Introduction
Multiple myeloma is a plasma cell malignancy that
often remains fatal despite the use of high dose chemotherapy with
hematopoietic stem cell transplantation (1). Most multiple myeloma patients are
elderly; therefore, severe adverse effects and complications such
as serious infection due to anticancer drugs are major problems in
clinical practice. Since 2000, novel agents such as thalidomide,
lenalidomide, and the proteasome inhibitor bortezomib have been
introduced for the treatment of this disease and have remarkably
improved patient outcome (2–4).
However, adverse events and complications of these agents are often
problematic in the clinical setting. In addition, prolonged use and
repeated disease relapse may lead to the development of drug
resistance in myeloma cells (5).
Therefore, novel effective and less toxic therapeutic strategies
are desired in order to improve clinical outcomes.
Extracts of the herb Tripterygium wilfordii
Hook F have been used for more than two centuries as the
traditional Chinese medicine to treat a variety of autoimmune and
inflammatory diseases including rheumatoid arthritis (6,7). In
addition, recent studies have demonstrated that triptolide has
potential antitumor properties by inhibiting cell growth and
inducing apoptotic cell death (8–11).
Furthermore, triptolide shows antitumor effects on various
hematological malignancies, and many studies have been performed to
elucidate the molecular mechanism of triptolide-induced antitumor
activities (12–15). Previous studies have shown that
triptolide induces apoptosis of multiple myeloma cells mediated
through the PI3K/Akt and NF-κB pathway and further associated with
the MAPK pathway via mitochondrial cell death signaling and caspase
activation (16,17). However, a more detailed mechanism
of triptolide-induced apoptotic cell death in multiple myeloma
cells remains unknown.
In the present study, we investigated the effects of
triptolide on myeloma cells and showed that triptolide at low
nanomolar concentrations induced apoptotic cell death in various
multiple myeloma cell lines. We also examined the molecular
mechanisms of triptolide-induced cell death in myeloma cells.
Materials and methods
Cells and cell culture
Human myeloma cell lines, HS-sultan, IM9, RPMI8226
and U266, were cultured in RPMI-1640 medium (Gibco BRL, Grand
Island, NY, USA) supplemented with 10% fetal bovine serum (FBS)
(Gibco BRL) in a humidified atmosphere with 5% CO2 at
37°C. These cell lines were obtained from the Japan Cancer Research
Resources Bank (Tokyo, Japan). Cell morphology was evaluated using
cytospin slide preparations with Giemsa staining and viability was
assessed by trypan blue dye exclusion.
Reagents
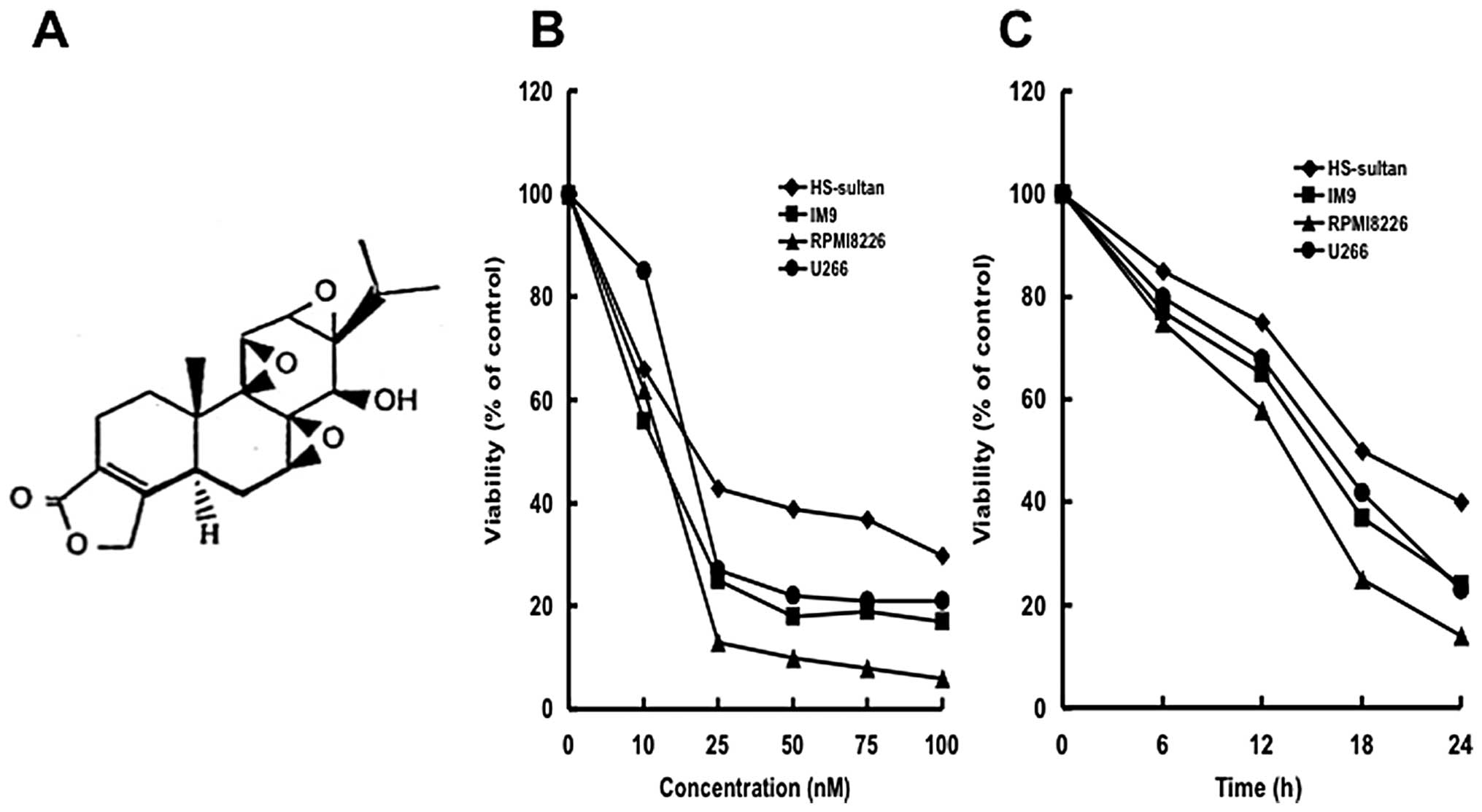
Triptolide (Fig.
1A) was purchased from Sigma-Aldrich Japan (Tokyo, Japan) and
was dissolved in PBS.
Cell proliferation assay
Cell proliferation was measured using an MTT
proliferation assay kit (Roche Molecular Biochemicals, Mannheim,
Germany). Cells were plated in 96-well culture plates at
5×104 cells/ml in a total volume of 100 ml with the
indicated reagents. After a 2-day incubation, cellular
proliferation was measured using the MTT assay. Mean and standard
deviation were calculated from triplicate experiments.
Assays for apoptotic cell death
Cell death was determined by assessing morphological
changes as well as by staining with Annexin V-FITC and PI labeling.
Apoptotic cells were quantified with Annexin V-FITC and PI double
staining by using a staining kit purchased from Pharmingen (San
Diego, CA, USA). In addition, induction of apoptotic cell death was
detected using a DNA fragmentation assay. Cells (1×106)
were harvested and incubated in a lysis buffer [10 mM Tris-HCl (pH
7.4), 10 mM EDTA, 0.5% Triton-X] at 4°C. After centrifugation,
supernatants were collected and incubated with RNase A (Sigma) at
50 mg/ml and proteinase K (Sigma) for 1 h at 37°C. The DNA samples
was elevtrophoresed on a 2% agarose gel and visualized with
ethidium bromide staining. The mitochondrial transmembrane
potential (Δψm) was determined by flow cytometry
(FACSCalibur; Becton-Dickinson, San Jose, CA, USA). Briefly, cells
were washed twice with PBS and incubated with 1 mg/ml Rhodamine-123
(Sigma) at 37°C for 30 min. Rhodamine-123 intensity was determined
by flow cytometry.
Cell cycle analysis
Cells (1×105) were suspended in hypotonic
solution [0.1% Triton X-100, 1 mM Tris-HCl (pH 8.0), 3.4 mM sodium
citrate, 0.1 mM EDTA] and stained with 50 mg/ml of PI. DNA content
was analyzed by flow cytometry and the population of cells in each
phase of the cell cycle was determined using ModiFIT software
(Becton-Dickinson).
Caspase activation assays
The activation of caspase-3 was analyzed using a
caspase-3 assay kit from BD Biosciences (San Jose, CA, USA).
Briefly, the FITC-conjugated antibody against the active form of
caspase-3 provided in the kit was used for FACS analysis according
to the manufacturer’s instructions (BD Biosciences).
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
Total cellular RNA was extracted using the RNeasy
Mini kit (Qiagen, Valencia, CA, USA) according to the
manufacturers’ instructions. Ten pmol of primers for Mcl-1
(forward, 5′-GCCAAGGACACAAAGCCAAT-3′; and reverse, 5′-AACT
CCACAAACCCATCCCA-3′) were used in the PCR reactions. Primer sets
for β-actin (forward, 5′-TCCTTCTGCATCCTG TCGGCA-3′; and reverse,
5′-CAAGAGATGGCCACGGCT GCT-3′) was used as the internal control.
After an initial denaturation at 94°C for 2 min, 30 cycles of 30
sec at 94°C, 30 sec at 54°C, 1 min at 72°C, and final extension at
72°C for 7 min were performed using the Superscript III
First-Strand Synthesis System for RT-PCR (Invitrogen Co., Carlsbad,
CA, USA). The PCR products were electrophoresed in 2% agarose
gels.
Cell lysate preparation and western
blotting
Cells were collected by centrifugation at 700 g for
10 min and the pellets were resuspended in lysis buffer (1% NP-40,
1 mM phenylmethylsulfonyl fluoride (PMSF), 40 mM Tris-HCl (pH 8.0),
150 mM NaCl), and incubated at 4°C for 15 min. Mitochondrial and
cytosolic fractions were prepared with digitonin-nagarse treatment.
Protein concentrations were determined using the protein assay DC
system (Bio-Rad, Richmond, CA, USA). Cell lysates (20 μg
protein per lane) were fractionated in 12.5% SDS-polyacrylamide
gels prior to transfer to membranes (Immobilon-P membranes,
Millipore, Bedford, MA, USA) using standard protocol. Antibody
binding was detected using an enhanced chemiluminescence kit for
western blotting detection with hyper-ECL film (Amersham,
Buckinghamshire, UK). Blots were stained with Coomassie brilliant
blue to confirm equal loading of protein extracts. The following
antibodies were used in this study: anti-caspase 3, anti-caspase-8,
anti-caspase-9, anti-cytochrome c (Pharmingen), Bcl-2,
anti-Bcl-XL, anti-Mcl-1, anti-cyclin D1, anti-β-actin
(Santa Cruz Biotech, Santa Cruz, CA, USA), anti-Bax, anti-Smac
(second mitochondria-derived activator of caspases)/DIABLO (MBL,
Nagoya, Japan), and anti-RNA polymerase II CTD (phosphor, serine 7)
(Funakoshi Co. Ltd., Tokyo, Japan).
Statistical analysis
All data are presented as the mean ± SD. Statistical
significance was examined using Student’s t-test analysis and
p<0.05 was considered statistically significant.
Results
Triptolide inhibits cellular
proliferation of various myeloma cells
We first examined whether triptolide inhibited
cellular growth of myeloma cells (HS-sultan, IM9, RPMI8226 and U266
cells). Triptolide inhibited the cellular growth of all myeloma
cells dose- (0–100 nM) and time (0–24 h)-dependently (Fig. 1B and C). Among the cells tested,
RPMI8226 cells were the most sensitive to triptolide with an
IC50 of 17 nM (Fig.
1B). Therefore, we used RPMI8226 cells for further experiments.
Interestingly, cell growth was suppressed as early as 6 h with low
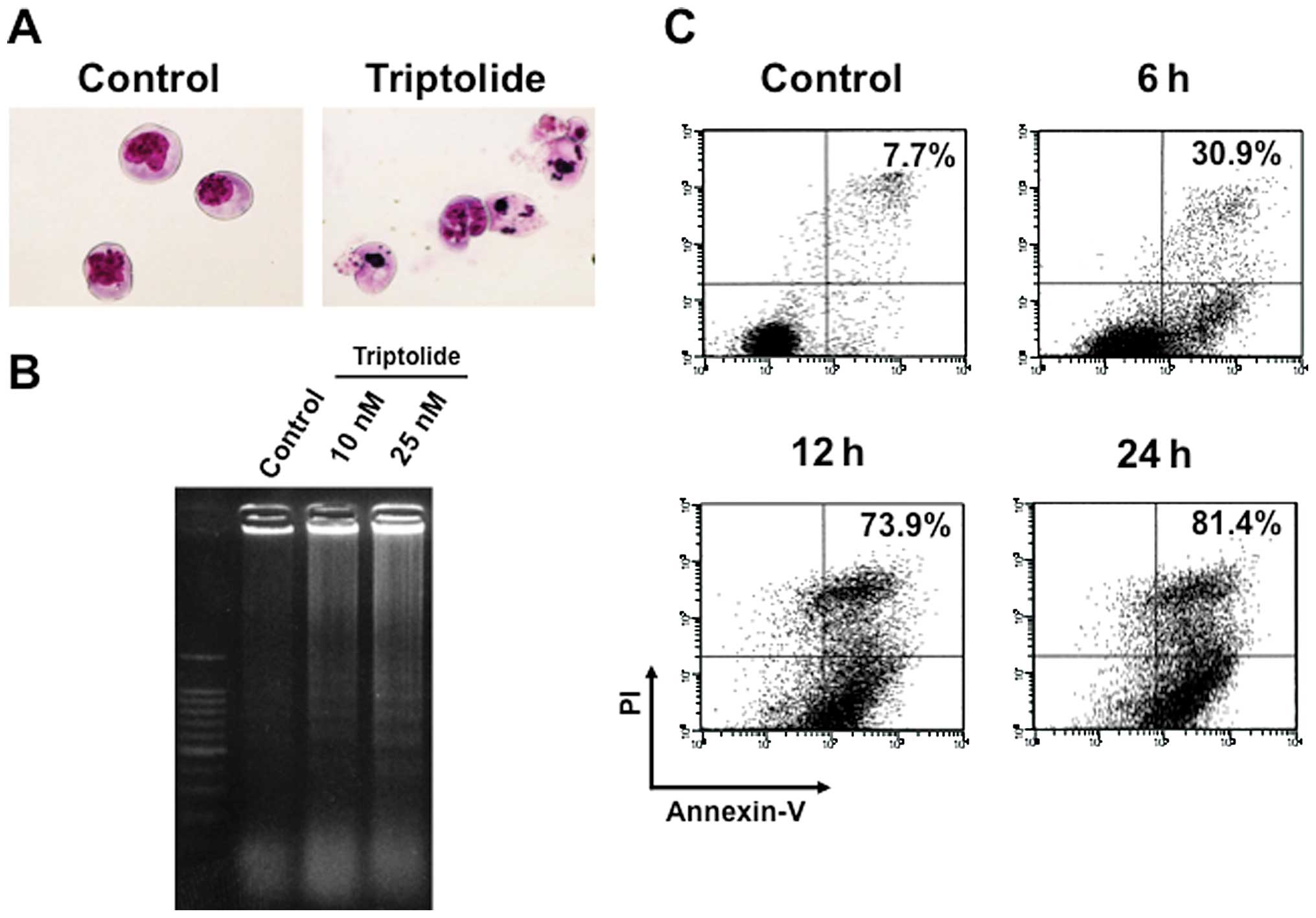
concentrations with typical apoptotic morphology observed,
including condensed chromatin and fragmented nuclei with apoptotic
bodies (Fig. 2A).
Triptolide induces G1 cell cycle arrest
followed by apoptotic cell death
The effects of triptolide on cell cycle progression
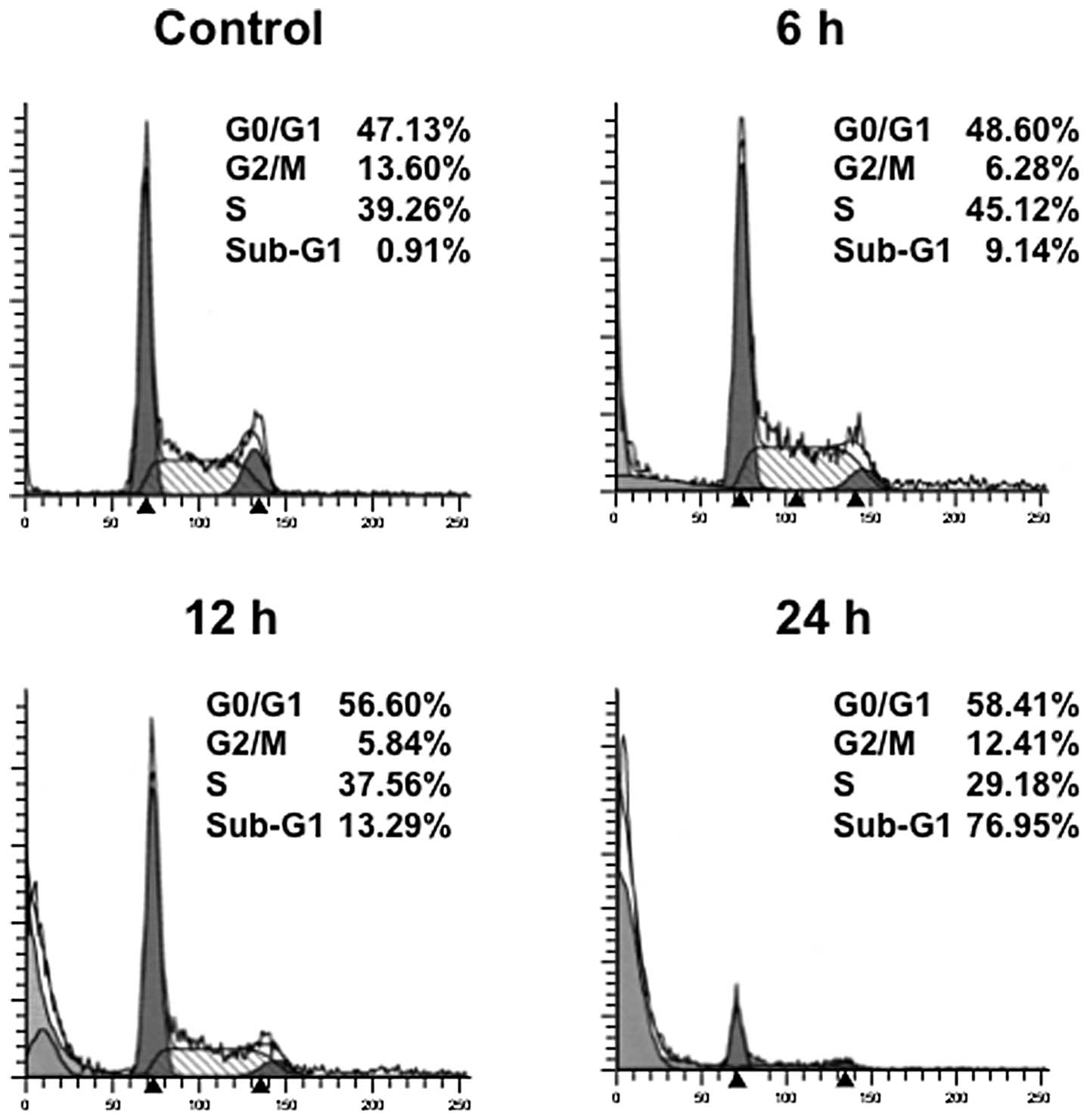
were investigated using RPMI8226 cells. The cells were treated with
25 nM triptolide for the indicated times and analyzed for cell
cycle distribution by means of flow cytometry. Cultivation with
triptolide increased the population of cells in the G0/G1 phase
with a reduction of cells in the S phase (Fig. 3). In addition, a strong induction
of apoptosis was shown by the appearance of a haplodiploid DNA peak
with sub-G1 DNA contents after triptolide treatment. These results
indicate that triptolide led to cell cycle arrest at the G1 phase
followed by apoptosis. We thus confirmed the induction of apoptosis
by triptolide by means of DNA ladder formation and Annexin V/PI
staining. DNA ladder formation was confirmed at time-points as
early as 6 h by electrophoresis of genomic DNA extracted from
RPMI8226 cells treated with 25 nM triptolide (Fig. 2B). Consistent with these results,
Annexinn V-positive cells dramatically increased in a
time-dependent manner (Fig. 2C),
indicating that triptolide rapidly induced apoptotic cell death in
RPMI8226 cells.
Triptolide induces caspase-dependent cell
death in myeloma cells
Caspases are believed to play a central role in
mediating various apoptotic responses. To address the apoptotic
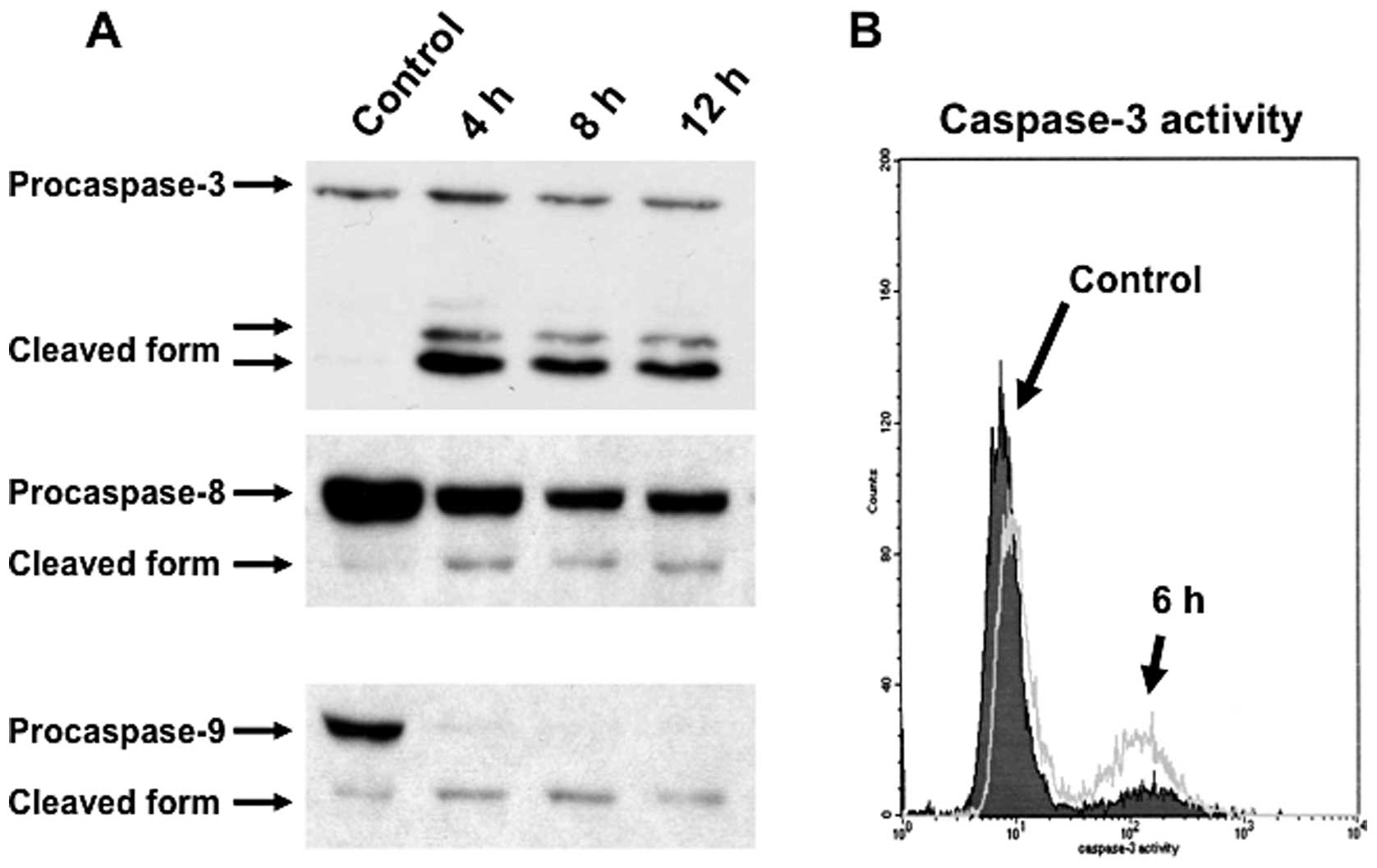
cell death pathway in triptolide-treated RPMI8226 cells, we next
examined the activation of caspases by western blot analysis. The
downregulation of procaspase-3, procaspase-9 and procaspase-8 were
detected after treatment with 25 nM triptolide for the indicated
times (Fig. 4A). To clarify the
activation of caspase-3, the percentage of cells expressing the
active form of caspase-3 was analyzed by FACS. After incubation
with 25 nM triptolide for 6 h, the percentage of RPMI8226 cells
expressing the active form of caspase-3 was increased (Fig. 4B). These results indicated that
triptolide induces apoptotic cell death of myeloma cells via a
caspase-dependent pathway.
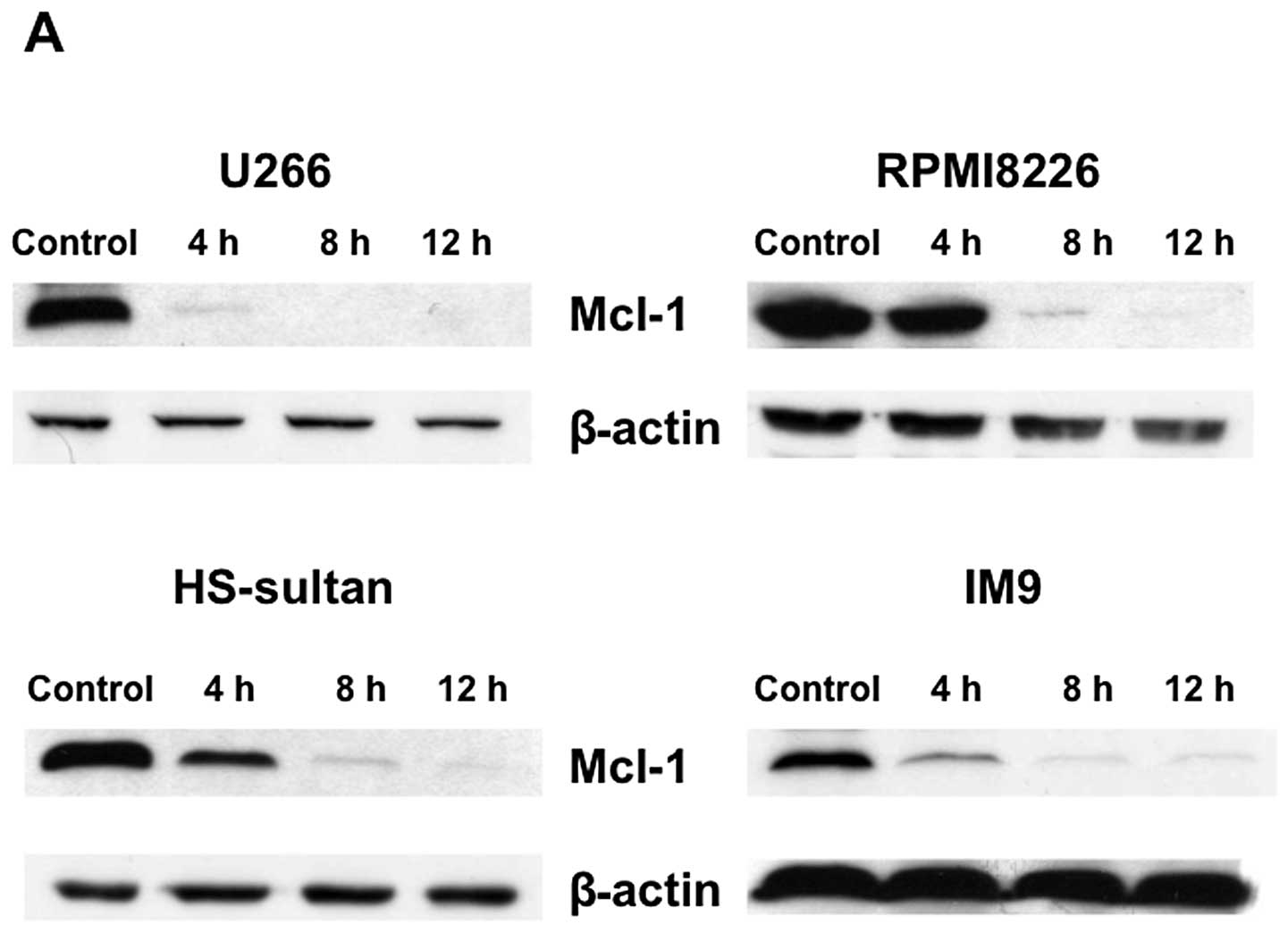
Triptolide induces cell death of myeloma
cells with downregulation of Mcl-1
To investigate the molecular mechanisms of
triptolide-induced apoptosis in RPMI8226 cells, the expression of
several apoptosis-associated proteins were examined. Mcl-1, a
critical survival factor for myeloma cells, was down-regulated with
triptolide treatment in various myeloma cells (Fig. 6A). In contrast, triptolide did not
modulate the levels of pro-apoptotic Bax or anti-apoptotic Bcl-2
and Bcl-XL proteins in RPMI8226 cells (data not
shown).
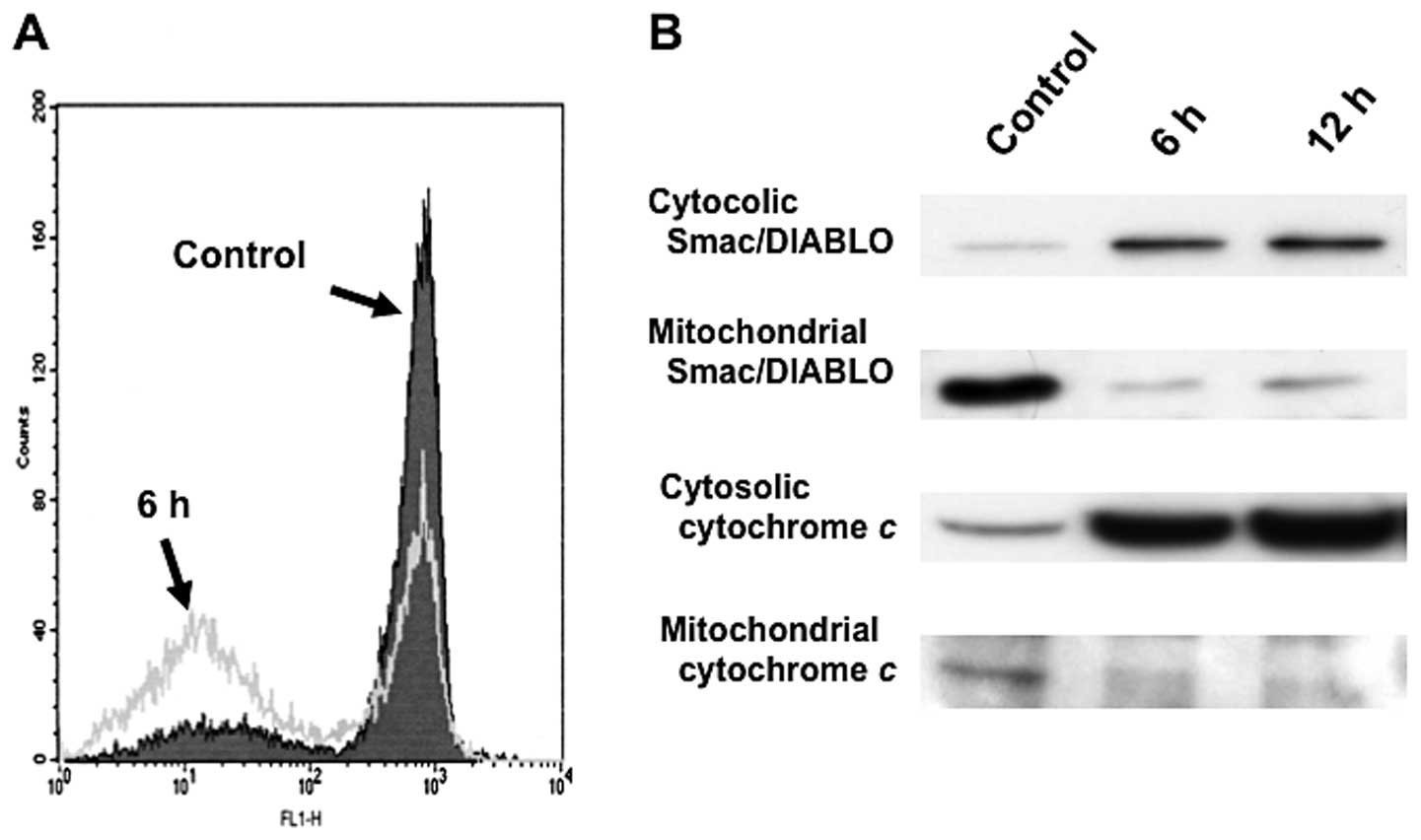
Triptolide-induced death signaling is
mediated through the mitochondrial pathway
Recent studies have suggested that mitochondria play
an essential role in death signal transduction. Mitochondrial
changes, including permeability transition pore opening and the
collapse of the mitochondrial Δψm, result in the release
of cytochrome c into the cytosol, which subsequently causes
cell death by the activation of caspases (19). After treatment with 25 nM
triptolide for 3 h, low Rh123 staining in RPMI8226 cells indicated
an increase in the loss of mitochondrial Δψm (Fig. 5A). The loss of Δψm
appeared in parallel with the activation of caspase-3 and
caspase-9, as well as with apoptosis. In addition, triptolide
induced a substantial release of various mitochondrial apoptogenic
proteins, cytochrome c, Smac/DIABLO from the mitochondria
into the cytosol in RPMI8226 cells (Fig. 5B). These results suggest that
mitochondrial dysfunction causes the release of cytochrome
c, Smac/DIABLO into the cytosol; caspase-9 and caspase-3
were then activated, thereby propagating the death signal.
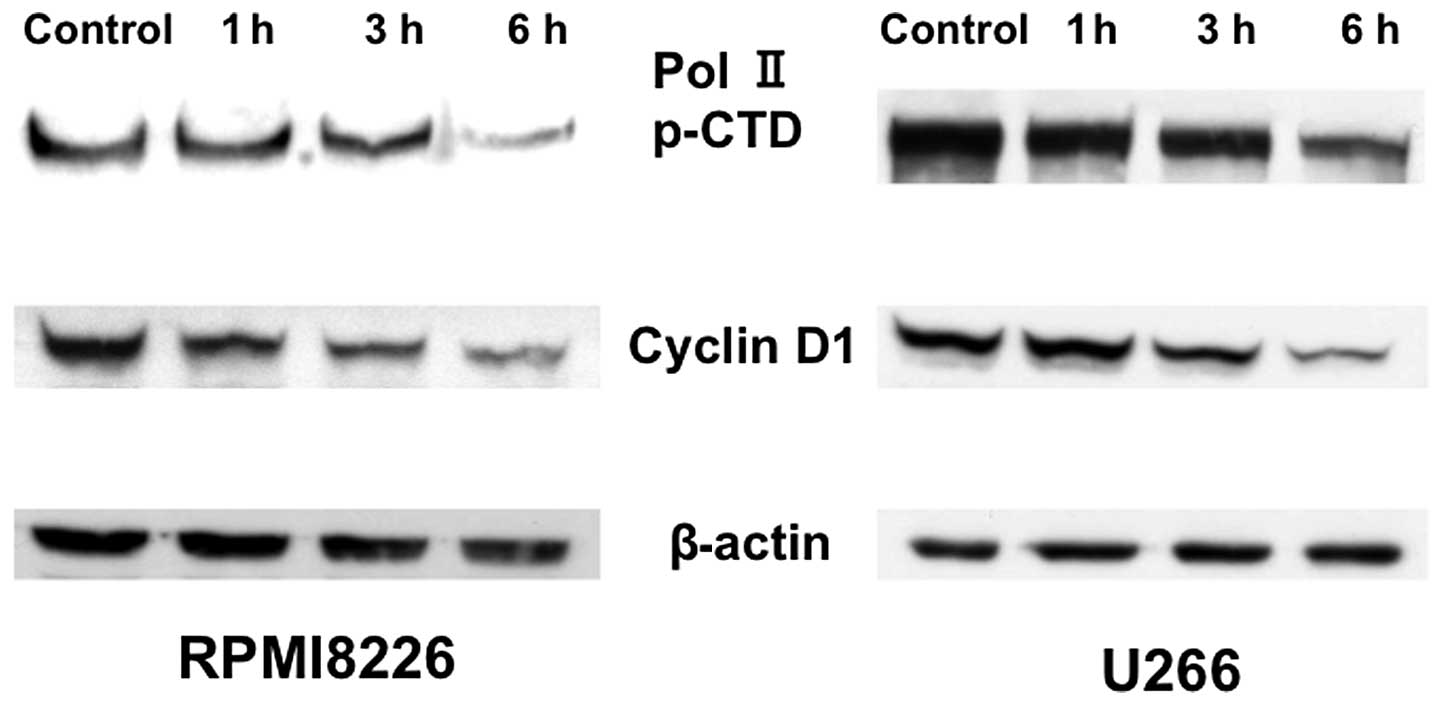
Triptolide downregulates Mcl-1 expression
through a transcriptional mechanism in association with the
inhibition of RNA polymerase II CTD phosphorylation
To address the mechanism underlying
triptolide-induced downregulation of Mcl-1, expression of Mcl-1
mRNA was examined using RT-PCR (Fig.
6B). Expression of Mcl-1 mRNA was decreased by triptolide
treatment in various myeloma cell lines (U266, RPMI8226, HS-sultan,
and IM9) in a time-dependent manner. Reductions in Mcl-1 mRNA
levels were observed at 25 nM triptolide by 2–4 h and roughly
paralleled the extent of protein downregulation (Fig. 6). We next examined the
phosphorylation of the RNA polymerase II C-terminal domain (CTD) by
triptolide treatment. Exposure to triptolide inhibited
phosphorylation of RNA polymerase II CTD at serine 7, consistent
with the inhibition of cyclin D1 expression (Fig. 7). These results suggest triptolide
blocks RNA polymerase II CTD phosphorylation to repress Mcl-1
transcription.
Discussion
Multiple myeloma is a plasma cell neoplasm derived
from clonal B cell lineage cells. The development of new agents
such as the proteasome inhibitor, bortezomib, and the
immunomodulatory drugs, thalidomide and lenalidomide, has led to
improved outcomes in patients with multiple myeloma (2–4).
However, a high proportion of patients cannot expect long-term
remission due to drug-resistance, minimal residual disease, or
complications such as severe infections (1–4).
Therefore, new potent therapeutic agents and substantial
therapeutic advances are needed for the treatment of multiple
myeloma.
Triptolide has been found to have potent
anti-inflammatory, immunosuppressive and antitumor properties
(6–11). Recent studies have suggested that
triptolide inhibits NF-κB activity via downregulation of Bcl-2
expression and the inhibition of p53 transcription activity in
various p53-wild-type human tumor cells (18–20).
It has also been reported that triptolide has antitumor activity
against hematological malignancies. Triptolide induces apoptosis in
chronic myeloid leukemia (CML) cells via downregulation of BCR-ABL
(21,22), and inhibits MDM2 in acute
lymphoblastic leukemia (ALL) cells through a p53-dependent pathway
(23). In multiple myeloma, it was
reported that triptolide inhibits NF-κB and induces apoptosis in
RPMI8226 and U266 myeloma cells (17). It has also been suggested that
triptolide enhances PS-341-induced apoptosis via the PI3K/Akt/NF-κB
pathways in human multiple myeloma cells (16) and overcomes dexamethasone
resistance in myeloma through upregulation of IL-6-independent
expression of glucocorticoid receptors (24). There have been many studies on the
antitumor activity of triptolide; however, its mechanism of action
remains unclear, especially with respect to multiple myeloma.
In the present study, we showed that triptolide
induced G0/G1 cell cycle arrest followed by apoptotic cell death at
low nanomolar concentrations in human multiple myeloma cells in
association with the downregulation of Mcl-1 at the level of mRNA
transcription, loss of mitochondrial transmembrane potentials
(Δψm), the release of mitochondrial apoptotic proteins
such as cytochrome c, Smac/DIABLO into the cytosol, and the
activation of caspase-3 and caspase-9.
Recent studies have reported that the Bcl-2 family
member, Mcl-1, plays a critical role in multiple myeloma
pathogenesis and drug-resistance by preventing the activation of
endogenous apoptotic pathways and may represent a promising
therapeutic target (25–28). In addition, it has been reported
that drug-induced generation of Mcl-1 fragments followed by c-Jun
upregulation may also be a novel therapeutic approach (29). In our study, Mcl-1 protein levels
rapidly decreased after triptolide treatment. This suggests that
the loss of Mcl-1 expression may play a significant role in
triptolide-induced apoptosis of myeloma cells. In addition,
triptolide inhibited phosphorylation of RNA polymerase II CTD at
serine 7, indicating that triptolide blocks RNA polymerase II CTD
phosphorylation to repress Mcl-1 transcription elongation.
Consistent with our data, it has been reported that triptolide
inhibits de novo total RNA transcription in leukemic cells,
and induces changes in the nuclear substructure that are associated
with decreased RNA polymerase II CTD serine phosphorylation
(30). Recently, the effects of
triptolide on gene expression was examined using whole human
genomic DNA microarrays and it was found that 4-h triptolide
treatment upregulated 160 genes and downregulated 1,511 genes in
the non-small cell lung cancer cell line A549 (31). Additionally, it was reported that
triptolide significantly suppresses apoptosis related genes, such
as XIAP, Bcl-2, Mcl-1, as well as cell cycle regulators including
CDC25A, Polo-like kinases, in various human tumor cells (9,10,32).
Taken together, our results and the previously published data
indicate that triptolide suppresses a broad range of gene
expression by inactivation of NF-κB and other signal transduction
pathways, as well as by global suppression of transcription
(33).
We also found that Smac was released from
mitochondria to the cytosol during triptolide-induced apoptotic
cell death in myeloma cells. Smac binds to XIAP and eliminates its
inhibitory effect on caspase-9. Various anti-myeloma agents trigger
the loss of Δψm, and the release of the mitochondrial
apoptogenic proteins cytochrome c and Smac/DIABLO (34,35).
It has also been reported that PS-341-induced apoptosis in myeloma
cells is associated with activation of JNK, translocation of JNK
from the cytosol to mitochondria, and release of Smac from
mitochondria to the cytosol (36).
Other studies showed that 2-methoxyestradiol (2ME2)-induced
apoptosis in multiple myeloma cells is mediated by JNK activation
and JNK-dependent release of Smac from mitochondria to the cytosol
(37). Various studies have shown
that stress-induced changes in Δψm correlate with an
increase in reactive oxygen species (ROS) and the release of
mitochondrial cytochrome c and Smac/DIABLO. The role of ROS
in mediating apoptosis in various cancer cells is well established
(34,38). The generation of ROS have been
linked to the release of Smac or cytochrome c from
mitochondria to the cytosol during apoptosis (39,40).
Therefore, it will be important to address the effects of ROS on
triptolide-induced apoptotic cell death in multiple myeloma
cells.
Many natural products have been used as anticancer
agents in a clinical setting, and have provided lead chemical
structures with which to develop new agents with enhanced
biological properties and decreased adverse effects (41). Natural compounds appear to be safer
than popular chemotherapeutic agents; therefore, they might be
useful in older patients or in immunocompromised patients because
of their safety and lack of known toxicity. Furthermore, it would
be useful to design clinical trials with myeloma patients to
evaluate their anti-myeloma effects.
In conclusion, triptolide may have potential as a
novel therapeutic agent to replace or augment the more cytotoxic
agents currently used to treat myeloma patients. Further studies
are warranted to evaluate this possibility.
Acknowledgements
We thank Chika Nakabayashi for her
helpful technical assistance. This study was supported in part by
the Ministry of Education, Culture, Sports, Science and Technology
of Japan, and by the National Cancer Center Research and
Development Fund of Japan (23-A-17).
References
|
1.
|
Kyle RA and Rajkumar SV: Multiple myeloma.
Blood. 111:2962–2972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Palumbo A and Anderson K: Multiple
myeloma. N Engl J Med. 364:1046–1060. 2011. View Article : Google Scholar
|
|
3.
|
Boyd KD, Pawlyn C, Morgan GJ and Davies
FE: Understanding the molecular biology of myeloma and its
therapeutic implications. Expert Rev Hematol. 5:603–617. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Watanabe R, Tokuhira M and Kizaki M:
Current approaches for the treatment of multiple myeloma. Int J
Hematol. 97:333–344. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Kumar S, Lee JH, Morgan G, et al: Risk of
progression and survival in multiple myeloma relapsing after
therapy with IMiDs and bortezomib: a multicenter international
myeloma working group study. Leukemia. 26:149–157. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Gu WZ and Brandwein SR: Inhibition of type
II collagen-induced arthritis in rats by triptolide. Int J
Immunopharmacol. 20:389–400. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Cibere J, Deng Z, Lin Y, et al: A
randomized double blind, placebo controlled trial of tropical
Tripterygium wilfordii in rheumatoid arthiritis: reanalysis
using logistic regression analysis. J Rheumatol. 30:465–467.
2003.PubMed/NCBI
|
|
8.
|
Shamon LA, Pezzuto JM, Graves JM, et al:
Evaluation of the mutagenic, cytotoxic, and antitumor potential of
triptolide, a highly oxygenated diterpene isolated from
Tripterygium wilfordii. Cancer Lett. 112:113–117. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Yang S, Chen J, Guo Z, et al: Triptolide
inhibits the growth and metastasis of solid tumors. Mol Cancer
Ther. 2:65–72. 2003.PubMed/NCBI
|
|
10.
|
Kiviharju TM, Lecane PS, Sellers RG and
Peehl DM: Antiproliferative and proapoptotic activities of
triptolide (PG490), a natural product entering clinical trials, on
primary cultures of human prostatic epithelial cells. Clin Cancer
Res. 8:2666–2674. 2002.
|
|
11.
|
Owa C, Messina ME Jr and Halaby R:
Triptolide induces lysosomal-mediated programmed cell death in
MCF-7 breast cancer cells. Int J Women’s Health. 5:557–569.
2013.PubMed/NCBI
|
|
12.
|
Carter BZ, Mak DH, Schober WD, et al:
Triptolide induces caspase-dependent cell death mediated via the
mitochondrial pathway in leukemic cells. Blood. 108:630–637. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Zhang C, Cui GH, Liu F, Wu QL and Chen Y:
Inhibitory effect of triptolide on lymph node metastasis in
patients with non-Hodgkin lymphoma by regulating SDF-1/CXCR4 axis
in vitro. Acta Phramacol Sin. 27:1438–1446. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Carter BZ, Mak DH, Schober WD, et al:
Triptolide sensitizes AML cells to TRAIL-induced apoptosis via
decrease of XIAP and p53-mediated increase of DR5. Blood.
111:3742–3750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Fuchs O: Transcription factor NF-κB
inhibitors as single therapeutic agents or in combination with
classical chemotherapeutic agents for the treatment of
hematological malignancies. Curr Mol Pharmacol. 3:98–122. 2010.
|
|
16.
|
Yang M, Huang J, Pan HZ and Jin J:
Triptolide overcomes dexamethasone resistance and enhanced
PS-341-induced apoptosis via PI3k/Akt/NF-κB pathways in human
multiple myeloma cells. Int J Mol Med. 22:489–496. 2008.PubMed/NCBI
|
|
17.
|
YinJun L, Jie J and YunGui W: Triptolide
inhibits transcription factor NF-κB and induces apoptosis of
multiple myeloma cells. Leuk Res. 29:99–105. 2005.
|
|
18.
|
Jiang XH, Wong BCY, Lin MCM, et al:
Functional p53 is required for triptolide-induced apoptosis and
AP-1 and NF-κB activation in gastric cancer cells. Oncogene.
20:8009–8018. 2001.PubMed/NCBI
|
|
19.
|
Lin J, Chen LY, Lin ZX and Zhao ML: The
effects of triptolide on apoptosis of glioblastoma multiforme (GBM)
cells. J Int Med Res. 35:637–643. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Zhou GX, Ding XL, Zhang H, et al:
Apoptosis of human pancreatic cells induced by triptolide. World J
Gastroenterol. 14:1504–1509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Lou YJ and Jin J: Triptolide
down-regulates bcr-abl expression and induces apoptosis in chronic
myelogenous leukemia cells. Leuk Lymphoma. 45:373–376. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Shi Y, Jin Y, Cheng H, et al: Triptolide
inhibits Bcr-Abl transcription and induces apoptosis in
STI571-resistant chronic myelogenous leukemia cells harboring T315I
mutation. Clin Cancer Res. 15:1686–1697. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Huang M, Zhang H, Jiu T, Tian D, Gu L and
Zhou M: Triptolide inhibits MDM2 and induces apoptosis in acute
lymphoblastic leukemia cells through a p53-independent pathway. Mol
Cancer Ther. 12:184–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yang M, Shen JK, Huang J, Du HP, Ma QL and
Jin J: Interleukin-6-independent expression of glucocorticoid
receptor is upregulated by triptolide in multiple myeloma. Leuk
Lymphoma. 50:802–808. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Jourdan M, Veyune JL, De Vos J, Redal N,
Couderc G and Klein B: A major role for Mcl-1 antiapoptotic protein
in the IL-6-induced survival of human myeloma cells. Oncogene.
22:2950–2959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Le Gouill S, Podar K, Harousseau JL and
Anderson KC: Mcl-1 regulation and its role in multiple myeloma.
Cell Cycle. 3:1259–62. 2004.PubMed/NCBI
|
|
27.
|
Le Gouill S, Podar K, Amiot M, et al: VEGF
induces Mcl-1 up-regulation and protects multiple myeloma cells
against apoptosis. Blood. 104:2886–2892. 2004.PubMed/NCBI
|
|
28.
|
Gomez-Bougie P, Wuillème-Toumi S, Ménoret
E, et al: Noxa up-regulation and Mcl-1 cleavage are associated to
apoptosis induction by bortezomib in multiple myeloma. Cancer Res.
67:5418–5424. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Fan F, Tonon G, Bashari MH, et al:
Targeting Mcl-1 for multiple myeloma (MM) therapy: drug-induced
generation of Mcl-1 fragment Mcl-1128-350 triggers MM
cell death via c-Jun upregulation. Cancer Lett. 28–Oct;2013.Epub
ahead of print.
|
|
30.
|
Leuenroth SJ and Crews CM:
Triptolide-induced transcriptional arrest is associated with
changes in nuclear substructure. Cancer Res. 68:5257–5266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Vispe S, DeVries L, Creancier L, et al:
Triptolide is an inhibitor of RNA polymerase I and II-dependent
transcription leading predominantly to down-regulation of
short-lived mRNA. Mol Cancer Ther. 8:2780–2790. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Wan CK, Wang C, Cheung HY, Yang M and Fong
WF: Triptolide induces Bcl-2 cleavage and mitochondria dependent
apoptosis in p53-dependent HL-60 cells. Cancer Lett. 241:31–41.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Pan J: RNA polymerase an important
molecular target of triptolide in cancer cells. Cancer Lett.
292:149–152. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Cai J and Jones DP: Mitochondrial redox
signaling during apoptosis. J Bioenerg Biomemb. 31:327–334. 1999.
View Article : Google Scholar
|
|
35.
|
Chauhan D, Hideshima T and Anderson KC:
Apaf-1/cytochrome c-independent and Smac-dependent induction
of apoptosis in multiple myeloma cells. J Biol Chem.
276:24453–24456. 2001.PubMed/NCBI
|
|
36.
|
Chauhan D, Li G, Hideshima T and Anderson
KC: JNK-dependent release of mitochondrial protein, Smac, during
apoptosis in multiple myeloma cells. J Biol Chem. 278:17593–17596.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Chauhan D, Li G, Hideshima T and Anderson
KC: 2-methoxyestradiol overcomes drug resistance in multiple
myeloma cells. Blood. 100:2187–2194. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Simon HU, Haj-Heyia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Hsu MJ, Sheu JR, Lin CH, Shen MY and Hsu
CY: Mitochondrial mechanisms in amyloid beta peptide-induced
cerebrovascular degeneration. Biochim Biophys Acta. 1800:290–296.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Monteiro JP, Oliveira PJ and Jurado AS:
Mitochondrial membrane lipid remodeling in pathophysiology: A new
target for diet and therapeutic interventions. Prog Lipid Res.
52:513–528. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Mann J: Natural products in cancer
chemotherapy: past, present and future. Nat Rev Cancer. 2:143–148.
2002. View
Article : Google Scholar : PubMed/NCBI
|