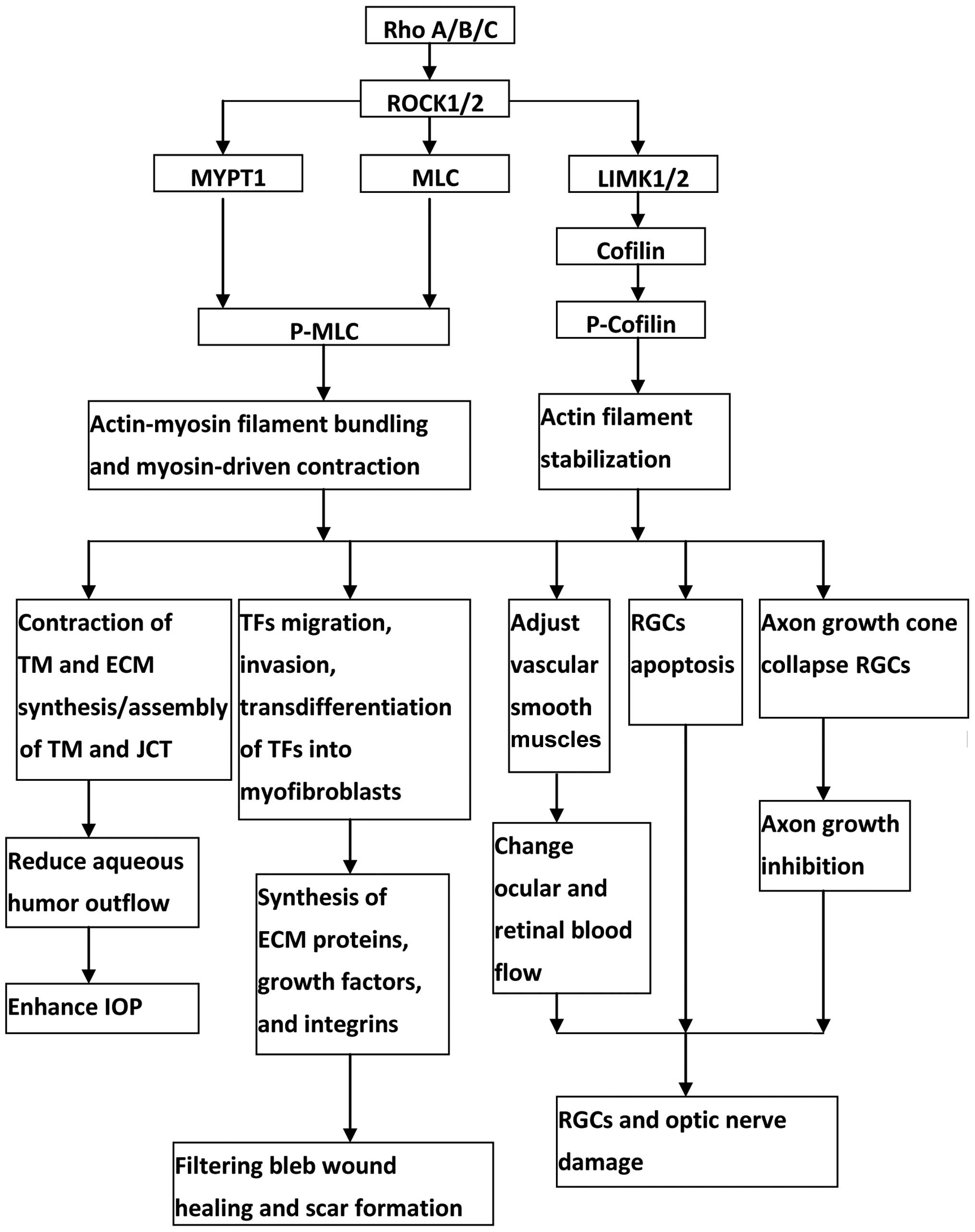
|
1.
|
Weinreb RN and Khaw PT: Primary open-angle
glaucoma. Lancet. 363:1711–1720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sommer A: Intraocular pressure and
glaucoma. Am J Ophthalmol. 107:186–188. 1989. View Article : Google Scholar
|
|
4.
|
Tielsch JM, Sommer A, Katz J, Royall RM,
Quigley HA and Javitt J: Racial variations in the prevalence of
primary open-angle glaucoma. The Baltimore Eye Survey. JAMA.
266:369–374. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Klein BE, Klein R, Sponsel WE, Franke T,
Cantor LB, Martone J and Menage MJ: Prevalence of glaucoma. The
Beaver Dam Eye Study. Ophthalmology. 99:1499–1504. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mitchell P, Smith W, Attebo K and Healey
PR: Prevalence of open-angle glaucoma in Australia. The Blue
Mountains Eye Study. Ophthalmology. 103:1661–1669. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Collaborative Normal-Tension Glaucoma
Study Group: Comparison of glaucomatous progression between
untreated patients with normal-tension glaucoma and patients with
therapeutically reduced intraocular pressures. Am J Ophthalmol.
126:487–497. 1998. View Article : Google Scholar
|
|
8.
|
Collaborative Normal-Tension Glaucoma
Study Group: The effectiveness of intraocular pressure reduction in
the treatment of normal-tension glaucoma. Am J Ophthalmol.
126:498–505. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
The AGIS Investigators: The advanced
glaucoma intervention study (AGIS): 7. The relationship between
control of intraocular pressure and visual field deterioration. Am
J Ophthalmol. 130:429–440. 2000. View Article : Google Scholar
|
|
10.
|
Lichter PR, Musch DC, Gillespie BW, Guire
KE, Janz NK, Wren PA and Mills RP: Interim clinical outcomes in the
Collaborative Initial Glaucoma Treatment Study comparing initial
treatment randomized to medications or surgery. Ophthalmology.
108:1943–1953. 2001. View Article : Google Scholar
|
|
11.
|
Gordon MO, Beiser JA, Brandt JD, Heuer DK,
Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK II,
Wilson MR and Kass MA: The Ocular Hypertension Treatment Study:
baseline factors that predict the onset of primary open-angle
glaucoma. Arch Ophthalmol. 120:714–720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kass MA, Heuer DK, Higginbotham EJ,
Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR and
Gordon MO: The Ocular Hypertension Treatment Study: a randomized
trial determines that topical ocular hypotensive medication delays
or prevents the onset of primary open-angle glaucoma. Arch
Ophthalmol. 120:701–713. 2002. View Article : Google Scholar
|
|
13.
|
Leske MC, Heijl A, Hussein M, Bengtsson B,
Hyman L and Komaroff E: Factors for glaucoma progression and the
effect of treatment: the early manifest glaucoma trial. Arch
Ophthalmol. 121:48–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hernandez MR: The optic nerve head in
glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye
Res. 19:297–321. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Burgoyne CF: A biomechanical paradigm for
axonal insult within the optic nerve head in aging and glaucoma.
Exp Eye Res. 93:120–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lutjen-Drecoll E: Functional morphology of
the trabecular meshwork in primate eyes. Prog Retin Eye Res.
18:91–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tan JC, Peters DM and Kaufman PL: Recent
developments in understanding the pathophysiology of elevated
intraocular pressure. Curr Opin Ophthalmol. 17:168–174.
2006.PubMed/NCBI
|
|
18.
|
Gabelt BT and Kaufman PL: Changes in
aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res.
24:612–637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Grant WM: Experimental aqueous perfusion
in enucleated human eyes. Arch Ophthalmol. 69:783–801. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Johnson M: What controls aqueous humour
outflow resistance? Exp Eye Res. 82:545–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tamm ER: The trabecular meshwork outflow
pathways: Structural and functional aspects. Exp Eye Res.
88:648–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kumar J and Epstein DL: Rho
GTPase-mediated cytoskeletal organization in Schlemm’s canal cells
play a critical role in the regulation of aqueous humor outflow
facility. J Cell Biochem. 112:600–606. 2011.PubMed/NCBI
|
|
23.
|
Ethier CR: The inner wall of Schlemm’s
canal. Exp Eye Res. 74:161–172. 2002.
|
|
24.
|
Wiederholt M, Thieme H and Stumpff F: The
regulation of trabecular meshwork and ciliary muscle contractility.
Prog Retin Eye Res. 19:271–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lutjen-Drecoll E: Morphological changes in
glaucomatous eyes and the role of TGF β2 for the pathogenesis of
the disease. Exp Eye Res. 81:1–4. 2005.
|
|
26.
|
Fuchshofer R and Tamm ER: The role of
TGF-β in the pathogenesis of primary open-angle glaucoma. Cell
Tissue Res. 347:279–290. 2012.
|
|
27.
|
Bradley JM, Vranka J, Colvis CM, Conger
DM, Alexander JP, Fisk AS, Samples JR and Acott TS: Effect of
matrix metalloproteinases activity on outflow in perfused human
organ culture. Invest Ophthalmol Vis Sci. 39:2649–2658.
1998.PubMed/NCBI
|
|
28.
|
Tian B, Geiger B, Epstein DL and Kaufman
PL: Cytoskeletal involvement in the regulation of aqueous humor
outflow. Invest Ophthalmol Vis Sci. 41:619–623. 2000.PubMed/NCBI
|
|
29.
|
Tian B, Gabelt BT, Geiger B and Kaufman
PL: The role of the actomyosin system in regulating trabecular
fluid outflow. Exp Eye Res. 88:713–717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Last JA, Pan T, Ding Y, Reilly CM, Keller
K, Acott TS, Fautsch MP, Murphy CJ and Russell P: Elastic modulus
determination of normal and glaucomatous human trabecular meshwork.
Invest Ophthalmol Vis Sci. 52:2147–2152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Erschbamer MK, Hofstetter CP and Olson L:
RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord,
sensory ganglia, and corticospinal tract neurons and long-lasting
specific changes following spinal cord injury. J Comp Neurol.
484:224–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
33.
|
Nakagawa O, Fujisawa K, Ishizaki T, Saito
Y, Nakao K and Narumiya S: ROCK-I and ROCK-II, two isoforms of
Rho-associated coiled-coil forming protein serine/threonine kinase
in mice. FEBS Lett. 392:189–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Leung T, Chen XQ, Manser E and Lim L: The
p160 RhoA-binding kinase ROK alpha is a member of a kinase family
and is involved in the reorganization of the cytoskeleton. Mol Cell
Biol. 16:5313–5327. 1996.PubMed/NCBI
|
|
36.
|
Coleman ML, Sahai EA, Yeo M, Bosch M,
Dewar A and Olson MF: Membrane blebbing during apoptosis results
from caspase-mediated activation of ROCK I. Nat Cell Biol.
3:339–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sebbagh M, Renvoizé C, Hamelin J, Riché N,
Bertoglio J and Bréard J: Caspase-3-mediated cleavage of ROCK I
induces MLC phosphorylation and apoptotic membrane blebbing. Nat
Cell Biol. 3:346–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sebbagh M, Hamelin J, Bertoglio J, Solary
E and Bréard J: Direct cleavage of ROCK II by granzyme B induces
target cell membrane blebbing in a caspase-independent manner. J
Exp Med. 201:465–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Van Aelst L and D’Souza-Schorey C: Rho
GTPases and signaling networks. Genes Dev. 11:2295–2322.
1997.PubMed/NCBI
|
|
40.
|
Ridley AJ, Paterson HF, Johnston CL,
Diekmann D and Hall A: The small GTP-binding protein rho regulates
growth factor-induced membrane ruffling. Cell. 70:401–410. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ridley AJ and Hall A: Signal transduction
pathways regulating Rho-mediated stress fibre formation:
requirement for a tyrosine kinase. EMBO J. 13:2600–2610.
1994.PubMed/NCBI
|
|
42.
|
Nobes C and Hall A: Regulation and
function of the Rho subfamily of small GTPases. Curr Opin Genet
Dev. 4:77–81. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Takai Y, Sasaki T, Tanaka K and Nakanishi
H: Rho as a regulator of the cytoskeleton. Trends Biochem Sci.
20:227–231. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Paterson HF, Self AJ, Garrett MD, Just I,
Aktories K and Hall A: Microinjection of recombinant p21rho induces
rapid changes in cell morphology. J Cell Biol. 111:1001–1007. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Hirata K, Kikuchi A, Sasaki T, Kuroda S,
Kaibuchi K, Matsuura Y, Seki H, Saida K and Takai Y: Involvement of
rho p21 in the GTP-enhanced calcium ion sensitivity of smooth
muscle contraction. J Biol Chem. 267:8719–8722. 1992.PubMed/NCBI
|
|
46.
|
Narumiya S: The small GTPase Rho: cellular
functions and signal transduction. J Biochem (Tokyo). 120:215–228.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Gong MC, Iizuka K, Nixon G, Browne JP,
Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV and Somlyo
AP: Role of guanine nucleotide-binding proteins - ras-family or
trimeric proteins or both - in Ca2+ sensitization of
smooth muscle. Proc Natl Acad Sci USA. 93:1340–1345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Narumiya S, Ishizaki T and Watanabe N: Rho
effectors and reorganization of actin cytoskeleton. FEBS Lett.
410:68–72. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Kaibuchi K, Kuroda S and Amano M:
Regulation of the cytoskeleton and cell adhesion by the Rho family
GTPases in mammalian cells. Annu Rev Biochem. 68:459–486. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Somlyo AP and Somlyo AV: Signal
transduction by G-proteins, rho-kinase and protein phosphatase to
smooth muscle and non-muscle myosin II. J Physiol. 522:177–185.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Fukata Y, Amano M and Kaibuchi K:
Rho-Rho-kinase pathway in smooth muscle contraction and
cytoskeletal reorganization of non-muscle cells. Trends Pharmacol
Sci. 22:32–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Nakayama M, Amano M, Katsumi A, Kaneko T,
Kawabata S, Takefuji M and Kaibuchi K: Rho-kinase and myosin II
activities are required for cell type and environment specific
migration. Genes Cells. 10:107–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Nakajima E, Nakajima T, Minagawa Y,
Shearer TR and Azuma M: Contribution of ROCK in contraction of
trabecular meshwork: proposed mechanism for regulating aqueous
outflow in monkey and human eyes. J Pharm Sci. 94:701–708. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Tan HB, Zhong YS, Cheng Y and Shen X:
Rho/ROCK pathway and neural regeneration: a potential therapeutic
target for central nervous system and optic nerve damage. Int J
Ophthalmol. 4:652–657. 2011.PubMed/NCBI
|
|
56.
|
Asano T, Ikegaki I, Satoh S, Suzuki Y,
Shibuya M, Takayasu M and Hidaka H: Mechanism of action of a novel
antivasospasm drug, HA1077. J Pharmacol Exp Ther. 241:1033–1040.
1987.PubMed/NCBI
|
|
57.
|
Asano T, Suzuki T, Tsuchiya M, Satoh S,
Ikegaki I, Shibuya M, Suzuki Y and Hidaka H: Vasodilator actions of
HA1077 in vitro and in vivo putatively mediated by the inhibition
of protein kinase. Br J Pharmacol. 98:1091–1100. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Honjo M, Inatani M, Kido N, Sawamura T,
Yue BY, Honda Y and Tanihara H: A myosin light chain kinase
inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes.
Exp Eye Res. 75:135–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Yoneda A, Multhaupt HA and Couchman JR:
The Rho kinases I and II regulate different aspects of myosin II
activity. J Cell Biol. 170:443–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Wang Y, Zheng XR, Riddick N, Bryden M,
Baur W, Zhang X and Surks HK: ROCK isoform regulation of myosin
phosphatase and contractility in vascular smooth muscle cells. Circ
Res. 104:531–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Hartshorne DJ: Myosin phosphatase:
subunits and interactions. Acta Physiol Scand. 164:483–493. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Pfitzer G: Invited review: regulation of
myosin phosphorylation in smooth muscle. J Appl Physiol.
91:497–503. 2001.PubMed/NCBI
|
|
63.
|
Harnett KM and Biancani P:
Calcium-dependent and calcium-independent contractions in smooth
muscles. Am J Med. 115(Suppl 3A): 24S–30S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Kimura K, Ito M, Amano M, Chihara K,
Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K,
Iwamatsu A and Kaibuchi K: Regulation of myosin phosphatase by rho
and rho-associated kinase (rho-kinase). Science. 273:245–248. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Wettschureck N and Offermanns S:
Rho/Rho-kinase mediated signaling in physiology and
pathophysiology. J Mol Med. 80:629–638. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosin II: modulated by
G proteins, kinases, and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003.
|
|
67.
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar
|
|
68.
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
69.
|
Jaffe AB and Hall A: Rho GTPases:
biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Spindler V, Schlegel N and Waschke J: Role
of GTPases in control of microvascular permeability. Cardiovasc
Res. 87:243–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Thieme H, Nuskovski M, Nass JU, Pleyer U,
Strauss O and Wiederholt M: Mediation of calcium-independent
contraction in trabecular meshwork through protein kinase c and
rho-A. Invest Ophthalmol Vis Sci. 41:4240–4246. 2000.PubMed/NCBI
|
|
72.
|
Honjo M, Tanihara H, Inatani M, Kido N,
Sawamura T, Yue BY, Narumiya S and Honda Y: Effects of
rho-associated protein kinase inhibitor Y-27632 on intraocular
pressure and outflow facility. Invest Ophthalmol Vis Sci.
42:137–144. 2001.PubMed/NCBI
|
|
73.
|
Rao PV, Deng PF, Kumar J and Epstein DL:
Modulation of aqueous humor outflow facility by the Rho
kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci.
42:1029–1037. 2001.PubMed/NCBI
|
|
74.
|
Goldhagen B, Proia AD, Epstein DL and Rao
PV: Elevated levels of RhoA in the optic nerve head of human eyes
with glaucoma. J Glaucoma. 21:530–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Epstein DL, Rowlette LL and Roberts BC:
Acto-myosin drug effects and aqueous outflow function. Invest
Ophthalmol Vis Sci. 40:74–81. 1999.PubMed/NCBI
|
|
76.
|
Rao PV, Deng P, Sasaki Y and Epstein DL:
Regulation of myosin light chain phosphorylation in the trabecular
meshwork: role in aqueous humour outflow facility. Exp Eye Res.
80:197–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Geiger B, Yehuda-Levenberg S and
Bershadsky AD: Molecular interactions in the submembrane plaque of
cell-cell and cell-matrix adhesions. Acta Anat (Basel). 154:46–62.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Rosenthal R, Choritz L, Schlott S,
Bechrakis NE, Jaroszewski J, Wiederholt M and Thieme H: Effects of
ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction
of bovine trabecular meshwork. Exp Eye Res. 80:837–845. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Renieri G, Choritz L, Rosenthal R,
Meissner S, Pfeiffer N and Thieme H: Effects of endothelin-1 on
calcium-independent contraction of bovine trabecular meshwork.
Graefes Arch Clin Exp Ophthalmol. 246:1107–1115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Lu Z, Overby DR, Scott PA, Freddo TF and
Gong H: The mechanism of increasing outflow facility by rho-kinase
inhibition with Y-27632 in bovine eyes. Exp Eye Res. 86:271–281.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Johnstone MA: The aqueous outflow system
as a mechanical pump: evidence from examination of tissue and
aqueous movement in human and non-human primates. J Glaucoma.
13:421–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Rao VP and Epstein DL: Rho GTPase/Rho
kinase inhibition as a novel target for the treatment of glaucoma.
BioDrugs. 21:167–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
WuDunn D: Mechanobiology of trabecular
meshwork cells. Exp Eye Res. 88:718–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Wiederholt M, Bielka S, Schweig F,
Lutjen-Drecoll E and Lepple-Wienhues A: Regulation of outflow rate
and resistance in the perfused anterior segment of the bovine eye.
Exp Eye Res. 61:223–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Honjo M, Inatani M, Kido N, Sawamura T,
Yue BY, Honda Y and Tanihara H: Effects of protein kinase
inhibitor, HA1077, on intraocular pressure and outflow facility in
rabbit eyes. Arch Ophthalmol. 119:1171–1178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Tokushige H, Inatani M, Nemoto S, Sakaki
H, Katayama K, Uehata M and Tanihara H: Effects of topical
administration of Y-39983, a selective rho-associated protein
kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest
Ophthalmol Vis Sci. 48:3216–3222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Tanihara H, Inatani M, Honjo M, Tokushige
H, Azuma J and Araie M: Intraocular pressure-lowering effects and
safety of topical administration of a selective ROCK inhibitor,
SNJ-1656, in healthy volunteers. Arch Ophthalmol. 126:309–315.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Fukunaga T, Ikesugi K, Nishio M, Sugimoto
M, Sasoh M, Hidaka H and Uji Y: The effect of the Rho-associated
protein kinase inhibitor, HA-1077, in the rabbit ocular
hypertension model induced by water loading. Curr Eye Res.
34:42–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Gottanka J, Chan D, Eichhorn M,
Lutjen-Drecoll E and Ethier CR: Effects of TGF-β2 in perfused human
eyes. Invest Ophthalmol Vis Sci. 45:153–158. 2004.
|
|
90.
|
Mettu PS, Deng PF, Misra UK, Gawdi G,
Epstein DL and Rao PV: Role of lysophospholipid growth factors in
the modulation of aqueous humor outflow facility. Invest Ophthalmol
Vis Sci. 45:2263–2271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Zhang M, Maddala R and Rao PV: Novel
molecular insights into RhoA GTPase-induced resistance to aqueous
humor outflow through the trabecular meshwork. Am J Physiol Cell
Physiol. 295:C1057–C1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92.
|
Waki M, Yoshida Y, Oka T and Azuma M:
Reduction of intraocular pressure by topical administration of an
inhibitor of the Rho-associated protein kinase. Curr Eye Res.
22:470–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93.
|
Wiederholt M and Stumpff H: The trabecular
meshwork and aqueous humor reabsorption. Curr Top Membr.
45:163–202. 1998. View Article : Google Scholar
|
|
94.
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
95.
|
Iizuka K, Yoshii A, Samizo K, Tsukagoshi
H, Ishizuka T, Dobashi K, Nakazawa T and Mori M: A major role for
the rho-associated coiled coil forming protein kinase in
G-protein-mediated Ca2+ sensitization through inhibition
of myosin phosphatase in rabbit trachea. Br J Pharmacol.
128:925–933. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Lütjen-Drecoll E, Futa R and Rohen JW:
Ultrahistochemical studies on tangential sections of the trabecular
meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis
Sci. 21:563–573. 1981.PubMed/NCBI
|
|
97.
|
Yue BY: The extracellular matrix and its
modulation in the trabecular meshwork. Surv Ophthalmol. 40:379–390.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Tamm ER and Fuchshofer R: What increases
outflow resistance in primary open-angle glaucoma? Surv Ophthalmol.
52(Suppl 2): S101–S104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99.
|
Acott TS and Kelley MJ: Extracellular
matrix in the trabecular meshwork. Exp Eye Res. 86:543–561. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
100.
|
Keller KE, Aga M, Bradley JM, Kelley MJ
and Acott TS: Extracellular matrix turnover and outflow resistance.
Exp Eye Res. 88:676–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Pattabiraman PP and Rao PV: Mechanistic
basis of Rho GTPase-induced extracellular matrix synthesis in
trabecular meshwork cells. Am J Physiol Cell Physiol. 298:C749–763.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Nakamura Y, Hirano S, Suzuki K, Seki K,
Sagara T and Nishida T: Signaling mechanism of TGF-β1-induced
collagen contraction mediated by bovine trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 43:3465–3472. 2002.
|
|
103.
|
Fleenor DL, Shepard AR, Hellberg PE,
Jacobson N, Pang IH and Clark AF: TGFβ2-induced changes in human
trabecular meshwork: implications for intraocular pressure. Invest
Ophthalmol Vis Sci. 47:226–234. 2006.
|
|
104.
|
Iyer P, Maddala R, Pattabiraman PP and Rao
PV: Connective tissue growth factor-mediated upregulation of
neuromedin U expression in trabecular meshwork cells and its role
in homeostasis of aqueous humor outflow. Invest Ophthalmol Vis Sci.
53:4952–4962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105.
|
Bershadsky AD, Balaban NQ and Geiger B:
Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol.
19:677–695. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Kaunas R, Nguyen P, Usami S and Chien S:
Cooperative effects of Rho and mechanical stretch on stress fiber
organization. Proc Natl Acad Sci USA. 102:15895–15900. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
107.
|
Shikata Y, Rios A, Kawkitinarong K,
DePaola N, Garcia JG and Birukov KG: Differential effects of shear
stress and cyclic stretch on focal adhesion remodeling,
site-specific FAK phosphorylation, and small GTPases in human lung
endothelial cells. Exp Cell Res. 304:40–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Ingber DE: Cellular mechanotransduction:
putting all the pieces together again. FASEB J. 20:811–827. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
109.
|
Tzima E: Role of small GTPases in
endothelial cytoskeletal dynamics and the shear stress response.
Circ Res. 98:176–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110.
|
Schlunck G, Han H, Wecker T, Kampik D,
Meyer-ter-Vehn T and Grehn F: Substrate rigidity modulates cell
matrix interactions and protein expression in human trabecular
meshwork cells. Invest Ophthalmol Vis Sci. 49:262–269. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
111.
|
Kameda T, Inoue T, Inatani M, Fujimoto T,
Honjo M, Kasaoka N, Inoue-Mochita M, Yoshimura N and Tanihara H:
The effect of Rho-associated protein kinase inhibitor on monkey
Schlemm’s canal endothelial cells. Invest Ophthalmol Vis Sci.
53:3092–3103. 2012.PubMed/NCBI
|
|
112.
|
Epstein DL, Freddo TF, Bassett-Chu S,
Chung M and Karageuzian L: Influence of ethacrynic acid on outflow
facility in the monkey and calf eye. Invest Ophthalmol Vis Sci.
28:2067–2075. 1987.PubMed/NCBI
|
|
113.
|
Ethier CR, Read AT and Chan DW: Effects of
latrunculin-B on outflow facility and trabecular meshwork structure
in human eyes. Invest Ophthalmol Vis Sci. 47:1991–1998. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
114.
|
Inoue T, Pattabiraman PP, Epstein DL and
Rao PV: Effects of chemical inhibition of N-WASP, a critical
regulator of actin polymerization on aqueous humor outflow through
the conventional pathway. Exp Eye Res. 90:360–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115.
|
Bill A and Svedbergh B: Scanning electron
microscopic studies of the trabecular meshwork and the canal of
Schlemm - an attempt to localize the main resistance to outflow of
aqueous humor in man. Acta Ophthalmol (Copenh). 50:295–320. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
116.
|
Liu Q, Wu K, Qiu X, Yang Y, Lin X and Yu
M: siRNA silencing of gene expression in trabecular meshwork: RhoA
siRNA reduces IOP in mice. Curr Mol Med. 12:1015–1027. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
117.
|
Tian B and Kaufman PL: Effects of the Rho
kinase inhibitor Y-27632 and the phosphatase inhibitor calyculinA
on outflow facility in monkeys. Exp Eye Res. 80:215–225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
118.
|
Yu M, Chen X, Wang N, Cai S, Li N, Qiu J,
Brandt CR, Kaufman PL and Liu X: H-1152 effects on intraocular
pressure and trabecular meshwork morphology of rat eyes. J Ocul
Pharmacol Ther. 24:373–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119.
|
Nishio M, Fukunaga T, Sugimoto M, Ikesugi
K, Sumi K, Hidaka H and Uji Y: The effect of the H-1152P, a potent
Rho-associated coiled coil-formed protein kinase inhibitor, in
rabbit normal and ocular hypertensive eyes. Curr Eye Res.
34:282–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120.
|
Whitlock NA, Harrison B, Mixon T, Yu XQ,
Wilson A, Gerhardt B, Eberhart DE, Abuin A and Rice DS: Decreased
intraocular pressure in mice following either pharmacological or
genetic inhibition of ROCK. J Ocul Pharmacol Ther. 25:187–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
121.
|
Lama PJ and Fechtner RD: Antifibrotics and
wound healing in glaucoma surgery. Surv Ophthalmol. 48:314–346.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
122.
|
Yoon PS and Singh K: Update on
antifibrotic use in glaucoma surgery, including use in
trabeculectomy and glaucoma drainage implants and combined cataract
and glaucoma surgery. Curr Opin Ophthalmol. 15:141–146. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
123.
|
Migdal C, Gregory W and Hitchings R:
Long-term functional outcome after early surgery compared with
laser and medicine in open-angle glaucoma. Ophthalmology.
101:1651–1656. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
124.
|
Khaw PT, Occleston NL, Schultz G, Grierson
I, Sherwood MB and Larkin G: Activation and suppression of
fibroblast function. Eye. 8:188–195. 1994. View Article : Google Scholar
|
|
125.
|
Occleston NL, Daniels JT, Tarnuzzer RW,
Sethi KK, Alexander RA, Bhattacharya SS, Schultz GS and Khaw PT:
Single exposures to antiproliferatives: long-term effects on ocular
fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci.
38:1998–2007. 1997.PubMed/NCBI
|
|
126.
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
127.
|
Hinz B, Mastrangelo D, Iselin CE,
Chaponnier C and Gabbiani G: Mechanical tension controls
granulation tissue contractile activity and myofibroblast
differentiation. Am J Pathol. 159:1009–1020. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
128.
|
Desmouliere A, Chaponnier C and Gabbiani
G: Tissue repair, contraction, and the myofibroblast. Wound Repair
Regen. 13:7–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
129.
|
Meyer-Ter-Vehn T, Gebhardt S, Sebald W,
Buttmann M, Grehn F, Schlunck G and Knaus P: p38 inhibitors prevent
TGF-β-induced myofibroblast transdifferentiation in human tenon
fibroblasts. Invest Ophthalmol Vis Sci. 47:1500–1509. 2006.
|
|
130.
|
Ehrlich HP and Rajaratnam JB: Cell
locomotion forces versus cell contraction forces for collagen
lattice contraction: an in vitro model of wound contraction. Tissue
Cell. 22:407–417. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
131.
|
Desmouliere A, Redard M, Darby I and
Gabbiani G: Apoptosis mediates the decrease in cellularity during
the transition between granulation tissue and scar. Am J Pathol.
146:56–66. 1995.PubMed/NCBI
|
|
132.
|
Darby IA and Hewitson TD: Fibroblast
differentiation in wound healing and fibrosis. Int Rev Cytol.
257:143–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133.
|
Meyer-ter-Vehn T, Sieprath S, Katzenberger
B, Gebhardt S, Grehn F and Schlunck G: Contractility as a
prerequisite for TGF-β-induced myofibroblast transdifferentiation
in human tenon fibroblasts. Invest Ophthalmol Vis Sci.
47:4895–4904. 2006.
|
|
134.
|
Miller MH, Grierson I, Unger WI and
Hitchings RA: Wound healing in an animal model of glaucoma
fistulizing surgery in the rabbit. Ophthalmic Surg. 20:350–357.
1989.PubMed/NCBI
|
|
135.
|
Desmouliere A, Geinoz A, Gabbiani F and
Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth
muscle actin expression in granulation tissue myofibroblasts and in
quiescent and growing cultured fibroblasts. J Cell Biol.
122:103–111. 1993. View Article : Google Scholar
|
|
136.
|
Watsky MA: Lysophosphatidic acid, serum,
and hyposmolarity activate Cl− currents in corneal keratocytes. Am
J Physiol. 269:C1385–C1393. 1995.PubMed/NCBI
|
|
137.
|
Cordeiro MF, Reichel MB, Gay JA,
D’Esposita F, Alexander RA and Khaw PT: Transforming growth
factor-beta1, -beta2, and -beta3 in vivo: effects on normal and
mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis
Sci. 40:1975–1982. 1999.PubMed/NCBI
|
|
138.
|
Cordeiro MF, Chang L, Lim KS, Daniels JT,
Pleass RD, Siriwardena D and Khaw PT: Modulating conjunctival wound
healing. Eye. 14:536–547. 2000. View Article : Google Scholar
|
|
139.
|
Wong TT, Mead AL and Khaw PT: Matrix
metalloproteinase inhibition modulates postoperative scarring after
experimental glaucoma filtration surgery. Invest Ophthalmol Vis
Sci. 44:1097–1103. 2003. View Article : Google Scholar
|
|
140.
|
Itoh K, Yoshioka K, Akedo H, Uehata M,
Ishizaki T and Narumiya S: An essential part for Rho-associated
kinase in the transcellular invasion of tumor cells. Nat Med.
5:221–225. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
141.
|
Harvey SA, Anderson SC and SundarRaj N:
Downstream effects of ROCK signaling in cultured human corneal
stromal cells: microarray analysis of gene expression. Invest
Ophthalmol Vis Sci. 45:2168–2176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
142.
|
Yoshizaki H, Ohba Y, Parrini MC,
Dulyaninova NG, Bresnick AR, Mochizuki N and Matsuda M: Cell
type-specific regulation of RhoA activity during cytokinesis. J
Biol Chem. 279:44756–44762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
143.
|
Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani
G, Martin MD, Haaksma CJ and Hinz B: Contraction of myofibroblasts
in granulation tissue is dependent on Rho/Rho kinase/myosin light
chain phosphatase activity. Wound Repair Regen. 14:313–320. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
144.
|
Parizi M, Howard EW and Tomasek JJ:
Regulation of LPA-promoted myofibroblast contraction: role of Rho,
myosin light chain kinase, and myosin light chain phosphatase. Exp
Cell Res. 254:210–220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
145.
|
Tangkijvanich P, Melton AC, Santiskulvong
C and Yee HF Jr: Rho and p38 MAP kinase signaling pathways mediate
LPA-stimulated hepatic myofibroblast migration. J Biomed Sci.
10:352–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
146.
|
Honjo M, Tanihara H, Kameda T, Kawaji T,
Yoshimura N and Araie M: Potential role of Rho-associated protein
kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest
Ophthalmol Vis Sci. 48:5549–5557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
147.
|
Lukas TJ, Miao H, Chen L, Riordan SM, Li
W, Crabb AM, Wise A, Du P, Lin SM and Hernandez MR: Susceptibility
to glaucoma: differential comparison of the astrocyte transcriptome
from glaucomatous African American and Caucasian American donors.
Genome Biol. 9:R1112008. View Article : Google Scholar : PubMed/NCBI
|
|
148.
|
Hathaway DR, March KL, Lash JA, Adam LP
and Wilensky RL: Vascular smooth muscle. A review of the molecular
basis of contractility. Circulation. 83:382–390. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
149.
|
Savineau JP and Marthan R: Modulation of
the calcium sensitivity of the smooth muscle contractile apparatus:
molecular mechanisms, pharmacological and pathophysiological
implications. Fundam Clin Pharmacol. 11:289–299. 1997. View Article : Google Scholar
|
|
150.
|
Mita M, Yanagihara H, Hishinuma S, Saito M
and Walsh MP: Membrane depolarization-induced contraction of rat
caudal arterial smooth muscle involves Rho-associated kinase.
Biochem J. 364:431–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
151.
|
Sward K, Mita M, Wilson DP, Deng JT,
Susnjar M and Walsh MP: The role of RhoA and Rho-associated kinase
in vascular smooth muscle contraction. Curr Hypertens Rep. 5:66–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
152.
|
Kandabashi T, Shimokawa H, Miyata K,
Kunihiro I, Kawano Y, Fukata Y, Higo T, Egashira K, Takahashi S,
Kaibuchi K and Takeshita A: Inhibition of myosin phosphatase by
upregulated rho-kinase plays a key role for coronary artery spasm
in a porcine model with interleukin-1beta. Circulation.
101:1319–1323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
153.
|
Sato M, Tani E, Fujikawa H and Kaibuchi K:
Involvement of Rho-kinase-mediated phosphorylation of myosin light
chain in enhancement of cerebral vasospasm. Circ Res. 87:195–200.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
154.
|
Iizuka K, Shimizu Y, Tsukagoshi H, Yoshii
A, Harada T, Dobashi K, Murozono T, Nakazawa T and Mori M:
Evaluation of Y-27632, a rho-kinase inhibitor, as a bronchodilator
in guinea pigs. Eur J Pharmacol. 406:273–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
155.
|
Chitaley K, Wingard CJ, Clinton Webb R,
Branam H, Stopper VS, Lewis RW and Mills TM: Antagonism of
Rho-kinase stimulates rat penile erection via a nitric
oxide-independent pathway. Nat Med. 7:119–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
156.
|
Takahashi R, Nishimura J, Hirano K, Seki
N, Naito S and Kanaide H: Ca2+ sensitization in
contraction of human bladder smooth muscle. J Urol. 172:748–752.
2004.
|
|
157.
|
Schubert R, Kalentchuk VU and Krien U: Rho
kinase inhibition partly weakens myogenic reactivity in rat small
arteries by changing calcium sensitivity. Am J Physiol Heart Circ
Physiol. 283:H2288–H2295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
158.
|
Cavarape A, Bauer J, Bartoli E, Endlich K
and Parekh N: Effects of angiotensin II, arginine vasopressin and
tromboxane A2 in renal vascular bed: role of rho-kinase. Nephrol
Dial Transplant. 18:1764–1769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
159.
|
Cavarape A, Endlich N, Assaloni R, Bartoli
E, Steinhausen M, Parekh N and Endlich K: Rho-kinase inhibition
blunts renal vasoconstriction induced by distinct signaling
pathways in vivo. J Am Soc Nephrol. 14:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
160.
|
Randriamboavonjy V, Busse R and Fleming I:
20-HETE-induced contraction of small coronary arteries depends on
the activation of Rho-kinase. Hypertension. 41:801–806. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
161.
|
Watabe H, Abe S and Yoshitomi T: Effects
of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on
isolated rabbit ciliary arteries. Jpn J Ophthalmol. 55:411–417.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
162.
|
Tokushige H: ROCK inhibitor and glaucoma.
Bio Clinica. 17:1191–1194. 2002.
|
|
163.
|
Sugiyama T, Shibata M, Kajiura S, Okuno T,
Tonari M, Oku H and Ikeda T: Effects of fasudil, a Rho-associated
protein kinase inhibitor, on optic nerve head blood flow in
rabbits. Invest Ophthalmol Vis Sci. 52:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
164.
|
Okamura N, Saito M, Mori A, Sakamoto K,
Kametaka S, Nakahara T and Ishii K: Vasodilator effects of fasudil,
a Rho-kinase inhibitor, on retinal arterioles in stroke-prone
spontaneously hypertensive rats. J Ocul Pharmacol Ther. 23:207–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
165.
|
Wolfrum S, Dendorfer A, Rikitake Y,
Stalker TJ, Gong Y, Scalia R, Dominiak P and Liao JK: Inhibition of
Rho-kinase leads to rapid activation of phosphatidylinositol
3-kinase/protein kinase Akt and cardiovascular protection.
Arterioscler Thromb Vasc Biol. 24:1842–1847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
166.
|
Anderson DR: Introductory comments on
blood flow auto-regulation in the optic nerve head and vascular
risk factors in glaucoma. Surv Ophthalmol. 43:S5–S9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
167.
|
Chung HS, Harris A, Evans DW, Kagemann L,
Garzozi HJ and Martin B: Vascular aspects in the pathophysiology of
glaucomatous optic neuropathy. Surv Ophthalmol. 43:S43–S50. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
168.
|
Flammer J, Orgul S, Costa VP, Orzalesi N,
Krieglstein GK, Serra LM, Renard JP and Stefansson E: The impact of
ocular blood flow in glaucoma. Prog Retin Eye Res. 21:359–393.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
169.
|
Ben Simon GJ, Moroz I, Goldenfeld M and
Melamed S: Scanning laser Doppler flowmetry of nonperfused regions
of the optic nerve head in patients with glaucoma. Ophthalmic Surg
Lasers Imaging. 34:245–250. 2003.PubMed/NCBI
|
|
170.
|
Hu Y, Cui Q and Harvey AR: Interactive
effects of C3, cyclic AMP and ciliaryneurotrophic factor on adult
retinal ganglion cell survival and axonal regeneration. Mol Cell
Neurosci. 34:88–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
171.
|
Kitaoka Y, Kitaoka Y, Kumai T, Lam TT,
Kuribayashi K, Isenoumi K, Munemasa Y, Motoki M, Kobayashi S and
Ueno S: Involvement of RhoA and possible neuroprotective effect of
fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in
the rat retina. Brain Res. 1018:111–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
172.
|
Bertrand J, Di Polo A and McKerracher L:
Enhanced survival and regeneration of axotomized retinal neurons by
repeated delivery of cell-permeable C3-like Rho antagonists.
Neurobiol Dis. 25:65–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
173.
|
Sheikh AM, Nagai A, Ryu JK, McLarnon JG,
Kim SU and Masuda J: Lysophosphatidylcholine induces glial cell
activation: role of rho kinase. Glia. 57:898–907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
174.
|
Buchi ER: Cell death in the rat retina
after a pressure-induced ischaemia-reperfusion insult: an electron
microscopic study. I Ganglion cell layer and inner nuclear layer.
Exp Eye Res. 55:605–613. 1992. View Article : Google Scholar
|
|
175.
|
Rosenbaum DM, Rosenbaum PS, Gupta A,
Michaelson MD, Hall DH and Kessler JA: Retinal ischemia leads to
apoptosis which is ameliorated by aurintricarboxylic acid. Vision
Res. 37:3445–3451. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
176.
|
Hirooka K, Miyamoto O, Jinming P, Du Y,
Itano T, Baba T, Tokuda M and Shiraga F: Neuroprotective effects of
D-allose against retinal ischemia-reperfusion injury. Invest
Ophthalmol Vis Sci. 47:1653–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
177.
|
Millán J and Ridley AJ: Free in PMC Rho
GTPases and leucocyte-induced endothelial remodelling. Biochem J.
385:329–337. 2005.PubMed/NCBI
|
|
178.
|
Wittchen ES, van Buul JD, Burridge K and
Worthylake RA: Trading spaces: Rap, Rac, and Rho as architects of
transendothelial migration. Curr Opin Hematol. 12:14–21. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
179.
|
Song H and Gao D: Fasudil, a
Rho-associated protein kinase inhibitor, attenuates retinal
ischemia and reperfusion injury in rats. Int J Mol Med. 28:193–198.
2011.PubMed/NCBI
|
|
180.
|
Tsujikawa A, Ogura Y, Hiroshiba N,
Miyamoto K, Kiryu J, Tojo SJ, Miyasaka M and Honda Y: Retinal
ischemia-reperfusion injury attenuated by blocking of adhesion
molecules of vascular endothelium. Invest Ophthalmol Vis Sci.
40:1183–1190. 1999.PubMed/NCBI
|
|
181.
|
Hirata A, Inatani M, Inomata Y, Yonemura
N, Kawaji T, Honjo M and Tanihara H: Y-27632, a Rho-associated
protein kinase inhibitor, attenuates neuronal cell death after
transient retinal ischemia. Graefes Arch Clin Exp Ophthalmol.
246:51–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
182.
|
Lingor P, Tönges L, Pieper N, Bermel C,
Barski E, Planchamp V and Bähr M: ROCK inhibition and CNTF interact
on intrinsic signalling pathways and differentially regulate
survival and regeneration in retinal ganglion cells. Brain.
131:250–263. 2008.PubMed/NCBI
|
|
183.
|
Tura A, Schuettauf F, Monnier PP,
Bartz-Schmidt KU and Henke-Fahle S: Efficacy of Rho-kinase
inhibition in promoting cell survival and reducing reactive gliosis
in the rodent retina. Invest Ophthalmol Vis Sci. 50:452–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
184.
|
Fournier AE, GrandPre T, Gould G, Wang X
and Strittmatter SM: Nogo and the Nogo-66 receptor. Prog Brain Res.
137:361–369. 2002. View Article : Google Scholar
|
|
185.
|
Hunt D, Coffin RS and Anderson PN: The
Nogo receptor, its ligands and axonal regeneration in the spinal
cord; a review. J Neurocytol. 31:93–120. 2002. View Article : Google Scholar
|
|
186.
|
McKerracher L and Winton MJ: Nogo on the
go. Neuron. 36:345–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
187.
|
Sandvig A, Berry M, Barrett LB, Butt A and
Logan A: Myelin-, reactive glia-, and scar-derived CNS axon growth
inhibitors: expression, receptor signaling, and correlation with
axon regeneration. Glia. 46:225–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
188.
|
Fawcett JW: Overcoming inhibition in the
damaged spinal cord. J Neurotrauma. 23:371–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
189.
|
Du J, Fu C and Sretavan DW: Eph/ephrin
signaling as a potential therapeutic target after central nervous
system injury. Curr Pharm Des. 13:2507–2518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
190.
|
Fabes J, Anderson P, Brennan C and Bolsove
S: Regeneration-enhancing effects of EphA4 blocking peptide
following corticospinal tract injury in adult rat spinal cord. Eur
J Neurosci. 26:2496–2505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
191.
|
Hou ST, Jiang SX and Smith RA: Permissive
and repulsive cues and signalling pathways of axonal outgrowth and
regeneration. Int Rev Cell Molec Biol. 267:125–181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
192.
|
Berry M, Ahmed Z, Lorber B, Douglas M and
Logan A: Regeneration of axons in the visual system. Restor Neurol
Neurosci. 26:147–174. 2008.PubMed/NCBI
|
|
193.
|
Bertrand J, Winton MJ, Rodriguez-Hernandez
N, Campenot RB and McKerracher L: Application of Rho antagonist to
neuronal cell bodies promotes neurite growth in compartmented
cultures and regeneration of retinal ganglion cell axons in the
optic nerve of adult rats. J Neurosci. 25:1113–1121. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
194.
|
Sagawa H, Terasaki H, Nakamura M, Ichikawa
M, Yata T, Tokita Y and Watanabe M: A novel ROCK inhibitor,
Y-39983, promotes regeneration of crushed axons of retinal ganglion
cells into the optic nerve of adult cats. Exp Neurol. 205:230–240.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
195.
|
Monnier PP, Sierra A, Schwab JM,
Henke-Fahle S and Mueller BK: The Rho/ROCK pathway mediates neurite
growth-inhibitory activity associated with the chondroitin sulfate
proteoglycans of the CNS glial scar. Mol Cell Neurosci. 22:319–330.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
196.
|
Lingor P, Teusch N, Schwarz K, Mueller R,
Mack H, Bahr M and Mueller BK: Inhibition of Rho kinase (ROCK)
increases neurite outgrowth on chondroitin sulphate proteoglycan in
vitro and axonal regeneration in the adult optic nerve in vivo. J
Neurochem. 103:181–189. 2007.PubMed/NCBI
|
|
197.
|
Ahmed Z, Berry M and Logan A: ROCK
inhibition promotes adult retinal ganglion cell neurite outgrowth
only in the presence of growth promoting factors. Mol Cell
Neurosci. 42:128–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
198.
|
Watanabe M: Regeneration of optic nerve
fibers of adult mammals. Dev Growth Differ. 52:567–576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
199.
|
Tan H, Zhong Y, Shen X, Cheng Y, Jiao Q
and Deng L: Erythropoietin promotes axonal regeneration after optic
nerve crush in vivo by inhibition of RhoA/ROCK signaling pathway.
Neuropharmacology. 63:1182–1190. 2012. View Article : Google Scholar : PubMed/NCBI
|