Introduction
In Southeast China, nasopharyngeal carcinoma (NPC)
is one of the most common malignancies of the head and neck that
can be effectively treated by radiotherapy (1,2).
However, a high proportion of patients with NPC exhibit
radioresistance, which is the main risk factor that contributes to
poor prognosis (3). Studies have
revealed that increased radioresistance may be associated with
various factors that participate in tumor development (4). Thus, the molecular mechanisms of
radioresistance should be understood to provide opportunities for
enhancing radiosensitivity and to develop a more effective
anticancer strategy of NPC radiotherapy (5).
Curcumin (diferuloylmethane; Cur), a polyphenol from
Curcuma longa rhizomes, is the major constituent of the
yellow spice turmeric, a flavoring agent commonly used in Asian
cooking (6). Cur also inhibits
proliferation and angiogenesis in tumor cells to induce apoptosis
or cell cycle arrest and cause tumor regression in pre-clinical
models (7–9). In NPC, Cur has potent antitumor
activity and radiosensitivity (10,11);
however, the exact molecular mechanism remains unclear.
Long non-coding RNAs (lncRNAs) are
non-protein-coding transcripts that are longer than 200 nucleotides
(12). They are pervasively
transcribed with spatially and temporally regulated expression
patterns (13). lncRNAs have
important functions in gene expression regulation, dosage
compensation, genomic imprinting, nuclear organization and
compartmentalization, and nuclear-cytoplasmic trafficking (14–18).
lncRNAs have continuously emerged as new contributing factors in
cancer because they are involved in diverse biological processes
and aberrantly expressed in various human cancers (17). lncRNAs also have potential
functions in oncogenic (18) and
tumor-suppressive pathways (19).
lncRNAs also regulate gene expression at transcriptional,
post-transcriptional, and epigenetic levels (20–22).
Altered lncRNA expression may potentially enhance oncogenesis by
altering some of these functions (14,23).
The differential lncRNA expressions can also indicate disease
progression and function as predictors of patient outcomes.
In the present study, we demonstrated that Cur
enhanced the radiosensitivity in NPC cell line CNE2 at an
appropriate MTT concentration or with a clonogenic survival test.
To determine the mechanism of radiosensitization, we performed a
chip assay for CNE2 treated with irradiation (IR) and/or Cur.
Numerous differentially expressed lncRNAs were identified, in which
six lncRNAs were verified by qPCR. We observed that this response
altered by IR was reversed by Cur in NPC cells. Our findings
provide novel information on lncRNA expression profiles, in which
Cur protected the cells from radiation toxicity, suggesting that
this natural product may be an effective radiosensitizer or
radioenhancer for managing patients with NPC.
Materials and methods
Cell culture
The present study was performed in human NPC cell
lines [CNE-2; obtained from Sun Yat-sen University and had been
described before (24)]. CNE-2 was
maintained in Roswell Park Memorial Institute 1640 medium
(RPMI-1640) supplemented with 10% fetal bovine serum (FBS;
Invitrogen, USA) at 37°C in 5% carbon dioxide. Cur (Sigma-Aldrich,
USA) was dissolved in 0.5% dimethyl sulphoxide (Sigma-Aldrich) and
diluted with RPMI-1640 medium to the desired concentrations before
use. The cells were divided into three groups [control group (CN);
IR group (CX); and IR + Cur group (JX)] and irradiated linearly
with X-rays at 6 MV to deliver the indicated doses (2 Gy) at room
temperature. The compensators used were 1.5 cm bolus. For the
microarray, the sample was pooled in each group and the experiment
was performed in triplicate.
Isolation of RNA
Total RNA was extracted using the TRIzol reagent
(Invitrogen) according to the manufacturer’s instructions. The RNA
integrity number was checked to inspect RNA integration by an
Agilent Bioanalyzer 2100 (Agilent Technologies, USA). Qualified
total RNA was further purified using RNeasy mini kit (Qiagen,
Germany) and RNase-free DNase set (Qiagen).
Preparation of array hybridization
The SBC 8×60K human lncRNA microarrays were custom
designed using the Agilent eArray program according to the
manufacturer’s recommendations (https://earray.chem.agilent.com/earray). The
microarray contained 31,171 mRNA probes, which were derived from
the probe sequence for mRNA in Agilent 8×60K Whole Human Genome
Oligo Microarray, and 29,971 lncRNA probes, which were designed by
using an eArray-based system. The lncRNA sequence was derived from
six databases, including LNCRNA-DB, NCBI_refseq, Ensembl, UCSC,
NCBI_unigene, and ncRNASCAN. After purification of labeled cRNAs,
each slide was hybridized and washed according to the
manufacturer’s instructions (Agilent Technologies).
Data anaysis
Raw data were normalized by quantile algorithm on
the Gene Spring 11.0 software (Agilent Technologies). lncRNAs and
mRNAs with ‘Present’ or ‘Marginal’ (All Targets Value) flags in all
of the groups were further subjected to data analysis.
Differentially expressed lncRNAs and mRNAs were identified by fold
change. Clustering was analyzed using the multi-experimental viewer
(MeV) 4.6 and functional enrichment analysis was performed using
DAVID’s Functional Annotation Tool (http://david.abcc.ncifcrf.gov) (25).
Confirmation test of real-time
quantitative RT-PCR
Total RNAs from tissues were extracted using TRIzol
(Invitrogen) according to the manufacturer’s instructions.
Qualified total RNA was further purified by RNase-free DNase set
(Qiagen). Reverse transcription was performed using a gene-specific
primer and quantification was performed using the Quantitect SYBR
Green PCR kit (Stratagene, USA) with an MX3005P multiplex
quantitative PCR (qPCR) system (Stratagene) according to the
manufacturer’s instructions. GAPDH, the human housekeeping gene,
was used for normalization. The relative lncRNA expression levels
were calculated using the comparative ΔΔCt method as previously
described (26). The fold changes
were calculated according to 2−ΔΔCt equation. All of the
primers used are listed in Table
I.
 | Table I.Primers for QPCR or cloning. |
Table I.
Primers for QPCR or cloning.
| Gene | Primer (5′-3′) | Product (bp) |
|---|
| GAPDH | Forward:
ATCATCAGCAATGCCTCCTG | 102 |
| Reverse:
ATGGACTGTGGTCATGAGTC |
| AF086415 | Forward:
AGCGCGACTTCTCTGTCTCT | 115 |
| Reverse:
GCAGAGGAGGAGACGCTGA |
| AK095147 | Forward:
ACGAGTGACCGAAGCTGAAC | 117 |
| Reverse:
GCACCATCCAGAGGGATTTA |
| RP1-179N16.3 | Forward:
CGCGTTAGGAGATTCTGGAG | 105 |
| Reverse:
AGGGTGGATACAGGCTCCTT |
| AK056098 | Forward:
GGCCTCGGGGTAGAACTTAC | 143 |
| Reverse:
CAAGCCTCCTGGTCTTTCTG |
| AK294004 | Forward:
GTGCAACCAGAAATGCACAG | 168 |
| Reverse:
ACGCTTTGTCTGTCGTGATG |
| MUDENG | Forward:
ACTTTGTGGCACCGTGAGAT | 194 |
| Reverse:
GGCCCACTAAATGCAGAGTC |
| CCND1 | Forward:
GATCAAGTGTGACCCGGACT | 129 |
| Reverse:
TCCTCCTCCTCTTCCTCCTC |
| Primer A | Forward:
gattatagaTGTAATTCTTGTAATTTTT | |
| Reverse:
gattatagaGCAGCAAACAATGTGAAAGA |
| Primer B | Forward:
gattatagaTCAACCATCCTGGCTGCGGCGT | |
| Reverse:
gattatagaTGCCGGTTACATGTTGGTGCT |
| Primer C | Forward:
gattatagaTGTAATTCTTGTAATTTTT | |
| Reverse:
gattatagaTGCCGGTTACATGTTGGTGCT |
| Primer D | Forward:
gatggtaccGCCGGTTACATGTTGGTGCT | |
| Reverse:
gatctcgagTGTAATTCTTGTAATTTTT |
RNA interference
In all, 20–30% confluent CNE2 cells were transfected
with 50 nM of siRNAs using Lipofectamine 2000 (Invitrogen)
following the manufacturer’s direction. Two individual small
interfering RNA (siRNAs) and scrambled negative control siRNA
(siRNA-NC) were obtained from Invitrogen. The target sequences of
AK294004 are the following: siRNA-1, 5-CUCCCUUCAACACUUCCUAAAUA-3
and siRNA-2, 5-AGCAGCAAACAAUGUGAAAGAGA-3. Thirty-six hours after
transfection, cells were harvested for qRT-PCR (27).
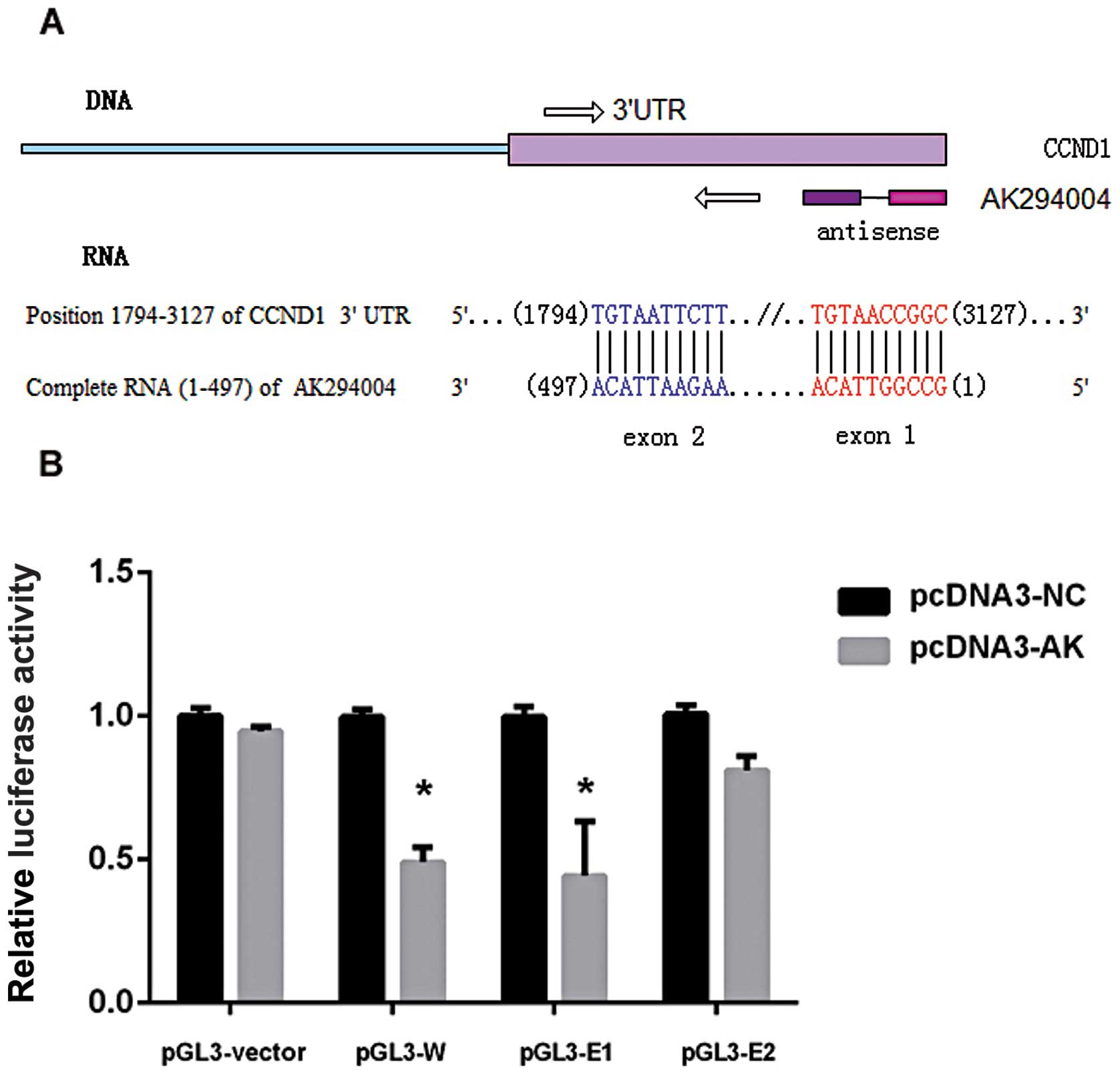
Luciferase reporter assay
A 1,334-bp (1,794–3,127) fragment of CCND1 3′UTR
containing whole complementry sequences of AK294004 two exons was
amplified using the primer pairs A, the sequence (1,794–2,486 bp)
of CCND1 3′UTR containing the complementry sequence of AK294004
exon 2 was amplified using the primer pairs B, and the sequence
(2,876–3,127 bp) of CCND1 3′UTR containing the complementry
sequence of AK294004 exon 1 was amplified using the primer pairs C
(Table I). Each fragment was
respectively cloned downstream of the Renilla luciferase
gene at the XbaI site in the pGL-3 promoter plasmid
(Promega, USA). The entire 497-bp fragment of AK294004 was
amplified using the the primer pairs D and was cloned at the
KpnI and XhoI sites in the pcDNA3.1+
plasmid (Promega). The pGL3 constructs were designated as pGL3-W
(whole sequence), pGL3-E1 (completed to exon 1) and pGL3-E2
(completed to exon 2) and the pcDNA3.1 construct was designated as
pcDNA3-AK.
To facilitate cloning into each expression plasmid,
the primers were designed to incorporate XbaI, KpnI
and XhoI sites at the 5′ end (underlined in the primers
above). HEK293 cells were co-transfected with 30 pmol of either
pcDNA3-AK or pcDNA3-NC (empty vector control) and each
pGL-construct using Lipofectamine 2000 (Invitrogen). Transfection
efficiency was normalized by co-transfection with a firefly
luciferase expressing plasmid. Luciferase activity was measured
using the Promega dual-luciferase assay kit, in accordance with the
instructions of the manufacturer. Relative protein levels were
expressed as Renilla/firefly luciferase ratios. Each
transfection was repeated twice in triplicates (27).
Results
LncRNA and mRNA microarray data
Array hybridization was performed using the SBC
8×60K human lncRNA microarrays. After quantile normalization of the
raw data, the expression profiles of 29,971 lncRNAs and 31,171
mRNAs were obtained from the cells in the three groups. We
identified differentially expressed genes among the matched groups
with a fold change >2. Table II
summarizes the differentially expressed genes in each group.
 | Table II.Summary of differently expressed
genes. |
Table II.
Summary of differently expressed
genes.
| CX vs. CN | JX vs. CN | JX vs. CX |
|---|
| Up | 865 (592) | 623 (445) | 734 (615) |
| Down | 777 (712) | 579 (588) | 859 (832) |
| Total | 1,642 (1,304) | 1,202 (1,033) | 1,593 (1,447) |
Altered and reversed lncRNAs and mRNA
expression
After clustering analysis was performed, JX and CN
revealed seemingly similar expression signatures. The lncRNAs were
altered by IR and Cur reversed this response and a similar trend
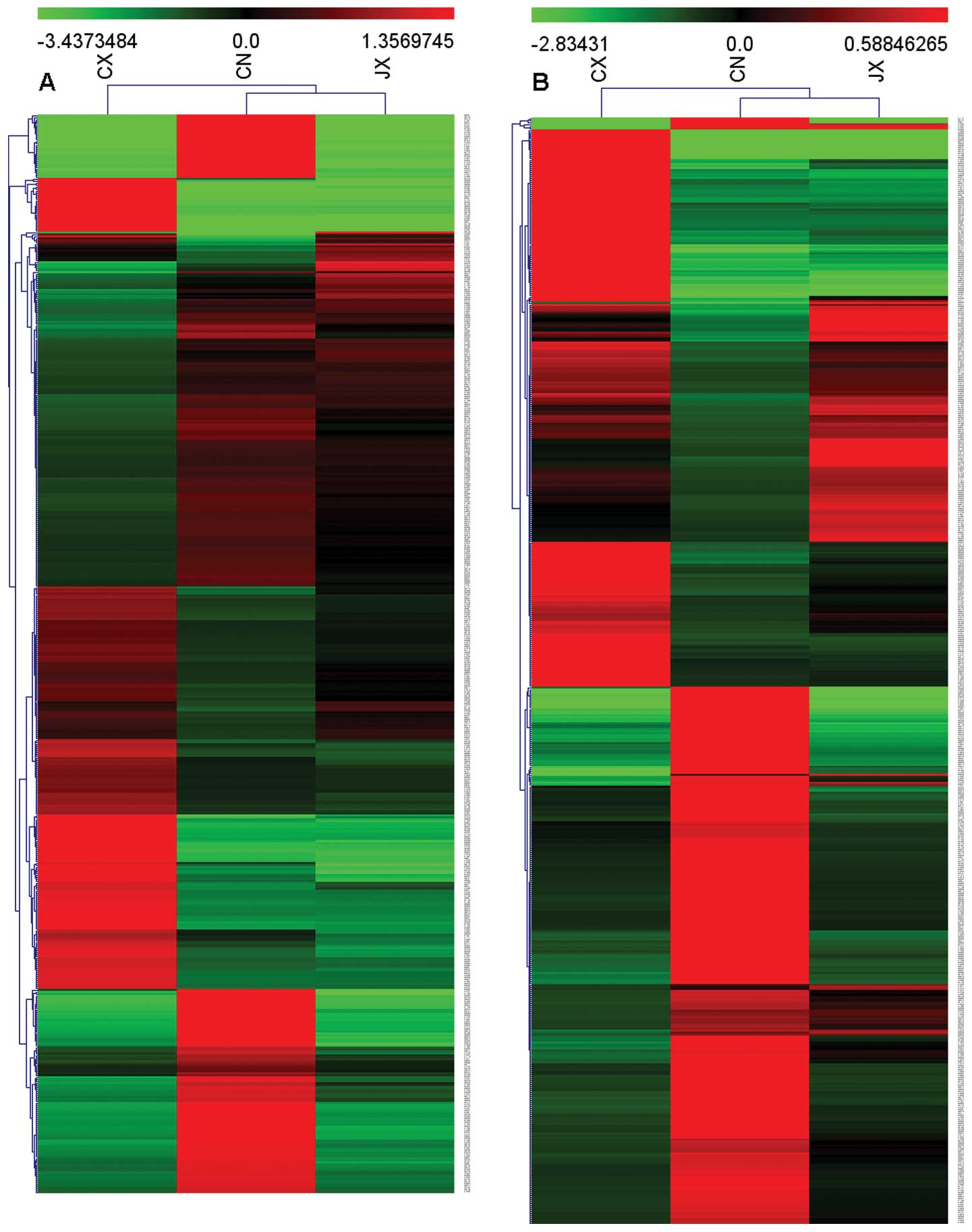
was observed in the mRNA expression profile. Fig. 1 shows the heat maps of the
expression ratios of lncRNAs and mRNAs among the JX, CX and CN
groups. In addition, we focused on those altered expression genes
induced by irradition while reversed by curcumin by a stronger raw
signal screening. We obtained 116 lncRNAs, in which 76 were
upregulated and 40 were downregulated in the CX group compared with
those in the CN group. lncRNAs in the JX group were completely or
partially reversed. We used the same screening method and obtained
178 differentially expressed mRNAs, in which 59 mRNAs were
upregulated and 119 mRNAs were downregulated. Functional enrichment
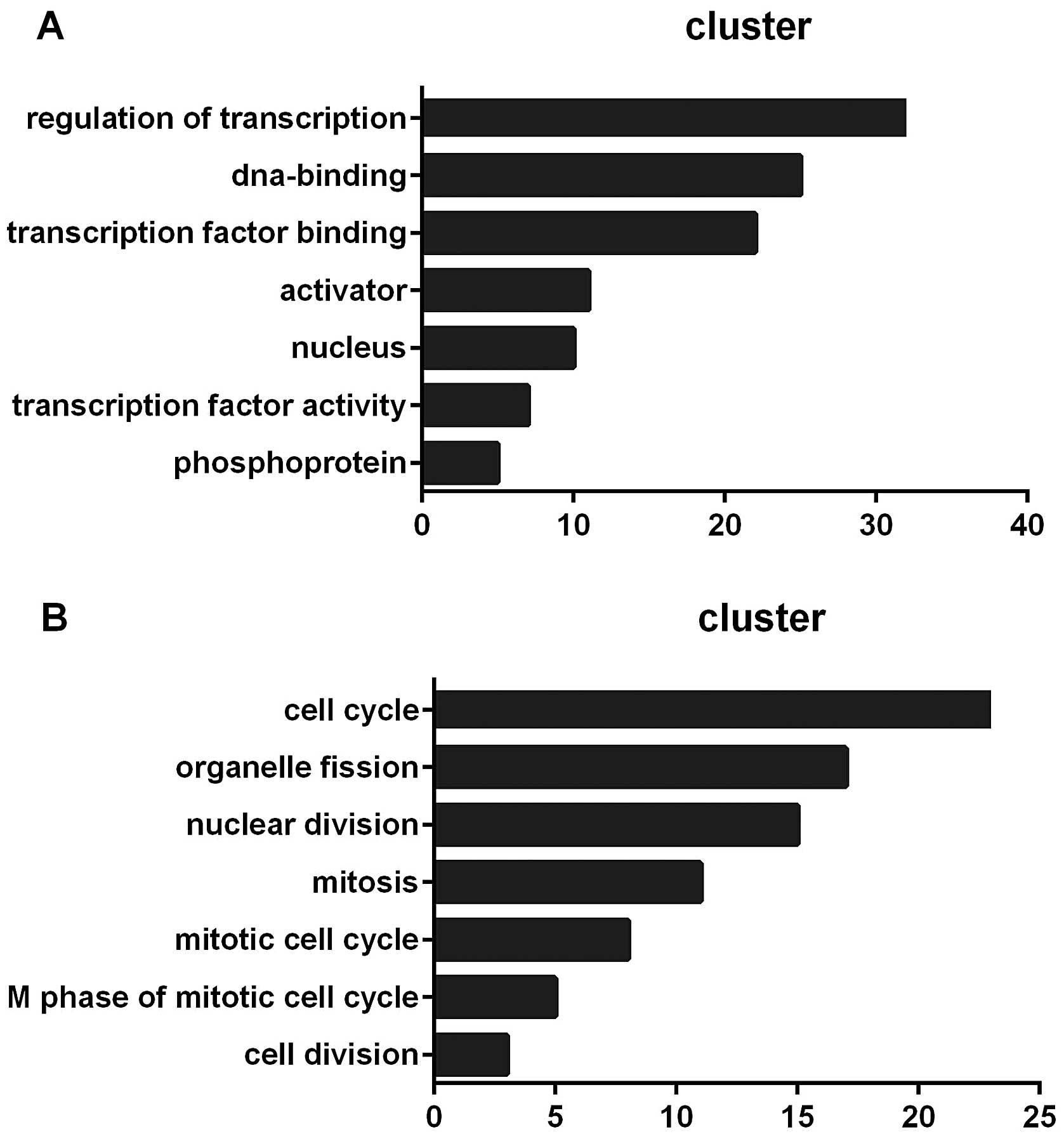
analysis of these differentially expressed genes was performed and
clustered by DAVID’s Functional Annotation Chart (25). Fig.
2 shows the functional annotation terms of the analysis.
Confirmation of some differentially
expressed lncRNAs
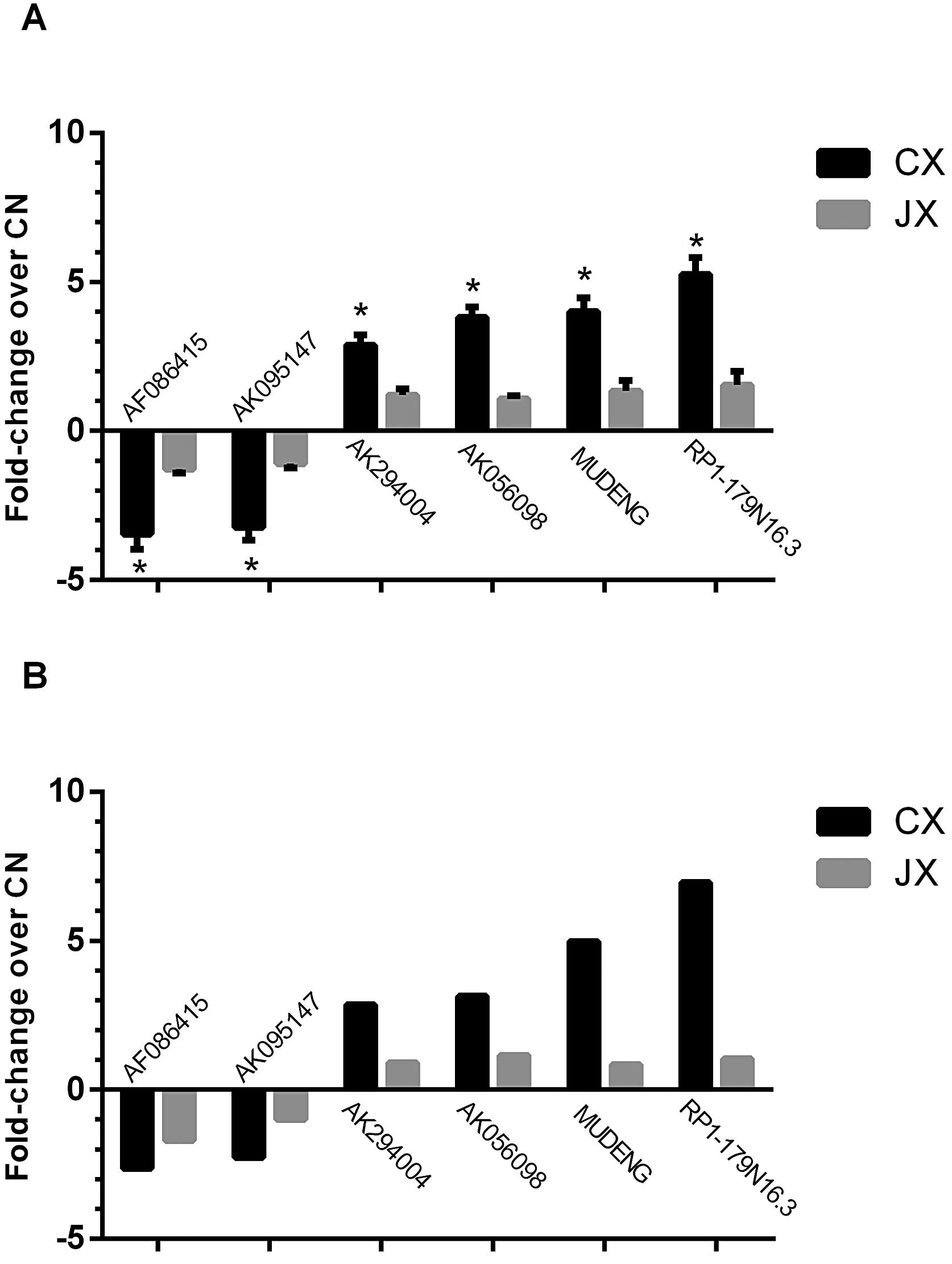
We performed qPCR assays to confirm the expression
patterns of some differentially expressed lncRNAs. qPCR results
were consistent with the microarray analysis results of six lncRNAs
(AF086415, AK095147, RP1-179N16.3, MUDENG, AK056098 and AK294004)
in terms of regulation direction and significance. In particular,
0.29-fold downregulation in CX and 0.78-fold reversal in JX were
observed in AF086415 (0.38- and 0.59-fold in microarray analysis,
respectively). For AK095147, 0.31-fold downregulation in CX and
0.92-fold of reversal in JX were observed (0.44- and 1.04-fold in
microarray analysis, respectively). For RP1-179N16.3, 5.25-fold
upregulation in CX and 1.54-fold reversal in JX were observed
(6.96- and 1.04-fold in microarray analysis, respectively). For
MUDENG, 4.01-fold upregulation in CX and 1.34-fold reversal in JX
were observed (4.98- and 0.85-fold in microarray analysis,
respectively). For AK056098, 3.81-fold upregulation in CX and
1.08-fold reversal in JX were observed (3.14- and 1.16-fold in
microarray analysis, respectively). For AK294004, 2.88-fold
upregulation in CX and 1.21-fold reversal in JX were observed
(2.86- and 0.91-fold in microarray analysis, respectively. All of
the fold changes were compared with CN (Fig. 3).
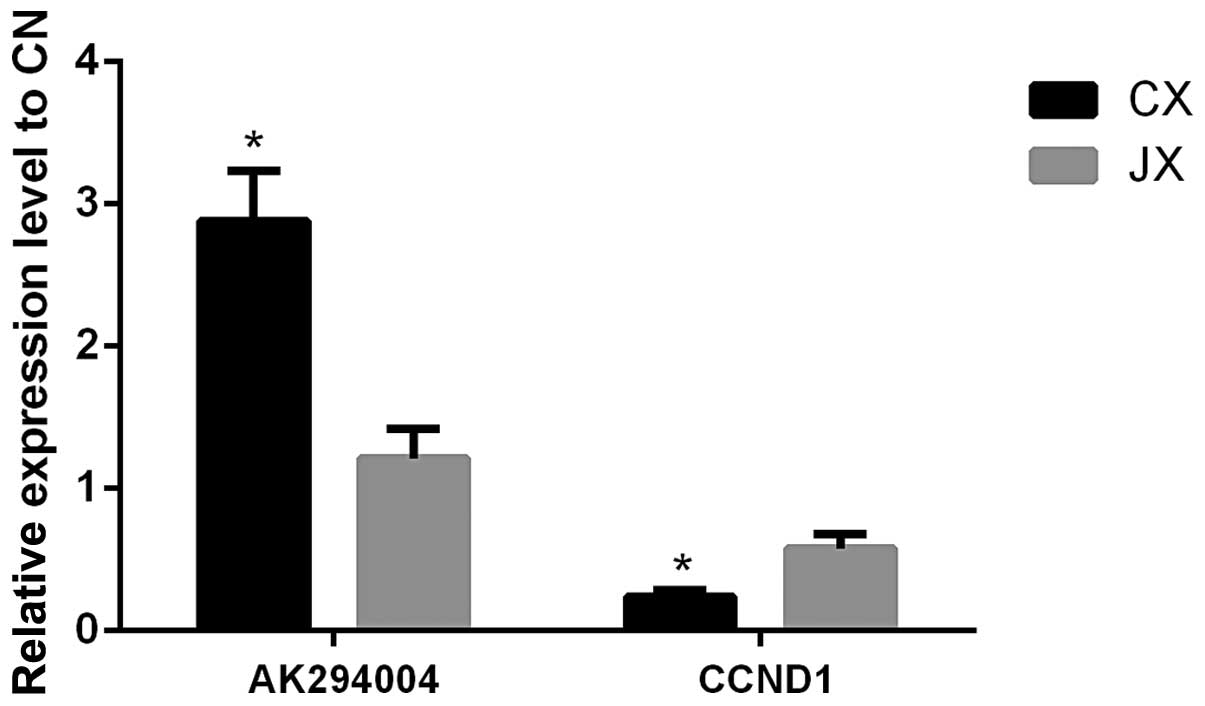
Effect of interaction between AK294004
and 3′UTR of CCND1
AK294004 exhibited a negative effect on CCND1. For
AK294004, 2.88-fold upregulation in CX and 1.21-fold reversal in JX
were observed by qPCR, and for CCND1, 0.26-fold downregulation in
CX and 0.61-fold reversal in JX were observed (compared with CN,
Fig. 4).
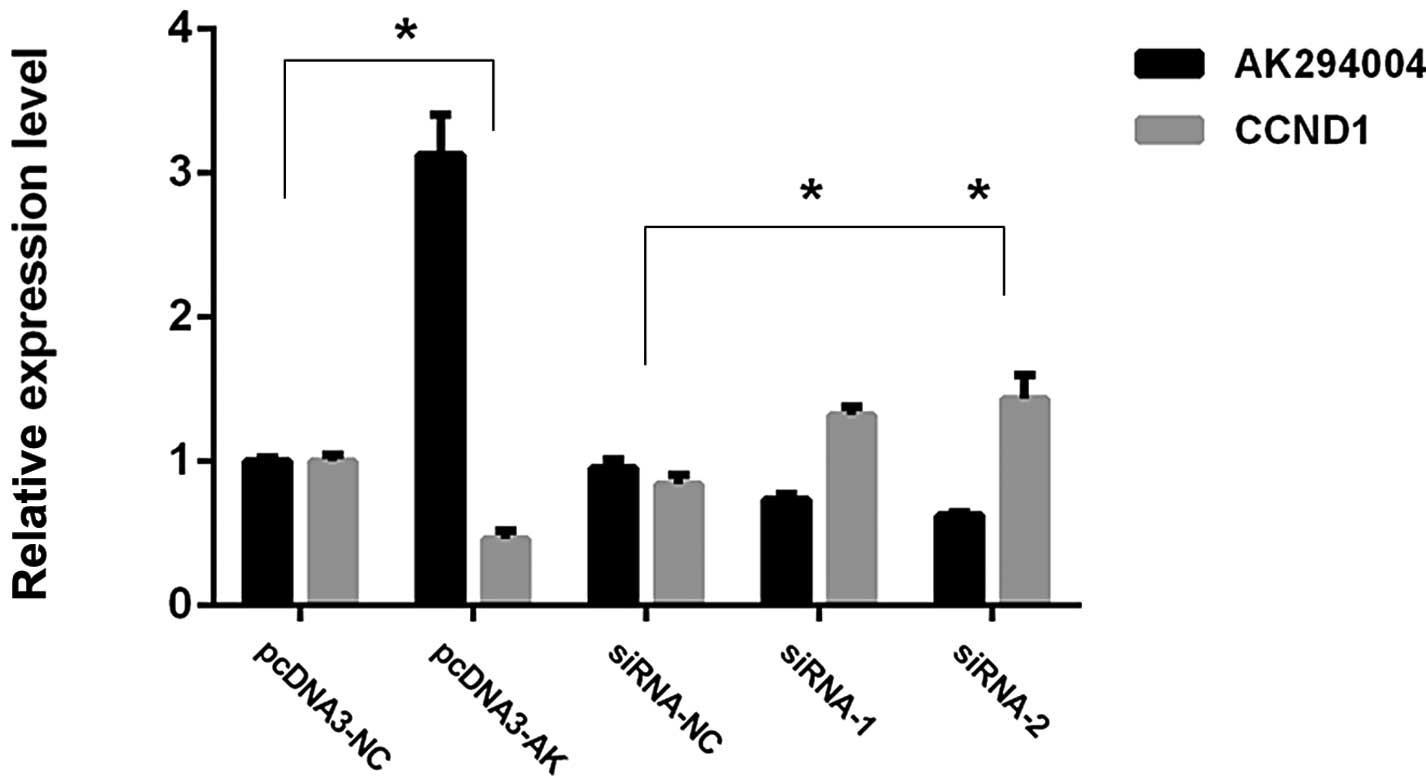
To investigate the functional effects of AK294004 in
NPC cells, we modulated its expression through RNA interference and
overexpression experiments. pCDNA3-AK and two individual AK294004
siRNAs were transfected into CNE2 cells. qPCR analysis of AK204004
and CCND1 levels was performed 36 h post-transfection. As shown in
Fig. 5, for overexpression
experiments, AK204004 expression was increased 3.16-fold while
CCND1 expression was decreased by 46% in pCDNA3-AK cells, compared
with control cells (pCDNA3-NC). For RNA interference experiments,
when compared with control cells (siRNA-NC), AK294004 expression
was knocked down 67% by siRNA-2, and 75% by siRNA-1, while CCND1
expression was increased 1.34- and 1.48-fold, respectively.
A luciferase-based reporter was constructed to
evaluate the effect of AK294004 direct binding to the putative
target sites on the 3′UTR of CCND1. To substantiate the assumption
that AK294004 can directly repress CCND1, the reporter construct
pGL3-vector or pGL3-W, pGL3-E1 and pGL3-E2 was co-transfected with
pcDNA3-AK and pcDNA3-NC to HEK293 cells. Luciferase activity was
then assayed. As shown in Fig. 6,
for pGL3-W or pGL3-E1 construct, pcDNA3-AK significantly lowered
luciferase activity compared with pcDNA3-NC. There was no different
luciferase activity observed between the pGL3-vector and pGL3-E2
constructs.
These findings support the hypothesis that lncRNA
AK294004 directly targets CCND1 expression by its exon 1 part, but
not the exon 2 part, thus leading to the decreased CCND1 expression
through some inhibition mechanism.
Discussion
Radiotherapy is considered one of the most effective
treatments for patients with NPC, and radioresistance is the main
risk factor that contributes to poor prognosis (2). Radioresistance occurs in primary IR
treatment and the survived cells may be more resistant to the
second IR treatment, thereby leading to the failure of radiotherapy
(2,28,29).
In this regard, the exact molecules and signaling pathway involved
in radiosensitization should be determined to develop target
therapy and enhance the efficacy of radiation. In this study, we
observed that IR-induced differentially expressed lncRNAs were
almost reversed by Cur. This result is consistent with our
hypothesis, in which Cur enhances radiosensitivity through the
reversal of effective molecules (7,30,31).
For example, AK294004, a natural antisense lncRNA, exhibited
2.86-fold upregulation in CX (compare with CN), whereas a reversal
at 0.32-fold downregulation by Cur was observed in JX (compare with
CX). This finding was further confirmed by qPCR. lncRNA may have an
important function in IR-induced radioresistance.
Cur regulates the gene expression involved in
survival, proliferation, angiogenesis, invasion and metastasis.
This phytochemical also modulates various mechanisms that are
associated with radioresistance, including the following:
downregulating COX-2, MRP, Bcl-2, and survivin expression;
inhibiting PI3K/AKT activation; suppressing growth factor signaling
pathways; and inhibiting STAT3 activation (32–34).
In this study, we demonstrated that Cur enhanced radiosensitivity
in the NPC cell line CNE2 at 10 μmol/l by MTT or clonogenic
survival test (35) before we
performed the array test (data not shown), although Cur exhibited
higher anti-proliferative effects when used alone at a
concentration of 20 or 40 μmol/l. Considering the cytotoxicity of
Cur and IR, a concentration of 10 μmol/l was more suitable as a
radioenhancer. Therefore, no significant data were obtained by
conjoint analysis with other groups at particular time-points,
although the array of JN (Cur group) was performed (data not
shown). Further analysis need to be performed to reveal other
chemical mechanisms for Cur. Furthermore, the optimal IR dosage of
2 Gy and the Cur pretreatment time of 6 h were confirmed for the
succeeding study (data not shown).
The mammalian genome clearly encodes numerous
lncRNAs that are highly conserved and biologically functional
(36). Expression patterns have
suggested that these lncRNAs are involved in diverse biological
processes, including cell cycle regulation, innate immunity, and
pluripotency (37), but current
understanding on the functions of lncRNAs is limited. In this
study, 116 differentially expressed lncRNAs were expressed
site-specifically, such as intergenic, intronic antisense, natural
antisense, bidirectional, and intron sense overlapping. We used the
DAVID Functional Annotation Chart (25) for the functional enrichment
analysis of these differentially expressed genes. In this study,
the most significant functional annotation terms of 116 lncRNAs
were transcription regulation, DNA binding, transcription factor
binding, activator and nucleus (Fig.
2A). For 178 mRNAs, the functional annotation terms were cell
cycle, organelle fission, nuclear division, mitosis, mitotic cell
cycle and M phase of the mitotic cell cycle (Fig. 2B). No direct relationship was found
between the altered lncRNA and mRNA expressions, indicating that
lncRNA performed a biological function via a complex regulatory
mechanism instead of directly targeting mRNA during the Cur-induced
radiosensitization involved in NPC.
AK294004, a natural antisense lncRNA that completely
complements the terminal end of the 3′ untranslated region of CCND1
mRNA, exhibited a negative effect on CCND1, an important molecule
of the cell cycle and DNA repair. CCND1 is downregulated during
IR-induced DNA damage (17,38).
In this study, we observed the IR-induced altered regulation and
the Cur-induced reversal of either AK294004 or CCND1 that were
consequently confirmed by qPCR (Fig.
4). Moreover, luciferase reporter assay and modulated
expression experiments indicated that CCND1 might be a direct
target of AK294004, however, it needed to be further determined how
these lcnRNAs and mRNAs interact with one another.
In general, the cells respond to IR-induced
biological process, such as DNA damage repair, cell cycle arrest,
and so on (33,39–41).
In this study, we performed the microarray assay at 3 h post-IR. We
also performed qPCR to validate the altered lncRNA expression at
different time-points until 48 h post-IR was reached in parallel
cell groups (data not shown). The microarray results were
consistent with the qPCR data, particularly at 3–12 h checkpoint
but slightly differed after 24 h. These differences in responses
may be attributed to different mechanisms of lncRNA
performance.
In conclusion, we demonstrated the mechanism by
which Cur enhanced radiosensitivity in NPC cells that involved
differentially expressed lncRNAs and provided better understanding
of chemically-mediated radiosensitization. The function of
Cur-induced lncRNA reversal should be fully understood to provide a
new and more effective radiotherapeutic treatment for patients with
NPC by using natural products.
Acknowledgements
This study was supported by National
Natural Science Foundation of China (grant no. 81173616) and New
Technology Project of Nanfang Hospital (grant no. 201103). We thank
medical personnel Jiabin Liu and Huarui Niu of NanFang Hospital for
providing X-ray radiation equipment.
References
|
1.
|
Ou J, Pan F, Geng P, et al: Silencing
fibronectin extra domain A enhances radiosensitivity in
nasopharyngeal carcinomas involving an FAK/Akt/JNK pathway. Int J
Radiat Oncol Biol Phys. 82:e685–e691. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Lu ZX, Ma XQ, Yang LF, et al: DNAzymes
targeted to EBV-encoded latent membrane protein-1 induce apoptosis
and enhance radiosensitivity in nasopharyngeal carcinoma. Cancer
Lett. 265:226–238. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Ou J, Luan W, Deng J, Sa R and Liang H: αv
integrin induces multicellular radioresistance in human
nasopharyngeal carcinoma via activating SAPK/JNK pathway. PLoS One.
7:e387372012.
|
|
4.
|
Bernier J: A multidisciplinary approach to
squamous cell carcinomas of the head and neck: an update. Curr Opin
Oncol. 20:249–255. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Feng XP, Yi H, Li MY, et al:
Identification of biomarkers for predicting nasopharyngeal
carcinoma response to radiotherapy by proteomics. Cancer Res.
70:3450–3462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Sandur SK, Deorukhkar A, Pandey MK, et al:
Curcumin modulates the radiosensitivity of colorectal cancer cells
by suppressing constitutive and inducible NF-kappaB activity. Int J
Radiat Oncol Biol Phys. 75:534–542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Shishodia S, Amin HM, Lai R and Aggarwal
BB: Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB
activation, induces G1/S arrest, suppresses proliferation, and
induces apoptosis in mantle cell lymphoma. Biochem Pharmacol.
70:700–713. 2005. View Article : Google Scholar
|
|
8.
|
Li L, Aggarwal BB, Shishodia S, Abbruzzese
J and Kurzrock R: Nuclear factor-kappaB and IkappaB kinase are
constitutively active in human pancreatic cells, and their
down-regulation by curcumin (diferuloylmethane) is associated with
the suppression of proliferation and the induction of apoptosis.
Cancer. 101:2351–2362. 2004. View Article : Google Scholar
|
|
9.
|
Bharti AC, Shishodia S, Reuben JM, et al:
Nuclear factor-kappaB and STAT3 are constitutively active in
CD138+ cells derived from multiple myeloma patients, and
suppression of these transcription factors leads to apoptosis.
Blood. 103:3175–3184. 2004.PubMed/NCBI
|
|
10.
|
Wang X, Xia X, Xu C, et al:
Ultrasound-induced cell death of nasopharyngeal carcinoma cells in
the presence of curcumin. Integr Cancer Ther. 10:70–76. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Wong TS, Chan WS, Li CH, et al: Curcumin
alters the migratory phenotype of nasopharyngeal carcinoma cells
through up-regulation of E-cadherin. Anticancer Res. 30:2851–2856.
2010.PubMed/NCBI
|
|
12.
|
Zhang X, Sun S, Pu JK, et al: Long
non-coding RNA expression profiles predict clinical phenotypes in
glioma. Neurobiol Dis. 48:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Kaikkonen MU, Lam MT and Glass CK:
Non-coding RNAs as regulators of gene expression and epigenetics.
Cardiovasc Res. 90:430–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Wang KC and Chang HY: Molecular mechanisms
of long noncoding RNAs. Mol Cell. 43:904–914. 2011. View Article : Google Scholar
|
|
16.
|
Nagano T and Fraser P: No-nonsense
functions for long noncoding RNAs. Cell. 145:178–181. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ozgur E, Mert U, Isin M, Okutan M, Dalay N
and Gezer U: Differential expression of long non-coding RNAs during
genotoxic stress-induced apoptosis in HeLa and MCF-7 cells. Clin
Exp Med. 13:119–126. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Barsyte-Lovejoy D, Lau SK, Boutros PC, et
al: The c-Myc oncogene directly induces the H19 noncoding RNA by
allele-specific binding to potentiate tumorigenesis. Cancer Res.
66:5330–5337. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Zhou Y, Zhong Y, Wang Y, et al: Activation
of p53 by MEG3 non-coding RNA. J Biol Chem. 282:24731–24742. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Mariner PD, Walters RD, Espinoza CA, et
al: Human Alu RNA is a modular transacting repressor of mRNA
transcription during heat shock. Mol Cell. 29:499–509. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Beltran M, Puig I, Pena C, et al: A
natural antisense transcript regulates Zeb2/Sip1 gene expression
during Snail1-induced epithelial-mesenchymal transition. Genes Dev.
22:756–769. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Gupta RA, Shah N, Wang KC, et al: Long
non-coding RNA HOTAIR reprograms chromatin state to promote cancer
metastasis. Nature. 464:1071–1076. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Huarte M and Rinn JL: Large non-coding
RNAs: missing links in cancer? Hum Mol Genet. 19:R152–R161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Wang J, Guo LP, Chen LZ, Zeng YX and Lu
SH: Identification of cancer stem cell-like side population cells
in human nasopharyngeal carcinoma cell line. Cancer Res.
67:3716–3724. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Huang da W, Sherman BT and Lempicki RA:
Bioinformatics enrichment tools: paths toward the comprehensive
functional analysis of large gene lists. Nucleic Acids Res.
37:1–13. 2009.PubMed/NCBI
|
|
26.
|
Fan Q, He M, Deng X, et al: Derepression
of c-Fos caused by MicroRNA-139 down-regulation contributes to the
metastasis of human hepatocellular carcinoma. Cell Biochem Funct.
31:319–324. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Liu Y, Cai H, Liu J, et al: A miR-151
binding site polymorphism in the 3′-untranslated region of the
cyclin E1 gene associated with nasopharyngeal carcinoma. Biochem
Biophys Res Commun. 432:660–665. 2013.PubMed/NCBI
|
|
28.
|
Pearce AG, Segura TM, Rintala AC,
Rintala-Maki ND and Lee H: The generation and characterization of a
radiation-resistant model system to study radioresistance in human
breast cancer cells. Radiat Res. 156:739–750. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Singh S and Aggarwal BB: Activation of
transcription factor NF-kappa B is suppressed by curcumin
(diferuloylmethane) [corrected]. J Biol Chem. 270:24995–25000.
1995.
|
|
31.
|
Li JY, Li YY, Jin W, Yang Q, Shao ZM and
Tian XS: ABT-737 reverses the acquired radioresistance of breast
cancer cells by targeting Bcl-2 and Bcl-xL. J Exp Clin Cancer Res.
31:1022012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Kunnumakkara AB, Diagaradjane P, Guha S,
et al: Curcumin sensitizes human colorectal cancer xenografts in
nude mice to gamma-radiation by targeting nuclear
factor-kappaB-regulated gene products. Clin Cancer Res.
14:2128–2136. 2008. View Article : Google Scholar
|
|
33.
|
Javvadi P, Segan AT, Tuttle SW and
Koumenis C: The chemo-preventive agent curcumin is a potent
radiosensitizer of human cervical tumor cells via increased
reactive oxygen species production and overactivation of the
mitogen-activated protein kinase pathway. Mol Pharmacol.
73:1491–1501. 2008. View Article : Google Scholar
|
|
34.
|
Narang H and Krishna M: Inhibition of
radiation induced nitration by curcumin and nicotinamide in mouse
macrophages. Mol Cell Biochem. 276:7–13. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hannoun-Levi JM, Chand-Fouche ME, Dejean C
and Courdi A: Dose gradient impact on equivalent dose at 2 Gy for
high dose rate interstitial brachytherapy. J Contemp Brachytherapy.
4:14–20. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Khalil AM, Guttman M, Huarte M, et al:
Many human large intergenic noncoding RNAs associate with
chromatin-modifying complexes and affect gene expression. Proc Natl
Acad Sci USA. 106:11667–11672. 2009. View Article : Google Scholar
|
|
37.
|
Guttman M, Amit I, Garber M, et al:
Chromatin signature reveals over a thousand highly conserved large
non-coding RNAs in mammals. Nature. 458:223–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Wang X, Arai S, Song X, et al: Induced
ncRNAs allosterically modify RNA-binding proteins in cis to inhibit
transcription. Nature. 454:126–130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Aravindan N, Veeraraghavan J,
Madhusoodhanan R, Herman TS and Natarajan M: Curcumin regulates
low-linear energy transfer gamma-radiation-induced
NFkappaB-dependent telomerase activity in human neuroblastoma
cells. Int J Radiat Oncol Biol Phys. 79:1206–1215. 2011. View Article : Google Scholar
|
|
40.
|
Forrester HB, Li J, Hovan D, Ivashkevich
AN and Sprung CN: DNA repair genes: alternative transcription and
gene expression at the exon level in response to the DNA damaging
agent, ionizing radiation. PLoS One. 7:e533582012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Lan ML, Acharya MM, Tran KK, et al:
Characterizing the radio-response of pluripotent and multipotent
human stem cells. PLoS One. 7:e500482012. View Article : Google Scholar : PubMed/NCBI
|