Introduction
High expression and over-secretion of cathepsin D
(cath D) have been shown in several independent studies to be
associated with an increased risk of recurrence and metastasis in
breast cancer (1–3). Since the inactive 52 kDa procathepsin
D is secreted by cancer cells both in culture under routine
conditions at pH 7.4 (4,5) and in pleural effusion of breast
cancer (6), its maturation in
acidic compartment is required to be proteolytically active. In
this investigation we raised two major questions. Are cancer cells,
cultured at the extracellular pH of hypoxic solid tumors (7,8) able
to stimulate the cath D activity, and in this case, would cath D be
able to activate the plasminogen activator system since a high uPA
concentration in breast cancer cytosol was associated with poor
prognosis (9,10)?
We therefore monitored serum-free culture conditions
at pH 6.6 approaching the acidity of tumor microenvironments
allowing the survival of invasive MDA-MB231 breast cancer cells
without cell lysis. We then tested the effects of medium
acidification mediated by cath D activity on the secretion of uPA,
tissue-type plasminogen activator (tPA) and PA inhibitor-1 (PAI-1).
The two secreted plasminogen activators and their activities were
analysed in the presence or absence of pepstatin, a specific
aspartyl protease inhibitor.
Materials and methods
Cell culture and preparation of
conditioned media
MDA-MB231 breast cancer cells were cultured in
duplicate 6-well plates in Dulbecco’s modified Eagle’s medium
(DMEM) with 10% fetal calf serum to 80% confluence. To test the
effect of an acidic pH on lysosome distribution and cath D routing,
cells were transferred on glass coverslips and medium was changed
to a 50 mM HEPES-buffered Ringer’s solution with 70 mM Na acetate
as described (11). To prepare
conditioned media during longer time of treatment, the cells were
transferred in duplicate in 6-well plates in DMEM solution as
described (12). Briefly, the
culture medium was DMEM without FCS, with Hank’s balanced salt
solution. It was adjusted to pH 6.6 or 7.4 using
HCO3/HCl according to the equation [HCO3] =
(1.52 mM of CO2) × [10 (pH 6.24)]. In experiments with
pepstatin A (50 or 100 μM), this aspartyl protease inhibitor
was dissolved in DMSO and its effect was compared to control media
containing the same amount of DMSO alone. The pH of conditioned
media measured before addition to the cells and at the end of
incubation were controlled to be constant. Under these conditions,
MDA-MB231 cells were viable for at least 4 days. Cell number was
evaluated by DNA assay using the DABA colorimetric method (13).
Media conditioned by these cells during increasing
periods of time were normalised to the number of cells (determined
by DNA assay), and centrifuged to remove cell debris. The
supernatant was then layered on a PAGE for zymography or western
blot analysis or were assayed for tPA activity and for tPA and
PAI-1 antigen concentrations.
Analysis of plasminogen activators (PA)
activity by casein-plasminogen zymography
PA activity was analysed after separation by
electrophoresis in 10% polyacrylamide SDS gel copolymerized with 1
mg/ml of casein (Sigma, St. Louis, MO, USA) and 30 μg/ml
human plasminogen (Sigma) under non-reducing conditions (without
mercaptoethanol) and in the absence of serine protease inhibitors,
as described (14,15). The caseinolytic bands were revealed
and quantified by scanning. Molecular weight markers (Amersham,
Piscataway, NJ, USA) were run in parallel.
Plasminogen activator enzymatic
assay
PA activity in conditioned media was determined in
triplicate using the human PA chromogenic enzymatic kit (Assay pro
ref CT1001, AssaySense, St. Charles, MO, USA) according to
instructions of this laboratory. This assay was not specific for
tPA or uPA. Briefly, plasmin produced from plasminogen activation
was quantified using a specific plasmin substrate releasing
para-nitroaniline, a yellow chromophore. Amiloride (1 mM), which
inhibits uPA activity, was used to discriminate tPA activities
(16). The plate was incubated at
37°C in a humid incubator for increasing periods of time. The
optical density at 405 nm determined using a microplate
spectrophotometer MRX (Dynatech Laboratories, Chantilly, VA, USA)
and checked to be proportional to PA activities.
Western blot analysis of tPA
concentration
The 3-day conditioned media were prepared from
MDA-MB231 cells as described above, supplemented with protease
inhibitors (Complete, Roche Diagnostics, Meylan, France), and 5 mM
mercaptoethanol and concentrated 20-fold using 10K Amicon Ultra-0.5
ml (Millipore, Molsheim, France). A total of 10 μl of
concentrated media were analysed on 7.5% SDS-PAGE and transferred
to nitrocellulose as described (17). Membranes were probed with a rabbit
polyclonal antibody to human tPA (ab28219 from Abcam, Paris,
France) with 1:1,000 dilution. After washing, membranes were
incubated with peroxidaseconjugated secondary antibody (dilution
1:5,000; Amersham) and revealed by bioluminescence with the ECL
detection system (Amersham). The apparent molecular weights were
estimated with pre-stained standard proteins (Bio-Rad,
Marnes-la-Coquette, France) run in parallel. Intracellular protein
amounts were quantified by the Bradford method.
tPA and PAI-1 ELISA assays
The Imubind tPA and PAI-1 ELISA kits (American
Diagnostica GmbH, Pfungstadt, Germany) were used according to
manufacturer’s instructions. Briefly, tPA or PAI-1 antigens were
retained on a goat polyclonal antibody anti-tPA or a mouse
monoclonal anti-PAI-1 adsorbed on the microtiter plate and
quantified by a second peroxidase-conjugated polyclonal anti-tPA or
anti-PAI-1 antibody. The concentrations of tPA and PAI-1 in the
samples were determined by plotting the values of peroxidase
activities to a standard curve obtained from purified antigens.
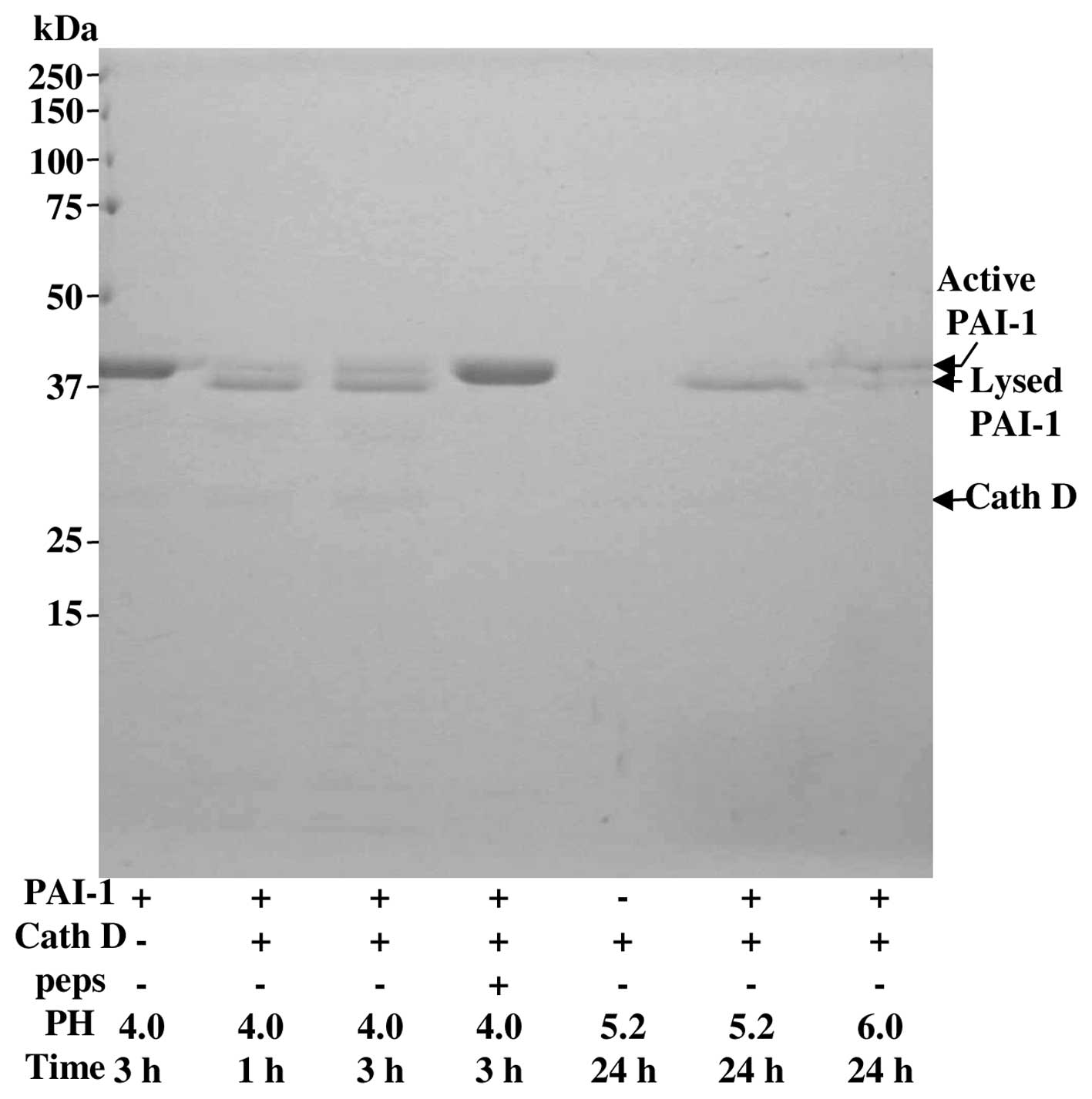
Degradation of PAI-1 by cath D
Recombinant active PAI-1 (∼43 kDa) and cath D from
human liver (∼34 kDa) were purchased from Sigma-Aldrich (Lyon,
France). The ability of cath D to cleave PAI-1 was investigated
with an enzyme:substrate ratio of 1:5 in a 100 μM sodium
acetate buffer at 37°C at pH 6.0, 5.2 or 4.0 as previously
described (23). Inhibition of
this digestion was performed with pepstatin at 0.1 μM. After
the indicated time of incubation, the reaction was stopped by
freezing. Samples were then boiled in electrophoresis buffer for 3
min, and fragments of PAI-1 were separated on a 12% polyacrylamide
SDS-PAGE. The SDS-PAGE was stained with Brilliant Blue.
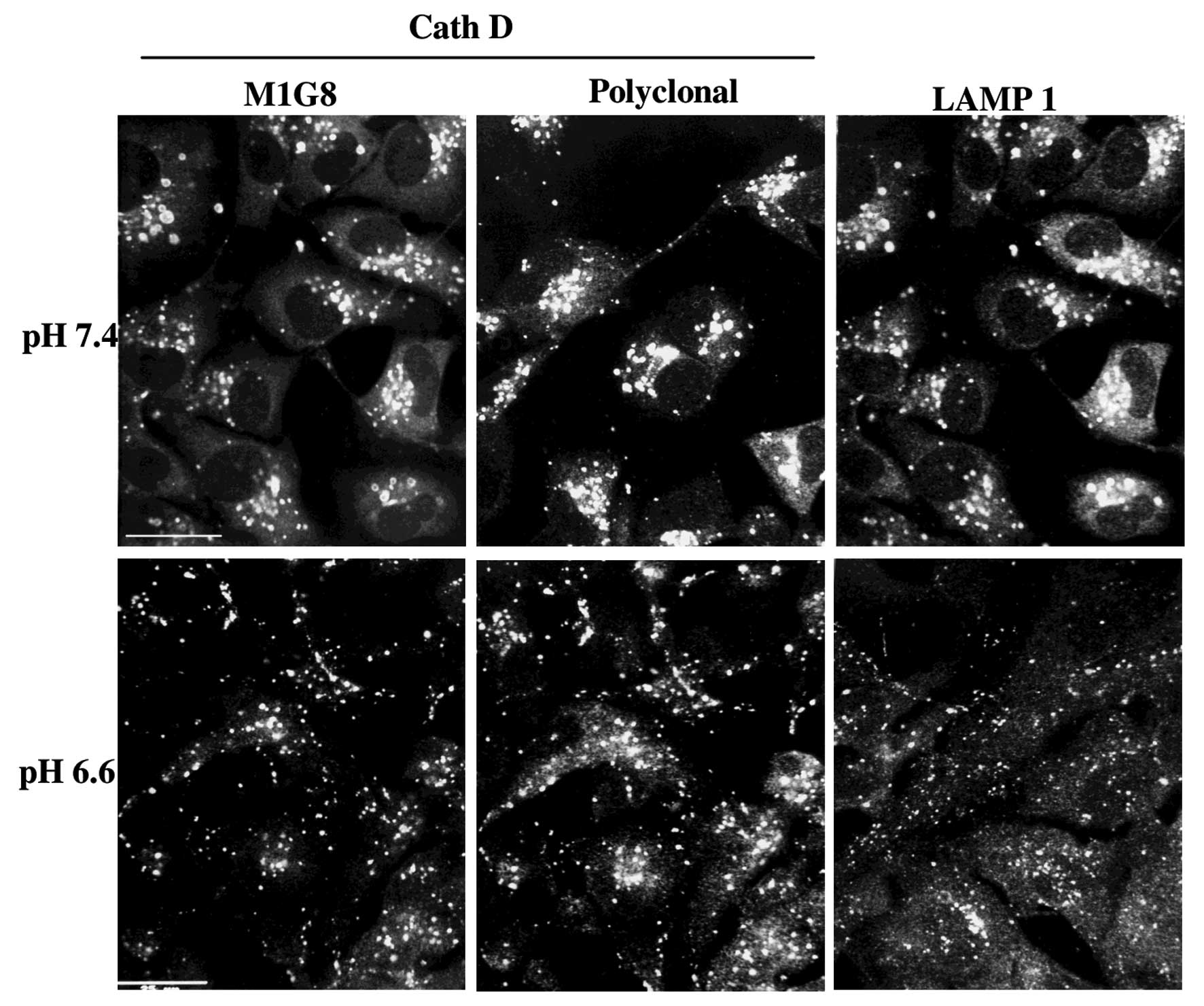
Immunolocalization of cath D and validity
of the cath D antibodies
MDA-MB231 cells were cultured on glass coverslips at
pH 7.4 or 6.6 for 3 h. They were then fixed with paraformaldehyde
and glutaraldehyde and permeabilized by Triton X-100. Lysosomes
vesicles were stained using the anti-cath D M1G8 mAb (18), the cath D swine polyclonal
antibodies (kindly provided by Dr M. Fusek) or the anti-LAMP1
antibody (Abcam). The M1G8 mAb used (Figs. 1 and 2) recognises all forms of cath D, while
the M2E8 mAb is specific of the pro-enzyme. These antibodies have
been characterized previously (18,19
and the references within).
Results
Effect of the acidic pH on MDA-MB231
cells, lysosome localization and cath D level
MDA-MB231 breast cancer cells were plated in 12-well
plates to reach 30% confluence and then incubated in the FCS free
media at pH 7.4 or 6.6 for up to 3 days. Their growth was decreased
by 35% at pH 6.6 compared to pH 7.4, but not altered by pepstatin
A, at either pH value (results not shown). We have also verified
that cells plated at 80% confluence, remained quiescent without
cell lysis at pH 6.6 for at least 36 h allowing the preparation of
conditioned media for secreted proteins analysis (data not
shown).
As shown on Fig. 1,
the lysosomes stained by different cath D antibodies or Lamp1
antibodies, were delocalised at the cell periphery by medium
acidification from pH 7.4 (30% at the cell periphery) to pH 6.6
(70% at periphery). The effect was progressive from pH 7.4 to 6.3
and was optimal at pH 6.6 which was chosen for further experiments.
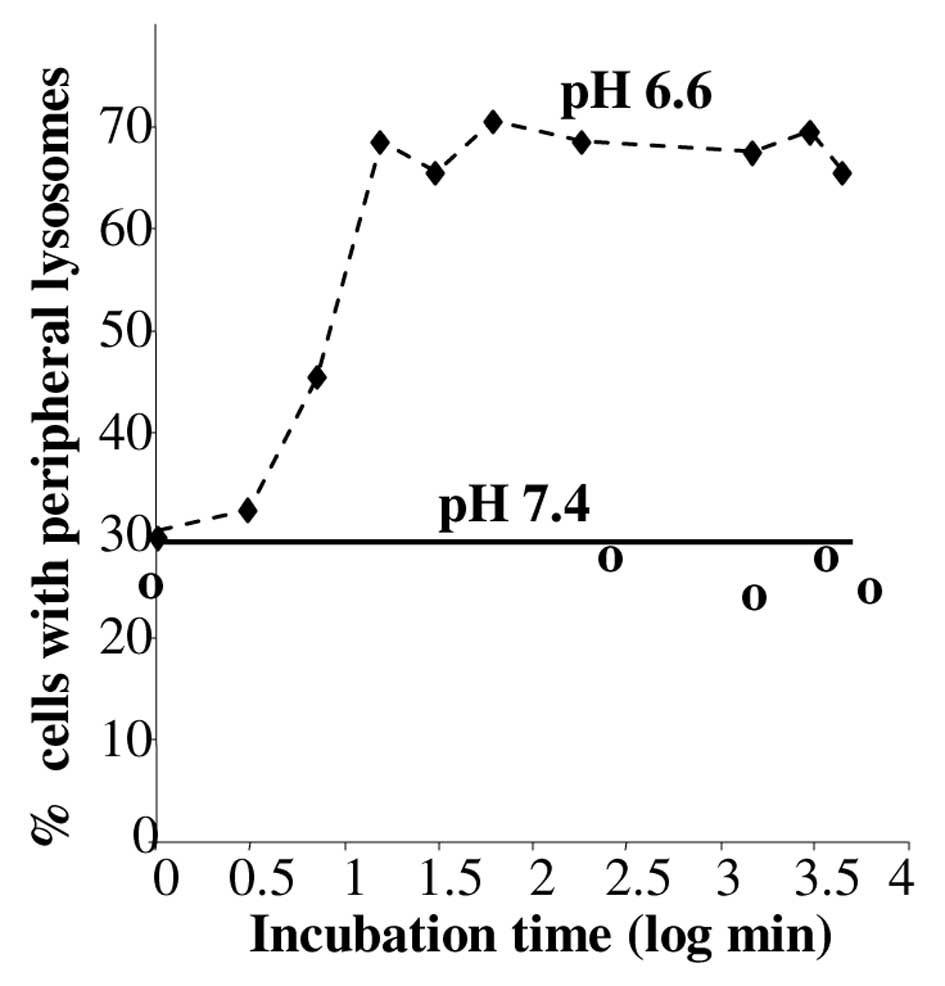
This peripheral location of lysosomes at pH 6.6 was rapid, within 7
min and stable for 3 days (Fig.
2). It was rapidly reversible at pH 7.4 indicating that the
cells were still viable (data not shown).
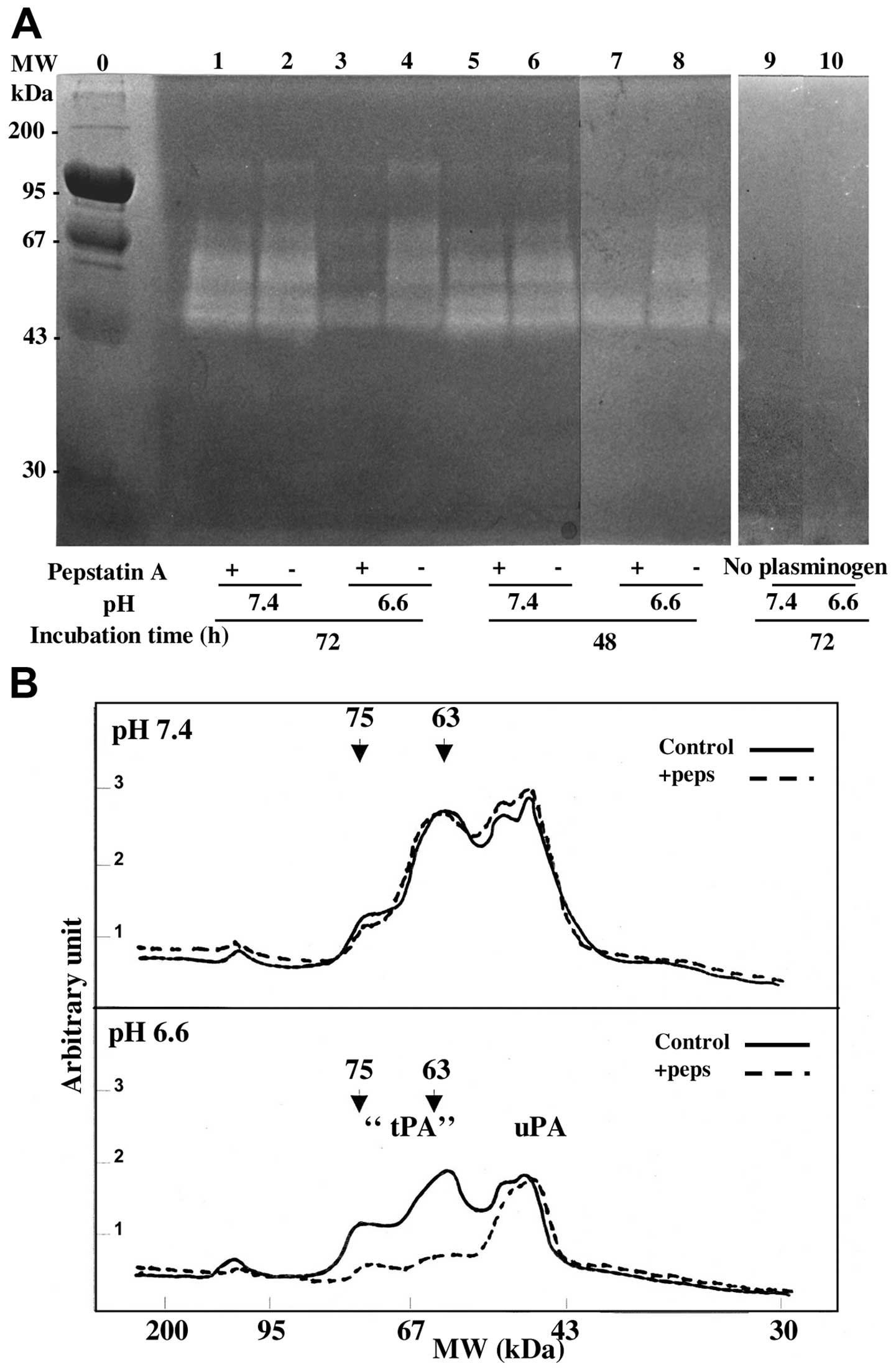
Zymography analysis of the cath D
stimulation of the proteolytic activity of secreted plasminogen
activators
The conditioned media of MDA-MB231 cells, collected
after 1 to 3 days of culture at pH 7.4 or 6.6, were analysed for PA
activities on a non-reducing SDS-PAGE containing plasminogen and
casein as described in Materials and methods. In acidic and neutral
media the same caseinolytic large bands were observed migrating
with apparent molecular weight of 110–130, 63–75 and 45 kDa.
However, when cells had been treated with pepstatin, the
caseinolytic bands of higher molecular weight (63–75 kDa) were
decreased in acidic conditions but 45 kDa bands were unaffected
(Fig. 3). The molecular weights of
the cath D stimulated bands were in agreement with those of the
unbound tPA (63–75 kDa) and of a PA/PAI-1 complex (110–130 kDa)
(16,20,21).
The absence of caseinolytic bands in PAGE analysis performed in the
same experiments without plasminogen (Fig. 3A, lanes 9 and 10) or without
conditioned media showed an effect due to the activation of
plasminogen rather than the activation of other proteases secreted
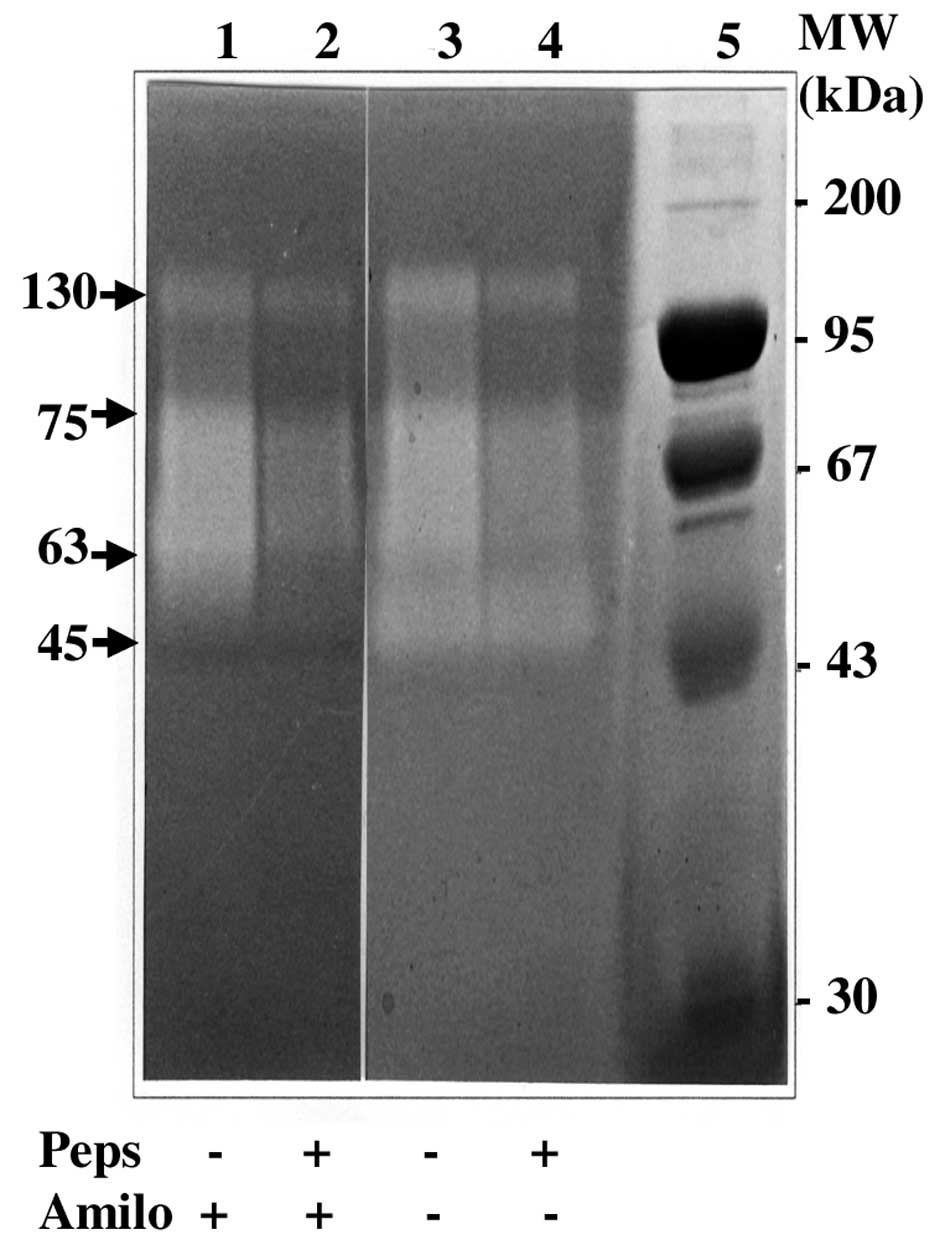
or present in the gel. The 45 kDa band (Fig. 3) corresponded to uPA on the basis
of its molecular weight and specific sensitivity to amiloride
(16,22) as shown in Fig. 4. This band was not modified by
pepstatin treatment suggesting that uPA activity was not stimulated
by cath D activity (Figs. 3 and
4). Quantification by scanning of
lanes 1 to 4 of the zymograph (Fig.
3A) showed that after 3 days of conditioning, the activity of
the different molecular weight PA forms, was decreased at the
acidic pH (Fig. 3B). This
decreased PA-induced caseinolytic activity at pH 6.6 compared to pH
7.4 was reproducibly observed in independent experiments (Fig. 5) and was associated with a decrease
of the secreted cath D (see previous section). Fig. 3B also shows that pepstatin
decreased specifically the 75 to 63 kDa activities at pH 6.6 but
not at pH 7.4 while the uPA activities were not altered. The effect
of pepstatin on the 63–75 kDa lytic bands was slow and optimal
after 3 days of treatment (Fig.
5).
Effect of pepstatin at pH 6.6 on the
activity and protein level of tPA and PAI-1 in the conditioned
media
The effect of pepstatin on the PA proteolytic
activity of conditioned media was also evaluated by a PA
colorimetric assay. After 3 days of culture at pH 6.6, the total PA
activity and the amiloride resistant tPA activity were partly
inhibited by pepstatin (Table I,
compare lanes a and b).
 | Table I.Effect of pepstatin on PA activities,
tPA and PAI-1 concentrations secreted by MDA-MB-231 cells at pH
6.6. |
Table I.
Effect of pepstatin on PA activities,
tPA and PAI-1 concentrations secreted by MDA-MB-231 cells at pH
6.6.
| Cell medium after
72 h culture at pH 6.6 | Cell medium after
72 h culture at pH 6.6 + 104 M pepstatin | Student’s t-test:
p-value |
|---|
| 1) PA activity %
control ± SD | 100.0±15.3 (2) | 60.0±7.3a (2) | 0.06 |
| 2) tPA activity
(amiloride resistant) % control ± SD | 100.0±29.5 (6) | 68.0±14.7a (6) | 0.048 |
| 3) tPA level in
ng/mg protein | 9.86±0.73 (3) | 11.02±0.52a (3) | 0.039 |
| 4) PAI-1 level in
ng/mg protein | 269.3±10.9 (3) | 405±17.0b (3) | 0.00001 |
In order to confirm that the effect of cath D was
not due to an increase of the secreted tPA, we estimated its
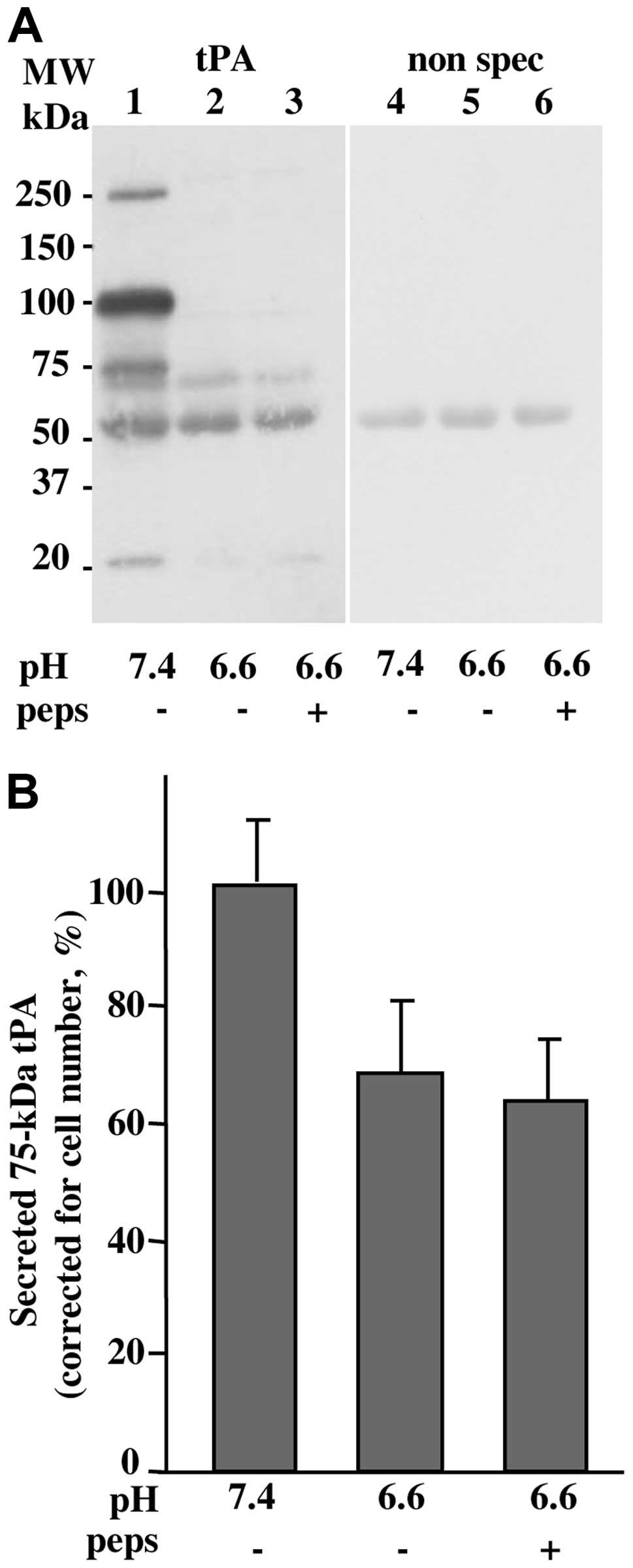
concentration using western immunoblot analysis. As shown in
Fig. 6A, the human tPA present as
a large 75 kDa band appeared unaffected in the presence of
pepstatin at pH 6.6. Excluding a non-specific 55 kDa band also
revealed with the secondary antibodies alone (lanes 4–6), the tPA
antibodies recognised at pH 7.4 a 75 kDa band corresponding to tPA
and a predominant 110 kDa band corresponding to tPA/PAI-1 inhibitor
complexes (lane 1) as previously described (20,21).
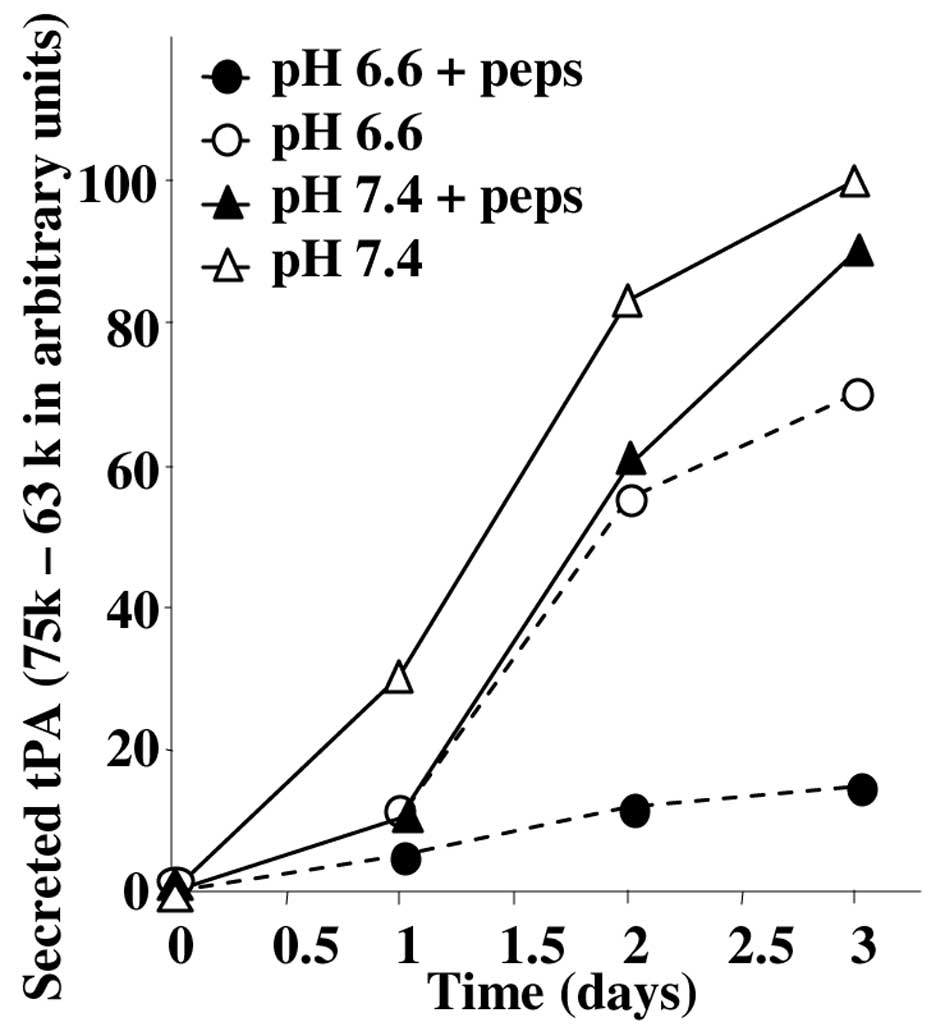
At pH 6.6 the amount of the secreted tPA was decreased compared to
pH 7.4, but pepstatin had no effect on the level of the 75 kDa band
(Fig. 6). While the 110 kDa entity
was predominant at pH 7.4, (lane 1) it was not seen at pH 6.6
(lanes 2 and 3) which is consistent with a dissociation of the
tPA/PAI-1 inhibitor complex facilitated at an acidic pH as shown
previously (14,15,25).
The contrast between the larger lytic bands (75 to 63 kDa) of the
zymography experiments, compared to the distinct 75 kDa bands of
the western blot analysis, might be due to the absence of added
serine protease inhibitors and mercaptoethanol in the zymograpy
experiments.
These data suggest that the mechanism of the cath D
activation of tPA was probably indirect and due to the degradation
of a tPA inhibitor by cath D. We demonstrate that pepstatin
markedly increased PAI-1 concentration in the same conditioned
media (Table I, line 4). PAI-1
degradation by cath D has been previously described in monocytes at
a more acidic condition (23). We
complete these data in Fig. 7 by
finding that cath D slowly degraded pure PAI-1 even at pH 6.0 in a
cell free system. Collectively, these data indicate that cath D
increased the activity of secreted plasminogen activators (PA) at
acidic pH and decreased in the same conditions the level of
PAI-1.
Discussion
This study aimed to specify whether the weak acidity
(pH 6.5 to 6.7) of the extracellular milieu of hypoxic solid tumors
(7,8) allows the activation of pro-cath D and
alters the secreted activities of plasminogen activators.
We show that under a weakly acidic condition,
MDA-MB231 cells were able to survive and to secrete a
proteolytically active cath D. Therefore, in addition to its
mitogenic activity as a ligand at a neutral pH (24), cath D can also be activated as a
protease when breast cancer cells are exposed to an extracellular
pH of 6.6. This mild acidic pH can be observed in vivo in
aggressive solid tumors (12,25).
At pH 6.6, cath D stimulated the liberation in the
culture medium of an active plasminogen activator resistant to
amiloride and corresponding most likely to tPA. The MDA-MB231 cells
are more aggressive and more efficient than MCF7 cells in
spontaneously acidifying an extracellular milieu (12). They overexpress constitutively cath
D (5) corresponding to a
basal-type breast cancer. By contrast the estrogen receptor
positive luminal type MCF7 cells require estradiol to express and
secrete both pro-cath D (5) and
tPA (26). We show here that the
MDA-MB231 cells secrete both tPA, uPA and PAI1 and that cath D can
increase PA activities via its proteolytic activity inhibited by
pepstatin.
The monolayer cell culture on plastic, used in our
study, is far from the in vivo condition. However, the cath
D effect on PA activity might even be more important in vivo
and within the microenvironment of a solid tumor. Actually both tPA
activity and tumor aggressiveness were markedly increased in
MDA-MB231 cells after their in vivo passage as orthotopic
xenograft tumors in mice, indicating that the rodent
microenvironment including stromal and endothelial cells,
cooperated to increase tPA activity (27).
We show here additional evidence that cath D can be
activated in vivo to behave as a protease. Previous reports
showed that cath D in MCF7 cells stimulated FGF2 cellular uptake
from an embedded extracellular matrix (28). Conversely, the addition of a KDEL
retention signal for endoplasmic reticulum to pre-procath D,
inhibited in vivo both cath D maturation and experimental
metastasis in mice (29)
underlining the requirement of a proteolytic activity to stimulate
metastasis.
The mechanisms of the presence of secreted active
forms of cath D and of its specific effect on PA activities are not
fully understood. Active secreted cath D could be due to the
auto-activation of procath D by removal of its pro-fragment
(30) or to the rapid displacement
of lysosomes at the cell periphery facilitating the secretion of
lysosomal active cath D (Figs. 1
and 2). Whether this rapid
lysosome delocalisation explains the secretion of mature cath D in
the medium as proposed for cath B secretion (31) was not investigated in the present
study.
The mechanism of the increased activity of the
secreted tPA by cath D is not due to an increased amount of tPA, as
shown by western blot analysis and immunoassay of the antigen.
Since the zymogen and the two-chains forms of tPA display similar
activity (32,33), the most likely explanation is that
the effect of cath D is indirect. In fact the secreted PAI-1 is in
large excess compared to PA in several cell lines (34) and modulation of tPA activity by
PAI-1 is central in endothelial cells (35). Moreover, PAI-1 is a specific
substrate of cath D, but not of cysteinyl cathepsins, as shown in
human monocytes (23). The fact
that we show here at pH 6.6 a significant increase of PAI-1 with
pepstatin and the proteolysis of recombinant PAI-1 by cath D
supports an indirect stimulation of the secreted PA activity via
PAI-1 proteolysis. It is however intriguing that the degradation of
PAI-1 by cath D stimulates specifically tPA activity measured by
zymography while cath D appears to stimulate both uPA and tPA
activities (compare lines 1 and 2 of Table I) when measured in the conditioned
media before separation of the two enzymes by electrophoresis
(Table I). This might be due to a
different affinity of PAI-1 for tPA and for uPA (36). However, we cannot exclude other
mechanisms at the cell surface where several receptors bind these
proteases, such as the Annexin II/plasminogen receptor (37) and the LRP receptor which can bind
both tPA/PAI-1 complex (38) and
cath D (39).
The cath D proteolytic activity facilitating
indirectly PA activation completes the scheme of a very complex
proteolytic network leading to the stimulation of invasion,
angiogenesis and tumor growth (40). Procath D can be autoactivated in
vitro at an acidic pH (4,5,30) to
trigger a proteolytic cascade when liberated with other proteases
in the extracellular milieu of tumors. Activation of procathepsin B
(41) and procathepsin L (42) by cath D had been shown in
vitro but at the more acidic pH found in lysosomes, thus
limiting its biological significance. This study introduces tPA as
an additional partner in this complex proteolytic cascade to
modulate invasion, metastasis and angiogenesis.
The significance and role of tPA and PAI-1 in cancer
is debated (43). On one hand,
several experimental studies indicate that tPA facilitates tumor
progression and several mechanisms have been proposed (27,39,44,45).
Gene invalidation in mouse showed that tPA cooperates with uPA in
stimulating cell survival (46).
However, clinical studies have underlined tPA level as a marker of
good prognosis in breast cancer (47,48)
in contrast to uPA (9). The reason
for these discrepancies is not currently understood. Moreover,
PAI-1 by inhibiting tPA activity has also been proposed to decrease
the stress-induced senescence activity of the wild-type p53
(49). Thus cath D, by degrading
PAI-1 and IGF-BP3 (50), might
also interfere with the PAI-1/IGF-BP3 cascade (51) to stimulate cancer cells.
To conclude, we show here that cath D in breast
cancer cells at pH 6.6, but not at neutral pH, stimulates as a
protease the activity of secreted plasminogen activators probably
by degrading PAI-1. This effect might also take place in
vivo in poorly vascularised regions of solid tumors to modulate
invasion and tumor growth.
Acknowledgements
This study was supported by INSERM and
University of Montpellier 1. R.F. was a recipient of the
Association pour la Recherche sur le Cancer. We are grateful to Dr
Y. Hayashido for his advice on zymography, to M. Gleizes for
technical assistance, to C. Viglianti and S. Roques for tPA
enzymatic assays and to J.-Y. Cance for the skilful preparation of
the figures.
References
|
1.
|
Spyratos F, Brouillet JP, Defrenne A, et
al: Cathepsin-D: an independant prognostic factor for metastasis of
breast cancer. Lancet. 8672:1115–1118. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Foekens JA, Look MP, Bolt-de Vries J,
Meijer-van Gelder M, van Putten WLJ and Klijn JGM: Cathepsin D in
primary breast cancer: prognostic evaluation involving 2810
patients. Br J Cancer. 79:300–307. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Rochefort H, Garcia M, Glondu M, Laurent
V, Liaudet E, Rey JM and Roger P: Cathepsin D in breast cancer:
mechanisms and clinical applications a 1999 overview. Clinica
Chimica Acta. 291:157–170. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Capony F, Rougeot C, Montcourier P,
Cavaillès V, Salazar G and Rochefort H: Increased secretion altered
processing, and glycosylation of pro-cathepsin D in human mammary
cancer cells. Cancer Res. 49:3904–3909. 1989.PubMed/NCBI
|
|
5.
|
Rochefort H, Capony F and Garcia M:
Cathepsin D: a protease involved in breast cancer metastasis.
Cancer Metastasis Rev. 9:321–331. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Veith FO, Capony F, Garcia M, Chantelard
J, Pujol H, Veith F, Zajdela A and Rochefort H: Release of
estrogen-induced glycoprotein with a molecular weight of 52,000 by
breast cancer cells in primary culture. Cancer Res. 43:1861–1868.
1983.PubMed/NCBI
|
|
7.
|
Stubbs M, McSheehy PM and Griffiths JR:
Causes and consequences of acidic pH in tumors: a magnetic
resonance study. Adv Enzyme Regul. 39:13–30. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Martin GR and Jain RK: Measurement of
interstitial pH profiles in normal and neoplastic tissue using
fluorescence ratio imaging microscopy. Cancer Res. 54:5670–5674.
1994.PubMed/NCBI
|
|
9.
|
Foekens JA, Peters HA, Look MP, et al: The
urokinase system of plasminogen activation and prognosis in 2,780
breast cancer patients. Cancer Res. 60:636–643. 2000.PubMed/NCBI
|
|
10.
|
Harbeck N, Schmitt M, Meisner C, et al:
Ten-year analysis of the prospective multicentre Chemo-N0 trial
validates American Society of Clinical Oncology (ASCO)-recommended
biomarkers uPA and PAI-1 for therapy decision making in
node-negative breast cancer patients. Eur J Cancer. 49:1825–1835.
2013.
|
|
11.
|
Heuser J: Changes in lysosome shape and
distribution correlated with changes in cytoplasmic pH. J Cell
Biol. 108:855–864. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Montcourrier P, Silver IA, Farnoud R, Bird
I and Rochefort H: Breast cancer cells have a high capacity to
acidify extracellular milieu by a dual mechanism. Clin Exp
Metastasis. 15:382–392. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Chalbos D, Vignon F, Keydar I and
Rochefort H: Estrogens stimulate cell proliferation and induce
secretory proteins in a human breast cancer cell line (T47D). J
Clin Endocrin Metab. 55:276–283. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Martínez-Zaguilán R, Seftor EA, Seftor RE,
Chu YW, Gillies RJ and Hendrix MJ: Acidic pH enhances the invasive
behavior of human melanoma cells. Clin Exp Metastasis. 14:176–186.
1996.PubMed/NCBI
|
|
15.
|
Heussen C and Dowdle EB: Electrophoretic
analysis of plasminogen activators in polyacrylamide gels
containing sodium dodecyl sulfate and copolymerized substrates.
Anal Biochem. 102:196–202. 1980. View Article : Google Scholar
|
|
16.
|
Vassalli JD and Belin D: Amiloride
selectively inhibits the urokinase-type plasminogen activator. FEBS
Lett. 214:187–191. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Maynadier M, Ramirez JM, Cathiard AM, et
al: Unliganded estrogen receptor alpha inhibits breast cancer cell
growth through interaction with a cyclin-dependent kinase inhibitor
(p21(WAF1)). FASEB J. 22:671–681. 2008. View Article : Google Scholar
|
|
18.
|
Garcia M, Capony F, Derocq D, Simon D, Pau
B and Rochefort H: Characterization of monoclonal antibodies to the
estrogen-regulated Mr 52,000 glycoprotein and their use in MCF7
cells. Cancer Res. 45:709–716. 1985.PubMed/NCBI
|
|
19.
|
Brouillet JP, Spyratos F, Hacene K, Fauque
J, Freiss G, Dupont F, Maudelonde T and Rochefort H:
Immunoradiometric assay of pro-cathepsin D in breast cancer
cytosol: relative prognostic value versus total cathepsin D. Eur J
Cancer. 29A:1248–1251. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Tissot JD, Hauert J and Bachmann F:
Characterization of plasminogen activators from normal human breast
and colon and from breast and colon carcinomas. Int J Cancer.
34:295–302. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Christensen L, Wiborg Simonsen AC,
Heegaard CW, Moestrup SK, Andersen JA and Andreasen PA:
Immunohistochemical localization of urokinase-type plasminogen
activator, type-1 plasminogen-activator inhibitor, urokinase
receptor and α2-macroglobulin receptor in human breast carcinomas.
Int J Cancer. 66:441–452. 1996.
|
|
22.
|
Jankun J and Skrzypczak-Jankun E:
Molecular basis of specific inhibition of urokinase plasminogen
activator by amiloride. Cancer Biochem Biophys. 17:109–123.
1999.PubMed/NCBI
|
|
23.
|
Simon DI, Xu H and Vaughan DE: Cathepsin
D-like aspartyl protease activity mediates the degradation of
tissue-type plasminogen activator/plasminogen activator inhibitor-1
complexes in human monocytes. Biochim Biophys Acta. 1268:143–145.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Rochefort H and Liaudet-Coopman E:
Cathepsin D in cancer metastasis: a protease and a ligand. Acta
Pathol Microb Immunol Scand. 107:86–95. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Rofstad K, Mathiesen B, Kindem K and
Galappathi K: Acidic extracellular pH promotes experimental
metastasis of human melanoma cells in athymic nude mice. Cancer
Res. 66:6699–6707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ryan TJ, Seeger JI, Kumar SA and Dickerman
HW: Estradiol preferentially enhances extracellular tissue
plasminogen activators of MCF-7 breast cancer cells. J Biol Chem.
259:14324–14327. 1984.PubMed/NCBI
|
|
27.
|
Jessani N, Humphrey M, McDonald WH, et al:
Carcinoma and stromal enzyme activity profiles associated with
breast tumor growth in vivo. Proc Natl Acad Sci USA.
101:13756–13761. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Briozzo P, Badet J, Capony F, Pieri I,
Montcourrier P, Barritault D and Rochefort H: MCF7 mammary cancer
cells respond to bFGF and internalize it following its release from
extracellular matrix: a permissive role of cathepsin D. Exp Cell
Res. 194:252–259. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Liaudet E, Garcia M and Rochefort H:
Cathepsin D maturation and its stimulatory effect on metastasis are
prevented by addition of KDEL retention signal. Oncogene.
9:1145–1154. 1994.PubMed/NCBI
|
|
30.
|
Richo G and Conner GE: Proteolytic
activation of human procathepsin D. Adv Exp Med Biol. 306:289–296.
1991. View Article : Google Scholar
|
|
31.
|
Rozhin J, Sameni M, Ziegler G and Sloane
BF: Pericellular pH affects distribution and secretion of cathepsin
B in malignant cells. Cancer Res. 54:6517–6525. 1994.PubMed/NCBI
|
|
32.
|
Tate KM, Higgins DL, Holmes WE, Winkler
ME, Heyneker HL and Vehar GA: Functional role of proteolytic
cleavage at arginine-275 of human tissue plasminogen activator as
assessed by site-directed mutagenesis. Biochemistry. 26:338–343.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Berg DT and Grinnell BW: Signal and
propeptide processing of human tissue plasminogen activator:
activity of a pro-tPA derivative. Biochem Biophys Res Commun.
179:1289–1296. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Cajot JF, Kruithof EK, Schleuning WD,
Sordat B and Bachmann F: Plasminogen activators, plasminogen
activator inhibitors and procoagulant analyzed in twenty human
tumor cell lines. Int J Cancer. 38:719–727. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Suzuki Y, Mogami H, Ihara H and Urano T:
Unique secretory dynamics of tissue plasminogen activator and its
modulation by plasminogen activator inhibitor-1 in vascular
endothelial cells. Blood. 113:470–478. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Rijken DC, Hoegee-de Nobel E, Jie AF,
Atsma DE, Schalij MJ and Nieuwenhuizen W: Development of a new test
for the global fibrinolytic capacity in whole blood. J Thromb
Haemost. 6:151–157. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sharma M, Ownbey RT and Sharma MC: Breast
cancer cell surface annexin II induces cell migration and
neoangiogenesis via tPA dependent plasmin generation. Exp Mol
Pathol. 88:278–286. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Krieger M and Herz J: Structures and
functions of multi-ligand lipoprotein receptors: macrophage
scavenger receptors and LDL receptor-related protein (LRP). Annu
Rev Biochem. 63:601–637. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Beaujouin M, Prébois C, Derocq D, et al:
Pro-cathepsin D interacts with the extracellular domain of the beta
chain of LRP1 and promotes LRP1-dependent fibroblast outgrowth. J
Cell Sci. 123:3336–3346. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Mason SD and Joyce JA: Proteolytic
networks in cancer. Trends Cell Biol. 4:228–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Pagano M, Capony F and Rochefort H:
Pro-cathepsin D can activate in vitro pro-cathepsin B secreted by
ovarian cancers. C R Acad Sci III. 309:7–12. 1989.(In French).
|
|
42.
|
Nishimura Y, Kawabata T, Furuno K and Kato
K: Evidence that aspartic proteinase is involved in the proteolytic
processing event of procathepsin L in lysosomes. Arch Biochem
Biophys. 271:400–406. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Danø K, Behrendt N, Hoyer-Hansen G,
Johnsen M, Lund LR, Ploug M and Romer J: Plasminogen activation and
cancer. Thromb Haemost. 93:676–681. 2005.
|
|
44.
|
Fredriksson L, Li H, Fieber C, Li X and
Eriksson U: Tissue plasminogen activator is a potent activator of
PDGF-CC. EMBO J. 23:37932004. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Seeds NW, Basham ME and Haffke SP:
Neuronal migration is retarded in mice lacking the tissue
plasminogen activator gene. Proc Natl Acad Sci USA. 96:14118–14123.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Carmeliet P, Schoonjans L, Kieckens L, et
al: Physiological consequences of loss of plasminogen activator
gene function in mice. Nature. 368:419–424. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Duffy MJ, O’Grady P, Devaney D, O’Siorain
L, Fennelly JJ and Lijnen HR: Tissue-type plasminogen activator, a
new prognostic marker in breast cancer. Cancer Res. 48:1348–1349.
1988.PubMed/NCBI
|
|
48.
|
Chappuis PO, Dieterich B, Sciretta V,
Lohse C, Bonnefoi H, Remadi S and Sappino AP: Functional evaluation
of plasmin formation in primary breast cancer. J Clin Oncol.
19:2731–2738. 2001.PubMed/NCBI
|
|
49.
|
Kortlever RM, Higgins PJ and Bernards R:
Plasminogen activator inhibitor-1 is a critical downstream target
of p53 in the induction of replicative senescence. Nature Cell
Biol. 8:877–884. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Conover CA and De Leon DD: Acid-activated
insulin-like growth factor-binding protein-3 proteolysis in normal
and transformed cells. Role of cathepsin D. J Biol Chem.
269:7076–7080. 1994.PubMed/NCBI
|
|
51.
|
Elzi DJ, Lai Y, Song M, Hakala K,
Weintraub ST and Shiio Y: Plasminogen activator inhibitor
1-insulin-like growth factor binding protein 3 cascade regulates
stress-induced senescence. Proc Natl Acad Sci USA. 109:12052–12057.
2012. View Article : Google Scholar : PubMed/NCBI
|