Introduction
Ameloblastoma is a benign tumor of the jaw that is
characterized by local invasion and a high tendency to recur. This
tumor is thought to arise from the odontogenic epithelial apparatus
or its remnant tissues and has a histological appearance similar to
the developing enamel organ (1–3).
However, in spite of numerous histological and biological studies,
the mechanisms underlying the proliferation of this tumor are still
poorly understood.
Fibroblast growth factors (FGFs) have important
proliferative functions in the development of limbs, lungs, hair
and feathers, as well as in wound healing and development of some
tumors (4–10). In mammals, the FGF family currently
consists of 23 structurally related growth factors (11), the biological effects of which are
mediated through 4 high-affinity tyrosine kinase receptors, called
fibroblast growth factor receptors (FGFR) 1–4. FGFR1-3 have RNA
isoforms produced through alternative splicing and have different
ligand-binding properties (12–14).
In the developing tooth germ, the proliferation and differentiation
are governed by interactions between the odontogenic epithelium and
the neural crest-derived ectomesenchyme including the FGF signaling
(15–18). Among the FGFs, FGF3, FGF7 and FGF10
are expressed in mesenchymal cells and have important roles in the
proliferation of odontogenic epithelial cells during tooth
development (19–21). FGF3 stimulates the proliferation of
inner enamel epithelial cells, FGF7 also stimulates the
proliferation of outer enamel epithelial cells and FGF10 maintains
dental epithelial stem cells (22,23).
FGF7 binds to FGFR2 only, while FGF3 and FGF10 bind to both FGFR1
and FGFR2 (24,25). Signaling through FGFR1 and FGFR2
causes activation of the mitogen-activated protein kinases (MAPKs)
and phosphoinositide 3-kinase (PI3K). When FGF binds its receptors,
MAPK are stimulated to phosphorylate Raf, MEK and ERK sequentially.
Finally, activated ERK translocates to the nucleus, activates
transcription factors and induces cell proliferation. PI3K is also
phosphorylated downstream of FGF, resulting in the phosphorylation
of Akt and the inhibition of apoptosis (26).
In the present study, we thus hypothesized that
FGF3, FGF7 and FGF10 may also affect the proliferation of
ameloblastoma cells with odontogenic epithelial characteristics and
show some evidences that FGF signaling involved in the
ameloblastoma proliferation through MAPK pathway.
Materials and methods
Tissue samples and AM-1 cell culture
Surgically resected ameloblastoma tissues, with
written consent, from 32 patients (20 men and 12 women), from the
mandibles (31 cases) and maxilla (1 case), were analysed. Tissue
samples were classified according to the World Health
Organization’s International Histological Typing of Odontogenic
Tumors, 2nd edition: 18 cases, follicular type; 8 cases, plexiform
type; 4 cases, basal cell type; and 2 cases, desmoplastic type.
Resected samples were fixed in a 4% paraformaldehyde (PFA) solution
for 24–48 h and embedded in paraffin. To prepare frozen samples,
tissues were embedded in OCT compound (Tissue-Tek; Sakura
Finetechnical Co., Tokyo, Japan), frozen in liquid nitrogen and
stored at −80°C. Paraffin sections were sliced at 4-μm
thickness and the frozen sections were sliced at 6-μm
thickness; sections were then mounted on poly-L-lysine-coated glass
slides for immunohistochemical studies. AM-1 cells, an immortalized
ameloblastoma cell line transfected with HPV-16 DNA, were used in
this study (27). They were
cultured in Keratinocyte-SFM medium (Gibco Invitrogen Corp.,
Carlsbad, CA, USA), which were calcium chloride free medium for
inhibiting cell differentiation, containing 1%
penicillin-streptomycin (Gibco), 0.2% fungizone amphotericin B
(Gibco) and bovine pituitary extract (Gibco BRL, Grand Island, NY,
USA) at 37°C in a humidified atmosphere containing 5%
CO2.
Immunohistochemistry
Paraffin-embedded tissue sections were
de-paraffinized, frozen sections were fixed in 4% PFA for 30 min
and AM-1 cells cultured on Lab-Tek Chamber Slides (Nalge Nunc
International, Naperville, IL, USA) and fixed with acetone: 99.5%
ethanol (1:1) for 15 min. Slides were then immersed in 3% hydrogen
peroxide for 30 min and 10% normal goat serum prepared in
phosphate-buffered saline (PBS) for 20 min. The primary antibodies
(Table I) were applied overnight
in a moist chamber at 4°C, labeled using a Histofine SAB-PO (R) kit
(Nichirei Co., Tokyo, Japan) and visualized with diaminobenzidine
(Nichirei). Negative controls were prepared by substituting PBS for
primary antibody. Tissue sections were counterstained with
hematoxylin. In each step, the samples were washed thrice with PBS
for 5 min. According to their immunoreactivity, these samples were
further classified into 3 groups; negative (−), weakly positive (+)
and strongly positive (++).
 | Table I.Primer sequences for RT-PCR. |
Table I.
Primer sequences for RT-PCR.
| Genes | Forward | Reverse |
|---|
| FGF7 |
GCTTGCAATGACATGACTCC |
TGCCATAGGAGAAAAGTGGG |
| FGF10 |
TGTCACCTGCCAAGCCCTT |
TACGGGCAGTTCTCCTTCTT |
| β-actin |
CATCCTGACCCTCAAGTACCC |
GTGGTGGTGAAGCTGTAGCC |
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was isolated from 10 cases of
ameloblastoma (follicular type, 7; plexiform type, 1; basal cell
type, 2) and AM-1 cells by using a QuickPrep Total RNA Extraction
kit (Amersham Biosciences, Piscataway, NJ, USA). mRNA was
transcribed to cDNA by using Ready-to-go You-Prime First-Strand
Beads (Amersham Biosciences) and random primers, according to the
manufacturer’s instructions. Primer sequences are listed in
Table II. PCR was performed using
Ready-To-Go PCR beads. Diethylpyrocarbonate water (26 μl),
forward primer (1 μl) and reverse primer (1 μl) were
added to the beads. PCR of FGF3, FGF7 and β-actin was carried out
for 30 cycles at 95°C for 30 sec, 55°C for 1 min and 72°C for 1
min, while that of FGF10 was carried out for 30 cycles at 95°C for
45 sec, 65°C for 45 sec and 72°C for 45 sec. The PCR products were
separated on 2% agarose gels and stained with ethidium bromide.
 | Table II.Primary antibodies for
immunohistochemistry and western blotting. |
Table II.
Primary antibodies for
immunohistochemistry and western blotting.
| Antibody (host,
clonality, Company) | Purpose | Dilution |
|---|
| FGFR1 (rabbit,
polyclonal, Santa Cruz Biotechnology, CA) | IHC | 1:150 |
| FGFR2 (rabbit,
polyclonal, Santa Cruz Biotechnology, CA) | IHC | 1:150 |
| FGF7 (rabbit,
polyclonal, Santa Cruz Biotechnology, CA) | IHC | 1:150 |
| FGF7 (rabbit,
polyclonal, Abcam, Cambridge, UK) | WB | 1:200 |
| FGF10 (rabbit,
polyclonal, Santa Cruz Biotechnology, CA) | IHC | 1:150 |
| FGF10 (goat,
polyclonal, Peprotech Inc., Rocky Hill, NJ) | WB | 1:200 |
| p-p44/42 MAPK
(Thr202/Tyr204) (rabbit, polyclonal, Cell Signaling, Beverly,
MA) | WB | 1:1,000 |
| Akt (rabbit,
polyclonal, Cell Signaling, Beverly, MA) | WB | 1:1,000 |
| p-Akt (Ser473)
(rabbit, polyclonal, Cell Signaling, Beverly, MA) | WB | 1:1,000 |
| Actin (mouse,
monoclonal, Amersham, Buckinghamshire, UK) | | |
Immunoblotting
Fresh tissue samples from 4 cases (follicular type,
2; plexiform type, 1; basal cell type, 1) of ameloblastoma and AM-1
cells were homogenized and lysed in a buffer containing 20 mM
HEPES/NaOH (pH 7.2), 150 mM NaCl, 2 mM EDTA, 1% Triton X-100, 50 mM
sodium fluoride, 2 mM sodium orthovanadate, 30 mM sodium
pyrophosphate and a mixture of protease inhibitors (10 μM
aprotinin, 10 μM pepstatin A, 10 μM leupeptin and 1
mM PMSF). For phosphorylation assays, AM-1 cells were seeded in
Keratinocyte-SFM with pituitary extract for 24 h, washed with PBS
and then incubated in Keratinocyte-SFM without bovine pituitary
extract for 24 h, before being stimulated with recombinant FGF7
(Peprotech Inc., Rocky Hill, NJ, USA) or recombinant FGF10 (R&D
Systems, Inc., Minneapolis, MN, USA) for the indicated time
periods. In some experiments, cells were pre-incubated for 1 h with
1 or 10 μM U0126 (a specific inhibitor of MEK1/2) (Cell
Signaling Technology, Danvers, MA, USA). U0126 was dissolved in
dimethyl sulfoxide (DMSO) and a similar concentration (0.1%) was
added to PBS as a control, before treatment with the growth factor.
The proteins were separated on a sodium dodecyl sulfate
(SDS)-polyacrylamide gel and transferred to a nitrocellulose
membrane. After the blocking of non-specific binding with 0.1% BSA
in PBS, the membrane was incubated with the primary antibodies
(Table I). Secondary antibodies
were horseradish peroxidase-conjugated donkey anti-rabbit IgG
antibodies (Amersham Biosciences) diluted 1:1,000 or donkey
anti-mouse IgG antibody (Amersham Biosciences) diluted 1:2,000. The
bound antibodies were visualized using the ECL system (Amersham
Biosciences).
Cell proliferation assay
AM-1 cells (2,500/well) were seeded in 96-well
plates and then incubated in Keratinocyte-SFM with pituitary
extract for 24 h. Cells were treated with FGF7 or FGF10, FGF7
(Abcam, Cambridge, UK) or FGF10 (Peprotech Inc.) neutralizing
antibody and MAPK inhibitor (U0126) were added to the culture
medium every 48 h. After treatment, the cells were enumerated using
a Cell-Counting kit (Dojindo, Tokyo, Japan) according to the
manufacturer’s instructions. Briefly, 10 μl of the reagent
was added to each well and incubated for 2 h. The reaction was
measured at 450 nm by using a microplate reader.
Statistical analyses
Results of proliferation assay were expressed as
mean ± standard error of mean (SE). Data were analyzed by unpaired
Student’s t-test. Statistical significance was set as p<0.05.
Analyses were applied to experiments carried out at least three
times.
Results
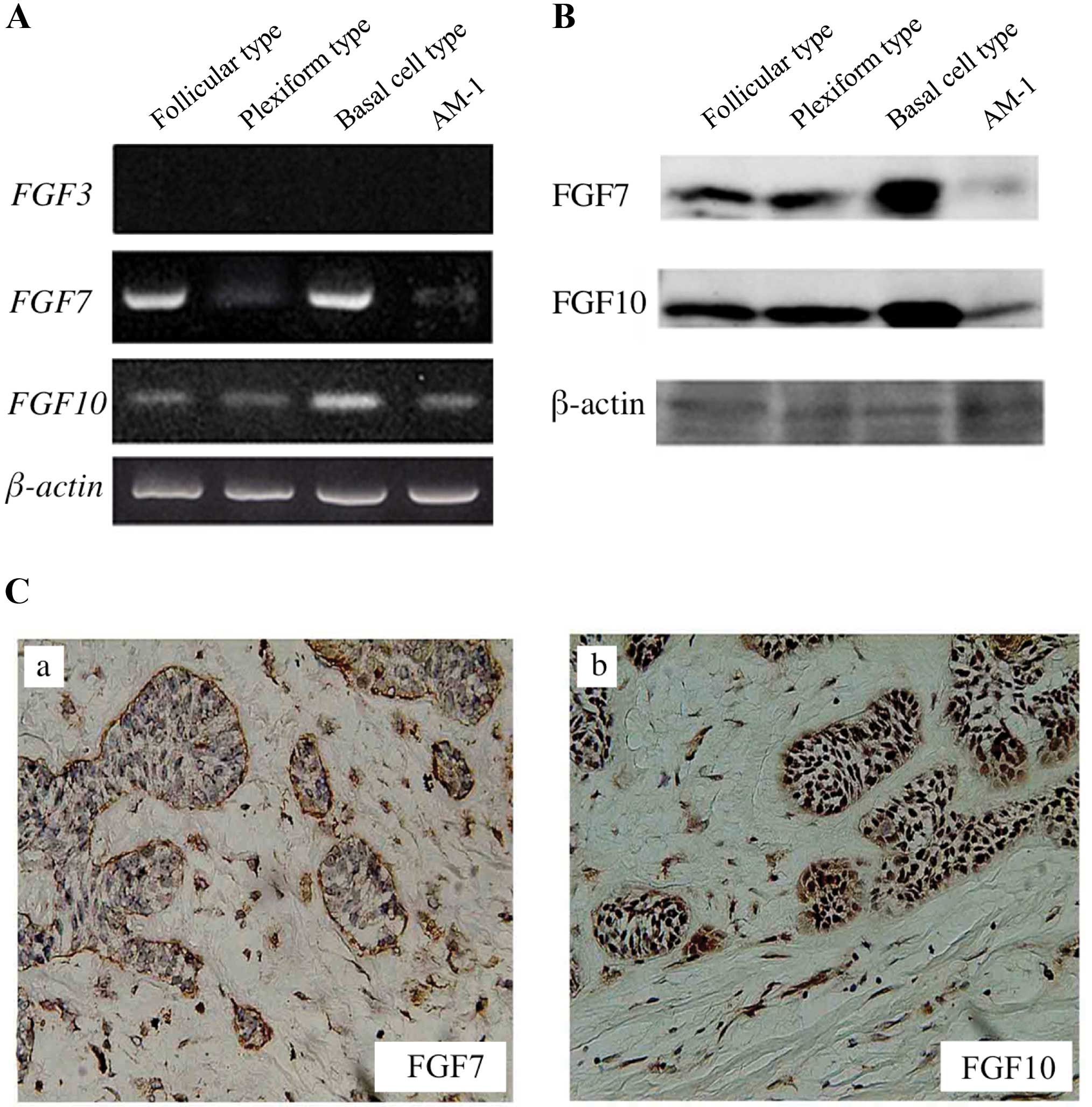
Expression of FGF7 and FGF10 in various
types of ameloblastoma and AM-1 cells
FGF7 and FGF10 were expressed in all
types of ameloblastoma and AM-1 cells, though expression of
FGF3 was not found (Fig.
1A). By western blot analyses, FGF7 and FGF10 were also
detected in the ameloblastoma tissues and AM-1 cells (Fig. 1B). Furthermore, immunohistochemical
location of FGF7 and FGF10 was investigated mainly in the stromal
cells rather than the tumor cells (Fig. 1C and Table III).
 | Table III.Results of immunohistochemical
staining in the ameloblastoma specimens. |
Table III.
Results of immunohistochemical
staining in the ameloblastoma specimens.
|
Immunoreactivity | FGF7 | FGF10 | FGFR1 | FGFR2 |
|---|
|
|
|
|
|---|
| − | + | ++ | − | + | ++ | − | + | ++ | − | + | ++ |
|---|
| Follicular type
(n=18) | | | | | | | | | | | | |
| Tumor cells | 0 (0) | 16 (88.9) | 2 (11.1) | 0 (0) | 14 (77.8) | 4 (22.2) | 0 (0) | 2 (11.1) | 16 (88.9) | 0 (0) | 0 (0) | 18 (100) |
| Stromal
cells | 0 (0) | 0 (0) | 18 (100) | 0 (0) | 0 (0) | 18 (100) | 0 (0) | 0 (0) | 18 (100) | 18 (100) | 0 (0) | 0 (0) |
| Plexiform type
(n=8) | | | | | | | | | | | | |
| Tumor cells | 0 (0) | 6 (75.0) | 2 (25.0) | 0 (0) | 6 (75.0) | 2 (25.0) | 0 (0) | 2 (25.0) | 6 (75.0) | 0 (0) | 0 (0) | 8 (100) |
| Stromal
cells | 0 (0) | 0(0) | 8 (100) | 0 (0) | 0 (0) | 8 (100) | 0 (0) | 4 (50.0) | 4 (50.0) | 8 (100) | 0 (0) | 0 (0) |
| Basal cell type
(n=4) | | | | | | | | | | | | |
| Tumor cells | 0 (0) | 2 (50.0) | 2 (50.0) | 0 (0) | 2 (50.0) | 2 (50.0) | 0 (0) | 1 (25.0) | 3 (75.0) | 0 (0) | 0 (0) | 4 (100) |
| Stromal
cells | 0 (0) | 0 (0) | 4 (100) | 0 (0) | 0 (0) | 4 (100) | 0 (0) | 0 (0) | 4 (100) | 4 (100) | 0 (0) | 0 (0) |
| Desmoplastic type
(n=2) | | | | | | | | | | | | |
| Tumor cells | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
| Stromal
cells | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) | 0 (0) | 2 (100) | 0 (0) |
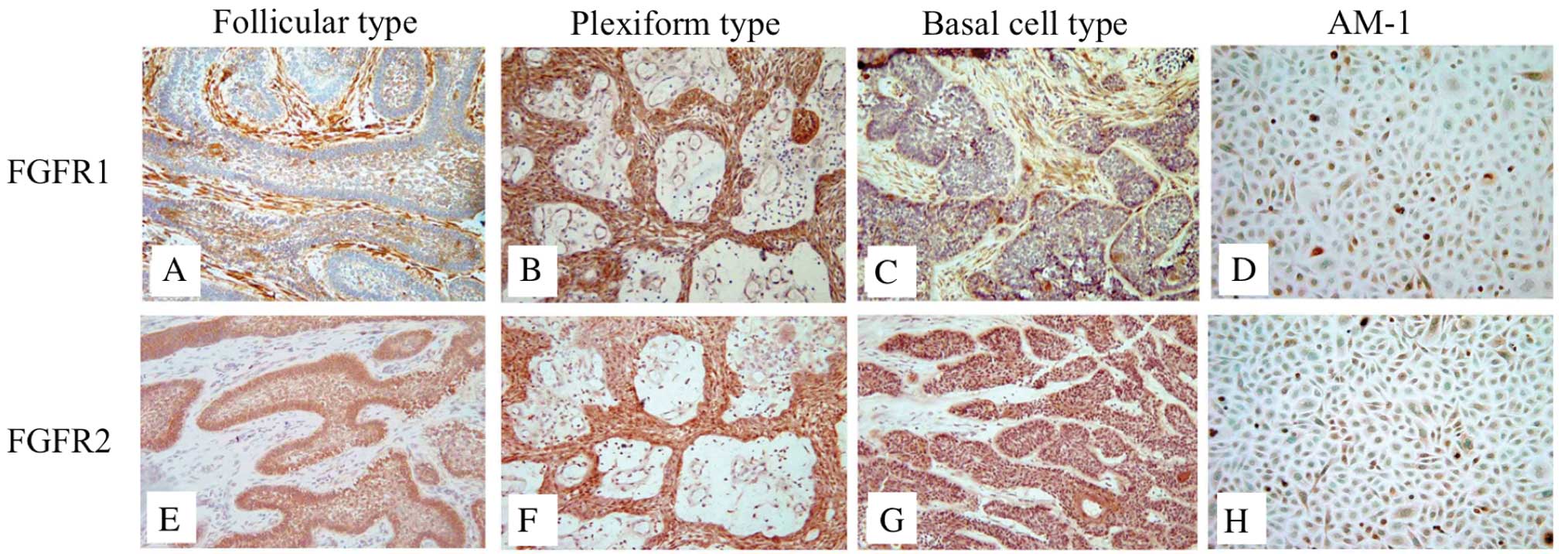
Localization of FGFR1 and FGFR2 in
various types of ameloblastoma and AM-1 cells
Next, we performed immunohistochemistry to analyze
the expression of FGFR1 and FGFR2, the specific receptors of FGF7
and FGF10, in the ameloblastoma specimens and in AM-1 cells. FGFR1
was expressed in the tumor and stromal cells of ameloblastoma, the
expression of FGFR2 was investigated only in the tumor cells
(Fig. 2). In the follicular
ameloblastoma, FGFR1 was strongly expressed in the stromal cells
(Table III). In AM-1 cells, the
expression of FGFR1 and FGFR2 was detected in the cytoplasm and
cell membrane (Fig. 2D and H).
FGFR1 and FGFR2 were weakly expressed in the desmoplastic type
tissues (Table III).
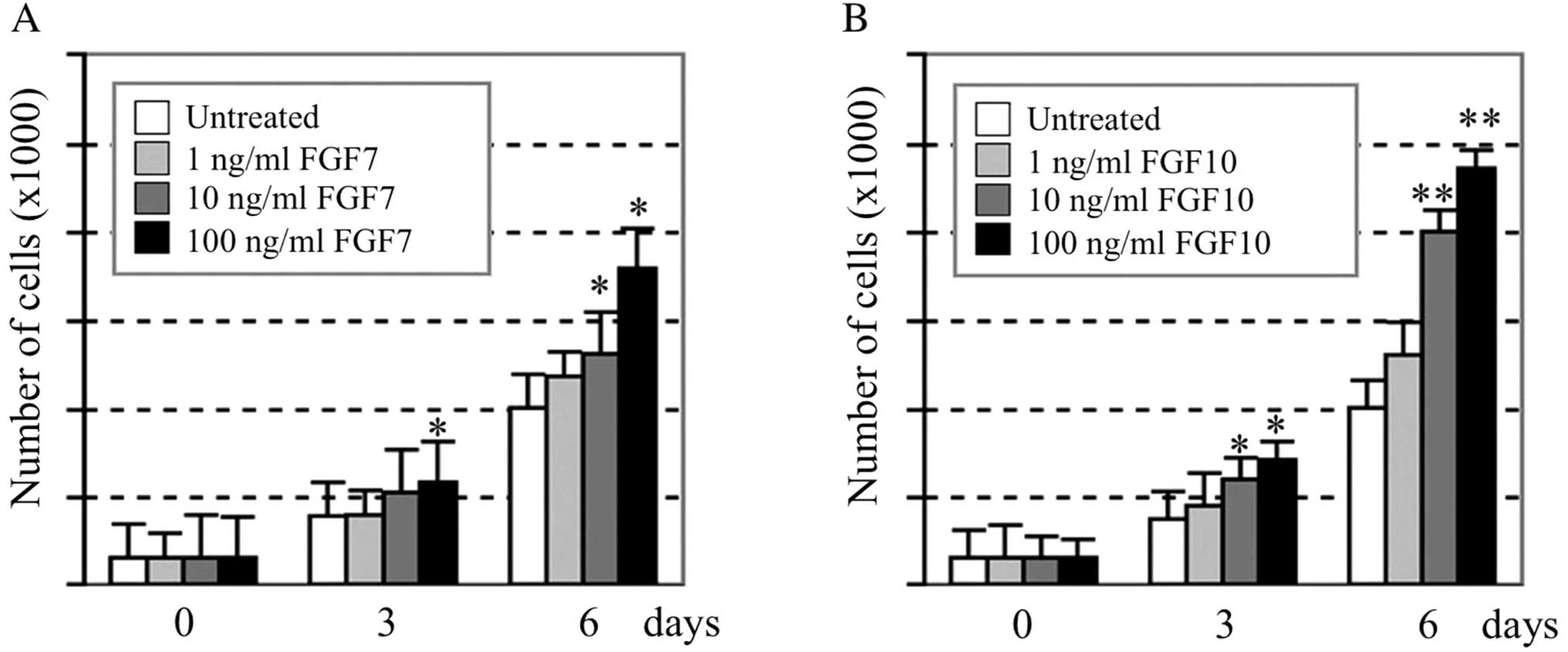
Effect of recombinant human FGF7 and
FGF10 proteins on cell proliferation and phosphorylation of p44/42
MAPK in AM-1 cells
When various concentrations (0, 1, 10 and 100 ng/ml)
of recombinant human FGF7 or FGF10 proteins were added to the
medium, AM-1 cells proliferated in a dose-dependent manner. At the
highest dose of FGF7 and FGF10 (100 ng/ml), the number of cells
increased to 176 and 247% of the control value, respectively
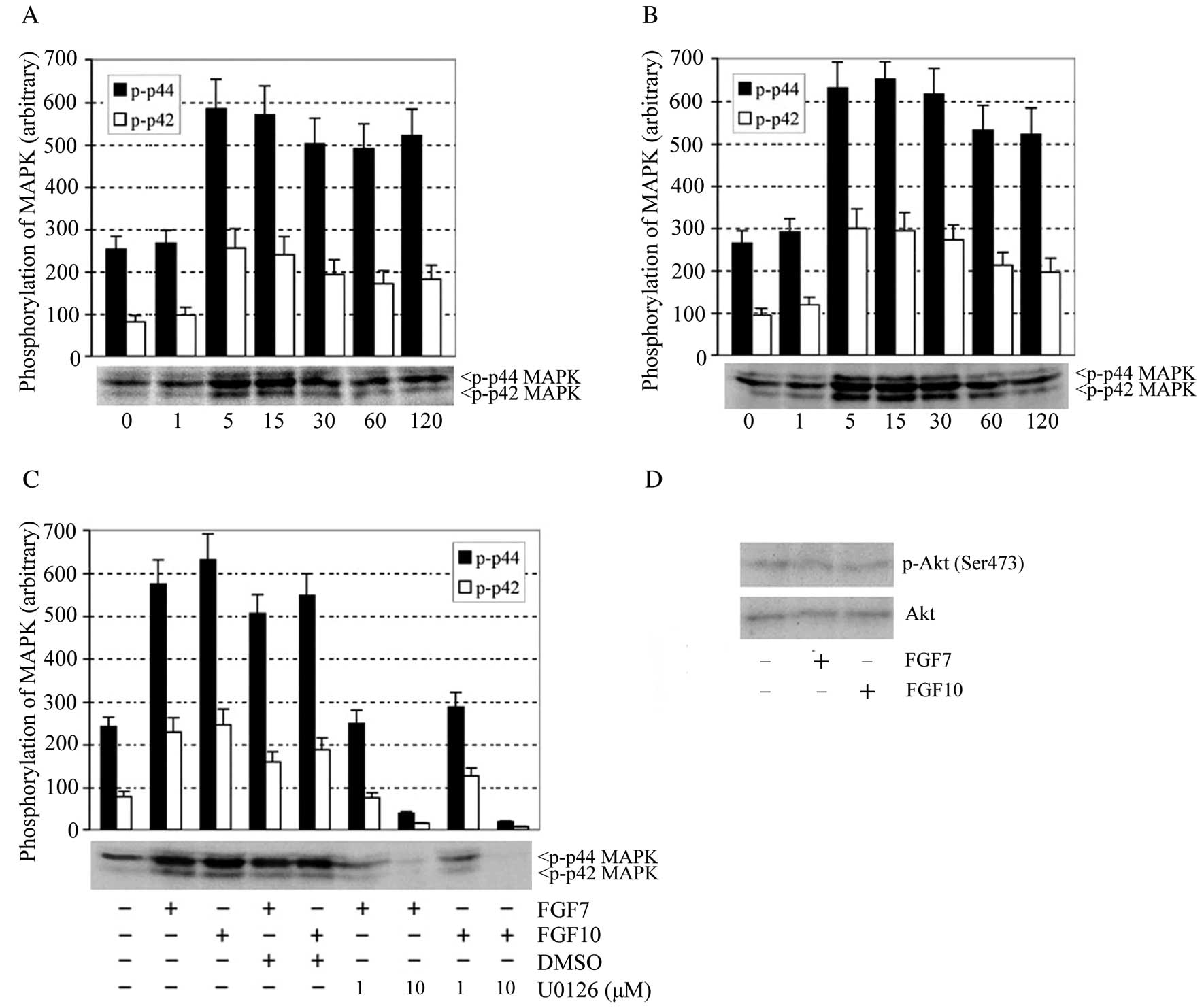
(Fig. 3). The time course of
p44/42 phosphorylation in AM-1 cells during treatment with FGF7 (10
ng/ml) and FGF10 (10 ng/ml) was examined using an
anti-phospho-p44/42 MAPK antibody. FGF7-mediated activation of
phospho-p44/42 MAPK peaked at 5 min and continued for up to 15 min
(Fig. 4A). FGF10 caused increased
p44/42 phosphorylation at 5 min that lasted for up to 30 min
(Fig. 4B). Pre-treatment with the
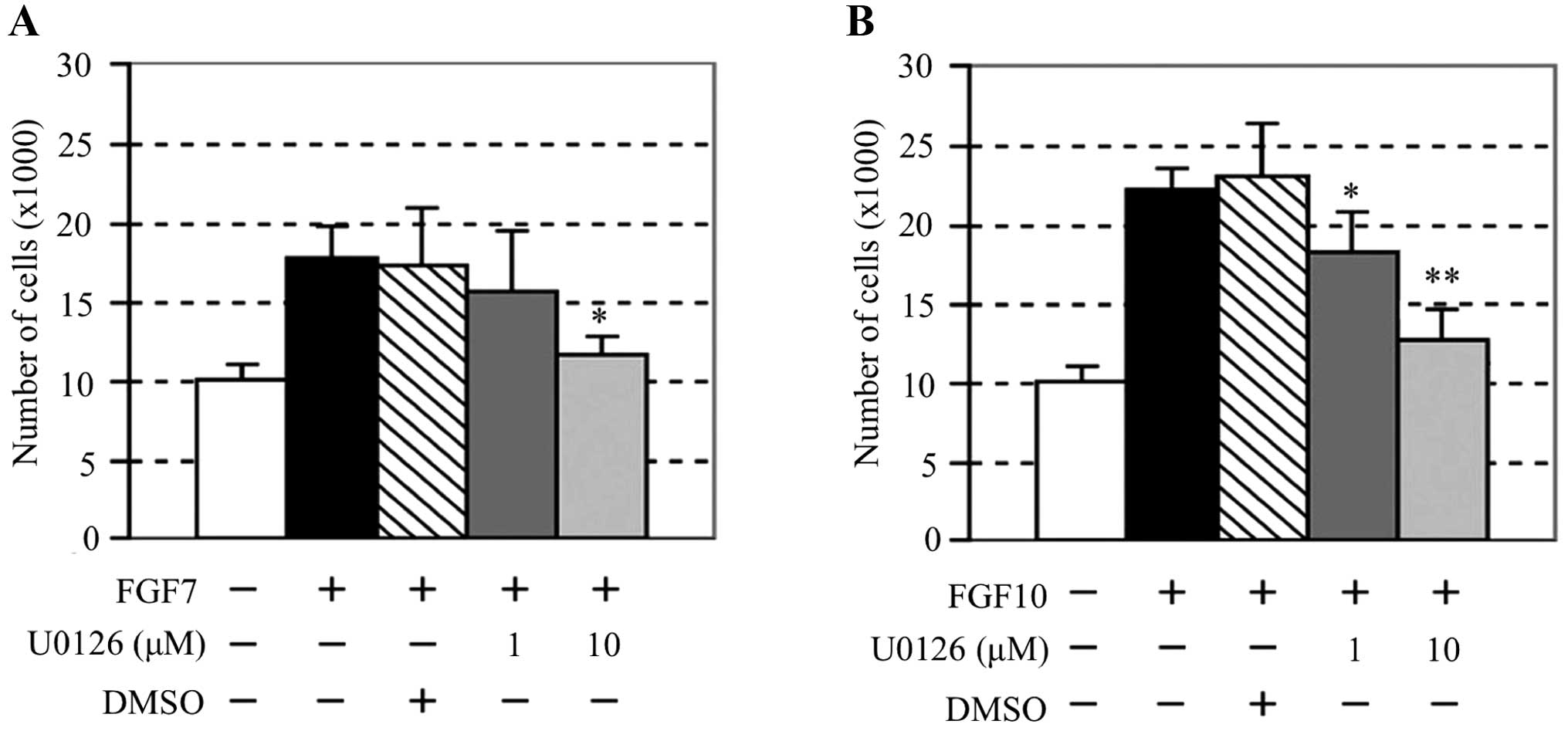
MAPK inhibitor U0126 completely inhibited the phosphorylation of
p44/42 MAPK by FGF7 and FGF10 (Fig.
4C). Interestingly, phosphorylation of Akt (Ser473) through
PI3K/Akt signaling pathway was not investigated by adding FGF7 or
FGF10 (Fig. 4D). U0126 also
inhibited proliferation of AM-1 cells stimulated with FGF7 or FGF10
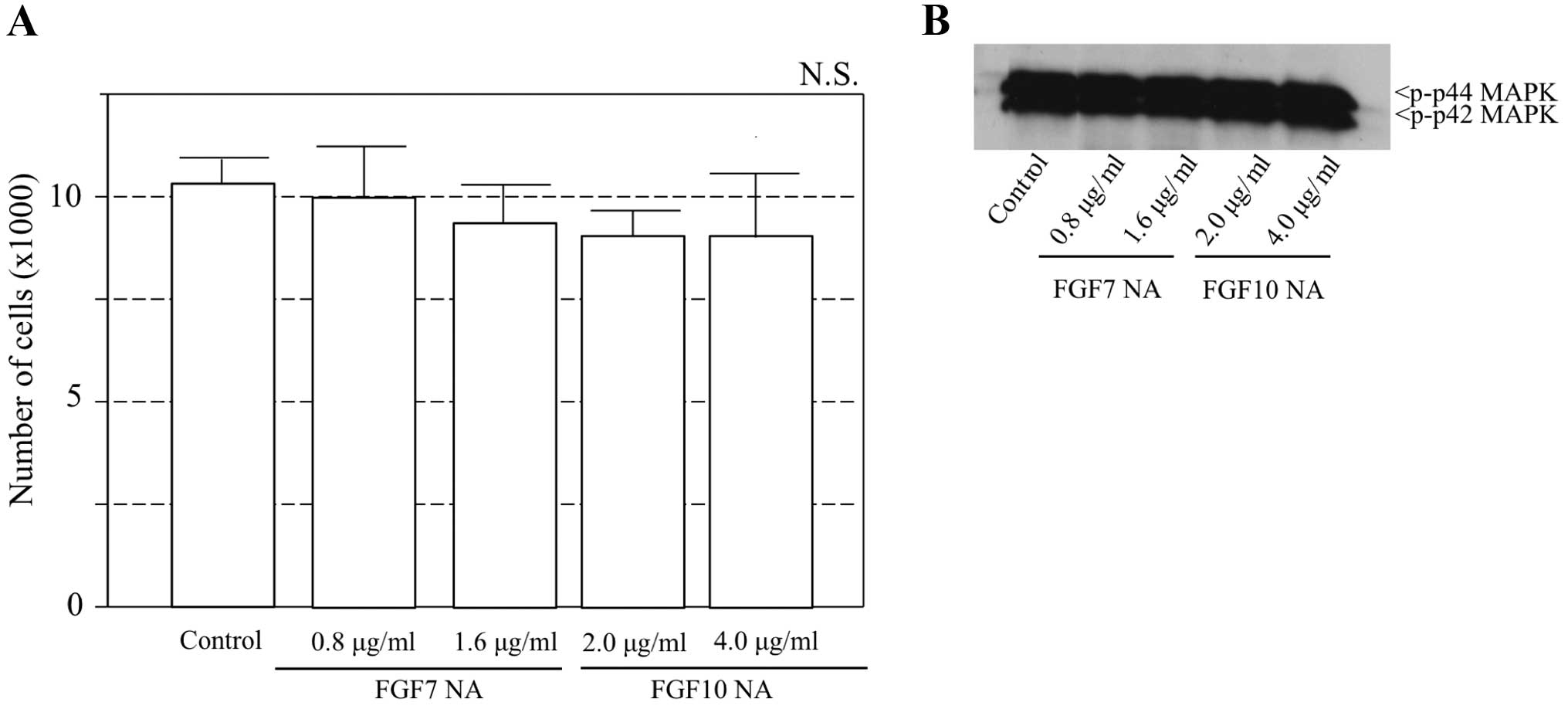
(Fig. 5). Furthermore, to examine
whether FGF7 and FGF10 secreted by AM-1 cells affect the
proliferation through autocrine stimulation, neutralized antibodies
for these FGFs were added to the culture of AM-1 cells. The
addition of FGF7 or FGF10 neutralizing antibody did not inhibit the
proliferation of AM-1 cells and the activation of phospho-p44/42
MAPK was not investigated (Fig.
6).
Discussion
The expression of FGF1, FGF2, FGFR2 and FGFR3 in
ameloblastoma was previously reported and it was concluded that
FGF1 and FGF2 may contribute to the growth and development of
ameloblastoma mediated by their receptors (27–29).
However, the function of FGF signaling in ameloblastoma is still
poorly understood, because these previous reports investigated only
the expression patterns in this tumor immunohistochemically. In
this study, we examined the expression of FGF3, FGF7, FGF10 and
their specific receptors in histologically various types of
ameloblastoma and analyzed the effect of these growth factors on
the proliferation of ameloblastoma using the ameloblastoma cell
line AM-1.
It is already known that FGF7 and FGF10 act as a
proliferative signal from mesenchyme to epithelium through the
FGFR1 and FGFR2 during tooth development (19). Immunohistochemical staining in the
present study also revealed the expression of FGF7 and FGF10 mainly
in the stromal cells rather than the tumor cells. Therefore, to
examine the effects of FGF7 and FGF10 on the proliferation of
ameloblastoma, cell proliferation assays were performed. The
addition of recombinant human FGF7 or FGF10 proteins to the culture
medium stimulated the proliferation of AM-1 cells in the
dose-dependent manner. Our previous study also showed that FGF10
was expressed in the mesenchymal cells stimulating the
proliferation of rat dental epithelial cells (21). Previously it was demonstrated that
FGF10 induces cell migration and invasion in pancreatic cancer
(30). Taken together, FGF7 and
FGF10 may play important roles in the proliferation and progression
of ameloblastoma.
In this study, the expression of FGF7 and FGF10 was
also detected in AM-1 cells. To examine the effects of autocrine
stimulation of FGF7 and FGF10 on the proliferation of AM-1,
neutralized antibodies for these FGFs were thus added to the
culture. However, adding FGF7 or FGF10 neutralizing antibody did
not inhibit the proliferation of AM-1 cells. These results suggest
that FGF7 and FGF10 may act as paracrine factors from stromal cells
rather than autocrine factors from tumor cells in the proliferation
of ameloblastoma.
MAPKs are evolutionarily conserved enzymes
connecting cell-surface receptors to critical regulatory targets
within the cells. In mammals, MAPK signaling cascades regulate
important cellular processes. ERK1 (p/44) and ERK2 (p/42) are
predominantly activated by mitogenic factors and they, in turn,
activate transcription factors such as elk, c-myc and c-fos, which
stimulate cell proliferation, differentiation and survival
(31–34). In this study, we found that FGF7
and FGF10 induced the phosphorylation of p44/42 MAPK in AM-1 cells
and U0126 inhibited this phosphorylation as well as the
proliferation of AM-1 cells. On the other hand, phosphorylation of
Akt was not confirmed. These results suggested that the MAPK
pathway might have an important role in the FGF7- and
FGF10-stimulated growth of ameloblastomas.
In conclusion, this study showed that FGF7 and FGF10
are expressed in ameloblastomas and that they play an important
role in the growth of ameloblastomas through the MAPK pathway.
Acknowledgements
We thank Emeritus Professor Masamichi
Ohishi for continuous advice and Dr Hidemitsu Harada for his
helpful comments and for providing the AM-1 cells used in this
research. This study was supported by a Grant-in-Aid (no. 23792358)
from the Japanese Ministry of Education, Culture, Sports, Science
and Technology of Japan.
References
|
1.
|
Lucas RB: Pathology of Tumors of the Oral
Tissues. 4th edition. Churchill Livingstone; Edinburgh: 1984
|
|
2.
|
Kramer IRH, Pingborg JJ and Shear M: WHO
Histological Typing of Odontogenic Tumors. 2nd edition.
Springer-Verlag; Berlin: 1992, View Article : Google Scholar
|
|
3.
|
Gorlin RJ, Chaundhry AP and Pingborg JJ:
Odontogenic tumors: classification, histopathology and clinical
behavior in man and domesticated animals. Cancer. 14:73–112. 1961.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Hebert JM, Rosenquist T, Gotz J, et al:
FGF5 as a regulator of the hair growth cycle: evidence from
targeted and spontaneous mutations. Cell. 78:1017–1025. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Crossley PH, Minowada G, Macarthur CA, et
al: Roles for FGF8 in the induction, initiation and maintenance of
chick limb development. Cell. 84:127–136. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Peters K, Werner S, Liao X, et al:
Targeted expression of a dominant negative FGF receptor blocks
branching morphogenesis and epithelial differentiation of the mouse
lung. EMBO J. 13:3296–3301. 1994.PubMed/NCBI
|
|
7.
|
Sekine K, Ohuchi H, Fujiwara M, et al:
Fgf10 is essential for limb and lung formation. Nat Genet.
21:138–141. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Widelitz RB, Jiang TX, Noven A, et al: FGF
induces new feather buds from developing avian skin. J Invest
Dermatol. 107:797–803. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Stephan B, Olivera S, Michael S, et al:
Growth factors and cytokines in wound healing. Wound Repair Regen.
16:585–601. 2008. View Article : Google Scholar
|
|
10.
|
Turner N and Grose R: Fibroblast growth
factor signaling: from development to cancer. Nat Rev Cancer.
10:116–129. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Cormier S, Leroy C, Delezoid AL, et al:
Expression of fibroblast growth factors 18 and 23 during human
embryonic and fetal development. Gene Expr Patterns. 5:569–573.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wilkie AO, Morriss-Kay GM, Jones EY, et
al: Functions of fibroblast growth factors and their receptors.
Curr Biol. 5:500–507. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Johnson DE and Williams LT: Structural and
functional diversity in the FGF receptor multigene family. Adv
Cancer Res. 60:1–41. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Ornitz DM, Xu J, Colvin JS, et al:
Receptor specificity of the fibroblast growth factor family. J Biol
Chem. 271:15292–15297. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kollar EJ and Baird G: Tissue interactions
in embryonic mouse tooth germs. II. The inductive role of dental
papillae. J Embyonal Exp Morphol. 24:173–186. 1970.PubMed/NCBI
|
|
16.
|
Mina M and Kollar EJ: The induction of
odontogenesis in nondental mesenchyme combined with early murine
mandibular arch epithelium. Arch Oral Biol. 32:123–127. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Klein OD, Lyons DB, Balooch G, et al: An
FGF signaling loop sustains the generation of differentiated
progeny from stem cells in mouse incisors. Development.
135:377–385. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Porntaveetus T, Otsuka-Tanaka Y, Albert
BM, et al: Expression of fibroblast growth factors (Fgfs) in murine
tooth development. J Anat. 218:534–543. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Kettunen P, Laurikkala J, Itaranta P, et
al: Association of FGF-3 and FGF-10 with signaling networks
regulating tooth morphogenesis. Dev Dyn. 219:322–332. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Harada H, Kettunen P, Jung H, et al:
Localization of putative stem cells in dental epithelium and their
association with Notch and FGF signaling. J Cell Biol. 147:105–120.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kawano S, Saito M, Handa K, et al:
Characterization of dental epithelial progenitor cells derived from
cervical-loop epithelium in a rat lower incisor. J Dent Res.
83:129–133. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Harada H, Toyono T, Toyoshima K, et al:
FGF10 maintains stem cell compartment in developing mouse incisors.
Development. 129:1533–1541. 2002.PubMed/NCBI
|
|
23.
|
Harada H and Ohshima H: New perspectives
on tooth development and the dental stem cell niche. Arch Histol
Cytol. 67:1–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Miki T, Bottaro DP, Fleming TP, et al:
Determination of ligand-binding specificity by alternative
spricing: two distinct growth factor receptors encoded by a single
gene. Proc Natl Acad Sci USA. 89:246–250. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Hoffman MP, Kidder BL, Steinberg ZL, et
al: Gene expression profiles of mouse submandibular gland
development: FGFR1 regulates branching morphogenesis in vitro
through BMP- and FGF-dependent mechanisms. Development.
129:5767–5778. 2002. View Article : Google Scholar
|
|
26.
|
Yatskievych T, Konieczka JH, Bobbs A, et
al: FGF signaling through RAS/MAPK and PI3K pathways regulates cell
movement and gene expression in the chicken primitive streak
without affecting E-cadherin expression. BMC Dev Biol. 11:202011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Harada H, Mitsuyasu T, Nakamura N, et al:
Establishment of ameloblastoma cell line, AM-1. J Oral Pathol Med.
27:207–212. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Myoken Y, Myoken Y, Okamoto T, et al:
Immunohistochemical localization of fibroblast growth factor-1
(FGF-1) and FGF-2 in cultured human ameloblastoma epithelial cells
and ameloblastoma tissues. J Oral Pathol Med. 24:387–392. 1995.
View Article : Google Scholar
|
|
29.
|
So F, Daley TD, Jackson L, et al:
Immunohistochemical localization of fibroblast growth factors FGF-1
and FGF-2 and receptors FGFR2 and FGFR3 in the epithelium of human
odontogenic cysts and tumors. J Oral Pathol Med. 30:428–433. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Nomura S, Yoshitomi H, Takano S, et al:
FGF10/FGFR2 signal induces cell migration and invasion in
pancreatic cancer. Br J Cancer. 99:305–313. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Taniguchi F, Harada T, Sakamoto Y, et al:
Activation of mitogen-activated protein kinase pathway by
keratinocyte growth factor or fibroblast growth factor-10 promotes
cell proliferation in human endometrial carcinoma cells. J Clin
Endocrinol Metab. 88:773–780. 2003. View Article : Google Scholar
|
|
32.
|
Renaud F, Desset S, Oliver L, et al: The
neurotrophic activity of fibroblast growth factor 1 (FGF1) depends
on endogenous FGF1 expression and is independent of the
mitogen-activated protein kinase cascade pathway. J Biol Chem.
271:2801–2811. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Chang L and Karin M: Mammalian MAP kinase
signaling cascades. Nature. 410:37–40. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Lovicu FJ and Mcavoy JW: FGF-induced lens
cell proliferation and differentiation is dependent on MAPK
(ERK1/2) signalling. Development. 128:5075–5084. 2001.PubMed/NCBI
|