Introduction
Bortezomib is a reversible 26S proteasome inhibitor
which was approved by the Food and Drug Administration in 2003,
2005 and 2008 for the treatment of relapsed/refractory, relapsed
and newly diagnosed MM, respectively. The initial rationale to use
bortezomib is inhibition of NF-κB activity, since NF-κB plays a
crucial role in the pathogenesis in many types of cancer cells,
including MM. The NF-κB complex is typically a dimer comprised of
different combinations of Rel family proteins, including p65
(RelA), RelB, c-Rel, p50 (NFκB1), and p52 (NFκB2). Previous studies
have revealed that NF-κB activity is mediated via two distinct,
canonical and non-canonical, pathways (1–4). In
the canonical pathway, NF-κB is typically a heterodimer composed of
p50 and p65 subunits (5), and its
activity is inhibited by association with IκB family proteins
(6). Following stimulation by
various factors, IκB protein is phosphorylated by IκB kinase (IKK),
typically IKKβ. Phosphorylated IκB is subsequently
poly-ubiquitinated and degraded by the 26S proteasome (7,8),
which allows p50/p65 NF-κB nuclear translocation. Bortezomib
inhibits degradation of IκBα and therefore blocks NF-κB
activity.
Although bortezomib shows remarkable antitumor
activities in preclinical (9–11)
and clinical studies (12–14) in MM, in most solid tumor
populations, including breast cancer, bortezomib as monotherapy has
not shown promising activity (15,16).
Bortezomib-based combination therapies have also been conducted
using capecitabin (17), pegylated
lisosomal doxorubicin (18),
docetaxel (19) or paclitaxel
(20). In these studies, 15 and
29% response rates were observed in combination with capecitabin
and docetaxel, respectively.
However, importantly, recent studies have shown that
bortezomib activates canonical NF-κB pathway both in vitro
and in a human MM cell mouse xenograft model, associated with
downregulation of IκBα. Moreover, IKKβ inhibitors augment
bortezomib-induced cytotoxicity (21). These results strongly suggest that
NF-κB may not be a major target of bortezomib in the treatment of
cancer cells. In this study, we therefore examined whether
bortezomib also activates NF-κB activity in breast cancer cells,
which may, at least in part, account for the insensitivity of these
cells to bortezomib. Although constitutive NF-κB activity was low,
bortezomib significantly induced the canonical NF-κB pathway, which
was blocked by IKKβ inhibitor, associated with enhanced
cytotoxicity of bortezomib.
Materials and methods
Cells
T47D and MCF7 breast cancer cells as well as RPMI
8226 multiple myeloma cells were obtained from the ATCC (Manassas,
VA). T47D and RPMI8226 cells were cultured in RPMI-1640 containing
10% fetal bovine serum (FBS, Sigma Chemical Co., St. Louis, MO), 2
μM L-glutamine, 100 U/ml penicillin and 100 μg/ml
streptomycin (Gibco-BRL, Grand Island, NY). MCF7 were cultured in
Dulbecco’s modified Eagle’s medium with the above supplements.
Reagents
Bortezomib was purchased from Toronto Research
Chemicals Inc. (North York, ON, Canada). IKKβ inhibitor BMS-345541
was purchased from Calbiochem (San Diego, CA).
Electrophoretic mobility shift analysis
(EMSA)
EMSA was carried out for detection of NF-κB
activity, as previously reported (4). Briefly, nuclear extracts from MM
cells were obtained using Nuclear Extraction Kit®
(Panomics, Fremont, CA). Double-stranded NF-κB oligonucleotide
probe (Promega, Madison, WI) were end-labeled with
[γ32P]ATP (10 mCi/ml, Perkin-Elmer, Boston, MA). Binding
reactions containing 0.035 pmol/μl of oligonucleotide and 10
μg of nuclear protein were conducted at room temperature for
30 min in binding buffer (10 mM Tris-HCl, pH 7.5, 50 mM NaCl, 1 mM
MgCl2, 0.5 mM EDTA, 0.5 mM DTT, 4% glycerol (v/v) and
0.5 μg poly (dI-dC) (Pharmacia, Peapack, NJ). The samples
were loaded onto a 4% polyacrylamide gel, transferred to Whatman
paper (Whatman International, Maidstone, UK) and visualized by
autoradiography. For supershift analysis, 1 μg of anti-p65,
RelB, c-Rel (Santa Cruz Biotechnology, Santa Cruz, CA), p50 (Abcam,
Cambridge, MA) or p52 (Rockland, Gilbertsville, PA) Abs were
incubated for 5 min prior to adding the reaction mixtures.
Cell proliferation assay
The inhibitory effect of bortezomib, alone or
combined with BMS-345541, on cell growth was assessed by measuring
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT,
Chemicon International, Temecula, CA) dye absorbance. Cells were
pulsed with 10 μl of 5 mg/ml MTT to each well for the last 4
of 24- and/or 48-h cultures, followed by 100 μl isopropanol
containing 0.04 N HCl. Absorbance was measured at 570/630 nm using
a spectrophotometer (Molecular Devices Corp., Sunnyvale, CA). All
experiments were performed 3 times in quadruplicate.
Immunoblot analysis
MM cells were harvested and lysed using lysis
buffer: 50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% NP-40, 5 mM EDTA,
5 mM NaF, 2 mM Na3VO4, 1 mM PMSF, 5
μg/ml leupeptine and 5 μg/ml aprotinin. Whole cell
lysates were subjected to SDS-PAGE and transferred to PVDF membrane
(Bio-Rad Laboratories, Hercules, CA). The Abs used for immunoblot
analysis included: anti-phospho (p)-RIP2 (Ser176), p-IKKα/β
(ser176/180), p-p65 (Ser536), p-IκBα (Ser32/36), IκBα and β-catenin
(Cell Signaling Technology, Danvers, MA); as well as anti-RIP2,
p65, p50, p52, RelB and GAPDH (Santa Cruz Biotechnology) Abs.
Immunofluorescence
Immunostaining was carried out according to the
manufacturer’s protocol. Briefly, T47D cells were cultured for 24 h
on Lab-Tek®II Chamber Slide System (Thermo Fisher
Scientific, Rochester, NY) prior to bortezomib treatment. T47 cells
were then treated with 10 nM Bortezomib for 16 h, fixed with 2%
formaldehyde-PBS and 100% methanol. After blocking with 5% rabbit
serum-PBS for 1 h, slides were incubated overnight with anti-p65
NF-κB Ab (Cell Signaling Technology, Danvers, MA). Cells were then
washed and incubated with fluorescence in isothiocyanate-conjugated
goat anti-rabbit IgG. Slides were analyzed using Yokogawa spinning
disk confocal/TIRE system with Nikon inverted Ti microscope.
Statistical analysis
Statistical significance of differences observed in
drug-treated versus control cultures was determined using the
Wilcoxon signed-rank test. The minimal level of significance was
p<0.05.
Results
MCF7 and T47D cells are relatively
resistant to bortezomib treatment
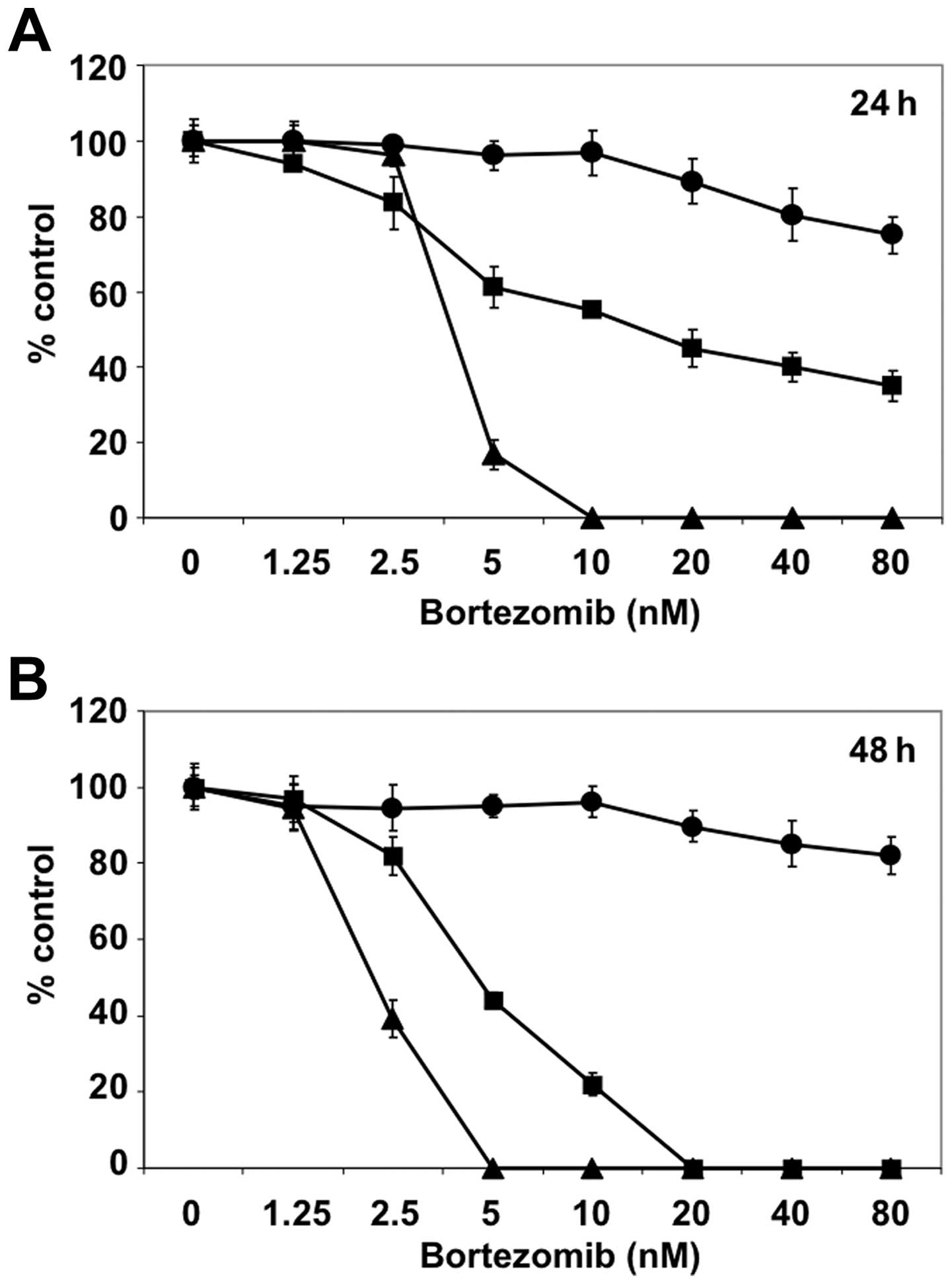
We first examined sensitivity of MCF7 and T47D cells
to bortezomib treatment. MCF7 and T47D cells were cultured in the
presence of different concentration of bortezomib (up to 80 nM) for
24 (Fig. 1A) and 48 h (Fig. 1B). RPMI8226 MM cells were employed
as positive control of bortezomib treatment. Compared to RPMI8226,
MCF7 and T47D cells were relatively resistant to bortezomib.
Especially, bortezomib could not reach the IC50 growth
inhibition dose in MCF7 cells in this setting.
Bortezomib downregulates the IκBα
protein
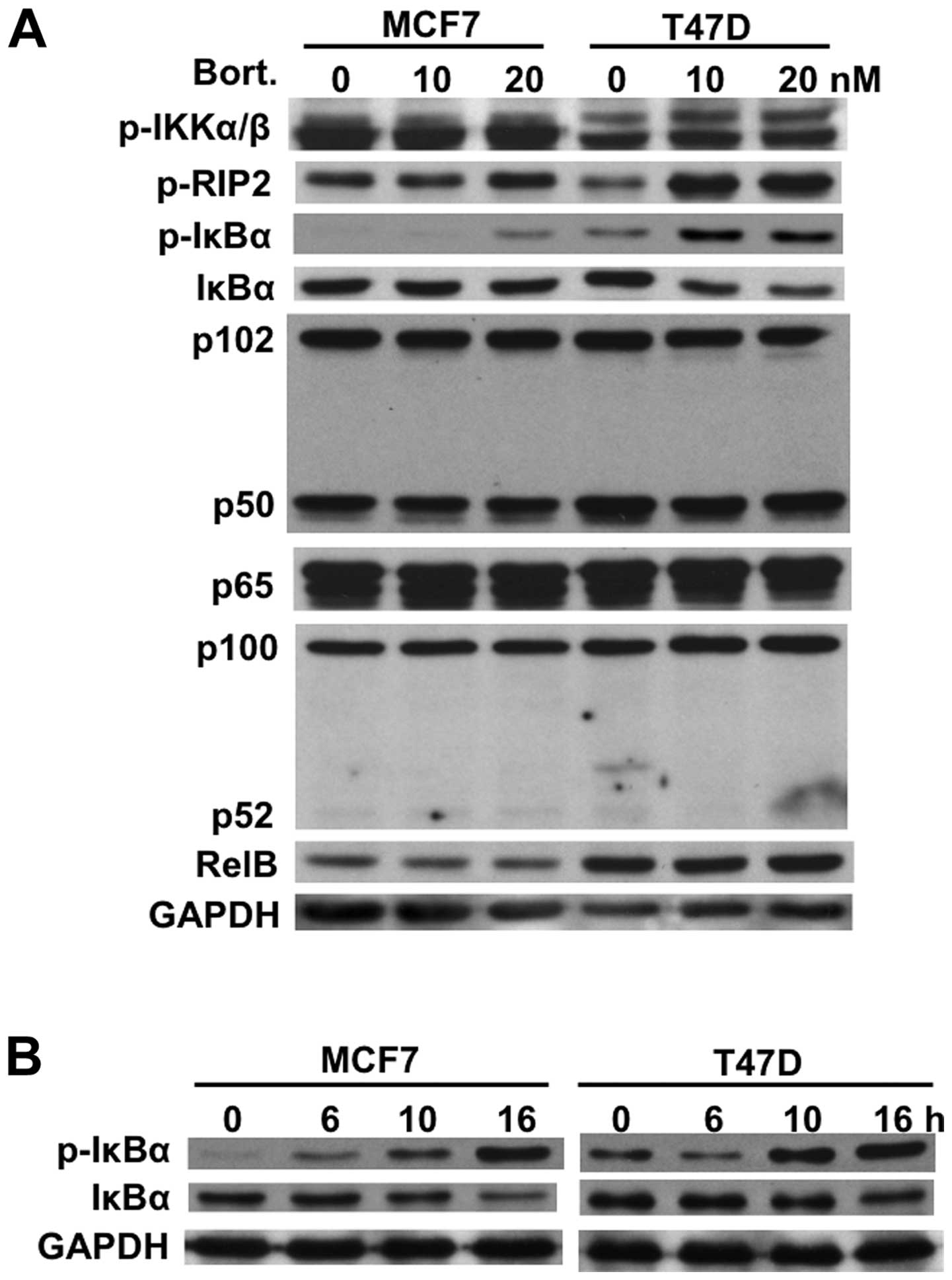
We next examined expression of IκBα and other Rel
family member proteins (p50, p52, p65, RelB) in both MCF7 and T47D
cells before and after bortezomib treatment. Similar to MM cells
(21), bortezomib induced
phosphorylation and downregulation of IκBα in both MCF7 and T47D
cells after 8-h treatment without alteration of other Rel family
member proteins. Interestingly, this effect was more pronounced in
T47D cells than in MCF7 cells (Fig.
2A). However, time-dependent study demonstrated that
phosphorylation and downregulation of IκBα were similarly observed
in both cell lines after 16-h treatment with bortezomib (Fig. 2B). These results strongly suggest
bortezomib may activate the canonical NF-κB activity.
Bortezomib triggers NF-κB activation
associated with enhanced p65 (RelA) nuclear translocation
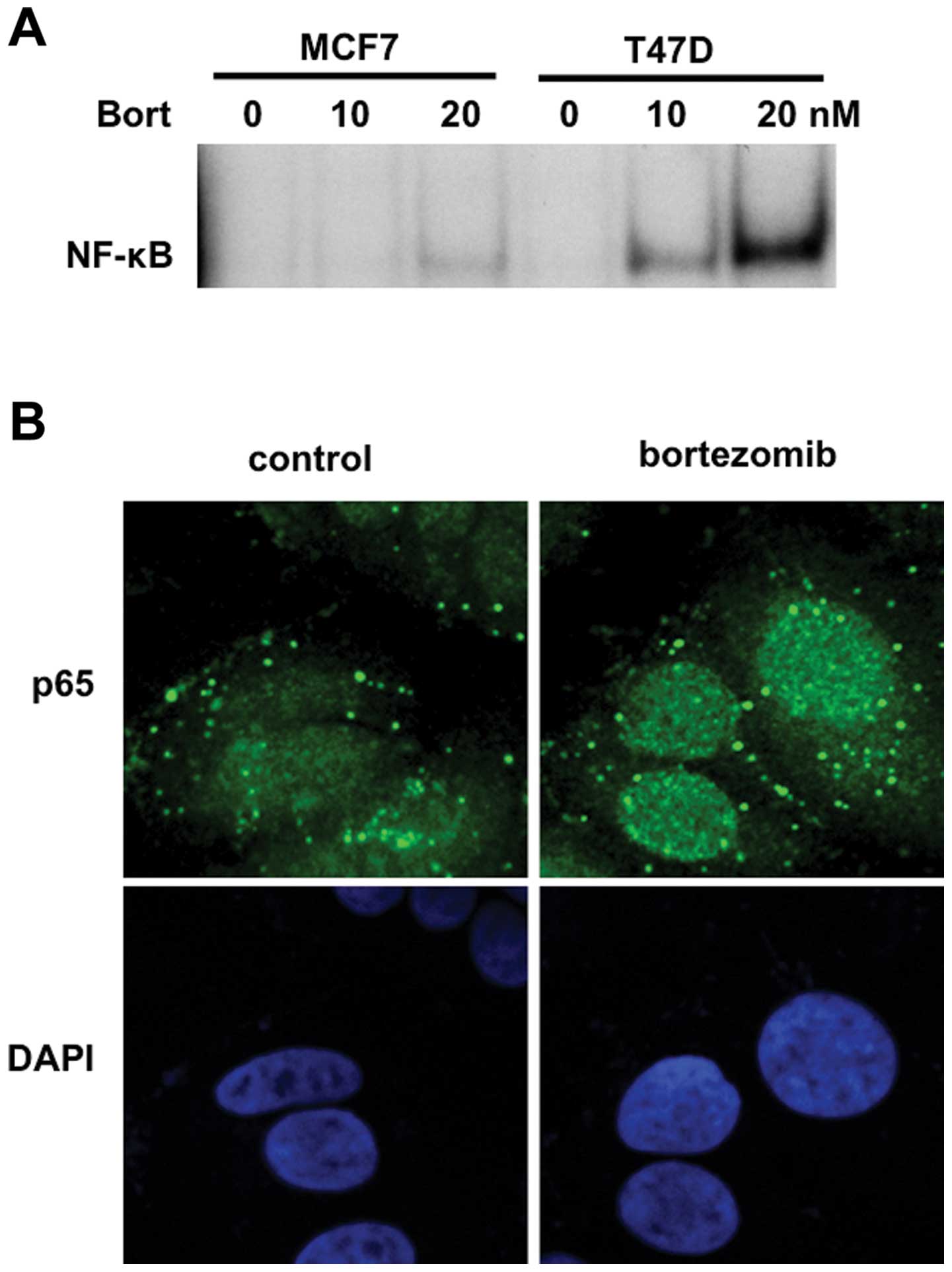
Since IκBα is an inhibitor of nuclear translocation
of p50/p65 heterodimer, we next examined whether bortezomib
triggered NF-κB activation in breast cancer cells. To obtain direct
evidence showing NF-κB activation by bortezomib, we carried out
EMSA. Consistent with downregulation of IκBα, bortezomib markedly
enhanced NF-κB activity in a dose-dependent fashion in MCF7 and
T47D cells (Fig. 3A). We further
examined nuclear p65 expression in T47D cells by
immunocytochemistry and confirmed that bortezomib markedly enhanced
nuclear translocation of p65 (Fig.
3B). These results indicated that NF-κB was activated by
bortezomib treatment in breast cancer cells.
Bortezomib activates the canonical NF-κB
pathway in breast cancer cell lines
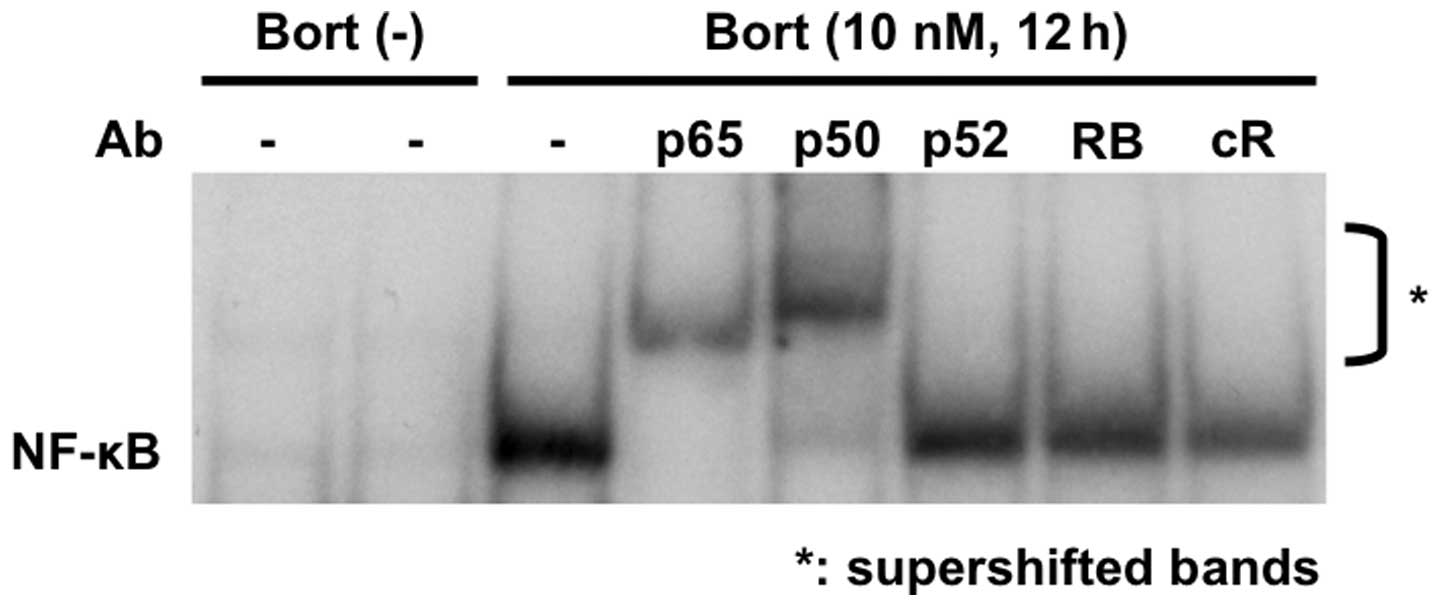
NF-κB activation is mediated via both canonical and
non-canonical pathways, and we further examined whether NF-κB
activation by bortezomib was solely via canonical NF-κB activation,
since IκBα is a major inhibitor of p50/65 nuclear translocation.
Supershift assays confirmed that bortezomib triggered canonical
NF-κB activation, evidenced by markedly enhanced supershifted bands
in the presence of anti-p65 and p50, but not p52 or RelB (RB) Abs
in T47D cells (Fig. 4). This
result, strongly indicating that bortezomib triggers activation of
the canonical NF-κB pathway.
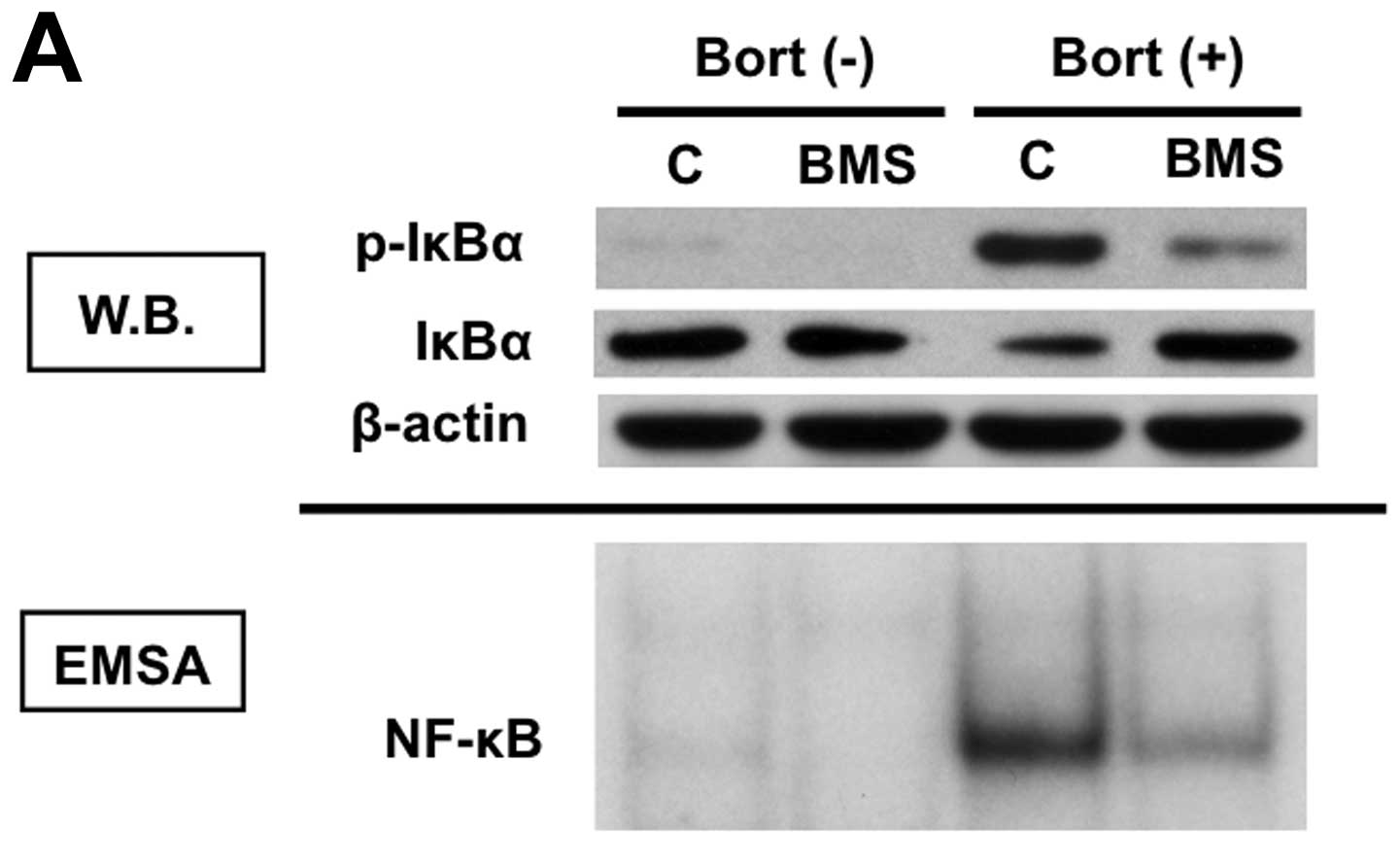
Inhibition of IKKβ blocks
bortezomib-induced IκBα down-regulation and NF-κB activation
Since bortezomib-triggered NF-κB canonical
activation is mediated via phosphorylation and downregulation of
IκBα, we next examined whether inhibition of upstream molecule
blocked bortezomib-induced NF-κB activation. T47D cells were
cultured with bortezomib in the presence and absence of IKKβ
inhibitor BMS-345541. BMS-345541 inhibited both phosphorylation and
protein expression of IκBα (Fig. 5A,
upper panel). Importantly, NF-κB activation induced by
bortezomib was completely blocked by BMS-345541 (Fig. 5A, lower panel), suggesting that
activation of IKKβ plays a key role in bortezomib-induced NF-κB
activation. Moreover, inhibition of canonical NF-κB activity by
BMS-345541 enhanced bortezomib-induced cytotoxicity in T47D cells
(Fig. 5B).
Discussion
NF-κB is a transcriptional factor of the Rel family
proteins, including p65 (RelA), RelB, c-Rel, p50 (NF-κB1) and p52
(NFκ-B2), which regulates cell proliferation, anti-apoptosis and
cytokine secretion in many cancers (22). In canonical pathway, NF-κB is
typically a heterodimer composed of p50 and p65 subunits and
constitutively present both in the cytosol and nucleus. In the
cytosol, p50/p65 nuclear translocation is blocked by IκB family
inhibitors; IκBα therefore has a crucial role in regulating NF-κB
activation (23). Upon stimulation
by various types of growth factors and cytokines (i.e., TNFα), IκBα
is phosphorylated by the upstream molecules, IκB kinases (IKKs).
IκBα is subsequently polyubiquitinated and degraded by proteasome,
allowing nuclear translocation of p50/65, where it binds to
specific DNA sequences in the promoters of target genes.
Bortezomib is a 26S proteasome inhibitor initially
used in MM treatment based upon expectation that bortezomib could
inhibit NF-κB activity by preventing proteasomal degradation of
IκBα. It demonstrates remarkable anti-MM activities in both
preclinical (9–11) and clinical (12–14)
studies, and was approved by FDA in 2003 for therapy of relapsed
refractory MM, in 2005 for treatment of relapsed MM, and in 2008
for initial treatment in MM. Although extensive molecular-based
studies have been done in MM, inhibition of constitutive NF-κB
activity by bortezomib has not been shown in either preclinical or
clinical studies. However, in most solid tumors, including breast
cancer, bortezomib, as monotherapy, has not shown promising
antitumor activity (15,16). Importantly, recent studies have
shown that bortezomib activates constitutive NF-κB in endothelial
cell carcinoma cells (24) and in
primary tumor cells from MM patients (21,25),
suggesting that inhibition of NF-κB does not solely account for its
antitumor activities. In this study, we therefore examined whether
bortezomib also modulates constitutive NF-κB activity regulating
cell proliferation and anti-apoptosis in breast cancer cells.
To determine the optimal dose of bortezomib to
assess NF-κB activity, we first examined sensitivity of MCF7 and
T47D to bortezomib treatment and observed that breast cancer lines,
especially MCF7, were relatively resistant to bortezomib. Previous
studies have shown that heat shock protein 27 (26,27)
and proteasome subunit β5 (PSMB5) gene mutation and overexpression
of PSMB5 protein decrease sensitivity to bortezomib in MM and
myelomonocytic THP1 cells, respectively (28). However, the mechanisms of action
decreasing the sensitivity to bortezomib in these breast cancer
cell lines remain unclear.
It has been shown that bortezomib inhibits
proteasomal degradation of IκBα induced by cytokines (i.e., TNFα)
(29); however, IκBα was
downregulated in both cell lines by bortezomib treatment,
associated with enhanced phosphorylation in breast cancer cells.
These results are similar to previous studies observed in MM cells
(21). Since IκBα regulates the
canonical NF-κB pathway, we further examined NF-κB activity in MCF7
and T47D cells after bortezomib treatment. Although constitutive
NF-κB activity was extremely low, bortezomib markedly enhanced
NF-κB activity in a dose-dependent fashion. This result was
consistent to down-regulated protein level of IκBα by bortezomib.
Interestingly, bortezomib-induced NF-κB activity in T47D was more
significant than that in MCF7 cells. As described above, MCF7 cells
are more resistant to bortezomib compared to T47D, these results
suggested that NF-κB activation may not be totally responsible for
resistance to bortezomib in these breast cancer cell lines.
Since NF-κB mediates cell survival and progression
of disease in breast cancer (30,31),
we hypothesized that the blockade of bortezomib-induced canonical
NF-κB activation could enhance its growth inhibitory effect.
Indeed, previous study has shown that doxorubicin activates the
NF-κB and that inhibition of IKK sensitizes breast cancer cells to
bortezomib (32). As expected,
IKKβ inhibitor almost completely blocked bortezomib-induced NF-κB
activation and significantly augmented its cytotoxicity. Taken
together our results provide the preclinical framework for
combination strategy of bortezomib with other agents inhibiting
IKKβ.
Acknowledgements
This study was supported by the
National Institute of Health Grants (SPORE-P50100707, P01 CA78378
and R01 CA50947; to K.C.A.).
References
|
1.
|
Jost PJ and Ruland J: Aberrant NF-κB
signaling in lymphoma: mechanisms, consequences, and therapeutic
implications. Blood. 109:2700–2707. 2007.
|
|
2.
|
Keats JJ, Fonseca R, Chesi M, Schop R,
Baker A, Chng WJ, Van Wier S, Tiedemann R, Shi CX, Sebag M, Braggio
E, Henry T, et al: Promiscuous mutations activate the noncanonical
NF-κB pathway in multiple myeloma. Cancer Cell. 12:131–144.
2007.PubMed/NCBI
|
|
3.
|
Annunziata CM, Davis RE, Demchenko Y,
Bellamy W, Gabrea A, Zhan F, Lenz G, Hanamura I, Wright G, Xiao W,
Dave S, Hurt EM, et al: Frequent engagement of the classical and
alternative NF-κB pathways by diverse genetic abnormalities in
multiple myeloma. Cancer Cell. 12:115–130. 2007.
|
|
4.
|
Hideshima T, Chauhan D, Kiziltepe T, Ikeda
H, Okawa Y, Podar K, Raje N, Protopopov A, Munshi NC, Richardson
PG, Carrasco RD and Anderson KC: Biologic sequelae of IκB kinase
(IKK) inhibition in multiple myeloma: therapeutic implications.
Blood. 113:5228–5236. 2009.
|
|
5.
|
Baldwin AS Jr: The NF-κB and IκB proteins:
new discoveries and insights. Annu Rev Immunol. 14:649–683.
1996.
|
|
6.
|
Beg AA and Baldwin AS Jr: The IκB
proteins: multifunctional regulators of Rel NF-κB transcription
factors. Genes Dev. 7:2064–2070. 1993.
|
|
7.
|
Zandi E, Chen Y and Karin M: Direct
phosphorylation of IkappaB by IKKα and IKKβ: discrimination between
free and NF-κB-bound substrate. Science. 281:1360–1363. 1998.
|
|
8.
|
DiDonato JA, Hayakawa M, Rothwarf DM,
Zandi E and Karin M: A cytokine-responsive IκB kinase that
activates the transcription factor NF-κB. Nature. 388:548–554.
1997.
|
|
9.
|
Hideshima T, Richardson P, Chauhan D,
Palombella V, Elliott P, Adams J and Anderson KC: The proteasome
inhibitor PS-341 inhibits growth, induces apoptosis and overcomes
drug resistance in human multiple myeloma cells. Cancer Res.
61:3071–3076. 2001.
|
|
10.
|
Mitsiades N, Mitsiades CS, Poulaki V,
Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA,
Treon SP, Munshi NC, Richardson PG, et al: Molecular sequelae of
proteasome inhibition in human multiple myeloma cells. Proc Natl
Acad Sci USA. 99:14374–14379. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hideshima T, Mitsiades C, Akiyama M,
Hayashi T, Chauhan D, Richardson P, Schlossman R, Podar K, Munshi
NC, Mitsiades N and Anderson KC: Molecular mechanisms mediating
anti-myeloma activity of proteasome inhibitor PS-341. Blood.
101:1530–1534. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Richardson PG, Barlogie B, Berenson J,
Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina
M, Alexanian R, Siegel D, Orlowski RZ, et al: A phase 2 study of
bortezomib in relapsed, refractory myeloma. N Engl J Med.
348:2609–2617. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Richardson PG, Sonneveld P, Schuster MW,
Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D,
Lonial S, Goldschmidt H, Reece D, San-Miguel JF, et al: Bortezomib
or high-dose dexamethasone for relapsed multiple myeloma. N Engl J
Med. 352:2487–2498. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
San Miguel JF, Schlag R, Khuageva NK,
Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT,
Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, et al:
Bortezomib plus melphalan and prednisone for initial treatment of
multiple myeloma. N Engl J Med. 359:906–917. 2008.
|
|
15.
|
Engel RH, Brown JA, Von Roenn JH, O’Regan
RM, Bergan R, Badve S, Rademaker A and Gradishar WJ: A phase II
study of single agent bortezomib in patients with metastatic breast
cancer: a single institution experience. Cancer Invest. 25:733–737.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Dees EC and Orlowski RZ: Targeting the
ubiquitin-proteasome pathway in breast cancer therapy. Future
Oncol. 2:121–135. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Schmid P, Kuhnhardt D, Kiewe P,
Lehenbauer-Dehm S, Schippinger W, Greil R, Lange W, Preiss J,
Niederle N, Brossart P, Freier W, Kummel S, et al: A phase I/II
study of bortezomib and capecitabine in patients with metastatic
breast cancer previously treated with taxanes and/or
anthracyclines. Ann Oncol. 19:871–876. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Dees EC, O’Neil BH, Lindley CM, Collichio
F, Carey LA, Collins J, Riordan WJ, Ivanova A, Esseltine D and
Orlowski RZ: A phase I and pharmacologic study of the combination
of bortezomib and pegylated liposomal doxorubicin in patients with
refractory solid tumors. Cancer Chemother Pharmacol. 63:99–107.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Awada A, Albanell J, Canney PA, Dirix LY,
Gil T, Cardoso F, Gascon P, Piccart MJ and Baselga J:
Bortezomib/docetaxel combination therapy in patients with
anthracycline-pretreated advanced/metastatic breast cancer: a phase
I/II dose-escalation study. Br J Cancer. 98:1500–1507. 2008.
View Article : Google Scholar
|
|
20.
|
Cresta S, Sessa C, Catapano CV, Gallerani
E, Passalacqua D, Rinaldi A, Bertoni F, Vigano L, Maur M, Capri G,
Maccioni E, Tosi D, et al: Phase I study of bortezomib with weekly
paclitaxel in patients with advanced solid tumours. Eur J Cancer.
44:1829–1834. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Hideshima T, Ikeda H, Chauhan D, Okawa Y,
Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD
and Anderson KC: Bortezomib induces canonical nuclear factor-κB
activation in multiple myeloma cells. Blood. 114:1046–1052.
2009.
|
|
22.
|
Karin M and Greten FR: NF-kappaB: linking
inflammation and immunity to cancer development and progression.
Nat Rev Immunol. 5:749–759. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Karin M and Ben-Neriah Y: Phosphorylation
meets ubiquitination: the control of NF-κB activity. Annu Rev
Immunol. 18:621–663. 2000.PubMed/NCBI
|
|
24.
|
Dolcet X, Llobet D, Encinas M, Pallares J,
Cabero A, Schoenenberger JA, Comella JX and Matias-Guiu X:
Proteasome inhibitors induce death but activate NF-κB on
endometrial carcinoma cell lines and primary culture explants. J
Biol Chem. 281:22118–22130. 2006.
|
|
25.
|
Markovina S, Callander NS, O’Connor SL,
Kim J, Werndli JE, Raschko M, Leith CP, Kahl BS, Kim K and Miyamoto
S: Bortezomib-resistant nuclear factor-κB activity in multiple
myeloma cells. Mol Cancer Res. 6:1356–1364. 2008.
|
|
26.
|
Chauhan D, Li G, Shringarpure R, Podar K,
Ohtake Y, Hideshima T and Anderson KC: Blockade of Hsp27 overcomes
Bortezomib/proteasome inhibitor PS-341 resistance in lymphoma
cells. Cancer Res. 63:6174–6177. 2003.PubMed/NCBI
|
|
27.
|
Hideshima T, Podar K, Chauhan D, Ishitsuka
K, Mitsiades C, Tai YT, Hamasaki M, Raje N, Hideshima H, Schreiner
G, Nguyen AN, Navas T, et al: p38 MAPK inhibition enhances PS-341
(bortezomib)-induced cytotoxicity against multiple myeloma cells.
Oncogene. 23:8766–8776. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Oerlemans R, Franke NE, Assaraf YG, Cloos
J, van Zantwijk I, Berkers CR, Scheffer GL, Debipersad K, Vojtekova
K, Lemos C, van der Heijden JW, Ylstra B, et al: Molecular basis of
bortezomib resistance: proteasome subunit beta5 (PSMB5) gene
mutation and overexpression of PSMB5 protein. Blood. 112:2489–2499.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Hideshima T, Chauhan D, Richardson P,
Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A,
Palombella V, Adams J and Anderson KC: NF-kB as a therapeutic
target in multiple myeloma. J Biol Chem. 277:16639–16647. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Garg AK, Hortobagyi GN, Aggarwal BB, Sahin
AA and Buchholz TA: Nuclear factor-κB as a predictor of treatment
response in breast cancer. Curr Opin Oncol. 15:405–411. 2003.
|
|
31.
|
Wu JT and Kral JG: The NF-κB/IκB signaling
system: a molecular target in breast cancer therapy. J Surg Res.
123:158–169. 2005.
|
|
32.
|
Tapia MA, Gonzalez-Navarrete I, Dalmases
A, Bosch M, Rodriguez-Fanjul V, Rolfe M, Ross JS, Mezquita J,
Mezquita C, Bachs O, Gascon P, Rojo F, et al: Inhibition of the
canonical IKK/NF κB pathway sensitizes human cancer cells to
doxorubicin. Cell Cycle. 6:2284–2292. 2007.PubMed/NCBI
|