heterogeneous and associated
with vascular infiltration, histological grades and cell
proliferativity in HCC and HC-CC. However, in conflict with our
findings were the reports of Effendi et al(14) and Wang et al(16), which indicated that Bmi-1
expression did not correlate with any clinicopathological
parameters, including tumour size, histological differentiation,
metastasis and recurrence. These differences across studies may be
due to the tissue samples being obtained from HCC patients with
different stages of disease or may reflect population differences.
Notably, the distribution of disease stages in these studies
differed. Another explanation for the discrepancies might be the
different protocols used for immunohistochemistry, including
antibody dilution, development time and the positive criteria
applied, especially the score used to discriminate positivity. For
example, in the study of Wang et al(16), cytoplasmic staining of Bmi-1 was
considered as positive as well; however, in the other three studies
including ours, cells were considered positive for Bmi-1 only when
nuclear staining was observed. To further understand the
significance of Bmi-1 expression in HCC, multi-centre studies and
additional samples are necessary.
Moreover, the Kaplan-Meier analysis showed that
patients with positive Bmi-1 expression had significantly worse
overall survival compared to patients with negative Bmi-1
expression, indicating that Bmi-1 protein may serve as a factor of
poor prognosis for patients with HCC. The multivariate analysis
found Bmi-1 expression could be an indicator of worse patient
outcome, independently of known clinical prognostic indicators such
as TNM stage. These data suggest that high Bmi-1 expression is
correlated with worse patient outcome and may serve as an
independent prognostic factor for patients with HCC, similar to
pancreatic cancer (13) and
nasopharyngeal carcinoma (20).
An important finding of our study was that Bmi-1 was
positively associated with metastasis and venous invasion of HCC.
To further investigate the role of increasing Bmi-1 expression on
HCC invasion, we stably knocked down Bmi-1 expression in two HCC
cell lines by transfection with lentiviral vectors expressing
Bmi-1-targeting shRNA. The suppression of Bmi-1 expression
significantly inhibited the invasion of HCC cells in vitro.
In breast cancer and nasopharyngeal cancer, silencing endogenous
Bmi-1 expression can reduce the motility and invasiveness of cancer
cells (20,29). Mouse xenograft studies indicate
that coexpression of Bmi-1 and H-Ras in breast cancer cells can
induce an aggressive and metastatic phenotype with an unusual
occurrence of brain metastasis (30). These findings indicate that Bmi-1
contributes to increased aggressive behaviour in cancer cells.
Tumour invasion and metastasis are complex,
multistage processes by which cancer cells undergo genetic
alternations that result in their acquisition of the ability to
degrade and migrate through the extracellular matrix (ECM)
(31). Of the several families of
ECM-degrading enzymes, the most extensive are matrix
metalloproteinases (MMPs), which are a large family of structurally
related zinc-endopeptidases that collectively degrade all essential
components of ECM, including type IV collagen, laminin,
proteoglycans and glycosaminoglycans (32). Among the previously reported human
MMPs, MMP-2 and MMP-9 play the most important roles in tumour
invasion and metastasis because of their specificity for degrading
the basement membrane (23,33).
Many studies indicate that MMP-2 and MMP-9 are correlated with an
aggressive, invasive or metastatic tumour phenotype and participate
in the invasion and metastasis of cancers, including HCC (34,35).
Another important molecule involved in tumour cell
invasion and metastasis is vascular endothelial growth factor
(VEGF). Angiogenesis is essential for carcinogenesis and tumour
growth and metastasis. The most potent tumour angiogenic factor,
VEGF, can stimulate the proliferation of endothelial cells in many
human cancers. VEGF expression is commonly upregulated in tumours
and plays a key role in invasion and migration of tumour cells
(36), including HCC (22).
These results indicate that MMP-2, MMP-9 and VEGF
play an important role in HCC cell invasion. Therefore, we
hypothesised that these metastasis-related proteins were involved
in Bmi-1-mediated invasion. To test this hypothesis, we
investigated the expression and activities of MMP-2, MMP-9 and
VEGF. Bmi-1 knockdown decreased the expression and activities of
MMP-2, MMP-9 and VEGF. These results suggest that Bmi-1 knockdown
inhibits HCC cell invasion by suppression of MMP-2, MMP-9 and VEGF.
Meng et al demonstrated that knockdown of Bmi-1 inhibits
lung adenocarcinoma cell migration and metastasis by diminishing
VEGF secretion via the PTEN/PI3K/Akt signalling pathway (37) and Jiang et al showed that
Bmi-1 promotes the aggressiveness of glioma by activating the
NF-κB/MMP-9 signalling pathway (38). However, the potential mechanisms of
interaction between Bmi-1, MMPs and VEGF in HCC invasion are poorly
understood.
It is known that the PI3K/Akt signalling pathway is
involved in many cellular processes including proliferation,
apoptosis, cell cycle progression, cell motility, angiogenesis,
invasion and metastasis (39). The
PI3K/Akt signalling pathway also regulates the expression of MMPs
and VEGF (26,27). In this study, Bmi-1 knockdown
reduced phosphorylated Akt levels, accompanied by inhibition of the
protein expression and activities of MMP-2, MMP-9 and VEGF. We
further found that inhibition of PI3K/Akt pathway with LY294002 in
HCC cells with Bmi-1 shRNA did not block the invasion ability of
these cells to a greater extent. Thus, downregulation of Bmi-1
leads to inhibition of the PI3K/Akt pathway and its downstream
targets (MMP-2, MMP-9 and VEGF) and ultimately reduces the invasion
of HCC cells.
The tumour suppressor gene PTEN is one of the most
commonly lost or mutated phosphatase genes in a variety of human
cancers, including HCC (40). PTEN
antagonises PI3K/Akt signalling, thereby negatively regulating
aggressive tumour behaviour. One previous study showed that
upregulation of Bmi-1 can activate the PI3K/Akt pathway by
downregulating the transcription of PTEN via a direct association
with the PTEN gene locus (24). We
also found that Bmi-1 knockdown increased the expression of
PTEN.
Taken together, Bmi-1 is upregulated in HCC tissues
compared to adjacent normal liver tissues and overexpression of
Bmi-1 is associated with tumour size, metastasis, venous invasion
and AJCC TNM stage. High Bmi-1 expression is associated with the
adverse prognosis of HCC and is an independent prognostic factor
for overall survival. Bmi-1 enhances the invasion of HCC cells
in vitro by inhibiting the expression of PTEN, thereby
activating the PI3K/Akt pathway and ultimately increasing the
expression and activity of MMP-2, MMP-9 and VEGF. Therefore,
inhibition of Bmi-1 could be useful as a therapeutic strategy to
inhibit invasion and improve survival in HCC.
Acknowledgwments
This study was supported by grants from the National
Natural Science Foundation of China (grants no. 81101820/H1617) and
the Major Program of the National Natural Science Foundation of
China (grants no. 81030010/H0318).
References
|
1
|
Tung-Ping PR, Fan ST and Wong J: Risk
factors, prevention and management of postoperative recurrence
after resection of hepatocellular carcinoma. Ann Surg. 232:10–24.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thomas MB and Zhu AX: Hepatocellular
carcinoma: the need for progress. J Clin Oncol. 23:2892–2899. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jacobs JJ and van Lohuizen M: Polycomb
repression: from cellular memory to cellular proliferation and
cancer. Biochim Biophys Acta. 1602:151–161. 2002.PubMed/NCBI
|
|
4
|
Kondo Y, Shen L, Cheng AS, et al: Gene
silencing in cancer by histone H3 lysine 27 trimethylation
independent of promoter DNA methylation. Nat Genet. 40:741–750.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Raaphorst FM: Deregulated expression of
Polycomb-group oncogenes in human malignant lymphomas and
epithelial tumors. Hum Mol Genet. 14:R93–R100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van Lohuizen M, Verbeek S, Scheijen B,
Wientjens E, van der Gulden H and Berns A: Identification of
cooperating oncogenes in E mu-myc transgenic mice by provirus
tagging. Cell. 65:737–752. 1991.PubMed/NCBI
|
|
7
|
Liu S, Dontu G, Mantle ID, et al: Hedgehog
signaling and Bmi-1 regulate self-renewal of normal and malignant
human mammary stem cells. Cancer Res. 66:6063–6071. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobs JJ, Kieboom K, Marino S, DePinho RA
and van Lohuizen M: The oncogene and Polycomb-group gene bmi-1
regulates cell proliferation and senescence through the ink4a
locus. Nature. 397:164–168. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dimri GP, Martinez JL, Jacobs JJ, et al:
The Bmi-1 oncogene induces telomerase activity and immortalizes
human mammary epithelial cells. Cancer Res. 62:4736–4745.
2002.PubMed/NCBI
|
|
10
|
Mihic-Probst D, Kuster A, Kilgus S, et al:
Consistent expression of the stem cell renewal factor BMI-1 in
primary and metastatic melanoma. Int J Cancer. 121:1764–1770. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim JH, Yoon SY, Jeong SH, et al:
Overexpression of Bmi-1 oncoprotein correlates with axillary lymph
node metastases in invasive ductal breast cancer. Breast.
13:383–388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin ZK, Yang JA, Ye YL, et al: Expression
of Bmi-1 is a prognostic marker in bladder cancer. BMC Cancer.
9:612009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song W, Tao K, Li H, et al: Bmi-1 is
related to proliferation, survival and poor prognosis in pancreatic
cancer. Cancer Sci. 101:1754–1760. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Effendi K, Mori T, Komuta M, Masugi Y, Du
W and Sakamoto M: Bmi-1 gene is upregulated in early-stage
hepatocellular carcinoma and correlates with ATP-binding cassette
transporter B1 expression. Cancer Sci. 101:666–672. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sasaki M, Ikeda H, Itatsu K, et al: The
overexpression of polycomb group proteins Bmi1 and EZH2 is
associated with the progression and aggressive biological behavior
of hepatocellular carcinoma. Lab Invest. 88:873–882. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Pan K, Zhang HK, et al: Increased
polycomb-group oncogene Bmi-1 expression correlates with poor
prognosis in hepatocellular carcinoma. J Cancer Res Clin Oncol.
134:535–541. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sparmann A and van Lohuizen M: Polycomb
silencers control cell fate, development and cancer. Nat Rev
Cancer. 6:846–856. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li DW, Tang HM, Fan JW, et al: Expression
level of Bmi-1 oncoprotein is associated with progression and
prognosis in colon cancer. J Cancer Res Clin Oncol. 136:997–1006.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu JH, Song LB, Zhang X, et al: Bmi-1
expression predicts prognosis for patients with gastric carcinoma.
J Surg Oncol. 97:267–272. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song LB, Zeng MS, Liao WT, et al: Bmi-1 is
a novel molecular marker of nasopharyngeal carcinoma progression
and immortalizes primary human nasopharyngeal epithelial cells.
Cancer Res. 66:6225–6232. 2006. View Article : Google Scholar
|
|
21
|
Jiang Y, Su B, Meng X, et al: Effect of
siRNA-mediated silencing of Bmi-1 gene expression on HeLa cells.
Cancer Sci. 101:379–386. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou L, Wang DS, Li QJ, Sun W, Zhang Y and
Dou KF: Downregulation of the Notch signaling pathway inhibits
hepatocellular carcinoma cell invasion by inactivation of matrix
metalloproteinase-2 and -9 and vascular endothelial growth factor.
Oncol Rep. 28:874–882. 2012.
|
|
23
|
Zheng H, Takahashi H, Murai Y, et al:
Expressions of MMP-2, MMP-9 and VEGF are closely linked to growth,
invasion, metastasis and angiogenesis of gastric carcinoma.
Anticancer Res. 26:3579–3583. 2006.PubMed/NCBI
|
|
24
|
Song LB, Li J, Liao WT, et al: The
polycomb group protein Bmi-1 represses the tumour suppressor PTEN
and induces epithelial-mesenchymal transition in human
nasopharyngeal epithelial cells. J Clin Invest. 119:3626–3636.
2009. View
Article : Google Scholar
|
|
25
|
Pore N, Liu S, Haas-Kogan DA, O’Rourke DM
and Maity A: PTEN mutation and epidermal growth factor receptor
activation regulate vascular endothelial growth factor (VEGF) mRNA
expression in human glioblastoma cells by transactivating the
proximal VEGF promoter. Cancer Res. 63:236–241. 2003.
|
|
26
|
Liu B, Wu X, Liu B, et al: MiR-26a
enhances metastasis potential of lung cancer cells via AKT pathway
by targeting PTEN. Biochim Biophys Acta. 1822:1692–1704. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen JS, Wang Q, Fu XH, et al: Involvement
of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in
hepatocellular carcinoma: association with MMP-9. Hepatol Res.
39:177–186. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gomaa AI, Khan SA, Toledano MB, Waked I
and Taylor-Robinson SD: Hepatocellular carcinoma: epidemiology,
risk factors and pathogenesis. World J Gastroenterol. 14:4300–4308.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Guo BH, Feng Y, Zhang R, et al: Bmi-1
promotes invasion and metastasis and its elevated expression is
correlated with an advanced stage of breast cancer. Mol Cancer.
10:102011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoenerhoff MJ, Chu I, Barkan D, et al:
BMI1 cooperates with H-RAS to induce an aggressive breast cancer
phenotype with brain metastases. Oncogene. 28:3022–3032. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Deryugina EI and Quigley JP: Matrix
metalloproteinases and tumor metastasis. Cancer Metastasis Rev.
25:9–34. 2006. View Article : Google Scholar
|
|
32
|
Egeblad M and Werb Z: New functions for
the matrix metalloproteinases in cancer progression. Nat Rev
Cancer. 2:161–174. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fingleton B: Matrix metalloproteinases:
roles in cancer and metastasis. Front Biosci. 11:479–491. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang Q, Chen X, Zhou J, et al: CD147,
MMP-2, MMP-9 and MVD-CD34 are significant predictors of recurrence
after liver transplantation in hepatocellular carcinoma patients.
Cancer Biol Ther. 5:808–814. 2006. View Article : Google Scholar
|
|
35
|
Giannelli G, Bergamini C, Marinosci F, et
al: Clinical role of MMP-2/TIMP-2 imbalance in hepatocellular
carcinoma. Int J Cancer. 97:425–431. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wey JS, Fan F, Gray MJ, et al: Vascular
endothelial growth factor receptor-1 promotes migration and
invasion in pancreatic carcinoma cell lines. Cancer. 104:427–438.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meng X, Wang Y, Zheng X, et al:
shRNA-mediated knockdown of Bmi-1 inhibit lung adenocarcinoma cell
migration and metastasis. Lung Cancer. 77:24–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang L, Wu J, Yang Y, et al: Bmi-1
promotes the aggressiveness of glioma via activating the
NF-kappaB/MMP-9 signaling pathway. BMC Cancer. 12:4062012.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chalhoub N and Baker SJ: PTEN and the
PI3-kinase pathway in cancer. Annu Rev Pathol. 4:127–150. 2009.
View Article : Google Scholar : PubMed/NCBI
|