Introduction
Colorectal cancer is the third most common cancer in
men (10.0% of the total, ∼663,000 cases) and the second in women
(9.4% of the total, ∼570,000 cases) worldwide (1). Approximately 608,000 colorectal
cancer deaths were estimated worldwide, accounting for 8% of all
cancer deaths, making it the fourth most common cause of death from
cancer. The traditional treatment of colorectal cancer is generally
drugs, radiotherapy and chemotherapy, but the effect of these
methods are not satisfactory, the mortality rate of colorectal
cancer still remains high. The study of Center et al
(2) indicated that the mortality
of colorectal cancer had also been increasing because of tumor
relapse and metastasis. However, carcinogenesis is a complicated
biological process, and the molecular mechanisms, metastasis
phenotype, pathways and regulating genes are not well known
(3). Therefore, better
understanding of molecular mechanisms underlying proliferation,
invasion and survival of colorectal cancer are critical for the
development of optimal therapeutic modalities.
CD147 (also called EMMPRIN, basigin, tumor cell
derived collagenase stimulatory factor, or human leukocyte
activation-associated M6 antigen), is a 43-66-kDa multifunctional
glycosylated transmembrane protein, which belongs to the
immunoglobulin superfamily (4–7). The
protein of CD147 is highly expressed on the cell surface of many
tumor cells such as, oral, breast, lung, bladder, kidney,
laryngeal, pancreatic, gastric, colorectal cancer, glioma, lymphoma
and melanoma (8–13), and was correlated with tumor
progression and invasion (14,15)
and could also stimulate tumor cells to produce matrix
metalloproteinases (MMPs), a family of zinc-dependent
endopeptidases including >25 members, specifically MMP-2 and
MMP-9 (5,16). The MMPs are one of the important
factors of tumor invasion and metastasis (17). Previous studies showed that CD147
could promote the generation of a MMP complex by endothelial cells,
which modified the tumor cell pericellular matrix concentrating at
tumor cell surface to promote tumor cell invasion (18,19).
In addition, CD147 was also able to influence lactate transport and
glycolysis by its association with lactate transporters
monocarboxylat transporter (MCT), specifically MCT-1 and MCT-4
(20). MCT-1 and MCT-4 are two
members of the proton-linked monocarboxylate (lactate) transporter
family, playing a fundamental role in metabolism. CD147 is
essential in transporting MCT1 and MCT4 to plasma membrane
(21). CD147 was also involved in
multidrug resistance of cancer cells via hyaluronan-mediated
activation of ErbB2 signaling and survival pathway activity, and
multidrug resistance and tumor invasiveness might be linked during
the progression of malignant disease (22,23),
but the function and mechanism of CD147 remain elusive on
proliferation, invasion, metastasis and multidrug resistance of
colorectal cancer.
The studies of Zhu et al (12) showed that CD147 expression was high
in colorectal cancer and and was associated with the colorectal
development, and with poor prognosis. The molecular mechanisms
involved and the role of CD147 in colorectal cancer, however,
remained poorly understood. To determine the role of CD147 in
invasiveness, metastasis, growth and survival of colorectal cancer,
we used RNA interference (RNAi) technique to knock down the
expression of CD147 in HT29 cells, and investigated its roles on
proliferation, invasion and the chemosensitivity of colorectal
cancer cells.
Materials and methods
Cell culture
HT29 cells, a human colorectal cancer cell line, was
provided from the Shanghai Cell Collection, the Chinese Academy of
Sciences. Cells were maintained in DMEM (Gibco BRL, Grand Island,
NY, USA) with 10% fetal bovine serum (FBS), 100 U/ml of penicillin
and 100 g/ml of streptomycin (Gibco BRL) in a 5% CO2
humidified atmosphere at 37°C.
Design of pYr-mir30-shRNA plasmid
construction
The vector pYr-mir30-shRNA was used to generate
short hairpin RNA (shRNA) specific for CD147 by selecting the
808-828 fragment as the RNAi target site, and the scrambled control
sequence was also synthesized as shown in Table I. These oligonucleotides were
annealed and subcloned into the BsaI sites of the vector. These
recombinant vectors were designated as pYr-mir30-shRNA-control and
pYr-mir30-shRNA, respectively. The vector of pYr-mir30-shRNA
included the EGFP gene sequence, so the EGFP protein expression can
reflect the CD147 protein expression. All the cloned genes were
confirmed by DNA sequencing.
 | Table I.Sequences of the designed CD147
specific shRNAs. |
Table I.
Sequences of the designed CD147
specific shRNAs.
| shRNAs | Sequence |
|---|
| shRNA-control |
5′-GATCCACTACCGTTGTTATAGGTGTTCAAGAGA |
|
CACCTATAACAACGGTAGTTTTTTTGGAAA-3′ |
| shRNA-control |
5′-AGCTTTTCCAAAAAAACTACCGTTGTTATAGGT |
|
GTCTCTTGAACACCTATAACAACGGTAGTG-3′ |
| shRNA |
5′-GATCCGTGACAAAGGCAAGAACGTCTTCAAGA |
|
GAGACGTTCTTGCCTTTGTCATTTTTTGGAAA-3′ |
| shRNA |
5′-AGCTTTTCCAAAAAATGACAAAGGCAAGAACG |
|
TCTCTCTTGAAGACGTTCTTGCCTTTGTCACG-3′ |
Transient and transfection screening
HT29 cells were plated in 6-well plates at a density
of 3×105 cells per well and incubated in 2 ml of growth
medium without antibiotics. When the cells reached 80% confluence
after 24-h incubation, cells were transferred with
pYr-mir30-CD147-shRNA-control and pYr-mir30-CD147-shRNA,
respectively, using Lipofectamine 2000 (Invitrogen-Life
Technologies, Carlsbad, CA, USA) according to the manufacturer’s
instructions. Forty-eight hours after transfection, HT29 cells were
diluted 1:10 for passage and neomycin resistance clones were
selected in the medium containing 600 μg/ml G418 (Gibco-BRL)
for 2 weeks. The positive clones were picked and expanded to
establish cell lines in 300 μg/ml G418. The stable
transfection cell clones, designated as HT29/shRNA-control,
HT29/shRNA, were verified by quantitative real-time RT-PCR and
western blot analysis.
Quantitative real-time PCR assay
Total cellular RNA was extracted using TRIzol
reagent (Invitrogen), according to the manufacturer’s instructions
and reverse transcribed into cDNA using PrimeScript RT reagent kit
Perfect Real (Takara). First the cDNA was quantified in a 1:10
dilution on a spectrophotometer. CD147 mRNA expression was
evaluated by RT-PCR on an ABI PRISM 7500 real-time PCR apparatus
(Applied Biosystems, USA) with SYBR Premix Ex TaqTM II.
The primer sequences used for CD147, MCT1, MCT4 and β-actin are
listed in Table II. The conditions
for real-time PCR were: 95°C for 30 sec, then 40 cycles at 95°C for
5 sec, and 60°C for 34 sec. The mRNA level for CD147 of each sample
was normalized to Ct values of the β-actin amplified from the same
sample, ΔCt=CtCD147 − Ctβ-actin and the
2−ΔΔCt method was used to calculate gene expression
change. Samples were measured in triplicates to ensure the
reproducibility of the results.
 | Table II.Primers of CD147, MCT1, MCT4 and
β-actin for real-time PCR. |
Table II.
Primers of CD147, MCT1, MCT4 and
β-actin for real-time PCR.
| Target | Primers |
|---|
| CD147 | Sense: |
5′-CCATGCTGGTCTGCAAGTCAG-3′ |
| Antisense: |
5′-CCGTTCATGAGGGCCTTGTC-3′ |
| MCT1 | Sense: |
5′-CACTTAAAATGCCACCAGCA-3′ |
| Antisense: |
5′-AGAGAAGCCGATGGAAATGA-3′ |
| MCT4 | Sense: |
5′-GTTGGGTTTGGCACTCAACT-3′ |
| Antisense: |
5′-GAAGACAGGGCTACCTGCTG-3′ |
| β-actin | Sense: |
5′-CTGGAACGGTGAAGGTGACA-3′ |
| Antisense: |
5′-AAGGGACTTCCTGTAACAACGCA-3′ |
Western blot analysis
Western blot analysis was performed to evaluate
CD147, MCT1 and MCT4 protein levels. The cultured tumor cells were
washed three times with ice-cold PBS, then the cells were suspended
in lysis buffer [150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1 mM
MgCl2, 100 μg/ml PMSF, 1.0% Triton X-100] on ice
for 30 min. Cell lysates were then collected after centrifugation
at 12,000 rpm for 5 min at 4°C. Equal amounts (30 μg) of
lysate proteins were separated on 10% SDS-PADE gels, and
transferred to a polyvinylidene difluoride (PVDF) membrane. After
blocking with 5% non-fat dry milk in TBST buffer (10 mM Tris-HCl,
pH 7.5, 150 mM NaCl, and 0.05% Tween-20) for 2 h at room
temperature, the membrane was probed with mouse anti-CD147 primary
antibodies (1:500), rabbit anti-MCT1 primary antibodies (1:500),
rabbit anti-MCT4 primary antibodies (1:500) and rabbit anti-human
β-actin primary antibodies (1:500) incubated at room temperature
for 2 h, followed by incubation in a 1:2,000 dilution of secondary
antibodies conjugated to horseradish peroxidase (Santa Cruz
Biotechnology, Santa Cruz, CA, USA) for 1 h at room temperature.
Protein bands were detected using ECL detection system (Boster,
Wuhan, China). Western blot experiments were performed at least
three times.
Gelatin zymography assay
Cells were cultured in serum-free DMEM medium for 24
h, and then harvested in conditioned medium. The gelatinolytic
activity of MMP-2 and MMP-9 in the conditioned medium was assayed
by electrophoresis on 10% polyacrylamide gels containing 1 mg/ml of
gelatin. PAGE gels were run at 100 V in stacking gels, and 100 mA
in separating gels, washed in 2.5% Triton X-100 twice every 40 min,
and then incubated for 16 h at 37°C in activation buffer (50 mM
Tris-HCl, pH 7.6, 5 mM CaCl2, 0.02% Brij-35). After
reaction, the gels were stained with Coomassie Brilliant Blue R-250
for 3 h and destained for 30 min in 20% methanol and 10% acetic
acid. White lysis zones indicating gelatin degradation were
revealed. This experiment was repeated at least three times.
Intracellular lactate concentration
assay
We used a lactic acid assay kit (KeyGen Biotech Co.,
Ltd., Nanjing, China) to assess the change of intracellular lactate
concentration in HT29 cells after CD147 silencing. Cells
(1×106) were harvested by centrifugation and were then
ruptured by hypotonic salt solution for 1 h at room temperature.
The supernatant was retained after centrifuging. The optical
density was read at 530 nm. This experiment was repeated at least
three times.
Cell proliferation assay
Cell proliferation in vitro was analyzed with
the formazan substrate,
2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5 (2,
4-disulfophenyl)-2H tetrazolium monosodium salt (WST-8). Cells were
plated in 96-well plates in 100 μl DMEM at a density of
1×104 cells per well. After 24, 48, 72, 96, 120 h of
culture, respectively, the medium was removed and replaced with
fresh 100 μl medium, then 10 μl of WST-8 was added to
each well and the plates were returned to standard tissue incubator
conditions for an additional 1.5 h. Colorimetric analysis was
performed at 450 nm wavelength on a micro plate reader. Each
analysis was done at least three times.
Invasion assay
Transwell plates (Corning Costar, Cambridge, MA,
USA) were coated with basement membrane Matrigel (20 mg/ml,
Becton-Dickinson, Franklin Lakes, NJ, USA) for 4 h at 37°C.
Serum-free DMEM containing 1×105 cells in 100 μl
was added into the upper chamber, the lower chamber received 500
μl of 10% FBS-containing medium and was incubated at 37°C
for 24 h. After 18 h, cells that migrated through the permeable
membrane were fixed with 100% methanol for 10 min. The membranes
with cells were soaked in 0.1% crystal violet for 10 min and then
washed with distilled water. The number of cells which attached to
the lower surface of the polycarbonate filter was counted at ×400
magnification under a light microscope. Results were expressed as
mean of triplicate experiments.
Drug sensitivity assay
To assess the antitumor drug chemo-sensitivity,
cells were seeded in triplicates on 96-well plates at
1×104 cells/well and incubated for 24 h. The medium was
then removed and added with fresh medium containing cisplatin,
paclitaxel, gemcitabine and oxaliplatin (Sigma) with varying
concentrations: 0.1, 1 and 10 μM. After 48 h, cells were
treated with MST-8 as described earlier. Spectrometric absorbance
at 450 nm was measured with a micro-plate reader. Each group was
repeated at least three times.
In vivo tumor progression assay
Tumor xenografts were established by subcutaneous
injection of 5×106 HT29, HT29/ shRNA-control, HT29/shRNA
cells into the right flank of 4–6-week-old female nude mice,
respectively. The experiments were approved by the Experimental
Animal Center of University of Yangzhou, Yangzhou, China. The size
of the transplanted tumors was measured every 5 days and the
average tumor volume was measured: volume = 1/2 × (length ×
width2). All animals were euthanized after 30 days
post-inoculation. Harvested tissues were fixed in 10% buffered
formalin, embedded in paraffin, sectioned at 4 μm, and
stained with H&E. Immunohistochemistry analysis used goat
anti-mouse CD147 polyclonal antibody (1:50 dilution, Santa Cruz
Biotechnology) to detect CD147 protein expression. Animal
experiments were performed in accordance with institutional
guidelines for animal care by Nanjing Medical University.
Statistical analysis
Statistical analysis was performed by the SPSS
software. Each assay was conducted at least three times. All
experimental data were expressed as the mean ± SD and assessed by
Student’s t-tests and one-way ANOVA at a significance level of
p<0.05.
Results
Selection of pYr-mir30-shRNA stable
expression transfectants
In our study, we established two vectors, the
pYr-mir30-shRNA-control and the pYr-mir30-shRNA. After 48 h of
transfect, the transfect cells were treated with 600 μg/ ml
G418 selection. After two weeks, the stable expression of
HT29/shRNA and HT29/shRNA-control cells were obtained. The EGFP
expression could be clearly observed as shown in Fig. 1.
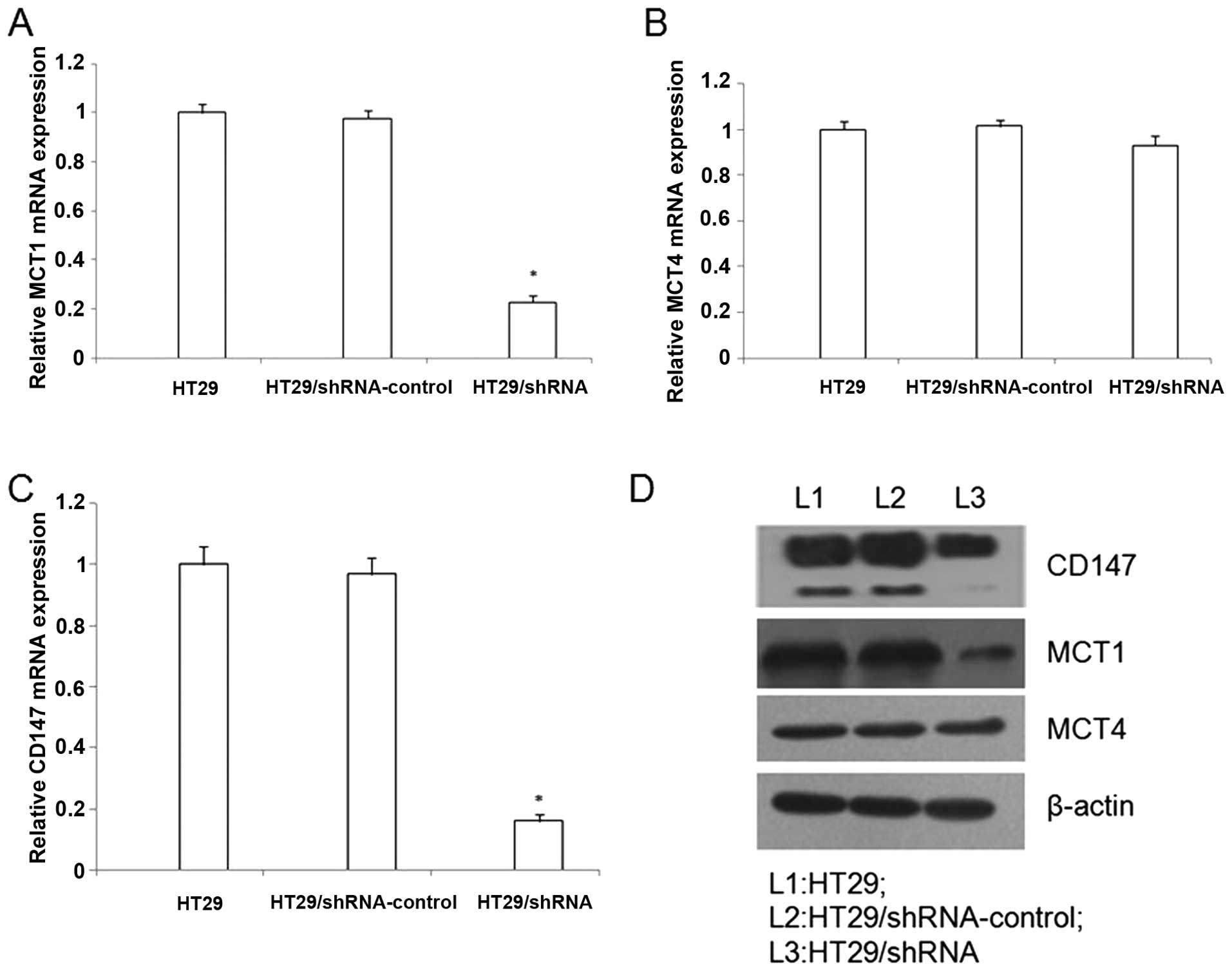
The pYr-mir30-shRNA-mediated gene
silencing inhibits CD147 expression in HT29 cells
To evaluate the expression of CD147 in HT29 cells,
we used the real-time RT-PCR (RT-PCR) and western blotting. The
gene of β-actin was selected as the internal gene. A significantly
reduced CD147 mRNA level for HT29/shRNA was achieved compared with
untreated HT29 cells, respectively (p<0.01) (Fig. 2). In addition, western blot
analysis confirmed the downregulation of CD147 protein by the
HT29/shRNA (Fig. 2).
The CD147 silencing inhibits MCT1 and
MCT4 expression in HT29 cells
Many studies have confirmed the expression of MCT1
and MCT4 are closely associated with CD147 in various cancers. To
detect whether the CD147 silencing could reduce the expression of
MCT1 and MCT4. We performed real-time RT-PCR and western blotting.
The real-time RT-PCR analysis, contrasted with the
HT29/shRNA-control, the HT29/shRNA mRNA expression of MCT1 was
down-regulated (p<0.01), but the MCT4 mRNA expression did not
significantly change in HT29 cells (p>0.05) (Fig. 2). In addition, western blot
analysis demonstrated that the MCT1 protein was downregulation by
HT29/shRNA (p<0.01), but the MCT4 protein did not significantly
alter in HT29 cells (p>0.05) (Fig.
2).
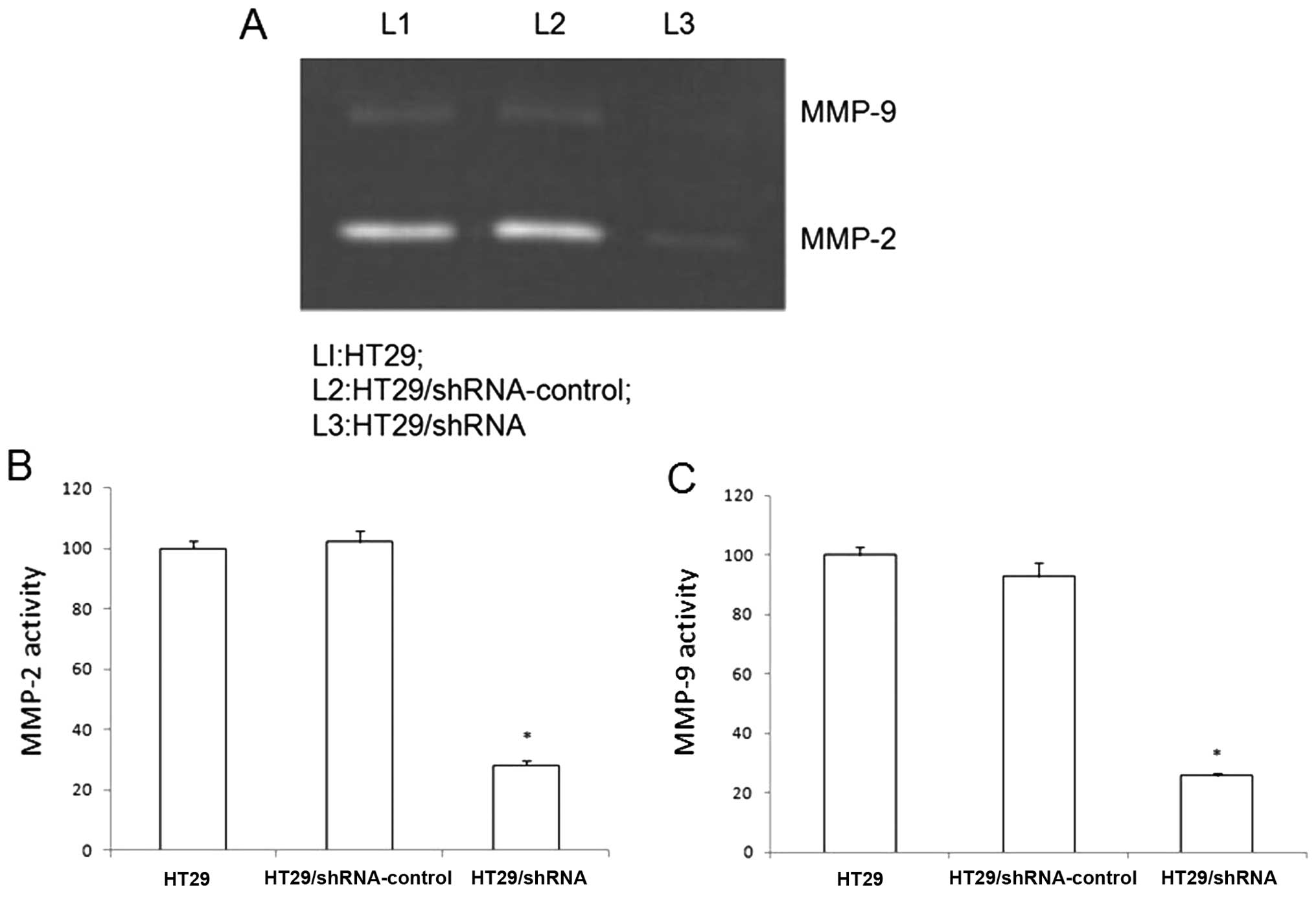
The CD147 silencing reduces the activity
of MMP-2 and MMP-9 in HT29 cells
CD147 contributed to tumor invasion and metastasis
by stimulating fibroblasts matrix metalloproteinase production. We
used gelatin zymography to investigate the effect of CD147
silencing on HT29 cells reducing the activity of MMP-2 and MMP-9.
As shown in Fig. 3, the secretion
levels of MMP-2 and MMP-9 in the HT29/shRNA cells show significant
difference in the HT29 cells (p<0.01). There was no significant
difference between HT29 and HT29/ shRNA-control (p>0.05).
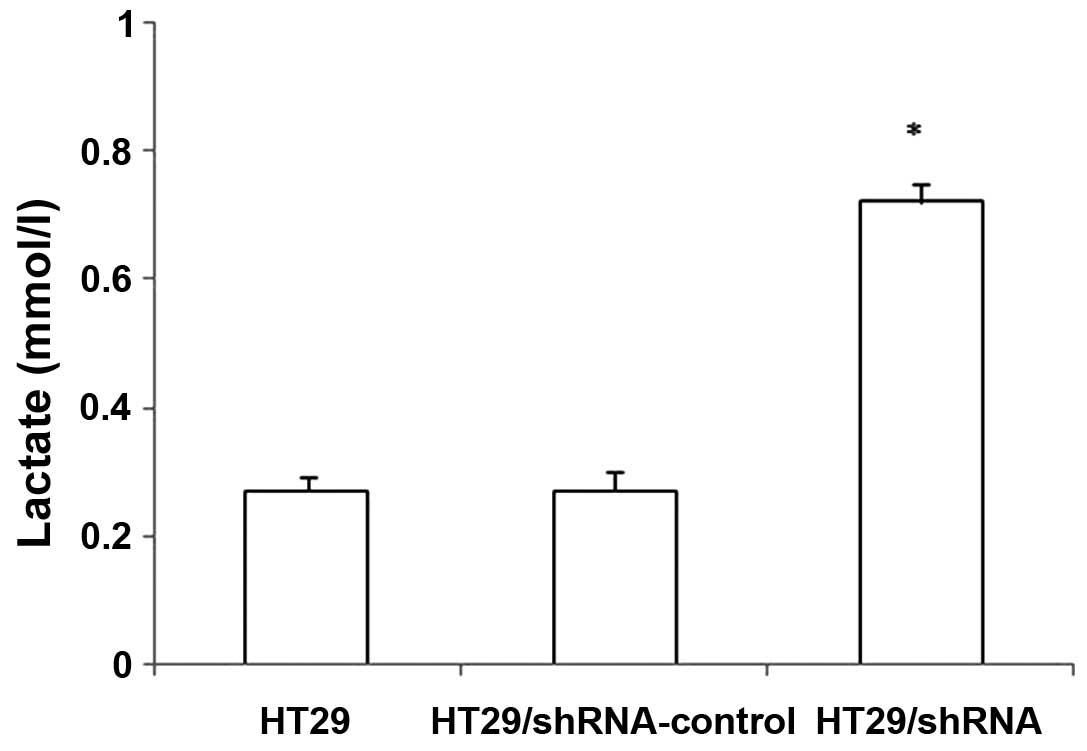
The CD147 silencing inhibits the function
of lactate transporters in HT29 cells
We examined whether CD147 silencing inhibited the
function of lactate transporters used in the lactic acid assay. As
shown in Fig. 4, the intracellular
lactate concentration of the HT29 cells were increasing after CD147
silencing. There was no significant difference between
HT29/shRNA-control and HT29 cells (p>0.05). These results
confirmed that the downregulation of MCT1 expression by HT29/shRNA
inhibits the function of these transporters in HT29 cells. Thus,
the decrease of MCT1 expression is associated with an increase in
intracellular lactate concentration.
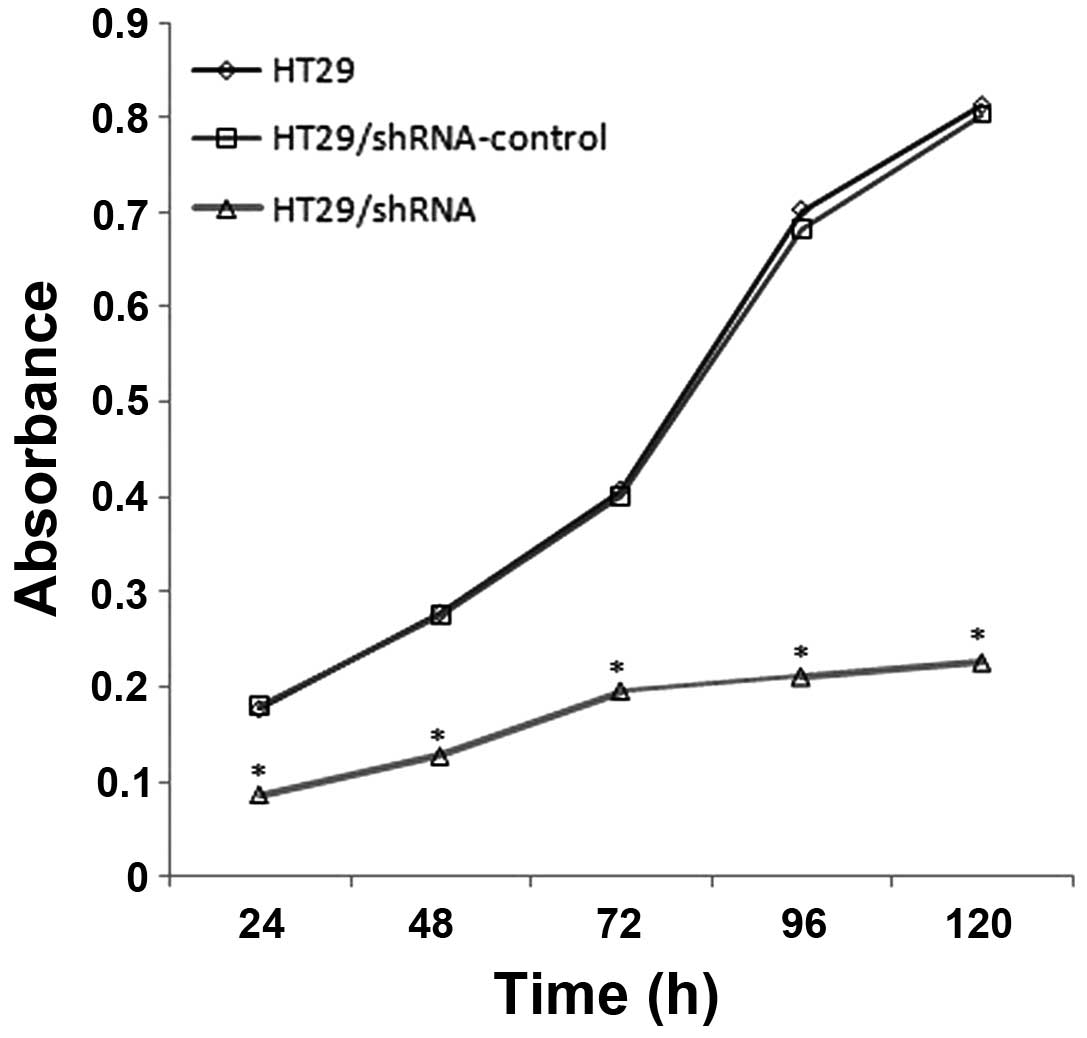
The CD147 silencing reduces the
proliferation of HT29 cells
In order to examine whether the CD147 silencing
affects cell proliferation, we used the WST-8 assay to determine
the proliferation of HT29, HT29/shRNA-control and HT29/ shRNA,
respectively. The results (Fig.
5), compared with HT29, show the proliferation of HT29/shRNA
was inhabited to 51.03 (p<0.01), 54.26 (p<0.01), 51.92
(p<0.01), 69.94 (p<0.01) and 72.24% (p<0.01) at 24, 48,
72, 96 and 120 h, respectively. There was no significant difference
between HT29 and HT29/ shRNA-control (p>0.05).
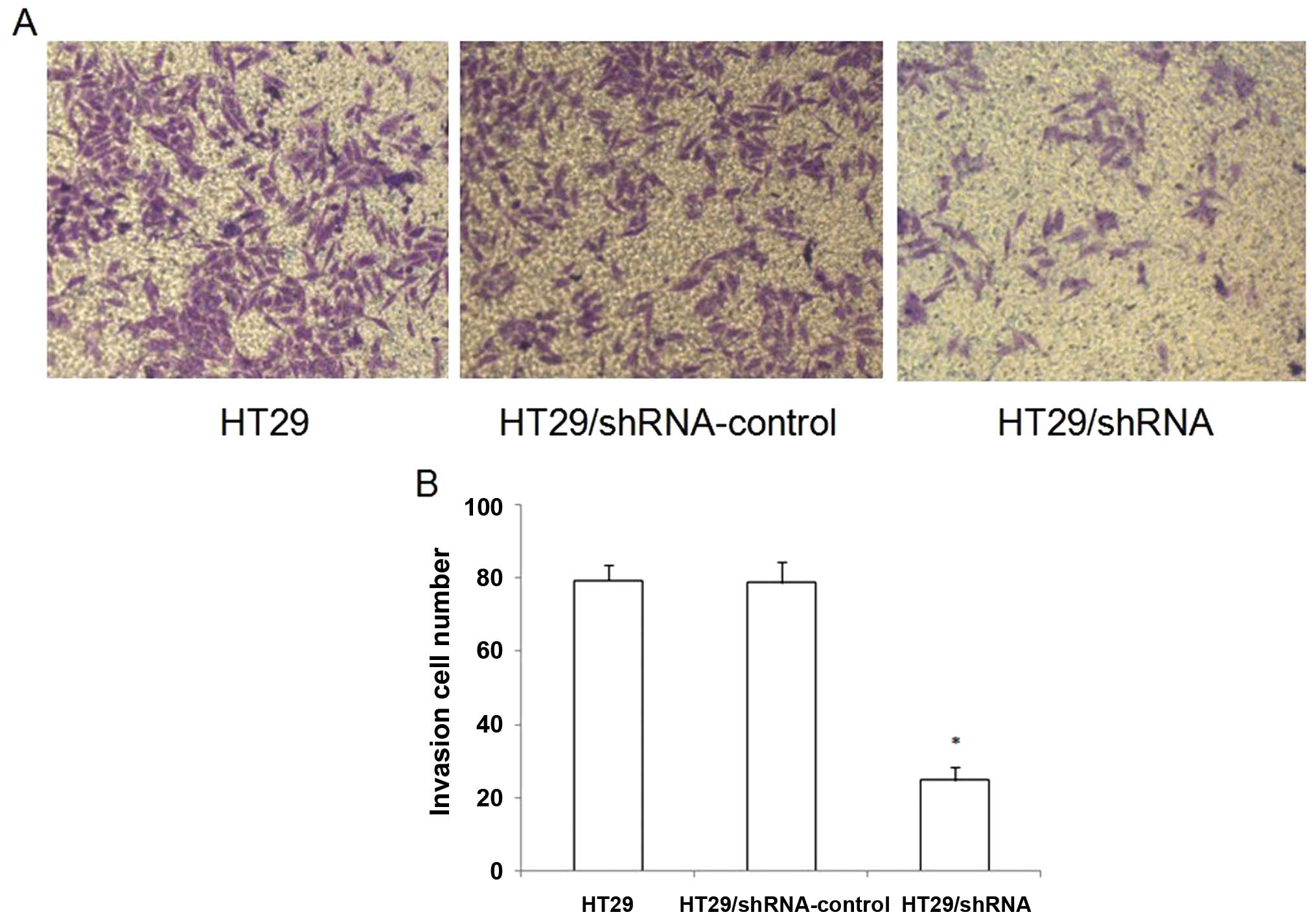
CD147 silencing reduces the invasive
ability of HT29 cells in vitro
To examine whether the downregulation of CD147 in
HT29 cells affected its invasive ability, we performed a Matrigel
Transwell analysis in vitro. The results showed that HT29
and HT29/shRNA-control cells had a similar ability to pass through
the Matrigel coated filter (Fig.
6). The number of HT29/shRNA cells passing through the Matrigel
was markedly lower than the numbers of HT29 and HT29/ shRNA-control
cells (p<0.05).
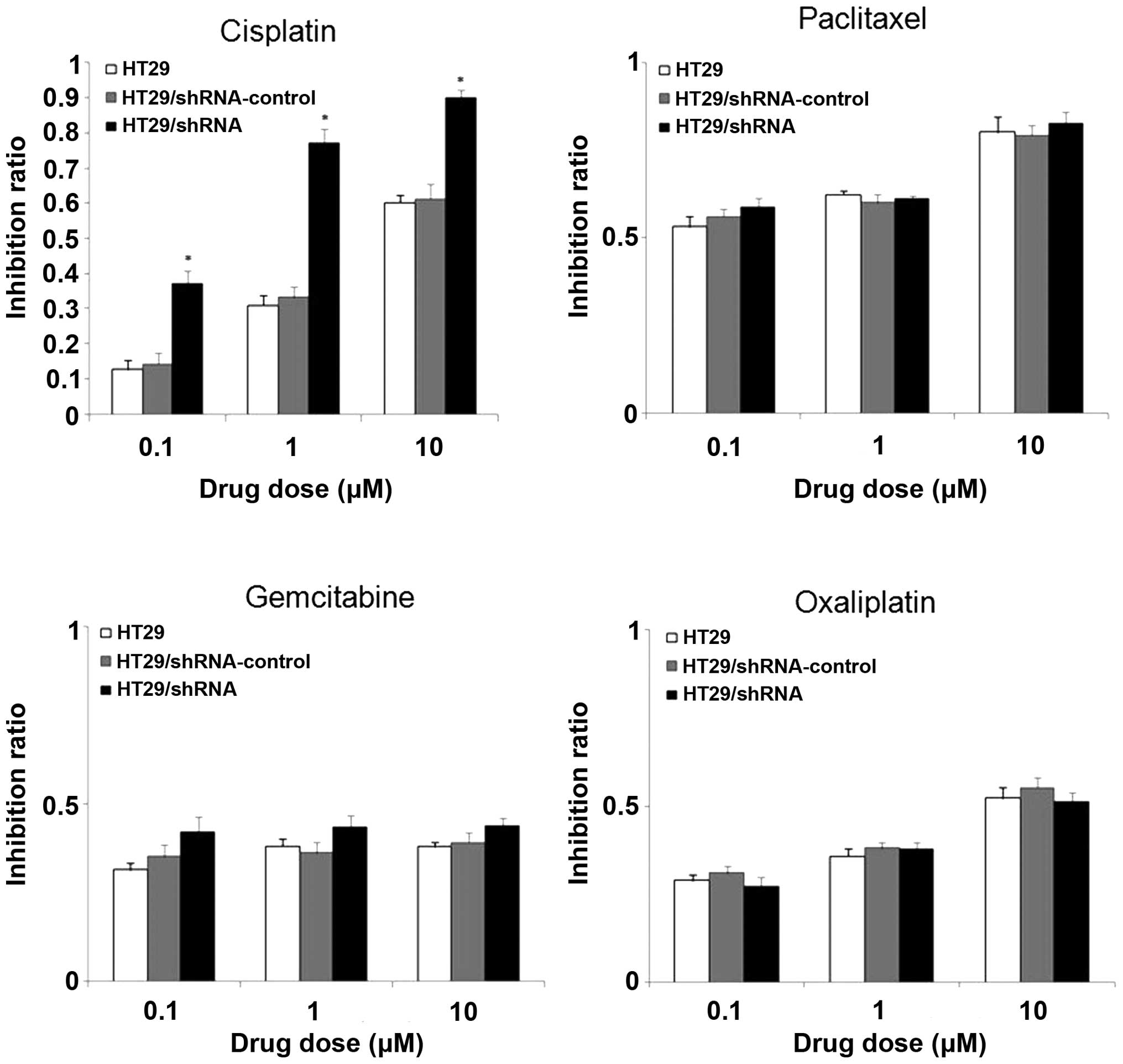
The CD147 silencing increases the
sensitivity to chemotherapeutic drugs in HT29 cells
CD147 was found to be overexpressed in multidrug
resistance tumor cells and could confer resistance to some
antitumor drugs. In order to test whether CD147 silencing affected
its sensitivity to chemotherapeutic drugs in HT29 cells, we used
WST-8 assay to investigate the sensitivity of HT29 cells to the
antitumor drug. As shown in Fig.
7, the chemosensitivity of HT29 cells was significantly
increased to the antitumor drug cisplatin (p<0.01), whereas to
paclitaxel, gemcitabine or oxaliplatin, there was no significant
difference between the HT29/shRNA and HT29/ shRNA-control after
CD147 silencing (p>0.05).
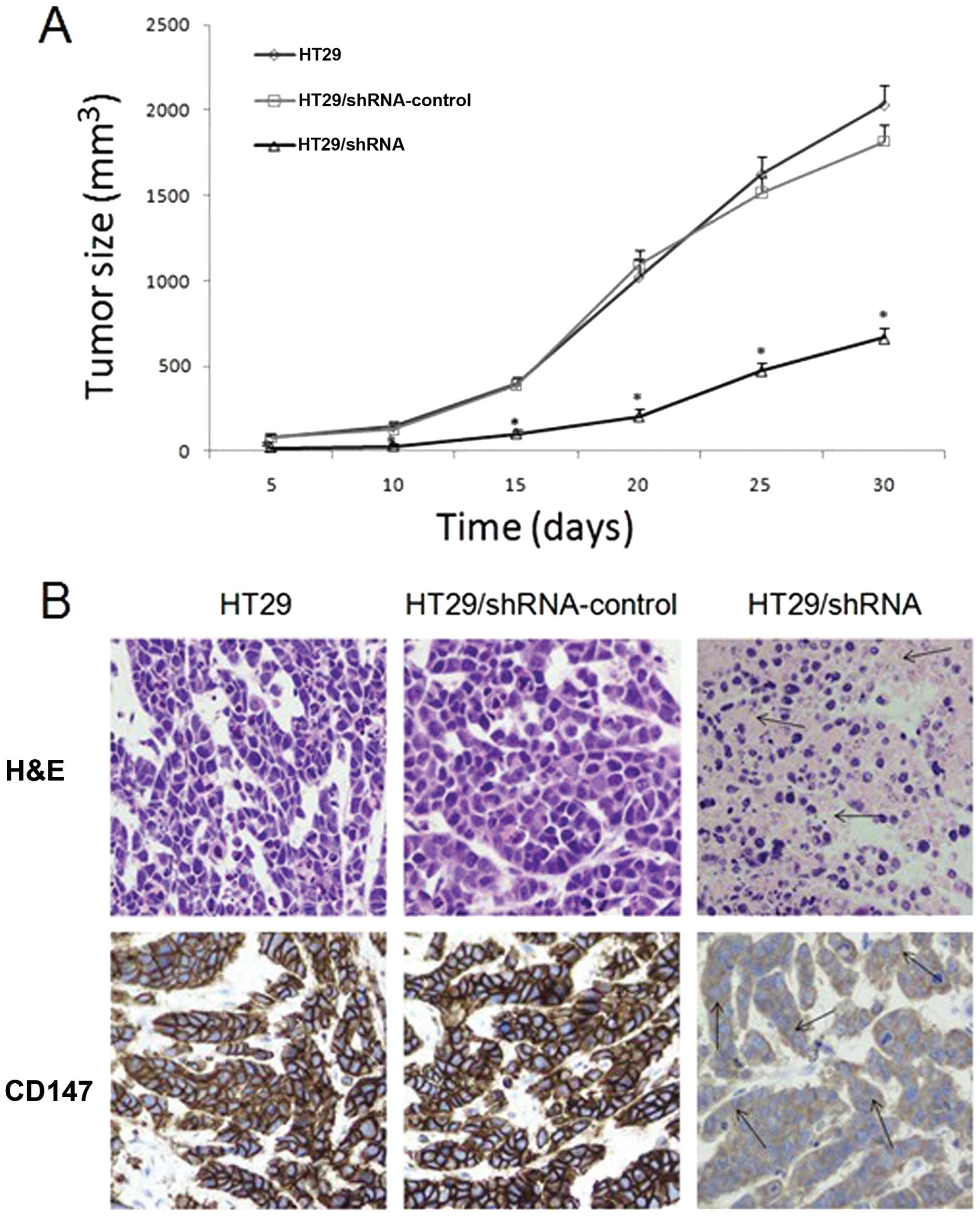
CD147 silencing inhibited the tumor
formation of HT29 cells in vivo
Using RNA interference can effectively reduce the
proliferative ability of HT29 colorectal cancer cells in
vitro, and we investigated its efficacy in vivo. From
the date of vaccination, and every 5 days, we measured the tumor
length and width and calculated their volume. As shown in Fig. 8A, the tumor growth was slower in
the HT29/shRNA group than that in other groups (p<0.01) and
there was no significant difference between HT29 and
HT29/shRNA-control group (p>0.05). Hematoxylin and eosin
(H&E)-stained examination did not reveal obvious morphological
changes among the tumors generated from the three groups, but there
were larger areas of necrosis in the tumors formed by injecting
with HT29/shRNA cells (Fig. 8B).
Immunohistochemistry analysis showed that CD147 protein expression
was high in HT29 and HT29/shRNA-control group mice, but was very
low in tumors treated with HT29/shRNA (p<0.01) (Fig. 8B).
Discussion
CD147 is a multifunctional glycoprotein forming
homo-oligomers in a cis-dependent manner in the plasma
membrane (24). Based on the high
CD147 expression reported in colorectal cancer and the association
with colorectal development, we constructed an RNA interference
vector, the pYr-mir30-shRNA. We used this vector to decline the
CD147 expression in colorectal cancer cell line HT29 and further
observed that the levels of CD147 mRNA and protein were
significantly reduced, and the proliferation, invasion and
metastasis of colorectal cancer HT29 cells were also reduced
significantly.
CD147 was reported to be more highly expressed on
the surface of most human carcinoma cells, and correlated with
tumor progression and invasion by stimulating peritumoral
fibroblasts to produce elevated levels of several MMPs (25). The present results showed that
CD147 silencing resulted in a clear reduction of MMP-2 and MMP-9
expression in colorectal cancer cells, supporting the concept that
CD147 was associated with increased expression of MMP-2 and MMP-9
(26). Data have suggested that
CD147 stimulated the synthesis of specific MMPs to participate in
tumor progression through peritumor fibroblasts (15). MMPs are believed to play important
roles in disrupting the balance between growth and anti-growth
signals in the tumor microenvironment (27,28).
Previous study reported that MMP-2 and MMP-9 were potential
prognostic biomarkers of colorectal cancer (29). Development of a new generation of
selective inhibitors of MMPs through the pharmacological targeting
to colorectal cancer is a promising and challenging area for future
research (30). In the present
study, we also detected the HT29 cell invasion ability changes
using transwell. The results showed that inhibition of CD147
expression reduced the ability of invasion in HT29 cells. The
possible mechanism was that CD147 silencing inhibited the secretion
of MMPs. In order to make the results of the experiment more
convincing, we carried out animal experiments. The nude mouse
experiments showed that inhibition of CD147 expression reduced
colorectal cell tumorigenicity. Immunohistochemical staining
suggested that CD147 protein expression was slightly detected in
tumor derived from HT29/shRNA cells. These results proved that
CD147 silencing could reduce the HT29 cell tumorigenicity, invasion
and once again confirmed that the MMPs were associated with cell
invasion.
CD147 was able to interact with certain lactate
transporters (MCT1 and MCT4) and facilitate their expression on the
cell surface. The present results showed that CD147 silencing
resulted in a clear reduction of MCT1 expression, supporting the
concept that CD147 was an ancillary protein required for the
expression of these MCTs (21).
Further evidence has demonstrated that the levels of CD147 in the
plasma membrane were controlled by silencing or overexpressing of
MCT4 (31). Our results showed
that the CD147 silencing resulted in a significant reduction of
MCT1, but the expression of MCT4 protein did not significantly
change in colorectal cancer cells. CD147 inserted
H+/lactate symporters, MCTs, in many organizations
control the stability and function of plasma membrane, which played
a determinant role in metabolic energy (32). In our study, CD147 silencing was
able to increase the lactate concentration which might be due to
the CD147 silencing leading to the MCT1 protein reduction, and the
increase in lactate concentration might reduce the cell growth or
other tumor-associated biological activities.
Multidrug resistance (MDR) occurred in tumor cells,
tumor stem cells and tumor metastases, which is the main cause of
failure in cancer therapy, and upregulated CD147 was observed in
many MDR cancer cells (22). In
colorectal cancer patients, MDR was also an important cause of
treatment failure and mortality. In many different ways, the
protein of CD147 could regulate the chemosensitivity of certain
chemotherapeutic drugs (33,34).
The anticancer drugs cisplatin, paclitaxel, oxaliplatin and
gemcitabine are widely used, and potent in head and neck cancer,
and also often used in treatment of colorectal cancer. In the
present study, the results revealed that CD147 silencing increased
the chemosensitivity to cisplatin, but not to gemcitabine,
oxaliplatin or paclitaxel in human colorectal cancer cell line
HT29, suggesting that CD147 was an adjuvant chemotherapy target for
colorectal cancer. Cisplatin was shown to be first efficacious
compound in the treatment of colorectal cancer, and oxaliplatin
consistently exerted antitumor activity in colorectal cancer
(35,36), the specific molecular mechanism of
resistence to cisplatin are unclear, but several mechanisms of the
resistance to cisplatin were proposed, such as reducing drug
uptake, increasing drug inactivation, increasing DNA adduct repair
and defecting apoptotic response, and we will investigate these
mechanisms in the future.
In conclusion, CD147 silencing by RNAi inhibited the
proliferation and invasion of cancer cells. The possible mechanism
was that CD147 silencing inhibited the MCT1 protein expression,
resulting in increased intracellular lactic acid and inhibition of
cell proliferation. However, CD147 silencing suppressed the
secretion of MMP proteins, thereby inhibited tumor cell invasion
and metastasis. CD147 is a key regulator of the multidrug
resistance of human colorectal cancer cell line HT29. The results
of this study provide new ideas for potential strategies of gene
target therapy in colorectal cancer.
Acknowledgements
This study was supported by National
Nature Science Foundation of China (no. 81172141), Nanjing Science
and Technology Committee project (no. 201108025), Nanjing Medical
Technology Development Project (no. ZKX11025), Nanjing Health Young
Talent Project, Jiangsu Provincial Key Medical Talents to S.K.W.,
Nanjing Medical Science and Technique Development Foundation to
Y.Q.P. (no. QRX11255) and B.S.H. (no. QRX11254).
References
|
1.
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Center MM, Jemal A, Smith RA and Ward E:
Worldwide variations in colorectal cancer. CA Cancer J Clin.
59:366–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Stein U and Schlag PM: Clinical,
biological, and molecular aspects of metastasis in colorectal
cancer. Recent Results Cancer Res. 176:61–80. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Polette M, Gilles C, Marchand V, et al:
Tumor collagenase stimulatory factor (TCSF) expression and
localization in human lung and breast cancers. J Histochem
Cytochem. 45:703–709. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Jiang JL, Zhou Q, Yu MK, Ho LS, Chen ZN
and Chan HC: The involvement of HAb18G/CD147 in regulation of
store-operated calcium entry and metastasis of human hepatoma
cells. J Biol Chem. 276:46870–46877. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Riethdorf S, Reimers N, Assmann V, et al:
High incidence of EMMPRIN expression in human tumors. Int J Cancer.
119:1800–1810. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Muramatsu T and Miyauchi T: Basigin
(CD147): a multifunctional transmembrane protein involved in
reproduction, neural function, inflammation and tumor invasion.
Histol Histopathol. 18:981–987. 2003.PubMed/NCBI
|
|
8.
|
Rosenthal EL, Shreenivas S, Peters GE,
Grizzle WE, Desmond R and Gladson CL: Expression of extracellular
matrix metalloprotease inducer in laryngeal squamous cell
carcinoma. Laryngoscope. 113:1406–1410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hanata K, Yamaguchi N, Yoshikawa K, et al:
Soluble EMMPRIN (extra-cellular matrix metalloproteinase inducer)
stimulates the migration of HEp-2 human laryngeal carcinoma cells,
accompanied by increased MMP-2 production in fibroblasts. Arch
Histol Cytol. 70:267–277. 2007. View Article : Google Scholar
|
|
10.
|
Sameshima T, Nabeshima K, Toole BP, et al:
Expression of emmprin (CD147), a cell surface inducer of matrix
metalloproteinases, in normal human brain and gliomas. Int J
Cancer. 88:21–27. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Stenzinger A, Wittschieber D, von
Winterfeld M, et al: High extracellular matrix metalloproteinase
inducer/CD147 expression is strongly and independently associated
with poor prognosis in colorectal cancer. Hum Pathol. 43:1471–1481.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Zhu S, Chu D, Zhang Y, et al:
EMMPRIN/CD147 expression is associated with disease-free survival
of patients with colorectal cancer. Med Oncol. 30:3692013.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Pan Y, He B, Song G, et al: CD147
silencing via RNA interference reduces tumor cell invasion,
metastasis and increases chemosensitivity in pancreatic cancer
cells. Oncol Rep. 27:2003–2009. 2012.PubMed/NCBI
|
|
14.
|
Lynch CC and Matrisian LM: Matrix
metalloproteinases in tumor-host cell communication.
Differentiation. 70:561–573. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Caudroy S, Polette M, Tournier JM, et al:
Expression of the extracellular matrix metalloproteinase inducer
(EMMPRIN) and the matrix metalloproteinase-2 in bronchopulmonary
and breast lesions. J Histochem Cytochem. 47:1575–1580. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Caudroy S, Polette M, Nawrocki-Raby B, et
al: EMMPRIN-mediated MMP regulation in tumor and endothelial cells.
Clin Exp Metastasis. 19:697–702. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Biswas C, Zhang Y, DeCastro R, et al: The
human tumor cell-derived collagenase stimulatory factor (renamed
EMMPRIN) is a member of the immunoglobulin superfamily. Cancer Res.
55:434–439. 1995.PubMed/NCBI
|
|
18.
|
Menashi S, Serova M, Ma L, Vignot S,
Mourah S and Calvo F: Regulation of extracellular matrix
metalloproteinase inducer and matrix metalloproteinase expression
by amphiregulin in transformed human breast epithelial cells.
Cancer Res. 63:7575–7580. 2003.
|
|
19.
|
Guo H, Li R, Zucker S and Toole BP:
EMMPRIN (CD147), an inducer of matrix metalloproteinase synthesis,
also binds interstitial collagenase to the tumor cell surface.
Cancer Res. 60:888–891. 2000.PubMed/NCBI
|
|
20.
|
Philp NJ, Ochrietor JD, Rudoy C, Muramatsu
T and Linser PJ: Loss of MCT1, MCT3, and MCT4 expression in the
retinal pigment epithelium and neural retina of the
5A11/basigin-null mouse. Invest Ophthalmol Vis Sci. 44:1305–1311.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Kirk P, Wilson MC, Heddle C, Brown MH,
Barclay AN and Halestrap AP: CD147 is tightly associated with
lactate transporters MCT1 and MCT4 and facilitates their cell
surface expression. EMBO J. 19:3896–3904. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Yang JM, Xu Z, Wu H, Zhu H, Wu X and Hait
WN: Overexpression of extracellular matrix metalloproteinase
inducer in multidrug resistant cancer cells. Mol Cancer Res.
1:420–427. 2003.PubMed/NCBI
|
|
23.
|
Misra S, Ghatak S, Zoltan-Jones A and
Toole BP: Regulation of multidrug resistance in cancer cells by
hyaluronan. J Biol Chem. 278:25285–25288. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yan L, Zucker S and Toole BP: Roles of the
multifunctional glycoprotein, emmprin (basigin; CD147), in tumour
progression. Thromb Haemost. 93:199–204. 2005.PubMed/NCBI
|
|
25.
|
Kanekura T, Chen X and Kanzaki T: Basigin
(CD147) is expressed on melanoma cells and induces tumor cell
invasion by stimulating production of matrix metalloproteinases by
fibroblasts. Int J Cancer. 99:520–528. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Zhu C, Pan Y, He B, et al: Inhibition of
CD147 gene expression via RNA interference reduces tumor cell
invasion, tumorigenicity and increases chemosensitivity to
cisplatin in laryngeal carcinoma Hep2 cells. Oncol Rep. 25:425–432.
2011.PubMed/NCBI
|
|
27.
|
Lochter A and Bissell MJ: An odyssey from
breast to bone: multi-step control of mammary metastases and
osteolysis by matrix metalloproteinases. APMIS. 107:128–136. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Roy R, Yang J and Moses MA: Matrix
metalloproteinases as novel biomarkers and potential therapeutic
targets in human cancer. J Clin Oncol. 27:5287–5297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Herszenyi L, Hritz I, Lakatos G, Varga MZ
and Tulassay Z: The behavior of matrix metalloproteinases and their
inhibitors in colorectal cancer. Int J Mol Sci. 13:13240–13263.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gallagher SM, Castorino JJ, Wang D and
Philp NJ: Monocarboxylate transporter 4 regulates maturation and
trafficking of CD147 to the plasma membrane in the metastatic
breast cancer cell line MDA-MB-231. Cancer Res. 67:4182–4189. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Le Floch R, Chiche J, Marchiq I, et al:
CD147 subunit of lactate/ H+ symporters MCT1 and hypoxia-inducible
MCT4 is critical for energetics and growth of glycolytic tumors.
Proc Natl Acad Sci USA. 108:16663–16668. 2011.
|
|
33.
|
Zou W, Yang H, Hou X, Zhang W, Chen B and
Xin X: Inhibition of CD147 gene expression via RNA interference
reduces tumor cell invasion, tumorigenicity and increases
chemosensitivity to paclitaxel in HO-8910pm cells. Cancer Lett.
248:211–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Jia L, Wang H, Qu S, Miao X and Zhang J:
CD147 regulates vascular endothelial growth factor-A expression,
tumorigenicity, and chemosensitivity to curcumin in hepatocellular
carcinoma. IUBMB Life. 60:57–63. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Raymond E, Faivre S, Chaney S, Woynarowski
J and Cvitkovic E: Cellular and molecular pharmacology of
oxaliplatin. Mol Cancer Ther. 1:227–235. 2002.
|
|
36.
|
Virag P, Perde-Schrepler M, Fischer-Fodor
E, et al: Superior cytotoxicity and DNA cross-link induction by
oxaliplatin versus cisplatin at lower cellular uptake in colorectal
cancer cell lines. Anticancer Drugs. 23:1032–1038. 2012. View Article : Google Scholar : PubMed/NCBI
|