Introduction
Epstein-Barr virus (EBV) is a human γ herpesvirus
commonly carried in the majority of the human population. It is
implicated in a variety of human malignancies that include
Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's lymphoma,
nasal T cell lymphoma, and immunoblastic lymphomas in
post-transplant and AIDS patients. EBV-encoded LMP1 is the only one
implicated in cell immortalization and transformation. It is
required for B-cell immortalization, together with the EBNA2,
EBNA3a and EBNA3c genes (1–4). In
addition, LMP1 has been shown to induce the transformation of
certain established rodent fibroblast cell lines, including Rat-1
and BALB/c 3T3 (5–7). Furthermore, LMP1 induces the
tumorigenicity of epithelial cell lines in severe combined
immunodeficient mice (8,9).
As a 60 kDa integral membrane protein, LMP1
functions as a constitutively active tumor necrosis factor receptor
(TNFR) and, in certain instances, can substitute for CD40 in
vivo, providing both growth and differentiation responses in B
lymphocytes (10). LMP1 also
contributes to multiple aspects of NPC development through
activating a number of signaling pathways including nuclear factor
NF-κB, activator protein-1 (AP-1), Janus kinase/signal transducer
and activator of transcription (JAK/STAT) (11–14).
Activation of NF-κB or AP-1 by LMP1 has been linked to the
upregulation of some cellular proteins and inhibition of apoptosis.
LMP1 has clear oncogenic properties in multiple cell backgrounds
and in transgenic mice. In NPCs, LMP1 expression is heterogeneous
and associated with metastasis (15), supported by studies in epithelial
cultures showing LMP1 induced changes in cell morphology, adhesion,
cell motility and exo-proteolytic activity (16). Like many classical oncogenes, the
growth promoting properties of LMP1 appear to be cell-type
specific. Whereas LMP1 expression promotes growth and proliferation
in rodent fibroblasts and certain human epithelial cell lines such
as C33A and HONE1, its expression in RHEK-1, SVK and Hep-G2 cells
is associated with growth inhibition and, under certain
circumstances, cytostasis or apoptosis (17).
Interference with LMP1 function eliminates
tumorigenic potential. Recently there have been some reports that
the RNA interference against LMP1 exhibited anti-proliferative and
anti-metastasis effects in the LMP1 expressing NPCs (18,19).
Downregulation of the LMP1 expression has been shown to induce
apoptosis, and sensitize the EBV-positive cells to cytotoxic agents
(20,21). Although the biological effects of
inhibition of LMP1 expression has been well demonstrated, its cell
biological basis is yet to be elucidated. In a previous study we
demonstrated that the ‘10–23’ DNAzymes specifically targeting the
LMP1 mRNA significantly downregulated the expression of LMP1 in the
nasopharyngeal carcinoma cell line CNE1-LMP1 which led to
sensitization of LMP1 positive cells to radiation (22). In this study we found that after
DNAzyme 1 treatment the CNE1-LMP1 cells accumulated in G1 phase and
the percentage of cells in G2/M phase was decreased. This cell
cycle change was accompanied by γ-H2AX, a DNA damage marker,
increased and the phospharylation of p53 decreased. However, the
mechanisms of the cell cycle arrest caused by down regulation of
LMP1 are unclear. In the present study, we attempt to examine the
expression feature of the molecules associated with the cell cycle
control in G1/S and G2/M checkpoints, following the silencing of
the LMP1 expression by the LMP1-targeted DNAzyme 1. We show that
both checkpoints contributed to the G1 phase arrest and cells in
G2/M phase decreased induced by the downregulation of LMP1. Our
results thus suggest that LMP1 is an important regulator of the
cell cycle in LMP1-positive NPC cells.
Materials and methods
Cell culture
CNE1 is an LMP1 negative, low differentiated
nasopharyngeal squamous carcinoma cell line. CNE1-LMP1 is a cell
line transfected with EBV-LMP1 (23) and shown to have accelerated cell
proliferation (24). The cells
were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum (FBS, Gibco), 100 IU/ml penicillin, 100 mg/ml
streptomycin, and 2 mM/l L-glutamine in a humidified atmosphere of
5% CO2 at 37°C.
DNAzyme transfection of CNE1-LMP1 or CNE1
cells
Briefly, the ‘10–23’ model was adopted by
incorporating 9 bp arms at each side of the catalytic motif. To
increase stability of DNAzymes in cells, two phosphorothioate
linkages were introduced to both ends of the arms. A DNAzyme
oligonucleotide control (ODN) was a scrambled sequence that
consists of the same nucleotide composition. To transfect cells
with DNAzymes, tetra (4-methyridyl) porphyrine (TMP) was used to
facilitate the cellular uptake of the catalytic oligonucleotides
(25). Prior to transfection,
cells were seeded in 6-well plates overnight. The DNAzymes/TMP
mixtures were made at a charge ratio (C/R) of 1 with 2 μM
DNAzyme oligonucleotides as described (26). The mixtures were incubated for 15
min at room temperature to form the transfection complex. After the
cells were rinsed twice with PBS, the transfection mixture was
added to the cells and incubated at 37°C for 4 h in 5%
CO2, followed by the addition of complete medium to the
wells and further incubation for the indicated time.
Cell cycle distribution analysis
Flow cytometry analysis of PI-stained cells was
performed to study the effect of the active DNAzyme on the cellular
cycle. Cells (1×106) were cultured with or without 2
μM of DNAzyme and collected 24 h later. Treated and
untreated cells were then washed with ice-cold PBS and suspended in
75% ethanol at −20°C overnight. Fixed cells were centrifuged and
washed with PBS twice. Before flow cytometry analysis, cells were
stained with 50 μg/ml of propidium iodide and 0.1% of RNase
A in 400 μl PBS in a light-proof tube at 25°C for 30 min.
Stained cells were assayed on FACSort (Becton-Dickinson) and the
cell cycle parameters and the percentage of apoptotic cells (sub-G1
peak) were determined using the CellQuest software program
(Becton-Dickinson).
Western blot analysis
Cells grown in 6-well plates were cultured without
or with DNAzymes. Cells were harvested at different time points,
washed with ice-cold PBS and lysed in the lysis buffer (10 mM
Tris-HCl, pH 8.0, 1 mM EDTA, 2% SDS, 5 mM DTT, 10 mM PMSF,
proteinase inhibitor mix) for 30 min on ice. The lysate was
centrifuged for 15 min at 13,000 rpm in a microfuge at 4°C. After
protein quantification by the BCA Assay Reagent (Pierce Chemical,
Inc), 50 μg of the total proteins from the treated or
untreated cells were mixed with the sample buffer and boiled for 5
min. The samples were then resolved on a 10% polyacrylamide SDS gel
and transferred onto a nitrocellulose membrane by electroblotting.
The membrane was incubated in blocking buffer (TBS containing 5%
skimed milk and 0.1% Tween-20) for 2 h, followed by incubation with
primary antibody diluted in the same buffer (1:1,000). The membrane
was washed in TBS containing 0.1% Tween-20 and further incubated
with a secondary antibody that could be detected using a
peroxidase-conjugated anti-IgG at 1:10,000. Protein expression were
determined using a supersignal chemiluminescence system (ECL,
Pierce) followed by exposure to autoradiographic film. The
antibodies used in the study include LMP1 (Dako), mouse monoclonal
anti-β-actin (Sigma), mouse monoclonal anti-α-tublin (Santa Cruz),
rabbit anti-phosphoserine (Zymed), rabbit polyclonal anti-cdc2 p34
(Santa Cruz), rabbit polyclonal anti-phospho-Thr161-cdc2 and rabbit
polyclonal anti-phospho-Tyr15-cdc2 (Cell Signaling), mouse
monoclonal anti-cyclin B1 (Santa Cruz), p-p53 (Ser15) (1:500, Santa
Cruz), and γ-H2AX (1:1,000, Millipore). Secondary antibodies were
horseradish peroxidase (HRP)-conjugated rabbit anti-mouse IgG
(Santa Cruz), HRP-conjugated goat anti-rabbit IgG (Santa Cruz).
Immunofluorescent analysis
CNE1 and CNE1-LMP1 cell lines were seeded on
Millicell EZ slides (Millipore), and subjected to the following
treatments: untreated, DNAzyme- or control ODN-treated for 24 h.
Cells were fixed in ice cold methanol for 10 min and washed with
PBS, then blocked with 5% donkey serum/PBS for 1 h. The cells were
subsequently incubated with the mouse anti-γ-H2AX (1:450,
Millipore) overnight. Followed by washing with PBS for 15 min,
AlexaFluor 488-conjugated goat anti-mouse secondary antibodies
(1:1,000, Molecular Probes, Eugene, OR) were used. All samples were
mounted in Prolong Gold antifade reagent with DAPI (Molecular
Probes) and fluorescence images were captured using Leica DMI3000 B
(Leica, Germany).
Immunoprecipitation kinase assay
The cyclin D1-CDK4 kinase assay was performed as
described with modifications (27). Cells were transfected with
LMP1-specific DNAzyme 1 or control ODN 24 h after the transfection,
cells were washed twice with ice-cold PBS and harvested with a
kinase buffer [25 mM Tris-HCl (pH 7.5), 5 mM glycero-phosphate, 2
mM DTT, 0.1 mM Na3VO4, 10 mM
MgCl2, and 1 mM PMSF]. Protein content was determined
using the BCA assay. Cyclin D1 was immunoprecipitated from 600
μg of the cell extract using an anti-cyclin D1 monoclonal
antibody diluted at 1:100 overnight. A total of 20 μl of
protein A/G PLUS-Agarose (Santa Cruz) was added, incubated at 4°C,
and then washed at 4°C four times with ice-cold kinase buffer.
Immunoprecipitated cyclin D1 was incubated with 1 μg Rb-C
fusion protein (Cell Signaling) and 200 μM ATP in a kinase
buffer at 30°C for 30 min. Phosphorylation of Rb-C fusion protein
was detected by western blot analysis with anti-phospho-Rb (Ser780)
polyclonal antibodies.
cdc2 Kinase Activity Assay
The cdc2 kinase activity assay was carried out
according to the instruction in the MESACUP Cdc2 Kinase Assay Kit.
Cells were lysed in a sample buffer (50 mM Tris-HCl, pH 7.5, 0.5 M
NaCl, 5 mM EDTA, 2 mM EGTA, 0.01% Brij35, 1 mM PMSF, 0.05 mg/ml
leupeptin, 50 mM β-glycerophosphate, 1 mM Na-orthovanadate). After
sonication, cell extracts were separated. The amount of 600
μg cell extracts were incubated with HCK-gel suspension
(MBL, Code no. 5236) on ice for 1 h. Followed by centrifugation,
the pellets were separated and used for an enzyme source. Reaction
reagents added into the HCK-gel pellets included 10 × cdc2 reaction
buffer, biotinylated MV peptide, ATP and distilled water. Following
incubation for 30 min with the sample buffer as a control, the
phosphorylation reaction was terminated to permit the cdc2 kinase
activity to be measured. The reaction mixture was transferred to a
microwell strip coated with monoclonal antibody (4A4), incubated at
25°C for 60 min, and washed with the washing solution. Peroxidase
(POD)-conjugated streptavidin was added and incubated, and then the
mixture was washed once again. POD substrate solution was added and
incubated for 3 to 5 min. Stop solution was then added, and the
optical density (OD) of each well was read at 490 nm with a
microplate reader.
Co-immunoprecipitation analysis
(Co-IP)
CDK4 was pulled down by anti-CDK4 antibody, and then
precipitated by binding with protein A Sepharose CL-4B beads
(Pharmacia, USA). Anti-cyclin D1 antibody was used as the primary
antibody to detect the levels of cyclin D1. A
co-immunoprecipitation (Co-IP)/ western blot assay was performed to
analyze the interaction of CDK4 with cyclin D1. The cell extract
was prepared for immunoprecipitation or Co-IP/western blot
analysis. The harvested cells were lysed in IP lysis buffer (50 mM
Tris-HCl, 150 mM NaCl, 10% Nonidet P-40, 1 mM EDTA, 10% glycerol,
10 mM NaF, 1 mM Na3 VO4, 1 mM DTT, 1 mM PMSF,
and phosphatase inhibitor cocktail). The supernatant was mixed with
protein A Sepharose CL-4B beads, incubated for 2 h, centrifuged for
2 min at 2,000 rpm for pre-clearing and incubated overnight with
CDK4 antibody and protein A Sepharose CL-4B beads, followed by
centrifugation for 2 min at 12,000 rpm. The immunoprecipitates were
collected, washed five times, and finally subjected to western blot
analysis.
Co-IP was used to analyze protein interaction with a
special antibody linked to protein A Sepharose CL-4B beads
(Pharmacia). Immunoprecipitates were separated for western blot
analysis.
MTS analysis
MTS assays were performed to assess the effect of
DNAzymes on cell proliferation. Briefly, 200 μl of
logarithmically growing cells were plated on a 96-well plate and
LMP1-targeted-DNAzyme 1 or control ODN were transfected as
described above. Then cells were incubated at 37°C and 100
μl of sterile MTS dye (30156802, Promega) were added to each
well at different time points. The plates were further incubation
at 37°C for 4 h. After that, spectrometric absorbance at the
wavelength of 570 nm was measured on a microplate reader (Bio-Tek
Inc). The background absorbance of medium in the absence of cells
was subtracted. All samples were assayed in triplicate, and the
mean for two experiments was used to calculate cell proliferation
rate expressed as percentage of control.
Statistical analyses
All data were shown as mean ± standard deviation.
The difference between 2 groups of data was examined by Student's
t-test. The statistical difference at p<0.05 was considered as
significantly and p<0.01 as very significant.
Results
EBV-LMP1-targeted DNAzyme 1 inhibits the
expression of EBV-LMP1 and affects the cell cycle distribution in
CNE1-LMP1 cells
In order to determine if the DNAzyme 1 could affect
the target gene expression, we used a cationic porphyrin TMP, as a
transfection reagent, for transfecting DNAzyme oligonucleotides
into CNE1-LMP1 cells. DZ1 (2 μM) was transfected into both
CNE1-LMP1 cells and CNE1 cells (as a negative target control). The
effect on the LMP1 expression at the protein level was assayed
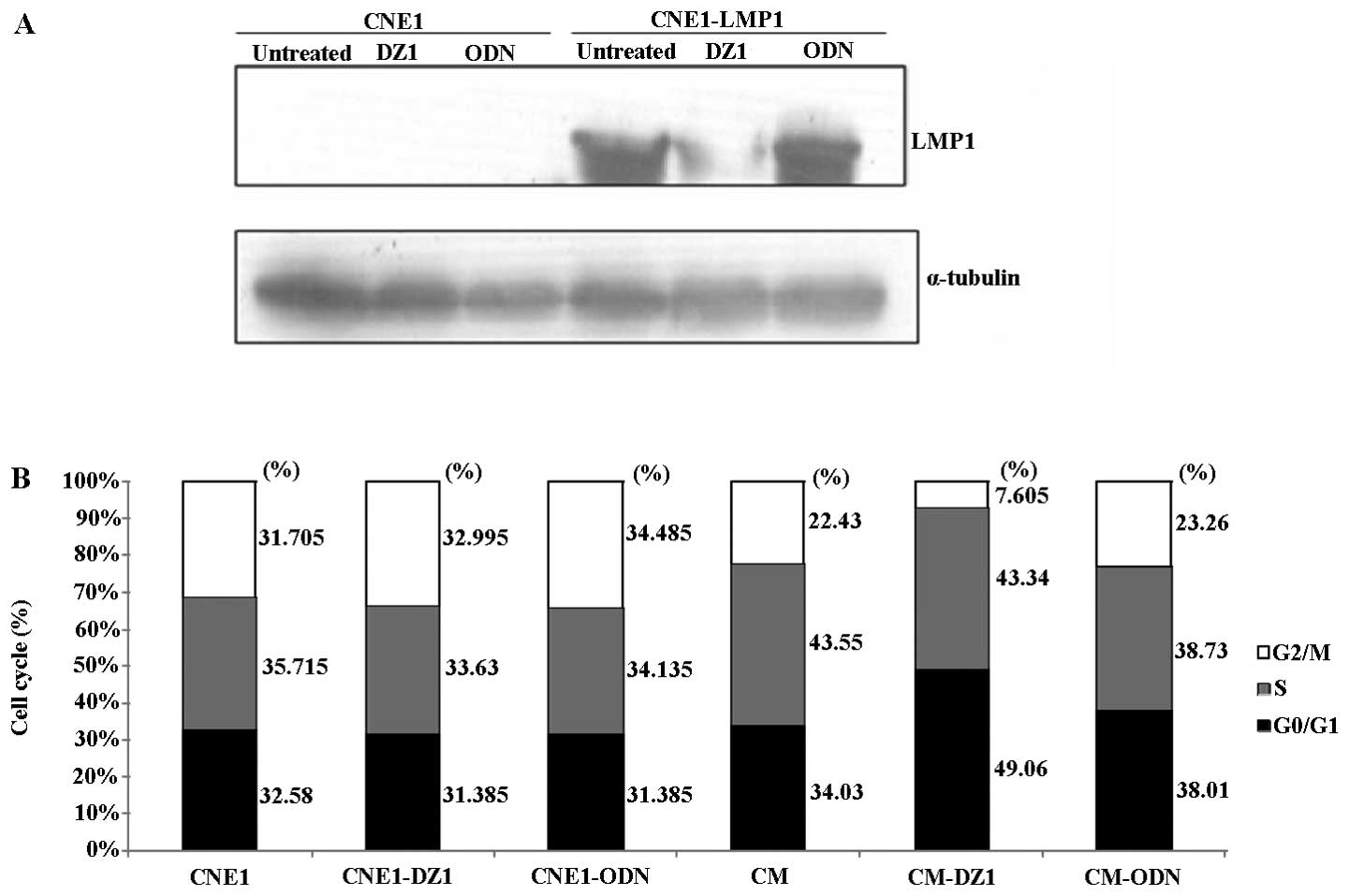
using western blot analysis. As shown in Fig. 1A, DZ1 significantly inhibited the
LMP1 protein expression in CNE1-LMP1 cells, while the control ODN
had no effect on the LMP1 expression.
Since LMP1 can promote cell growth and accelerate
cell cycle progression, we next examined the effect of
down-regulation of LMP1 on cell cycle. DZ1 was transfected into
CNE1-LMP1 and CNE1 cells after overnight serum starvation and the
cell cycle distribution was assessed by flow cytometry, it was
shown that DZ1 significantly increased the percentage of cells in
G1 phase (49.06±3.12) compared to the untreated (33.83±4.96) or
control ODN (38.01±2.18) in LMP1-positive cells. This increase in
the G1 phase was coupled with a significant decrease in the
percentage of cells in G2/M phase (7.61±3.84 of DZ1 treated
LMP1-positive cells vs 22.43±1.41 of untreated or 23.26±1.85 of
control ODN treated LMP1-positive cells) after 24 h of
transfection. Whereas, such an effect was not observed on the cell
cycle of LMP1-negative tumor cell (CNE1) (Fig. 1B). These data confirmed that DZ1
could down-regulate the LMP1 expression, resulting in G1 phase
arrest of CNE1-LMP1 cells and a decreased cell number in G2/M
phase.
EBV-LMP1-targeted DNAzyme 1 induces DNA
damage in CNE1-LMP1 cells and inhibits cell proliferation
Since DNA damage is an early event of cell cycle
arrest, we examined whether DZ1 could induce DNA damage in
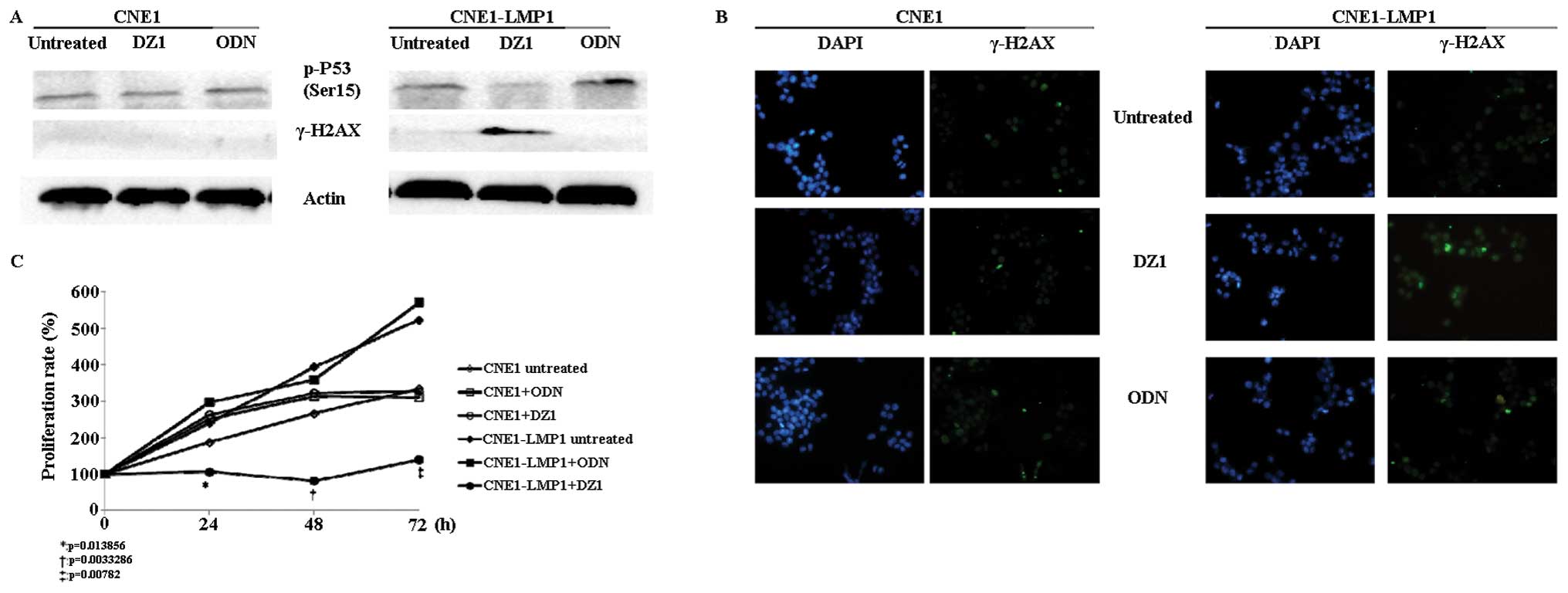
CNE1-LMP1 cells through inhibiting LMP1. As shown in Fig. 2A, the expression level of γ-H2AX, a
marker of DNA damage, was significantly increased by DZ1 in
CNE1-LMP1 cells compared with the untreated or control ODN treated
cells. This result was also confirmed by immunofluorescence
(Fig. 2B). When LMP1 was
inhibited, the γ-H2AX level was highly induced in CNE1-LMP1
cells.
It is known that p53 is required to promote the DNA
damage induced response. One key event for p53-mediated DNA damage
response is phosphorylation of serine 15 (Ser15) in human p53
(28). To evaluate whether the
LMP1-targeted DNAzyme mediated the DNA damage response, we examined
the level of p-p53 (Ser15) by western blot analysis. It was shown
that the phosphorylation of Ser15 was downregulated in DZ1-treated
CNE1-LMP1 cells (Fig. 2A).
To explore whether the LMP1-targeted DNAzyme 1 could
affect cell proliferation, MTS was performed. The results showed
that CNE1-LMP1 cells grew faster than CNE1 cells. However, when
CNE1-LMP1 is transfected with DZ1, they have a significant slower
growth rate than the cells transfected with negative control or
untreated cells from 24 to 72 h (Fig.
2C).
EBV-LMP1-targeted DNAzyme 1 affects the
expression of the genes that regulate G1/S progression in CNE1-LMP1
cells
Cells entering the proliferation phase go first
through the G1/S checkpoint, which is mainly regulated by CDK4 and
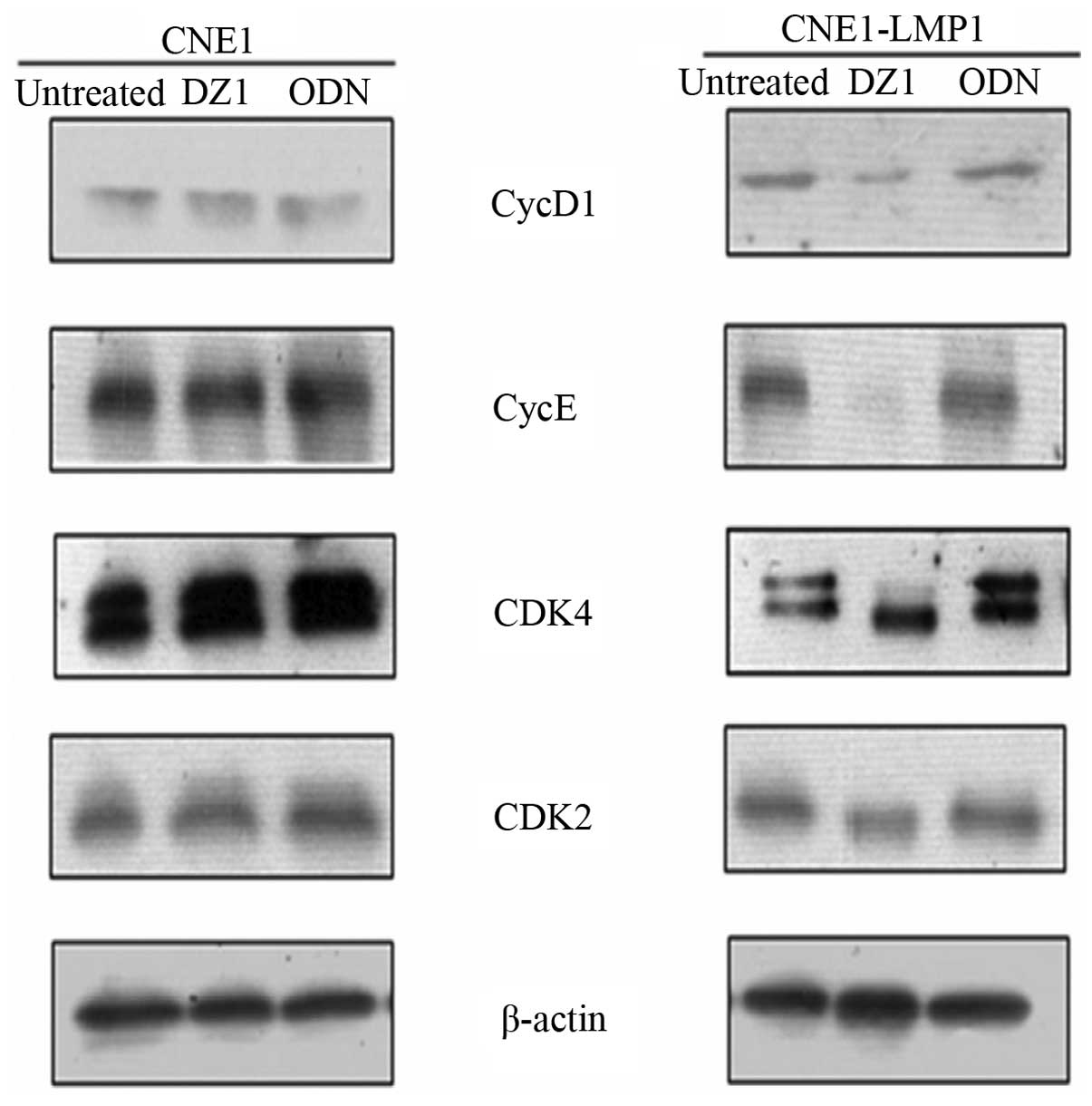
cyclin D1 and other CDKs and cyclins. We transfected the DZ1 to the
CNE1-LMP1 and CNE1 cells, and examined the expression of the G1/S
checkpoint-related genes. It was found that DZ1 markedly decreased
the expression of CDK4 and cyclin D1, as well as CDK2 and cyclin E.
These changes were not found in the LMP1 negative CNE1 cells
(Fig. 3). The results suggested
that DZ1 specifically inhibited the LMP1 expression resulting in
the downregulation of the expression of the G1/S checkpoint related
cyclins and CDKs.
It is necessary for CDKs and cyclins to interact
directly to function in the cell cycle control. Western blot
analysis demonstrated that inhibition of LMP1 expression led to
downregulation of cyclin D1 and CDK4. To investigate if the effect
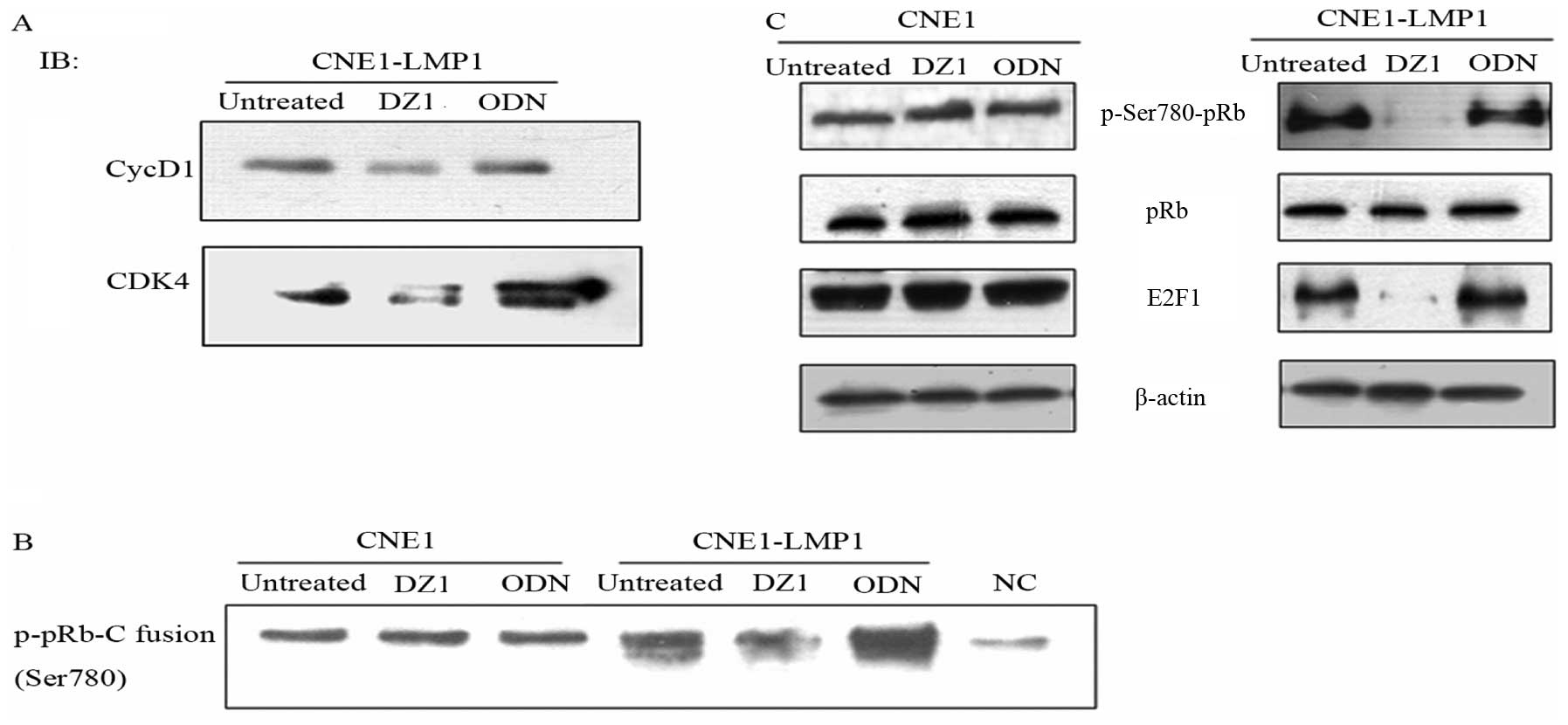
was due to a weaker interaction between CDK4 and cyclin D1, Co-IP
analysis was used to assay such an interaction. CDK4 was pulled
down by excess anti-CDK4 antibody, a co-immunoprecipitation (Co-IP)
assay performed to analyze cyclin D1. Analysis of total cell
lysates showed that the DZ1-mediated inhibition of LMP1 resulted in
a decrease of the level of CDK4 and also the associated cyclin D1,
which suggested a decrease of the interaction between cyclin D1 and
CDK4 (Fig. 4A).
pRb is a key regulator in the transition from G1 to
S and its activity is modulated by cyclin D-CDK complexes. In pRb,
the Ser780 site is specifically phosphorylated by the cyclin D/CDK4
complex both in vivo and in vitro (29). Having shown that the downregulation
of LMP1 expression by DZ1 lessened the CDK4/cyclin D1 complex, we
next examined if the activity of the CDK4/cyclin D1 complex was
affected in the context of pRb phosphorylation. Using the
Immunoprecipitation Kinase Assay, it was shown that the
downregulation of the LMP1 expression by DZ1 led to a significant
reduction of the phosphorylation of Ser780 in pRb (Fig. 4B). The result indicated that the
LMP1-specific DNAzyme 1 could slow down the G1 to S transition
through the suppression of activation of cyclin D1-CDK4.
The CDK4/cyclin D1 complex phosphorylates pRb that
transcriptionally activates some important transcriptional factors
such as E2Fs, which promotes the expression of the downstream
proteins such as cyclin A. Entry into S phase is controlled, in
part, by the level of active E2F (30). To explore whether LMP1 could
regulate the pRb signaling pathway, we investigated the change of
phosphorylation status of pRb induced by LMP1-specific DNAzyme 1
and the level of the pRb-regulated E2F. As shown in Fig. 4C, DZ1 mediated suppression of LMP1
could markedly decrease the level of phosphorylated pRb, while
total pRb level remained unchanged. This decreased level of pRb
phosphorylation directly impacted on the E2F1 expression.
EBV-LMP1-targeted DNAzyme 1 does not
affect expression of cdc2, but downregulates levels of
phosphorylation of cdc2 on Thr161 and cdc2 kinase activity
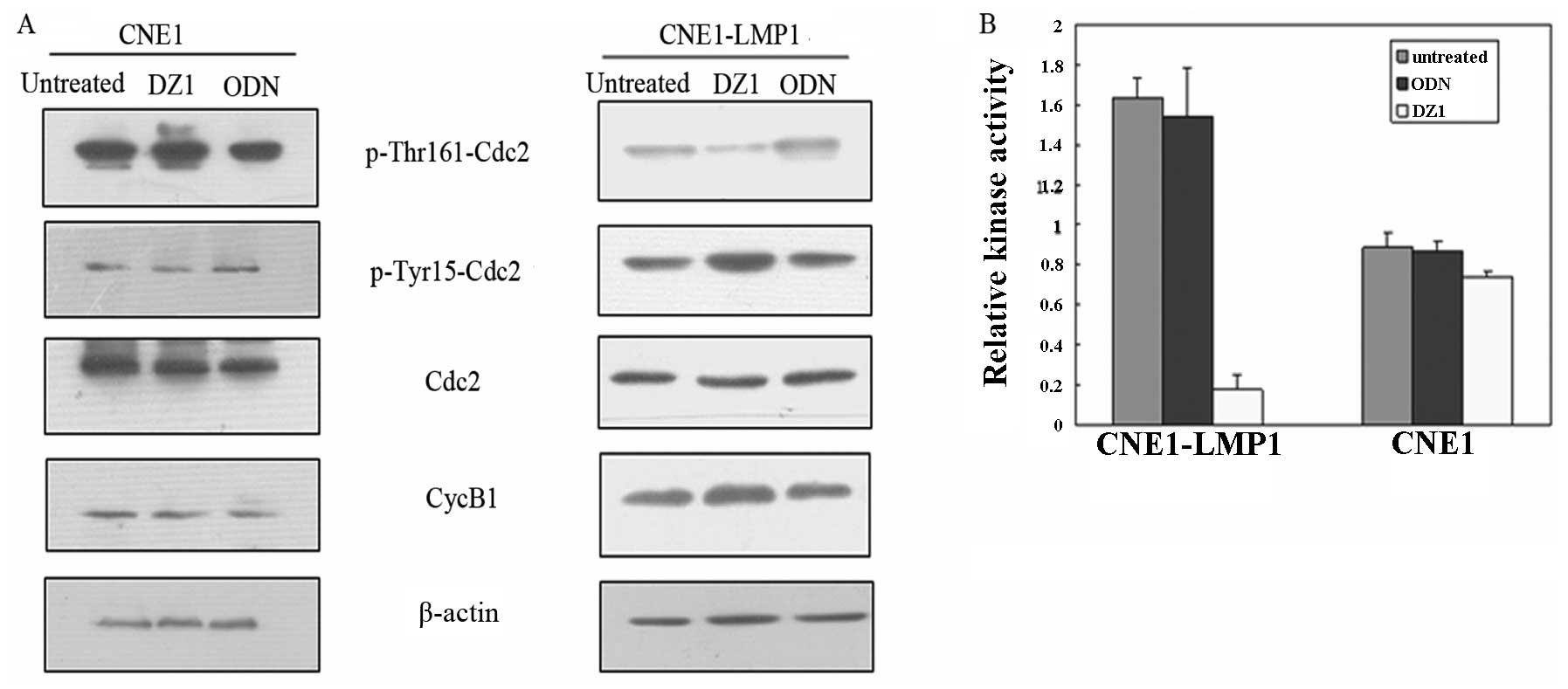
Cdc2 is a critical regulator during the G2 phase
progression. Its activation depends on the Thr161 phosphorylation
and dephosphorylation of the Thr14 and Tyr15 (31,32).
Thus, the status of Thr161 phosphorylation of cdc2 partially
reflects cdc2 kinase activity. To further examine whether the G2/M
checkpoint could contribute to the decrease of the number of cells
in G2/M phase, cdc2 kinase assay was performed.
The results showed that the level of phosphorylation
of the Thr161 was decreased and the dephosphorylation of Tyr15 was
increased in DZ1 treated CNE1-LMP1 cells, whereas ODN had no effect
(Fig. 5A). There was no difference
in the level of cdc2 and cyclin B1 expression in CNE1-LMP1 cells
with or without DZ1 treatment. ELISA results demonstrated that cdc2
kinase activity in untreated CNE1-LMP1 cells was nearly 5-fold
higher than that in the cells treated with DZ1. Together the
LMP1-specific DNAzyme 1 significantly suppressed the LMP1
expression and attenuated cdc2 kinase activity (Fig. 5B).
Discussion
LMP1, as a key oncogenic protein in NPC, plays
important roles in several signal transduction pathways that are
involved in cell proliferation, oncogenesis and apoptosis (33,34).
There are some reports showing that the silencing of EBV-LMP1 by
siRNA slowed the cell cycle progression and enhance
chemosensitivity (35). Here we
show that EBV-LMP1 may through two cell cycle checkpoints, regulate
the cell cycle distribution affecting the DNA damage response. This
was evidenced by the present data showing that the LMP1-targeted
DNAzyme 1 induced cell accumulation in G1 phase and decreased the
number of cells in G2/M phase. H2AX is one of several genes coding
for histone H2A. H2AX becomes phosphorylated on Ser139, then called
γ-H2AX, as a reaction on DNA double-strand breaks (DSB). The
modification can happen in the response to ionizing radiation. As a
sensitive target for looking at DSBs in cells, γ-H2AX is considered
as a DNA damage marker. We found that the DNA damage marker γ-H2AX
was significantly increased by DZ1 in CNE1-LMP1 cells shown by
western blot and immunofluorescent analyses. The DNA damage
response factor p-p53 was downregulated by DZ1 and the
proliferation of LMP1-positive cells was also inhibited by DZ1.
Taken together silencing of EBV-LMP1 induced DNA damage and caused
cell cycle arrest in LMP1 positive cells.
Cell cycle change is sometimes controlled by the
cycling checkpoints. We found downregulation of LMP1 could inhibit
expression of cyclin D1 and CDK4 as well as cyclin E and CDK2;
suppress cyclin D1 and CDK4 interaction and the activity of the
complex; and decrease the Rb protein phosphorylation at Ser780
site. E2F1 is an important regulator of cell entry to S phase
(36). Our results showed the DZ1
could suppress the Rb/E2F1 pathway and markedly downregulate the
expression of E2F1. In addition, the DZ1-mediated suppression of
LMP1 could markedly decrease the cdc2 kinase activity. Taken
together, our data suggested that the G1 phase arrest caused by the
LMP1 targeted DNAzyme 1 is likely through the G1/S and G2/M
checkpoints.
In NPC cells, it has been shown that LMP1 impacted
on cell proliferation and growth at three levels: stimulation of
quiescent cells to re-enter the cell cycle, acceleration of cycling
cells through the G1 phase and increase of cycling cells in the
G2/M phases (37,38). In our previous study we
demonstrated that LMP1 expression could enhance phosphorylation of
p105-Rb to upregulate E2F1 expression and increase E2F1 ability to
transactivate the downstream signal molecules through inhibiting
p16INK4A expression. In the present study, we provided further
evidence showing that LMP1 plays critical roles in controlling the
cell cycle. First, downregulation of the expression of LMP1 could
decrease the expression of the G1/S related molecules and disrupt
their interactions and activities, which consequentially suppressed
the pRb-E2F pathway leading to cell cycle arrest through the
restriction point in G1 phase. Second, downregulation of LMP1 could
inhibit the cdc2 kinase activity, which led to cell cycle arrest
through the restriction point in G2 phase. Therefore, LMP1 could be
a central regulator in oncogenesis of EBV-positive cancers, which
further support the notion that LMP1 could potentially be an
important therapeutic target for NPC.
It has been well established that different types of
cells underwent apoptosis from different cell cycle stages under
different stimulus (39,40). For example, in HeLa cells, UV
caused apoptosis from G1 phase, while the camptothecin (CPT)
induced apoptosis from S phase. Several other previously reported
cancer-preventive agents seem to act in a manner similar to
LMP1-targeted DNAzyme 1. For example, dactylone and inositol
hexaphosphate were reported to decrease CDK4 and cyclin D1 protein
levels and also showed an inhibitory effect on Rb phosphorylation
at Ser780, Ser807 and Ser811, causing G1 arrest and apoptotic death
of human prostate carcinoma LNCaP cells (41). In a previous study, we reported
that the LMP1 targeted DNAzymes in combination with radiation could
enhance radiosensitivity to NPC both in vitro and in
vivo (22,42). Thus, the G1 phase arrest induced by
DNAzyme accompanied by DNA damage may cause the NPC cells to enter
apoptosis.
In conclusion, LMP1 plays an important role in onco
genesis of the EBV-positive cancers. We presented data suggesting
that downregulation of EBV-LMP1 by DNAzyme 1 induced DNA damage
leading to G1 phase arrest. Our data imply that the G1 phase arrest
caused by the LMP1 targeted DNAzyme 1 is likely through the G1/S
and G2/M checkpoints. The biological significance of G1 phase
arrest could relate to the sensitization of the EBV-positive cancer
cells to either radiotherapy or chemotherapy. Thus, targeting LMP1
by DNAzyme might present a novel strategy to sensitize the
EBV-LMP1-positive cells to improve the current treatment
efficacy.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (30930101, 81072220
and 81201171), the National Basic Research Program of China
(2009CB521801 and 2011CB504300), and the Fundamental Research Funds
for the Central Universities.
References
|
1.
|
Ponce RA, Gelzleichter T, Haggerty HG, et
al: Immunomodulation and lymphoma in humans. J Immunotoxicol. Jun
7–2013.(Epub ahead of print).
|
|
2.
|
Ghosh SK, Perrine SP, Williams RM and
Faller DV: Histone deacetylase inhibitors are potent inducers of
gene expression in latent EBV and sensitize lymphoma cells to
nucleoside antiviral agents. Blood. 119:1008–1017. 2012. View Article : Google Scholar
|
|
3.
|
Brocqueville G, Ndour PA, Ouk TS, et al:
LMP1-induced cell death may contribute to the emergency of its
oncogenic property. PLoS One. 8:e607432013. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Smuk G, Illes A, Keresztes K, et al:
Pheno- and genotypic features of Epstein-Barr virus associated
B-cell lymphoproliferations in peripheral T-cell lymphomas. Pathol
Oncol Res. 16:377–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Diduk SV, Smirnova KV, Pavlish OA and
Gurtsevitch VE: Functionally significant mutations in the
Epstein-Barr virus LMP1 gene and their role in activation of cell
signaling pathways. Biochemistry (Mosc). 73:1134–1139. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Everly DN Jr, Mainou BA and Raab-Traub N:
The ID proteins contribute to the growth of rodent fibroblasts
during LMP1-mediated transformation. Virology. 376:258–269. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wu ZZ, Chow KP, Kuo TC, Chang YS and Chao
CC: Latent membrane protein 1 of Epstein-Barr virus sensitizes
cancer cells to cisplatin by enhancing NF-kappaB p50 homodimer
formation and downregulating NAPA expression. Biochem Pharmacol.
82:1860–1872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Lu JH, Tang YL, Yu HB, et al: Epstein-Barr
virus facilitates the malignant potential of immortalized
epithelial cells: from latent genome to viral production and
maintenance. Lab Invest. 90:196–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Gan R, Xie X, He J, et al: Gene analysis
of Epstein-Barr virus-associated lymphomas in Hu-Pbl/SCID chimeras.
Tumori. 96:465–472. 2010.PubMed/NCBI
|
|
10.
|
Arcipowski KM and Bishop GA: TRAF binding
is required for a distinct subset of in vivo B cell functions of
the oncoprotein LMP1. J Immunol. 189:5165–5170. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yoshizaki T, Kondo S, Wakisaka N, et al:
Pathogenic role of Epstein-Barr virus latent membrane protein-1 in
the development of nasopharyngeal carcinoma. Cancer Lett. 337:1–7.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zheng H, Li LL, Hu DS, Deng XY and Cao Y:
Role of Epstein-Barr virus encoded latent membrane protein 1 in the
carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol.
4:185–196. 2007.PubMed/NCBI
|
|
13.
|
Tao YG, Tan YN, Liu YP, et al:
Epstein-Barr virus latent membrane protein 1 modulates epidermal
growth factor receptor promoter activity in a nuclear factor kappa
B-dependent manner. Cell Signal. 16:781–790. 2004. View Article : Google Scholar
|
|
14.
|
Wang Z, Luo F, Li L, et al: STAT3
activation induced by Epstein-Barr virus latent membrane protein1
causes vascular endothelial growth factor expression and cellular
invasiveness via JAK3 And ERK signaling. Eur J Cancer.
46:2996–3006. 2010. View Article : Google Scholar
|
|
15.
|
Horikawa T, Yang J, Kondo S, et al: Twist
and epithelial-mesenchymal transition are induced by the EBV
oncoprotein latent membrane protein 1 and are associated with
metastatic nasopharyngeal carcinoma. Cancer Res. 67:1970–1978.
2007. View Article : Google Scholar
|
|
16.
|
Dawson CW, Laverick L, Morris MA,
Tramoutanis G and Young LS: Epstein-Barr virus-encoded LMP1
regulates epithelial cell motility and invasion via the ERK-MAPK
pathway. J Virol. 82:3654–3664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sheu LF, Chen A, Wei YH, et al:
Epstein-Barr virus LMP1 modulates the malignant potential of
gastric carcinoma cells involving apoptosis. Am J Pathol.
152:63–74. 1998.PubMed/NCBI
|
|
18.
|
Li G, Li XP, Peng Y, Liu X and Li XH:
Effect of inhibition of EBV-encoded latent membrane protein-1 by
small interfering RNA on EBV-positive nasopharyngeal carcinoma cell
growth. Di Yi Jun Yi Da Xue Xue Bao. 24:241–246. 2004.PubMed/NCBI
|
|
19.
|
Li XP, Li G, Peng Y, Kung HF and Lin MC:
Suppression of Epstein-Barr virus-encoded latent membrane protein-1
by RNA interference inhibits the metastatic potential of
nasopharyngeal carcinoma cells. Biochem Biophys Res Commun.
315:212–218. 2004. View Article : Google Scholar
|
|
20.
|
Ndour PA, Brocqueville G, Ouk TS, et al:
Inhibition of latent membrane protein 1 impairs the growth and
tumorigenesis of latency II Epstein-Barr virus-transformed T cells.
J Virol. 86:3934–3943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yang L, Lu Z, Ma X, Cao Y and Sun LQ: A
therapeutic approach to nasopharyngeal carcinomas by DNAzymes
targeting EBV LMP-1 gene. Molecules. 15:6127–6139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Lu ZX, Ma XQ, Yang LF, et al: DNAzymes
targeted to EBV-encoded latent membrane protein-1 induce apoptosis
and enhance radiosensitivity in nasopharyngeal carcinoma. Cancer
Lett. 265:226–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Glaser R, Zhang HY, Yao KT, et al: Two
epithelial tumor cell lines (HNE-1 and HONE-1) latently infected
with Epstein-Barr virus that were derived from nasopharyngeal
carcinomas. Proc Natl Acad Sci USA. 86:9524–9528. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Zhao Y, Tao YG, Luo FJ, Tang FQ, Tang M
and Cao Y: Interference effect of epigallocatechin-3-gallate on
targets of nuclear factor kappaB signal transduction pathways
activated by EB virus encoded latent membrane protein 1. Int J
Biochem Cell Biol. 36:1473–1481. 2004.
|
|
25.
|
Benimetskaya L, Takle GB, Vilenchik M,
Lebedeva I, Miller P and Stein CA: Cationic porphyrins: novel
delivery vehicles for antisense oligodeoxynucleotides. Nucleic
Acids Res. 26:5310–5317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Lu ZX, Ye M, Yan GR, et al: Effect of EBV
LMP1 targeted DNAzymes on cell proliferation and apoptosis. Cancer
Gene Ther. 12:647–654. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sugimoto M, Martin N, Wilks DP, et al:
Activation of cyclin D1-kinase in murine fibroblasts lacking both
p21(Cip1) and p27(Kip1). Oncogene. 21:8067–8074. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ling S and Lin WC: EDD inhibits
ATM-mediated phosphorylation of p53. J Biol Chem. 286:14972–14982.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Kitagawa M, Higashi H, Jung HK, et al: The
consensus motif for phosphorylation by cyclin D1-Cdk4 is different
from that for phosphorylation by cyclin A/E-Cdk2. EMBO J.
15:7060–7069. 1996.PubMed/NCBI
|
|
30.
|
Hansen U, Owens L and Saxena UH:
Transcription factors LSF and E2Fs: tandem cyclists driving G0 to
S? Cell Cycle. 8:2146–2151. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Zhang XH, Zou ZQ, Xu CW, Shen YZ and Li D:
Myricetin induces G2/M phase arrest in HepG2 cells by inhibiting
the activity of the cyclin B/Cdc2 complex. Mol Med Rep. 4:273–277.
2011.PubMed/NCBI
|
|
32.
|
Tan M, Jing T, Lan KH, et al:
Phosphorylation on tyrosine-15 of p34(Cdc2) by ErbB2 inhibits
p34(Cdc2) activation and is involved in resistance to taxol-induced
apoptosis. Mol Cell. 9:993–1004. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Dawson CW, Port RJ and Young LS: The role
of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the
pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol.
22:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Tulalamba W and Janvilisri T:
Nasopharyngeal carcinoma signaling pathway: an update on molecular
biomarkers. Int J Cell Biol. 2012:5946812012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Mei YP, Zhou JM, Wang Y, et al: Silencing
of LMP1 induces cell cycle arrest and enhances chemosensitivity
through inhibition of AKT signaling pathway in EBV-positive
nasopharyngeal carcinoma cells. Cell Cycle. 6:1379–1385. 2007.
View Article : Google Scholar
|
|
36.
|
Korotayev K, Chaussepied M and Ginsberg D:
ERK activation is regulated by E2F1 and is essential for
E2F1-induced S phase entry. Cell Signal. 20:1221–1226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Deng L, Yang J, Zhao XR, et al: Cells in
G2/M phase increased in human nasopharyngeal carcinoma cell line by
EBV-LMP1 through activation of NF-kappaB and AP-1. Cell Res.
13:187–194. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Zhao XR, Gu HH, Weng XX, Yi W, Deng XY and
Cao Y: The primary study on expression and function of D-type
cyclins in nasopharyngeal carcinoma cell lines. Sheng Wu Hua Xue Yu
Sheng Wu Wu Li Xue Bao (Shanghai). 32:192–196. 2000.PubMed/NCBI
|
|
39.
|
Liu DX and Greene LA: Neuronal apoptosis
at the G1/S cell cycle checkpoint. Cell Tissue Res. 305:217–228.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Tao D, Wu J, Feng Y, Qin J, Hu J and Gong
J: New method for the analysis of cell cycle-specific apoptosis.
Cytometry A. 57:70–74. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Agarwal C, Dhanalakshmi S, Singh RP and
Agarwal R: Inositol hexaphosphate inhibits growth and induces G1
arrest and apoptotic death of androgen-dependent human prostate
carcinoma LNCaP cells. Neoplasia. 6:646–659. 2004. View Article : Google Scholar
|
|
42.
|
Ma X, Yang L, Xiao L, et al:
Down-regulation of EBV-LMP1 radio-sensitizes nasal pharyngeal
carcinoma cells via NF-kappaB regulated ATM expression. PLoS One.
6:e246472011. View Article : Google Scholar : PubMed/NCBI
|