Introduction
The overall incidence of laryngeal cancer has
remarkably increased in recent decades and has become the second
most common cancer of the respiratory system in China (1,2). In
the United States, over 13,000 new cases were detected and
diagnosed in 2007 (3). The
establishment of organ preservation approaches in recent years has
improved the life quality of early cancer patients. However, the
survival rate of patients with laryngeal cancer has decreased
compared with the survival rate of patients with all other types of
cancers as a whole (4). Therefore,
the molecular pathogenesis of laryngeal cancer must be elucidated
and novel strategies for treating laryngeal cancer must be
developed.
The Src homologue phosphotyrosine phosphatase 2
(SHP2) is an intracellular tyrosine phosphatase with two tandem
repeated Src homology 2 domains and has emerged as a major
regulator of receptor tyrosine kinase, cytokine receptor, and
hormone signaling (5,6). Constitutively activating mutations in
human gene PTPN11 encoding for SHP2 have been detected in
almost 50% of Noonan syndrome patients who are at high risk for
juvenile myelomonocytic leukemia. Somatic mutations resulting in
constitutively active SHP2 have also been found in several types of
leukemia (7). Notably, SHP2 and
its binding scaffolding/adaptor protein Gab2 are overexpressed in a
significant portion of breast cancer patients to promote mammary
cell hyperproliferation (8).
Furthermore, SHP2 is a major target of Helicobacter pylori
CagA, which plays a key role in the pathogenesis of gastric
carcinoma by activating SHP2 in a tyrosine
phosphorylation-dependent manner (9,10).
Collectively, these findings indicate the involvement of SHP2 in
the initiation and development of a variety of human malignancies
(11). Therefore, PTPN11 is
proposed to be the first proto-oncogene that encodes a tyrosine
phosphatase (12).
In this study, we investigated the role of SHP2 in
laryngeal cancer and its correlation with the prognosis of
laryngeal cancer patients. Our findings provided a basis for the
development of SHP2-targeted laryngeal cancer prevention and
therapy.
Materials and methods
Ethics statement
All patients were from China and received treatment
in the First Hospital of China Medical University. All clinical
investigations were approved by the China Medical University Ethics
Committee, and all patients involved in this study signed pertinent
consent forms.
All animal studies were approved by the China
Medical University Ethics Committee, and all animal experiments
were conducted in the Experimental Animal Center of China Medical
University according to International Guiding Principles for
Biomedical Research Involving Animals. All animals were housed in
virus-free facilities and maintained in a temperature and light
(12/12-h light/dark cycle)-controlled environment. Mice were
permitted ad libitum access to water and standard chow.
Animal experiments
For the growth assay, 18 eight-week-old male nude
mice were divided into two groups and inoculated with Hep2
Control/siSHP2 cells (2×106). All mice were sacrificed
by anesthesia overdose 8 weeks after inoculation. Tumors were
measured by a Vernier caliper, weighed, and photographed. A portion
of tumor tissues was collected, fixed in 10% formaldehyde, and
embedded in paraffin for tumor pathological analysis.
Patients and microarray tissue
samples
All human laryngeal tissues were obtained from
surgical resection specimens of laryngeal cancer patients in China
Medical University (Shenyang, China). For prognostic analysis, a
tissue microarray was made from 112 laryngeal cancer samples with
corresponding peritumor tissues collected from laryngeal cancer
patients who underwent curative resection in China Medical
University (Shenyang, China) from September 2005 to May 2012. For
real-time PCR analysis, another 68 laryngeal cancer and
corresponding peritumor frozen tissues were collected.
Immunohistochemistry and tissue
microarray analysis
IHC analysis of tumor sections and tissue microarray
were performed using the antibodies listed in Table I. The sections were incubated with
primary antibodies at 4°C overnight, followed by horseradish
peroxidase-conjugated secondary antibody at 37°C for 30 min. The
sections were then incubated with diaminobenzidine and
counterstained with hematoxylin for detection. Assessment of tissue
microarray staining was based on the percentage of positively
stained cells, and staining intensity was measured by Image Scope
(Aperio Technologies, Inc.) software. The expression levels of
SHP2, p-Raf, p-MEK, and p-Erk in all 112 pairs of samples (cancer
tissues and corresponding non-cancer adjacent tissues) were
quantified (13). The ratio of
SHP2, p-Raf, p-MEK, and p-Erk expression between each tumor and
corresponding non-cancer surrounding tissue was calculated.
 | Table I.Information for antibodies. |
Table I.
Information for antibodies.
| Name | Isotype | Company |
|---|
| p-MEK1/2 | Rabbit | Cell Signaling
Technology |
| MEK1/2 | Rabbit | Cell Signaling
Technology |
| GAPDH | Mouse | Santa Cruz
Biotechnology, Inc. |
| p-Erk1/2 | Mouse | Cell Signaling
Technology |
| Erk1/2 | Rabbit | Cell Signaling
Technology |
| p-Raf | Rabbit | Santa Cruz
Biotechnology, Inc. |
| Raf | Rabbit | Santa Cruz
Biotechnology, Inc. |
| SHP2 | Rabbit | Cell Signaling
Technology |
Cell lines and recombinant virus
Laryngeal cancer cell line Hep2 was purchased from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Lentivirus expressing siSHP2 or control was generated
using Lenti-X Expression system (Clontech Laboratories, Inc.).
Cell proliferation assay
Hep2 siSHP2 and their control cells
(1×103) were cultured in 96-well plates for various time
periods. ATP activity was measured using a Cell Counting Kit-8
(Dojindo, Kumamoto, Japan) with a Synergy 2 microplate reader
following the manufacturer’s instructions. The results are
presented as proliferation indices relative to control cells.
Real-time PCR
The original amount of specific transcripts was
measured by real-time PCR using an ABI PRISM 7300 sequence detector
(Applied Biosystems). The primer sequences are provided in Table II.
 | Table II.Sequence of primers for real-time
PCR. |
Table II.
Sequence of primers for real-time
PCR.
| Primer | Sequence
(5′→3′) |
|---|
| Shp2 forward
primer |
CTGCCTCCACACCAGTGATA |
| Shp2 reverse
primer |
GGAGCCTGAGCAAGGAGC |
| 18s forward
primer |
CGGCTACCACATCCAAGGAA |
| 18s reverse
primer |
GCTGGAATTACCGCGGCT |
| c-Jun forward
primer |
ACCGACGAGCAGGAGGGCTT |
| c-Jun reverse
primer |
CAGCGCACCCGGGTTGAAGT |
| c-myc forward
primer |
GGCCGCTGCCAAACTGGTCT |
| c-myc reverse
primer |
TGGGCGAGCTGCTGTCGTTG |
Western blot analysis
The Hep2 cell extract was separated on
polyacrylamide-SDS gels, transferred, and probed with a specific
primary antibody. The protein band, specifically bound to the
primary antibody, was detected using an IRDye 800CW-conjugated
secondary antibody and LI-COR imaging system (LI-COR Biosciences).
The manufacturer information of the primary antibodies is provided
in Table I.
Statistical analysis
Overall survival was defined as the interval between
the dates of surgery and death. All statistical analyses were
performed with SPSS version 18.0 software. The χ2 test
or Fisher’s exact test was used to compare qualitative variables,
whereas continuous variables were compared using Student’s t-test
or Mann-Whitney test for variables with abnormal distribution.
Survival curves were calculated using the Kaplan-Meier method and
compared with a log-rank test. The Cox proportional hazards model
was used to determine the independent factors affecting survival
based on variables selected by univariate analysis. P<0.05 was
considered statistically significant.
Results
SHP2 expression is upregulated in
laryngeal cancer compared with other head and neck tumors
To explore the role of SHP2 in head and neck tumor,
we first evaluated SHP2 expression in various head and neck tumors.
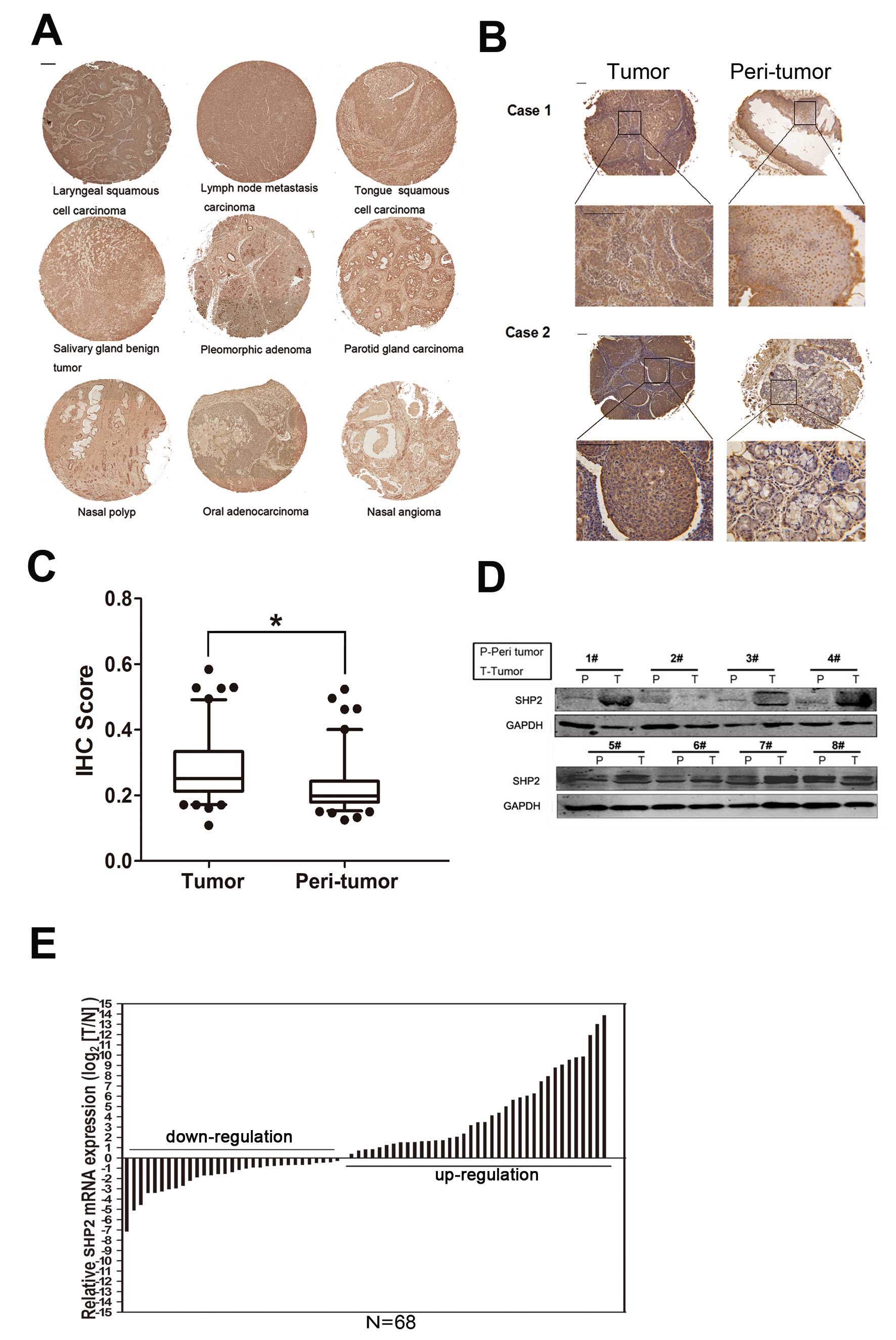
Fig. 1A shows a tissue array
containing different head and neck tumor samples subjected to
immunohistochemical staining. SHP2 expression was more elevated in
laryngeal cancer and its neck lymph nodes than in other tumors.
This finding prompted us to investigate whether SHP2 played an
important role in laryngeal cancer tumorigenesis. A tissue array
containing 112 laryngeal cancer samples relative to corresponding
non-cancer tissues in patients was used for immunohistochemical
staining (Fig. 1B and C). The
results were further confirmed by western blotting (Fig. 1D) and real-time PCR (Fig. 1E) analyses. We found that SHP2
expression was remarkably enhanced in laryngeal cancer.
SHP2 overexpression was associated with
poor prognosis of laryngeal cancer
To investigate the clinical significance of SHP2
overexpression in laryngeal cancer, we further studied the role of
SHP2 in laryngeal cancer at the molecular level. We found that SHP2
expression was correlated with the survival of laryngeal cancer
patients. Higher SHP2 expression was associated with poorer
prognosis of laryngeal cancer, whereas lower SHP2 expression
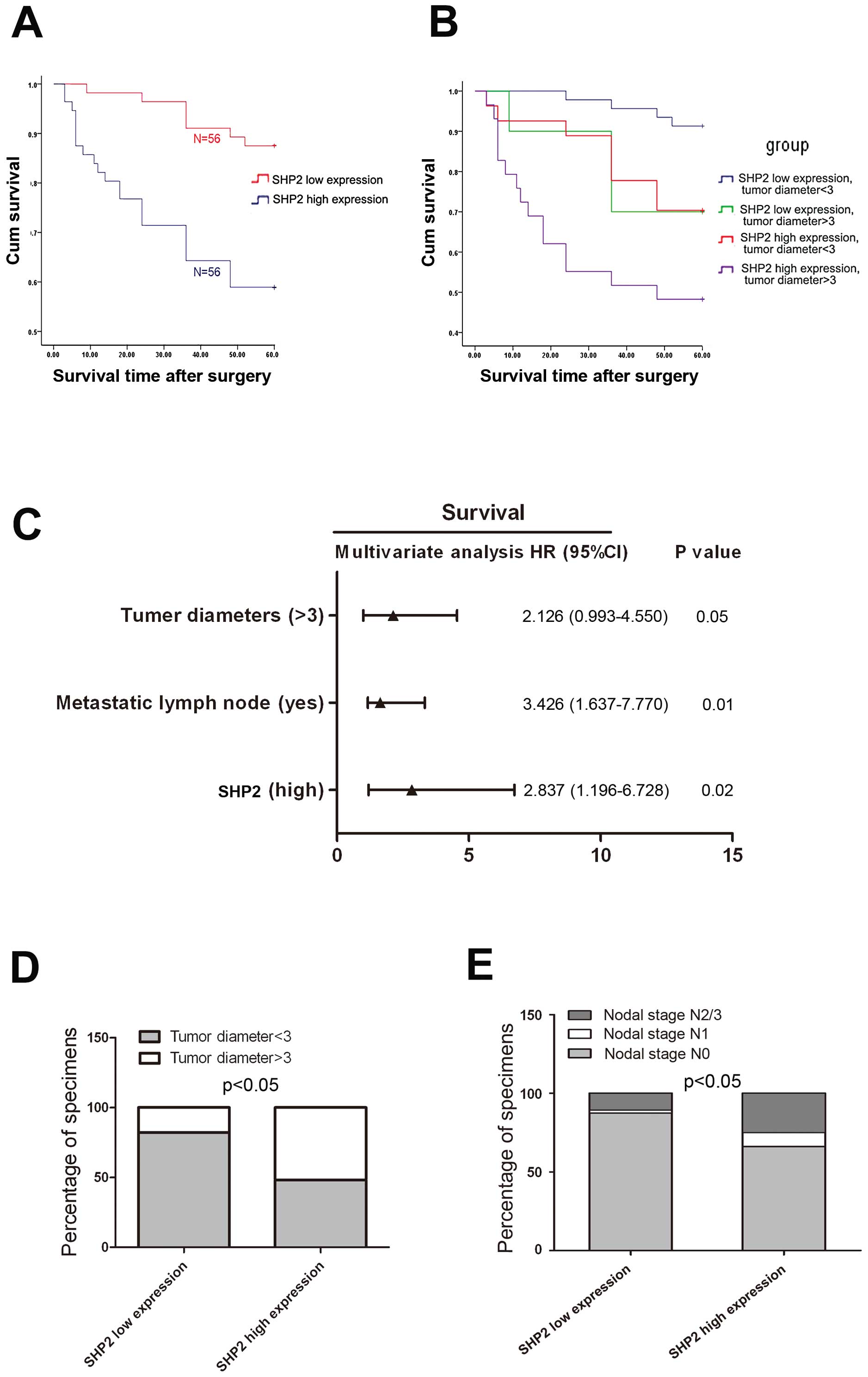
corresponded to higher survival rates (Fig. 2A). We then examined the
relationship between SHP2 expression in tumor tissues and the
clinicopathological characteristics of the 112 patients (Table III). Correlation of regression
analysis indicated that prognosis was correlated with several
individual parameters, including SHP2 expression, tumor stage,
nodal stage, tumor diameter, growth pattern, and surgery (Table IV). These individual parameters
were further analyzed with a multivariate Cox proportional hazard
model. Results indicated that tumor diameter, nodal stage, and SHP2
expression level were independent and significant factors that
affected the survival of laryngeal cancer patients (Fig. 2C and Table IV). Among these factors, SHP2
expression level had a significant hazard ratio (HR) value for
cumulative survival (HR, 2.837; 95% confidence interval,
1.196–6.728; P=0.02). We also analyzed the percentage of tumor
diameters and nodal stage between low- and high-level SHP2
expression specimens. We found that patients with high-level SHP2
expression tended to have larger tumors and poorer nodal stages
(Fig. 2D and E). These results
suggested that SHP2 may function as a proto-oncogene.
 | Table III.Clinicopathological
characteristics. |
Table III.
Clinicopathological
characteristics.
| Number and value
(range)
|
|---|
| Variable | SHP2-high
expression (n=56) | SHP2-low expression
(n=56) | P-value |
|---|
| Median age (range,
years) | 63 (40–85) | 64 (58–79) | |
| Gender | | | |
| Male | 45 (47.9%) | 49 (52.1%) | 0.441 |
| Female | 11 (61.1%) | 7 (38.9%) | |
| Locationa | | | |
| Supraglottic | 35 (60.3%) | 23 (39.7%) | 0.027 |
| Glottic | 17 (35.4%) | 31 (64,6%) | |
| Subglottic | 4 (66.7%) | 2 (33.3%) | |
| Tumor stagea | | | |
| T1 | 2 (18.2%) | 9 (81.8%) | 0.000 |
| T2 | 8 (81.0%) | 34 (19.0%) | |
| T3 | 33 (78.6%) | 9 (21.4%) | |
| T4 | 13 (23.5%) | 4 (76.5%) | |
| Nodal stagea | | | |
| N0 | 37 (43.0%) | 49 (57.0%) | 0.023 |
| N1 | 5 (83.3%) | 1 (16.7%) | |
| N2/N3 | 14 (70.0%) | 6 (30.0%) | |
| Tumor diameter
(cm)a | | | |
| ≤3 | 29 (38.7%) | 46 (61.3%) | 0.001 |
| >3 | 27 (73.0%) | 10 (27.0%) | |
| Growth
patterna | | | |
| Exophytic | 28 (39.4%) | 43 (60.6%) | 0.001 |
| Expansive | 7 (46.7%) | 8 (53.3%) | |
| Ulcerative | 21 (80.8%) | 5 (19.2%) | |
| Histological
grade | | | |
| Well | 37 (52.9%) | 33 (42.1%) | 0.271 |
| Moderately | 11(37.9%) | 18 (62.1%) | |
| Poorly | 8 (61.5%) | 5 (38.5%) | |
| Surgerya | | | |
| Partial
laryngectomy | 17 (34.7%) | 32 (65.3%) | 0.007 |
| Total
laryngectomy | 39 (61.9%) | 24 (38.1%) | |
| Smoking index | | | |
| None | 6 (66.7%) | 3 (33.3%) | 0.453 |
| 0–400 | 12 (42.9%) | 16 (57.1%) | |
| >400 | 38 (50.7%) | 37 (49.3%) | |
| Alcohol abuse | | | |
| None | 28 (57.1%) | 21 (42.9%) | |
| Mild | 4 (30.8%) | 9 (69.2%) | 0.223 |
| Heavy | 24 (48%) | 26 (52.0%) | |
 | Table IV.Univariate and multivariate analyses
of the primary cohort. |
Table IV.
Univariate and multivariate analyses
of the primary cohort.
| Variable | n | Time
(months)b | P-value as
univariate | P-value as
multivariate |
|---|
| Shp2
expressiona | | | | |
| High | 56 | 43.857±21.855 | 0.000 | 0.012 |
| Low | 56 | 56.804±9.780 | | |
| Location | | | | |
| Supraglottic | 58 | 48.862±19.776 | 0.167 | - |
| Glottic | 48 | 53.958±13.043 | | |
| Subglottic | 6 | 35.500±27.783 | | |
| Tumor stage | | | | |
| T1 | 11 | 57.818±7.236 | 0.000 | 0.357 |
| T2 | 42 | 55.381±12.069 | | |
| T3 | 42 | 49.667±18.153 | | |
| T4 | 17 | 34.647±25.409 | | |
| Nodal stagea | | | | |
| N0 | 86 | 55.012±12.193 | 0.000 | 0.020 |
| N1 | 6 | 20.500±17.841 | | |
| N2/N3 | 20 | 39.150±25.415 | | |
| Tumor diameter
(cm)a | | | | |
| ≤3 | 73 | 55.575±11.860 | 0.000 | 0.042 |
| >3 | 39 | 40.513±23.130 | | |
| Growth pattern | | | | |
| Exophytic | 71 | 52.225±16.272 | 0.042 | 0.740 |
| Expansive | 15 | 56.800±8.445 | | |
| Ulcerative | 26 | 41.423±23.449 | | |
| Histological
grade | | | | |
| Well | 70 | 50.643±17.878 | 0.477 | - |
| Moderately | 29 | 52.724±15.736 | | |
| Poorly | 13 | 43.308±23.135 | | |
| Surgery | | | | |
| Partial
laryngectomy | 49 | 55.102±12.754 | 0.023 | 0.769 |
| Total
laryngectomy | 63 | 46.619±20.648 | | |
| Smoking index | | | | |
| None | 9 | 46.667±21.378 | 0.458 | - |
| 0–400 | 28 | 48.893±20.977 | | |
| >400 | 75 | 51.307±16.619 | | |
| Alcohol abuse | | | | |
| None | 49 | 49.510±18.808 | 0.545 | - |
| Mild | 13 | 57.231±7.190 | | |
| Heavy | 50 | 49.340±19.125 | | |
Transfection of SHP2 into human laryngeal
cancer cells promotes cell growth
To validate our findings that SHP2 acted as a
proto-oncogene in laryngeal cancer, we used a human laryngeal
cancer cell line to examine the effects of SHP2 expression on cell
growth. The identification of Hep2 cell lines with over- or
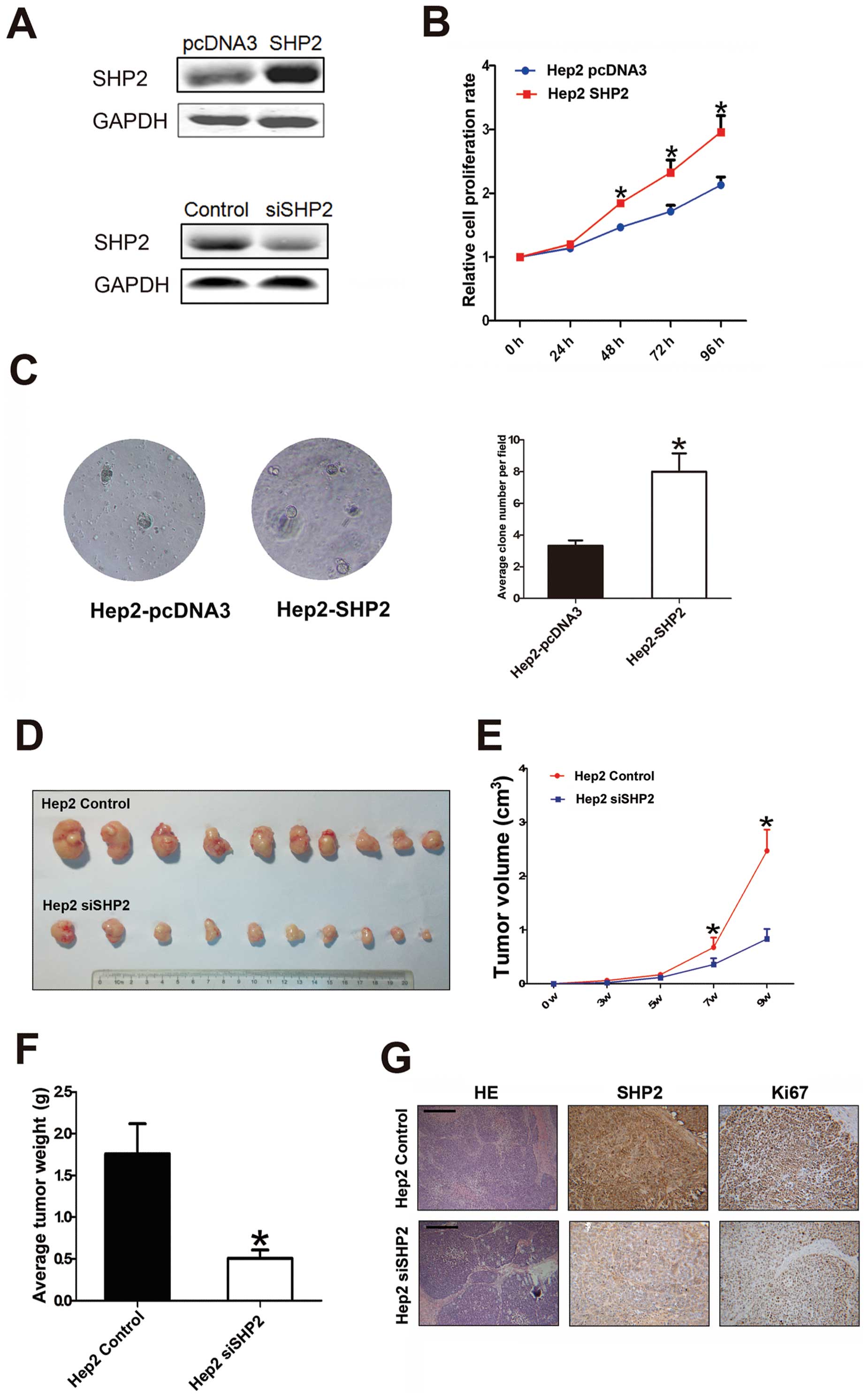
low-expressing SHP2, is shown in Fig.
3A. Using a cell-counting kit assay, we found that SHP2
influenced Hep2 (parental transfectant) cell growth (Fig. 3B). This result was further
supported by the results of an anchorage-independent cell growth
assay. Using a soft agar colony formation assay, we found that
SHP2-overexpressing Hep2 (Hep2-SHP2) cells grew in the absence of
anchorage, suggesting that increased SHP2 expression resulted in
anchorage-independent cell growth (Fig. 3C). To determine the effects of
elevated SHP2 expression on laryngeal cell growth in vivo,
we established two stably transfected cell lines, namely,
Hep2-siSHP2 and Hep2 control cells (Fig. 3A). Then, 2×106
Hep2-siSHP2 and Hep2 control cells were subcutaneously inoculated
into nude mice for the xenograft assay. Fig. 3D shows that compared with the
control cells, Hep2 cells with low-level SHP2 expression exhibited
weaker tumorigenicity in nude mice both in terms of volume and
weight (Fig. 3E and F). SHP2
expression in the tumors was identified by IHC staining.
Representative images stained with hematoxylin and eosin are shown
in Fig. 3G. Ki67 staining in
tumors showed that SHP2 expression was lower in tissue sections
from low SHP2 expression tumors than that in high SHP2 expression
xenografts (Fig. 3G), suggesting
an important role of SHP2 in Hep2 cell growth. Overall, the results
in Fig. 3 suggested that SHP2 may
be crucial to human laryngeal cancer growth.
SHP2 promotes laryngeal cancer growth by
activating the Ras/Raf/MAPKs signaling pathway
To identify the molecular mechanism of laryngeal
cancer growth promoted by SHP2, we assessed the expression profile
of related genes in SHP2-expressing cells and analyzed the relevant
regulatory signaling pathways. The Ras/Raf/MAPK signaling pathway
is a predominant regulatory mechanism activated by SHP2 in leukemia
(12). Therefore, the
phosphorylation levels of Raf, MEK, and Erk were examined to
determine whether phosphorylation was required in SHP2-mediated
laryngeal cancer growth. Fig. 4A
shows that the phosphorylation levels of Raf, MEK and Erk were
significantly upregulated in tissues of laryngeal cancer patients.
In the subcutaneous xenografts, we confirmed this result in
Hep2-siSHP2 cells (Fig. 4B).
Furthermore, the phosphorylation levels of Raf, MEK and Erk were
significantly downregulated after stimulation with EGF (50 ng/ml)
in low SHP2-expressing Hep2 cells compared with the levels in the
control cells (Fig. 4C).
Downregulation of the downstream transcription factors of this
pathway such as c-Jun and c-Myc was also observed in low
SHP2-expressing cells (Fig. 4D).
SHP2 was closely correlated with p-Raf in the 112 laryngeal cancer
tissues samples (r=0.799, p<0.001) (Fig. 4E). In addtion, the combination
analysis of SHP2 expression and p-Raf expression could assess the
overall survival rates of 112 laryngeal cancer patients more
accurate (Fig. 4F).
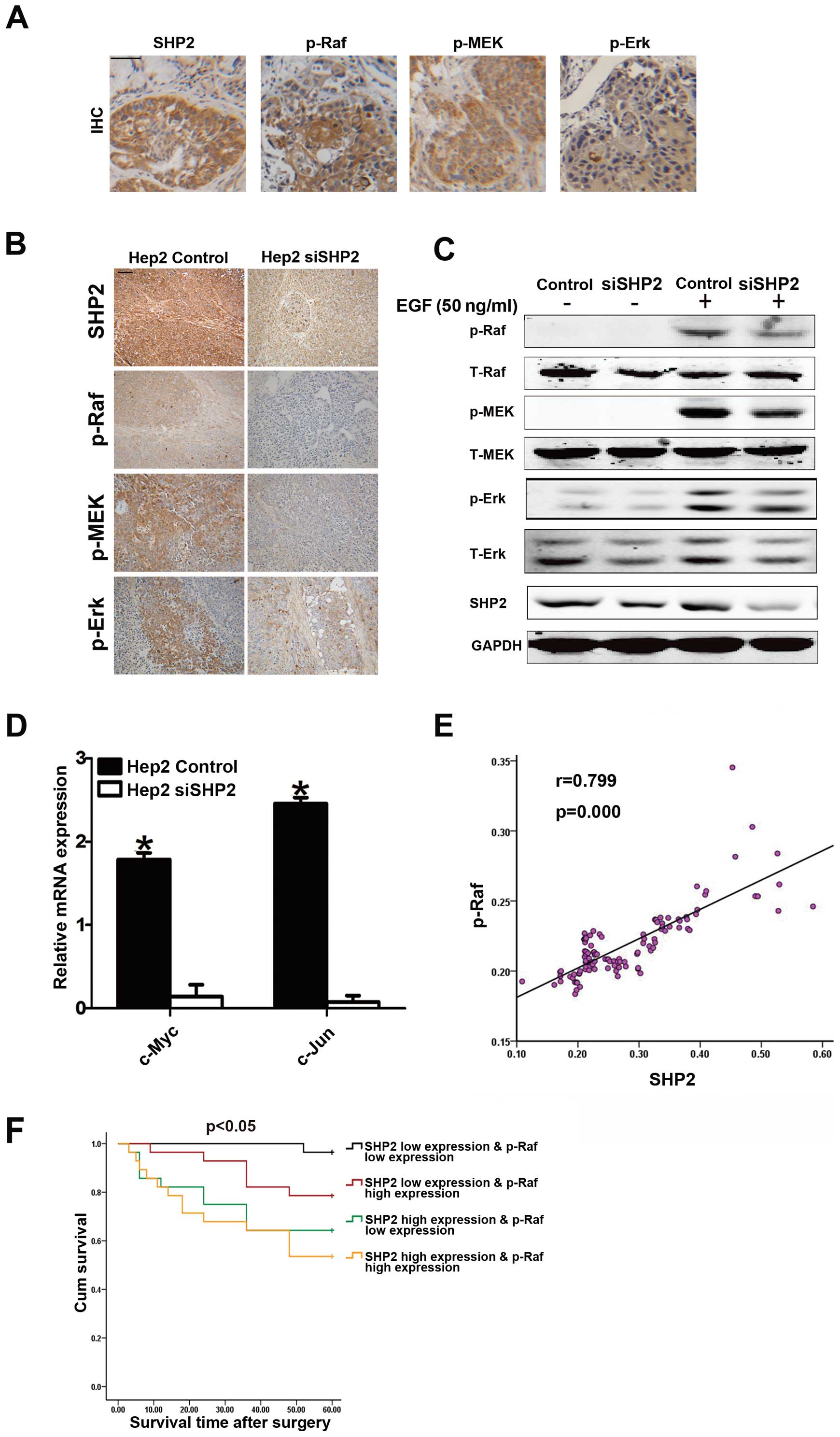 | Figure 4.SHP2 promotes laryngeal cancer growth
by activating the Ras/Raf/MAPKs signaling pathway. (A)
Representative images of immunohistochemical staining of SHP2,
p-Raf, p-MEK and p-Erk in tissue sections from laryngeal cancer
patients. Black scale bars, 100 μm. (B) Representative
images of immunohistochemical staining of SHP2, p-Raf, p-MEK and
p-Erk in tissue sections from tumors of xenografts. Black scale
bars, 100 μm. (C) Hep2-siSHP2 and Hep2 control stably
transfected cells were subjected to western blot analysis after
stimulated with EGF (50 ng/ml). (D) Quantitative RT-PCR was
performed to determine the mRNA levels of c-Jun and c-myc in
Hep2-siSHP2 and Hep2 control cells as indicated. (E) The
relationship (Pearson r=0.799, P<0.001) between SHP2 and p-Raf
IHC score analyzed among the 112 patients by tissue microarray. (F)
Laryngeal cancer patients were divided into 4 groups, SHP2 high
expression and p-Raf high expression group, SHP2 high expression
and p-Raf low expression group, SHP2 low expression and p-Raf high
expression group, SHP2 low expression and p-Raf low expression
group, the overall survival rates of the 112 laryngeal cancer
patients were compared among different groups. |
Discussion
Laryngeal carcinoma accounts for 90% of carcinomas
found in the head and neck region. This cancer is often fatal
despite current treatment protocols involving surgery, radiation
and chemotherapy (14,15). Even with new treatment protocols,
no significant improvement in 5-year overall survival rates has
been observed (15). Novel
therapeutic strategies specifically targeting growth pathways
utilized by tumor cells have shown great potential in treating this
disease.
SHP2 encoded by the PTPN11 gene is a protein
tyrosine phosphatase (PTP) containing two tandem repeated Src
homology 2 domains in its N-terminal region (15). In contrast to many other protein
phosphatases, SHP2 promotes rather than inhibits cellular processes
such as cell proliferation and motility (16). Germline gain-of-function mutations
in PTPN11 have been reported in 50% of patients with Noonan
syndrome, a developmental disorder characterized by facial
abnormalities, short stature, congenital heart defects and
increased risk of hematological malignancies, notably juvenile
myelomonocytic leukemia (17).
Known as the first bona fide PTP oncogenic regulator
(12), SHP2 overexpression has
been detected in numerous tumors, such as several types of leukemia
and breast cancer (18,19). SHP2 overexpression also triggers
the activation of several cytoplasmic kinase cascade pathways, such
as the Ras-Raf-Erk (20), JAK/STAT
(21) and PI3K/AKT pathways
(22). Moreover, studies on mouse
genetics, gene silencing, and sequencing reveal a broad role of
SHP2 in cell fate and tumor development (18,19,23,24).
In the present study, we showed that SHP2 expression
increased at both mRNA and protein levels in laryngeal cancer, and
this elevated expression was associated with malignant
clinicopathological characteristics. The correlation between SHP2
expression and surgical outcomes was further investigated in a
retrospective study of 112 laryngeal cancer patients. We found that
tumor survival rates substantially differed between patients with
high- and low-level expressions of SHP2 in tumor tissues.
Multivariate analysis revealed that SHP2 expression was an
independent and significant risk factor affecting survival rate
after curative resection, with the greatest HR value for survival.
More importantly, SHP2 overexpression showed enhanced accuracy in
predicting surgical outcome in patients with laryngeal cancer at
early stages. To gain insight into the mechanism regulating
laryngeal cancer tumorigenesis, we established a stable cell line
expressing low-level SHP2 by recombinant lentivirus. We further
found that SHP2 could promote laryngeal cancer cell growth and
tumor formation, and under the stimulation of EGF (50 ng/ml), SHP2
could activate the Ras/Raf/Erk pathway which play an important role
in tumor growth (25,26). Moreover, SHP2 expression is closely
correlated with p-Raf expression in laryngeal cancer, and the
combination analysis of SHP2 expression and p-Raf expression would
help to assess the prognosis of laryngeal cancer patients. These
data strongly suggested that SHP2 had clinical significance by
targeting the SHP2-activated signaling pathway in the
individualized therapy of laryngeal cancer patients. The data also
provided new insight into the prognosis of laryngeal tumorigenesis
and the individualized therapy of patients. Therefore, we believe
that SHP2 functioned as a proto-oncogene, and its elevated
expression activated the Ras/Raf/Erk pathway and contributed to
complicated malignant progression.
In conclusion, methods traditionally used to
determine the clinical management of laryngeal cancer, one of the
most common malignancies in the head and neck, are now considered
inadequate for current clinical protocols (27). Although attempts have been made to
improve the prediction of patient outcome using new markers such as
p53, p21, and EGFR (28–30), results are not satisfactory.
However, SHP2 as a novel regulator of laryngeal cancer may have an
important role in laryngeal cancer tumorigenesis through the
Ras/Raf/MEK/Erk signaling pathway and may serve as a valuable
prognostic biomarker and potential therapeutic target. Thus, our
study revealed a novel function of SHP2 and the molecular mechanism
by which SHP2 promoted laryngeal cancer tumorigenesis.
Acknowledgements
The authors would like to thank Ying
Li for her technical assistance and sincerely thank G.S. Feng and
H.Y. Wang for their selfless guidance. This study was supported by
grants from National Natural Science Foundation of China, 81000931;
Medical Peak Construction Special Fund of Liaoning Province,
4010218; Ministry of Science and Technology key program,
2012ZX10002-009.
References
|
1.
|
Li XY, Guo X, Feng S, et al: Relationship
between a family history of malignancy and the incidence of
laryngeal carcinoma in the Liaoning province of China. Clin
Otolaryngol. 34:127–131. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar
|
|
3.
|
Loyo M and Pai SI: The molecular genetics
of laryngeal cancer. Otolaryngol Clin North Am. 41:v657–v672. 2008.
View Article : Google Scholar
|
|
4.
|
Hoffman HT, Porter K, Karnell LH, et al:
Laryngeal cancer in the United States: changes in demographics,
patterns of care, and survival. Laryngoscope. 116:1–13. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Neel BG, Gu H and Pao L: The ‘Shp’ing
news: SH2 domain-containing tyrosine phosphatases in cell
signaling. Trends Biochem Sci. 28:284–293. 2003.
|
|
6.
|
Lai LA, Zhao C, Zhang EE and Feng GS: The
Shp-2 tyrosine phosphatase. Protein Phosphatases. Arino J and
Alexander D: Springer-Verlag; Berlin, Heidelberg: pp. 275–299.
2004, View Article : Google Scholar
|
|
7.
|
Tartaglia M and Gelb BD: Germ-line and
somatic PTPN11 mutations in human disease. Eur J Med Genet.
48:81–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Bentires-Alj M, Gil SG, Chan R, et al: A
role for the scaffolding adapter GAB2 in breast cancer. Nat Med.
12:114–121. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Higuchi M, Tsutsumi R, Higashi H and
Hatakeyama M: Conditional gene silencing utilizing the lac
repressor reveals a role of SHP-2 in cagA-positive Helicobacter
pylori pathogenicity. Cancer Sci. 95:442–447. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Higashi H, Tsutsumi R, Muto S, et al:
SHP-2 tyrosine phosphatase as an intracellular target of
Helicobacter pylori CagA protein. Science. 295:683–686.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Takahashi A, Tsutsumi R, Kikuchi I, et al:
SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor
suppressor to an oncogenic driver. Mol Cell. 43:45–56. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Chan RJ and Feng GS: PTPN11 is the first
identified proto-oncogene that encodes a tyrosine phosphatase.
Blood. 109:862–867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Wen W, Han T, Chen C, et al: Cyclin G1
expands liver tumor-initiating cells by Sox2 induction via Akt/mTOR
signaling. Mol Cancer Ther. 12:1796–1804. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Broich G, Lavezzi AM, Biondo B and
Pignataro LD: PCNA - a cell proliferation marker in vocal cord
cancer. Part II: recurrence in malignant laryngeal lesions. In
Vivo. 10:175–178. 1996.PubMed/NCBI
|
|
15.
|
Dobrossy L: Epidemiology of head and neck
cancer: magnitude of the problem. Cancer Metastasis Rev. 24:9–17.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Manes S, Mira E, Gomez-Mouton C, Zhao ZJ,
Lacalle RA and Martinez AC: Concerted activity of tyrosine
phosphatase SHP-2 and focal adhesion kinase in regulation of cell
motility. Mol Cell Biol. 19:3125–3135. 1999.PubMed/NCBI
|
|
17.
|
Tartaglia M, Mehler EL, Goldberg R, et al:
Mutations in PTPN11, encoding the protein tyrosine phosphatase
SHP-2, cause Noonan syndrome. Nat Genet. 29:465–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Aceto N, Sausgruber N, Brinkhaus H, et al:
Tyrosine phosphatase SHP2 promotes breast cancer progression and
maintains tumor-initiating cells via activation of key
transcription factors and a positive feedback signaling loop. Nat
Med. 18:529–537. 2012. View
Article : Google Scholar
|
|
19.
|
Grossmann KS, Rosario M, Birchmeier C and
Birchmeier W: The tyrosine phosphatase Shp2 in development and
cancer. Adv Cancer Res. 106:53–89. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Cunnick JM, Meng S, Ren Y, et al:
Regulation of the mitogen-activated protein kinase signaling
pathway by SHP2. J Biol Chem. 277:9498–9504. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Dittrich A, Quaiser T, Khouri C, Gortz D,
Monnigmann M and Schaper F: Model-driven experimental analysis of
the function of SHP-2 in IL-6-induced Jak/STAT signaling. Mol
Biosyst. 8:2119–2134. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tseng PC, Huang WC, Chen CL, et al:
Regulation of SHP2 by PTEN/AKT/GSK-3beta signaling facilitates
IFN-gamma resistance in hyperproliferating gastric cancer.
Immunobiology. 217:926–934. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Chan G, Kalaitzidis D and Neel BG: The
tyrosine phosphatase Shp2 (PTPN11) in cancer. Cancer Metastasis
Rev. 27:179–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Feng GS: Shp2-mediated molecular signaling
in control of embryonic stem cell self-renewal and differentiation.
Cell Res. 17:37–41. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Wu R, Duan L, Ye L, et al: S100A9 promotes
the proliferation and invasion of HepG2 hepatocellular carcinoma
cells via the activation of the MAPK signaling pathway. Int J
Oncol. 42:1001–1010. 2013.PubMed/NCBI
|
|
26.
|
Chung EJ, Urick ME, Kurshan N, et al:
MEK1/2 inhibition enhances the radiosensitivity of cancer cells by
downregulating survival and growth signals mediated by EGFR
ligands. Int J Oncol. 42:2028–2036. 2013.PubMed/NCBI
|
|
27.
|
Pradier R, Gonzalez A, Matos E, et al:
Prognostic factors in laryngeal carcinoma. Experience in 296 male
patients. Cancer. 71:2472–2476. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jin YT, Kayser S, Kemp BL, et al: The
prognostic significance of the biomarkers p21WAF1/CIP1,
p53, and bcl-2 in laryngeal squamous cell carcinoma. Cancer.
82:2159–2165. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tandon S, Tudur-Smith C, Riley RD, Boyd MT
and Jones TM: A systematic review of p53 as a prognostic factor of
survival in squamous cell carcinoma of the four main anatomical
subsites of the head and neck. Cancer Epidemiol Biomarkers Prev.
19:574–587. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Fujii S, Uryu H, Akashi K, et al: Clinical
significance of KRAS gene mutation and epidermal growth factor
receptor expression in Japanese patients with squamous cell
carcinoma of the larynx, oropharynx and hypopharynx. Int J Clin
Oncol. 118:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|