Introduction
The majority of acute promyelocytic leukemia (APL)
patients harbor the t(15;17) translocation leading to the
expression of the fusion protein promyelocytic leukemia-retinoic
acid receptor α (PML-RARα) (1,2). The
oncogenic fusion protein PML-RARα can recruit corepressor (CoR)
complexes containing nuclear receptor CoR, histone deacetylases
(HDACs), resulting in myeloid differentiation arrest observed in
APL (3,4). All-trans retinoic acid (ATRA)
induces differentiation of APL cells through not only dissociating
CoR from PML-RARα oncoprotein, but also recruiting coactivators
that possess histone acetylase activity (3–5). Due
to its good clinical outcomes, ATRA is used as a first-line
administration for de novo APL patients. Nevertheless, an
approximately 30% of the patients relapse and often become
resistant to the conventional treatment (6).
Recent clinical studies have demonstrated that
following remission induction with arsenic trioxide (ATO)-based
regimens in patients with relapsed APL, consolidation with
autologous stem cell transplantation (SCT) is associated with a
significantly superior clinical outcome as compared with other
maintenance regimens (7,8). However, relapsed APL patients
ineligible for autologous SCT usually have poor prognosis (8). Therefore, it would be logical to
consider more efficacious treatment strategy employing ATRA in
combination with other drugs to cure the disease in the initial
treatment. Since HDACs play a key role in transcriptional
regulation and pathogenesis of cancer (9,10),
its inhibitors (HDACi) are currently being developed for therapy of
several types of cancer including leukemia (11). Valproic acid (VPA) belongs to the
class I HDACi and shows potential anti-leukemic activities either
alone or in combination with other anti-leukemic agents (9,10,12,13).
Furthermore, aberrant recruitment of HDACs through expression of
PML-RARα has been implicated as an initiating tumorigenic event in
APL (3–5). Therefore, there is a logical
rationale for use of HDACi such as VPA in combination with ATRA in
the initial treatment of APL. However, the efficacy of the
combination therapy has been investigated mostly in non-APL AML and
myelodysplastic syndromes (MDS) (10,13–15),
and remains largely unexplored in APL.
Transcription factors, including members of CCAAT/
enhancer-binding proteins (C/EBPs) and PU.1, are critical for
normal myelopoiesis, granulocytic maturation and being repressed in
APL (4,16–21).
Although a number of studies have been conducted to explore the
molecular mechanism underlying the effects of ATRA on these
transcription factors associated with differentiation induction
(16,18,19,22),
the effects of VPA alone or in combination with ATRA on human APL
cell line harboring PML-RARα remain largely unclear.
In the current study, the effects of ATRA and VPA,
alone and in combination, were investigated by focusing on
differentiation and cell viability in the APL cell line NB4
(PML-RARα positive). The expression profiles of transcription
factors, C/EBP(α, β, ɛ) and PU.1 were further evaluated in the
cells after treatment with ATRA and VPA.
Materials and methods
Reagents
ATRA was purchased from Sigma (St. Louis, MO, USA)
and dissolved in ethanol to obtain a final concentration of 2 mM
and stored at −20°C in the dark. The vehicle reagent, ethanol
(final concentration <0.05%), did not affect cell viability and
differentiation. VPA was purchased from Wako Pure Chemical
Industries (Miyazaki, Japan) and dissolved in phosphate-buffered
saline (PBS) to obtain a final concentration of 1 M, sterilized by
filtration (0.22 μM filter), and used as the stock solution.
Primary antibodies [phycoerythrin (PE)-conjugated mouse anti-human
CD11b IgG, fluorescein isothiocyanate (FITC)-conjugated mouse
anti-human CD10 IgG], and control antibodies [non-binding mouse
IgG-PE isotype antibody and non-binding mouse IgG-FITC isotype
antibody] were obtained from BD Transduction Laboratories (San
Diego, CA, USA) and were used for assessment of differentiation
induction. Rabbit polyclonal antibodies against human C/EBPα,
C/EBPβ, C/EBPɛ and PU.1 were purchased from Abnova (Taipei,
Taiwan). FITC-conjugated goat anti-rabbit polyclonal IgG secondary
antibody was obtained from Kirkegaard and Perry Laboratories
(Gaithersburg, MD, USA).
Cell culture and treatment
NB4, a human APL cell line with t(15;17), was
purchased from Deutsche Sammalung von Mikroorganismen und
Zellkulturen GmbH (Braunschweig, Germany) and cultured in RPMI-1640
medium (Sigma, St. Louis, MO, USA) supplemented with 10%
heat-inactivated fetal bovine serum (FBS) (Gibco-BRL, Rockville,
MD, USA), 100 U/ml penicillin, and 100 μg/ml streptomycin
(Gibco-BRL, Gaithersburg, MD, USA) at 37°C in a humidified
atmosphere (5% CO2 in air). Cells were seeded at a
density of 1×105 cells/ml and treated with 1 μM
ATRA and various concentrations of VPA (0.1, 0.3, 1, 3, 10 mM),
alone or in combination.
Trypan blue exclusion assay
After the treatment with ATRA and/or VPA, cell
viability of NB4 cells was investigated by trypan blue exclusion
assay. Trypan blue negative and positive cells were considered as
viable and dead cells, respectively. The number of total cells was
calculated as the sum of viable and dead cells. The percent of
viable cells were expressed as the ratio of the number of viable
cells of each treatment group against those of control group. The
percent of trypan blue positive cells were calculated using the
following formula: the percent of trypan blue positive cells = the
number of trypan blue positive cells/the number of total cells.
Growth inhibition assay
Cell growth inhibition by ATRA and/or VPA was
investigated by XTT dye-reduction assays according to the method
previously described with slight modifications (23). Briefly, the cells were seeded in
96-well plates (Iwaki, Tokyo, Japan) at a density of
1×104 cells per well in 0.1 ml cell culture medium.
Cultures in triplicate were treated with 1 μM ATRA, 1 mM
VPA, alone or in combination. After 48 h of treatment,
2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[(phenylamino)carbonyl]-2H-tetrazolium
hydroxide (XTT) (Sigma, MD, USA) and phenazine methosulfate (Wako
Pure Chemical Industries, Osaka, Japan) were added into each well
at final concentrations of 0.2 mg/ml and 1 mM, respectively. After
incubation at 37°C for 2 h, the plates were mixed, and the
absorbance at 450 nm was measured with a microplate reader (Safire,
Tecan, Switzerland). The relative cell viability was expressed as
the ratio of the absorbance of each treatment group against those
of the corresponding untreated control group.
Differentiation analysis
Differentiation induction was confirmed by
morphology and expression of surface markers. For morphological
assessment, cytospin preparations of treated cells stained with
Wright-Giemsa were evaluated by light microscopy as previously
described (24). Furthermore, the
numbers of cells with differentiation-associated morphological
changes such as apparent lobulated nuclei, multi-lobulated nuclei
were counted and presented as the percent of differentiated cells.
Myeloid maturation with cell surface marker was analyzed by
FACSCanto flow cytometer (BD Immunocytometry System) using
antibodies for CD11b and CD10 as previously described with minor
modifications (24). In brief,
approximately 1×106 cells were washed with PBS
containing 2.5% FBS and 0.5% NaN3 (PBSF) and stained
with PE-conjugated mouse anti-human CD11b IgG or FITC-conjugated
mouse anti-human CD10 IgG for 30 min at 4°C in the dark. Cells were
then washed three times with PBSF and analyzed by flow cytometry
with a minimum acquisition of 10,000 events. Non-binding mouse
IgG-PE isotype antibody or non-binding mouse IgG-FITC isotype
antibody was used as controls.
Expression profiles of transcription
factors in NB4 cells
The expression levels of transcription factors,
including C/EBPα, C/EBPβ, C/EBPɛ and PU.1 were evaluated by flow
cytometry (Cyto ACE-150, Jasco) using antibody for each as
previously described with minor modifications (24). In brief, approximately
1×106 cells were washed with PBS, and fixed with 4%
formaldehyde for 10 min at 37°C. Then, cells were permeabilized
with 90% ice-cold methanol for over 2 h at −20°C. After washing
with PBS, cells were stained with primary antibodies against
C/EBPα, C/EBPβ, C/EBPɛ and PU.1 for 30 min at 4°C, followed by
staining with FITC-conjugated secondary antibody for 30 min at 4°C
in the dark. Cells were then washed three times with PBS and
analyzed by flow cytometry with a minimum acquisition of 10,000
events.
Western blot analysis
Protein samples were separated on an SDS-PAGE,
followed by transferring to a nitrocellulose membrane as described
previously (25). Proteins bands
were detected using the following primary antibodies: rabbit
polyclonal antibodies against human C/EBPα, C/EBPβ, C/EBPɛ and PU.1
(1:1,000 dilution). Blotted protein bands were detected with
horseradish peroxidase-conjugated secondary antibody and an
enhanced chemiluminescence (ECL) western blot analysis system
(Amersham Pharmacia Biotech, Buckinghamshire, UK).
Statistical analysis
Experiments were independently repeated at least
three times and results are shown as mean ± standard deviation
(SD). Data were analyzed using Student's t-test and p<0.05 was
considered as statistically significant.
Results
Cell viability of NB4 cells treated with
ATRA and VPA, alone or in combination
Since 1 μM ATRA has been commonly used to
induce differentiation of NB4 cells (16,18,26),
the concentration was used in the current study to evaluate the
differentiation-inducing activity of a combination of ATRA and VPA.
In order to determine the appropriate concentration of VPA for the
combinatorial treatment with 1 μM ATRA, cell viability was
first determined by trypan blue exclusion assay after treatment
with 1 μM ATRA and various concentrations of VPA (0.1, 0.3,
1, 3 and 10 mM), alone or in combination, for 48 h. No alteration
was observed in the number of viable cells after treatment with 1
μM ATRA and relatively low concentrations of VPA (0.1 and
0.3 mM), alone or in combination, as compared to control (Fig. 1A). A slight but not significant
decrease in the number of viable cells was observed when NB4 cells
were treated with 1 mM VPA alone or in combination with 1 μM
ATRA (Fig. 1A). Furthermore,
analysis of growth inhibition by the XTT dye-reduction assay
demonstrated that treatment with 1 mM VPA alone, instead of 1
μM ATRA alone, significantly inhibited growth of NB4 cells,
and that treatment with 1 mM VPA in combination of 1 μM ATRA
further strengthened the growth inhibition as compared to each
alone (Fig. 1C). On the other
hand, treatment with relatively high concentrations of VPA (3 and
10 mM) alone or in combination with 1 μM ATRA resulted in
substantial decrease in the number of viable cells along with a
marked increase in the number of trypan blue positive cells
(Fig. 1A and B). Therefore, the
concentrations of VPA (3 and 10 mM) were not used for further
investigation of differentiation induction.
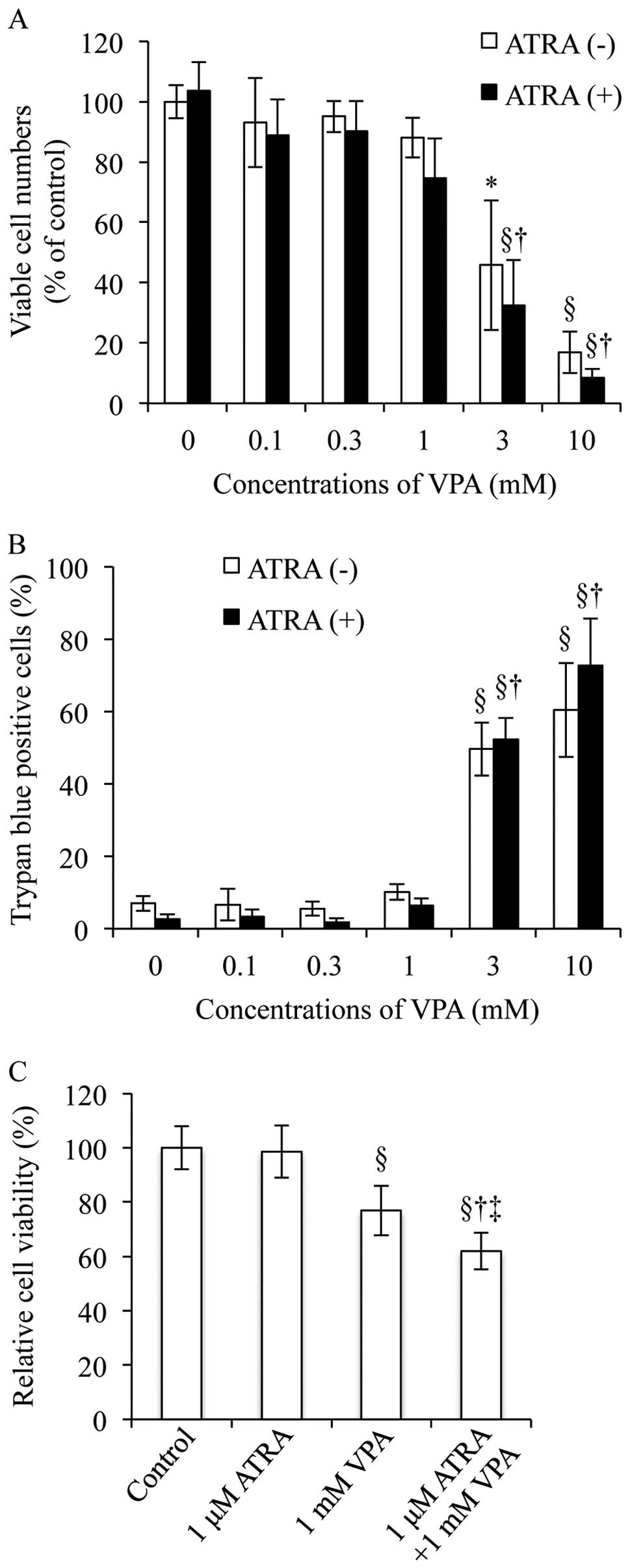 | Figure 1.Cell viability of NB4 cells treated
with ATRA and VPA, alone or in combination. After the treatment
with 1 μM ATRA and various concentrations of VPA (0.1, 0.3,
1, 3 and 10 mM), alone or in combination, for 48 h, cell viability
[(A) viable cells, and (B) dead cells] and (C) growth inhibition
were investigated by trypan blue exclusion assay and XTT
dye-reduction assay, respectively, as described in Materials and
methods. Experiments were independently repeated at least three
times and results are shown as mean ± SD. *p<0.05 vs.
control; §p<0.01 vs. control; †p<0.01
vs. 1 μM ATRA; ‡p<0.01 vs. VPA. |
Differentiation of NB4 cell induction by
ATRA and VPA, alone or in combination
NB4 cells have been demonstrated to differentiate
toward granulocytic lineages after exposure to retinoic acid
(19,27). Morphological changes such as
condensation of chromatin, lobulation of nuclei, and increased
expression of CD11b and CD10 have been used as markers of the
differentiation of NB4 cells (16,28–30).
Therefore, the differentiation-inducing activity of 1 μM
ATRA alone or in combination with various concentrations of VPA
(0.1, 0.3 and 1.0 mM) was first assessed by examining the
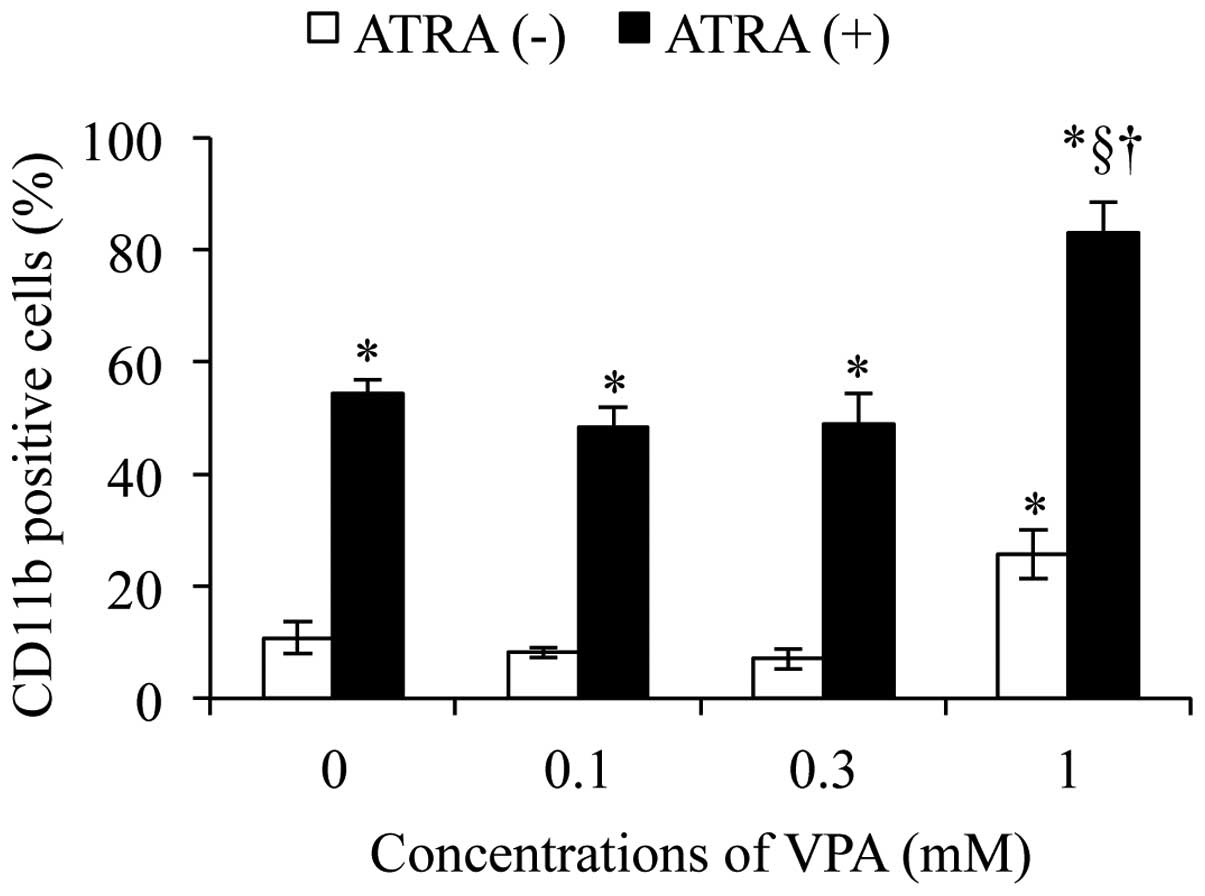
alterations in CD11b expression level in NB4 cells. A significant
upregulation of the expression level of CD11b was observed in NB4
cells when treated with 1 mM VPA alone, but not 0.1 and 0.3 mM VPA
alone, for 48 h (Fig. 2). Much
higher level of CD11b expression was observed in NB4 cells treated
with 1 μM ATRA alone as compared to that observed when
treated with 1 mM VPA alone (Fig.
2). Furthermore, a significant increase in
differentiation-inducing activity was observed only in NB4 cells
treated with a combination of 1 μM ATRA and 1 mM VPA when
compared with the other two combinatorial treatment groups
(Fig. 2). Therefore, the following
experiments on morphological changes, the alterations in CD11b and
CD10 expression levels were subsequently conducted by exposing NB4
cells to the combination of 1 μM ATRA and 1 mM VPA for 48
h.
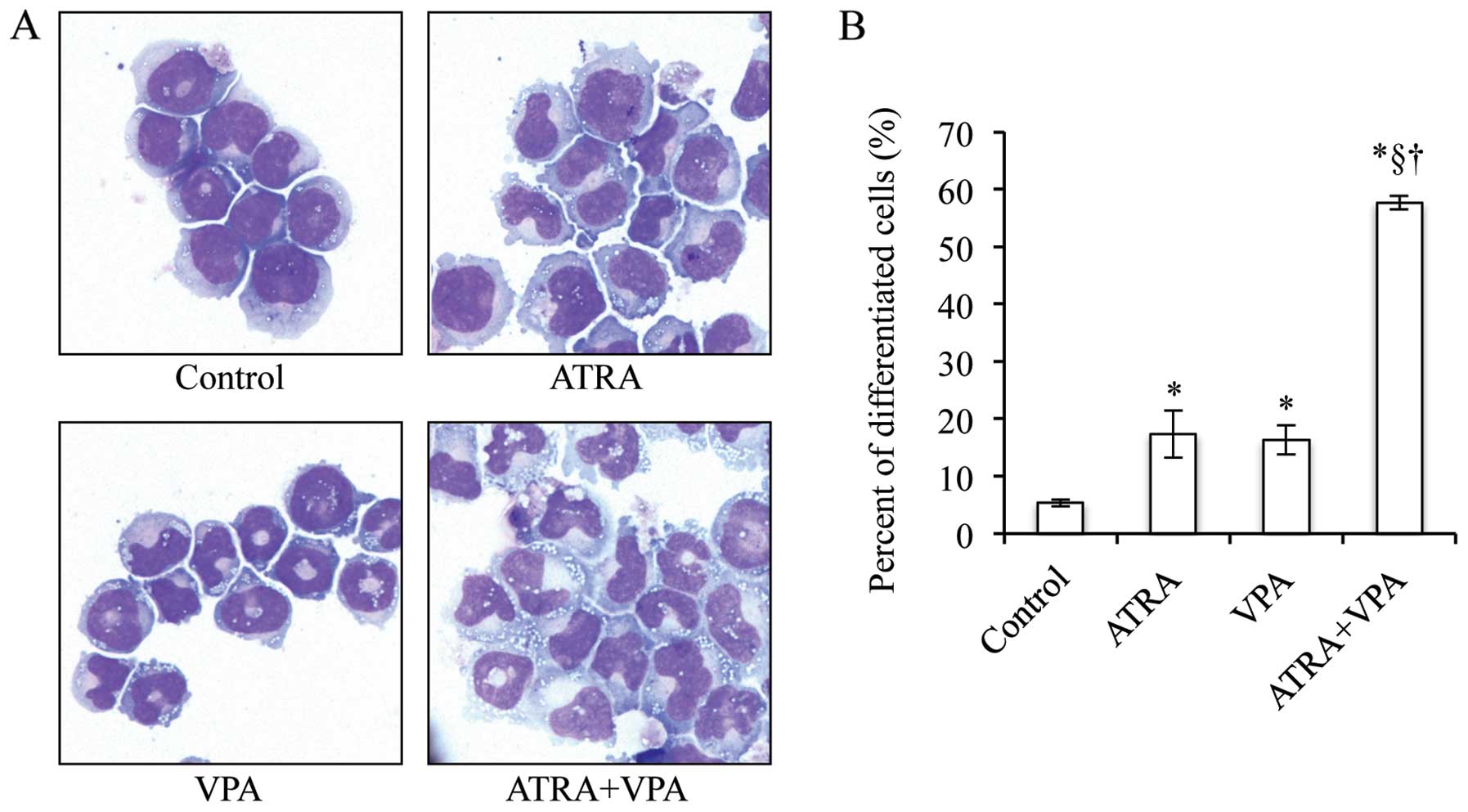
NB4 cells treated with 1 μM ATRA alone
underwent remarkable differentiation-associated changes with
condensation and lobulation of nuclei (jelly bean-shaped nuclei,
known as a stage before multi-lobulated nuclei) (control: 5.3±0.6
vs. 1 μM ATRA: 17.3±4.2, p<0.01) (Fig. 3), and a significant increase in
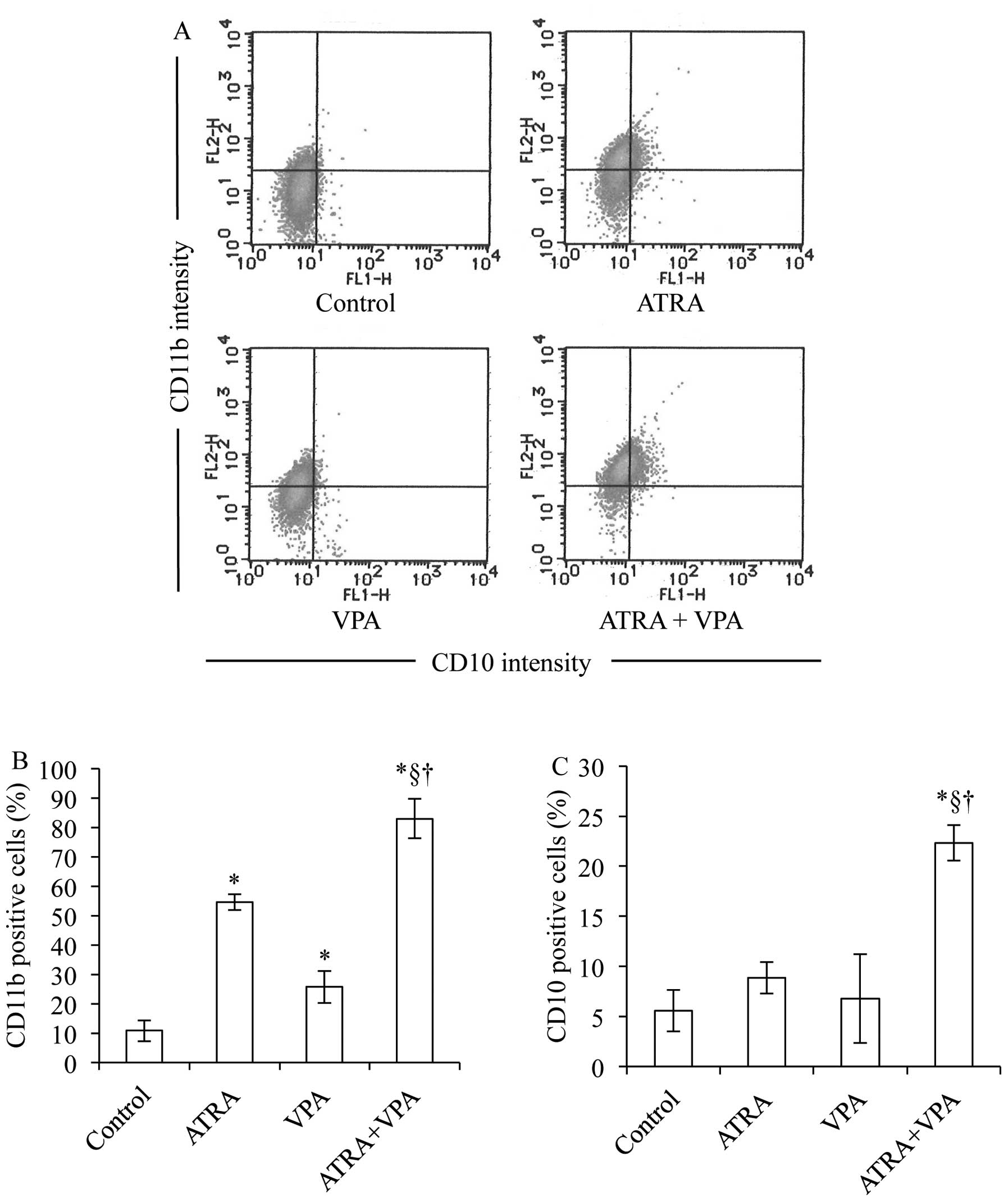
CD11b expression concurred with the morphological changes (Fig. 4A and B). Treatment with 1 mM VPA
alone also induced significant morphological changes (control:
5.3±0.6 vs. 1 mM VPA: 16.3±2.5, p<0.01) (Fig. 3) and upregulation of CD11b
expression levels (Fig. 4A and B).
Furthermore, the number of cells containing multi-lobulated nuclei
prominently increased when the cells were treated with the
combination of ATRA and VPA as compared to that treated with each
alone (1 μM ATRA: 17.3±4.2; 1 mM VPA: 16.3±2.5 vs. 1
μM ATRA + 1 mM VPA: 57.7±1.2, p<0.01) (Fig. 3). The increase in
differentiation-inducing activities due to combination treatment
was further confirmed by a significant increase in CD11b and CD10
expression level as compared to each alone (Fig. 4).
Expression profiles of C/EBPs and PU.1 in
NB4 cells treated with ATRA and VPA, alone or in combination
After the treatment with 1 μM ATRA and 1 mM
VPA, alone or in combination, for 48 h, the expression levels of
C/EBPs and PU.1 were evaluated using FACS and western blot
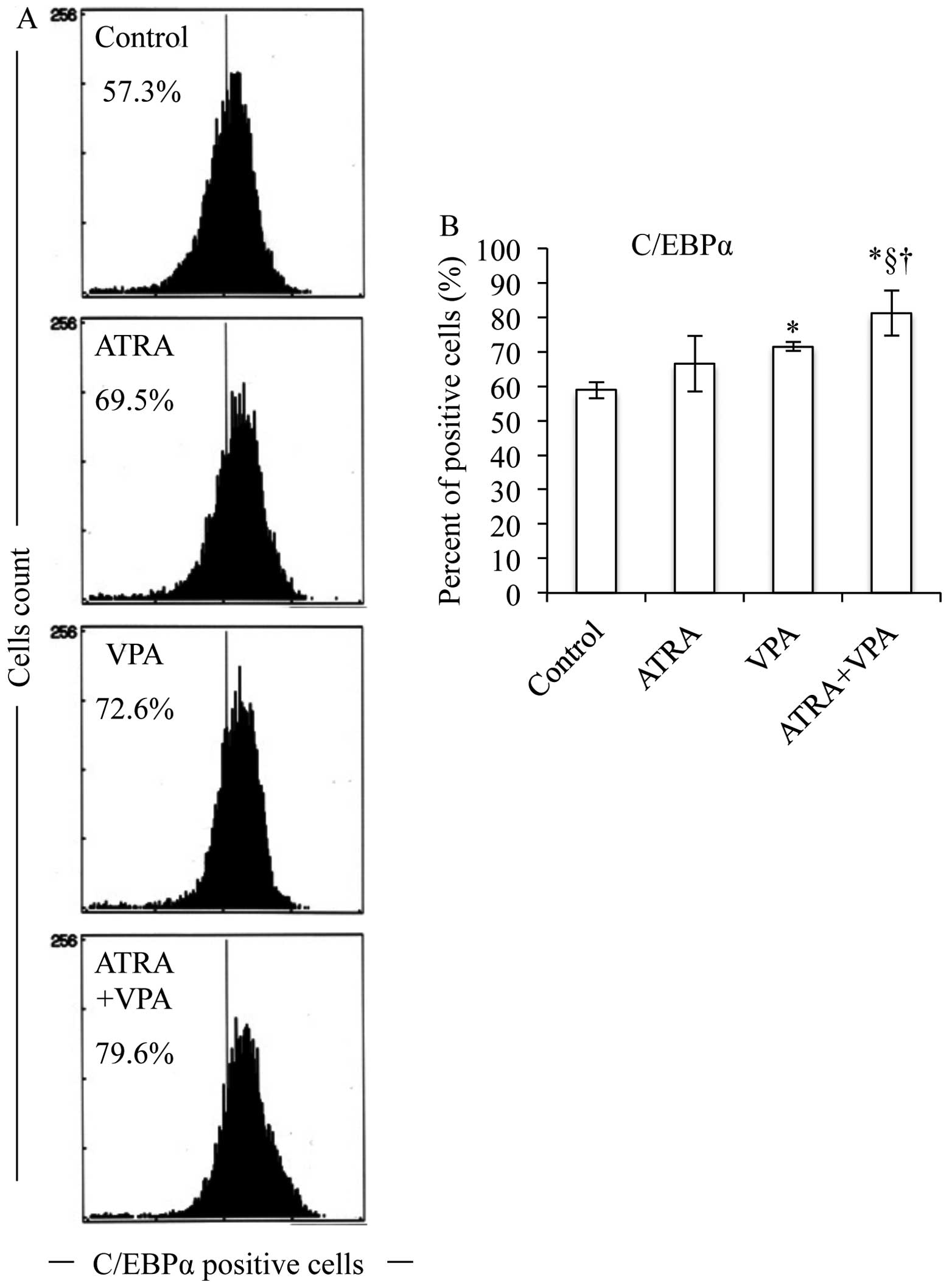
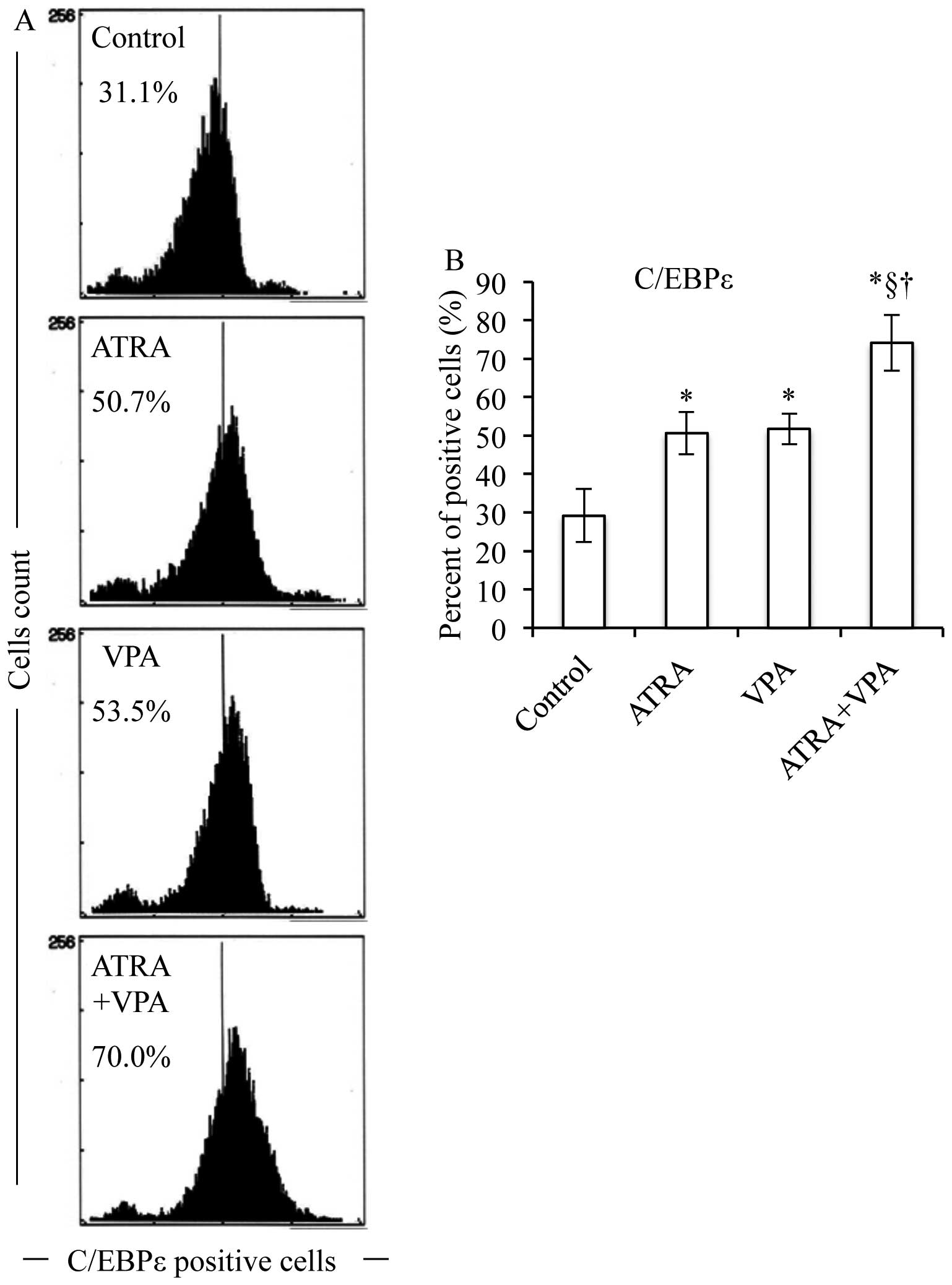
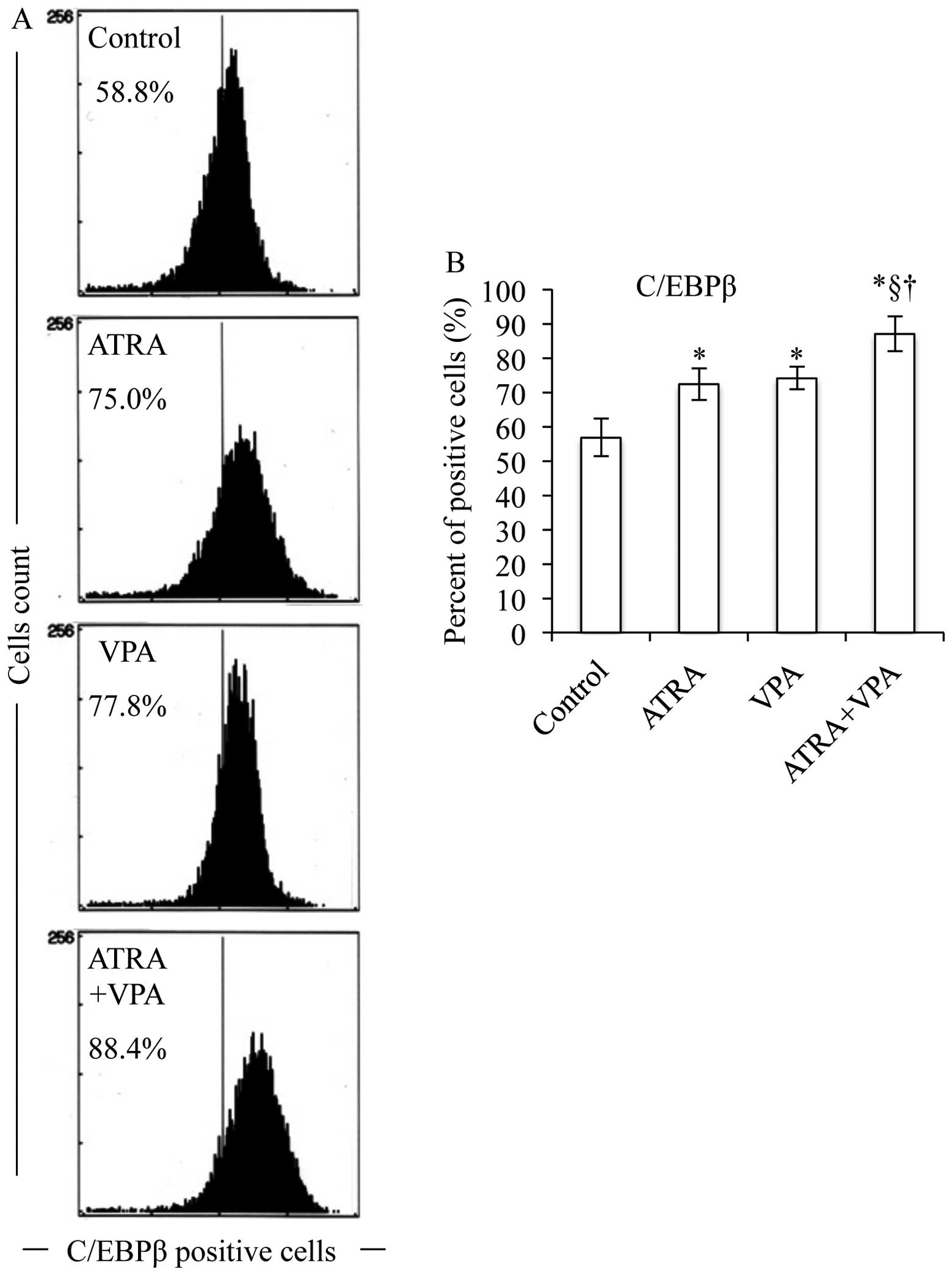
analysis. ATRA alone significantly upregulated C/EBP β and ɛ,
whereas VPA alone significantly upregulated C/EBP α, β and ɛ
(Figs. 5–7). Of note, the degree of upregulation in
C/EBP β and ɛ induced by ATRA and VPA is almost the same (Figs. 6 and 7). It is of note that both ATRA and VPA
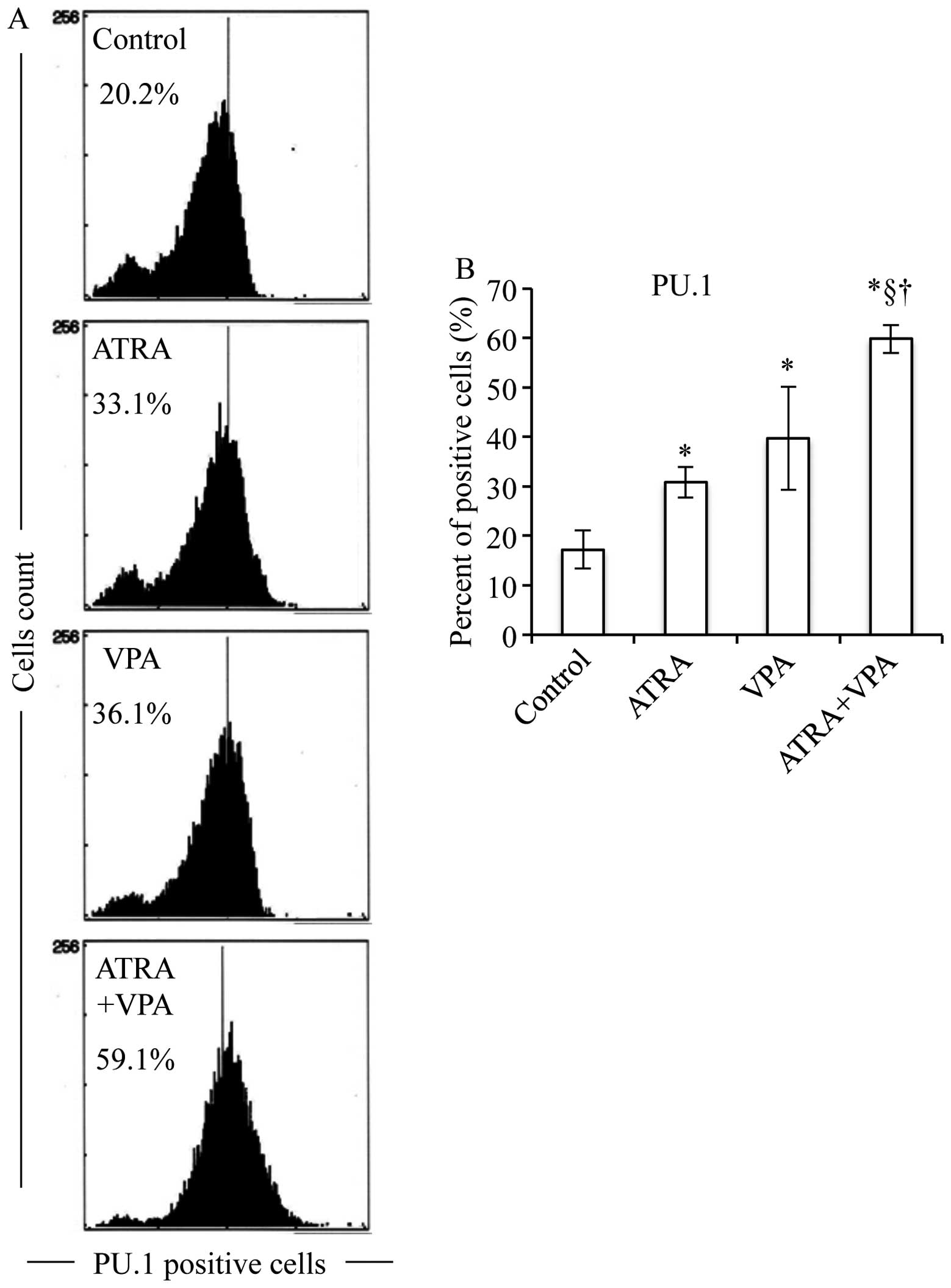
significantly upregulated PU.1 expression (Fig. 8). Furthermore, combinational
treatment of ATRA and VPA significantly upregulated the expression
level of C/EBP α, β, ɛ and PU.1 as compared to that treated with
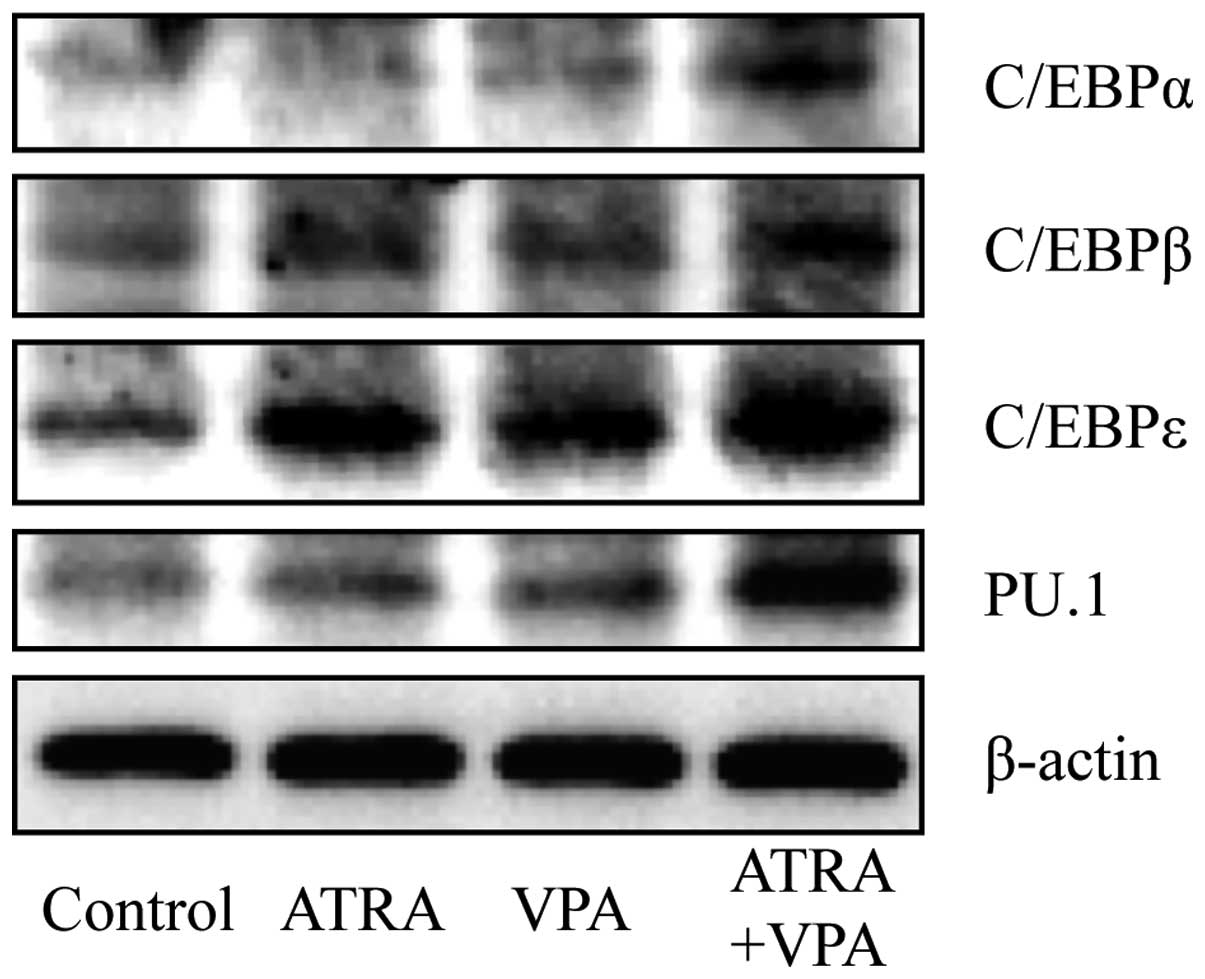
each alone (Figs. 5–8). Moreover, the expression levels of
these transcription factors demonstrated by western blot analysis
are in good agreement with those demonstrated by FACS analysis
(Fig. 9).
Discussion
It has been demonstrated that VPA inhibits the
growth of NB4, HL-60 and U937 cells by causing cell cycle arrest at
G0/G1 phase (12). VPA has also been demonstrated to
induce apoptosis in other human leukemia cells by stimulating both
caspase-dependent and -independent apoptotic signaling pathways
(12,31). In the current study, we first
demonstrated that a slight but not significant decrease in the
number of viable cells was observed when NB4 cells were treated
with 1 mM VPA alone or in combination with 1 μM ATRA
(Fig. 1A). We further demonstrated
that a significant growth inhibition was observed after treatment
with 1 mM VPA, and that the growth inhibition was strengthened by
the addition of 1 μM ATRA (Fig.
1C). Furthermore, no alteration in the number of trypan blue
positive cells was observed in the same treatment, whereas
treatment with relatively high concentrations of VPA (3 and 10 mM)
alone or in combination with 1 μM ATRA resulted in
substantial increase in the number of trypan blue positive cells
(Fig. 1B). Taken together, results
suggest that proliferation arrest, rather than apoptosis, is a
plausible mechanism responsible for the growth inhibition induced
by VPA or VPA/ATRA. Furthermore, it is possible that ATRA-mediated
differentiation contributes to the enhancement of the growth
inhibition, although further investigation is still needed to draw
a concrete conclusion.
Next, we demonstrated that not only ATRA but also
VPA induced differentiation in NB4 cells (Figs. 2–4). The combination of ATRA and VPA
further augmented the differentiation activity as compared to that
treated with each alone. Similar to our results, a previous report
demonstrated that VPA induced differentiation in not only NB4 cells
but also HL-60 and U937 cells, although there were some differences
in the degree of differentiation among these leukemia cells
(12). Kosugi et al has
also demonstrated that trichostatin A, another HDACi,
synergistically induced differentiation in NB4 and HL-60 cells as
well as their ATRA-resistant sublines in combination with ATRA
(26). Furthermore, it has been
demonstrated that VPA per se induced differentiation in
PML-RARα and promyelocytic leukemia zinc-finger protein
(PLZF)-RARα-transformed mouse hematopoietic progenitor cells, and
enhances ATRA-induced differentiation in these cells (32). These findings thus suggest that
differentiation-inducing activities of these reagents do not appear
to be associated with a specific cytogenetic subtype of AML, and
that a larger scale study must be launched in order to draw a solid
conclusion.
We further demonstrated that treatment with ATRA
alone resulted in the upregulation of C/EBP(β, ɛ), but not C/EBPα
in NB4 cells (Figs. 5–7), suggesting that C/EBP(β, ɛ) play more
critical roles in the ATRA-induced differentiation. The notion was
supported by several previous reports showing that ATRA-induced
differentiation of APL cells might be mediated by C/EBP factors,
most notably C/EBPβ and C/EBPɛ (19,22).
Indeed, electrophoretic mobility shift assay of nuclear extract
from NB4 cells after ATRA stimulation revealed an increase in the
binding activity of C/EBP(β, ɛ), but not that of C/EBPα (16). Interestingly, VPA alone
significantly upregulated C/EBP(α, β, ɛ) expression levels
(Figs. 5–7). Data are scarce on whether C/EBPs or
PU.1 are involved in VPA-induced differentiation of NB4 cells
(PML-RARα positive), although a considerable amount of studies
dealing with the differentiation-inducing activity of VPA in
non-APL HL-60 cells (PML-RARα negative) has been conducted
(12,26,33).
To the best of our knowledge, this is the first report to
demonstrate the effects of VPA on the PML-RARα positive APL cells
by focusing on differentiation associated with the expression of
transcription factors, C/EBPs and PU.1.
We also demonstrated that both ATRA and VPA
significantly induced PU.1 expression level (Fig. 8). It has been demonstrated that
ATRA resolves the differentiation block in APL cell lines and
primary blasts by restoring PU.1 expression (18). Furthermore, ATRA-induced activation
of PU.1 in these cells is mediated by upregulation of the C/EBPs,
especially C/EBPβ (18). In
agreement with our results, Zapotocky et al also
demonstrated that VPA increased the expression of PU.1, resulting
in differentiation induction in t(8;21)/AML1-ETO-positive leukemic
cells (34). More importantly, we
further demonstrated that combination treatment with ATRA and VPA
resulted in the upregulation of C/EBP(α, β, ɛ) and PU.1 (Figs. 5–8) as compared to that treated with each
alone, suggesting that synergistic or additive effects of these
reagents on differentiation induction are attributed to the
restoration of the normal function of the myeloid cell
transcriptional machinery. Given the importance of C/EBP(α, β, ɛ)
and PU.1 in myeloid development, these studies suggest that
restoring the expression of these transcriptional factors may
represent a possible therapeutic modality leading to
differentiation of APL cells. Therefore, efforts to clarify the
potential clinical significance of the combination of ATRA and VPA
in patients with not only non-APL AML and MDS but also APL are
warranted.
In conclusion, our findings demonstrating growth
inhibition, enhanced differentiation and upregulation of
transcription factors in NB4 cells treated with combination of VPA
and ATRA provide novel insight into a possible combinational
therapeutic approach to APL patients. It has been suggested that
ATO/ATRA degrade PML-RARα oncoprotein, resulting in eradication of
leukemia-initiating cells (32,35,36).
Therefore, as a new therapeutic approach, a multi-target therapy
based on a combination of ATRA, ATO and VPA would be useful and
worth evaluating further for its beneficial clinical effects.
Acknowledgements
The authors thank Mr. Fumitaka Takemae
and Ms. Eiko Ishizuka for their technical assistance. This study
was supported in part by grants from the Ministry of Education,
Culture, Sports, Science and Technology and by the Promotion and
Mutual Aid Corporation for Private Schools of Japan.
References
|
1.
|
Grignani F, De Matteis S, Nervi C,
Tomassoni L, Gelmetti V, Cioce M, Fanelli M, Ruthardt M, Ferrara
FF, Zamir I, Seiser C, Grignani F, Lazar MA, Minucci S and Pelicci
PG: Fusion proteins of the retinoic acid receptor-alpha recruit
histone deacetylase in promyelocytic leukaemia. Nature.
391:815–818. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lin RJ, Nagy L, Inoue S, Shao W, Miller WH
Jr and Evans RM: Role of the histone deacetylase complex in acute
promyelocytic leukaemia. Nature. 391:811–814. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Fang J, Chen SJ, Tong JH, Wang ZG, Chen GQ
and Chen Z: Treatment of acute promyelocytic leukemia with ATRA and
As2O3: a model of molecular target-based cancer therapy. Cancer
Biol Ther. 1:614–620. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Wang ZY and Chen Z: Acute promyelocytic
leukemia: from highly fatal to highly curable. Blood.
111:2505–2515. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Zhang JW, Wang JY, Chen SJ and Chen Z:
Mechanisms of all-trans retinoic acid-induced differentiation of
acute promyelocytic leukemia cells. J Biosci. 25:275–284. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Melnick A and Licht JD: Deconstructing a
disease: RARalpha, its fusion partners, and their roles in the
pathogenesis of acute promyelocytic leukemia. Blood. 93:3167–3215.
1999.PubMed/NCBI
|
|
7.
|
Tallman MS and Altman JK: Curative
strategies in acute promyelocytic leukemia. Hematology Am Soc
Hematol Educ Program. 1:391–399. 2008. View Article : Google Scholar
|
|
8.
|
Thirugnanam R, George B, Chendamarai E,
Lakshmi KM, Balasubramanian P, Viswabandya A, Srivastava A, Chandy
M and Mathews V: Comparison of clinical outcomes of patients with
relapsed acute promyelocytic leukemia induced with arsenic trioxide
and consolidated with either an autologous stem cell transplant or
an arsenic trioxide-based regimen. Biol Blood Marrow Transplant.
15:1479–1484. 2009. View Article : Google Scholar
|
|
9.
|
Göttlicher M, Minucci S, Zhu P, Krämer OH,
Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG
and Heinzel T: Valproic acid defines a novel class of HDAC
inhibitors inducing differentiation of transformed cells. EMBO J.
20:6969–6978. 2001.PubMed/NCBI
|
|
10.
|
Quintás-Cardama A, Santos FP and
Garcia-Manero G: Histone deacetylase inhibitors for the treatment
of myelodysplastic syndrome and acute myeloid leukemia. Leukemia.
25:226–235. 2011.PubMed/NCBI
|
|
11.
|
Bolden JE, Peart MJ and Johnstone RW:
Anticancer activities of histone deacetylase inhibitors. Nat Rev
Drug Discov. 5:769–784. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Cheng YC, Lin H, Huang MJ, Chow JM, Lin S
and Liu HE: Downregulation of c-Myc is critical for valproic
acid-induced growth arrest and myeloid differentiation of acute
myeloid leukemia. Leuk Res. 31:1403–1411. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Cimino G, Lo-Coco F, Fenu S, Travaglini L,
Finolezzi E, Mancini M, Nanni M, Careddu A, Fazi F, Padula F,
Fiorini R, Spiriti MA, Petti MC, Venditti A, Amadori S, Mandelli F,
Pelicci PG and Nervi C: Sequential valproic acid/all-trans retinoic
acid treatment reprograms differentiation in refractory and
high-risk acute myeloid leukemia. Cancer Res. 66:8903–8911. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Kuendgen A, Schmid M, Schlenk R, Knipp S,
Hildebrandt B, Steidl C, Germing U, Haas R, Dohner H and Gattermann
N: The histone deacetylase (HDAC) inhibitor valproic acid as
monotherapy or in combination with all-trans retinoic acid in
patients with acute myeloid leukemia. Cancer. 106:112–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Kuendgen A, Strupp C, Aivado M, Bernhardt
A, Hildebrandt B, Haas R, Germing U and Gattermann N: Treatment of
myelodysplastic syndromes with valproic acid alone or in
combination with all-trans retinoic acid. Blood. 104:1266–1269.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Duprez E, Wagner K, Koch H and Tenen DG:
C/EBPbeta: a major PML-RARA-responsive gene in retinoic
acid-induced differentiation of APL cells. EMBO J. 22:5806–5816.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Guibal FC, Alberich-Jorda M, Hirai H,
Ebralidze A, Levantini E, Di Ruscio A, Zhang P, Santana-Lemos BA,
Neuberg D, Wagers AJ, Rego EM and Tenen DG: Identification of a
myeloid committed progenitor as the cancer-initiating cell in acute
promyelocytic leukemia. Blood. 114:5415–5425. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Mueller BU, Pabst T, Fos J, Petkovic V,
Fey MF, Asou N, Buergi U and Tenen DG: ATRA resolves the
differentiation block in t(15;17) acute myeloid leukemia by
restoring PU.1 expression. Blood. 107:3330–3338. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Park DJ, Chumakov AM, Vuong PT, Chih DY,
Gombart AF, Miller WH Jr and Koeffler HP: CCAAT/enhancer binding
protein epsilon is a potential retinoid target gene in acute
promyelocytic leukemia treatment. J Clin Invest. 103:1399–1408.
1999. View
Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Rosmarin AG, Yang Z and Resendes KK:
Transcriptional regulation in myelopoiesis: Hematopoietic fate
choice, myeloid differentiation, and leukemogenesis. Exp Hematol.
33:131–143. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zhang K, Li J, Meng W, Xing H and Yang Y:
C/EBPbeta and CHOP participate in tanshinone IIA-induced
differentiation and apoptosis of acute promyelocytic leukemia cells
in vitro. Int J Hematol. 92:571–578. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Tenen DG: Disruption of differentiation in
human cancer: AML shows the way. Nat Rev Cancer. 3:89–101. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Yoshino Y, Yuan B, Kaise T, Takeichi M,
Tanaka S, Hirano T, Kroetz DL and Toyoda H: Contribution of
aquaporin 9 and multidrug resistance-associated protein 2 to
differential sensitivity to arsenite between primary cultured
chorion and amnion cells prepared from human fetal membranes.
Toxicol Appl Pharmacol. 257:198–208. 2011. View Article : Google Scholar
|
|
24.
|
Iriyama N, Yuan B, Hatta Y, Horikoshi A,
Yoshino Y, Toyoda H, Aizawa S and Takeuchi J: Granulocyte
colony-stimulating factor potentiates differentiation induction by
all-trans retinoic acid and arsenic trioxide and enhances arsenic
uptake in the acute promyelocytic leukemia cell line HT93A. Oncol
Rep. 28:1875–1882. 2012.
|
|
25.
|
Yuan B, Ohyama K, Takeichi M and Toyoda H:
Direct contribution of inducible nitric oxide synthase expression
to apoptosis induction in primary smooth chorion trophoblast cells
of human fetal membrane tissues. Int J Biochem Cell Biol.
41:1062–1069. 2009. View Article : Google Scholar
|
|
26.
|
Kosugi H, Towatari M, Hatano S, Kitamura
K, Kiyoi H, Kinoshita T, Tanimoto M, Murate T, Kawashima K, Saito H
and Naoe T: Histone deacetylase inhibitors are the potent
inducer/enhancer of differentiation in acute myeloid leukemia: a
new approach to anti-leukemia therapy. Leukemia. 13:1316–1324.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Morosetti R, Park DJ, Chumakov AM,
Grillier I, Shiohara M, Gombart AF, Nakamaki T, Weinberg K and
Koeffler HP: A novel, myeloid transcription factor, C/EBP epsilon,
is upregulated during granulocytic, but not monocytic,
differentiation. Blood. 90:2591–2600. 1997.PubMed/NCBI
|
|
28.
|
Inazawa Y, Saeki K and Yuo A: Granulocyte
colony-stimulating factor-induced terminal maturation of human
myeloid cells is specifically associated with up-regulation of
receptor-mediated function and CD10 expression. Int J Hematol.
77:142–151. 2003. View Article : Google Scholar
|
|
29.
|
Zhao Q, Tao J, Zhu Q, Jia PM, Dou AX, Li
X, Cheng F, Waxman S, Chen GQ, Chen SJ, Lanotte M, Chen Z and Tong
JH: Rapid induction of cAMP/PKA pathway during retinoic
acid-induced acute promyelocytic leukemia cell differentiation.
Leukemia. 18:285–292. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Zhang K, Guo QL, You QD, Yang Y, Zhang HW,
Yang L, Gu HY, Qi Q, Tan Z and Wang X: Wogonin induces the
granulocytic differentiation of human NB4 promyelocytic leukemia
cells and up-regulates phospholipid scramblase 1 gene expression.
Cancer Sci. 99:689–695. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Kawagoe R, Kawagoe H and Sano K: Valproic
acid induces apoptosis in human leukemia cells by stimulating both
caspase-dependent and -independent apoptotic signaling pathways.
Leuk Res. 26:495–502. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Leiva M, Moretti S, Soilihi H, Pallavicini
I, Peres L, Mercurio C, Dal Zuffo R, Minucci S and de Thé H:
Valproic acid induces differentiation and transient tumor
regression, but spares leukemia-initiating activity in mouse models
of APL. Leukemia. 26:1630–1637. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Deubzer H, Busche B, Rönndahl G, Eikel D,
Michaelis M, Cinatl J, Schulze S, Nau H and Witt O: Novel valproic
acid derivatives with potent differentiation-inducing activity in
myeloid leukemia cells. Leuk Res. 30:1167–1175. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Zapotocky M, Mejstrikova E, Smetana K,
Stary J, Trka J and Starkova J: Valproic acid triggers
differentiation and apoptosis in AML1/ETO-positive leukemic cells
specifically. Cancer Lett. 319:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Ito K, Bernardi R, Morotti A, Matsuoka S,
Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J and
Pandolfi PP: PML targeting eradicates quiescent
leukaemia-initiating cells. Nature. 453:1072–1078. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Yuan B, Yoshino Y, Kaise T and Toyoda H:
Application of arsenic trioxide therapy for patients with leukemia.
Biological Chemistry of Arsenic, Antimony and Bismuth. Sun H: John
Wiley & Sons, Ltd; Chichester: pp. 263–292. 2011
|