Introduction
The widely used chemotherapeutic agent,
5-fluorouracil (5-FU), is a key drug for oral cancer treatment and
is known to be a potent radiosensitizer (1). Clinical studies have shown that
5-FU-based chemotherapy and chemoradiotherapy improve the survival
of patients with head and neck cancer, including oral squamous cell
carcinoma (OSCC) (2–4).
However, chemoresistance continues to be a major
clinical obstacle to the successful treatment of OSCC. For example,
patients with progressive and recurrent OSCC exhibit a poor
prognosis (5,6). This is often due to treatment failure
in the setting of progressive, recurrent disease that is resistant
to 5-FU-based chemotherapy (7,8). On
the other hand, in many cancers sensitive to 5-FU, resistance is
ultimately acquired through continuous drug administration
(9–11). In such cases, the drug induces
alterations in the gene expression and signaling cascades that
mediate resistance (12,13).
Several investigators have shown that the tumor
microenvironment can modulate drug resistance (14), and it has been reported that the
extracellular matrix (ECM) plays a pivotal role in cancer
progression and the response to therapy (15). Integrins comprise a large family of
heterodimeric ECM receptors that transmit critical signals by
interacting with the ECM (16).
The α subunit typically confers specificity for the ligand, whereas
the β subunit couples to downstream signaling pathways (17). β1 integrin is the main receptor
subunit, while α4β1 and α5β1 integrins are some of the major
cellular receptors for the ECM protein fibronectin (FN) (16). β1 integrin signaling has been shown
to play a significant role in mediating resistance to cytotoxic
chemotherapy by enhancing cell survival in patients with
hematologic malignancies and solid tumors (18–21).
Increased adhesion of β1 integrin to FN triggers the activation of
the essential β1 integrin signaling mediator, integrin-linked
kinase (ILK), which in turn activates Akt and NF-κB, thus
contributing to cell survival (22). The form of drug resistance mediated
by cell-ECM contact is called cell adhesion-mediated drug
resistance (CAM-DR) (23).
However, little is known about the contribution of CAM-DR in the
pathogenesis of OSCC.
FNIII14, a 22-mer peptide derived from the 14th type
III module of FN, has a significant inhibitory effect on β1
integrin-mediated adhesion to FN (24). Thus far, the potential of
preventing cell-FN interactions using FNIII14 has been confirmed in
the setting of hematologic malignancies (24–26),
and it has been reported that combination therapy consisting of
anticancer drugs plus FNIII14 is a promising treatment for
improving the prognosis of acute myelogenous leukemia patients
(25). However, the inhibitory
effects of FNIII14 in solid tumor cells have not been fully
elucidated.
In the present study, in order to identify novel
targets implicated in CAM-DR in 5-FU-resistant OSCC, we identified
ECM molecules that are commonly upregulated in two 5-FU-resistant
OSCC cell lines. Based on the results, we found that the resistant
cells overexpressed FN and that cell adhesion to FN confers CAM-DR
against 5-FU in OSCC cells via the activation of ILK/Akt/NF-κB
survival signaling. Furthermore, we demonstrated that FNIII14
sensitizes resistant cells to 5-FU with enhanced apoptosis by
suppressing ILK/Akt/NF-κB signaling.
Materials and methods
Cell line and cell culture
Human OSCC cell lines derived from primary tumors,
Ca9-22 (lower gingival cancer) and SAS (tongue cancer), were
obtained from the RIKEN BioResource Center (Ibaraki, Japan) and
cultured with DMEM supplemented with 10% FBS and maintained under
humidified 5% CO2 incubation at 37°C. The experiments
carried out to analyze the effects of cell adhesion to FN were
performed using FN-coated dishes (BD Bioscience, Bedford, MA,
USA).
Establishment of 5-FU-resistant OSCC cell
lines
To establish 5-FU-resistant cell lines, Ca9-22 cells
were continuously exposed to increasing concentrations of 5-FU over
two years. The surviving cells were cloned, and one of the most
5-FU-resistant sublines, designated Ca9-22/FR2, was selected. The
Ca9-22/FR2 cell line can survive exposure to 2.0 μg/ml of
5-FU. To ensure continued resistance, the cell line was maintained
in a culture in DMEM containing 2.0 μg/ml of 5-FU. In order
to eliminate the effects of 5-FU on the experimental outcomes, the
resistant cells were cultured in a drug-free medium for at least
two weeks before all experiments. Another 5-FU-resistant cell line,
SAS/FR2, was previously established by us in the same way (27).
Cell proliferation assay
To assess the normal degree of proliferation, viable
cells treated without 5-FU were quantified every 24 h using the
Cell Counting Kit-8 (Dojindo, Kumamoto, Japan).
Drug sensitivity assays
The cells (3×103/well) were seeded onto
24-well plates and incubated in DMEM with 10% FBS at 37°C. After 24
h, DMEM containing various concentrations (0.2, 0.4, 0.8, 1.6, 3.2,
6.4, 12.5, 25.0 and 50.0 μg/ml) of 5-FU was added to each
well, and the cells were incubated at 37°C for another 72 h. For
the assay, WST-8 (Cell Counting Kit-8, Dojindo) was added to each
well, and the plate was incubated for an additional 2 h at 37°C.
The absorbance was measured at 450 nm using a microplate reader
(Model 680, Bio-Rad, Hercules, CA, USA). Nine wells were used for
each drug concentration and the experiment was performed in
triplicate. The 50% inhibitory concentration (IC50) was
calculated from the survival curve.
Gene expression microarrays
The cRNA was amplified, labeled and hybridized to an
Agilent Human GE 4×44K v2 Microarray (Agilent Technologies, Santa
Clara, CA, USA) according to the manufacturer’s instructions. All
hybridized microarrays were scanned by an Agilent scanner, and the
signals of all probes were calculated using the Feature Extraction
software (9.5.1.1) program (Agilent Technologies).
Data analysis and filter criteria
The raw signal intensities and flags for each probe
were calculated from the hybridization intensities and spot
information, according to the procedures recommended by Agilent. In
addition, the raw signal intensities of two samples were
log2-transformed and normalized using a quantile algorithm
(28) on a Bioconductor (29,30).
We selected probes that identified the ‘P’ flag in both the control
and experimental samples. To identify up- or downregulated genes,
we calculated the Z-scores (30)
and ratios (non-log scaled fold change) from the normalized signal
intensity of each probe. We thereafter established the criteria for
the regulated genes: (upregulated genes) Z-score ≥2.0 and ratio
≥1.5-fold, (downregulated genes) Z-score ≤−2.0 and ratio ≤0.66.
Total RNA extraction and real-time qPCR
(RT-qPCR)
Total RNA was isolated using the RNeasy Plus mini
kit (Qiagen, Hilden, Germany) according to the instructions
provided by the manufacturer and reverse transcribed into cDNA
using the ReverTra Ace®qPCR RT Kit (Toyobo, Osaka,
Japan).
For real-time quantitative PCR (qRT-PCR), each
reaction mixture was diluted 5-fold with DNase/RNase-free water
(Life Technologies, Carlsbad, CA, USA), and 4 μl of each
mixture was subjected to PCR. The reactions were run using the
Thunderbird SYBR qPCR Mix (Toyobo) on a Light Cycler 1.5 (Roche
Diagnostics, Indianapolis, IN, USA). The comparative Ct (ΔΔCt)
method was used to determine the fold changes in the expressions
using glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Each sample
was run in triplicate. The following primers were used: fibronectin
(forward, 5′-AGCCGCCACGTGCCAGGATTAC-3′; reverse, 5′-CTTA
TGGGGGTGGCCGTTGTGG-3′); GAPDH (forward, 5′-CAA
CAGCCTCAAGATCATCAGC-3′; reverse, 5′-TTCTAGACG GCAGGTCAGGTC-3′). The
cycling conditions were: initial denaturation at 98°C for 5 min
followed by 45 cycles at 98°C for 15 sec, 58°C for 30 sec and 72°C
for 60 sec. The experiments were performed in triplicate.
Western blot analysis
Whole-cell proteins were separated using 5.0 or
10.0% SDS-PAGE, transferred onto nitrocellulose membranes and
probed with antibodies against fibronectin (1:3,000; Acris
Antibodies, San Diego, CA, USA), ILK (1:1,000; Cell Signaling
Technology, Danvers, MA, USA), phospho-ILK (Thr173) (1:200; Santa
Cruz Biotechnology, Santa Cruz, CA, USA), Akt (1:1,000; Cell
Signaling Technology), phospho-Akt (Ser473) (1:2,000; Cell
Signaling Technology), NF-κB p65 (1:1,000; Epitomics/Abcam,
Burlingame, CA, USA), phospho-NF-κB p65 (Ser536) (1:1,000; Cell
Signaling Technology), caspase-3 (1:1,000; Cell Signaling
Technology), cleaved caspase-3 (1:1,000; Cell Signaling
Technology), PARP (1:1,000; Cell Signaling Technology) and β-actin
(1:10,000; Sigma, St Louis, MO, USA). Following overnight
incubation, the membranes were washed and incubated wit horseradish
peroxidase-conjugated secondary antibodies (Dako, Glostrup,
Denmark). Finally, the membranes were washed and visualized using
the ECL Plus detection kit (GE Healthcare, Buckinghamshire,
UK).
Enzyme-linked immunosorbent assay (ELISA)
for the detection of secreted FN
The cell lines were cultured in 10% serum containing
DMEM with 2.0 μg/ml of 5-FU or culture medium alone. At 24,
48, 60 and 72 h after treatment, the conditioned media were
collected, and the concentration of FN was measured using the
directions of the manufacturer’s in the human fibronectin ELISA kit
(Biomedical Technologies, Stoughton, MA, USA). The concentration of
FN was calibrated from a dose response curve based on reference
standards. The experiments were performed in triplicate.
Flow cytometric analysis of the cell
surface integrin expression
The cell surface integrin expression was analyzed
using a FACSVerse (Becton-Dickinson, Franklin Lakes, NJ, USA). All
of the following antibodies used in the flow cytometric analysis
were obtained from BioLegend (San Diego, CA, USA). The expression
of integrin subunits was determined using fluorescein
isothiocyanate (FITC)-conjugated anti-CD49d (α4 integrin),
FITC-conjugated anti-CD49e (α5 integrin) and phycoerythrin
(PE)-conjugated anti-CD29 (β1 integrin) antibodies. FITC-conjugated
IgG1, IgG2b isotype control and non-labeled IgG2b isotype control
were used as negative controls. The data were analyzed using the
FlowJo software program (Treestar, Ashland, OR, USA).
FNIII14
A synthetic peptide, FNIII14, corresponding to
residues 1835–1855 of FN (31) was
obtained from Operon Biotechnologies (Tokyo, Japan). In the
experiments performed to assess the effects of blocking cell-FN
contact with FNIII14, the cells were treated with FNIII14 at a
concentration of 100 μg/ml.
Statistical analysis
The differences in the mean values between the two
groups were statistically analyzed using Student’s t-test. All
p-values were based on two-tailed statistical analyses, and a
p-value of <0.05 was considered to be statistically significant
(*p<0.05; **p<0.01). All statistical
analyses were performed using the JMP 9 software program (SAS
Institute Inc., Cary, NC).
Results
Growth of the 5-FU-resistant OSCC cell
lines and the cytotoxic effects of 5-FU in the cells
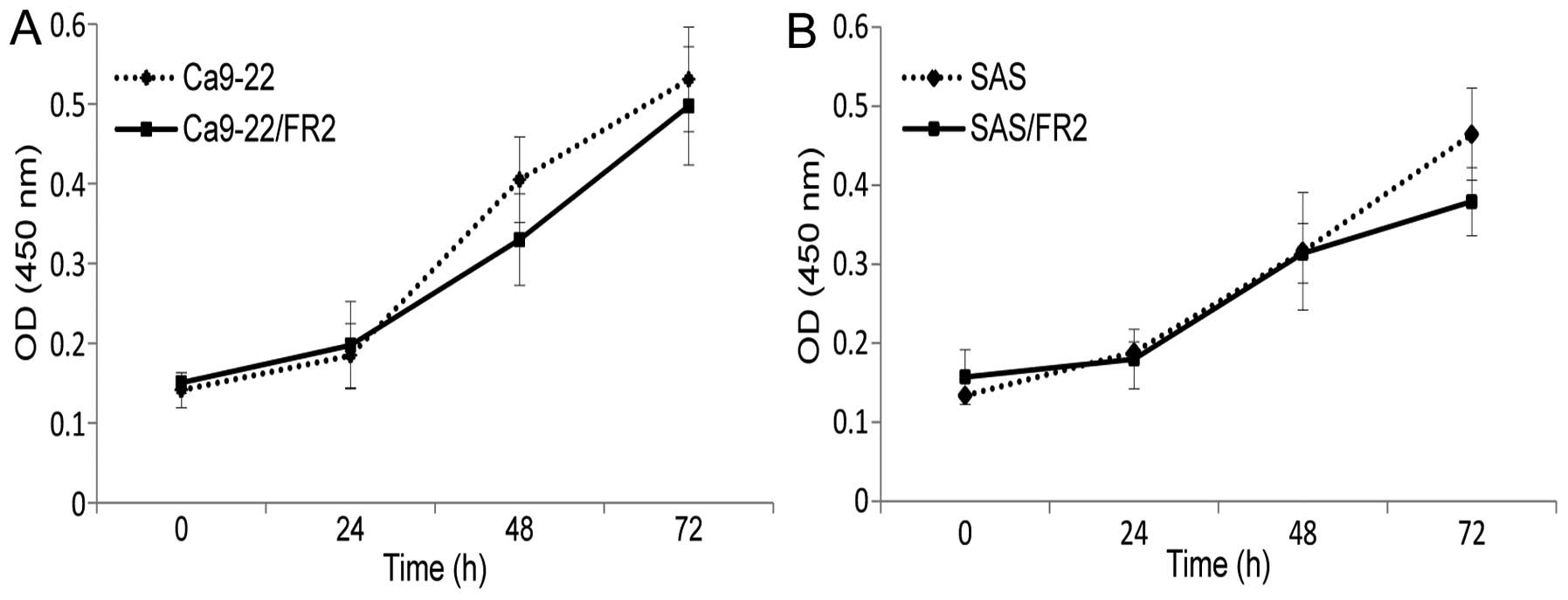
The cellular growth activities of the two
5-FU-resistant cell lines treated without 5-FU were evaluated for
72 h. No significant differences were found between the cellular
growth of the 5-FU-sensitive (SAS, Ca9-22) and -resistant (SAS/FR2,
Ca9-22/FR2) cell lines (Fig. 1A and
B), thus suggesting that the 5-FU resistance of OSCC cells is
not due to increased cell proliferation. We next examined the
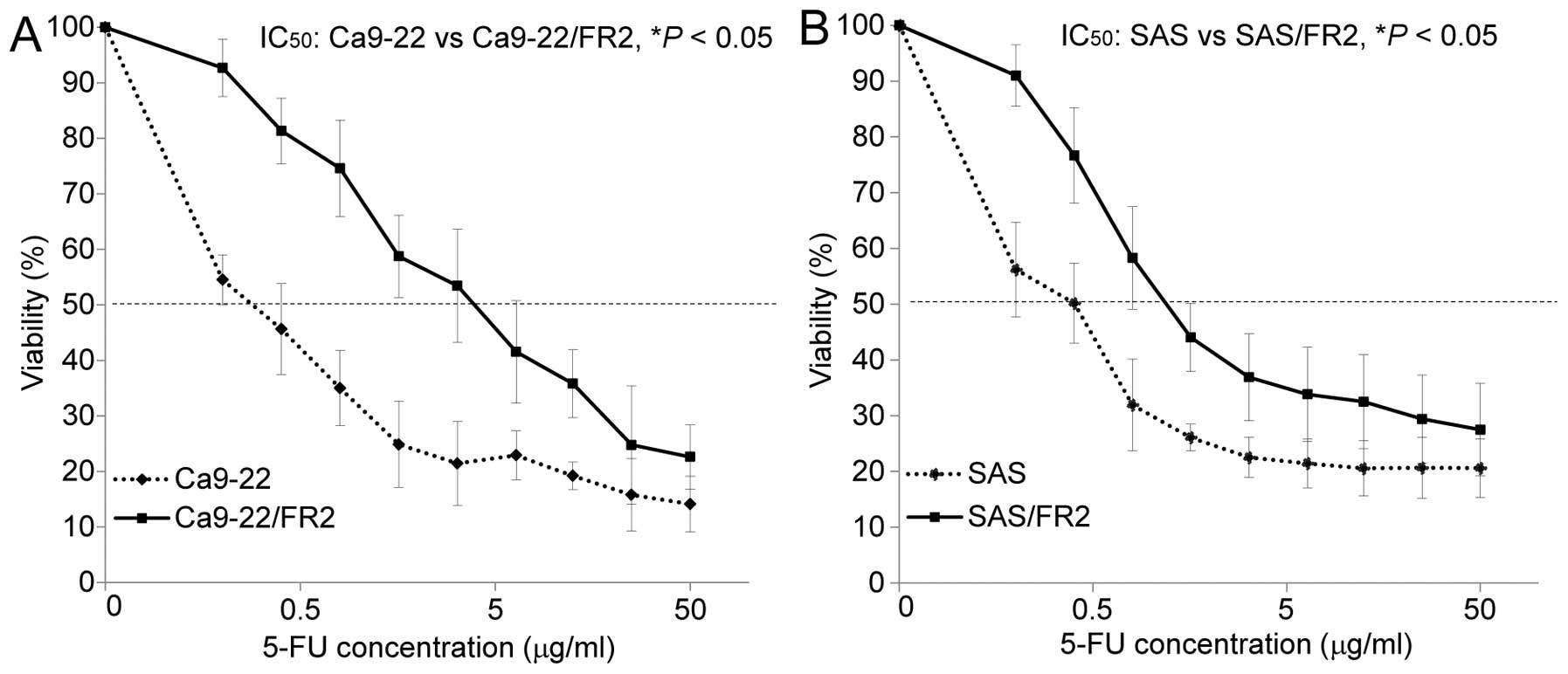
cytotoxic effects of 5-FU in the 5-FU-sensitive and -resistant
cells. Fig. 2A and B show the drug
sensitivity curves for the two sets of cell lines after 72 h of
incubation with various concentrations of 5-FU. After 72 h of
incubation with 2.0 μg/ml of 5-FU, increased apoptotic cell
changes (shrinkage and rounding-up of the cells) were noted in the
5-FU-sensitive cells compared with that observed in the
5-FU-resistant cells under phase-contrast microscopy (Fig. 2C and D). A comparison of the
IC50 value for 5-FU revealed that Ca9-22/FR2 and SAS/FR2
showed significantly higher resistance (13.7- and 3.0-fold,
respectively) to 5-FU than the parent cells.
DNA microarray analysis and the
upregulation of FN in the 5-FU-resistant OSCC cell lines
To identify genes that are differentially expressed
between 5-FU-sensitive and -resistant cell lines, a DNA microarray
analysis that contains 34,127 oligonucleotide-based probe sets was
performed. The results of the analysis showed that the expression
levels of 546 genes were elevated, while those of 442 genes were
decreased, in the Ca9-22/FR2 cells compared with those observed in
the parental Ca9-22 cells. On the other hand, the expression levels
of 598 genes were elevated and the expression levels of 447 genes
were decreased in the SAS/FR2 cells compared with those observed in
the parental SAS cells. Among these genes, we narrowed our search
to ECM molecules and found that the expression level of the FN gene
was remarkably increased in the Ca9-22/FR2 cells (ratio, 27.9-fold;
Z-score, 5.7) and that an increased expression of the FN gene was
further confirmed in the SAS/FR2 cells (ratio, 7.2-fold; Z-score,
2.5) (Table I). In addition, FN
was the only ECM molecule that was significantly upregulated in
both the resistant cell lines. It has been reported that cell
adhesion to ECM proteins, such as FN, regulates apoptosis and cell
survival in a wide variety of cell types (18,32–35).
Therefore, we focused on the analysis of FN in the present study.
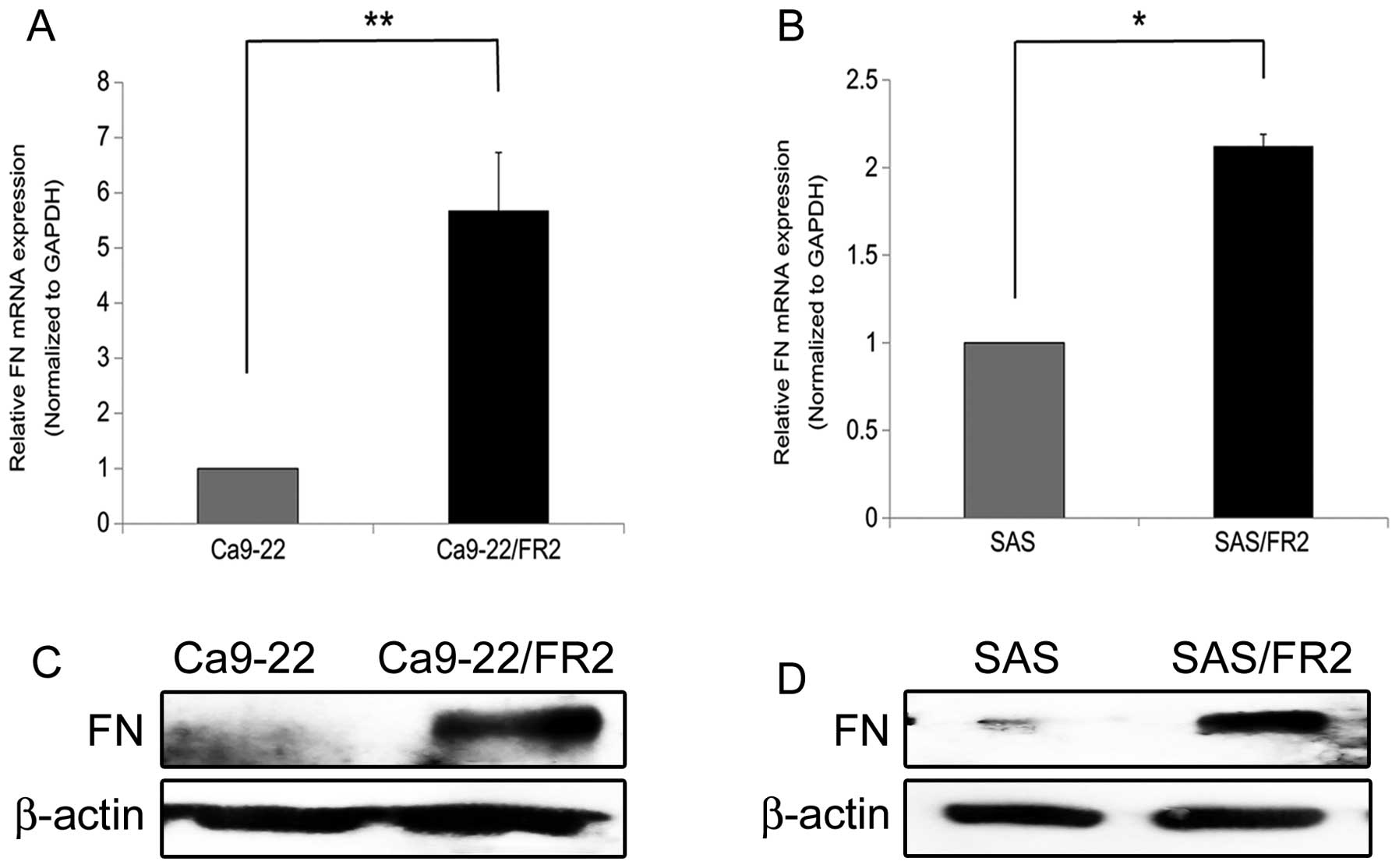
We first confirmed the expression levels of FN in the
5-FU-sensitive and -resistant cells at both the gene and protein
levels (Fig. 3A–D). Consistent
with the data obtained from the DNA microarray analysis, the
5-FU-resistant cells clearly expressed higher levels of FN than the
parental 5-FU-sensitive cells.
 | Table I.Comparison of FN genes between the
5-FU-sensitive and -resistant OSCC cell lines using a DNA
microarray analysis. |
Table I.
Comparison of FN genes between the
5-FU-sensitive and -resistant OSCC cell lines using a DNA
microarray analysis.
| Cell line | Ratio | Z-score |
|---|
| Ca9-22/FR2 | 27.9 | 5.7 |
| SAS/FR2 | 7.2 | 2.5 |
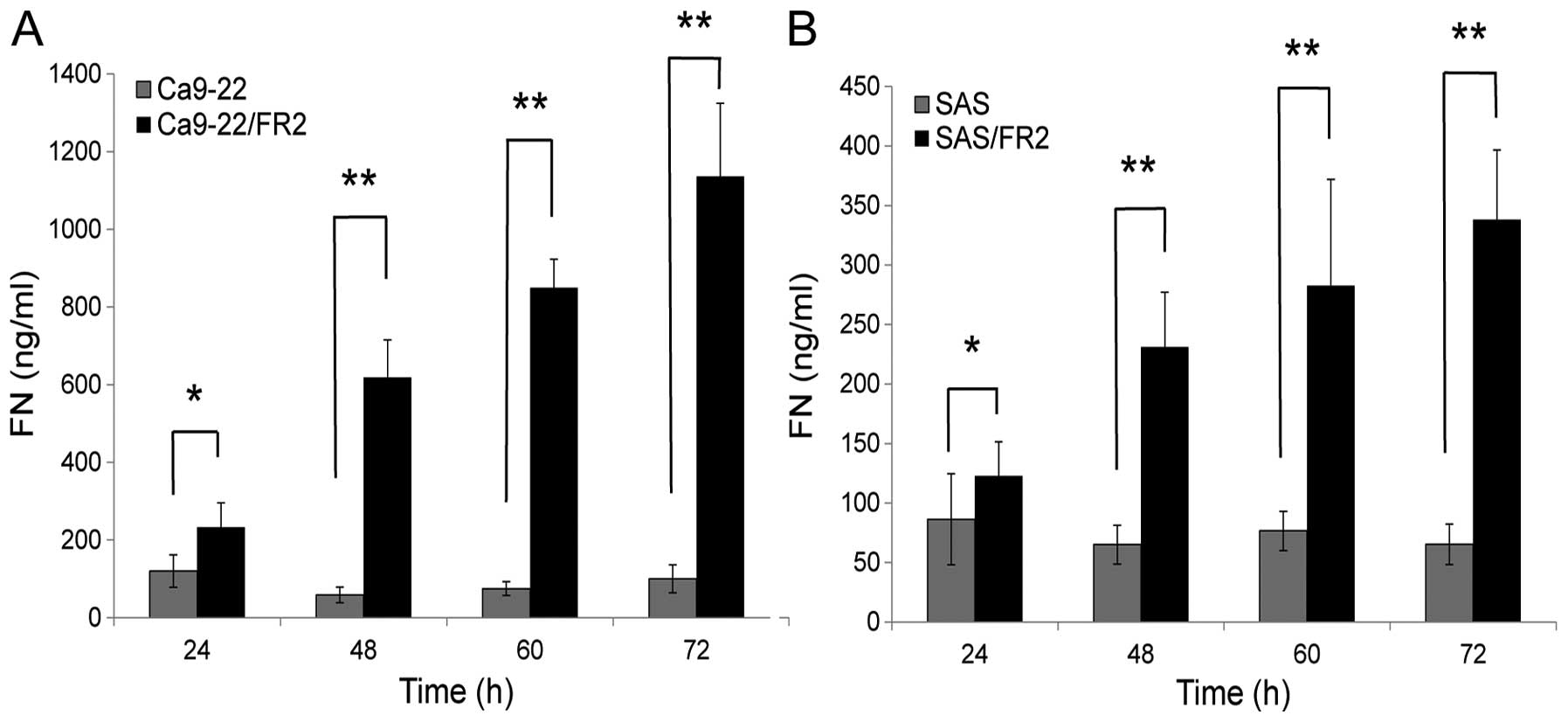
The release of FN in the 5-FU-resistant
OSCC cells was enhanced compared with that observed in the
5-FU-sensitive OSCC cells
To determine whether the upregulated FN in the
resistant cells is extracellularly released, we measured the amount
of FN in the conditioned media of the 5-FU-sensitive and -resistant
cells using ELISA kits after 24, 48, 60 and 72 h of incubation
without 5-FU treatment (Fig. 4A and
B). Considering that there were no differences in cell
proliferation between the 5-FU-sensitive and 5-FU-resistant cells,
it was confirmed that the release of FN in the resistant cells was
significantly increased compared with that observed in the
sensitive cells. This result suggests that resistant cells possess
the capacity to create the tumor-associated microenvironment that
protects tumor cells from therapy.
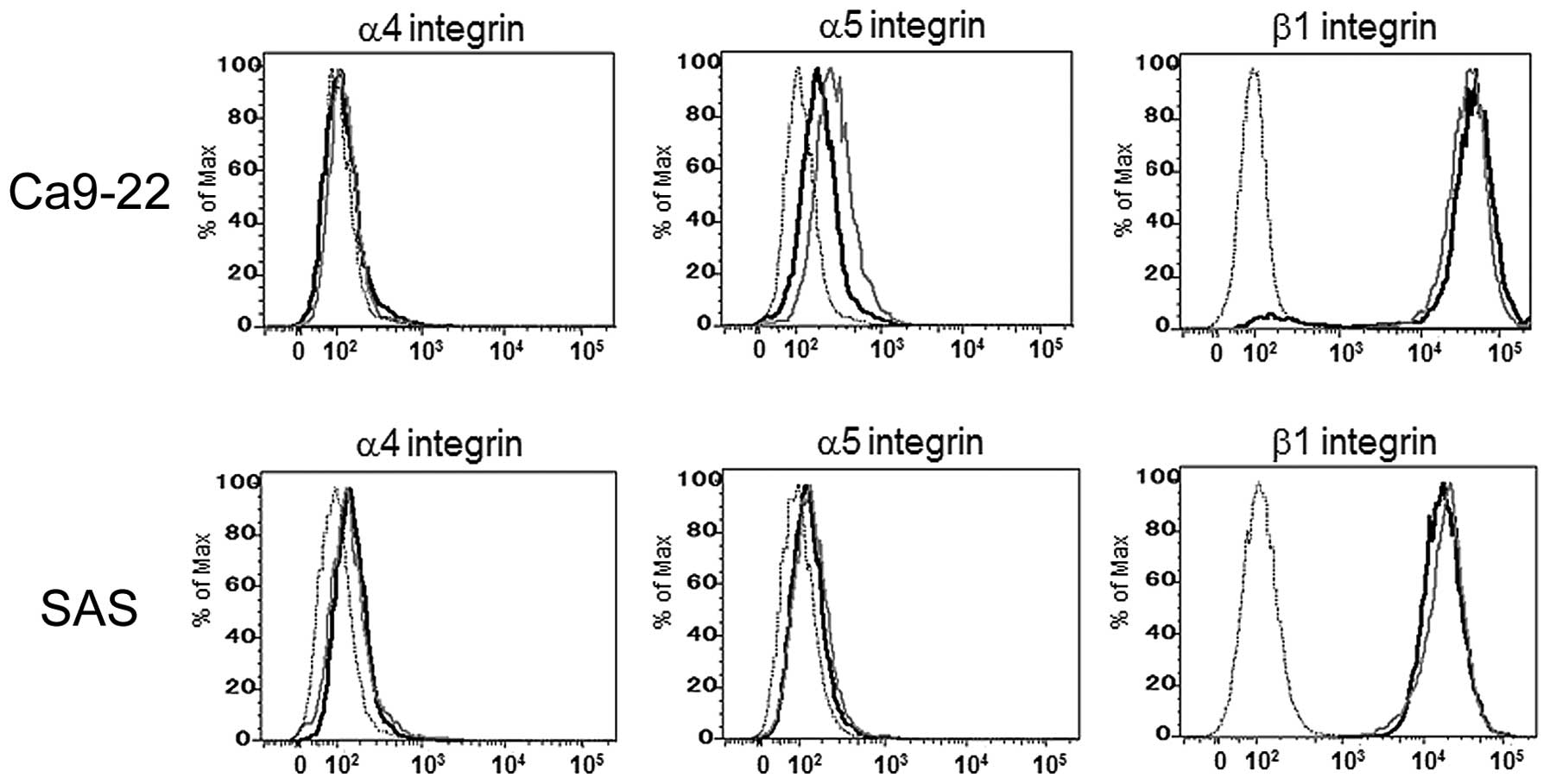
Expression levels of cell surface FN
receptors in the 5-FU-sensitive and -resistant OSCC cells
To elucidate the influence of the cell surface FN
receptor expression on 5-FU resistance, we next examined the
expression levels of the α4, α5 and β1 integrin subunits using a
flow cytometric analysis. As shown in Fig. 5, there were no differences in the
expression levels of these FN receptor components between the
5-FU-sensitive and -resistant cells.
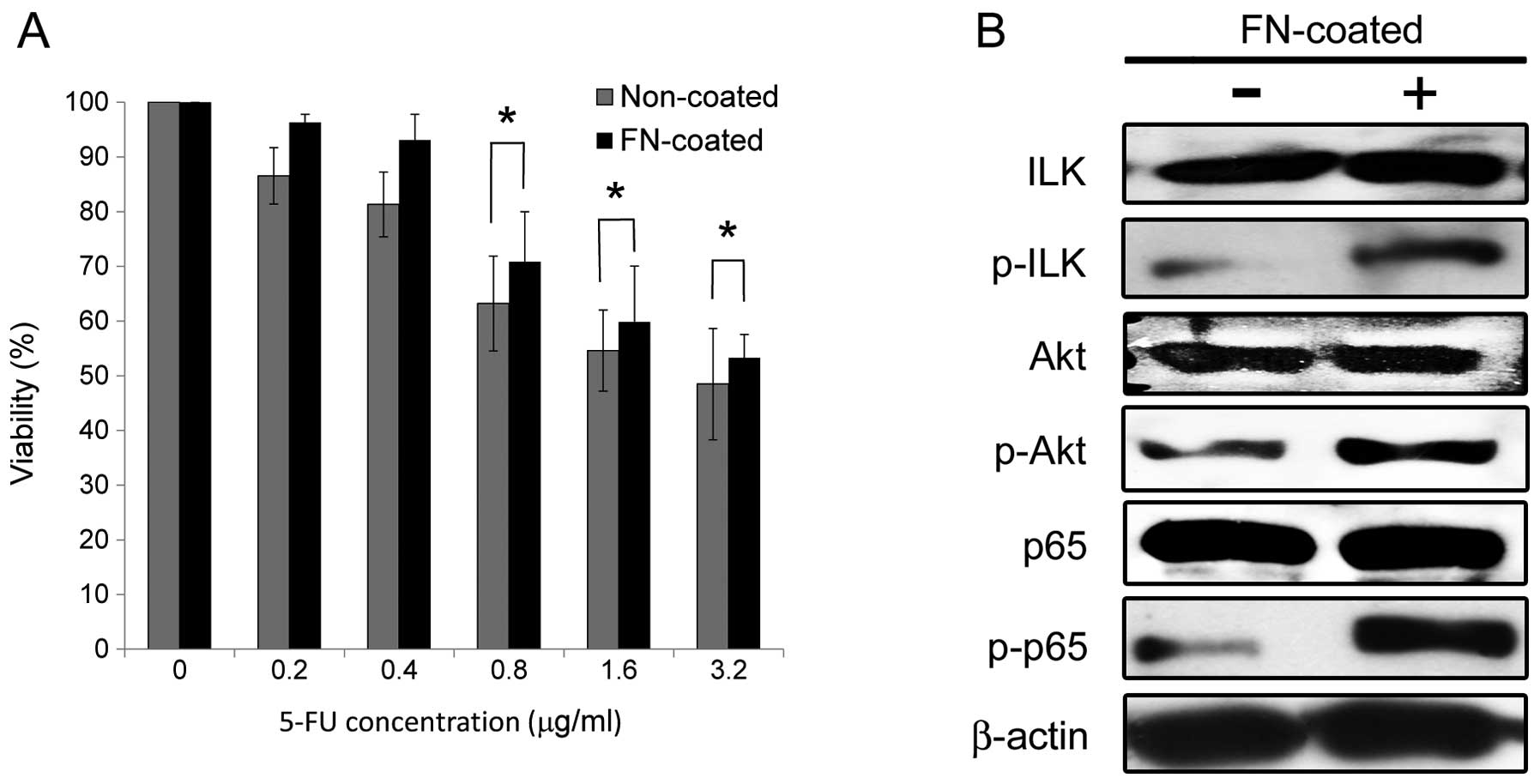
Culture on FN-coated dishes enhances 5-FU
resistance and activates integrin-mediated ILK/Akt/NF-κB survival
signaling in the 5-FU-resistant OSCC cells
The Ca9-22/FR2 cells exhibited much higher elevation
of 5-FU resistance and FN production than the SAS/FR2 cells
compared with each parental cell line, as shown in Figs. 2–4, suggesting that cell adhesion to FN
exerts protective effects against 5-FU more potently in Ca9-22/FR2
cells than in SAS/FR2 cells. Therefore, we conducted further
experiments using Ca9-22/FR2 cells. To investigate the effects of
cell adhesion to FN on 5-FU resistance in OSCC cells, we performed
drug sensitivity assays using the cells cultured on non-coated or
FN-coated dishes. As shown in Fig.
6A, cell adhesion to FN significantly enhanced resistance to
5-FU under treatment with 0.8, 1.6 and 3.2 μg/ml of 5-FU in
the 5-FU-resistant cells. In addition, a western blot analysis
revealed that ILK, Akt and NF-κB (p65) were activated in the cells
cultured on FN-coated dishes (Fig.
6B). These results suggest that enhanced 5-FU resistance by
cell adhesion to FN is implicated in the activation of
integrin-mediated ILK/Akt/NF-κB survival signaling.
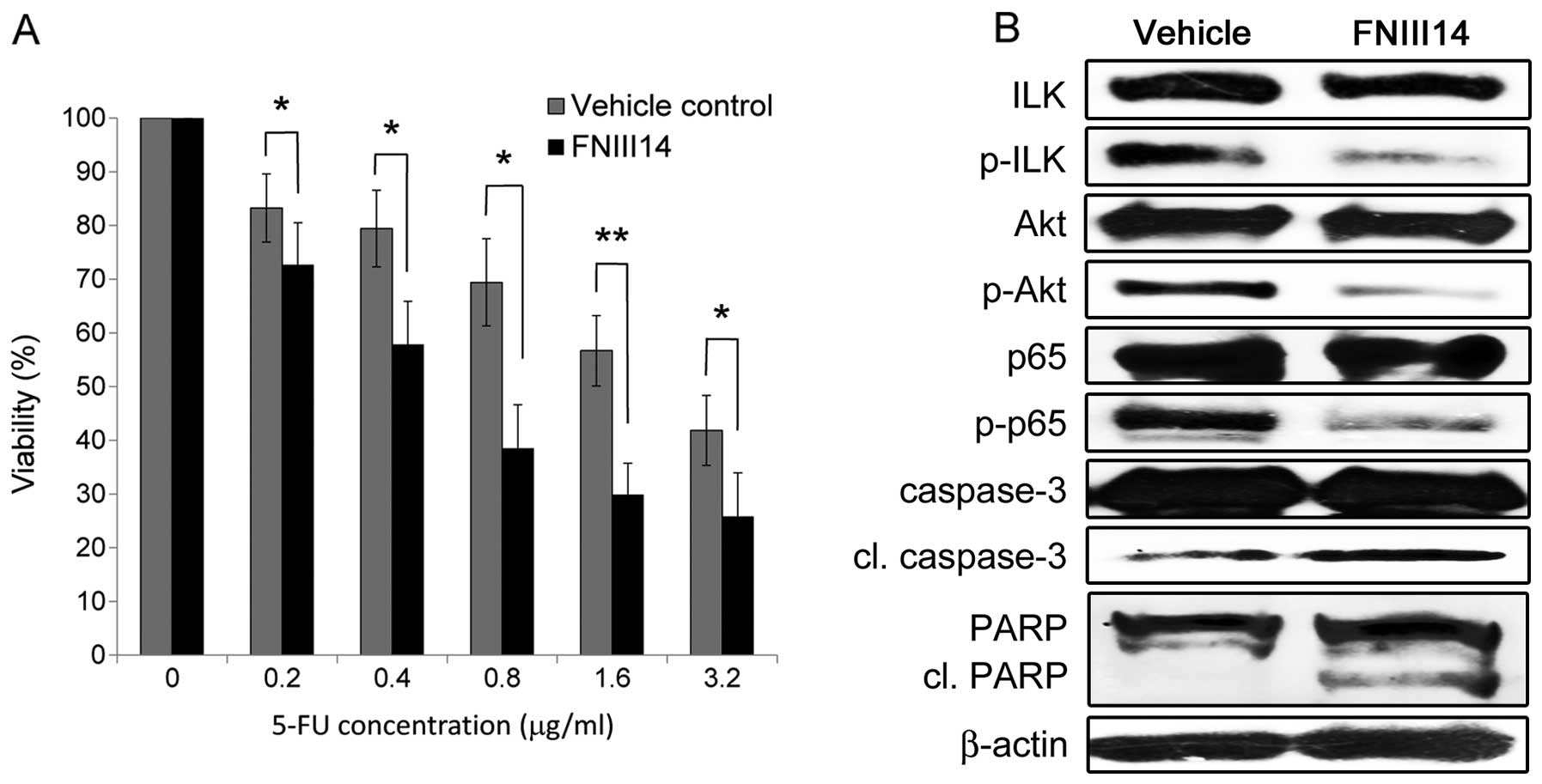
Inhibition of cell adhesion to FN by
FNIII14 enhances chemosensitivity and apoptosis by suppressing
integrin-mediated ILK/Akt/NF-κB signaling in the 5-FU-resistant
OSCC cells
We next examined the effects of blocking cell
adhesion to FN on 5-FU resistance using FNIII14, which has been
shown to have a strong inhibitory effect on the β1
integrin-mediated adhesion to FN. Consequently, FNIII14 treatment
significantly increased the chemosensitivity of the 5-FU-resistant
cells compared with that observed following treatment with the
vehicle control (Fig. 7A). We also
examined alterations in the expressions of integrin-mediated
signaling molecules and apoptosis-related molecules using a western
blot analysis (Fig. 7B). The
inhibition of cell adhesion to FN by FNIII14 decreased the
phosphorylation of ILK, Akt and NF-κB and increased the expression
levels of cleaved caspase-3 and cleaved PARP, thus leading to
enhanced apoptosis by suppressing integrin-mediated ILK/Akt/NF-κB
signaling in the 5-FU-resistant cells.
Discussion
5-FU is a key drug in the treatment of many solid
tumors, including OSCC, although some tumors exhibit 5-FU resistance. Therefore, it is
extremely important to understand the molecular mechanisms of
resistance in order to develop better treatment options. Hence,
establishing 5-FU-resistant cell lines is absolutely essential for
obtaining novel insights into resistance mechanisms. We recently
reported that we had established an 5-FU-resistant OSCC cell line,
SAS/FR2, for the first time, over a two-year period (27). In the present study, we used two
5-FU-resistant cell lines, including the newly established
Ca9-22/FR2 line, that demonstrate higher resistance to 5-FU than
SAS/FR2. We believe that the establishment of another cell line,
Ca9-22/FR2, largely contributed to obtaining novel findings in this
study.
Among the data obtained in the DNA microarray
analysis, we narrowed our search to ECM molecules produced by
cancer cells in order to identify targets implicated in CAM-DR
using two sets of cell lines. As a result, we focused on FN as a
key regulator exerting CAM-DR in 5-FU-resistant OSCC. Therefore,
selecting novel targets based on data found commonly in two
5-FU-resistant cell lines appears to be a rational approach.
Consistent with our results, previous reports based
on microarray analyses of human cancers have shown a link between
ECM overexpression and chemoresistance (36,37).
Furthermore, ECM overexpression has been demonstrated to not only
enhance chemoresistance, but also act as a negative prognostic
factor (21). These findings
indicate that drug-resistant cells can alter the composition of the
ECM in order to accelerate the acquisition of CAM-DR, thus
resulting in a more favorable microenvironment for tumor cells.
This phenomenon represents an autocrine-like survival enhancement
response, as reported by Morin (38).
To date, the importance of CAM-DR achieved via cell
adhesion to FN has been reported in various malignancies, including
small cell lung cancer (21),
myeloma (32), breast cancer
(18), colon cancer (33), acute myelogenous leukemia (35) and pancreatic cancer (39). Therefore, it is conceivable that
the overexpression of FN in cancer cells confers CAM-DR in patients
with OSCC. To the best of our knowledge, no other report has
demonstrated the contribution of FN overexpression to the
development of CAM-DR against 5-FU.
CAM-DR functions as a powerful stimulus that
triggers several signal transduction pathways, leading to a
decreased sensitivity to apoptosis. Cell adhesion to FN via
integrin receptors has been demonstrated to protect both
hematological and solid tumor cells from a number of apoptotic
stimuli (23). FN associates with
the major FN receptors α4β1 and α5β1 integrins and has been
demonstrated to mediate prosurvival effects in several cell systems
(40). However, as shown in
Fig. 5, the expressions of these
FN receptor components were not elevated in the 5-FU-resistant
cells. These data suggest that the upregulation of FN receptors is
not essential for exerting CAM-DR in OSCC cells.
ILK, an important β1 integrin signaling mediator,
promotes the phosphorylation of Akt (41) and its consequent activation of
downstream anti-apoptotic pathways mediated through NF-κB
activation (42,43). In the present study, as expected
based on the results of previous studies, our data demonstrated
that the activation of ILK, Akt and NF-κB in 5-FU-resistant cells
was enhanced by culture on FN-coated dishes. In addition, a
previous report indicates that ILK is overexpressed in SCC of the
head and neck (SCCHN) tumor specimens and that targeting ILK
induced apoptosis in the SCCHN cell lines (44). Collectively, the ILK/Akt/NF-κB
signaling pathway appears to play an important role in apoptosis
resistance, thus contributing to the development of CAM-DR in the
setting of OSCC.
We investigated whether FNIII14, which potently
impairs the interaction of FN with β1 integrin, is able to overcome
CAM-DR in 5-FU-resistant cells. An impairment of cell adhesion to
FN by FNIII14 enhanced the chemosensitivity of the 5-FU-resistant
cells cultured on FN-coated dishes, and this effect was accompanied
by the suppression of ILK/Akt/NF-κB signaling. These results
demonstrate that combination therapy consisting of 5-FU and FNIII14
can be used to effectively overcome CAM-DR in 5-FU-resistant cells.
However, the major limitation of our study is that these novel
findings were obtained based on in vitro data only.
Therefore, further studies are required to confirm the effects of
combination therapy with FNIII14 and 5-FU on 5-FU-resistant OSCC
cells using in vivo models.
In conclusion, we herein highlighted the potential
importance of CAM-DR achieved via cell adhesion to FN in
5-FU-resistant OSCC cells. Our data indicate that FN is a
potentially useful biomarker and therapeutic target for improving
the treatment of OSCC, particularly in the setting of 5-FU
resistance.
Abbreviations:
|
N
|
fibronectin;
|
|
5-FU
|
5-fluorouracil;
|
|
CAM-DR
|
cell adhesion-mediated drug
resistance;
|
|
OSCC
|
oral squamous cell carcinoma
|
Acknowledgements
This study was supported by a
Grant-in-Aid for Scientific Research (no. 21792017; to H.N.) from
the Japan Society for the Promotion of Science. The authors thank
Dr Kaori Yasuda and Dr Atsushi Doi (Cell Innovator Inc.) for their
skilled technical support regarding the microarray gene expression
analysis and their helpful discussions. We also thank Professor
Brian Quinn for critical reading of the manuscript.
References
|
1.
|
Lawrence TS, Tepper JE and Blackstock AW:
Fluoropyrimidine-radiation interactions in cells and tumors. Semin
Radiat Oncol. 7:260–266. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Pignon JP, Bourhis J, Domenge C and
Designe L: Chemotherapy added to locoregional treatment for head
and neck squamous-cell carcinoma: three meta-analyses of updated
individual data. MACH-NC Collaborative Group. Meta-analysis of
chemo-therapy on head and neck cancer. Lancet. 355:949–955. 2000.
View Article : Google Scholar
|
|
3.
|
Adelstein DJ, Saxton JP, Rybicki LA, et
al: Multiagent concurrent chemoradiotherapy for locoregionally
advanced squamous cell head and neck cancer: mature results from a
single institution. J Clin Oncol. 24:1064–1071. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Tsukuda M, Ishitoya J, Matsuda H, et al:
Randomized controlled phase II comparison study of concurrent
chemoradiotherapy with docetaxel, cisplatin, and 5-fluorouracil
versus CCRT with cisplatin, 5-fluorouracil, methotrexate and
leucovorin in patients with locally advanced squamous cell
carcinoma of the head and neck. Cancer Chemother Pharmacol.
66:729–736. 2010.
|
|
5.
|
Shingaki S, Takada M, Sasai K, et al:
Impact of lymph node metastasis on the pattern of failure and
survival in oral carcinomas. Am J Surg. 185:278–284. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bell RB, Kademani D, Homer L, Dierks EJ
and Potter BE: Tongue cancer: Is there a difference in survival
compared with other subsites in the oral cavity? J Oral Maxillofac
Surg. 65:229–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Colevas AD: Chemotherapy options for
patients with metastatic or recurrent squamous cell carcinoma of
the head and neck. J Clin Oncol. 24:2644–2652. 2006. View Article : Google Scholar
|
|
8.
|
Gibson MK, Li Y, Murphy B, et al:
Randomized phase III evaluation of cisplatin plus fluorouracil
versus cisplatin plus paclitaxel in advanced head and neck cancer
(E1395): an intergroup trial of the Eastern Cooperative Oncology
Group. J Clin Oncol. 23:3562–3567. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kang HC, Kim IJ, Park JH, et al:
Identification of genes with differential expression in acquired
drug-resistant gastric cancer cells using high-density
oligonucleotide microarrays. Clin Cancer Res. 10:272–284. 2004.
View Article : Google Scholar
|
|
10.
|
Herrmann R: 5-Fluorouracil in colorectal
cancer, a never ending story. Ann Oncol. 7:551–552. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Yoo BC, Jeon E, Hong SH, Shin YK, Chang HJ
and Park JG: Metabotropic glutamate receptor 4-mediated
5-fluorouracil resistance in a human colon cancer cell line. Clin
Cancer Res. 10:4176–4184. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Wang W, Cassidy J, O’Brien V, Ryan KM and
Collie-Duguid E: Mechanistic and predictive profiling of
5-fluorouracil resistance in human cancer cells. Cancer Res.
64:8167–8176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Petersen SL, Peyton M, Minna JD and Wang
X: Overcoming cancer cell resistance to Smac mimetic induced
apoptosis by modulating cIAP-2 expression. Proc Natl Acad Sci USA.
107:11936–11941. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Meads MB, Gatenby RA and Dalton WS:
Environment-mediated drug resistance: a major contributor to
minimal residual disease. Nat Rev Cancer. 9:665–674. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Denys H, Braems G, Lambein K, et al: The
extracellular matrix regulates cancer progression and therapy
response: implications for prognosis and treatment. Curr Pharm Des.
15:1373–1384. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Cukierman E and Bassi DE: The mesenchymal
tumor microenvironment: a drug-resistant niche. Cell Adh Migr.
6:285–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hynes RO: Integrins: bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Aoudjit F and Vuori K: Integrin signaling
inhibits paclitaxel-induced apoptosis in breast cancer cells.
Oncogene. 20:4995–5004. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Damiano JS: Integrins as novel drug
targets for overcoming innate drug resistance. Curr Cancer Drug
Targets. 2:37–43. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lewis JM, Truong TN and Schwartz MA:
Integrins regulate the apoptotic response to DNA damage through
modulation of p53. Proc Natl Acad Sci USA. 99:3627–3632. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Sethi T, Rintoul RC, Moore SM, et al:
Extracellular matrix proteins protect small cell lung cancer cells
against apoptosis: a mechanism for small cell lung cancer growth
and drug resistance in vivo. Nat Med. 5:662–668. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hannigan G, Troussard AA and Dedhar S:
Integrin-linked kinase: a cancer therapeutic target unique among
its ILK. Nat Rev Cancer. 5:51–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Shain KH and Dalton WS: Cell adhesion is a
key determinant in de novo multidrug resistance (MDR): new targets
for the prevention of acquired MDR. Mol Cancer Ther. 1:69–78.
2001.PubMed/NCBI
|
|
24.
|
Kato R, Ishikawa T, Kamiya S, et al: A new
type of anti-metastatic peptide derived from fibronectin. Clin
Cancer Res. 8:2455–2462. 2002.PubMed/NCBI
|
|
25.
|
Matsunaga T, Fukai F, Miura S, et al:
Combination therapy of an anticancer drug with the FNIII14 peptide
of fibronectin effectively overcomes cell adhesion-mediated drug
resistance of acute myelogenous leukemia. Leukemia. 22:353–360.
2008. View Article : Google Scholar
|
|
26.
|
Fukai F, Kamiya S, Ohwaki T, et al: The
fibronectin-derived anti-adhesive peptide III14-2 suppresses
adhesion and apoptosis of leukemic cell lines through
down-regulation of protein-tyrosine phosphorylation. Cell Mol Biol
(Noisy-le-grand). 46:145–152. 2000.PubMed/NCBI
|
|
27.
|
Nagata M, Nakayama H, Tanaka T, et al:
Overexpression of cIAP2 contributes to 5-FU resistance and a poor
prognosis in oral squamous cell carcinoma. Br J Cancer.
105:1322–1330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Bolstad BM, Irizarry RA, Astrand M and
Speed TP: A comparison of normalization methods for high density
oligonucleotide array data based on variance and bias.
Bioinformatics. 19:185–193. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Gentleman RC, Carey VJ, Bates DM, et al:
Bioconductor: open software development for computational biology
and bioinformatics. Genome Biol. 5:R802004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Quackenbush J: Microarray data
normalization and transformation. Nat Genet. 32(Suppl): 496–501.
2002. View
Article : Google Scholar
|
|
31.
|
Fukai F, Hasebe S, Ueki M, et al:
Identification of the anti-adhesive site buried within the
heparin-binding domain of fibronectin. J Biochem. 121:189–192.
1997.PubMed/NCBI
|
|
32.
|
Hazlehurst LA, Damiano JS, Buyuksal I,
Pledger WJ and Dalton WS: Adhesion to fibronectin via beta1
integrins regulates p27kip1 levels and contributes to cell adhesion
mediated drug resistance (CAM-DR). Oncogene. 19:4319–4327. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Kouniavsky G, Khaikin M, Zvibel I, et al:
Stromal extracellular matrix reduces chemotherapy-induced apoptosis
in colon cancer cell lines. Clin Exp Metastasis. 19:55–60. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Uhm JH, Dooley NP, Kyritsis AP, Rao JS and
Gladson CL: Vitronectin, a glioma-derived extracellular matrix
protein, protects tumor cells from apoptotic death. Clin Cancer
Res. 5:1587–1594. 1999.
|
|
35.
|
Matsunaga T, Takemoto N, Sato T, et al:
Interaction between leukemic-cell VLA-4 and stromal fibronectin is
a decisive factor for minimal residual disease of acute myelogenous
leukemia. Nat Med. 9:1158–1165. 2003. View
Article : Google Scholar
|
|
36.
|
Helleman J, Jansen MP, Ruigrok-Ritstier K,
et al: Association of an extracellular matrix gene cluster with
breast cancer prognosis and endocrine therapy response. Clin Cancer
Res. 14:5555–5564. 2008. View Article : Google Scholar
|
|
37.
|
Jansen MP, Foekens JA, van Staveren IL, et
al: Molecular classification of tamoxifen-resistant breast
carcinomas by gene expression profiling. J Clin Oncol. 23:732–740.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Morin PJ: Drug resistance and the
microenvironment: nature and nurture. Drug Resist Updat. 6:169–172.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Miyamoto H, Murakami T, Tsuchida K, Sugino
H, Miyake H and Tashiro S: Tumor-stroma interaction of human
pancreatic cancer: acquired resistance to anticancer drugs and
proliferation regulation is dependent on extracellular matrix
proteins. Pancreas. 28:38–44. 2004. View Article : Google Scholar
|
|
40.
|
Shain KH, Landowski TH and Dalton WS: The
tumor micro-environment as a determinant of cancer cell survival: a
possible mechanism for de novo drug resistance. Curr Opin Oncol.
12:557–563. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Persad S, Attwell S, Gray V, et al:
Regulation of protein kinase B/Akt-serine 473 phosphorylation by
integrin-linked kinase: critical roles for kinase activity and
amino acids arginine 211 and serine 343. J Biol Chem.
276:27462–27469. 2001. View Article : Google Scholar
|
|
42.
|
Nicholson KM and Anderson NG: The protein
kinase B/Akt signalling pathway in human malignancy. Cell Signal.
14:381–395. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Tan C, Mui A and Dedhar S: Integrin-linked
kinase regulates inducible nitric oxide synthase and
cyclooxygenase-2 expression in an NF-kappa B-dependent manner. J
Biol Chem. 277:3109–3116. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Younes MN, Yigitbasi OG, Yazici YD, et al:
Effects of the integrin-linked kinase inhibitor QLT0267 on squamous
cell carcinoma of the head and neck. Arch Otolaryngol Head Neck
Surg. 133:15–23. 2007. View Article : Google Scholar : PubMed/NCBI
|