Introduction
Capsosiphon fulvescens (Cf) is green seaweed
that grows mainly in the clean areas of the sea off the coast of
Korea. It is commonly used as a traditional health food, and
various bioactive effects of Cf have been reported (1). A compositional analysis of Cf showed
that it contains high levels of proteins, carbohydrates and amino
acids (2). Specific substances in
Cf are known to boost the immune system, bio-activity and have
anticancer activities (3–5). We previously observed the induction
of apoptosis in AGS human gastric cancer cells by a glycoprotein of
Cf (6,7). This glycoprotein has also been
reported to inhibit cell invasion (8).
Transforming growth factor (TGF)-β1 is a
cytokine associated with various human cancers (9,10)
involving macrophages, brain cells and keratinocytes. Previous
studies have reported that TGF-β1 modulates cell
migration, invasion and proliferation in gastric cancer cells
(11). TGF-β1 induces
the overexpression of growth factor focal adhesion kinase (FAK)
protein in several cancers, activates phosphatidylinositol 3-kinase
(PI3K)/AKT and small GTPase proteins (12), and upregulates integrin proteins.
The small GTPases, which include Rho A, Rho B, Rac-1 and Cdc 42,
are involved in the signaling pathways associated with diverse
cellular functions, including cell proliferation and migration, in
response to different growth factor receptors (13). The small GTPases also activate
nuclear transcription factor-κB (NF-κB), which upregulates the
expression of integrin receptor proteins, thereby contributing to
cell migration.
Integrin receptors are located on the cell surface,
where they are responsible for the adhesion of cells to
extracellular matrix (ECM) proteins such as fibronectin and the
transduction of extracellular signals to the cells (14). Integrins exist as hetero dimers of
two distinct transmembrane glycoprotein chains, called α and β
subunits, that are non-covalently linked. The integrin family
consists of 24 different αβ heterodimers (15). The binding of an integrin receptor
to its extracellular ligand causes a signal to be relayed into the
cell, resulting in the regulation of specific gene expression
(16). The binding of integrin
receptors to ECM molecules also produces cell adhesion, which is
critical for cell migration, proliferation and differentiation.
The present study investigated the possible
relationship between the anticancer activity of the C.
fulvescens glycoprotein (Cf-GP) and the downregulation of
integrin expression via the TGF-β1-activated
PI3K/AKT/small GTPases pathway in AGS human gastric cancer cells.
First, we established the effect of Cf-GP treatment on the
proliferation, migration and apoptosis of AGS cells. Then, to
investigate the mechanism by which Cf-GP may exert its anticancer
activity, we examined the effect of Cf-GP on the expression levels
of TGF-β1, the small GTPases, and integrins in the AGS
cells.
Materials and methods
Preparation of Cf-GP
The C. fulvescens used in this experiment was
purchased in 2010 in Republic of Korea. The Cf powder (40 g) was
diluted with water (1 liter) and stirred for 3 h at 80°C in a
heating mantle. The solution was clarified by centrifugation at
1,500 × g for 15 min at 4°C. Three volumes of 95% ethanol were
added to the solution, and precipitates were removed by vacuum
filtration. The supernatant was mixed with 80% ammonium sulfate and
stirred for 24 h, followed by dialysis (Por Membrane MW 3,500 Da,
Spcectrum Laboratories Inc., Rancho Dominguez, CA, USA) for one day
at 4°C to remove salts. The concentrated solution was distributed
into 1.5-ml tubes and stored at −70°C until use. These samples were
named Cf-GP.
Cell culture
AGS human gastric cancer cell line (American Type
Culture Collection, Manassas, VA, USA) was cultured in RPMI-1640
medium with 10% fetal bovine serum (FBS; Hyclone, Logan, UT, USA),
100 U/ml penicillin, and 100 mg/ml streptomycin, at a temperature
of 37°C in a humidified atmosphere of 5% CO2. The cells
were cultured to 80% confluence in 100-mm dishes. The medium was
replaced daily.
Cell proliferation assay
AGS cell proliferation was measured using a
CellTiter 96® aqueous non-radioactive cell proliferation
assay (Promega, Madison, WI, USA), which is based on the cleavage
of
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfonyl)-2H-tetrazolium
(MTS) into a formazan product soluble in tissue culture medium. The
cells were seeded onto 96-well plates at 2×104
cells/well and the medium was replaced with serum-free medium (SFM)
after culture for 24 h. After another 24 h, the medium was replaced
with SFM containing Cf-GP (5, 10 and 20 μg/ml), followed by
incubation for 24 h. For the assay, MTS solution was added to the
cells in each well and allowed to react for 30 min at 37°C. The
absorbance of the solution in each well was measured at 490 nm
using a microplate reader (Benchmark microplate reader; Bio-Rad
Laboratories, Hercules, CA, USA).
Cell migration assay
AGS cells were seeded onto 100-mm dishes and grown
to 80% confluence. The medium was replaced with SFM, and the cells
were cultured for 24 h, after which the cells were wounded by
scraping with a pipette tip. The medium was replaced with SFM
containing Cf-GP (5, 10 and 20 μg/ml), and the cells were
cultured for 24 h. Wound closure was determined from photographs
taken using a microscope at ×200 magnification.
Apoptosis assay
The level of apoptosis induced by Cf-GP treatment
was determined using a Muse™ Annexin V and Dead Cell kit (EMD
Millipore Co., Hayward, CA, USA). Cells were cultured in 6-well
seeds to 60% confluency, and then the medium was replaced with SFM
or SFM containing Cf-GP (5, 10 and 20 μg/ml). After 24 h,
the cells were collected in 1% FBS-RPMI-1640 medium, mixed with the
Muse Annexin V and Dead Cell Reagent, and analyzed using a Muse
Cell Analyzer (EMD Millipore Co.).
mRNA expression assay
The mRNA expression levels of specific genes were
evaluated by reverse-transcription polymerase chain reaction
(RT-PCR). AGS cells were seeded onto 6-well plates at
2×104 cells/well and were cultured for 24 h, after which
the medium was replaced with SFM containing Cf-GP (5, 10 and 20
μg/ml) for 24 h. Total RNA was isolated from the cells using
TRIzol reagent (Invitrogen Co., Carlsbad, CA, USA), and total RNA
was converted to cDNA using oligo(dT) primers (iNtRON Biotechnology
Inc., Seongnam, Korea). For PCR amplification, the cDNA and
specific primers (Table I) were
added to 2X TOPsimple™ DyeMIX-nTaq (Enzynomics, Inc., Daejoen,
Korea) and 0.1% diethylpyrocarbonate (DEPC) water. The amplified
products were analyzed on 1% agarose gels stained with RedSafe™
nucleic acid staining solution (iNtRON Biotechnology, Inc.).
 | Table I.Oligonucleotide sequences of the
primer pairs used for RT-PCR. |
Table I.
Oligonucleotide sequences of the
primer pairs used for RT-PCR.
| Name | Sequence of primers
(5′→3′) |
|---|
|
TGF-β1 | S:
GCA-GAA-CCC-AAA-AGC-CAG-AGT-G |
| A:
CCA-TAA-CTA-CCG-TGG-AGG-TTG-A |
| FAK | S:
TTC-ATT-ATT-TTG-AAA-GCA-ATA-GT |
| A:
CAA-CCC-AAC-TTC-AAA-GCA-ATT-TC |
| Rho A | S:
CTC-ATA-GTC-TTC-AGC-AAG-GAC-CAG-TT |
| A:
ATC-ATT-CCG-AAG-ATC-CTT-CTT-ATT |
| Rho B | S:
ATG-GCG-GCC-ATC-CGC-AAG-AAG-C |
| A:
TCA-TAG-CAC-CTT-GCA-GCA-GTT-G |
| Rac-1 | S:
GGA-CAC-AGC-TGG-ACA-AGA-AGA |
| A:
GGA-CAG-AGA-ACC-GCT-CGG-ATA |
| Cdc 42 | S:
CGA-CCG-CTA-AGT-TAT-CCA-CAG |
| A:
GCA-GCT-AGG-ATA-GCC-TCA-TCA |
| PI3K | S:
AGG-AGC-GGT-ACA-GCA-AAG-AA |
| A:
GCC-GAA-CAC-CTT-TTT-GAG-TC |
| Akt | S:
CAA-CTT-CTC-TGT-GGC-GCA-GTG |
| A:
GAC-AGG-TGG-AAG-AAC-AGC-TCG |
| IκB | S:
TGG-ATG-AAC-TGC-GTG-GTG-CAG |
| A:
GCA-GAA-GTG-TCC-CTG-TTC-CAG |
| NF-κB | S:
TCA-GGG-AAT-ATC-CAC-CTA-TCA-CTT-CAG |
| A:
CAT-CAG-CAG-CAG-CCA-TGT-ACT-CTT-CAC |
| Integrin
αν | S:
GAA-GCT-TCA-TCT-CCA-GTC-CCT |
| A:
TGG-GTA-GGG-CTG-TTT-GTC-ATC-ATA |
| Integrin
β1 | S:
GAC-CTG-CCT-TGG-TGT-CTG-TGC |
| A:
AGC-AAC-CAC-ACC-AGC-TAC-AAT |
| Integrin
β3 | S:
CCC-TCG-AAA-ACC-CCT-GCT-AT |
| A:
TTA-GCG-TCA-GCA-CGT-GTT-TGT-AG |
| Integrin
β5 | S:
GGC-TGG-GAC-GTC-ATT-CAG-AT |
| A:
AGC-TGG-AAG-GTG-GTC-TTG-TCA |
| β-actin | S:
CGT-ACC-ACT-GGC-ATC-GTG |
| A:
GTG-TTG-GCG-TAC-AGG-TCT-TTG |
Western blot analysis
AGS cells in 100-mm dishes were cultured to 80%
confluence and then incubated in SFM for 4 h. Fresh SFM containing
Cf-GP (5, 10 and 20 μg/ml) was added to the cells, and the
incubation continued for 24 h, after which the cells were washed
with phosphate-buffered saline (PBS) and mixed with lysis
extraction buffer [20 mM Tris (pH 7.5), 150 mM NaCl, 1% Triton
X-100, 1 mM EDTA, 1 mM EGTA, 2.5 mM sodium pyrophosphate, 1 mM
β-glycerophosphate, 1 mM sodium orthovanadate, 1 μg/ml
aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin A,
0.25% Na-deoxycholate, and 1 mM PMSF]. For western blot analysis,
the cell lysates were electrophoresed in 10–15% polyacrylamide
gels, and the resolved proteins were transferred to Immobilon-P
transfer membrane (Millipore Co., Billerica, MA, USA). The
membranes were blocked with 1% bovine serum albumin in TBS-T [10 mM
Tris-HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween-20] at room
temperature, followed by incubation with the following specific
primary antibodies (diluted 1:1,000): anti-TGF-β1,
anti-FAK, anti-PI3K, anti-AKT, anti-Rho A, anti-Rho B, anti-Rac-1,
anti-Cdc 42, anti-NF-κB, anti-IκB, anti-integrin αν,
anti-integrin β1, anti-integrin β3, and
anti-integrin β5. All primary antibodies were purchased
from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). The
secondary antibody was horse-radish peroxidase conjugated goat,
mouse or rabbit antibody (1:10,000) (GE Healthcare Bio-Sciences,
Piscataway, NJ, USA). Immunoreactive bands were detected with
SuperSignal West Pico Chemiluminescent substrate (Thermo Fisher
Scientific Inc., Rockford, IL, USA) and visualized on Kodak X-ray
film.
Statistical analysis
Data were analyzed using ANOVA. Values of p<0.05
on Duncan’s multiple range test indicated a significant difference
between each groups. The results are presented as means ± SD. All
analyses were performed with SPSS software (ver. 10.0; SPSS Inc.,
Chicago, IL, USA).
Results
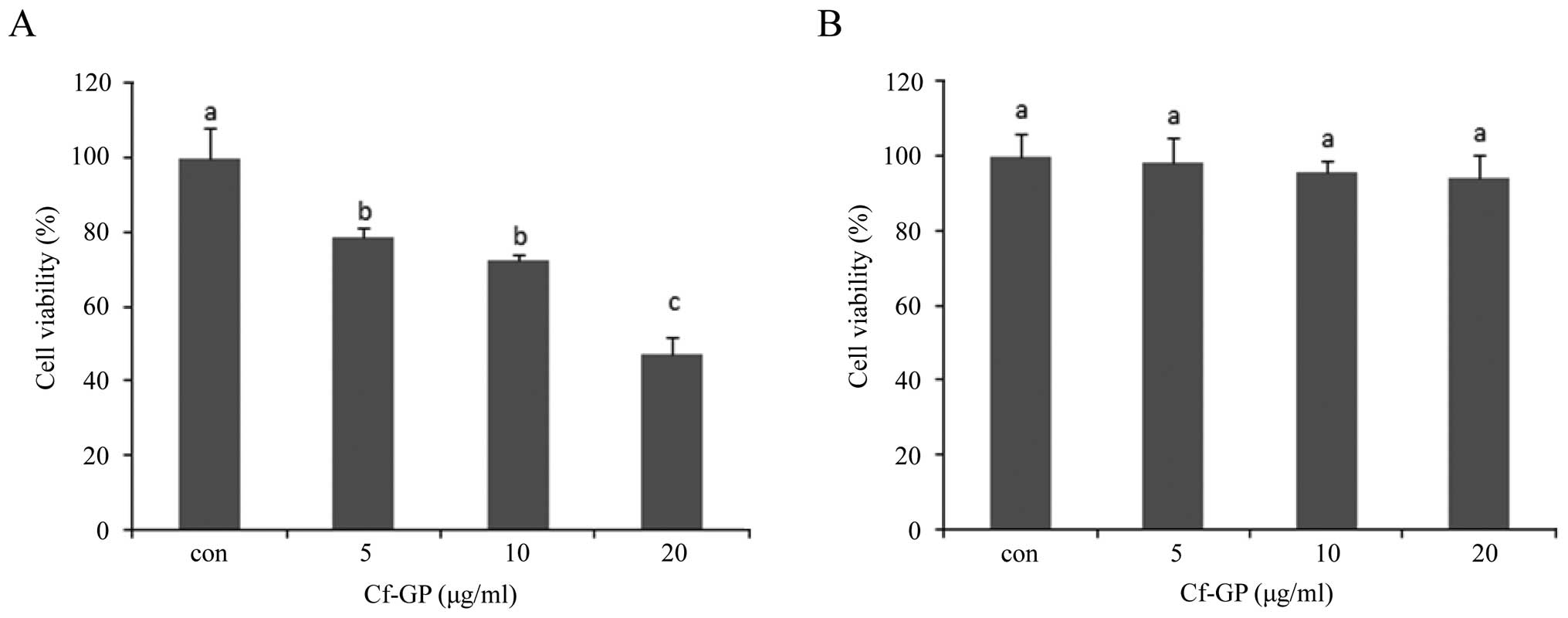
Cf-GP inhibits AGS cell
proliferation
The effects of Cf-GP on AGS cell proliferation were
examined using the MTS assay. AGS cells treated with Cf-GP at 5, 10
and 20 μg/ml showed dose-dependent inhibition of cell
proliferation, with a maximum inhibition of 50% at 20 μg/ml
(Fig. 1A). We found Cf-GP
dose-dependent effect of AGS cell growth inhibition by MTS assay.
In addition we determined the toxicity of the normal cells.
Treatment with Cf-GP had no toxic effects on the normal human
intestinal epithelial IEC-6 cells (Fig. 1B).
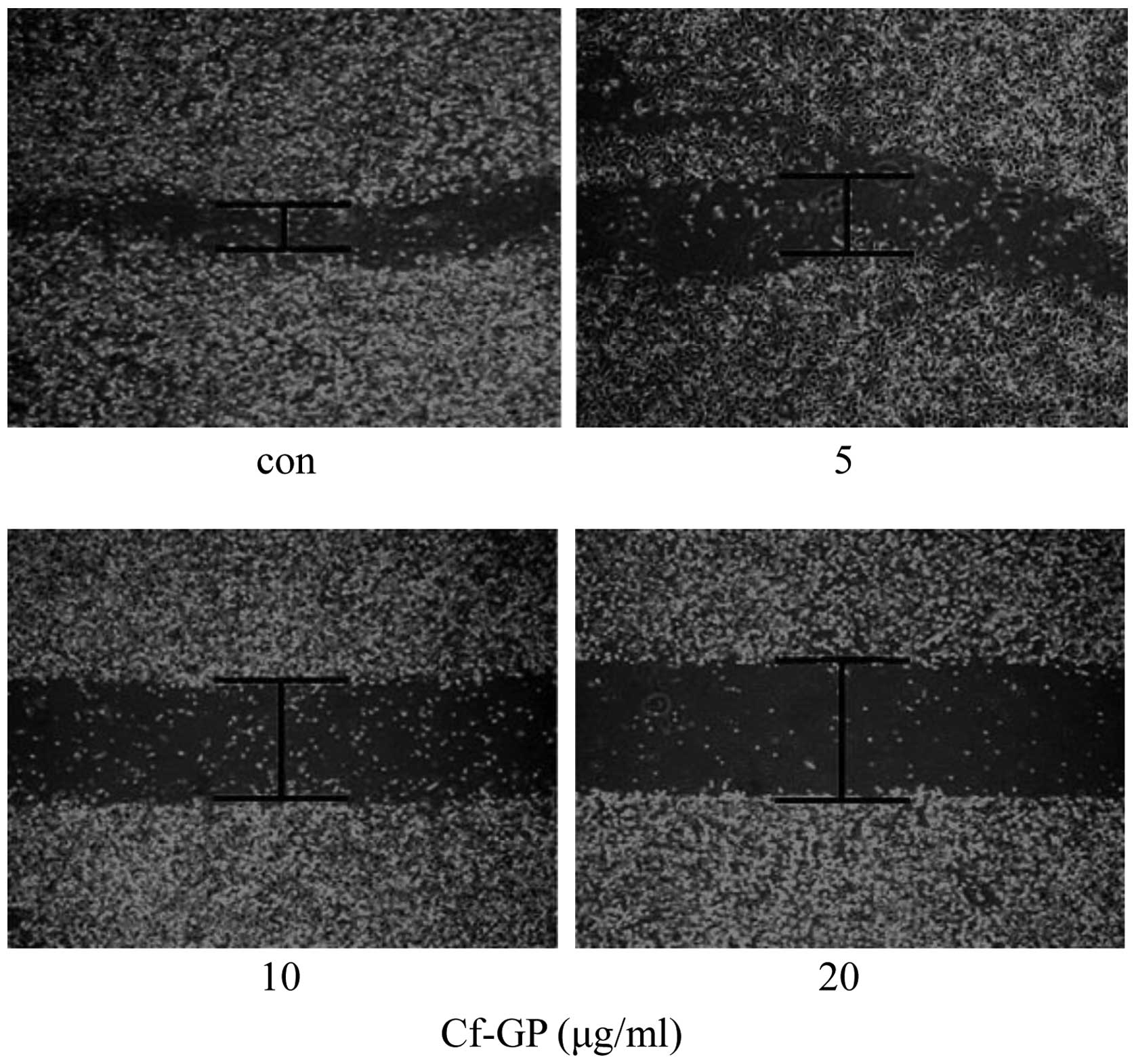
Cf-GP inhibits AGS cell migration
The effect of Cf-GP on AGS cell migration was
examined using a wound-healing assay. As results, in the case of
control AGS cells group over time, the mobility of cells in the
wounded area was found to increase. But, AGS cells treated with
Cf-GP (5, 10 and 20 μg/ml) exhibited dose-dependent
inhibition of migration into the cell-wounded zone on 100-mm
dishes, indicating a Cf-GP-induced inhibition of cell mobility
(Fig. 2).
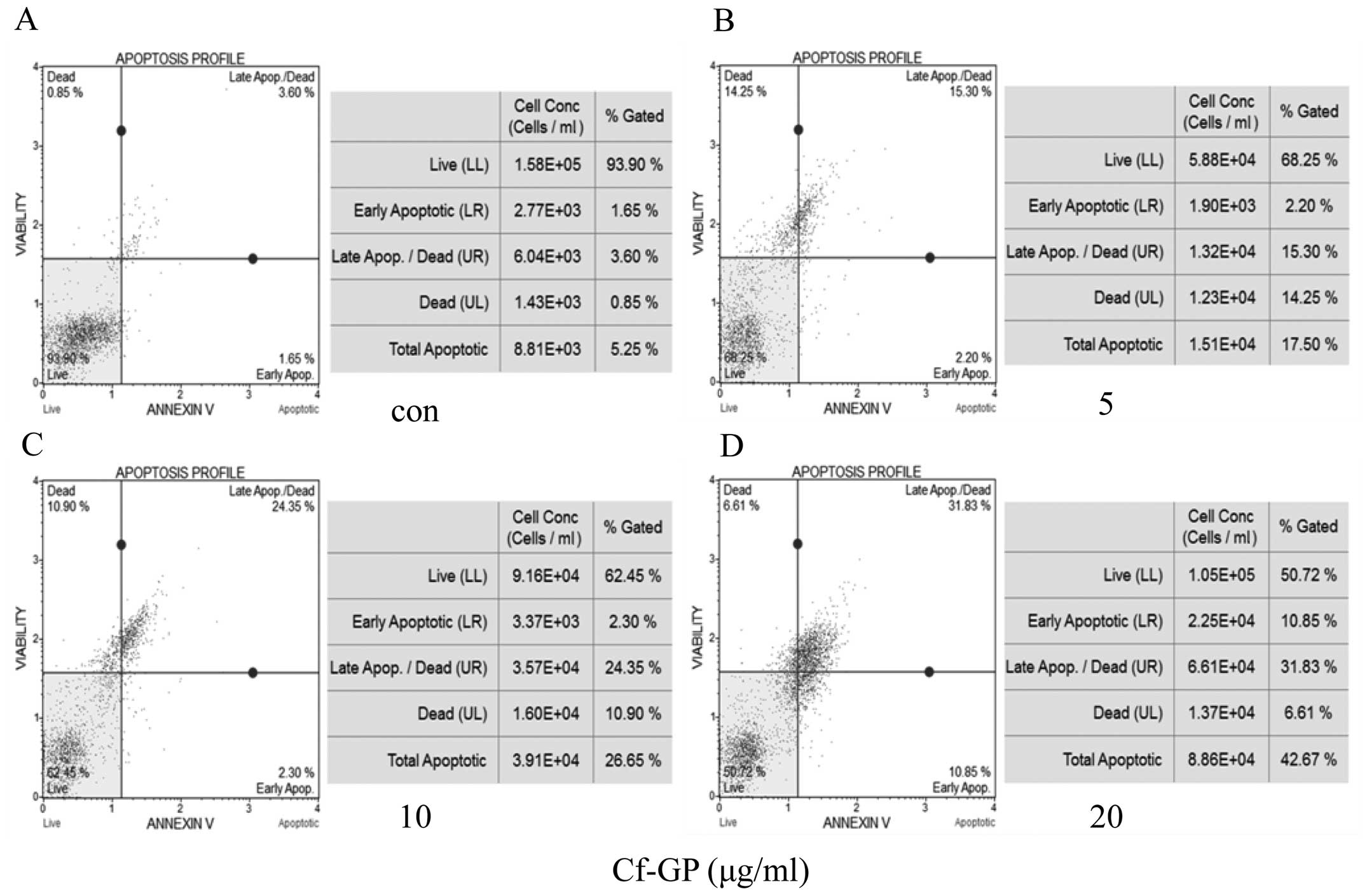
Cf-GP dose-dependently increases cellular
apoptosis in AGS cells
AGS cells were treated with Cf-GP (5, 10 and 20
μg/ml), and apoptotic and necrotic cells were detected by
Annexin V and 7-aminoactinomycin D (AAD) staining, respectively
(17). In this assay, cells in the
early stage of apoptosis are Annexin V-positive and 7-AAD-negative,
and those in late apoptosis are Annexin V-positive and
7-AAD-positive. In the present study, Cf-GP treatment increased the
percentage of apoptotic AGS cells, in a dose-dependent manner.
Control cells comprised 5.25% apoptotic cells, 0.85% necrotic cells
and 93.90% living cells (Fig. 3).
Treatment with 5, 10 and 20 μg/ml Cf-GP for 24 h resulted in
17.50, 26.65 and 42.68% apoptotic cells, respectively.
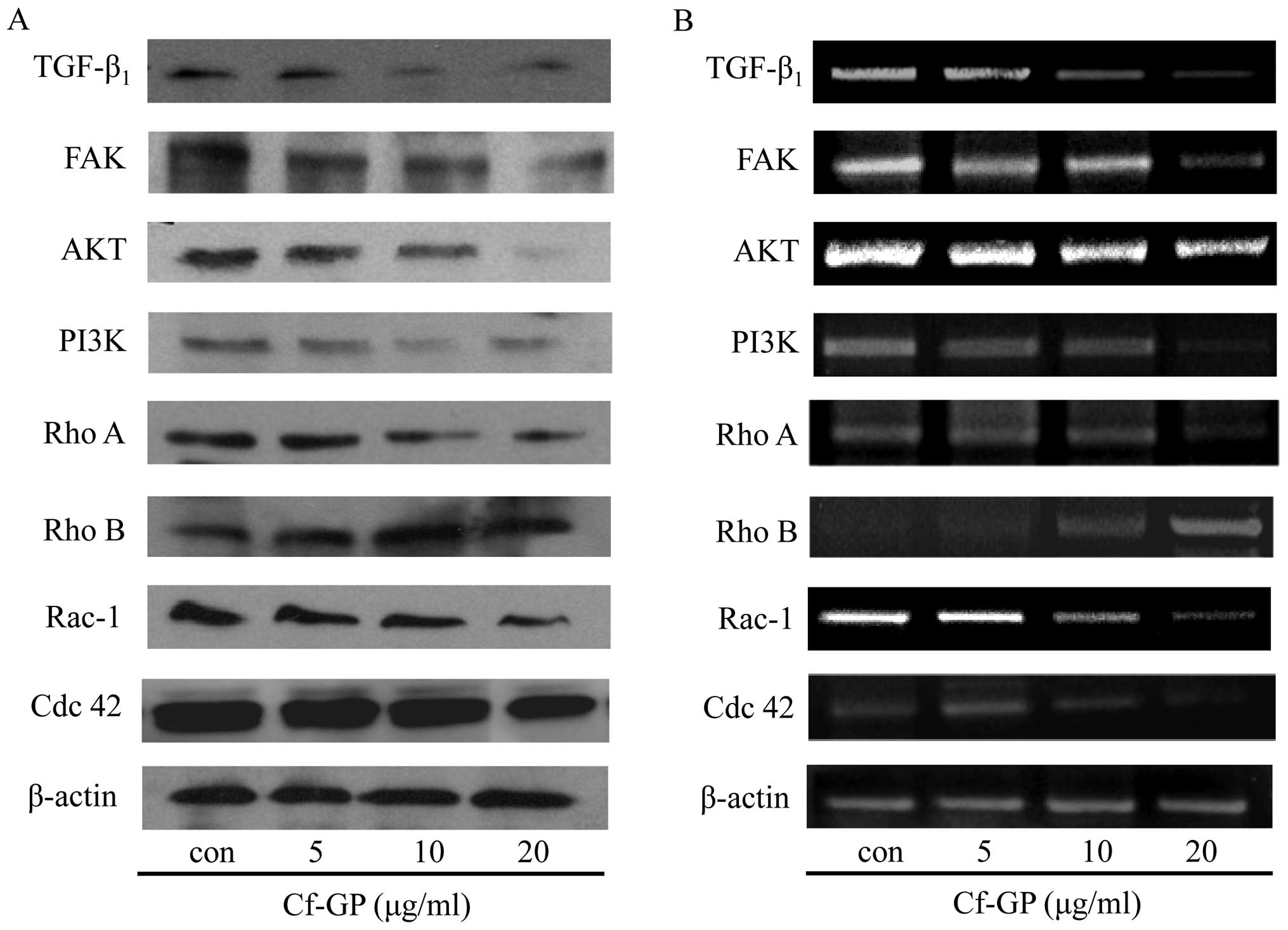
Cf-GP dose-dependently alters the
expression of TGF-β1 and small GTPases
The TGF-β1 receptor activates small
GTPases and the FAK/PI3K/AKT pathways. The overexpression of
FAK/PI3K/AKT can be induced by several other growth factors as
well, and the Rho family of small GTPases plays a critical role in
cancer cell growth and migration (18,19).
Thus, the altered expression of proteins associated with growth
factors regulates cancer cell growth and migration (20). In the present study, the protein
and mRNA expression levels of TGF-β1, FAK, PI3K, AKT and
the small GTPases Rho A, Rho B, Rac-1 and Cdc 42 were determined by
western blot analysis and RT-PCR analysis of AGS cells treated with
Cf-GP (5, 10 and 20 μg/ml) for 24 h. Treatment with Cf-GP
dose-dependently downregulated the protein (Fig. 4A) and mRNA expression levels
(Fig. 4B) of TGF-β1,
FAK, PI3K, AKT and the small GTPases Rho A, Rac-1 and Cdc 42. In
contrast, Cf-GP treatment upregulated the protein and mRNA
expression levels of Rho B, a small GTPase that is involved in the
inhibition of cancer cell growth.
Cf-GP dose-dependently downregulates the
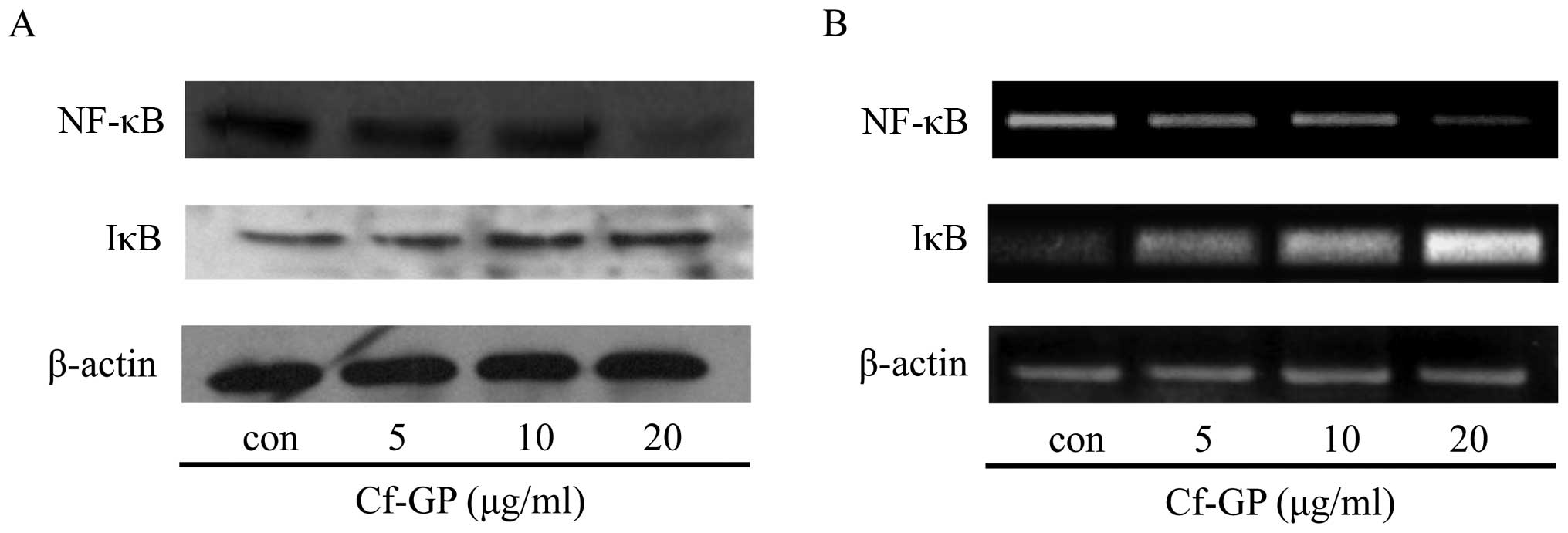
expression of NF-κB and IκB
NF-κB activation is necessary for cancer cell growth
and migration (21). NF-κB
inhibits activity through forming an NF-κB/IκB complex by
upregulation of IκB, however, almost all cancer cells show
downregulation IκB. Thus, degradation of NF-κB/IκB induced nuclear
translocation of NF-κB movement. The protein and mRNA expression
levels of the transcription factor NF-κB and its regulatory protein
IκB in AGS cells treated with Cf-GP (5, 10 and 20 μg/ml) for
24 h were analyzed by western blot analysis and RT-PCR. Cf-GP
treatment downregulated the expression of NF-κB and upregulation of
IκB (Fig. 5) in a dose-dependent
manner. This indicates that the Cf-GP induced inhibits degradation
of IκB. Therefore, inhibition of the translocated NF-κB is by the
stable NF-κB/IκB complex.
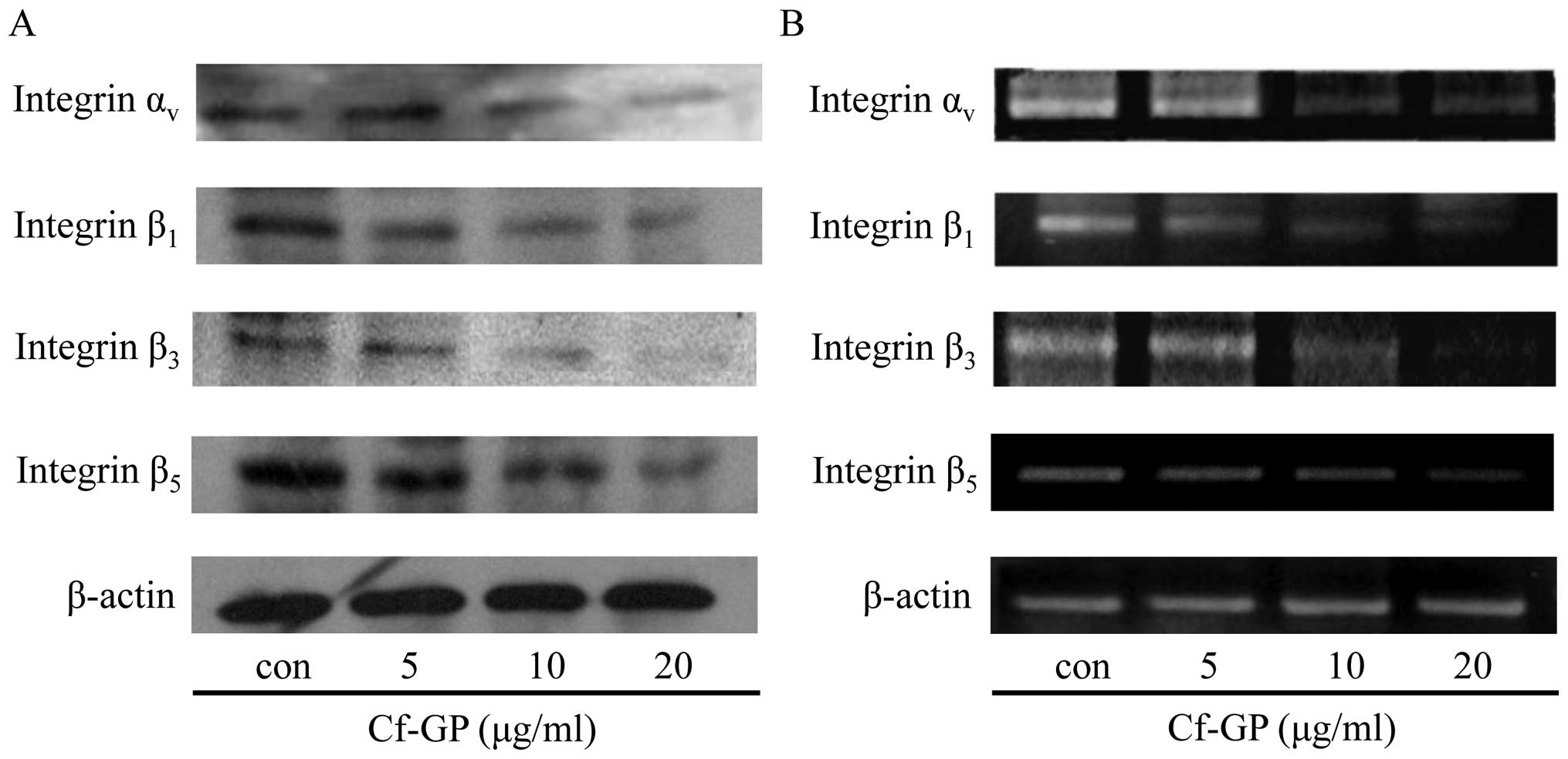
Cf-GP dose-dependently downregulates the
expression of integrins
Integrin proteins play important roles in cancer
cells growth and migration. Also, involvment in cancer cells
angiogenesis, invasion and incessant proliferation was induced.
Integrins are a family of heterodimers of α and β subunit, and
cancer cells of the specific expression of each subunit have been
reported (22). Among them,
integrin αν, β1, β3 and
β5 are known to be overexpressed in gastric cancer cells
(23). We have confirmed
inhibition of growth related factor and transcription factor via
reduction of TGF-β1 by Cf-GP. Therefore, we performed
expression of integrin proteins and mRNA levels through western
blot analysis and RT-PCR. The same conditions as in the treatment
by Cf-GP (5, 10 or 20 μg/ml) for 24 h, were used. The
protein (Fig. 6A) and mRNA
expression levels (Fig. 6B) of
integrins αν, β1, β3 and
β5 were reduced by Cf-GP treatment, in a dose-dependent
manner.
Discussion
Gastric cancer is common in many countries,
particularly in Asia, and is caused by irregular eating habits,
stress and environmental factors. Several studies (24–27)
have reported the anticancer effects of various marine algae,
including the green alga C. fulvescens, which is commonly
consumed in Asia. We previously observed that a glycoprotein of
C. fulvescens (Cf-GP) induced apoptosis in AGS human gastric
cancer cells through the Fas signaling pathway (6,7).
In the present study, we showed that Cf-GP also
inhibits the migration and proliferation of AGS cells, as well as
down-regulates the expression of several growth-related proteins,
including TGF-β1, FAK, PI3K, AKT and the small GTPases
Rho A, Rac-1 and Cdc 42. Cf-GP treatment also resulted in the
downregulated expression of NF-κB, IκB, and integrins
αν, β1, β3 and β5.
Conversely, Cf-GP upregulated the expression of the small GTPase
Rho B, which is involved in the inhibition of cell growth and the
induction of apoptosis.
TGF-β1 is a secreted cytokine that
participates in the regulation of cell migration, growth, apoptosis
and differentiation. TGF-β1 acts through its receptor to
induce FAK expression in several cancers, to activate the
PI3K/AKT/small GTPase pathway (13), and to upregulate integrin receptor
expression (28). The small
GTPases Rho A, Rho B, Rac-1, and Cdc 42 are involved in signaling
pathways associated with cell proliferation and migration in
response to various growth factor receptors (13). They also activate NF-κB, which
induces the expression of integrin receptors, thereby contributing
to cell migration. Integrin receptors are critical for cell
adhesion and cell attachment-dependent growth, migration, invasion
and metastasis (29,30). In particular, integrin has α and β,
two subunits of hetero-copolymer and these are associated with the
progression of a variety of human cancer cells. Attachment in
independent growth is a characteristic of transformed cells, but
cancer cell growth and migration depend on the interaction of the
cell adhesion receptor integrins and matrix. Therefore, integrins
promoted cell proliferation by attachment-dependent effects.
Specific integrin heterodimers are expressed in
different cancer cells, and the growth and migration of the cells
depend on the interactions between the integrins and their
extracellular ligands.
First, we observed the effects of Cf-GP in AGS cell
viability by MTS assay. Treatment by Cf-GP (5, 10 or 20
μg/ml) for 24 h inhibited the AGS cell growth and at the
highest concentration of 20 μg/ml in the apoptotic cells by
approximately 50% reduction compared to control group and no
toxicity to IEC-6 normal cell (Fig.
1). We used a wound-healing assay to confirm cell migration and
observed the denuded zone through a microscope. The gap (denuded
zone) between the cells was inhibited dose-dependently by Cf-GP
(Fig. 2). In addition, we
performed Annexin V staining assay for cell apoptosis rate by Muse
Annexin V and Dead Cell kit. The apoptosis rate increased 42.68% in
final Cf-GP concentration (20 μg/ml) compared with the
control group (Fig. 3). We found
TGF-β1 decreased FAK/PI3K/AKT/small GTPase expression by
using western blot analysis and RT-PCR (Fig. 4). This result shows that the
inhibition of FAK inhibited TGF-β1. Increased cancer
cell proliferation was observed when FAK/PI3K/AKT/small GTPase were
activated. Cf-GP-treated cells exhibited significant downregulation
of FAK/PI3K/AKT/small GTPase proteins (Rho A, Rac-1, Cdc 42) and
mRNA levels compared to the control group, but activation of Rho B
induced cancer cells apoptosis. In this study, Rho B increased by
Cf-GP (Fig. 4). The results of the
transcription factor NF-κB and IκB decreased due to inhibition of
these growth factors. Activation of the NF-κB and IκB induces
promotion of cancer cell migration and variety of intracellular
factors (31). We found
upregulation of IκB induced downregulation NF-κB through inhibition
of the growth factor and TGF-β1 by the Cf-GP effects
(Fig. 5). The integrin
αν pair with multiple integrin subunit β (β1,
β3, β5) and upregulation of integrin proteins
induced migration of cancer cells. The integrin expression through
the activation of transcription factors to increase was seen from
previous experiment, thus, the reduction of the NF-κB and increase
of IκB were indentified. Therefore, we performed expression of
integrins, and confirmed that Cf-GP induced downregulation of
integrin αν, β1, β3 and
β5 (Fig. 6).
Collectively, our findings suggest that Cf-GP
inhibits AGS gastric cancer cell migration and proliferation by
downregulating integrin expression via the inhibition of
TGF-β1-activated FAK/PI3K/AKT pathways. Cf-GP may be an
important factor in the development of functional foods and
therapeutic agents.
Acknowledgements
This research was supported by the
Basic Science Research Program through the National Research
Foundation of Korea (NRF) funded by the Ministry of Education,
Science and Technology (2012R1A6A1028677).
References
|
1.
|
Park HY, Lim CW, Kim YK, Yoon HD and Lee
KJ: Immunostimulating and anticancer activities of hot water
extract from Capsosiphon fulvescens. J Korean Soc Appl Biol
Chem. 49:343–348. 2006.
|
|
2.
|
Jung KJ, Jung CH, Pyeun JH and Choi YJ:
Changes of food components in Mesangi (Capsosiphon
fulvescens), Gashiparae (Enteromorpha prolifera), and
Cheonggak (Codium fragile) depending on harvest times. J
Korean Soc Food Sci Nutr. 34:687–693. 2005.
|
|
3.
|
Na YS, Kim WJ, Kim SM, Park JK, Lee MS,
Kim SO, Synytsya A and Park YI: Purification, characterization and
immunostimulating activity of water-soluble polysaccharide isolated
from Capsosiphon fulvescens. Int Immunopharmacol.
10:364–370. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Karnjanapratum S, Tabarsa M, Cho M and You
S: Characterization and immunomodulatory activities of sulfated
polysaccharides from Capsosiphon fulvescens. Int J Biol
Macromol. 51:720–729. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Hwang HJ, Kwon MJ, Kim IH and Nam TJ: The
effect of polysaccharide extracted from the marine alga
Capsosiphon fulvescens on ethanol administration. Food Chem
Toxicol. 46:2653–2657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Kim YM, Kim IH and Nam TJ: Induction of
apoptosis signaling by a glycoprotein of Capsosiphon
fulvescens in AGS cell. Kor J Fish Aquat Sci. 44:216–224.
2011.
|
|
7.
|
Kim YM, Kim IH and Nam TJ: Induction of
apoptosis signaling by glycoprotein of Capsosiphon
fulvescens in human gastric cancer (AGS) cells. Nutr Cancer.
64:761–769. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Kim YM, Kim IH and Nam TJ: Inhibition of
AGS human gastric cancer cell invasion and proliferation by
Capsosiphon fulvescens glycoprotein. Mol Med Rep. 8:11–16.
2013.PubMed/NCBI
|
|
9.
|
Levy L and Hill CS: Alterations in
components of the TGF-beta superfamily signaling pathways in human
cancer. Cytokine Growth Factor Rev. 17:41–58. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Serra R and Crowley MR: Mouse models of
transforming growth factor beta impact in breast development and
cancer. Endocr Relat Cancer. 12:749–760. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hua F, Zhongliang H, Jifang W, Kuansong W
and Ying L: TGF-beta promotes invasion and metastasis of gastric
cancer cells by increasing fascin1 expression via ERK and JNK
signal pathways. Acta Biochim Biophys Sin (Shanghai). 41:648–656.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Roarty K, Baxley SE, Crowley MR, Frost AR
and Serra R: Loss of TGF-β or Wnt5a results in an increase in
Wnt/β-catenin activity and redirects mammary tumor phenotype.
Breast Cancer Res. 11:R192009.
|
|
13.
|
Lee YC, Cheng TH, Lee JS, Chen JH, Liao
YC, Fong Y, Wu CH and Shih YW: Nobiletin, a citrus flavonoid,
suppresses invasion and migration involving FAK/PI3K/Akt and small
GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell
Biochem. 347:103–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Humphries MJ: Integrin structure. Biochem
Soc Trans. 28:311–339. 2000. View Article : Google Scholar
|
|
15.
|
Fong YC, Hsu SF, Wu CL, Li TM, Kao ST,
Tsai FJ, Chen WC, Liu SC, Wu CM and Tang CH: Transforming growth
factor-β1 increases cell migration and β1 integrin up-regulation in
human lung cancer cells. Lung Cancer. 64:13–21. 2009.
|
|
16.
|
Woodhouse EC, Chuaqui RF and Liotta LA:
General mechanisms of metastasis. Cancer. 80:1529–1537. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Krysho DV, Vanden BT, D’Herde K and
Vandenabeele P: Apoptosis and necrosis: detection, discrimination
and phagocytosis. Methods. 44:205–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Sahai E and Marshall CJ: Rho-GTPases and
cancer. Nat Rev Cancer. 2:133–142. 2002. View Article : Google Scholar
|
|
19.
|
Fritz G, Just I and Kaina B: Rho GTPases
are over-expressed in human tumors. Int J Cancer. 81:682–687. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Leivonen SK and Kähäri VM: Transforming
growth factor-beta signaling in cancer invasion and metastasis. Int
J Cancer. 121:2119–2124. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Boukerche H, Su ZZ, Emdad L, Sarkar D and
Fisher PB: mda-9/Syntenin regulates the metastatic phenotype in
human melanoma cells by activating nuclear factor-kappaB. Cancer
Res. 67:1812–1822. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Peng C, Liu X, Liu E, Xu K, Niu W, Chen R,
Wang J, Zhang Z, Lin P, Wang J, Agrez M and Niu J: Norcantharidin
induces HT-29 colon cancer cell apoptosis through the
ανβ6-extracellular signal-related kinase
signaling pathway. Cancer Sci. 100:2302–2308. 2009.PubMed/NCBI
|
|
23.
|
Zhang ZY, Xu KS, Wang JS, Yang GY, Wang W,
Wang JY, Niu WB, Liu EY, Mi YT and Niu J: Integrin
ανβ6 acts as a prognostic indicator in
gastric carcinoma. Clin Oncol (R Coll Radiol). 20:61–66. 2008.
|
|
24.
|
Go H, Hwang HJ and Nam TJ: A glycoprotein
from Laminaria japonica induces apoptosis in HT-29 colon
cancer cells. Toxicology In Vitro. 24:1546–1553. 2010.
|
|
25.
|
Hwang HJ, Kim IH and Nam TJ: Effect of a
glycoprotein from Hizikia fusiformis on
acetaminophen-induced liver injury. Food Chem Toxicol.
46:3475–3481. 2008.PubMed/NCBI
|
|
26.
|
Cho EK, Yoo SK and Choi YJ: Inhibitory
effects of maesaengi (Capsosiphon fulvescens) extracts on
angiotensin converting enzyme and α-glucosidase. Korea J Life Sci.
21:811–818. 2011.
|
|
27.
|
Kown MJ and Nam TJ: A polysaccharide of
the marine alga Capsosiphon fulvescens induces apoptosis in
AGS gastric cancer cells via an IGF-IR-mediated PI3K/Akt pathway.
Cell Biol Int. 31:768–775. 2007.
|
|
28.
|
Wei YY, Chen YJ, Hsiao YC, Huang YC, Lai
TH and Tang CH: Osteoblasts-derived TGF-β1 enhance motility and
integrin upregulation through Akt, ERK, and NF-κB-dependent pathway
in human breast cancer cells. Mol Carcinog. 47:526–537. 2008.
|
|
29.
|
Cooper CR, Chay CH and Pienta KJ: The role
of ανβ3 in prostate cancer progression.
Neoplasia. 4:191–194. 2002.
|
|
30.
|
Kumar CC: Integrin
ανβ3 as a therapeutic target for blocking
tumor-induced angiogenesis. Curr Drug Targets. 4:123–131. 2003.
|
|
31.
|
Helbig G, Christopherson KW II,
Bhat-Nakshatri P, Kumar S, Kishimoto H, Miller KD, Broxmeyer HE and
Naksharti H: NF-κB promotes breast cancer cell migration and
metastasis by inducing the expression of the chemokine receptor
CXCR4. J Biol Chem. 278:21631–21638. 2003.
|