Introduction
Breast cancer is one of the most common malignant
tumors and is the leading cause of cancer-related deaths in women
worldwide (1). Five-year survival
rate for tumor confined to the breast has increased to ∼80–90% over
the last decade. However, approximately one-third of breast cancer
patients still die from the disease once tumor metastasized to
other organs (2).
Major treatment strategies for breast cancer
consist, either separately or in combination of, radio therapy,
surgery and chemotherapy (3). Many
agents used to treat breast cancer are often associated with severe
systemic toxicities. Acquired tumor drug resistance also limits
their use in the treatment of breast cancer. Therefore, novel
non-toxic therapeutic agents active against breast cancer are under
investigation, with the need to develop new agents acting on novel
targets.
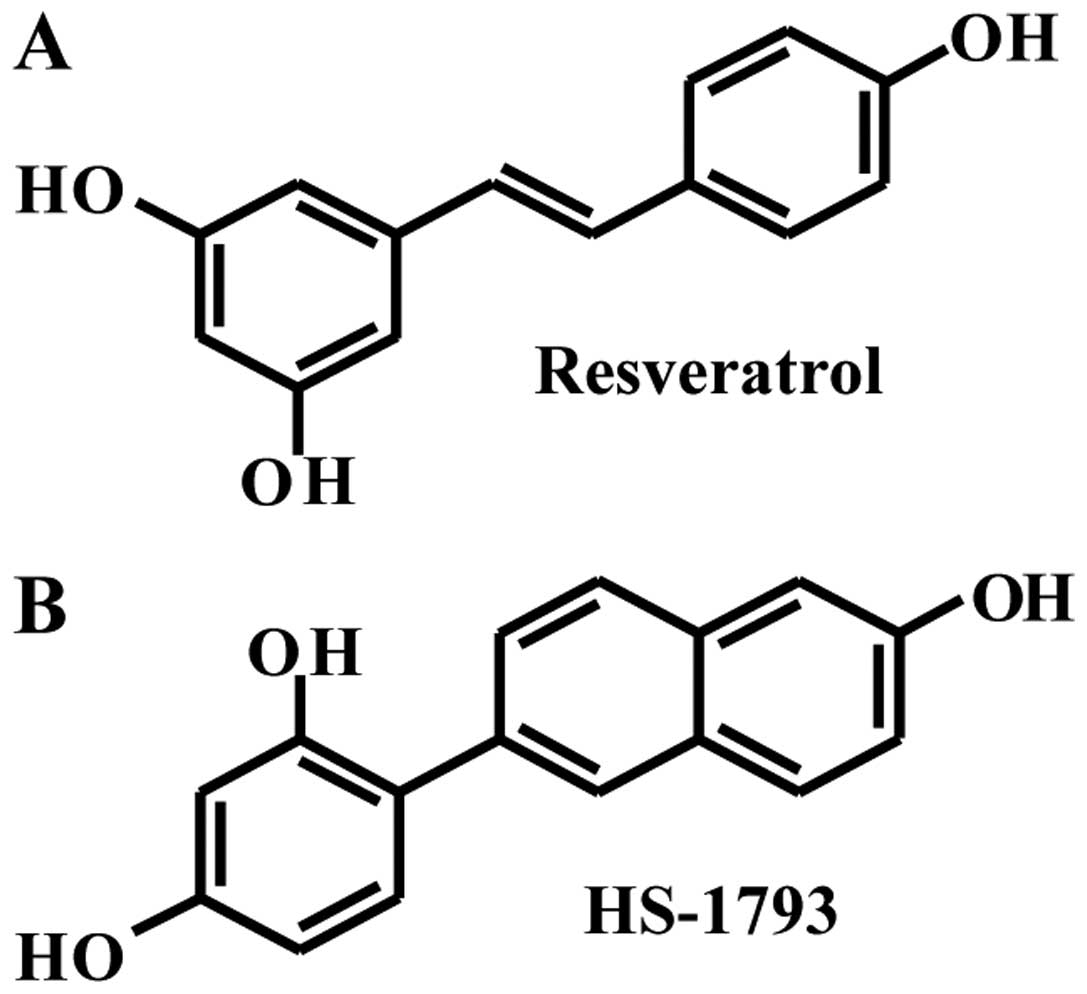
Resveratrol (trans-3,4,5'-trihydroxydtilbene,
Fig. 1A) is a natural polyphenol
compound (4,5). It has been reported to exhibit a wide
range of biological and pharmacological properties. It exists in
two isoforms: trans-resveratrol and cis-resveratrol;
however, the trans-isomer is more stable than
cis-resveratrol. Resveratrol-glucuronide is the major form
absorbed when compared to the very minute amounts of unconjugated
resveratrol and resveratrol sulfate are absorbed (6). Resveratrol has been reported to
induce apoptosis in various cancerous or transformed cells in
culture, chemically induced mouse skin tumors, and in transplanted
tumors in nude mice by activating both extrinsic and intrinsic
pathways of cell death machinery (7,8).
Resveratrol has shown to inhibit three major stages of
carcinogenesis: initiation, promotion and progression (9). However, exposure to high doses of
resveratrol is required to induce apoptosis in cancer cells. In
addition, resveratrol is photosensitive and metabolically unstable,
with stilbene double bonds being readily oxidized (3,10).
In previous studies, the resveratrol analog HS-1793
(Fig. 1B) overcame the resistance
conferred by Bcl-2 in U937 leukemia cells (11). In addition, HS-1793 induced higher
anti-tumor activity in various cancer cell lines (12–14)
and inhibited hypoxia-induced HIF-1 and VEGF expressions (15). However, detailed apoptosis
mechanisms at work are not yet well understood. Therefore, the
purpose of the present study was to investigate the
anti-proliferation and anti-apoptotic potential of HS-1793 and to
evaluate the signal pathway involved in relation to wild-type or
mutant p53 protein in human breast cancer cells, such as MCF-7
(wild-type p53) and MDA-MB-231 (mutant p53) cells.
Materials and methods
Chemicals
trans-3,4,5'-Trihydroxystilbene (resveratrol)
was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
4-(6-Hydroxy-2-naphthyl)-1,3-benzendiol (HS-1793) was supplied by
Professor Hongsuk Suh (Pusan National University, Busan, Korea),
and dissolved at a concentration of 100 mM in ethanol as a stock
solution, and stored −20°C. The stock solution was diluted with
cell culture medium to the desired concentration prior to use. The
maximal concentration of ethanol did not exceed 0.1% (v/v) in the
treatment range, where there was no influence on the cell
growth.
Cell culture
MCF-7 (wild-type p53) and MDA-MB-231 (mutant type
p53) cells were obtained from American Type Culture Collection
(Manassas, VA, USA). MCF-7 and MDA-MB-231 cells were maintained in
DMEM medium (HyClone, Logan, UT, USA) in humidified atmosphere of
37°C with 5% CO2. DMEM supplemented with 10%
heat-inactivated fetal bovine serum (FBS, HyClone), 2 mM glutamine
(Sigma-Aldrich), 100 U/ml penicillin (HyClone) and 100 μg/ml
streptomycin (HyClone).
MTT assay and growth inhibition
Cell survival was determined by colorimetric
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay which measures mitochondrial activity in viable cells. Cells
(1.5×105) were plated in each well of a 6-well plate,
allowed to adhere overnight and then the culture medium was
replaced with fresh media. Cells were treated with resveratrol or
HS-1793 at concentrations of 12.5, 25 and 50 μM for 24 h.
Control groups were treated with ethanol equal to the highest
percentage of (<0.1%) solvent used in experimental conditions
for MTT assay. After 24 h the medium was replaced with fresh
medium. MTT was freshly prepared at 5 mg/ml in PBS and passed
through a filter (pore size, 0.2 μm). An aliquot of 2 ml of
MTT stock solution was added to each well, and the plate was
incubated at 37°C for 4 h in humidified 5% CO2
incubator. After 4 h, media were removed and formazan crystals were
dissolved in 2 ml of DMSO for 10 min with gentle agitation. The
optical density of each well was measured with a spectrophotometer
equipped with a 540-nm filter.
Protein preparation and western blot
analysis
Cells were harvested and solubilized in lysis buffer
(40 mM Tris, pH 8.0, 120 mM NaCl, 0.5% NP-40, 0.1 mM sodium
orthovanadate, 2 μg/ml aprotinin, 2 μg/ml leupeptin
and 100 μg/ml phenylmethylsulfonyl fluoride), and the
supernatant was collected and protein concentrations were then
measured with a Bio-Rad protein assay kit (Bio-Rad, Hercules, CA,
USA) with bovine serum albumin as the standard. Equal amount of
protein extracts were denatured by boiling at 100°C for 5 min in
sample buffer (0.5 M Tris-Cl, pH 6.8, 4% SDS, 20% glycerol, 0.1%
bromophenol blue, 10% β-mercaptoethanol) in ratio of 1:1. Equal
amount of the total proteins were subjected to 6–15%
SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to
polyvinylidene difluoride (PVDF) membrane. The membranes were
blocked with 5% non-fat dry milk in TBS-T buffer (20 mM Tris, 100
mM NaCl, pH 7.5 and 0.1% Tween-20) for 1 h at room temperature. The
membrane was incubated with diluted primary antibody in TBS-T
buffer at 4°C overnight. The membranes were washed once for 10 min
3 times with TBS-T buffer and incubated for 1 h with secondary
antibody in TBS-T buffer at room temperature.
Hoechst staining and observation of
nuclear structure
MCF-7 and MDA-MB-231 cells were incubated with 12.5,
25 and 50 μM resveratrol or HS-1793 for 24 h and then cells
were washed twice with PBS containing 1% bovine serum albumin
(PBS-B) and fixed with 70% ethanol containing 0.5% Tween-20 at 4°C
for 30 min. Fixed cells were washed with PBS-B, and stained with 4
μg/ml Hoechst 33342 for 30 min at room temperature. The
stained cells were washed twice with PBS-B and observed under Zeiss
Axiophot microscope (Göttingen, Germany) at ×400 magnification.
Flow cytometric analysis
Cells were trypsinized, washed with PBS, and fixed
in 95% ethanol with Tween-20 at 4°C overnight. Prior to analyses,
cells were again washed with PBS, suspended in cold propidium
iodide (PI, Sigma-Aldrich) solution, and incubated at room
temperature for 30 min in the dark. Before analysis cell
suspensions were filtered with 40 μM pore nylon mesh to
remove debris. Flow cytometry analysis was performed on a FACScan
(Becton Dickinson, San Jose, CA, USA).
Immunoprecipitation and western blot
analysis
Total cell lysates were lysed in lysis buffer. The
supernatant was collected and protein concentration determined with
Bio-Rad protein assay kit (Bio-Rad). For immunoprecipitation, cell
extracts were incubated with immunoprecipitating antibody in lysis
buffer at 4°C for 1 h. The immuno-complexes were precipitated with
protein A-sepharose beads (Sigma-Aldrich) for 1 h, and washed five
times with extraction buffer prior to boiling in SDS sample buffer.
Immunoprecipitated proteins or aliquots containing 40 μg
protein were separated on SDS-PAGE and transferred to PVDF
membranes. Western blot analysis was performed. Primary antibodies
to p53, MDM2, p21WAF1/CIP1, cyclin D1, CDK4,
CDK6, cyclin B1, Cdc2, Cdc25C, Fas, Fas-L, PARP, Bax, Bcl-2,
ERK1/2, pERK, JNK and pJNK were purchased from Santa Cruz
Biotechnology Inc. (Santa Cruz, CA, USA). Primary antibody to
β-actin was purchased from Sigma-Aldrich. The proteins were
visualized with enhanced chemiluminescence (ECL) detection system
(GE Healthcare Biosciences, Pittsburgh, PA, USA).
Caspase activity assay
Cells were harvested and washed twice in PBS at 4°C.
Total cells were lysed with the lysis buffer at 4°C for 30 min with
vortexing. Cell lysate (200 μg) was mixed with assay buffer
in a final volume of 100 μl, followed by addition of 10
μl of 2 mM of p-nitroaniline (pNA)-conjugated caspase-8
(Z-IETD-pNA), caspase-9 (Ac-LEHD-pNA), or caspase-3 (Z-DEVD-pNA)
substrates, respective, for the caspase assay. The reaction mixture
was incubated at 37°C for 30 min and the liberated pNA was measured
at 405 nm.
Statistical analysis
Results are expressed as the mean ± SD of three
separate experiments and analyzed by Student's t-test. Means were
considered significantly different at p<0.05 or p<0.01.
Results
HS-1793 suppresses proliferation of MCF-7
and MDA-MB-231 cells
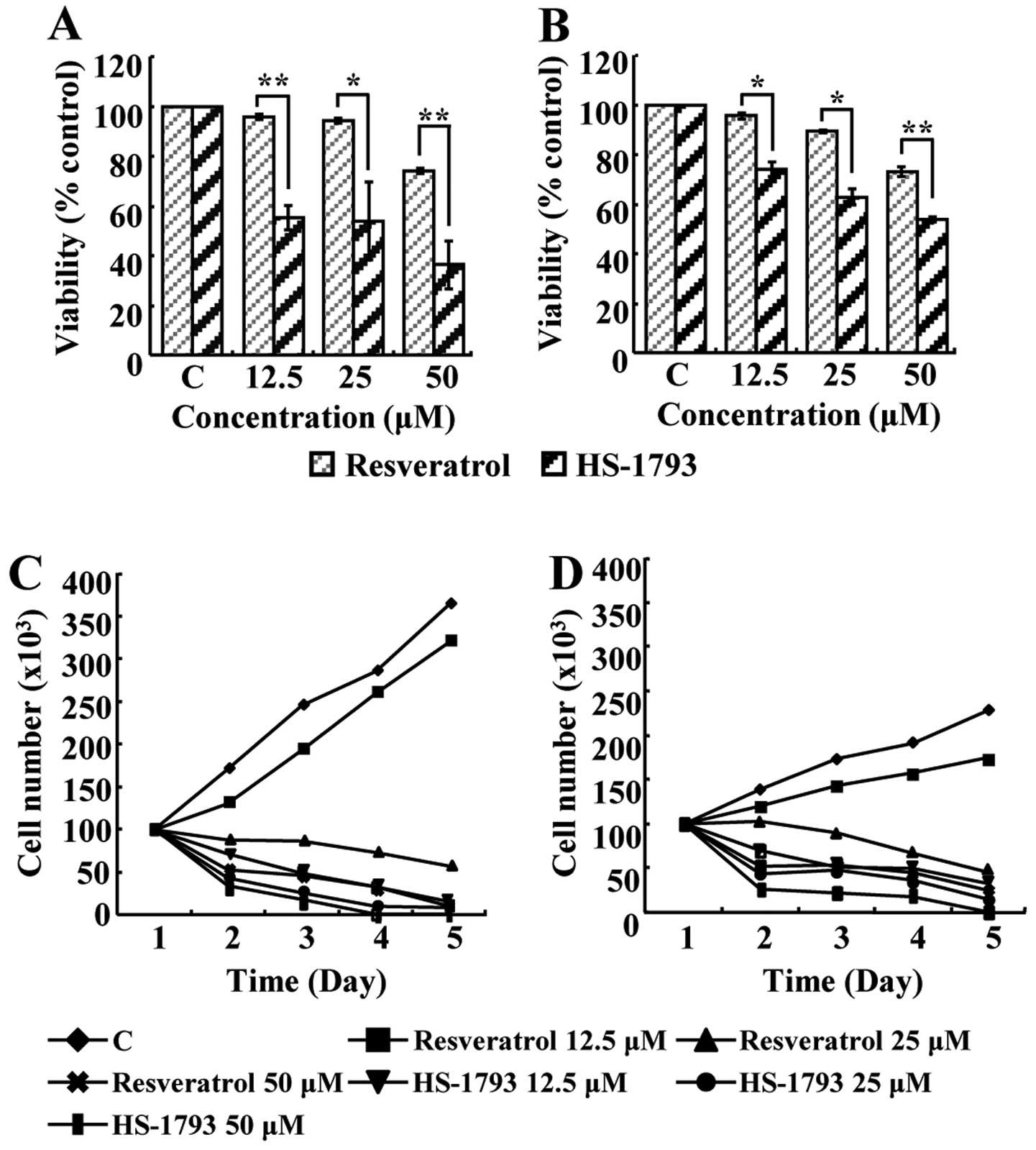
To investigate the effects of resveratrol and
HS-1793 on the viability of MCF-7 and MDA-MB-231 cells, the MTT
assay was performed. Resveratrol did not show any prominent
effects, with IC50 values not being measurable at the
concentration of 12.5, 25 and 50 μM. However, the
IC50 values of HS-1793 in MCF-7 and MDA-MB-231 cells
were 25 μM and 50 μM, respectively (Fig. 2A and B). Therefore, HS-1793 seems
to induce more efficient inhibition of cell viability than
resveratrol. Cell proliferation was also evaluated by counting dead
and live cell numbers by the trypan blue exclusion method. Results
indicated that resveratrol and HS-1793 exerted time- and
concentration-dependent inhibition of cell proliferation in both
MCF-7 and MDA-MB-231 cells (Fig. 2C
and D). Although both resveratrol and HS-1793 exhibited
anti-proliferative effect on both breast cancer cells, HS-1793 was
more potent than resveratrol.
HS-1793 modulates the cell cycle in MCF-7
and MDA-MB-231 cells
We next investigated whether resveratrol and HS-1793
affect cell cycle progression. Cells were treated with either
resveratrol (12.5, 25 and 50 μM) or HS-1793 (3, 6.25 and
12.5 μM) for 24 h and then the cell cycle was analyzed by
flow cytometric analysis. As shown in Table I, HS-1793 inhibited the cell growth
through G2/M phase arrest, while resveratrol led to G1 phase arrest
in MCF-7 (16) and MDA-MB-231
(17) cells as already
published.
 | Table I.Effect of resveratrol or HS-1793 on
cell cycle phase distribution of MCF-7 and MDA-MB-231
cells.a |
Table I.
Effect of resveratrol or HS-1793 on
cell cycle phase distribution of MCF-7 and MDA-MB-231
cells.a
| Cells | Phase | Resveratrol | HS-1793 |
|---|
|
|
|---|
| Control | 12.5 μM | 25 μM | 50 μM | Control | 3.0 μM | 6.25 μM | 12.5 μM |
|---|
| MCF-7 | G0/G1 (%) | 52.6 | 28.4 | 63.1 | 70.5 | 52.6 | 84.0 | 62.2 | 13.8 |
| S (%) | 41.7 | 71.6 | 35.5 | 29.5 | 41.7 | 10.0 | 20.1 | 18.8 |
| G2/M (%) | 5.7 | 0 | 1.4 | 0 | 5.7 | 6.0 | 17.7 | 67.4 |
| MDA-MB-231 | G0/G1 (%) | 54.0 | 36.6 | 55.0 | 69.1 | 54.0 | 50.4 | 39.0 | 9.3 |
| S (%) | 40.8 | 62.6 | 45.0 | 30.9 | 40.8 | 36.5 | 30.8 | 26.5 |
| G2/M (%) | 5.2 | 0.8 | 0 | 0 | 5.2 | 13.1 | 30.2 | 64.2 |
HS-1793 modulates the cell cycle
regulatory proteins in MCF-7 and MDA-MB-231 cells
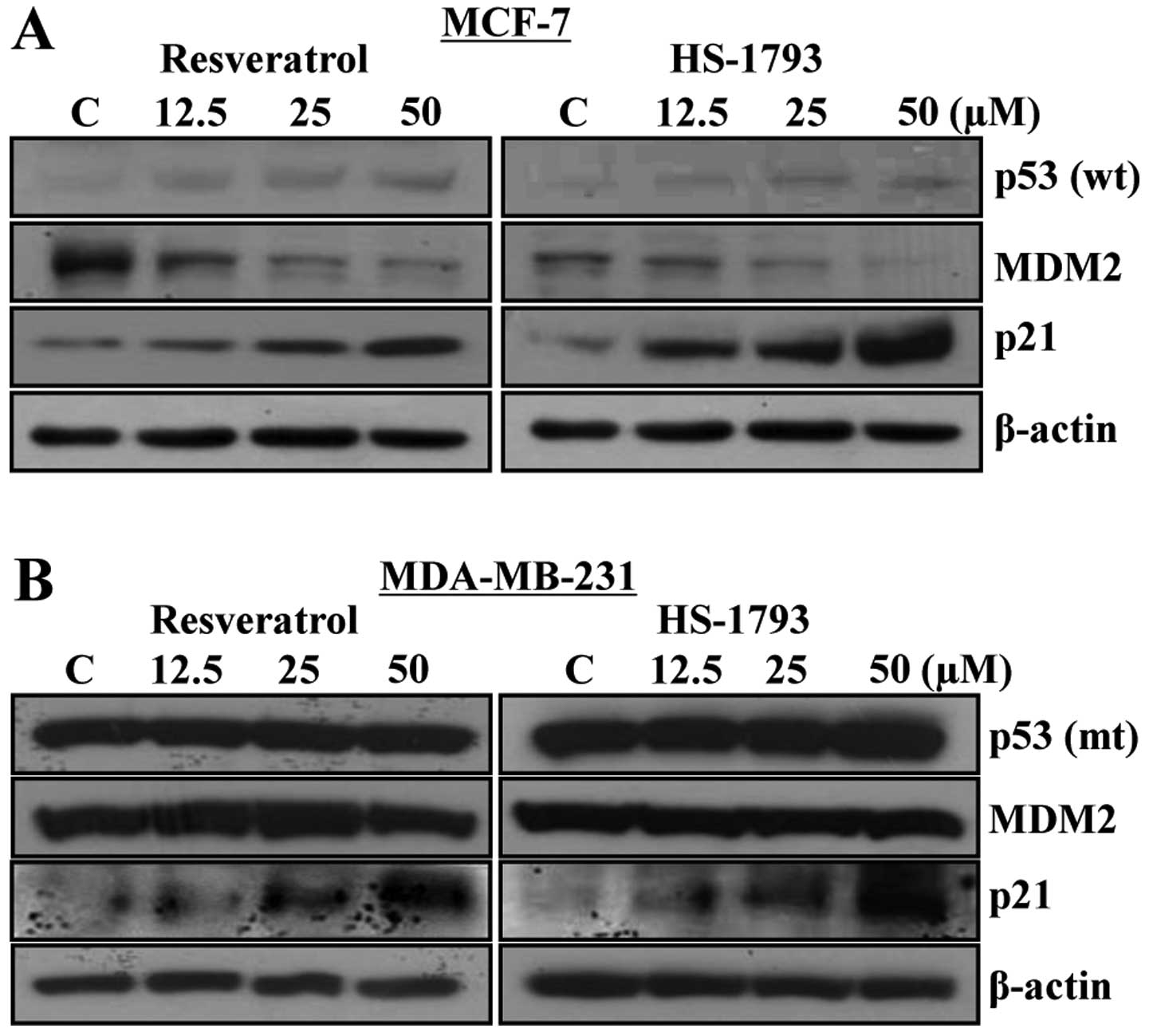
Generally, p53 is known as a tumor suppressor gene
and controls the expression of p21WAF1/CIP1, a
potent cyclin-dependent kinase (CDK) inhibitors in G1 and G2/M
phases. MDM2, an oncogene, negatively regulates p53 through
inhibiting the transactivation activity of p53 by binding to its
transactivation domain. MDM2 has also a ubiquitin ligase activity
that leads to the degradation of p53 (18,19).
In MCF-7 cells, p53 and p21WAF1/CIP1 were
increased while MDM2 was decreased by both resveratrol and HS-1793.
In contrast, in MDA-MB-231 cells, p21WAF1/CIP1
was increased without a change in the level of MDM2. Given that
MDA-MB-231 cells are p53 mutant, there might be a p53-independent
stimulus inducing the increase in p21WAF1/CIP1
(Fig. 3A and B). Western blot
analyses were conducted to further characterize the molecular
mechanisms by which resveratrol or HS-1793 inhibits cell growth.
The levels of intracellular proteins of G1 phase, such as cyclin
D1, CDK4 and CDK6, were not significantly changed (Fig. 4A). Immunoprecipitation was
conducted to investigate binding activity of the cyclin D1-CDK4
complex which showed a decrease of the complex in both cell types
(Fig. 4B). Cyclin B1, Cdc2 and
Cdc25C, which are proteins of G2 phase, were decreased in both cell
lines by HS-1793 treatment in a concentration-dependent manner
(Fig. 4C). Cdc2 and cyclin B1
interaction was also inhibited by HS-1793 treatment (Fig. 4D).
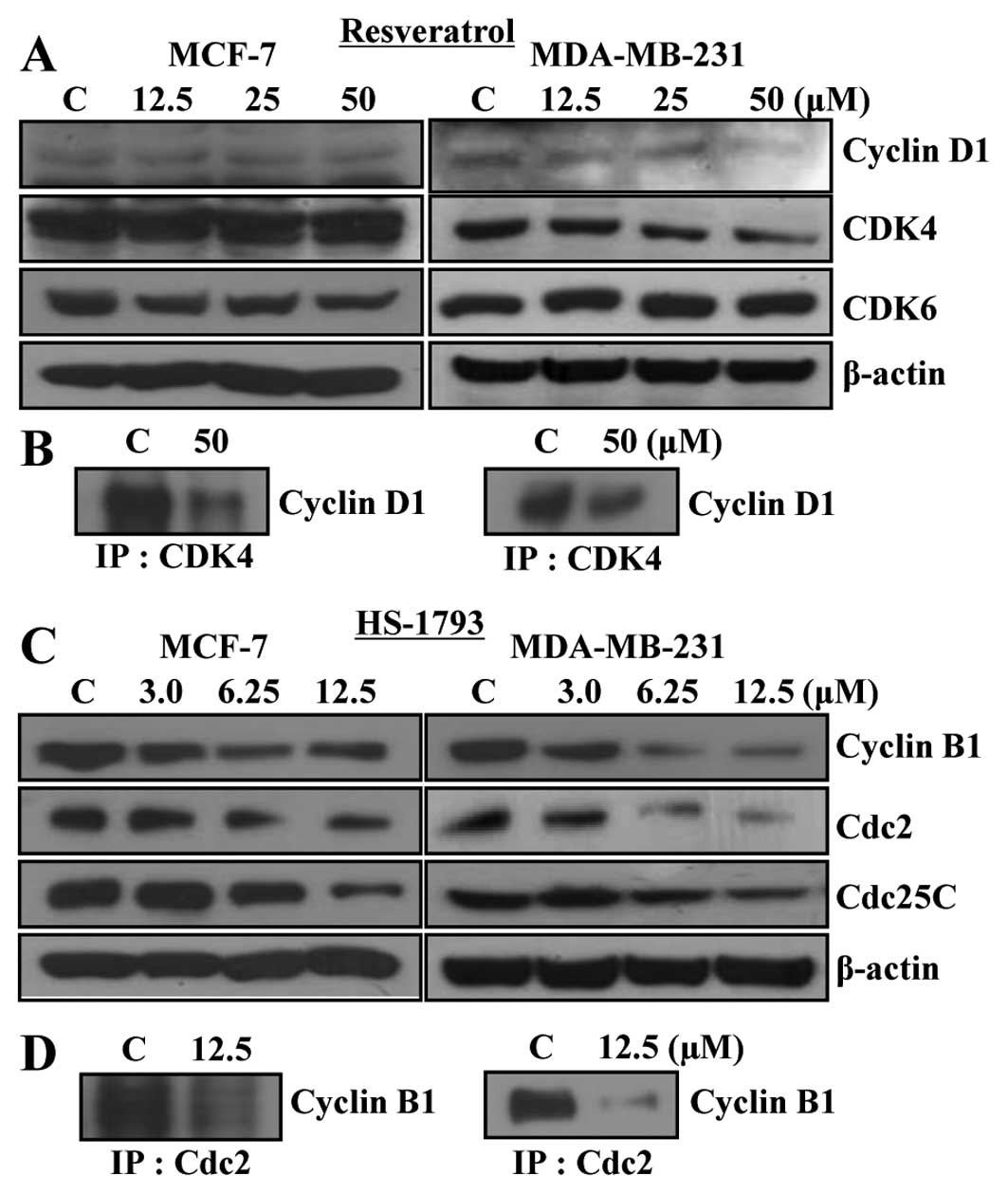 | Figure 4.Effects of resveratrol and HS-1793 on
the protein levels of cyclins and CDKs in MCF-7 and MDA-MB-231
cells. (A) To detect the protein levels of cell cycle regulators in
G1 phase such as cyclin D1, CDK4 and CDK6, MCF-7 and MDA-MB-231
cells were treated with various concentration of resveratrol for 24
h, collected, lysed and then cellular proteins were separated and
immunoblotted. (B) After treatment with resveratrol for 24 h, cell
lysates were immunoprecipitated with anti-CDK4 antibody, separated
on SDS-PAGE, transferred on to PVDF membrane, cyclin D1 protein
levels were detected with anti-cyclin D1 antibody and ECL detection
system. (C) To detect the protein levels of cell cycle regulators
in G2/M phase such as cyclin B1, Cdc2 and Cdc25C, MCF-7 and
MDA-MB-231 cells were treated with various concentration of HS-1793
for 24 h, collected, lysed and then cellular proteins were
separated and immunoblotted. (D) After treatment with HS-1793 for
24 h, cell lysates were immunoprecipitated with anti-Cdc2 antibody,
separated on SDS-PAGE, transferred to PVDF membranes, cyclin B1
protein levels were detected with anti-cyclin B1 antibody and ECL
detection system. Representative results from three independent
experiments are shown. Actin was used as a loading control. C,
control. |
HS-1793 induces apoptotic cell death in
MCF-7 and MDA-MB-231 cells
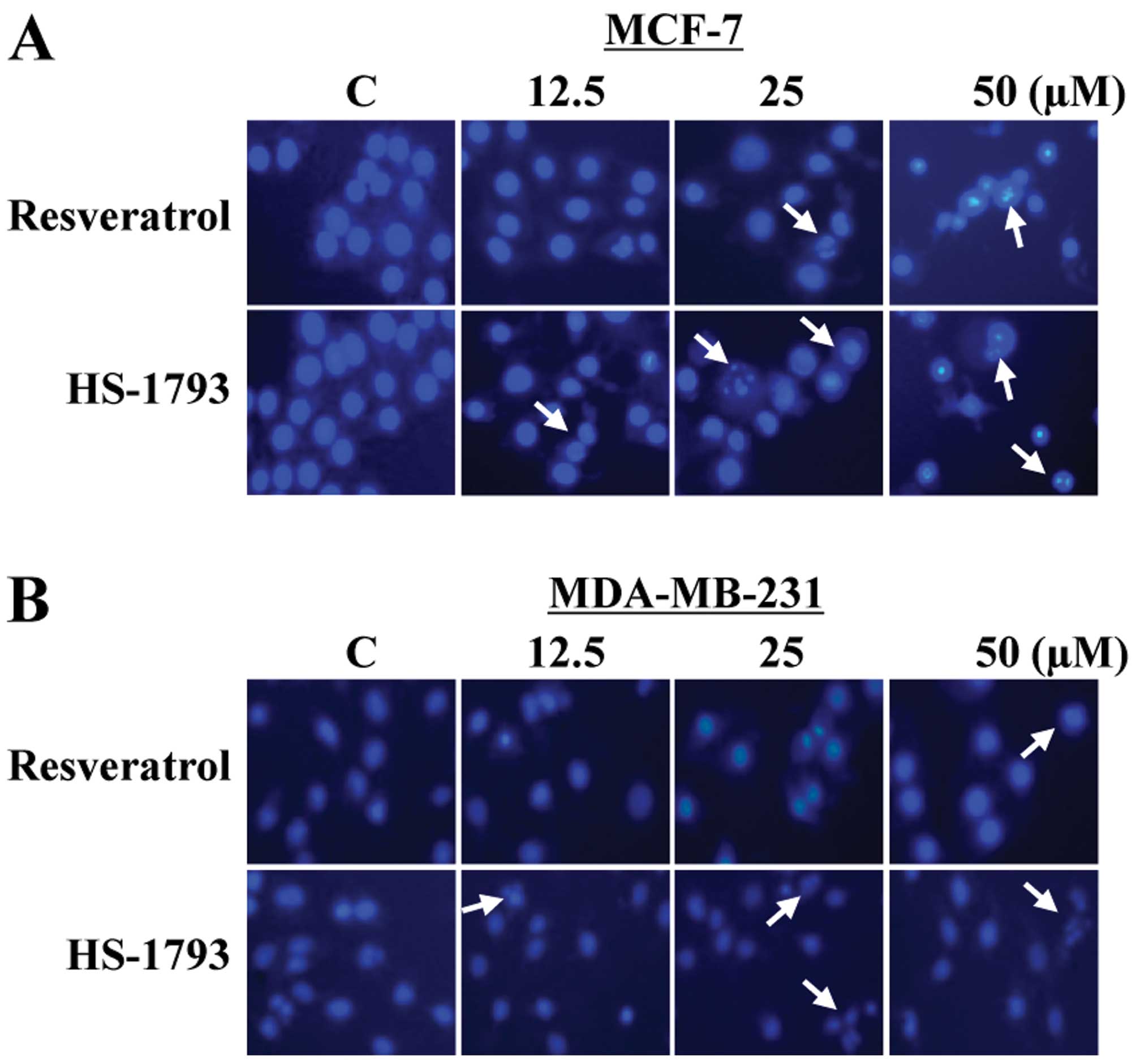
To determine whether the growth inhibitory effects
of resveratrol and HS-1793 could be attributed to apoptotic cell
death in MCF-7 and MDA-MB-231 cells, the morphological changes were
assessed with Hoechst 33342 staining (Fig. 5A and B). The control cells
displayed typical normal nuclear structure in a
concentration-dependent manner, while cells treated with
resveratrol and HS-1793 exhibited chromosomal condensation and
formation of apoptotic bodies, indicating apoptotic cell death. At
12.5 μM, HS-1793 was effective in inducing chromosomal
condensation in both cell types, whereas resveratrol did not
(Fig. 5A and B). Therefore, these
results also showed that HS-1793 exhibited more efficient induction
of apoptosis than resveratrol in both cell lines.
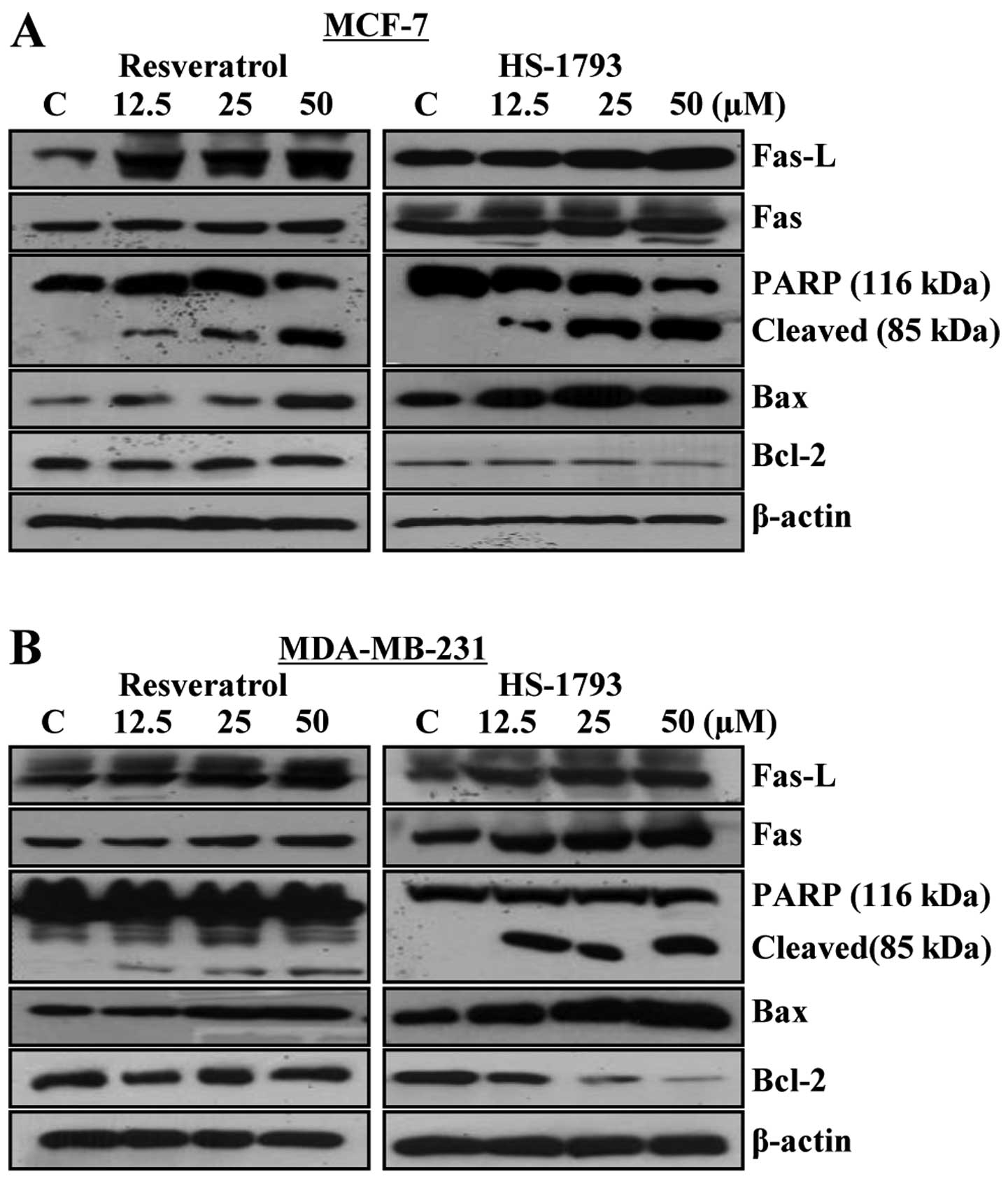
HS-1793 modulates the expression levels
of apoptosis-related proteins in MCF-7 and MDA-MB-231 cells
The degradation of polypeptides, such as
poly(ADP-ribose) polymerase (PARP), was examined to further study
the possible involvement of apoptosis-associated caspases in the
induction of apoptotic cell death (Fig. 6). Treatment with resveratrol and
HS-1793 caused increase in cleavage of PARP in both cell types
(Fig. 6A and B). To determine
whether the expression levels of death receptors and death
receptor-mediated apoptotic proteins were changed by resveratrol
and HS-1793, western blot analysis was performed and expression
levels of Fas, Fas-ligand (Fas-L), Bcl-2 and Bax were measured. The
expression of Fas and Fas-L was increased in a
concentration-dependent manner in both cell lines. In addition, in
both HS-1793 treated cell lines, the expression level of Bcl-2
protein was markedly downregulated, while Bax was upregulated in a
concentration-dependent manner. These data suggest that resveratrol
and HS-1793 induce apoptosis through the alteration in expression
levels of death receptor proteins as well as altered the expression
ratio of Bax/Bcl-2 protein (Fig. 6A
and B).
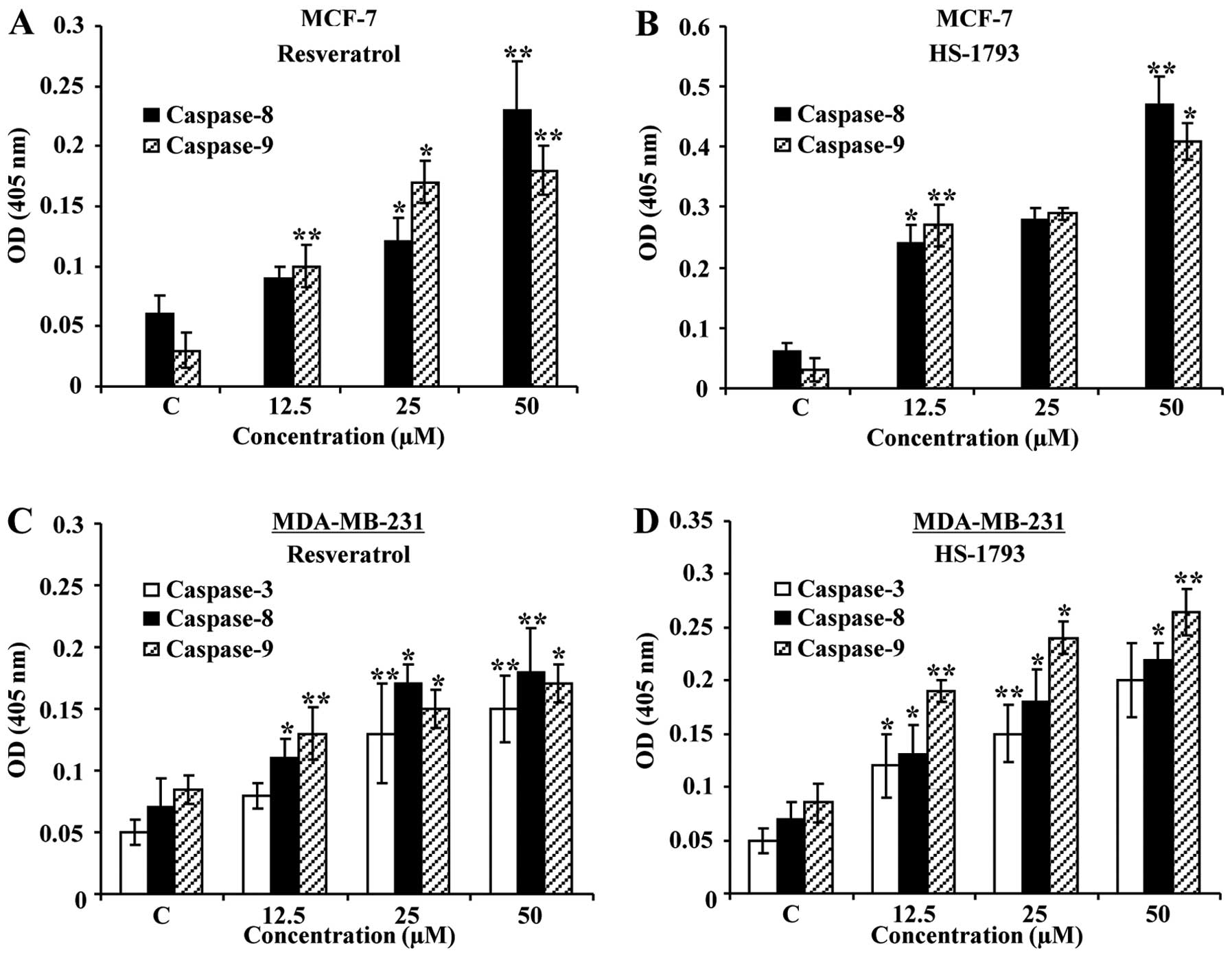
HS-1793 increases the caspase activity in
MCF-7 and MDA-MB-231 cells
In an attempt to further characterize the molecular
mechanisms of apoptosis induced by resveratrol or HS-1793, the
activity of caspases (-3, -8, and -9) was determined by
colorimetric assay. In case of MCF-7 cells (caspase-3 null type),
the activity of caspase-8 and -9 was increased with the treatment
of resveratrol or HS-1793 (Fig. 7A and
B). In MDA-MB-231 cells, however, caspase-3, -8, and -9 were
all activated with the treatment of both compounds (Fig. 7C and D). Overall, these results
implicate that both HS-1793 and resveratrol induce
caspase-dependent apoptotic cell death in MCF-7 and MDA-MB-231
cells.
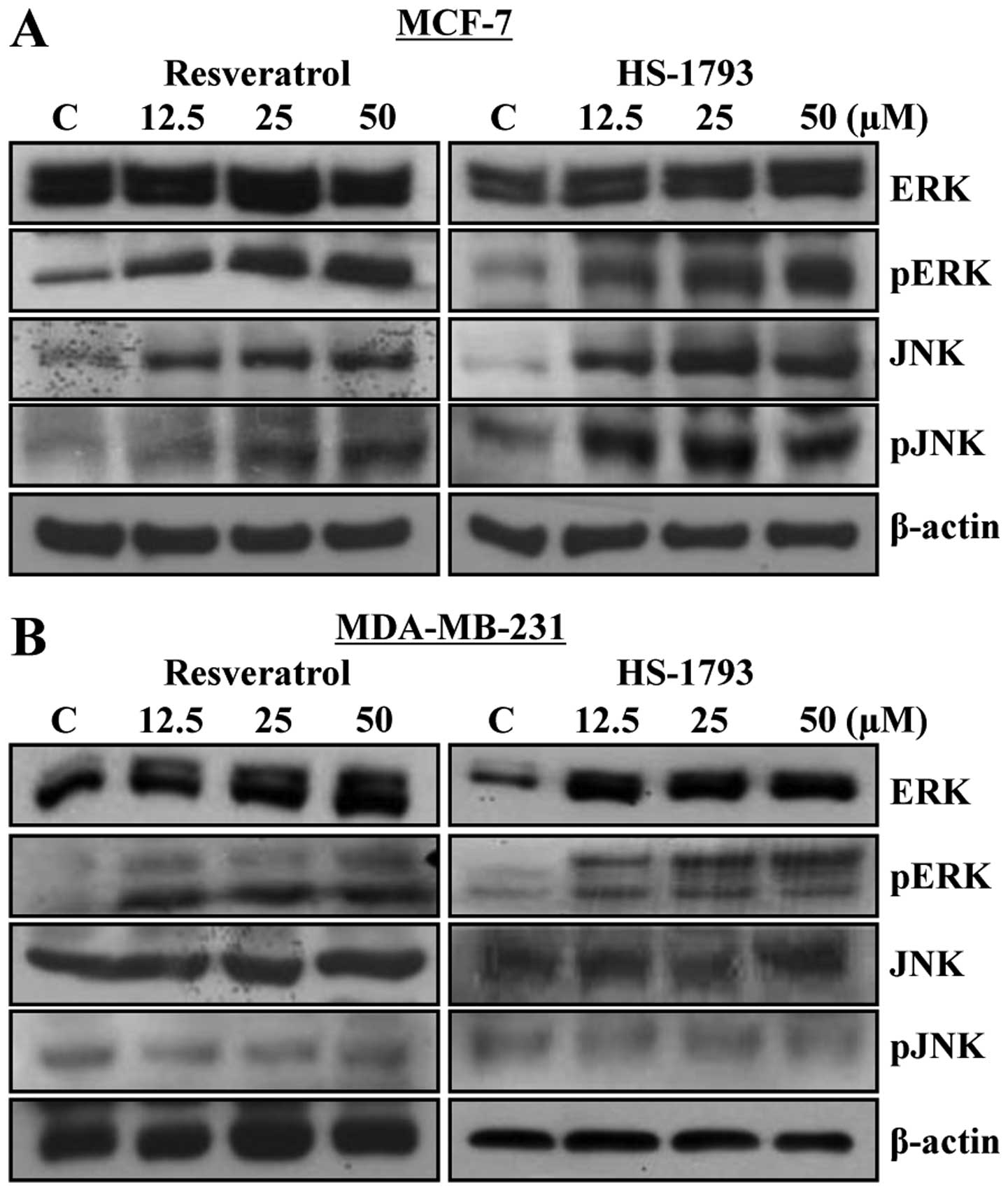
HS-1793 modulates the expression of MAP
kinases in MCF-7 and MDA-MB-231 cells
The mitogen-activated protein kinase (MAPK)
signaling pathway has been shown to play important roles in cell
cycle and apoptosis (20,21). Thus, to investigate whether the
MAPK pathway is involved in HS-1793 and resveratrol-induced
apoptosis, MCF-7 and MDA-MB-231 cells were treated with resveratrol
or HS-1793 at 12.5, 25 and 50 μM for 24 h and then the
expression levels of MAPKs [i.e., extracellular signal-regulated
kinase (ERK) and c-Jun N-terminal kinase (JNK)] were compared by
western blot analysis. As shown in Fig. 8, both resveratrol and HS-1793
induced phosphorylation of ERK and JNK in MCF-7 cells (Fig. 8A). In contrast to this, only ERK
phosphorylation was increased in MDA-MB-231 cells (Fig. 8B). These results suggest that
apoptosis induced by resveratrol or HS-1793 may be mediated by
different pathways in p53 wild and mutant type cell lines.
Discussion
This study was conducted to investigate and compare
the effects of resveratrol and HS-1793 on the proliferation and
apoptotic cell death in MCF-7 and MDA-MB-231 human breast cancer
cells. The resveratrol or HS-1793 treatment in both cell lines
efficiently inhibited cell growth in a concentration-dependent
manner. At equimolar concentrations, HS-1793 showed more potent
effects than resveratrol. Flow cytometric analysis revealed that
HS-1793 induced cell cycle arrest more efficiently than resveratrol
in both cell types. Resveratrol modulated the cell cycle
progression and caused G1 phase arrest in both cell lines. However,
HS-1793 treatment induced G2/M arrest and apoptosis by
downregulating cyclins and CDKs with upregulations of Bax, p53 and
p21WAF1/CIP1 in both cell lines.
The progression of eukaryotic cell cycle involves
sequential activation of CDKs whose association with corresponding
regulatory cyclins is necessary for their activations. For
instance, the G1/S transition is regulated by complexes formed by
cyclin D and CDK4 or CDK6 (22).
The CDK inhibitors can negatively regulate cell cycle progression
by competing with cyclin D1 for binding with CDK4 or CDK6 complexes
and inhibiting the kinase activities of CDKs/cyclin complexes
(23). In this study, the
intracellular protein levels of G1 phase regulatory proteins such
as cyclin D1, CDK4 and CDK6 were downregulated in both cell lines
by resveratrol. We found that G2 phase regulatory protein such as
cyclin B1, Cdc2 and Cdc25C were downregulated in both cell lines by
HS-1793. The resveratrol analogue HS-1793 also inhibited formation
of the Cdc2/cyclin B complex. Binding to cyclin B and
phosphorylation at threonine 161 by CDK-activating kinase are
required to activate Cdc2 during G2 and the Cdc2/cyclin B complex
is kept inactive by phosphorylation on tyrosine 15 and threonine 14
of Cdc2 by the kinases Wee1 and Myt1, respectively (24). Although detailed mechanism of
HS-1793 on Cdc2/cyclin B complex or each component was not
investigated, it is likely that HS-1793, either directly or through
downregulation of protein level, targets the Cdc2/cyclin B complex.
The tumor suppressor protein p53, was increased in MCF-7 cells by
both resveratrol and HS-1793. However, treatments with resveratrol
or HS-1793 upregulated the expression level of the CDK inhibitor
p21WAF1/CIP1 in a p53-dependent and -independent
manner in MCF-7 and MDA-MB-231 cells, respectively.
The resveratrol or HS-1793 treatment also induced
apoptosis as demonstrated by the formation of apoptotic bodies and
cleavages of PARP. Cellular p53 accumulation induces Fas-mediated
apoptosis by transcriptional activation of Fas gene and by cell
surface trafficking of Fas (25).
In this study, induction of apoptosis by resveratrol and HS-1793
was associated with the upregulation of Fas and Fas-L in MCF-7 and
MDA-MB-231 cells. The Bcl-2 family proteins play critical roles in
the induction of apoptosis. Treatment with resveratrol and HS-1793
induced alterations in expression ratio of Bax protein and Bcl-2 in
both cell types. During apoptosis, a series of proteolytic
cleavages of various intracellular polypeptides are initiated by
the action of a unique family of cysteine-dependent proteases
called caspases (26). We observed
induction of caspase activity in both cell lines. Induction of the
JNK and p38 MAPK-governed phosphorylative cascades has been
reported to be involved in the mechanisms of apoptosis triggered by
resveratrol (27). We found that
pJNK and pERK were increased by resveratrol and HS-1793 in MCF-7
cells, whereas only pERK was increased in MDA-MB-231 cells.
However, further experiments are required to clarify the detailed
molecular mechanisms of action in both cell lines.
In conclusion, this study demonstrated that HS-1793
was capable of inhibiting cell proliferation and inducing apoptosis
in MCF-7 and MDA-MB-231 cells harboring different p53 status.
HS-1793 induced G2/M arrest and apoptosis by downregulating cyclins
and CDKs with upregulation of Bax, p53, and
p21WAF1/CIP1 in both cell lines. The effects were
mediated via either a p53-dependent or -independent pathway.
Moreover, HS-1793 showed more potent effect than resveratrol on the
cytotoxicity of MCF-7 and MDA-MB-231 breast cancer cells.
Collectively, these results imply that HS-1793 could be a good
candidate as a new potent chemotherapeutic agent against human
breast cancer.
Acknowledgements
This study was supported by Basic
Science Research Program through the National Research Foundation
of Korea (NRF) funded by the Ministry of Education, Science and
Technology (2012R1A1A2006753).
References
|
1.
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2.
|
DeSantis C, Siegel R, Bandi P and Jemal A:
Breast cancer statistics, 2011. CA Cancer J Clin. 61:409–418. 2011.
View Article : Google Scholar
|
|
3.
|
Kim HJ, Yang KM, Park YS, et al: The novel
resveratrol analogue HS-1793 induces apoptosis via the
mitochondrial pathway in murine breast cancer cells. Int J Oncol.
41:1628–1634. 2012.PubMed/NCBI
|
|
4.
|
Baur JA and Sinclair DA: Therapeutic
potential of resveratrol: the in vivo evidence. Nat Rev Drug
Discov. 5:493–506. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Marques FZ, Markus MA and Morris BJ:
Resveratrol: cellular actions of a potent natural chemical that
confers a diversity of health benefits. Int J Biochem Cell Biol.
41:2125–2128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Khan N, Afaq F and Mukhtar H: Cancer
chemoprevention through dietary antioxidants: progress and promise.
Antioxid Redox Signal. 10:475–510. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Fulda S and Debatin KM:
Resveratrol-mediated sensitisation to TRAIL-induced apoptosis
depends on death receptor and mitochondrial signalling. Eur J
Cancer. 41:786–798. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Garvin S, Ollinger K and Dabrosin C:
Resveratrol induces apoptosis and inhibits angiogenesis in human
breast cancer xenografts in vivo. Cancer Lett. 231:113–122. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Jang M, Cai L, Udeani GO, et al: Cancer
chemopreventive activity of resveratrol, a natural product derived
from grapes. Science. 275:218–220. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Cai YJ, Wei QY, Fang JG, et al: The
3,4-dihydroxyl groups are important for trans-resveratrol analogs
to exhibit enhanced antioxidant and apoptotic activities.
Anticancer Res. 24:999–1002. 2004.PubMed/NCBI
|
|
11.
|
Jeong SH, Lee JS, Jeong NY, et al: A novel
resveratrol analogue HS-1793 treatment overcomes the resistance
conferred by Bcl-2 and is associated with the formation of mature
PML nuclear bodies in renal clear cell carcinoma Caki-1 cells. Int
J Oncol. 35:1353–1360. 2009.
|
|
12.
|
Jeong MH, Yang KM, Choi YJ, et al:
Resveratrol analog, HS-1793 enhance anti-tumor immunity by reducing
the CD4+CD25+ regulatory T cells in FM3A
tumor bearing mice. Int Immunopharmacol. 14:328–333. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Jeong NY, Yoon YG, Rho JH, et al: The
novel resveratrol analog HS-1793-induced polyploid LNCaP prostate
cancer cells are vulnerable to downregulation of Bcl-xL. Int J
Oncol. 38:1597–1604. 2011.PubMed/NCBI
|
|
14.
|
Jeong SH, Song IS, Kim HK, et al: An
analogue of resveratrol HS-1793 exhibits anticancer activity
against MCF-7 cells via inhibition of mitochondrial biogenesis gene
expression. Mol Cells. 34:357–365. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kim DH, Hossain MA, Kim MY, et al: A novel
resveratrol analogue, HS-1793, inhibits hypoxia-induced HIF-1alpha
and VEGF expression, and migration in human prostate cancer cells.
Int J Oncol. 43:1915–1924. 2013.PubMed/NCBI
|
|
16.
|
Ma Z, Molavi O, Haddadi A, Lai R, Gossage
RA and Lavasanifar A: Resveratrol analog trans
3,4,5,4'-tetramethoxystilbene (DMU-212) mediates anti-tumor effects
via mechanism different from that of resveratrol. Cancer Chemother
Pharmacol. 63:27–35. 2008.
|
|
17.
|
Kotha A, Sekharam M, Cilenti L, et al:
Resveratrol inhibits Src and Stat3 signaling and induces the
apoptosis of malignant cells containing activated Stat3 protein.
Mol Cancer Ther. 5:621–629. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Momand J, Zambetti GP, Olson DC, George D
and Levine AJ: The mdm-2 oncogene product forms a complex with the
p53 protein and inhibits p53-mediated transactivation. Cell.
69:1237–1245. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Honda R, Tanaka H and Yasuda H:
Oncoprotein MDM2 is a ubiquitin ligase E3 for tumor suppressor p53.
FEBS Lett. 420:25–27. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Makin G and Dive C: Modulating sensitivity
to drug-induced apoptosis: the future for chemotherapy? Breast
Cancer Res. 3:150–153. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Wilkinson MG and Millar JB: Control of the
eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J.
14:2147–2157. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bates S, Bonetta L, MacAllan D, et al:
CDK6 (PLSTIRE) and CDK4 (PSK-J3) are a distinct subset of the
cyclin-dependent kinases that associate with cyclin D1. Oncogene.
9:71–79. 1994.PubMed/NCBI
|
|
23.
|
Ellis M, Chew YP, Fallis L, et al:
Degradation of p27(Kip) cdk inhibitor triggered by Kaposi's sarcoma
virus cyclin-cdk6 complex. EMBO J. 18:644–653. 1999.PubMed/NCBI
|
|
24.
|
Taylor WR and Stark GR: Regulation of the
G2/M transition by p53. Oncogene. 20:1803–1815. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Bennett M, Macdonald K, Chan SW, Luzio JP,
Simari R and Weissberg P: Cell surface trafficking of Fas: a rapid
mechanism of p53-mediated apoptosis. Science. 282:290–293. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Earnshaw WC, Martins LM and Kaufmann SH:
Mammalian caspases: structure, activation, substrates, and
functions during apoptosis. Annu Rev Biochem. 68:383–424. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Filomeni G, Graziani I, Rotilio G and
Ciriolo MR: trans-Resveratrol induces apoptosis in human breast
cancer cells MCF-7 by the activation of MAP kinases pathways. Genes
Nutr. 2:295–305. 2007. View Article : Google Scholar : PubMed/NCBI
|