Introduction
Lung cancer is the leading cause of cancer-related
death worldwide (1) and can be
classified into two major groups, non-small cell lung cancer
(NSCLC) and small cell lung cancer (SCLC). NSCLC mainly consists of
squamous cell carcinoma (SC), adenocarcinoma (AC) and large-cell
carcinoma (LCC). The prognosis of lung cancer depends on
pathological stages and histological types, and the prognosis of
LCC is the worst in NSCLC (2).
Among subtypes of LCC, the prognosis of large-cell neuroendocrine
carcinoma (LCNEC) was poorer than others even if at early stages
(3–5) such as SCLC. A better therapeutic drug
for this kind of lung cancer is thus urgently required to
drastically reduce the number of deaths.
Histone deacetylase (HDAC) inhibitors have garnered
significant attention as anticancer drugs. These therapeutic agents
have been clinically validated with the market approval of
vorinostat (SAHA, Zolinza) for treatment of cutaneous T-cell
lymphoma (6,7). Suberoylanilide hydroxamic acid (SAHA)
is a potent, reversible pan-histone deacetylase (HDAC) inhibitor.
It inhibits both class I and class II HDACs, altering gene
transcription and inducing cell cycle arrest and/ or apoptosis in a
wide variety of transformed cells (8). It has been reported that SAHA
inhibited cell proliferation, induced apoptosis and had antitumor
activities in various human cancer cells (9–11),
including NSCLC cells (12,13).
SAHA also exhibited anticancer activities by regulating a variety
of signaling pathways (14). The
evidence suggested that SAHA might act as an oral anticancer agent
in LCNEC although few studies have yet been reported.
NCI-H460 is an LCC cell line with neuroendocrine
features (15,16). The present study focused on the
anticancer effects of SAHA on NCI-H460 cells in vitro and
in vivo. We aimed to investigate whether SAHA exhibits
anticancer effects by suppressing cell proliferation, cell
apoptosis, influencing cell cycle distribution, regulating cell
signaling in NCI-H460 cells, and next to confirm the antitumor
activity of SAHA in a nude mouse xenograft model of NCI-H460
cells.
Materials and methods
Antibodies and regents
Suberoylanilide hydroxamic acid (SAHA, Vorinostat)
with a purity of >98% was purchased from Sigma-Aldrich (St.
Louis, MO, USA). It was dissolved in dimethyl sulfoxide (DMSO) and
stored at −20°C, then thawed before used. DMSO,
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT),
propidium iodide (PI) were purchased from Sigma-Aldrich. Human
lymphocyte separation liquid Ficoll-Hypaque was purchased from TBD
Science (Tianjin, China). Hoechst 33342 was purchased from Beyotime
(Shanghai, China). Antibodies of AKT, ERK1/2, phosphor-AKT,
phosphor-ERK1/2, HIF-1α, VEGF were purchased from Cell Signaling
Technology (Danvers, MA, USA). β-actin was purchased from Santa
Cruz Biotechnology (Santa Cruz, CA, USA).
Isolation of peripheral blood mononuclear
cells and cell culture
The peripheral blood mononuclear cells (PBMCs) were
fractionated from peripheral blood (PB) of healthy volunteers by
Ficoll-Hypaque density gradient centrifugation. The isolated PBMCs
were washed for 3 times and suspended with RPMI-1640 medium
(Hyclone, UT, USA) containing 10% fetal bovine serum human (FBS)
(Gibco, USA). Human large-cell lung carcinoma cell line NCI-H460
was obtained from the China Center for Typical Culture Collection
(Wuhan, China) and maintained in RPMI-1640 medium with 10% FBS. All
cells were seeded onto 50 cm2 culture bottle at 37°C, 5%
CO2, in an incubator.
Cell proliferation assay
NCI-H460 cells (2×103) and PBMCs
(2×103) were cultured in RPMI-1640 medium with 10% FBS
in 96-well plates. 1, 2.5, 5 and 10 μM of SAHA were added
respectively and cells were incubated for 24, 48 and 72 h. The
anti-proliferative effect of SAHA was determined by using the MTT
dye uptake method. MTT solution (20 μl) (5 mg/ml) was added
to each well. After incubation for 4 h at 37°C, the supernatants
were removed and 150 μl DMSO was added to each well. Optical
density (OD) was detected with a microplate reader (Biotech, NY,
USA). IC50 was taken as the concentration that caused
50% inhibition of cell proliferation. Cell proliferation inhibited
(%) = [1-(OD of the experimental sample/OD of the control)] × 100%
(n=5).
Cell apoptosis assay
NCI-H460 cells were exposed to increasing
concentration of SAHA and the proportion of apoptotic cells was
quantified by Annexin V-FITC/PI dual staining (Beyotime).
SAHA-treated and untreated cells cultured for 12 h and resuspended
in 100 μl of binding buffer. Then cells were stained with 5
μl Annexin V-FITC and 10 μl PI for 15 min in the dark
at room temperature, then analyzed by flow cytometry (BD
Biosciences, CA, USA) within 1 h.
Hoechst 33342 staining
Cells were exposed to various concentration of SAHA
(5, 10 and 20 μM) for 12 h and cells without SAHA treatment
served as control. Cells were fixed in 4% paraformaldehyde for 10
min and permeabilized with 0.1% Triton X-100 for 5 min, then
stained with 10 μl Hoechst 33342 for 10 min. Finally cells
were viewed by a confocal microscope (Olympus, Tokyo, Japan).
Cell cycle assay
After 24-h treatment with 2.5 and 5 μM SAHA
in NCI-H460 cells, we performed DNA flow cytometric analysis to
study cell cycle distribution. Cell cycle distribution was
determined by DNA staining with PI. Briefly, cells were washed in
PBS and fixed in 70% ethanol overnight after treated with SAHA, the
next day cells were collected and resuspended in PBS containing 40
μg/ml PI, 0.1 mg/ml RNase (Beyotime), and 5% Triton X-100
and incubated at 37°C for 30 min. Finally cells were analyzed by
flow cytometry (BD Biosciences).
Western blot analysis
Lysates were prepared from 1×107 cells by
dissolving cell pellets in 100 μl of lysis buffer. Lysates
were centrifuged 12,000 g for 15 min at 4°C and the supernatants
were collected. Sodium dodecylsulfate-polyacrylamide gel
electrophoresis (SDS-PAGE) sample buffer was added and lysates were
heated at 100°C for 5 min and 50 μg of protein was loaded
into each well of 10% SDS-PAGE gels. Proteins were
electrophoretically transferred to nitrocellulose membranes blocked
with 5% non-fat milk or 5% BSA, and incubated overnight at 4°C with
the primary antibodies (AKT 1:500, p-AKT 1:500, p-ERK1/2 1:500,
ERK1/2 1:1,000, VEGF 1:400; HIF-1α 1:500 and β-actin 1:1,000). The
blots were washed, exposed for 1 h to corresponding HRP-conjugated
secondary antibodies, and detected using ECL (Pierce Biotechnology,
Rockford, IL, USA).
Nude mouse xenograft model
Four-week-old female BALB/c nude mice were purchased
from the Jackson Laboratory (Vital River, Beijing, China).
Experimental animals were performed according to the protocols
approved by the Institutional Animal Care and Use Committee of
Tongji Medical College. Mice were injected subcutaneously with
1×107 NCI-H460 cells per mouse into the right armpit.
When the maximum diameter of tumor reached 5 mm, mice were randomly
grouped into a blank group, a vehicle control group (DMSO) and SAHA
group. The tumors were observed and measured every day. The SAHA
group and DMSO group, respectively, received intraperitoneal
injection of SAHA (50 mg/kg/day, SAHA was first dissolved in DMSO,
then normal saline diluted to 0.2 ml per mouse, was added, the
final concentration of DMSO is 40%) or normal saline (0.2 ml/day,
40% DMSO) for 10 days.
Statistical analysis
All data are expressed as the mean ± SD of at least
3 independent experiments. Statistical differences between
experimental groups were analyzed by Student’s t-test, and ANOVA
(GraphPad Prism 5.0), P<0.05 was considered a statistically
significant difference.
Results
Effects of SAHA on the proliferation of
NCI-H460 cells and PBMCs
To explore the effect of SAHA on the growth of
NCI-H460 cells, MTT assay was used to assess the cell viability.
NCI-H460 cells were treated with increasing concentration of 1,
2.5, 5 and 10 μM SAHA for 24, 48 and 72 h, respectively.
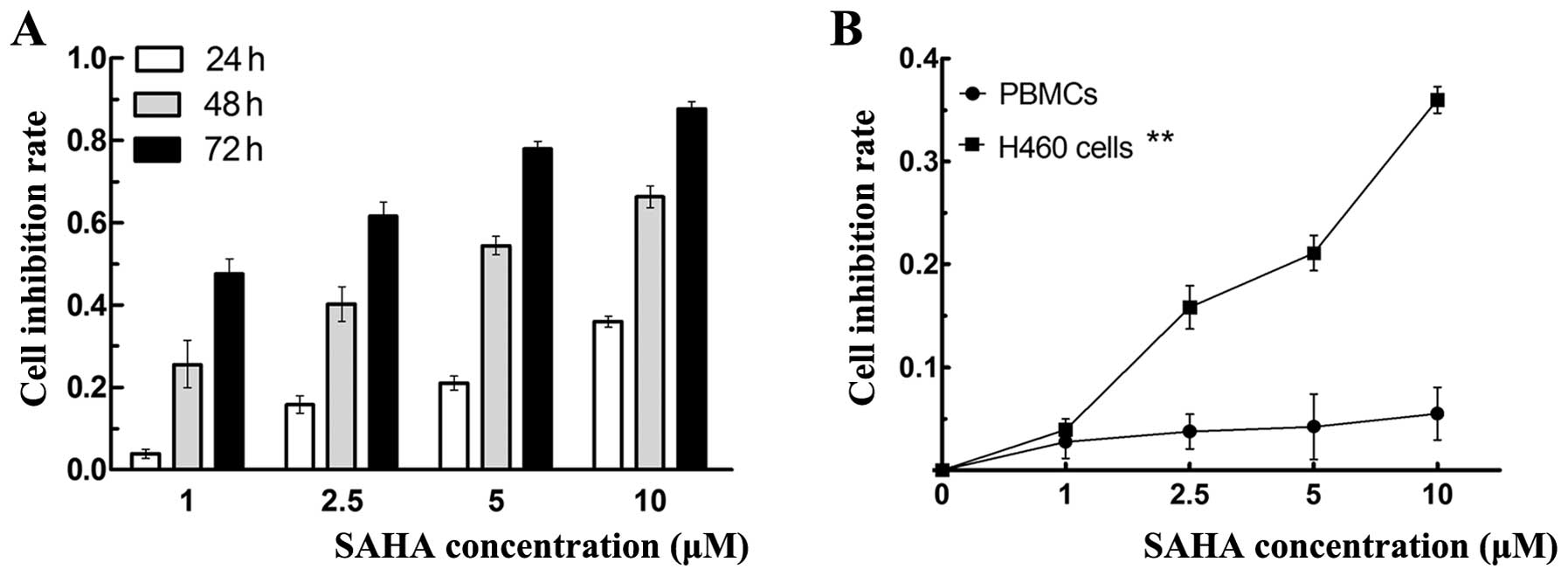
SAHA caused inhibition of cell viability in a time- and
dose-dependent manner as shown in Fig.
1A. The 24, 48 and 72 h IC50 of SAHA in NCI-460
cells were 43.23, 4.07 and 1.21 μM, respectively. The
IC50 values decreased gradually with time passing.
Fig. 1B shows the curve of growth
inhibition of SAHA on NCI-H460 cells and PBMCs. When treated with
various concentrations of SAHA for 24 h, SAHA inhibited the
proliferation of NCI-H460 cells in a dose-dependent manner, but had
no inhibitory effect on PBMCs.
Effects of SAHA on cell apoptosis of
NCI-H460 cells
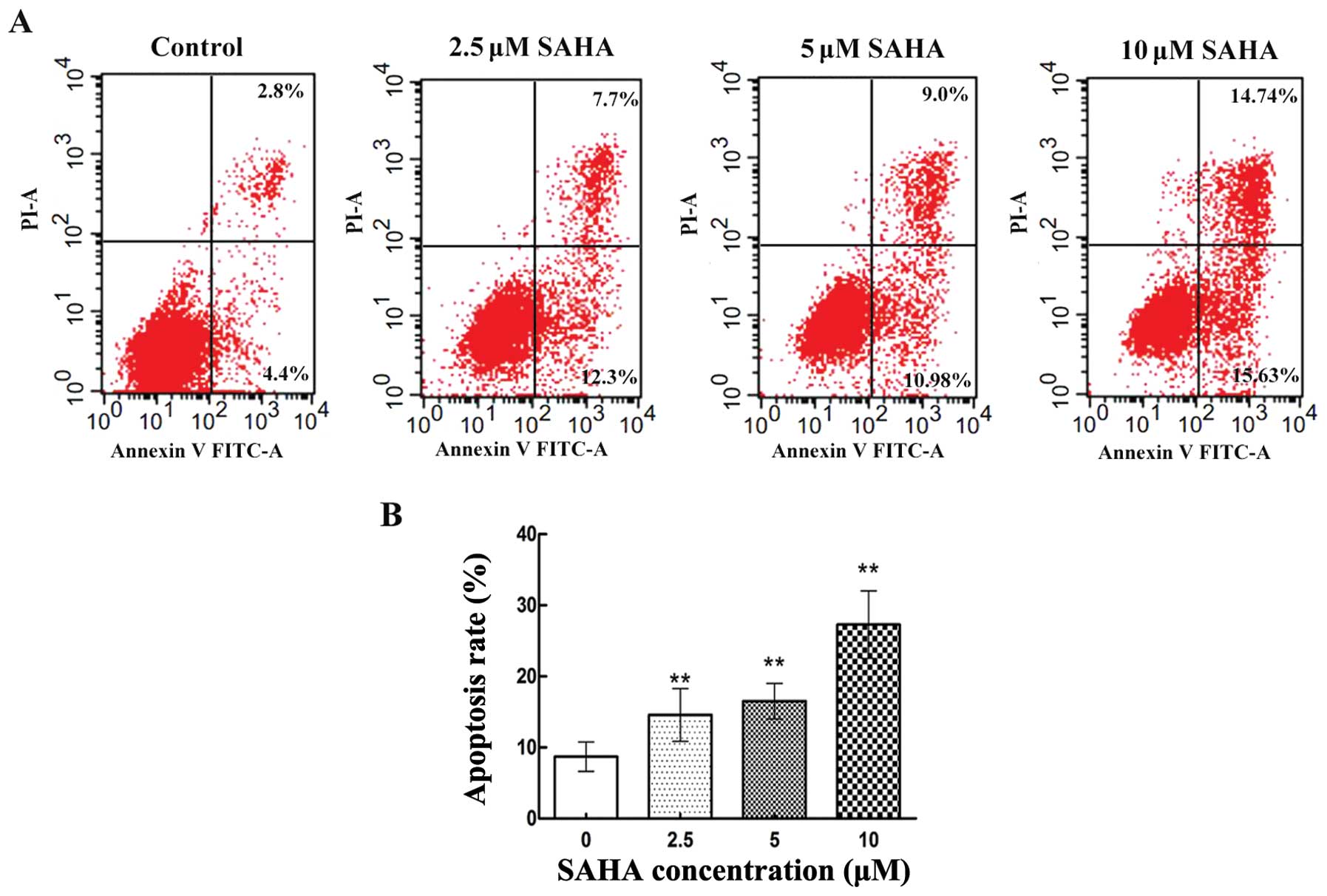
To determine whether the impaired cell viability of
NCI-H460 cells induced by SAHA involved apoptosis, NCI-H460 cells
were exposed to increasing concentration of SAHA. The proportion of
apoptotic cells was quantified by Annexin V-FITC/PI dual staining
cytometry. As shown in Fig. 2A,
untreated NCI-H460 cells exhibited little Annexin V staining. In
contrast, after treated with SAHA at 2.5, 5 and 10 μM for 12
h, NCI-H460 cells showed an increasing degree of Annexin V
staining. The apoptosis rate was 14.6±3.72, 16.5±2.49 and
27.3±4.74% respectively, which was statistically different from the
untreated cells (Fig. 2B). In
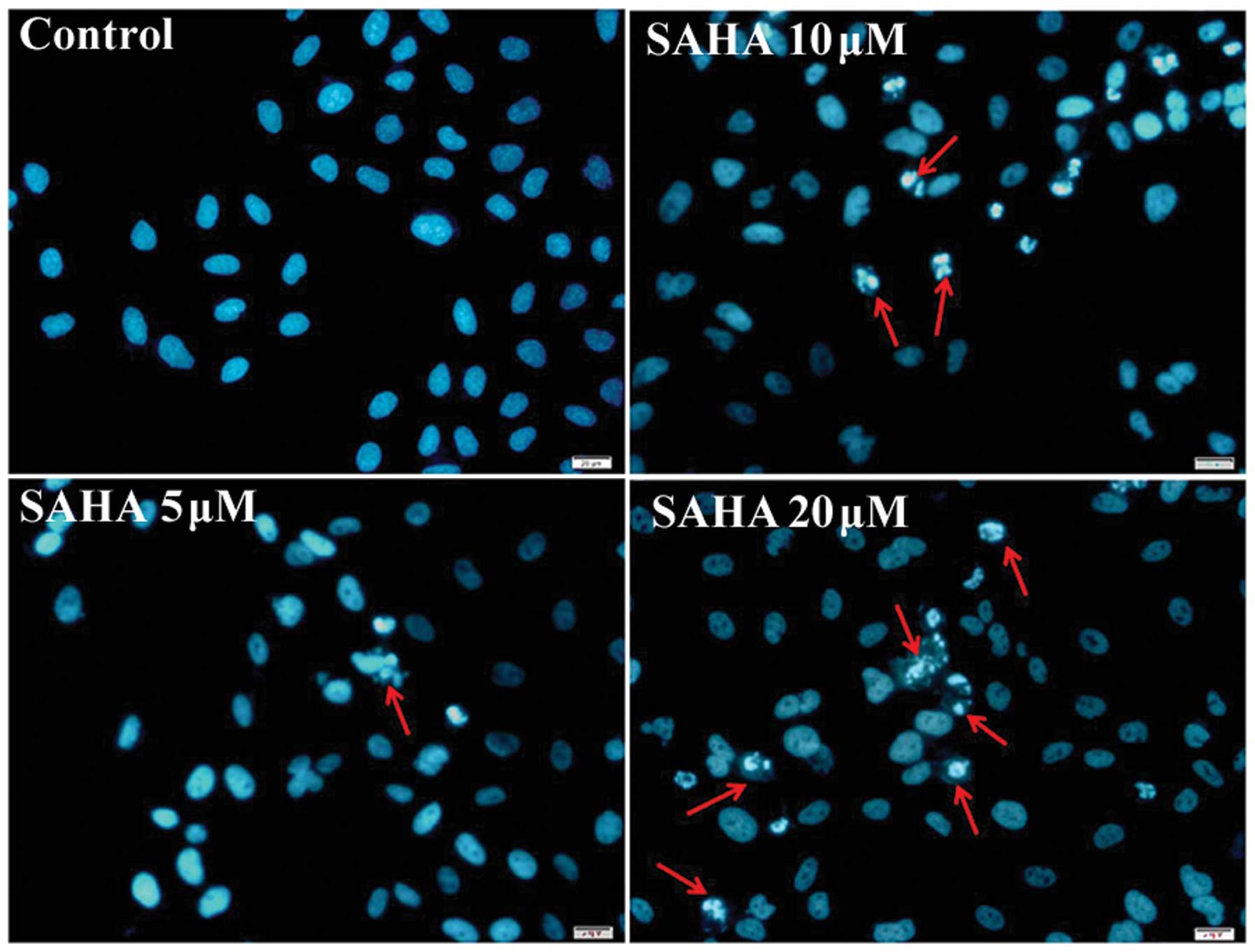
addition, we also assessed the effect of SAHA on apoptosis of
NCI-H460 cells by fluorescent Hoechst 33342 staining of cell
nucleus. Nucleus of cells in control was regular in shape, but a
part of the nucleus SAHA-treated cells were fragmented and
condensed, being typical apoptotic morphology (Fig. 3). The number of cells with
apoptotic morphology was increased progressively with SAHA
concentration.
Effects of SAHA on cell cycle of NCI-H460
cells
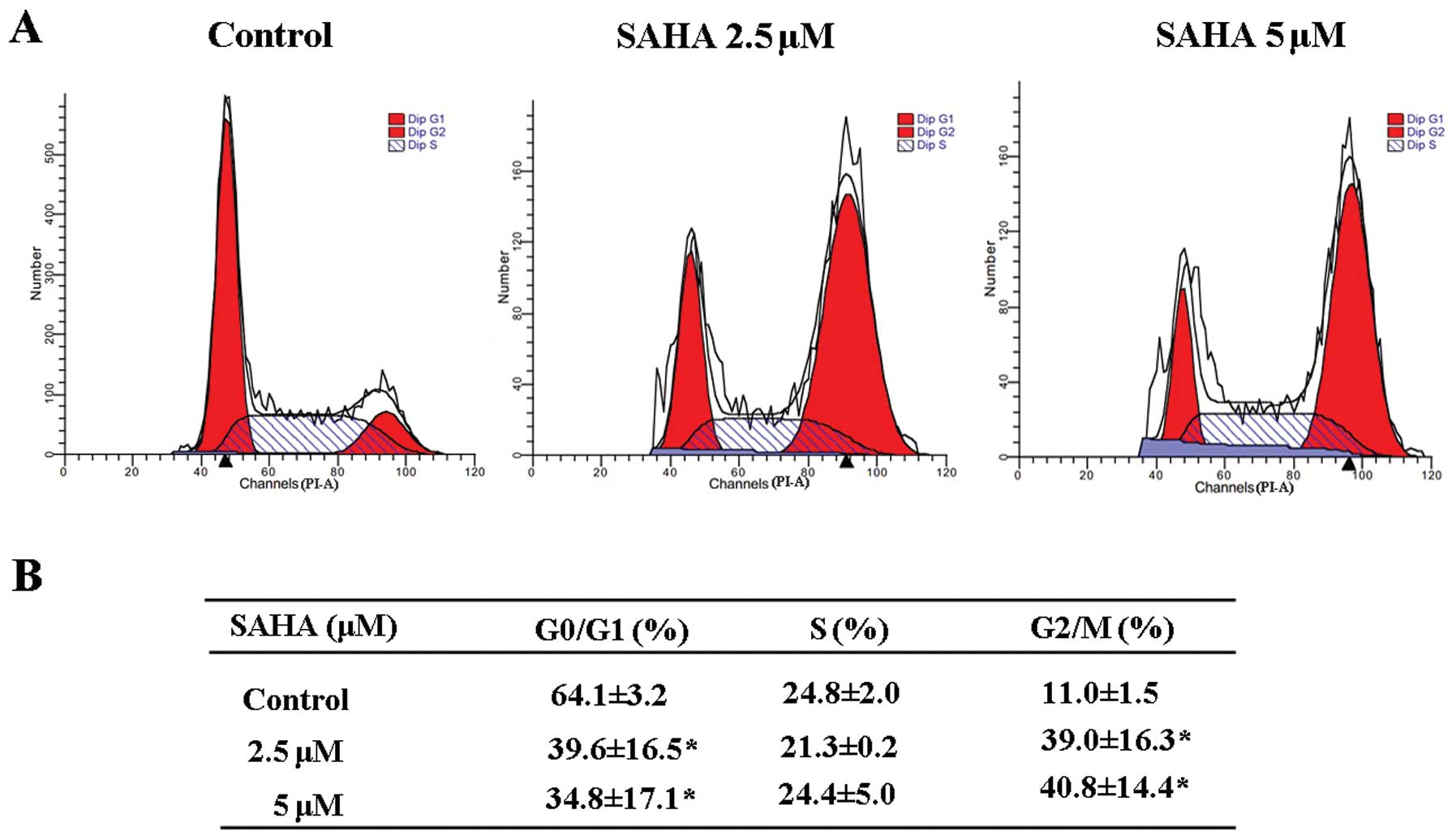
To assess whether SAHA affects the cell cycle, we
performed DNA flow cytometric analysis to study cell cycle
distribution. After 24-h treatment with 2.5 and 5 μM SAHA in
NCI-H460 cells, the cell proportion in the G2/M phase
increased gradually in a dose-dependent manner. The rate of
G0/G1 phase cells decreased accordingly and
the cells in S phase had no significant change (Fig. 4). This result indicated SAHA could
induce G2/M cell accumulation and cause G2/M
phase cell cycle arrest.
Effects of SAHA on the expression of AKT
and ERK signaling
Extensive studies showed that the activation of AKT
(phospho-AKT) and ERK1/2 (phospho-ERK1/2) play a crucial role in
tumor cell growth and survival, which regulated many related
factors for anti-apoptotic functions. To investigate whether SAHA
could affect the activation of AKT and ERK1/2, NCI-H460 cells were
treated with SAHA of 2.5, 5 and 10 μM for 24 h to detect the
protein expression. As a result, constitutively activated AKT
(phospho-AKT) and ERK (phospho-ERK1/2) were seen in untreated
cells. SAHA treatment induced a dose-dependent decline of
phospho-AKT and phospho-ERK1/2, while total AKT and total ERK1/2
proteins had no significant change (Fig. 5A).
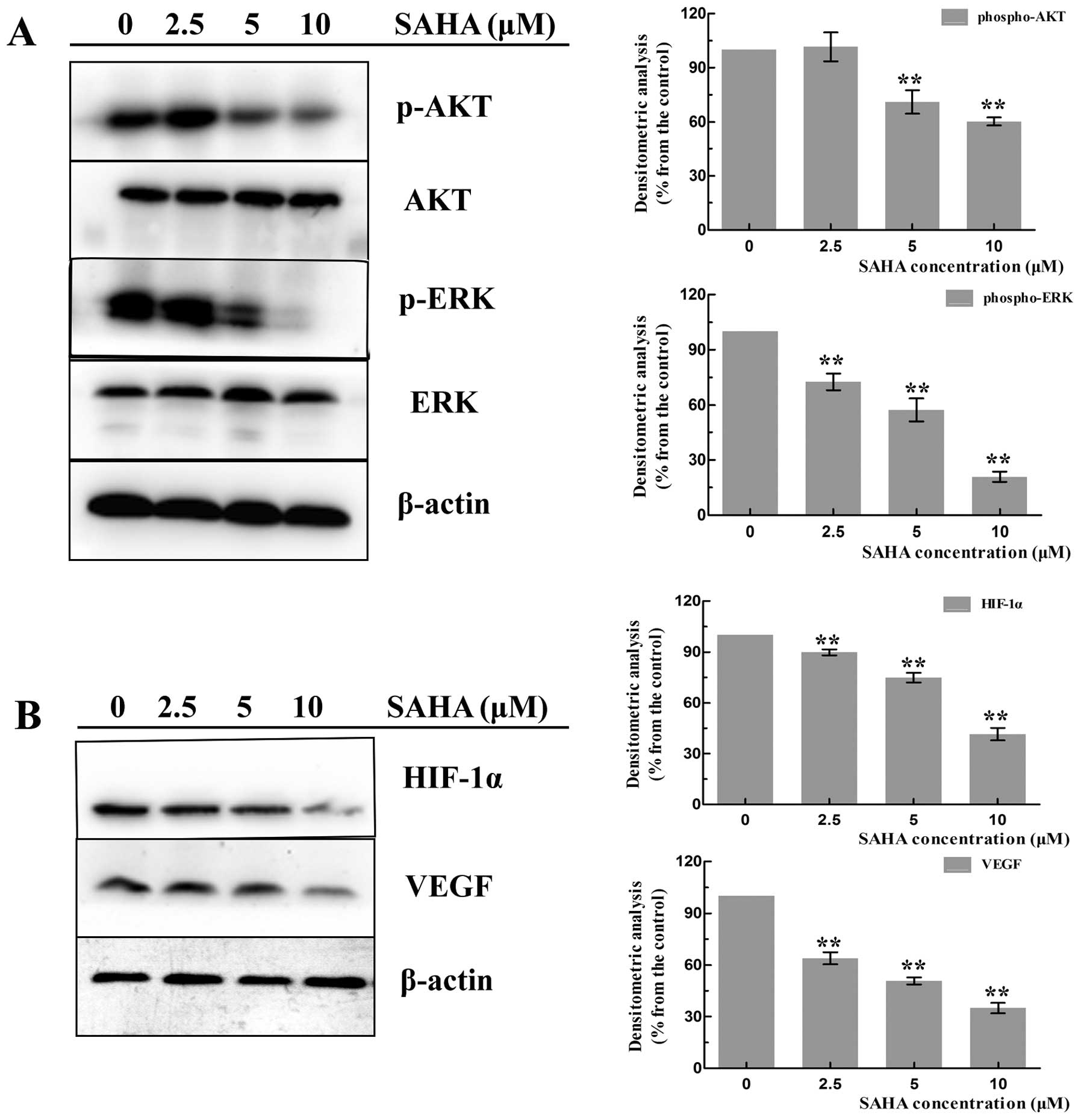 | Figure 5.Effects of SAHA on the
phosphorylation of AKT, ERK1/2 and VEGF, HIF-1α. NCI-H460 cells
were treated with increasing concentrations (0, 2.5, 5 and 10
μM) of SAHA for 18 h, then cell proteins were subjected to
western blotting with anti-phospho-AKT, anti-phospho-ERK1/2,
anti-total-AKT, anti-total-ERK1/2, anti-VEGF, anti-HIF-1α, β-actin
antibodies. (A) A representative western blotting of phospho-AKT,
phospho-ERK1/2, total-AKT, total-ERK1/2. The expression of
phospho-AKT and phospho-ERK1/2 proteins in SAHA-treated and
untreated cells was quantified by densitometry. Data represent the
mean ± SD of at least three separated experiments
(**P<0.01). (B) A representative western blotting of
VEGF, and HIF-1α. The expression of two proteins in SAHA-treated
and untreated cells were quantified by densitometry. Data represent
the mean ± SD of at least three separated experiments
(**P<0.01). |
Effects of SAHA on the expression of
HIF-1α and VEGF
We next determined the effects of SAHA on the
expression of proangiogenic factors. HIF-1α and VEGF are the two
important components in tumor angiogenesis. Many investigations
have found that SAHA could inhibit angiogenesis to play an
antitumor effect. To test this hypothesis in NCI-H460 cells, we
detected the expression of HIF-1α and VEGF in SAHA-treated and
untreated cells. As shown in Fig.
5B, SAHA-treated cells exhibited significant decreased levels
of HIF-1α and VEGF in a dose-dependent manner compare with the
untreated cells.
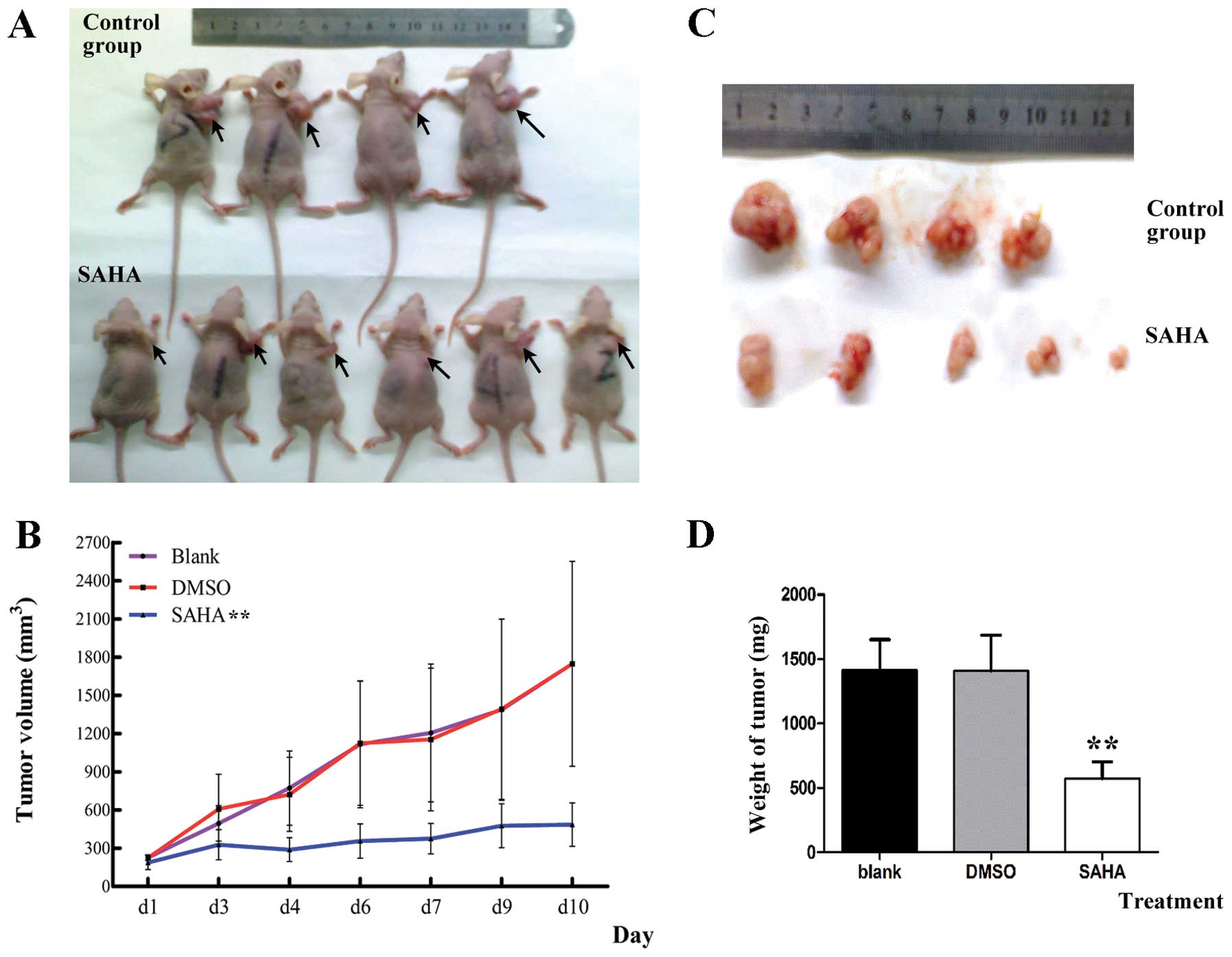
Effects of SAHA on the growth of NCI-H460
cell xenograft in vivo
To assess the therapeutic efficiency of SAHA in
vivo, a xenograft murine model of NCI-H460 cells was
established. After cell injections for 7 days, NCI-H460 cells
quickly developed a tumor at the site of subcutaneous injection. As
shown in Fig. 6A and B, compared
with vehicle control group (DMSO) and blank group, SAHA group
significantly reduced the tumor size (P<0.01). DMSO group had
almost no effect on the tumor and the tumor growth between control
and blank groups had no significant difference (P=0.99). The mice
were weighed every day, and no significant difference occurred in
their weights (data not shown). The mice were euthanized and tumors
were carefully dissected and weighed (Fig. 6C). Tumor weight of SAHA treatment
group was significantly less than control group and blank group
(P<0.01) (Fig. 6D).
Discussion
Chromatin protein acetylation is part of a complex
signaling system that is largely involved in the control of gene
expression (17). Histone
acetyltransferases and HDACs act in opposing manner to control the
acetylation state of nucleosomal histones. Epigenetic modification
by small-molecule HDAC inhibitors is a promising new
anti-neoplastic approach for various solid and hematological
malignancies (18). SAHA, a second
generation hybrid polar compound, is a potent pan-HDAC inhibitor
and has been clinically approved for treatment of cutaneous T-cell
lymphoma (CTCL) (18,19). HDACs act not only on histones, but
rather have many different cellular substrates and target proteins
involving physiological and pathological conditions, therefore SAHA
is able to exert a variety of anticancer activities in many tumor
types as a pan-HDAC inhibitor (20). It has been reported that SAHA
exhibited antitumor effects by prompting tumor cells to enter
apoptosis, interfering with the cell cycle, inducing DNA damage,
disturbing cell signaling, inhibiting tumor angiogenesis in many
solid and hematological tumors (20,21),
but few studies have been reported in large-cell lung carcinoma
(LCC).
LCC has the worst prognosis in NSCLC (2). Among subtypes of LCC, large-cell
neuroendocrine carcinoma (LCNEC) is the common type and the
prognosis of it was poorer than others even if at early stages
(22,23). The 5-year overall survival is
∼15–25% (23). The optimal
treatment of LCNEC has not been established. Because it is an
uncommon malignancy, prospective, randomized trials have not been
performed (23). Thus, there is an
urgent need for new drug that can target this kind of tumor cells
and benefit LCNEC patients. Since SAHA is able to inhibit tumor
progress and has been approved for treatment of CTCL, we proposed
that SAHA can also exert antitumor effects on LCNEC. For
confirmation, we chose the large-cell lung carcinoma neuroendocrine
cell line NCI-H460 (15,16) as our experimental cells and the
NCI-H460 cell nude mouse xenograft model to further verify the
effects of SAHA in vitro and in vivo.
We show that SAHA acted as potent chemotherapeutic
agent against LCNEC. Our data indicated that SAHA inhibited the
proliferation of NCI-H460 cells significantly, while had low
toxicity on human peripheral blood monocular cells (PBMCs). Its
biological effects on NCI-H460 cell growth varied with drug
concentration and duration of exposure. Cell cycle and apoptosis
detected by flow cytometry demonstrated that SAHA arrested NCI-H460
cells at G2/M phase and induced apoptosis in a
dose-dependent manner. With the increasing dose of SAHA (2.5, 5 and
10 μM for 12 h), the apoptosis rate was 14.6±3.72, 16.5±2.49
and 27.3±4.74%, respectively. NCI-H460 cells treated with SAHA also
manifested typical apoptotic morphological alterations with cell
nucleus presenting chromatin condensation or fragmented into
smaller structures by Hoechst 33342. Along with the drug dose
elevation, the cells of apoptotic morphology increased in number
accordingly. Further investigation indicated that since activities
of phospho-AKT and phospho-ERK1/2 both decreased in a
dose-dependent manner, inhibition of AKT and ERK1/2 signaling
seemed to be the potential mechanism of SAHA-induced
anti-proliferation effects. We also found that SAHA could inhibit
the expression of HIF-1α, VEGF of NCI-H460 cells dose-dependently.
Finally, we confirmed SAHA could significantly suppress the tumor
progression in the xenograft model, possibly as a result of
anti-proliferation and anti-angiogenesis effects of SAHA.
Extracellular growth factor-mediated signaling,
which is essential for cell proliferation, is frequently disrupted
in cancers. Activation of growth factor receptors leads to the
stimulation of numerous downstream pathways that modulate cell
metabolism, control gene transcription, and affect the cell cycle.
Of these pathways, the phosphatidylinositol 3-kinase (PI3K)/AKT and
mitogen-activated protein kinase (MAPK) pathways play a central
role (24). PI3K/AKT pathway is a
key signal transduction pathway that mediates cell growth and
blocks apoptosis (25). AKT, is
also called protein kinase B (PKB), plays an important role in cell
survival (26). Increased
activation of this survival cascade is a characteristic feature of
a large variety of human malignancies and has been associated with
carcinogenesis. Activation of AKT induce tumor cell proliferation
by regulating transcription factors which modulate distinct sets of
genes involved in cell cycle, apoptosis and DNA repair (27). MAPK cascades are also key signaling
pathways involved in the regulation of normal cell proliferation,
survival and differentiation. Aberrant regulation of MAPK cascades
contribute to cancer and other human diseases (28). ERK1/2 (extracellular signal
regulating kinase 1/2) are two isoforms that belong to the family
of MAPKs, which include ERK5, the c-Jun-NH2-terminal kinases
(JNK1/2/3) and the p38 MAP kinases (p38 MAPK). These enzymes are
activated through a sequential phosphorylation cascade that
amplifies and transduces signals (29). Our study showed that inhibition of
cell proliferation, intervention in the cell cycle and induction of
apoptosis by SAHA was accompanied by a downregulation of ERK1/2 and
AKT signaling, suggesting that SAHA by inhibiting HDACs in LCNEC
targets multiple proliferation and growth regulatory pathways. The
overexpression of ERK1/2 and AKT also confirmed that cell survival
signaling pathways may be the cause of LCNEC cell growth out of
control.
Angiogenesis is essential for the growth,
progression, and metastasis of solid tumors and efficient
inhibition of angiogenesis is considered a promising strategy for
the treatment of cancer (30).
Hypoxia induced factor-1α (HIF-1α) and vascular endothelial growth
factor (VEGF) are important pro-angiogenic factors in tumor
angiogenesis. HIF-1α plays a key role in tumor angiogenesis by
regulating the expression of angiogenic factors, including VEGF
(31). HIF-1α overexpression has
been implicated in many human cancers and is associated with
increased vascularization, drug resistance and poor diagnosis
(32,33). HIF-1α is acetylated and
hydroxylated in normal conditions, interacting with the
von-Hippel-Lindau and rapidly triggers degradation, but HIF-1α
accumulated in tumor hypoxia condition (33). SAHA can promote HIF-1α degradation
in tumor cells (20). It has been
reported that SAHA strongly impaired the hypoxia-induced secretion
of VEGF by neuroblastoma cells (34) and downregulated HIF-1α and VEGF in
a breast cancer cell line xenograft model (35). Our data showed SAHA could
downregulate the expression HIF-1α and VEGF in LCNEC and to
suppress tumor xenograft growth by its anti-proliferation and
anti-angiogenesis effects.
In conclusion, our data provide a novel
mechanism-based therapeutic intervention for LCC. SAHA can block
pathogenesis of aggressive LCC and may be utilized to treat LCC,
particularly in LCNEC patients.
Acknowledgements
This study was supported by the
Natural Science Foundation of China (no. 81172150) and the
Fundamental Research Funds for the Central Universities of China.
The authors would like to thank the Cancer Center, Union Hospital,
Tongji Medical College, Huazhong University of Science and
Technology, Wuhan, China, for offering relevant experimental
facilities and technical support.
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2.
|
Brambilla E: [Classification of
broncho-pulmonary cancers (WHO 1999)]. Rev Mal Respir. 19:455–466.
2002.(In French).
|
|
3.
|
Varlotto JM, Medford-Davis LN, Recht A, et
al: Should large cell neuroendocrine lung carcinoma be classified
and treated as a small cell lung cancer or with other large cell
carcinomas? J Thorac Oncol. 6:1050–1058. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sun JM, Ahn MJ, Ahn JS, et al:
Chemotherapy for pulmonary large cell neuroendocrine carcinoma:
similar to that for small cell lung cancer or non-small cell lung
cancer? Lung Cancer. 77:365–370. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Swarts DRA, Ramaekers FCS and Speel E-JM:
Molecular and cellular biology of neuroendocrine lung tumors:
evidence for separate biological entities. Biochim Biophys Acta.
1826:255–271. 2012.PubMed/NCBI
|
|
6.
|
Duvic M, Talpur R, Ni X, et al: Phase 2
trial of oral vorinostat (suberoylanilide hydroxamic acid, SAHA)
for refractory cutaneous T-cell lymphoma (CTCL). Blood. 109:31–39.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Olsen EA, Kim YH, Kuzel TM, et al: Phase
IIb multicenter trial of vorinostat in patients with persistent,
progressive, or treatment refractory cutaneous T-cell lymphoma. J
Clin Oncol. 25:3109–3115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Marchion D and Munster P: Development of
histone deacetylase inhibitors for cancer treatment. Expert Rev
Anticancer Ther. 7:583–598. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Fiskus W, Hembruff SL, Rao R, et al:
Co-treatment with vorinostat synergistically enhances activity of
Aurora kinase inhibitor against human breast cancer cells. Breast
Cancer Res Treat. 135:433–444. 2012. View Article : Google Scholar
|
|
10.
|
Lautz TB, Jie C, Clark S, et al: The
effect of vorinostat on the development of resistance to
doxorubicin in neuroblastoma. PLoS One. 7:e408162012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Muscal JA, Scorsone KA, Zhang L, Ecsedy JA
and Berg SL: Additive effects of vorinostat and MLN8237 in
pediatric leukemia, medulloblastoma, and neuroblastoma cell lines.
Invest New Drugs. 31:39–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Karelia N, Desai D, Hengst JA, Amin S,
Rudrabhatla SV and Yun J: Selenium-containing analogs of SAHA
induce cytotoxicity in lung cancer cells. Bioorg Med Chem Lett.
20:6816–6819. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Witta S: Histone deacetylase inhibitors in
non-small-cell lung cancer. J Thorac Oncol. 7:S404–S406. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kovacic P and Edwards CL: Hydroxamic acids
(therapeutics and mechanism): chemistry, acyl nitroso, nitroxyl,
reactive oxygen species, and cell signaling. J Recept Signal
Transduct Res. 31:10–19. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Osada H: ASH1 gene is a specific
therapeutic target for lung cancers with neuroendocrine features.
Cancer Res. 65:10680–10685. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lee M, Draoui M, Zia F, et al: Epidermal
growth factor receptor monoclonal antibodies inhibit the growth of
lung cancer cell lines. J Natl Cancer Inst Monogr. 117–123.
1992.PubMed/NCBI
|
|
17.
|
Backs J and Olson EN: Control of cardiac
growth by histone acetylation/deacetylation. Circ Res. 98:15–24.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Rangwala S, Zhang C and Duvic M: HDAC
inhibitors for the treatment of cutaneous T-cell lymphomas. Future
Med Chem. 4:471–486. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Marks PA: Discovery and development of
SAHA as an anticancer agent. Oncogene. 26:1351–1356. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
New M, Olzscha H and La Thangue NB: HDAC
inhibitor-based therapies: can we interpret the code? Mol Oncol.
6:637–656. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Khan O and La Thangue NB: HDAC inhibitors
in cancer biology: emerging mechanisms and clinical applications.
Immunol Cell Biol. 90:85–94. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Fernandez FG and Battafarano RJ:
Large-cell neuroendocrine carcinoma of the lung. Cancer Control.
13:270–275. 2006.PubMed/NCBI
|
|
23.
|
Gollard R, Jhatakia S, Elliott M and Kosty
M: Large cell/neuroendocrine carcinoma. Lung Cancer. 69:13–18.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Worster DT, Schmelzle T, Solimini NL, et
al: Akt and ERK control the proliferative response of mammary
epithelial cells to the growth factors IGF-1 and EGF through the
cell cycle inhibitor p57Kip2. Sci Signal. 5:ra192012.
View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Hsieh AC, Truitt ML and Ruggero D:
Oncogenic AKTivation of translation as a therapeutic target. Br J
Cancer. 105:329–336. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Engelman JA: Targeting PI3K signalling in
cancer: opportunities, challenges and limitations. Nat Rev Cancer.
9:550–562. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Cagnol S and Chambard JC: ERK and cell
death: mechanisms of ERK-induced cell death - apoptosis, autophagy
and senescence. FEBS J. 277:2–21. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Carmeliet P, Dor Y, Herbert JM, et al:
Role of HIF-1alpha in hypoxia-mediated apoptosis, cell
proliferation and tumour angiogenesis. Nature. 394:485–490. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Semenza GL: Evaluation of HIF-1 inhibitors
as anticancer agents. Drug Discov Today. 12:853–859. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar
|
|
34.
|
Muhlethaler-Mottet A, Meier R, Flahaut M,
et al: Complex molecular mechanisms cooperate to mediate histone
deacetylase inhibitors anti-tumour activity in neuroblastoma cells.
Mol Cancer. 7:552008. View Article : Google Scholar
|
|
35.
|
Shankar S, Davis R, Singh KP, Kurzrock R,
Ross DD and Srivastava RK: Suberoylanilide hydroxamic acid
(Zolinza/ vorinostat) sensitizes TRAIL-resistant breast cancer
cells orthotopically implanted in BALB/c nude mice. Mol Cancer
Ther. 8:1596–1605. 2009. View Article : Google Scholar
|