Introduction
Currently, intensity modulated radiotherapy (IMRT)
is a widely applied conformal radiotherapy modality; in contrast to
conventional radiotherapy, IMRT uses multiple beams across the
target field of treatment. This reduces the volume of tissues
receiving high doses, but a greater volume of normal tissues still
receives low doses of radiation (1–3). It
is estimated that IMRT can contribute to 1.5% increased risk of
secondary cancers by 10 years following treatment (4). However, these figures were considered
to be overestimated because the calculations of these risks were
based on the long-term data obtained from the follow-up of atomic
bomb survivors. This population was exposed to a single whole body
dose, while IMRT patients receive fractionated doses to specific
body parts (5). Besides, other
studies moderated the therapeutic effect of IMRT over its potential
health side effects (6,7).
Microarrays and DNA damage studies, through
measuring the Ser 139 phosphorylated form of histone H2AX (γ-H2AX),
are emerging applications in the field of radiation biology and
biodosimetry. Gene expression studies improved the knowledge on
cellular responses to both high and low radiation doses (8–11).
On the other hand, γ-H2AX foci immunodetection has been described
as useful quantitative biomarker of human low-level radiation
exposure (12).
In this study, we address the question of
understanding the whole blood tissue biological responses in
prostate cancer patients receiving low doses of ionizing radiation
during IMRT. It is the first study that combines DNA damage and
microarray investigations on whole blood samples collected in
vivo from patients receiving low doses over a large part of the
body. It highlights the mechanisms and the possible health effects
involved in response to low doses of ionizing radiation. For the
DNA damage assessment γ-H2AX foci were scored. For the analysis of
the microarray data, we applied a holistic approach, namely Gene
Set Enrichment Analysis (GSEA) that it is known to overcome many of
the limitations in individual gene pathway analysis, discussed
thoroughly by Subramanian and colleagues (13). In addition, we used differentially
expressed genes for Exploratory Gene Association Networks (EGAN)
analysis.
Materials and methods
Patients and sample collection
The study population consisted of 8 prostate cancer
patients treated with step and shoot-IMRT (ss-IMRT) (Elekta Synergy
linear accelerator) at the Department of Radiation Oncology (Ghent
University Hospital, Belgium) between March and May 2013. A dose
per fraction to the tumor was 2.09 Gy. After obtaining written
approval of the ethics committee at Ghent University Hospital and
signed informed consent, blood samples were taken at different
time-points. Blood sampling for the γ-H2AX foci was performed in
heparin vacutainer tubes before and 30 min after the first
fraction, blood sampling for the whole genome expression analysis
was performed in EDTA vacutainer tubes before the first and second
fraction, 18–24 h after the first fraction.
Dose calculation
The equivalent total body blood dose (DETB) was
calculated for each patient based on the treatment planning data.
To this end, the mean dose within the skin contour of the scanned
volume was normalised to the patient mass. As liver, heart/large
blood vessels and lungs contain together 38.5% of the total blood
volume it was assumed that 61.5% of the blood pool is distributed
uniformly over the rest of the body.
γ-H2AX scoring
The procedure for the γ-H2AX foci assay on
T-lymphocytes is described in detail in a previous report (14). Foci analysis was performed with the
Cytovision v.2.8 Software 2002 (Applied Imaging, USA) and an
Olympus BX60 fluorescent microscope was used with a 100x/1.30 oil
lens. Several images of one slide were captured with a digital
camera (Applied Imaging), 10 Z-stacks with 1.03 μm spacing
was used.
RNA isolation for microarray gene
expression studies
Collected venous blood (4-ml/time-point) was passed
through a LeukoLOCK™ filter (Life Technologies, USA), washed with
PBS and leukocytes were stabilized with RNAlater®.
Filters were capped and stored at −20°C. RNA was isolated using
LeukoLOCK™ Isolation System (Life Technologies) according to the
manufacturer’s instructions. RNA was quantified using a Nanodrop
2000 (Thermo Scientific, USA) spectrophotometer, the quality was
assessed with Agilent 2100 Bioanalyzer. All RNA samples had ≥8.5 as
an integrity number.
Microarray assay
Using the Ambion® WT Expression kit
(Ambion, USA), cDNA was prepared from 10 μg of purified
cRNA, originally synthesized and purified from 0.25 μg of
total RNA, following the manufacturer’s instructions. The cDNA
(2.75 μg) was used for fragmentation and labeling using
GeneChip® Terminal Labeling kit (Affymetrix, USA). Using
GeneChip® Hybridization, Wash and Stain kit
(hybridization module) (Affymetrix), and hybridization controls
(Affymetrix), fragmented and labeled cDNA was hybridized to Human
Gene 1.0 ST arrays (Affymetrix). After hybridization with rotation
for 16 h at 45°C, arrays were washed and stained, according to the
manufacturer’s instructions, using GeneChip®
Hybridization, Wash and Stain kit (stain module) (Affymetrix).
Finally, arrays were scanned immediately using Affymetrix
GeneChip® Scanner.
Microarray data processing
Raw Affymetrix data were preprocessed using Partek
Genomics Suite v6.6 (Partek Inc., USA). Briefly, Robust Multichip
Average (RMA) was used for background correction followed by
quantile normalization and summarization of multiple probe
intensities for each probeset using the median polish approach
(15). Gene expression values were
obtained by the one-step Tukey method.
Functional analysis - GSEA
GSEA calculates an enrichment score (ES) reflecting
the overrepresentation of a certain gene set at the top or bottom
of a ranked list of genes found in the expression dataset of two
classes. This method applies the Kolmogorov-Smirnov test to find
deviation between two distributions. Information on GSEA was
reported previously (13).
Briefly, genes are ranked using signal-to-noise ratio. Using
Kolmogorov-Smirnov statistics, pre-defined sets of genes are scored
and significance is tested by empirical permutation followed by
correction for multiple hypotheses. The Reactome database was used
as reference background for the implemented analysis. In total, the
data were analyzed against 674 gene sets downloaded from the
Molecular Signature Database (MSigDB) (http://www.broadinstitute.org/gsea/msigdb/index.jsp).
The GSEA software parameters were set to their default values. The
statistical significance of the normalized enrichment score (NES)
associated to each gene set was assessed through 1,000 random
permutations of the phenotypic labels. FDR (false discovery rate)
value <0.05 was used as a cut-off value for assessing the
statistical significance of the estimates. For gene set networks,
we used the Enrichment Map plug-in (16) for Cytoscape Desktop program
(http://baderlab.org/Software/EnrichmentMap/). Gene
sets with FDR values <0.05 and having ≥50% overlapping genes are
represented in the network.
Functional analysis - Exploratory Gene
Association Networks
To test for differential expression between
different irradiated conditions and reference conditions (no
irradiation) we used repeated measures ANOVA. Differentially
expressed genes were defined with a p-value cutoff with a false
discovery rate of <0.05. Differentially expressed genes were
analyzed using Exploratory Gene Association Networks (EGAN, The
Regents of the University of California) software to determine
differentially regulated pathways. P-values were corrected using
Westfall-Young minP method. P-values <0.05 were considered
significant. For clearer illustrations, not all genes belonging to
each pathway are shown in the figures.
Quantitative RT-PCR validation
For quantitative real-time (RT-PCR) confirmation, we
selected nine different genes that were shown to be differentially
expressed and contributed to the pathway enrichment of immune
signaling, DNA damage and repair and cell cycle progression.
Briefly, cDNA was prepared from 0.25 μg of total RNA using
Ambion® WT Expression kit (Ambion) following the
manufacturer’s instructions. RT-PCR was performed using
TaqMan® Gene Expression assays (Applied Biosystems,
USA). Each TaqMan assay was run in duplicate for each diluted cDNA
sample using TaqMan® Fast Advanced Master Mix (Applied
Biosystems). The reactions were run on ABI 7500 Fast RT-PCR system
following the manufacturer’s recommended PCR program: 95°C for 20
sec, followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec.
Relative expression values were calculated by Pfaffl (17) method normalized to PGK1
levels. Relative expression levels were tested for statistical
significance using paired t-test, genes having p-values <0.05
were considered significant.
Results
Based on the treatment planning data the equivalent
total body dose of one fraction amounted to 30.97±8.12 mGy
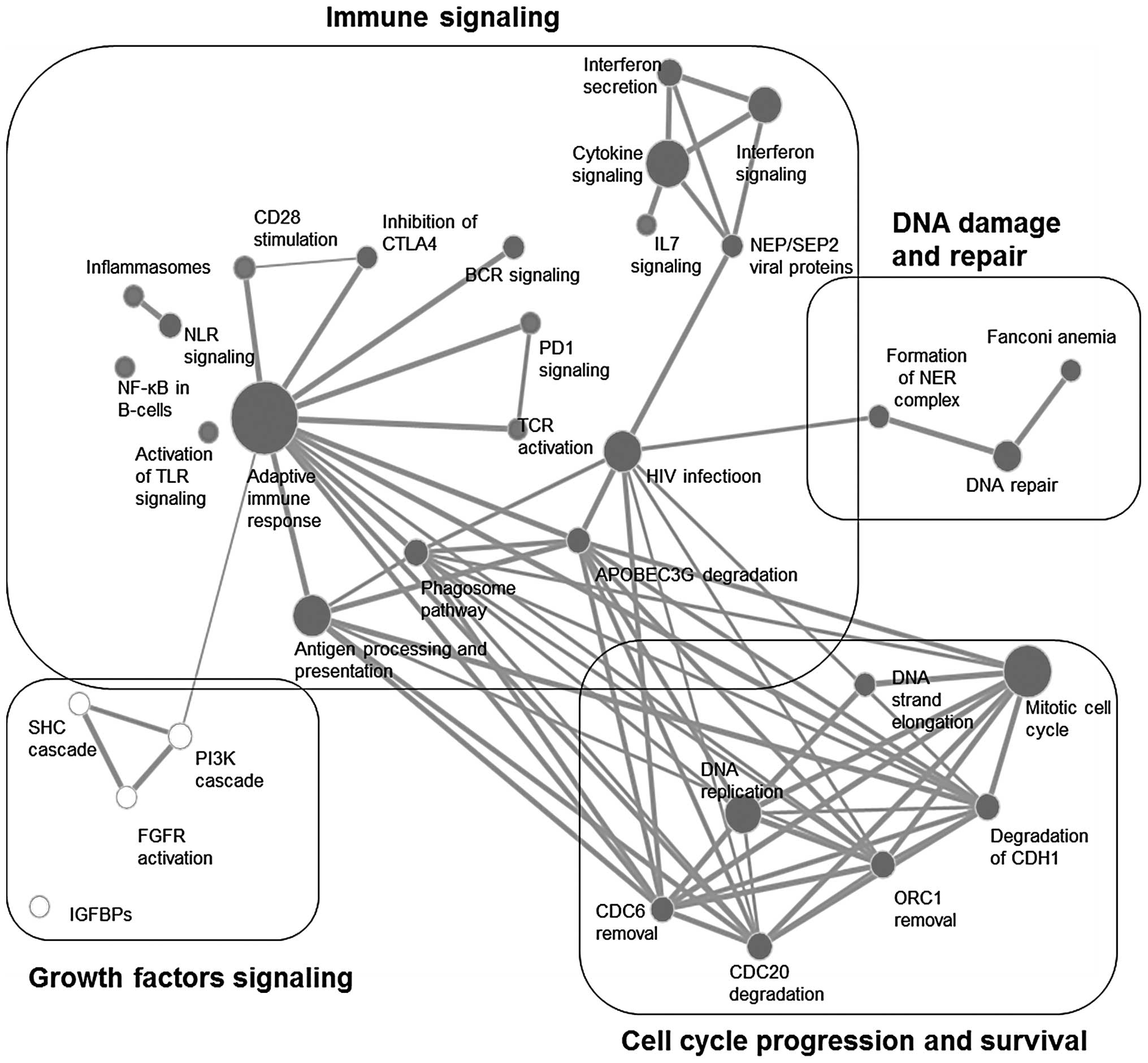
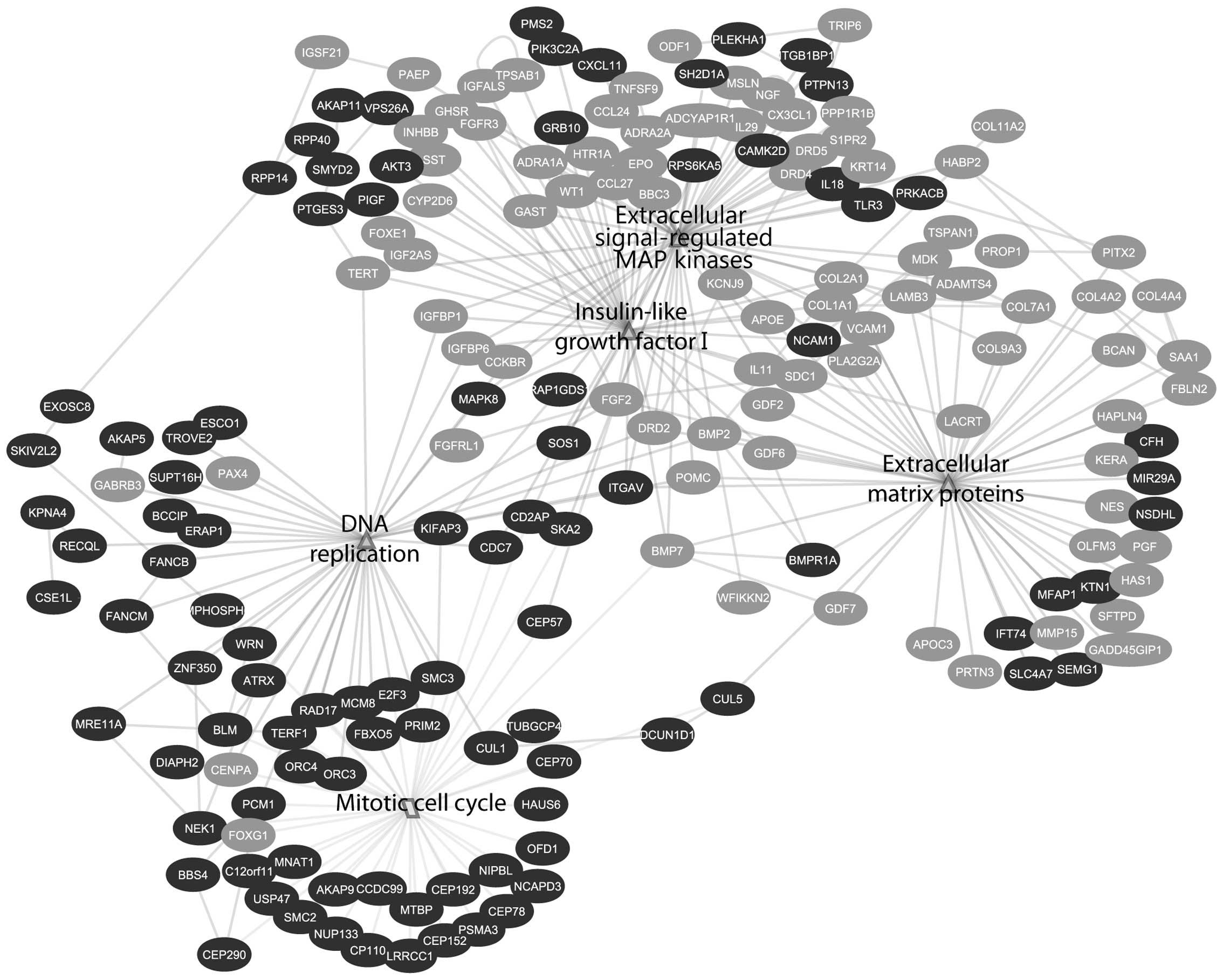
(Table III). GSEA enrichment map
analysis showed interconnections of 4 different signal transduction
categories; these are immune signaling, growth factors signaling,
cell cycle progression and survival, as well as DNA damage and
repair (Fig. 1). On the other
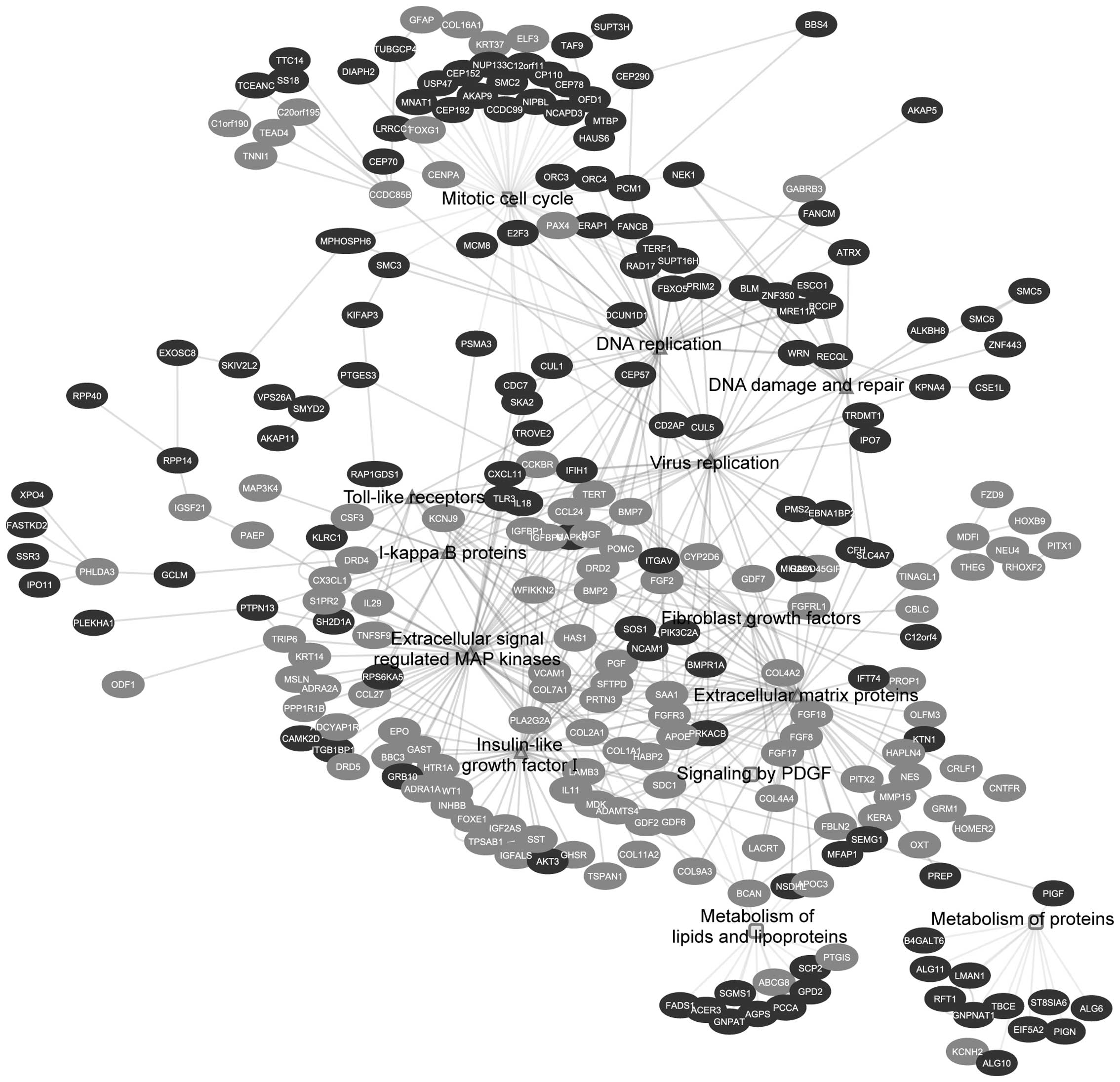
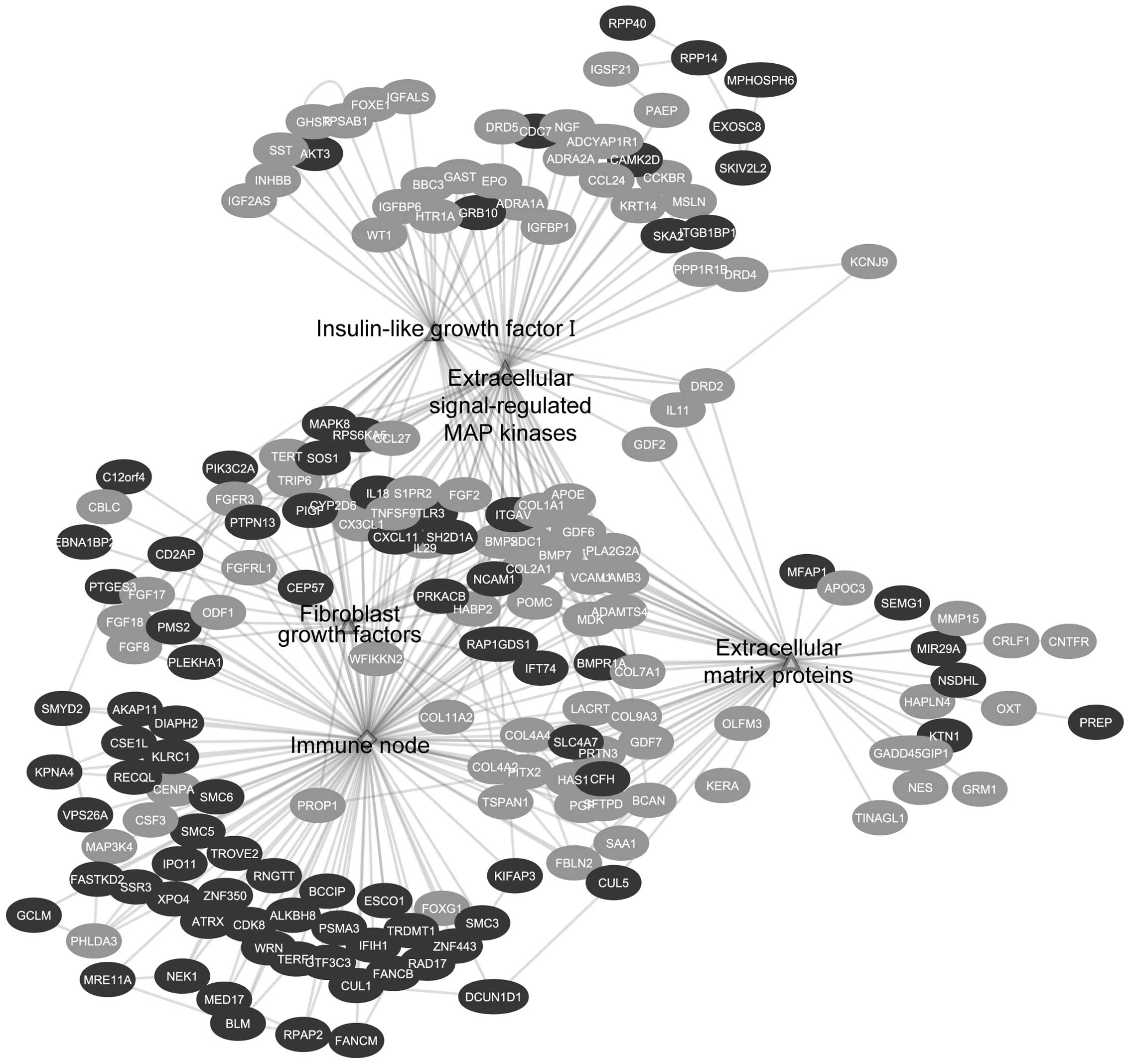
hand, EGAN analysis showed the biological response is divided into
three different categories: growth factors and cell cycle
progression, viral and immune signaling and metabolism (Table II and Fig. 2).
 | Table III.Number of induced γ-H2AX foci and the
equivalent total body dose (ETBD) in the eight prostate cancer
patients 30 min post-IMRT. |
Table III.
Number of induced γ-H2AX foci and the
equivalent total body dose (ETBD) in the eight prostate cancer
patients 30 min post-IMRT.
| Induced
foci/cell | ETBD (mGy) |
|---|
| Patient 1 | 0.622 | 28.01 |
| Patient 2 | 0.584 | 46.34 |
| Patient 3 | 0.267 | 30.24 |
| Patient 4 | 0.674 | 33.79 |
| Patient 5 | 0.583 | 37.94 |
| Patient 6 | 0.561 | 25.54 |
| Patient 7 | 0.28 | 22.07 |
| Patient 8 | 0.194 | 23.86 |
 | Table II.Enriched pathways of the
differentially expressed genes. |
Table II.
Enriched pathways of the
differentially expressed genes.
| Pathway | p-value |
|---|
| Growth factors
signaling and cell cycle progression | |
| Extracellular
matrix proteins | 1.16E-19 |
| Extracellular
signal regulated MAP kinases | 2.90E-16 |
| Mitotic cell
cycle | 8.70E-16 |
| Insulin growth
factor I | 2.30E-15 |
| Fibroblast growth
factors | 3.45E-11 |
| DNA
replication | 1.98E-10 |
| Signaling by
platelet derived growth factor | 2.80E-04 |
| Viral and immune
response | |
| Virus
replication | 2.80E-06 |
| DNA damage and
repair | 4.90E-06 |
| Toll-like
receptors | 5.40E-06 |
| IκB proteins | 1.90E-05 |
| Metabolism | |
| Metabolism of
lipids and lipoproteins | 1.40E-05 |
| Metabolism of
proteins | 5.40E-04 |
| RNA
degradation | 6.40E-04 |
Low doses of ionizing radiation induces
pro-inflammatory response via the activation of viral, adaptive and
innate immune signaling
HIV infection and gene sets belonging to the
adaptive immune response contributed mainly to the enrichment of
the immune signaling cluster (Table
I). The involvement of the viral infection response, along with
interferon signaling and secretion and APOBEC3G degradation denotes
the induction of an inflammatory response accompanied by DNA
damage; the HIV infection node shared a common edge with the DNA
damage and repair gene sets (Fig.
1). Furthermore, the enrichment map analysis showed involvement
of adaptive immune response activation, particularly CD28
stimulation that works in an opposite way with CTLA4, leading to
T-cell receptor activation and cytokine secretion (Table I and Fig. 1). In addition, innate immune gene
sets were significantly modulated; these include phagosome pathway,
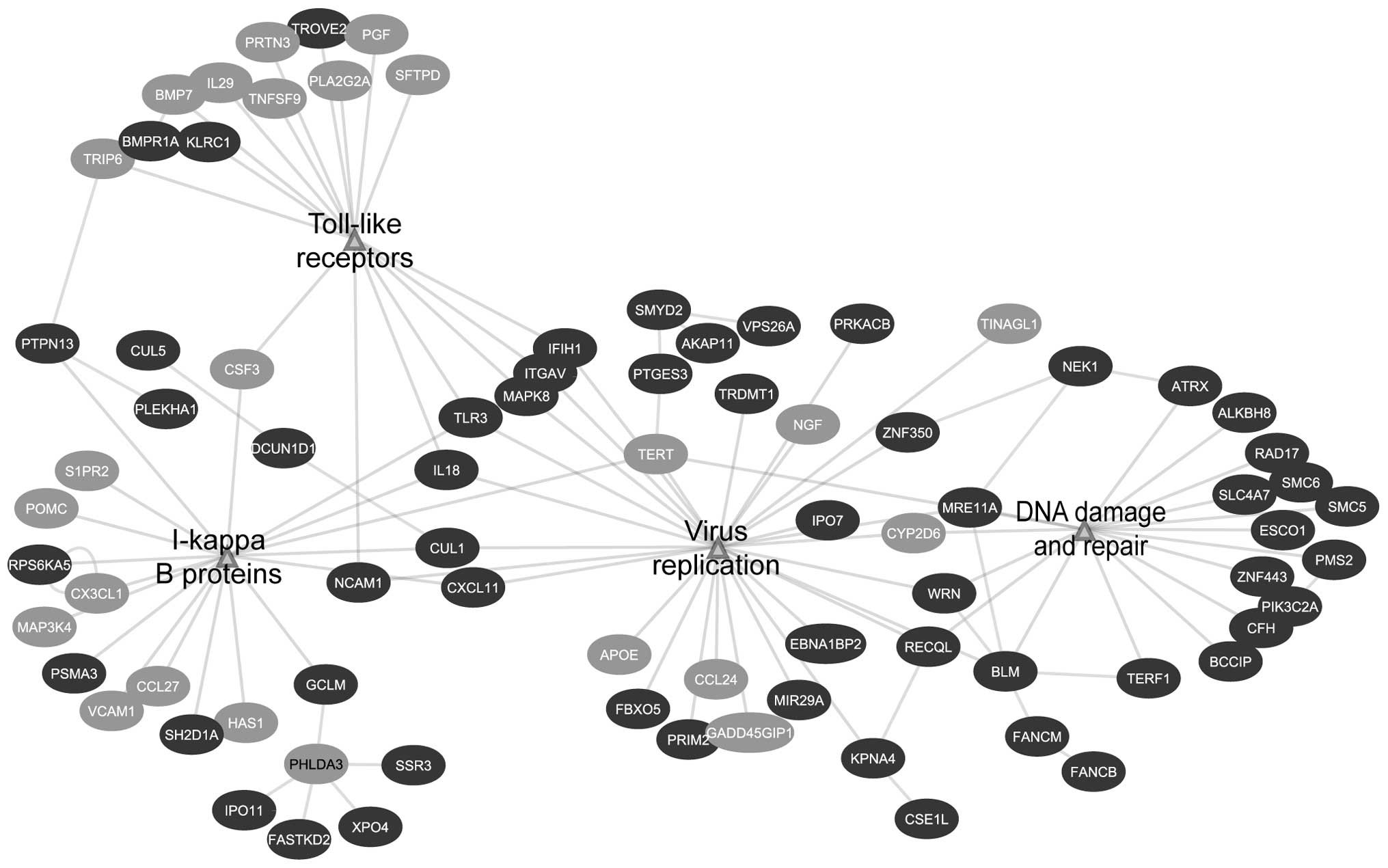
inflammasomes, toll-like receptors and NOD-like receptors (Table I and Fig. 1). Similar to GSEA, EGAN analysis
showed the enrichment of signaling involved in viral immune
responses. The viral signaling network was composed of several
immune-related pathways, namely virus replication, IκB proteins and
toll-like receptors. Furthermore, it showed connection with DNA
damage and repair node, which is a characteristic of a viral
response (Fig. 3 and Table II).
 | Table I.Statistical significance of GSEA
Reactome database gene sets. |
Table I.
Statistical significance of GSEA
Reactome database gene sets.
| Category | Gene set | Size of gene
set | FDR q-value |
|---|
| Immune
signaling | HIVa infection | 181 | <0.0001 |
| Interferon
secretion | 63 | <0.0001 |
| NEPb/SEP2c viral proteins | 25 | <0.0001 |
| Activation of
APOE3Gd degradation
via VIFe | 47 | 0.001 |
| CD28f stimulation | 56 | 0.004 |
| Antigen processing
and presentation | 183 | 0.006 |
| BCRg activation | 115 | 0.02 |
| TCRh activation | 13 | 0.009 |
| Phagosome
pathway | 55 | 0.01 |
| Adaptive immune
response | 460 | 0.013 |
| Inhibition of
CTLA4i | 20 | 0.013 |
| Inflammasomes | 15 | 0.02 |
| Activation of
NFκB | 60 | 0.031 |
| Activation of
TLRj signaling | 11 | 0.03 |
| Cytokine
signaling | 237 | 0.041 |
| NLRk signaling | 38 | 0.042 |
| IL7 signaling | 10 | 0.045 |
| PD1l signaling | 15 | 0.047 |
| Cell cycle | Mitotic cell
cycle | 278 | <0.0001 |
| Degradation of
mitotic proteins via CDC20m | 61 | <0.0001 |
| Degradation of
CDH1n | 54 | <0.0001 |
| Removal of
CDC6o | 45 | <0.0001 |
| DNA
replication | 170 | <0.0001 |
| Chromosome
maintenance | 100 | 0.028 |
| ORC1p removal | 58 | 0.003 |
| DNA damage and
repair | DNA repair | 91 | <0.0001 |
| Formation of
NERq complex | 17 | 0.0009 |
| Fanconi anemia | 16 | 0.001 |
| Growth
signaling | FGFRr activation | 21 | <0.0001 |
| SHCs cascade | 25 | 0.001 |
| PI3Kt cascade | 51 | 0.029 |
| IGFBPsu | 14 | 0.023 |
| Metabolism | Amino acids
metabolism | 16 | <0.0001 |
| Metabolism of
lipids | 19 | 0.001 |
| Metabolism of
proteins | 24 | 0.002 |
| TCAv cycle | 105 | 0.006 |
| Glucose
transport | 36 | 0.031 |
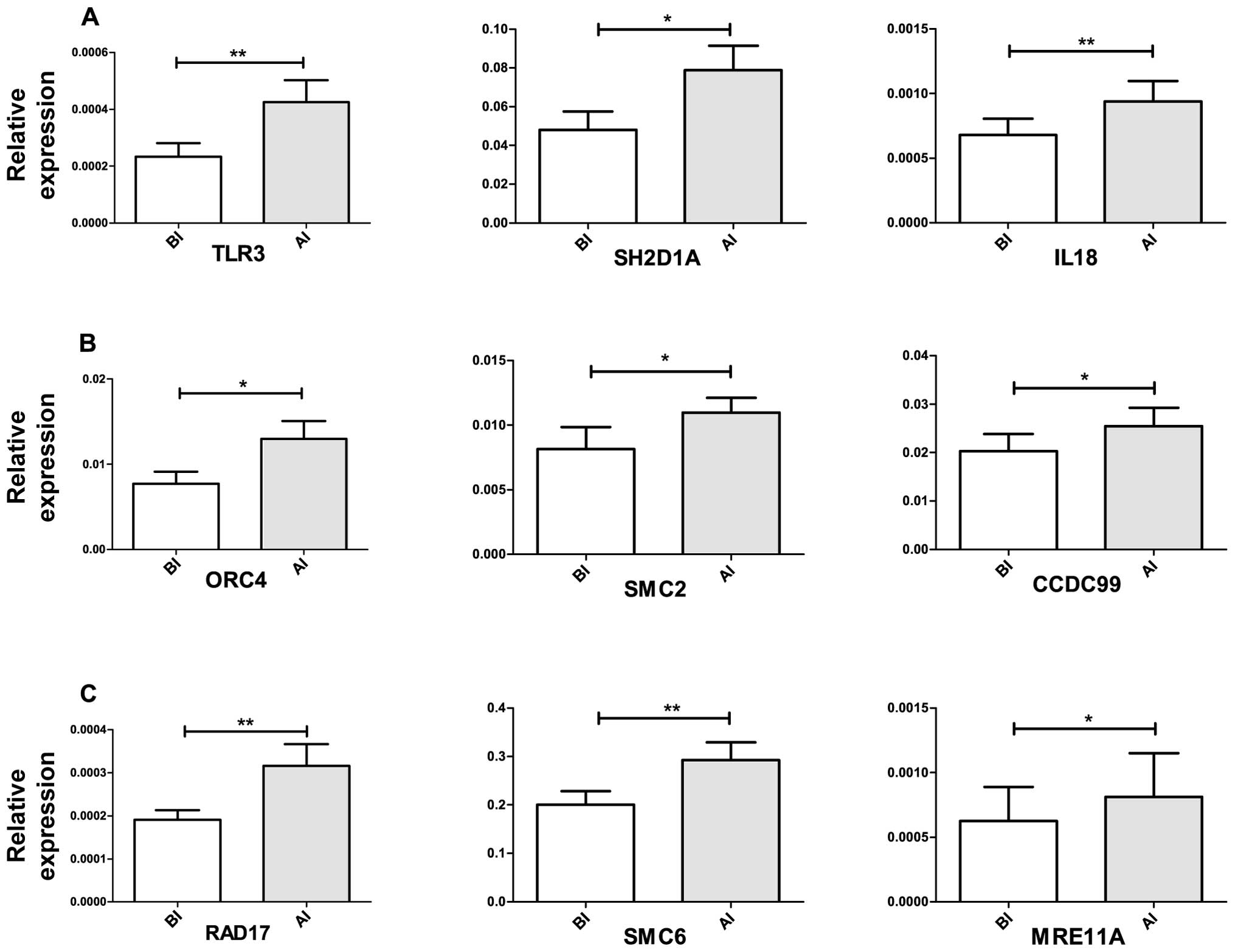
Among the upregulated genes that contributed to the
positive regulation of inflammatory response are SH2D1A,
TLR3 and IL18 (Figs. 3
and 7A).
Low doses of ionizing radiation induces
pro-survival response via immune-stimulation and cell cycle
progression responses downstream growth factors signaling
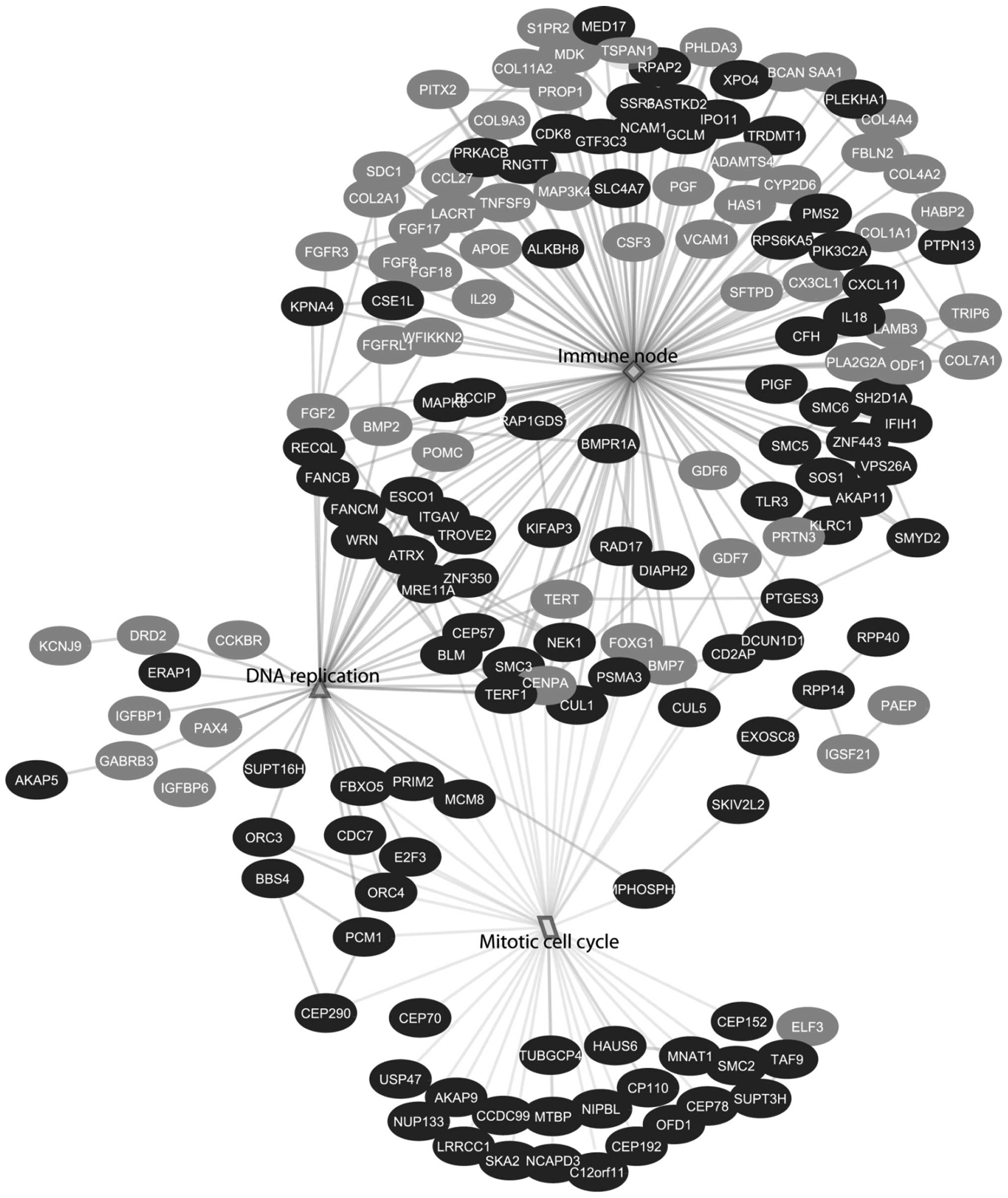
Individual gene pathways analysis showed the
downregulation of several growth factors like fibroblast growth
factors (FGFs), insulin growth factor I (IGF-I), extracellular
proteins and platelet-derived growth factor signaling (PDGF)
(Table II). Downstream to growth
factor signaling, DNA replication and mitotic cell cycle networks
were shown to be upregulated (Fig.
4).
Similarly, GSEA and EGAN showed downregulation of
gene sets involved in fibroblast growth factor signaling. These
nodes showed a connection with the adaptive immune response gene
set and immune-related network, respectively (Figs. 1 and 5). Downstream immune-related nodes were
connected to the positive cell cycle progression and survival via
the induction of the gene sets involved in CDC20, ORC1 and CDH1
degradation and promotion of DNA replication (Figs. 1 and 6). Among the upregulated genes
contributing to the positive regulation of cell cycle progression
are CCDC99, ORC4 and SMC2 (Figs. 4 and 7B).
Low doses of ionizing radiation induces
increased DNA damage
For all patients an increase of the γ-H2AX foci
yield was observed: 0.47±0.19 foci/cell (Table III). Furthermore, after 18–24 h,
significantly upregulated enriched gene sets were determined, and
differently expressed genes that are linked to DNA damage and
repair signaling like RAD17, MRE11A, and SMC6 were found (Figs. 3 and 7C).
Discussion
We investigated in vivo the biological
responses to low doses of ionizing radiation. To this end, we
assessed DNA damage, through scoring of γ-H2AX foci, and performed
whole genome analysis followed by qRT-PCR validation (Fig. 7) on whole blood samples collected
from prostate cancer patients undergoing IMRT. Whole blood samples
were collected from prostate cancer patients before, and at 30 min
(for γ-H2AX studies), and 18–24 h (for microarray studies) after
the first fraction of irradiation. We chose to perform the
experiments on whole blood samples as these are composed of a
complex combination of different cell types; therefore, it allows
the study of a collective tissue response. On the other hand, blood
is a circulating tissue, thus it reflects the response to the
calculated equivalent total body dose.
Prostate cancer patients show induction
of pro-inflammatory response via the activation of viral
signaling
Previously, we demonstrated that low doses of
ionizing radiation induce a unique gene expression profile compared
to high doses (11). The low doses
are characterized by the induction of stimulatory immune response
through the activation of chemokine and cytokine signaling, while
high doses are characterized by a damaging response through p53
signaling. In agreement with these results, current GSEA showed the
enrichment of several immune signaling pathways; top ranked gene
sets were related to viral signaling, in specific human
immunodeficiency virus (HIV) infection signaling and interferon
secretion (Table I). Viral
response is composed of signaling network between NF-κB, ERK 1/2
MAP kinase and p38 MAP kinase pathways. Furthermore, it is known
that ionizing radiation is able to activate HIV promoter and gene
expression in T cells. The gene expression of HIV viral infections
are regulated by various cell signaling events that combine
mitogens, cytokines, stress, and DNA damage (18). In other words, the enrichment of
the HIV-infection and interferon gene sets in our data suggests a
‘communication network’ between DNA damage and central pathways in
the immune response (Figs. 1 and
3). In addition, to that, other
viral-related gene sets were also shown to be upregulated; these
are NEP/SEP viral proteins, subset of the HIV-infection gene set,
and degradation of APOBEC3G via VIF (viral infectivity factor).
APOBEC3G is a protein that plays a role in activating an antiviral
response; its degradation denotes an amplification of viral and
inflammatory response (19). One
of the key genes that plays a role in response to viral infections
is the toll-like receptor 3 (TLR3) (Fig. 7A), after viral infection TLR3
recognizes double strand RNA (dsRNA) that leads to downstream
activation of type I interferons and NF-κB, a proinflammatory and
prosurvival pathway (20,21). TLR3 was reported also to be
activated upon interaction with exogenous and endogenous RNA
molecules (22). Furthermore, GSEA
showed the enrichment of TLR cascade gene set (Table I), where TLR3, 7 and 8, involved in
viral signaling, contributed to the enrichment score (23). In addition, several genes playing a
role in viral signaling and interferon induction such as IFIH1,
MAPK8, KPNA4 and IL18 genes were shown to be upregulated
(Fig. 3).
Downstream of the activation of TLRs and interferons
is the NF-κB signaling pathway, where EGAN analysis showed
deregulation of IκB proteins (Table
II). Overexpression of SH2D1A and IL18 (Fig. 7A), and CUL1 may indicate the
positive regulation of NF-κB signaling (Fig. 3) (24–26).
Prostate cancer patients show induction
of pro-inflammatory response via the activation of adaptive and
innate immune signaling
Previously, we have demonstrated that low doses
induce the activation of T- and B-cell receptors and innate-related
gene set, such as toll-like receptors, NOD-like receptors and
RIG-like receptors (11). In
agreement with these results, GSEA showed the enrichment of several
gene sets that are involved in the stimulation of the immune
response via the activation of both adaptive and innate immune
responses. The second ranked immune gene set was CD28 stimulation,
which is related also to the CTLA4 inhibition gene set (Table I). T cell activation is dependent
on the opposing signaling from two cell receptors CD28 and CTL4A.
Liu and colleagues (27) have
reported that stimulation of CD28 is dose-dependent and specific to
low doses of ionizing radiation. Furthermore, the same group
reported upregulation in CD28 and downregulation of CTLA4 in
lymphocytes isolated from mouse blood exposed to 0.075 Gy whole
body irradiation. They showed also that the interaction between
antigen presenting cells and T cells is suppressed after exposure
of mice to 2-Gy whole body irradiation as a result of CTLA4
upregulation (28). In addition,
programmed death 1 (PD1) signaling was shown to be upregulated; PD1
is a surface membrane protein that plays a role in attenuating
autoimmune responses, thus it acts in response to the increased
activity of the T cell signaling (29). Other gene sets related to innate
immune response and inflammation were shown to be activated as
well; these include phagosome pathway and inflammasome
formation.
Prostate cancer patients show induction
of pro-survival response via immune-stimulation and cell cycle
progression responses downstream the growth factor signaling
There is growing evidence that low doses of ionizing
radiation have a proliferative and pro-survival responses through
the involvement of growth factors (11,30–32).
Our data showed downregulation in several growth factors pathways,
e.g. FGF, IGF-I and PDGF and several molecules involved in
extracellular matrix (ECM) molecules (e.g. LAMB3, COL20A1
and COL9A3) that are involved in growth signaling. This
could be related to the late time-point. GSEA and EGAN analysis
showed that the growth factor signaling cluster showed a connection
with the adaptive immune response node and the viral response node;
growth factors, such as FGF were previously shown to be involved in
an immune-stimulatory reaction in response to lipopolysaccharide
(LPS) stimulation (33,34). Furthermore, genes playing a role in
the positive regulation of ERK, MAPK and NF-κB signaling were shown
to be upregulated, such as the induction of SOS1, ITGAV, AKT3,
PIK3C2A, MAPK8, SH2D1A and IL18 (Figs. 3 and 5).
Furthermore, both analyses showed that viral and
immune response gene sets were connected to the nodes of the cell
cycle progression and DNA replication (Figs. 1, 6 and 7C). Previously, it was reported that
regulation of cell cycle is a characteristic of a low dose response
24 h post-irradiation (9,10). In contrast to our expectation, cell
cycle was not arrested and cell cycle checkpoints were not
activated, probably due to the low doses received by the patients
and the cell cycle positive regulation of the downstream growth
factor and immune stimulation.
Prostate cancer patients show increased
DNA damage and anti-apoptotic response post-IMRT
DNA damage signaling was induced 30 min
post-irradiation (Table III) and
did not terminate 18–24 h later (Figs.
3 and 7B). Taking into account
that the cell cycle arrest was not activated (Fig. 4), and was shown not to be launched
under a threshold of 200 mGy; this might increase the possibility
of carrying unrepaired or misrepaired DNA breaks through the cell
division process, thus induction of cancers would be more probable
(35,36). In addition, p53 signaling, which is
known to be a central player in response to ionizing radiation
(37), was not enriched in either
analysis approach. The DNA damage and repair response was
accompanied by an anti-apoptotic response; where genes involved in
stabilization of p53 were downregulated (PHLDA3) (38) while others involved in its
degradation were upregulated (MTBP) (39). BBC3, belongs to the BH3-only
pro-apoptotic genes, and was also downregulated.
In conclusion, our study demonstrated that
immune-stimulatory signaling played a central role in response to
low doses of ionizing radiation. These results are in agreement
with those reported previously in our in vitro whole genome
analysis (11). Furthermore, we
showed that responses to low doses are a communication network
between growth factors and cell cycle progression pathways
stimulated by immune signaling. Moreover, we report that remaining
unrepaired DNA damage still exists after 18–24 h.
Our study addresses the need for reconsideration of
the health risks from the out-of-field low doses of ionizing
radiation exposed to the normal tissues when undergoing IMRT.
Inflammatory and DNA damage responses may carry the risk of
development of systematic inflammations and secondary cancers,
respectively; there is accumulating number of studies that show
advantages of using particle therapy over treatments that use
X-rays. It is demonstrated that the healthy surrounding tissues are
spared from out-field radiation. However, other studies have
reported that secondary neutrons can carry the risk of developing
secondary cancers during particle therapy. There are still no
clear-cut answers for a ‘perfect’ radiotherapy approach, and
further inter-disciplinary research is still required (7,40).
Acknowledgements
The authors are thankful to Ghent
University Hospital patients who kindly accepted to participate in
this study. Also, we appreciate Dr P. Willems [Federal Agency for
Nuclear Control (FANC), Belgium] for the fruitful scientific
discussions carried out through the preparation of the study. H.
El-Saghire was supported by a doctoral SCK·CEN/Ghent University
grant. This study was funded by the FANC CT-SCAN contract
(CO-90-09-2329-00) and by the FP7 EU EPI-CT contract (grant
agreement 269912).
References
|
1.
|
Ahmad SS, Duke S, Jena R, Williams MV and
Burnet NG: Advances in radiotherapy. BMJ. 345:e77652012. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Hall EJ and Wuu CS: Radiation-induced
second cancers: the impact of 3D-CRT and IMRT. Int J Radiat Oncol
Biol Phys. 56:83–88. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Purdy JA: Dose to normal tissues outside
the radiation therapy patient’s treated volume: a review of
different radiation therapy techniques. Health Phys. 95:666–676.
2008.
|
|
4.
|
Hall EJ: Intensity-modulated radiation
therapy, protons, and the risk of second cancers. Int J Radiat
Oncol Biol Phys. 65:1–7. 2006. View Article : Google Scholar
|
|
5.
|
Ruben JD, Davis S, Evans C, et al: The
effect of intensity-modulated radiotherapy on radiation-induced
second malignancies. Int J Radiat Oncol Biol Phys. 70:1530–1536.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ost P, Speleers B, De Meerleer G, et al:
Volumetric arc therapy and intensity-modulated radiotherapy for
primary prostate radiotherapy with simultaneous integrated boost to
intraprostatic lesion with 6 and 18 MV: a planning comparison
study. Int J Radiat Oncol Biol Phys. 79:920–926. 2011. View Article : Google Scholar
|
|
7.
|
Murray L, Henry A, Hoskin P, Siebert FA
and Venselaar J: BRAPHYQS/PROBATE group of the GEC ESTRO: Second
primary cancers after radiation for prostate cancer: a review of
data from planning studies. Radiat Oncol. 8:1722013. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Wyrobek AJ, Manohar CF, Krishnan VV, et
al: Low dose radiation response curves, networks and pathways in
human lymphoblastoid cells exposed from 1 to 10 cGy of acute gamma
radiation. Mutat Res. 722:119–130. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yunis R, Albrecht H, Kalanetra KM, Wu S
and Rocke DM: Genomic characterization of a three-dimensional skin
model following exposure to ionizing radiation. J Radiat Res.
53:860–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Ray M, Yunis R, Chen X and Rocke DM:
Comparison of low and high dose ionising radiation using
topological analysis of gene coexpression networks. BMC Genomics.
13:1902012. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
El-Saghire H, Thierens H, Monsieurs P,
Michaux A, Vandevoorde C and Baatout S: Gene set enrichment
analysis highlights different gene expression profiles in whole
blood samples X-irradiated with low and high doses. Int J Radiat
Biol. 89:628–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Pernot E, Hall J, Baatout S, et al:
Ionizing radiation biomarkers for potential use in epidemiological
studies. Mutat Res. 751:258–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Subramanian A, Tamayo P, Mootha VK, et al:
Gene set enrichment analysis: a knowledge-based approach for
interpreting genome-wide expression profiles. Proc Natl Acad Sci
USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Werbrouck J, De Ruyck K, Beels L, et al:
Prediction of late normal tissue complications in RT treated
gynaecological cancer patients: potential of the gamma-H2AX foci
assay and association with chromosomal radiosensitivity. Oncol Rep.
23:571–578. 2010.
|
|
15.
|
Irizarry RA, Hobbs B, Collin F, et al:
Exploration, normalization, and summaries of high density
oligonucleotide array probe level data. Biostatistics. 4:249–264.
2003. View Article : Google Scholar
|
|
16.
|
Merico D, Isserlin R, Stueker O, Emili A
and Bader GD: Enrichment map: a network-based method for gene-set
enrichment visualization and interpretation. PLoS One.
5:e139842010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Pfaffl MW: A new mathematical model for
relative quantification in real-time RT-PCR. Nucleic Acids Res.
29:e452001. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Oakley JD, Taher MM, Hershey CM, Aggarwal
PC, Estwani IB and Valerie K: Triggering of apoptosis is not
sufficient to induce human immunodeficiency virus gene expression.
IUBMB Life. 55:415–427. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Nowarski R, Wilner OI, Cheshin O, et al:
APOBEC3G enhances lymphoma cell radioresistance by promoting
cytidine deaminase-dependent DNA repair. Blood. 120:366–375. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhu J, Ghosh A, Coyle EM, et al:
Differential effects of phenethyl isothiocyanate and
D,L-sulforaphane on TLR3 signaling. J Immunol. 190:4400–4407. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Amarante MK and Watanabe MA: Toll-like
receptor 3: involvement with exogenous and endogenous RNA. Int Rev
Immunol. 29:557–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Bauernfeind F, Ablasser A, Kim S, Bartok E
and Hornung V: An unexpected role for RNA in the recognition of DNA
by the innate immune system. RNA Biol. 7:151–157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Vercammen E, Staal J and Beyaert R:
Sensing of viral infection and activation of innate immunity by
toll-like receptor 3. Clin Microbiol Rev. 21:13–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Chuang HC, Wang JM, Hsieh WC, Chang Y and
Su IJ: Up-regulation of activating transcription factor-5
suppresses SAP expression to activate T cells in hemophagocytic
syndrome associated with Epstein-Barr virus infection and immune
disorders. Am J Pathol. 173:1397–1405. 2008. View Article : Google Scholar
|
|
25.
|
Surjit M, Varshney B and Lal SK: The ORF2
glycoprotein of hepatitis E virus inhibits cellular NF-kappaB
activity by blocking ubiquitination mediated proteasomal
degradation of IkappaBalpha in human hepatoma cells. BMC Biochem.
13:72012. View Article : Google Scholar
|
|
26.
|
Hayden MS and Ghosh S: NF-kappaB, the
first quarter-century: remarkable progress and outstanding
questions. Genes Dev. 26:203–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Liu SZ, Jin SZ, Liu XD and Sun YM: Role of
CD28/B7 costimulation and IL-12/IL-10 interaction in the
radiation-induced immune changes. BMC Immunol. 2:82001. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Shan YX, Jin SZ, Liu XD, Liu Y and Liu SZ:
Ionizing radiation stimulates secretion of pro-inflammatory
cytokines: dose-response relationship, mechanisms and implications.
Radiat Environ Biophys. 46:21–29. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Riley JL: PD-1 signaling in primary T
cells. Immunol Rev. 229:114–125. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kim SJ, Dix DJ, Thompson KE, et al:
Effects of storage, RNA extraction, genechip type, and donor sex on
gene expression profiling of human whole blood. Clin Chem.
53:1038–1045. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Liang X, So YH, Cui J, et al: The low-dose
ionizing radiation stimulates cell proliferation via activation of
the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J
Radiat Res. 52:380–386. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Sofia Vala I, Martins LR, Imaizumi N, et
al: Low doses of ionizing radiation promote tumor growth and
metastasis by enhancing angiogenesis. PLoS One.
5:e112222010.PubMed/NCBI
|
|
33.
|
Marcinkowska E, Superat K and Wiedlocha A:
FGF-1 as a possible carrier for targeted drug delivery. Oncol Res.
16:27–34. 2006.PubMed/NCBI
|
|
34.
|
Shi M, Lin TH, Appell KC and Berg LJ: Cell
cycle progression following naive T cell activation is independent
of Jak3/common gamma-chain cytokine signals. J Immunol.
183:4493–4501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Fernet M, Megnin-Chanet F, Hall J and
Favaudon V: Control of the G2/M checkpoints after exposure to low
doses of ionising radiation: implications for
hyper-radiosensitivity. DNA Repair. 9:48–57. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Wood ME, Vogel V, Ng A, Foxhall L, Goodwin
P and Travis LB: Second malignant neoplasms: assessment and
strategies for risk reduction. J Clin Oncol. 30:3734–3745. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Rashi-Elkeles S, Elkon R, Shavit S, et al:
Transcriptional modulation induced by ionizing radiation: p53
remains a central player. Mol Oncol. 5:336–348. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Aviv Y and Kirshenbaum LA: Novel
phosphatase PHLPP-1 regulates mitochondrial Akt activity and
cardiac cell survival. Circ Res. 107:448–450. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Alam MJ, Fatima N, Devi GR, Ravins and
Singh RK: The enhancement of stability of p53 in MTBP induced
p53-MDM2 regulatory network. Biosystems. 110:74–83. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Newhauser WD and Durante M: Assessing the
risk of second malignancies after modern radiotherapy. Nat Rev
Cancer. 11:438–448. 2011. View Article : Google Scholar : PubMed/NCBI
|