Introduction
Until recently, cancer stem cells (CSCs) have been
identified in multiple solid tumors, represented by
CD44+/CD24− breast cancer cells and
CD133+ brain tumor cells (1–3).
However, the ways in which CSCs are maintained and regulated
remains to be discovered. Increasing evidence demonstrates that
CSCs are protected and regulated by a specialized tumor
microenvironment niche, which plays a crucial role in the
maintenance of the CSC biological properties, including
self-renewal, differentiation, invasion, metastasis, therapeutic
resistance, and genetic instability (4–6).
Although the CSC niche is still poorly understood, components of
the niche have been shown to dominate CSC maintenance and one of
the most important components is hypoxia.
Similar to the ways in which hypoxia maintains the
physiological functions of normal stem cells (7), recent advances have shown that
hypoxic stress also plays a critical role in the maintenance of
CSCs in solid tumors (8–10). Research into breast cancer shows
that hypoxic tumors induced by anti-angiogenic agents contain a
significantly higher percentage of CSCs (11), and prostate cancer data indicate
that prostate cancer cells under hypoxic conditions possess greater
stem-like properties (12).
Ovarian cancer cells under hypoxia upgrade their stem-like
properties through the upregulation of stemness-related factors and
behave more aggressively when returned to a higher oxygen
environment (13). Similarly,
hypoxia maintains the undifferentiated state of primary glioma
cells, slows down their growth to a relatively quiescent stage,
increases their colony forming efficiency and migration, and
elevates the expression of stem cell markers (14).
As one of the most common malignancies of the head
and neck region, laryngeal cancer is frequently encountered with
hypoxic stress. Our previous investigations have identified CD133
as one of the CSC markers of laryngeal cancer, and demonstrated
that CD133+ laryngeal cancer cells harbored more
stem-like properties than CD133− subpopulation (15–17),
indicating that laryngeal cancer also contains a CSC subpopulation.
However, whether hypoxia can regulate the stem-like biological
properties of laryngeal cancer cells remains unknown; therefore, we
investigated the influence of hypoxia on the stemness of laryngeal
cancer cells. Our findings demonstrate that hypoxia upgrades the
stem-like biological properties of laryngeal cancer cell lines by
increasing the CD133+ stem cell fraction, and the most
likely mechanism is discussed.
Materials and methods
Cell lines and cell culture
The human laryngeal cancer cell lines, Hep-2 and
AMC-HN-8, were obtained from the Cell Bank of Type Culture
Collection at the Chinese Academy of Sciences (CBTCCCAS, Shanghai,
China). Unless otherwise noted, the cells were maintained in
Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Grand
Island, NY, USA), supplemented with 10% fetal bovine serum (FBS)
(Invitrogen) and 1% penicillin/streptomycin (Invitrogen). For
normoxic cell culture, the cells were maintained in a humidified
incubator in an atmosphere of 21% O2, 5% CO2,
and 74% N2 at 37°C. For hypoxic cell culture, the ProOx
C21 hypoxia cell culture chamber (BioSpherix, Lacona, NY, USA) was
used to obtain the required atmosphere of 1% O2, 5%
CO2, and 94% N2 at 37°C.
Flow cytometry
The Hep-2 and AMC-HN-8 cells cultured at 21% or 1%
O2 for 24 h were harvested and fixed dropwise with 70%
ice-cold ethanol overnight at 4°C. Then, RNase (Kayon, Shanghai,
China) was added to the samples for a final concentration of 20
μg/ml, and incubated for 30 min at 37°C. This was followed
by the addition of propidium iodide (Sigma, St. Louis, MO, USA) for
a final concentration of 15 μmol/l. The samples were
filtered to acquire single-cell suspensions, and analyzed using an
Aria II flow cytometer (Becton Dickinson, San Jose, CA, USA).
CD133 marker detection
The Hep-2 and AMC-HN-8 cells cultured at 21% or 1%
O2 for 48 h were harvested, and the
phycoerythrin-conjugated CD133/1 (AC133) antibody (Miltenyi
Biotech, Gladbach, Germany) was used to label CD133, since our
previous investigations identified CD133 as one of the laryngeal
CSC surface markers in the Hep-2 cell line (15–17).
The incubation followed the protocol described by Chen et al
(17), and the mouse IgG1 (κ)
(eBioscience, San Diego, CA, USA) incubated under the same
condition served as the isotypic control to exclude nonspecific
staining. Samples were analyzed using the Epics Altra flow
cytometer (Beckman Coulter, Fullerton, CA, USA).
Quantitative real-time polymerase chain
reaction (qRT-PCR)
At the time points of 6, 12, 24, 48 and 72 h, the
total RNA was extracted from the Hep-2 and AMC-HN-8 cells
maintained at 1% O2 by using the TRIzol® reagent
(Invitrogen). Reverse transcription was performed using the
PrimeScript® RT reagent kit with the gDNA Eraser
(Takara, Dalian, China), and the qRT-PCR was done with the
SYBR® Premix Ex Taq™ kit (Takara) in a Lightcycler 480
instrument (Roche Diagnostics, Rotkreuz, Switzerland). Thermal
cycling included an initial denaturation at 95°C for 30 sec,
followed by 40 cycles at 95°C for 5 sec and 60°C for 30 sec. Cells
maintained at 21% O2 served as a control, while β-actin
(ACTB) was used as an endogenous control. The formula
2−ΔΔCT was used to calculate the relative mRNA
expression of 1% O2 vs. 21% O2. The primers
used for amplification are listed in Table I.
 | Table I.Primer sequences used for qRT-PCR and
chromosomal location. |
Table I.
Primer sequences used for qRT-PCR and
chromosomal location.
| Gene | Primer Sequence
(5′→3′) | Accession no. | Product (bp) | Location |
|---|
| OCT4 | F:
GTATTCAGCCAAACGACCAT | NM_001173531 | 100 | 6p21.31 |
| R:
CTTCCTCCACCCACTTCT | | | |
| SOX2 | F:
TGTCAAGGCAGAGAAGAG | NM_003106 | 223 | 3q26.3-q27 |
| R:
AGAGGCAAACTGGAATCA | | | |
| NANOG | F:
CTATAACTGTGGAGAGGAAT | NM_024865 | 124 | 12p13.31 |
| R:
AGTGGTCTGCTGTATTAC | | | |
| HIF-1α | F:
AGTGTACCCTAACTAGCCGAGGAA | NM_001243084 | 113 | 14q23.2 |
| R:
CTGAGGTTGGTTACTGTTGGTATCA | | | |
| HIF-2α | F:
ATGGTAGCCCTCTCCAACAAG | NM_001430 | 134 | 2p21-p16 |
| R:
AGGTTCTTCATCCGTTTCCAC | | | |
| ACTB | F:
TGACGTGGACATCCGCAAAG | NM_001101 | 205 | 7p22 |
| R: CTGGAA
GTGGACAGCGAGG | | | |
Western blotting
The Hep-2 and AMC-HN-8 cells maintained at 1%
O2 were harvested at 6, 12, 24, 48, and 72 h, while the
cells maintained at 21% O2 served as the control.
Approximately 30 μg of total protein from the lysates of
Hep-2 and AMC-HN-8 cells was analyzed by electrophoresis using
sodium dodecyl sulfate-polyacrylamide gel (Beyotime, Shanghai,
China), electrotransferred to polyvinylidene fluoride membranes
(Millipore, Billerica, MA, USA), and probed overnight with primary
antibodies (Epitomics, Burlingame, CA, USA) for OCT4 (1:1,000;
2876-1), SOX2 (1:1,000; 2683-1), NANOG (1:1,000; 3369-1), HIF-1α
(1:1000; 2015-1), and HIF-2α (1:1,000; 8551-1). Horseradish
peroxidase-conjugated goat anti-rabbit IgG (H+L) (1:10,000)
(Jackson ImmunoResearch, West Grove, PA, USA) was then applied,
followed by signal detection using BeyoECL Plus (Beyotime). The
ACTB antibody (1:5,000; R1207-1) (HuaAn Biotechnology, Hangzhou,
China) was used to normalize the amount of sample loaded.
Immunofluorescence staining
The Hep-2 and AMC-HN-8 cells maintained at 21%
O2 were harvested and replanted onto glass coverslips,
and when they adhered to the dish, they were maintained at 21% or
1% O2 for 48 h. The cells were then fixed with 4%
paraformaldehyde for 20 min, and incubated in 1% bovine serum
albumin/10% normal goat serum/0.5% Triton X-100 (Boster, Wuhan,
China) at room temperature for 40 min to permeabilize the cells and
block nonspecific interactions, followed by incubation with the
primary antibodies (Epitomics) of OCT4 (1:250), SOX2 (1:100), and
NANOG (1:100) overnight at 4°C. After being rinsed in phosphate
buffered saline (PBS), the cells were incubated in fluorescein
isothiocyanate-conjugated goat anti-rabbit IgG (H+L) (1:100)
(Jackson ImmunoResearch) in dark conditions for 1 h. Following
this, 4’,6-diamidino-2-phenylindole (DAPI) (Boster) was used to
stain the cell nuclei.
Proliferation assay
The cell counting kit-8 (CCK-8) (Dojindo
Laboratories, Kumamoto, Japan) was used to evaluate the cell
proliferation according to the manufacturer’s instructions. To
assess the influence of hypoxia on proliferation, Hep-2 and
AMC-HN-8 cells were cultured under 1% O2 for 48 h. The
cells were then re-harvested and seeded in a 96-well plate at a
density of 2,000 cells/well in 0.2 ml of DMEM, supplemented with
10% FBS at 21% O2 for 120 h. At 0, 24, 48, 72, 96 and
120 h after seeding, the cells were tested for their proliferation
capacities using CCK-8. The results were compared with the cells
maintained consistently at 21% O2, all the CCK-8
incubations lasted 3.5 h at 37°C. Each sample in the group was
repeated in 6 wells, while the medium alone (without cells) served
as the blank control. The ultraviolet absorbance was measured at
450 nm.
Matrigel invasion assay
The invasion assay was performed using 24-well
transwell chambers (pore size, 8 μm) (Corning Inc., Corning,
NY, USA) coated with Matrigel (BD Biosciences, Bedford, MA, USA)
(1:8 dilution in serum-free medium). After being maintained under
1% O2 for 48 h, harvested 2×104 Hep-2 and
AMC-HN-8 cells were suspended in 200 μl of serum-free DMEM
and plated in the upper chamber. The lower chamber was filled with
DMEM (600 μl) supplemented with 10% FBS, and the plates were
then incubated at 21% O2 for 24 h. The cells on the top
membrane surface were removed with a cotton swab, and those cells
that migrated through the Matrigel were fixed with methanol for 15
min, stained with 0.1% crystal violet for 15 min, rinsed with PBS,
and counted by microscopy. Ten low power fields were randomly
selected for counting, and the cells maintained consistently at 21%
O2 served as the control.
Sphere formation assay
The Hep-2 and AMC-HN-8 cells, after being maintained
under 1% O2 for 48 h, were harvested and seeded at a
density of 5,000 cells/ml at 21% O2 using the StemPro
NSC SFM kit (A10509-01) (Invitrogen), with serum-free medium
containing DMEM/F12, 20 ng/ml fibroblast growth factor, and 20
ng/ml epidermal growth factor. In addition, 1% 200 mmol/l
L-glutamine (25,030) (Invitrogen) was added. Ten days later, the
spheres (containing over 30 cells) were counted under a
phase-contrast microscope. Sphere formation efficiency was
calculated using the formula: sphere number/seeded cell number
×100%. The results were compared with the cells maintained
consistently at 21% O2.
Colony forming assay
After being maintained in 1% O2 for 48 h,
the Hep-2 and AMC-HN-8 cells were harvested and seeded in 6-well
plates at a density of 300 cells/well. The plates were then placed
in an incubator with 21% O2 for two weeks, after which
the samples were washed twice with PBS, fixed in methanol for 15
min, and stained with 0.1% crystal violet for 15 min. The dye was
gently washed out with PBS, and the clones containing over 50 cells
were counted. Colony formation efficiency was calculated using the
formula: colony number/300 ×100%. The cells maintained consistently
at 21% O2 served as a control.
Statistical analysis
Data are reported as mean ± standard deviation (SD)
of at least three independent experiments. The GraphPad Prism
software version 5.00 for Windows (GraphPad Software, San Diego,
CA, USA) was used for data analysis. The statistical analysis was
performed using the Student’s t-test, and a p-value of <0.05 was
considered to be statistically significant.
Results
Influence of hypoxia on the cell
cycle
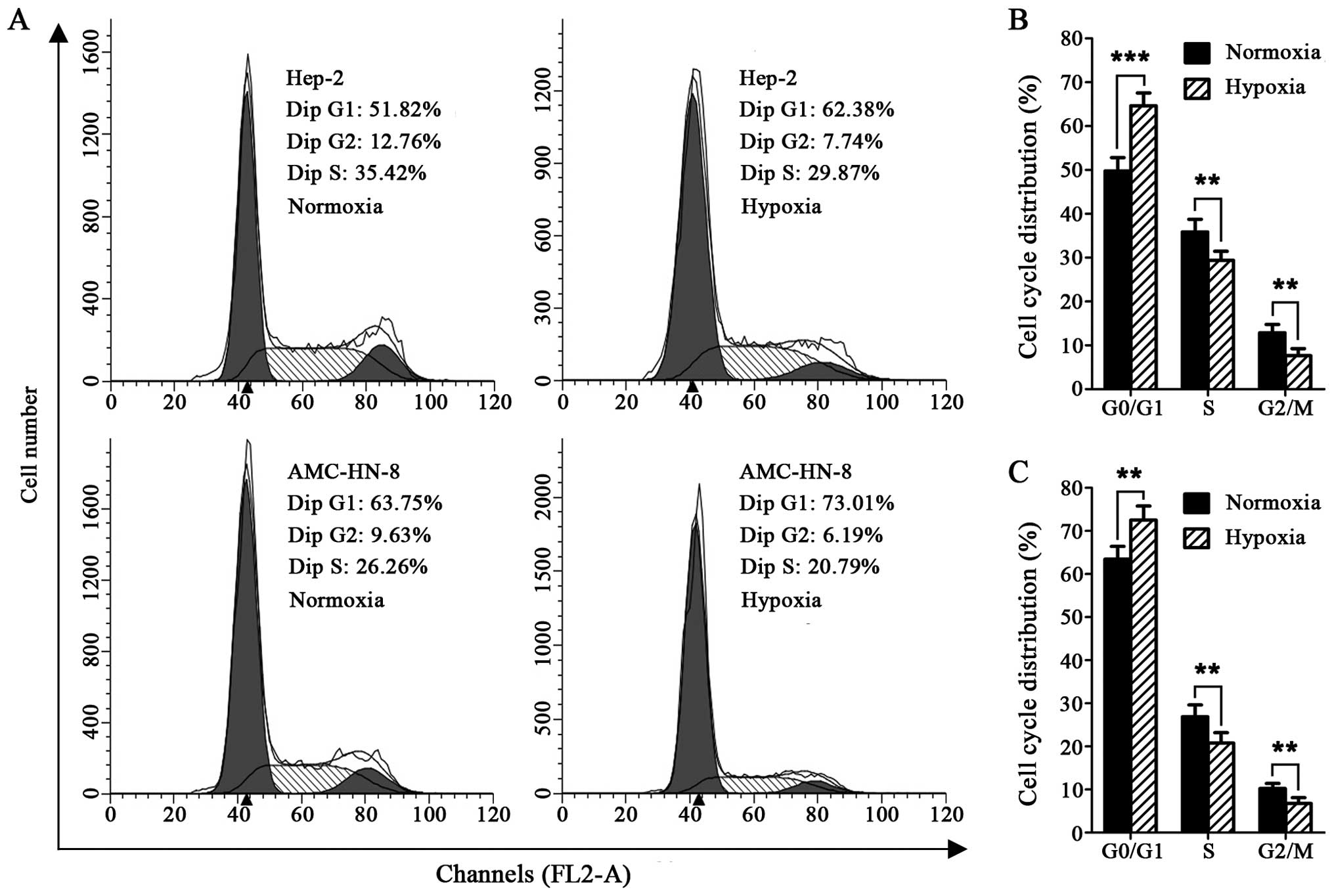
The cell cycle detection showed that exposure to 1%
O2 for 24 h resulted in significantly expanded G0/G1
phase cells in the Hep-2 and AMC-HN-8 cells, compared with the
cells maintained at 21% O2, whereas, the cells in the S
and G2/M phases were markedly reduced (Fig. 1A–C).
Preferential expression of laryngeal CSC
marker under hypoxia
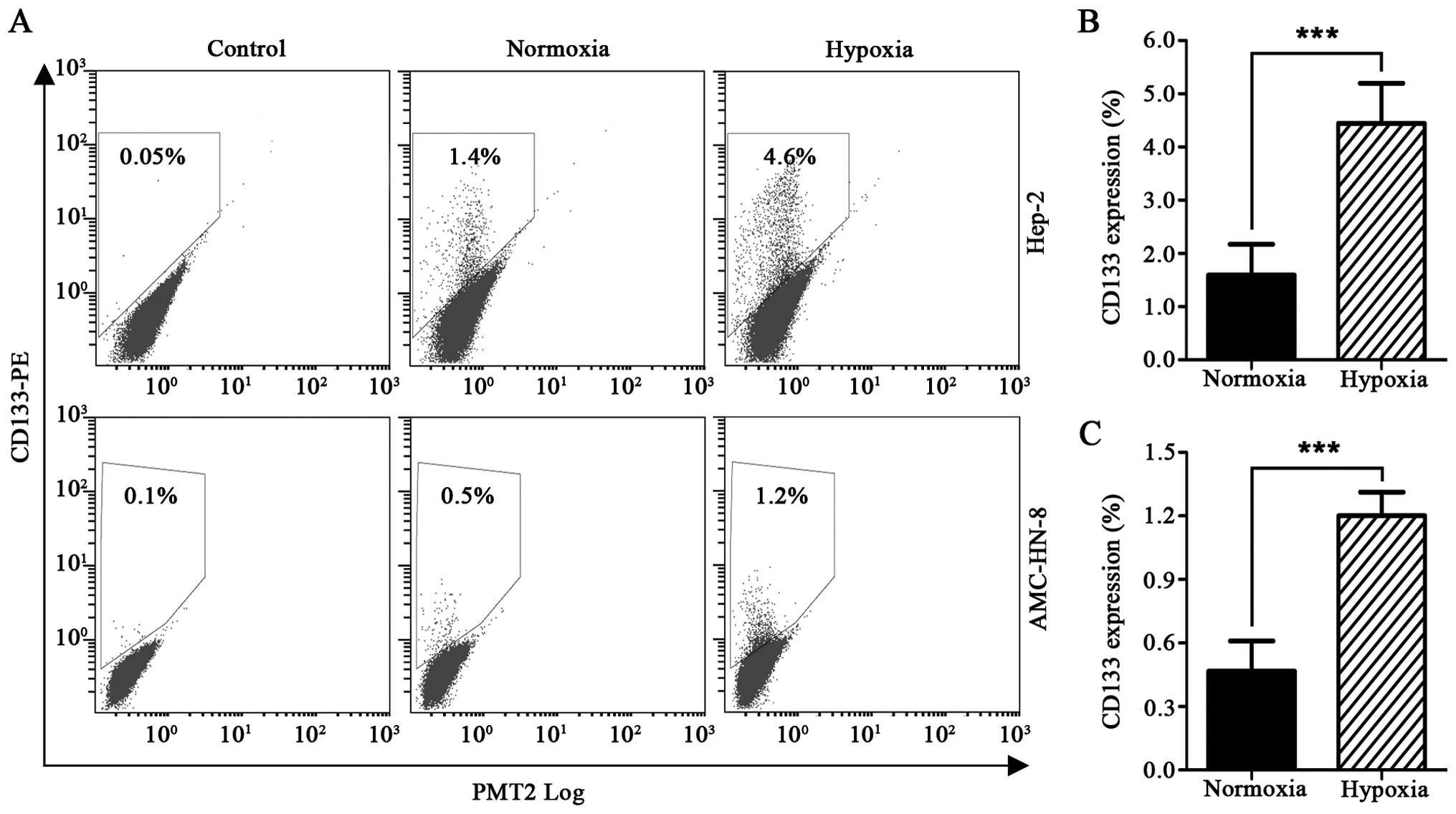
Detection by flow cytometry revealed that exposure
to 1% O2 for 48 h resulted in a significantly increased
percentage of CD133+ Hep-2 cells, from 1.59±0.58% to
4.45±0.75%. The percentage of CD133+ AMC-HN-8 cells also
increased from 0.47±0.14% to 1.20±0.11% (Fig. 2A–C).
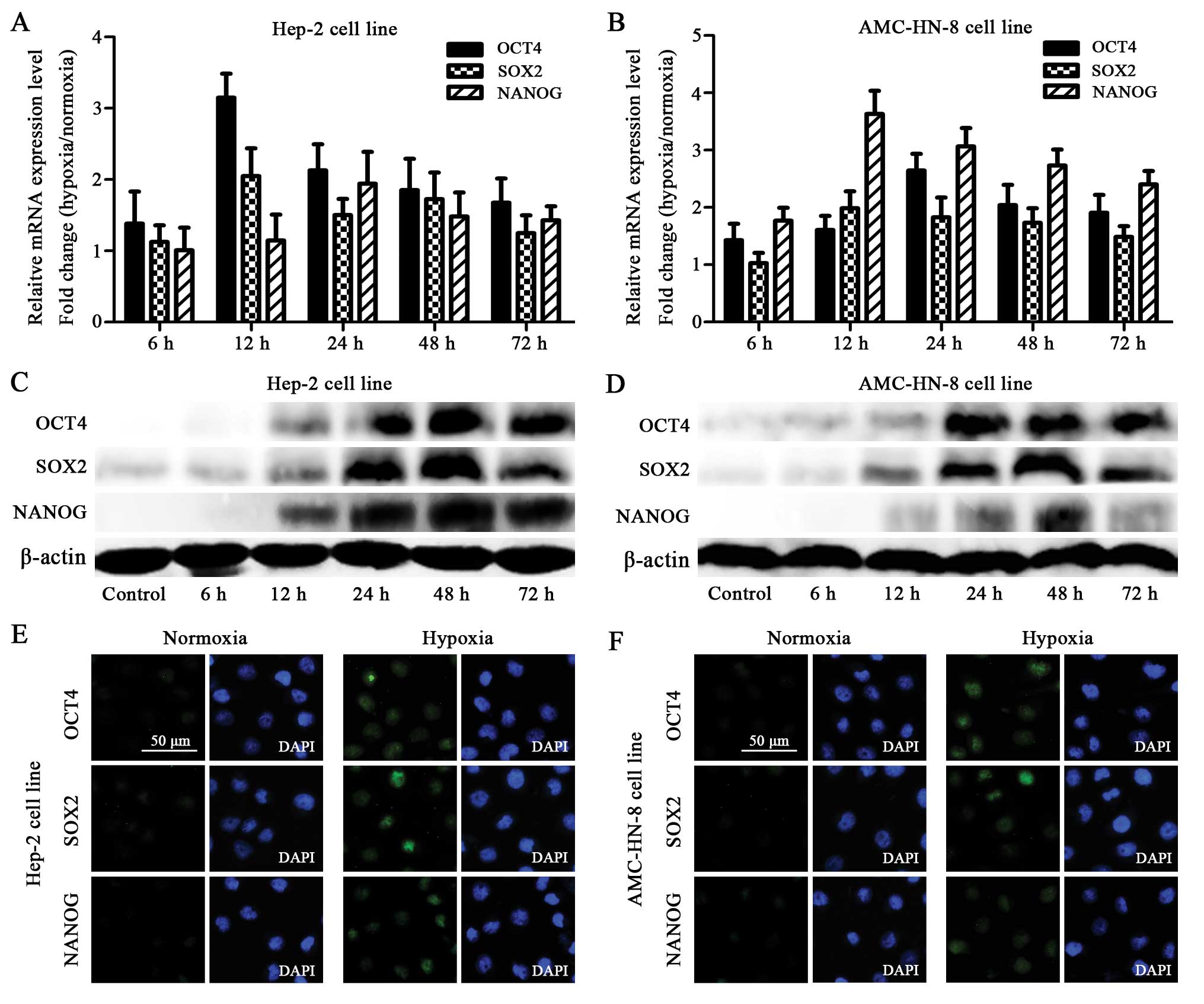
Upregulated mRNA level of stem cell genes
under hypoxia
The qRT-PCR assay showed that the Hep-2 and AMC-HN-8
cells manifested an increased mRNA expression of OCT4, SOX2 and
NANOG after being exposed to 1% O2 for 6 h. The mRNA
expression reached the highest level at 12 or 24 h (Fig. 3A and B).
Elevated protein level of stem cell genes
under hypoxia
The increased mRNA expression of OCT4, SOX2 and
NANOG in the hypoxia-treated cells was confirmed using the western
blot. Although the hypoxia-treated cells showed gradually increased
protein levels starting at 12 h, striking differences were observed
at 24 h, which was delayed compared to the mRNA expression.
Furthermore, most of the proteins reached the highest expression
level at 48 h, which was also delayed when compared with the
highest mRNA level (Fig. 3C and
D).
Enhanced immunochemical staining of stem
cell genes under hypoxia
The protein expression of OCT4, SOX2 and NANOG was
also detected by immunocytochemistry. The Hep-2 and AMC-HN-8 cells
treated with hypoxia for 48 h showed enhanced staining of OCT4,
SOX2 and NANOG (to different extents) compared to the normoxia
treated control cells (Fig. 3E and
F).
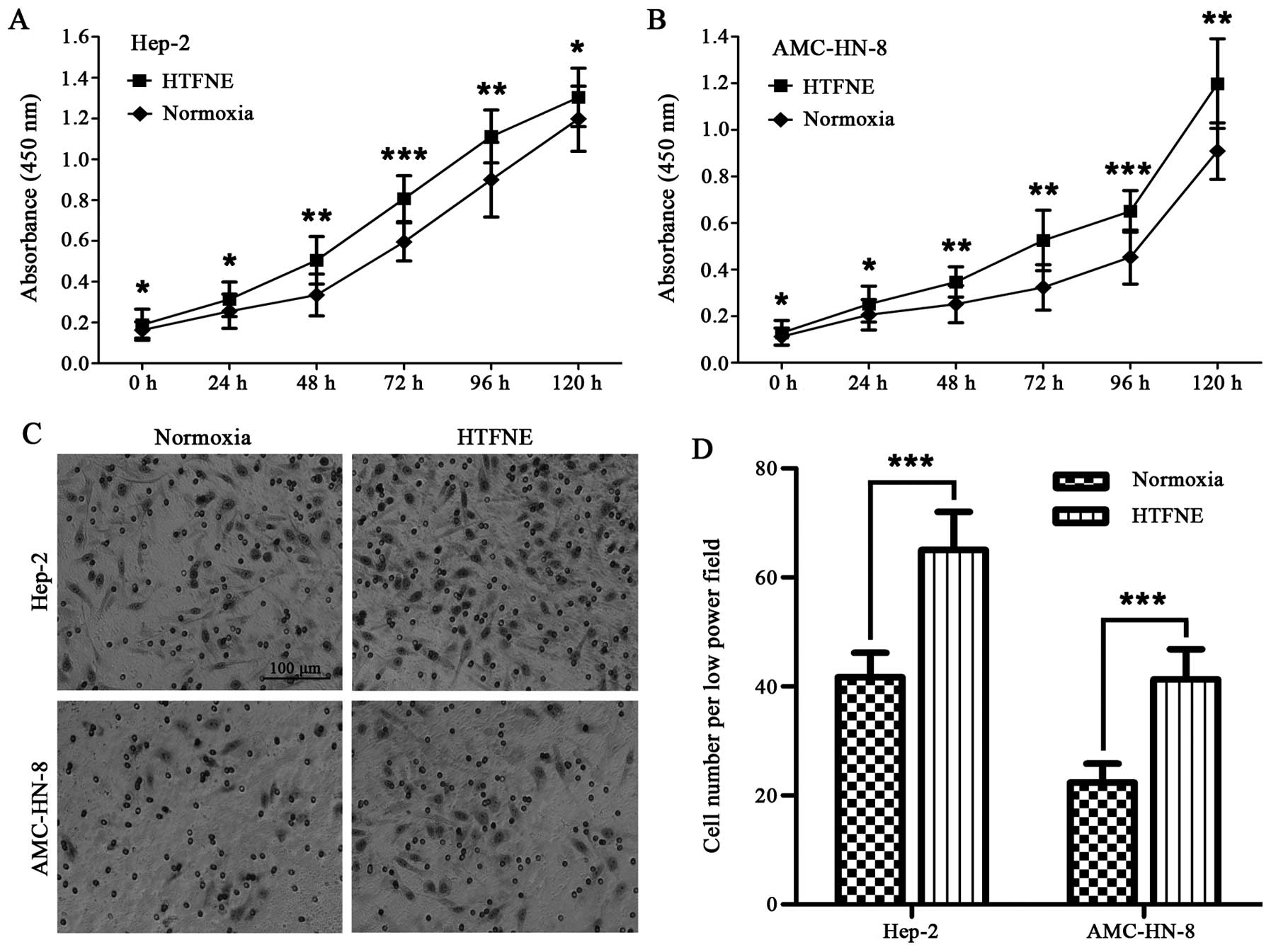
Hypoxia treatment followed by normoxia
exposure (HTFNE) exhibits upgraded proliferation and invasion
capacity
Although the Hep-2 and AMC-HN-8 cells maintained
consistently under hypoxia grew slower than those maintained under
normoxia in the first 48 h, the hypoxia-treated cells followed by
normoxia exposure grew faster than the control cells cultured
consistently under normoxia (Fig. 4A
and B). The transwell assay showed that both the
hypoxia-treated Hep-2 and AMC-HN-8 cells, when brought back to
normoxia, had a significantly increased number of cells migrating
across the Matrigel compared to the control cells (Fig. 4C and D).
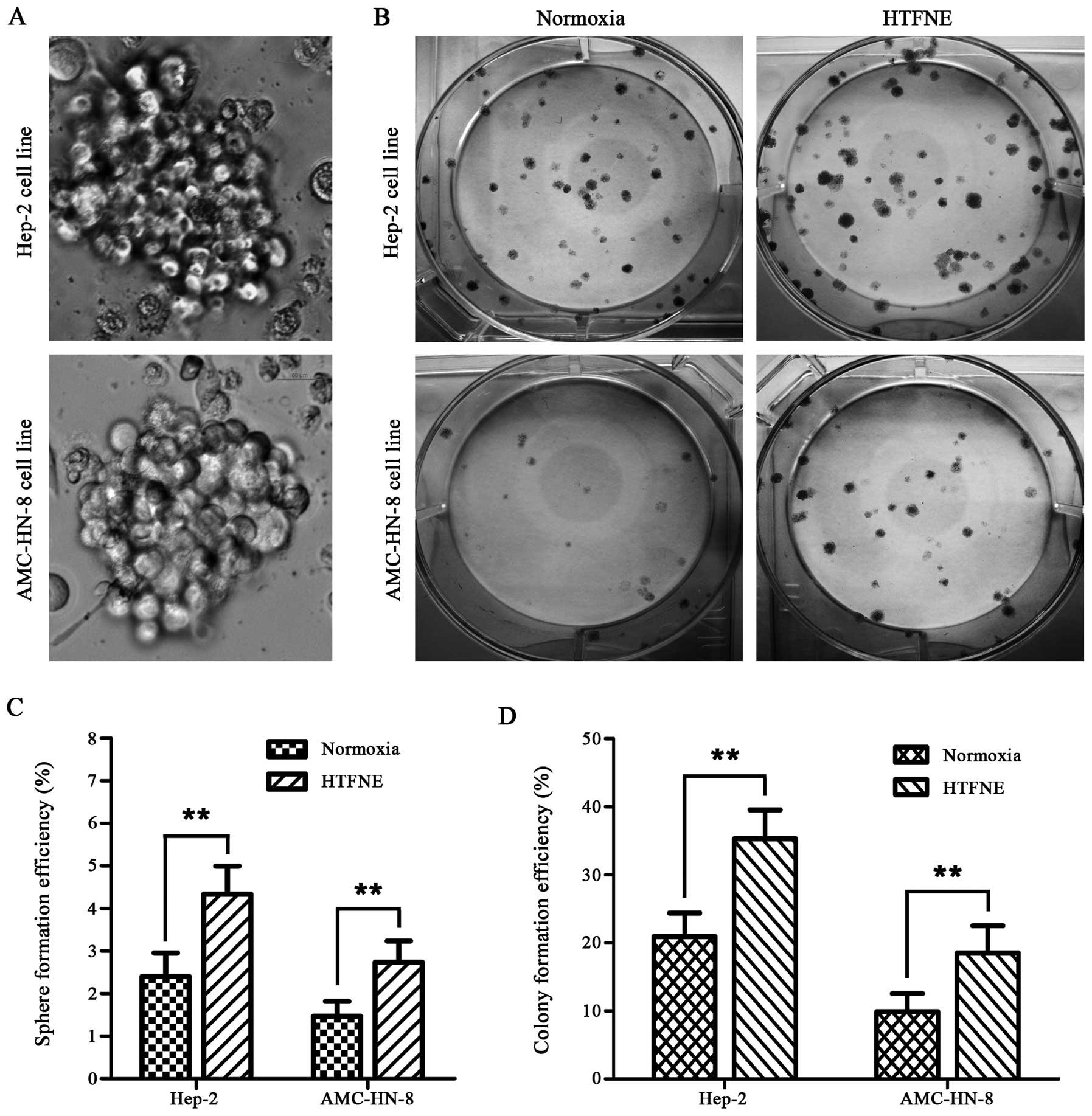
HTFNE presents enhanced sphere and colony
formation capability
In the sphere formation assay, when compared with
the cells maintained under normoxia, more spheres were observed in
both the hypoxia treated cell lines followed by normoxia exposure
(Fig. 5A and C). Furthermore, the
hypoxia treated cells, when brought back to normoxia, formed more
colonies than the control cells, with a 1.7-fold increase in the
Hep-2 cells and a 1.9-fold increase in the AMC-HN-8 cells,
respectively (Fig. 5B and D).
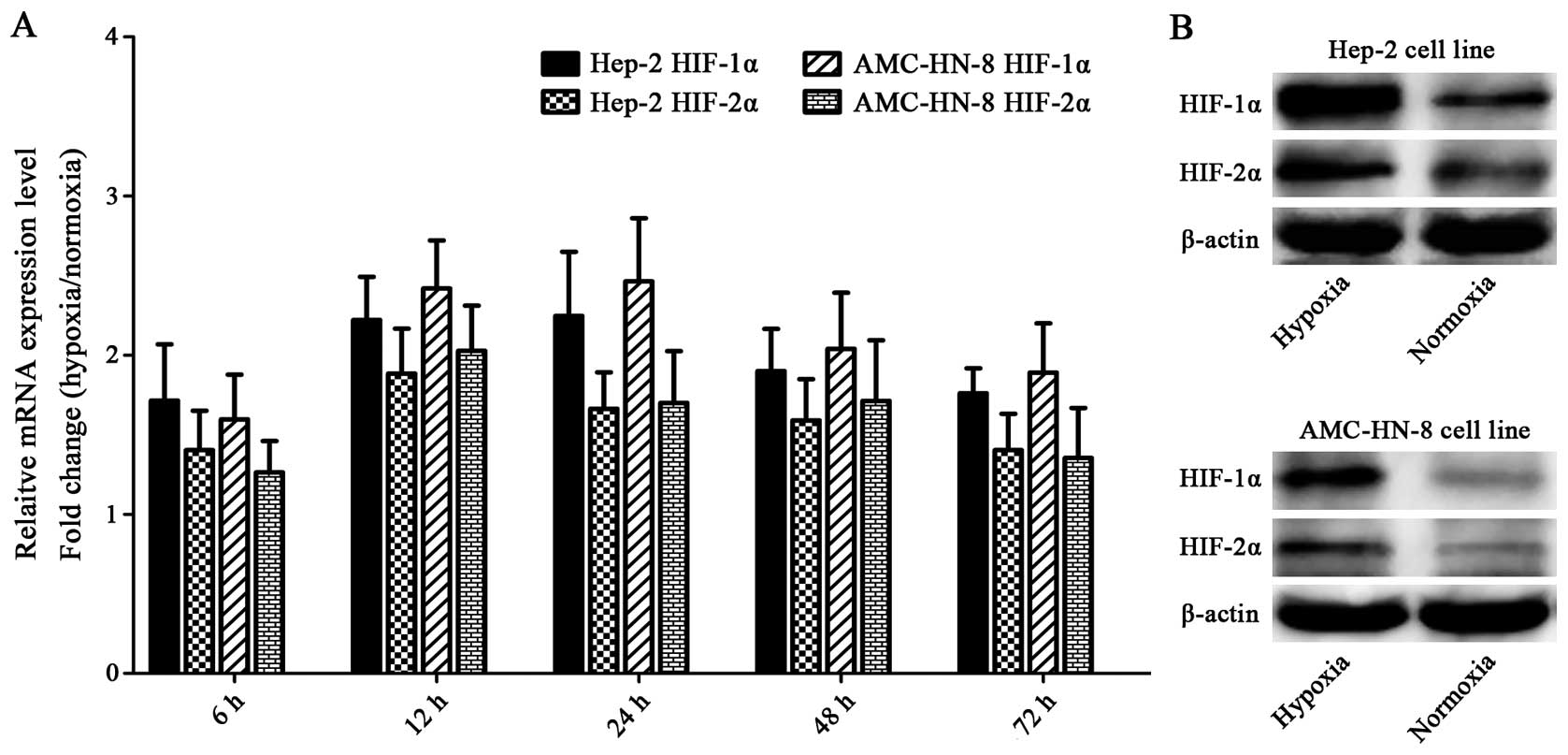
Hypoxia upregulates HIFs
The Hep-2 and AMC-HN-8 cells manifested increased
mRNA expressions of HIF-1α and HIF-2α after being exposed to
hypoxia for 6 h, and the mRNA expression reached the highest level
at 12 or 24 h. Both of the two cell lines cultured under hypoxia
for 48 h showed significantly enhanced protein levels of HIF-1α and
HIF-2α compared with the control cells. The elevation of HIF-1α was
greater than that of HIF-2α in both mRNA and protein levels
(Fig. 6).
Discussion
It is widely accepted that cancer cells are hypoxic
in solid tumors when compared to normal tissue because the
micro-circulation is compromised and tissue oxygenation is not
regulated according to metabolic demand. Hypoxia is an inherent
feature of solid tumors, and when the tumor volume reaches 2 ml,
the oxygen tension could be close to 0 mmHg (18). Recent studies show that hypoxia, as
a critical component of the tumor microenvironment, can directly
regulate the stem-like properties of CSCs in ways such as
self-renewal, differentiation, migration and invasion, colony and
sphere formation, and therapeutic resistance (12–14,19).
In this study, we investigated whether hypoxia is able to regulate
the stem-like biological properties of laryngeal cancer cells and
discussed the possible mechanisms involved.
The transient and long-term quiescence (G0/G1 phase
in cell cycle) are generally believed to be fundamental features of
stem cells (20). On the basis of
this premise, we analyzed the cell cycle distribution of the cells
cultured under hypoxia, and found that both the Hep-2 and AMC-HN-8
cell lines exhibited significantly expanded G0/G1 phases and
reduced S and G2/M phases after being exposed to hypoxia for 24 h
(Fig. 1A–C), consistent with the
previous studies that hypoxia can invert the vigorously divided
cancer cells into a quiescent state (21,22),
which is one of the inherent features of CSCs.
We subsequently focused on the expression of CD133,
the CSC marker of laryngeal cancer cells identified in our previous
investigations (15–17), in the hypoxia-exposed cancer cells.
We found that exposure to 1% O2 for 48 h resulted in
increased CD133 expression, ∼2.8-fold increase in the Hep-2 cell
line and 2.5-fold increase in the AMC-HN-8 cell line (Fig. 2A–C), in line with the previous
studies on pancreatic cancer (23), glioma (24), and ovarian cancer (13). The hypoxia-induced expansion of
CD133+ CSC subpopulation may reasonably explain the
enhanced stem-like properties of laryngeal cancer cells in the
subsequent investigation.
Evidence from different experimental systems has
shown that some transcriptional factors are critical participants
in the regulation of self-renewal and differentiation of stem
cells, such as OCT4 (25), SOX2
(26), and NANOG (27), and manipulating the expression of
OCT4 and SOX2 contribute to the induction of pluripotent stem cells
from differentiated mouse/human fibroblasts (28,29).
In addition, hypoxia is reported to influence the expression of a
panel of stem cell transcriptional factors (30). In this study, we also found that
the Hep-2 and AMC-HN-8 cells began to show increased mRNA
expression of OCT4, SOX2 and NANOG after being exposed to 1%
O2 for 6 h, and the mRNA expression reached the highest
level at 12 or 24 h (Fig. 3A and
B). Furthermore, we verified the upregulation of these three
genes by using the western blot and immunofluorescence staining. In
the western blot assay, we found a striking elevation in protein
expression at 24 h and the highest protein level at 48 h, both of
which were delayed compared to the changes at the mRNA level
(Fig. 3C and D). We also found
enhanced immunofluorescence staining of OCT4, SOX2 and NANOG (to
different extents) compared with the control cells (Fig. 3E and F). These findings were in
agreement with previous studies (12–14),
indicating that the influence of hypoxia on the stem-like property
may be exerted through changes in the expression of these stem cell
transcriptional factors.
Since hypoxia influenced the stem cell phenotypes,
such as the quiescent status and expression of stem cell genes and
laryngeal CSC marker, we then focused on whether hypoxia could
influence the stem-like biological behavior of these cell lines. To
our disappointment, we found that these two cell lines maintained
consistently under hypoxia did not exhibit significantly enhanced
stem-like biological properties, such as proliferation, invasion,
and sphere/colony formation (data not shown), than the cells
maintained consistently under normoxia. Such phenomenon was also
reported by Liang et al in ovarian cancer research; however,
they further observed that hypoxia-treated ovarian cancer cells
behave more aggressively when brought back to higher oxygen
environment (13). Enlightened by
their findings, we then investigated whether hypoxia treatment
followed by normoxia exposure could help us visualize the enhanced
biological stemness induced by hypoxia.
In the proliferation assay, we found that the
hypoxia treated laryngeal cancer cells, when brought back to
normoxia, propagated significantly faster compared to the cells
maintained under normoxia (Fig. 4A and
B). Additionally, we observed that when brought back to
normoxia, the hypoxia treated laryngeal cancer cells exhibited
significantly higher capacity for invasion (Fig. 4C and D) and colony and sphere
formation (Fig. 5) when compared
to the cells maintained consistently under hypoxia. These results
indicated that hypoxia did exert an important influence on the
stem-like properties of laryngeal cancer cells. However, the
enhanced stemness may be classified into two categories: the first
one is static, such as expanded G0/G1 phase and upregulated CSCs
marker and stem cell genes, which can be directly measured by using
cells cultured consistently under hypoxia; the second one is
dynamics, such as enhanced stem-like biological behavior of
proliferation, invasion and sphere/colony formation, which can only
be visualized by bringing the hypoxia-cultured cells back to
normoxia. This characteristic is reminiscent of tumor metastasis,
where invasive cancer cells migrated into the blood vessel (sudden
exposure to higher oxygen stress compared with the hypoxic tumor
microenvironment), and may exhibit more aggressive biological
properties.
Since we have verified that hypoxia upregulates the
stem cell transcriptional factors (OCT4, SOX2 and NONOG) of Hep-2
and AMC-HN-8 cells to different extent, which may contribute to the
expansion of CD133+ laryngeal CSC subpopulation because
a recent study revealed that hypoxia-induced OCT4 and SOX2
upregulate CD133 expression by directly binding to the P1 promoter
(31). Next we investigated the
possible mechanism whereby hypoxia regulated these transcriptional
factors. A previous study shows that HIF-2α specifically regulates
the expression of OCT4 by directly binding to its promoter, which
identifies OCT4 as a HIF-2α-specific target gene (32). In contrast, there are still no
reports suggesting that SOX2 and NANOG are direct (or indirect)
HIFs targets, although it is certainly possible. However, it has
been shown that it is through HIFs that hypoxia induces the
expression of pluripotent stem cell inducers, OCT4, NANOG, SOX2,
KLF4 and MYC, in 11 cancer cell lines, although the exact mechanism
remains to be clarified (33),
indicating that NANOG and SOX2 are also direct or indirect target
genes of HIFs. Therefore, next we investigated the expression
changes of HIFs.
We found that the expression of both HIF-1α and
HIF-2α was elevated in Hep-2 and AMC-HN-8 cells upon hypoxia
exposure, and the elevation of HIF-1α was greater than that of
HIF-2α (Fig. 6), indicating that
although both HIF-1α and HIF-2α are involved in the regulation of
laryngeal CSCs, HIF-1α may dominate. These findings may be helpful
in explaining the relevant results from our recent study (34) where HIF-1α was highly expressed in
laryngeal cancer tissues and significantly related to the clinical
stage and lymph node metastasis, a possible explanation may be that
advanced and metastatic cancer tissues contained a larger
proportion of CSCs.
However, the relative importance of HIF-1α and
HIF-2α in CSC maintenance varies in different cancer types. It has
been reported that HIF-2α maintains the undifferentiated and
aggressive phenotype of neuroblastoma CSCs (35). However, Soeda et al showed
that it is mainly through HIF-1α that hypoxia regulates the in
vitro self-renewal and differentiation of glioma CSCs (24). Although much remains to be
clarified, our findings solidify the central concept that
hypoxia-induced HIFs contribute to the maintenance of the CSC
phenotype by directly or indirectly regulating the stem cell
transcriptional factors.
Since hypoxia does not occur early in tumors, the
hypoxia-induced influence on cancer cells often results in poor
prognosis for cancer patients and resistance to radiotherapy and
chemotherapy, which has been validated by increasing studies
(36–38). However, the exact mechanism may
vary in different cancer types and remains to be clarified.
In conclusion, our observations demonstrate that the
hypoxic microenvironment may upgrade the stem-like biological
properties of laryngeal cancer cell lines by increasing the
CD133+ stem cell fraction. The most possible mechanism
may be the HIFs-induced upregulation of OCT4, SOX2 and NANOG, which
then directly bind to the CD133 promoter 1 and induce its
expression. This hypothesis needs further clarification.
Furthermore, the hypoxia-treated laryngeal cancer cells, which
acquire enhanced stemness, behave more aggressively when brought
back to normoxia. However, the study of hypoxia-induced influence
on laryngeal CSCs relatively lags behind other solid tumors, such
as prostate cancer and breast cancer, and we are still in the early
stages of understanding the exact mechanism by which hypoxia
maintains and regulates the laryngeal CSCs. Further studies are
needed to accelerate the pace of research on hypoxia-regulated CSCs
in laryngeal cancer.
Acknowledgements
We would like to thank Yan-Ping Zhang
for her assistance in the molecular biology experiments. This study
was partially funded by grants from the Shanghai Science and
Technology Foundation of China (12JC1402101) and the Ming Dao
Doctoral Sustentation Fund of Fudan University.
References
|
1.
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Akhtar K, Bussen W and Scott SP: Cancer
stem cells - from initiation to elimination, how far have we
reached? (Review). Int J Oncol. 34:1491–1503. 2009.PubMed/NCBI
|
|
3.
|
Bhaijee F, Pepper DJ, Pitman KT and Bell
D: Cancer stem cells in head and neck squamous cell carcinoma: a
review of current knowledge and future applications. Head Neck.
34:894–899. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sneddon JB and Werb Z: Location, location,
location: the cancer stem cell niche. Cell Stem Cell. 1:607–611.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Borovski T, De Sousa E, Melo F, Vermeulen
L and Medema JP: Cancer stem cell niche: the place to be. Cancer
Res. 71:634–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Takakura N: Formation and regulation of
the cancer stem cell niche. Cancer Sci. 103:1177–1181. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sun QJ, Li XM, Lu XY and Di B: Cancer stem
cells may be mostly maintained by fluctuating hypoxia. Med
Hypotheses. 76:471–473. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Lin Q and Yun Z: Impact of the hypoxic
tumor microenvironment on the regulation of cancer stem cell
characteristics. Cancer Biol Ther. 9:949–956. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Hill RP, Marie-Egyptienne DT and Hedley
DW: Cancer stem cells, hypoxia and metastasis. Semin Radiat Oncol.
19:106–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Conley SJ, Gheordunescu E, Kakarala P, et
al: Antiangiogenic agents increase breast cancer stem cells via the
generation of tumor hypoxia. Proc Natl Acad Sci USA. 109:2784–2789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Ma YY, Liang DM, Liu J, et al: Prostate
cancer cell lines under hypoxia exhibit greater stem-like
properties. PLoS One. 6:e291702011. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Liang DM, Ma YY, Liu J, Trope CG, Holm R,
Nesland JM and Suo ZH: The hypoxic microenvironment upgrades
stem-like properties of ovarian cancer cells. BMC Cancer.
12:2012012. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Li PC, Zhou C, Xu LS and Xiao HL: Hypoxia
enhances stemness of cancer stem cells in glioblastoma: an in vitro
study. Int J Med Sci. 10:399–407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Zhou L, Wei XD, Cheng L, Tian J and Jiang
JJ: CD133, one of the markers of cancer stem cells in Hep-2 cell
line. Laryngoscope. 117:455–460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Wei XD, Zhou L, Cheng L, Tian J, Jiang JJ
and MacCallum J: In vivo investigation of CD133 as a putative
marker of cancer stem cells in Hep-2 cell line. Head Neck.
31:94–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Chen H, Zhou L, Dou TH, Wan GL, Tang HQ
and Tian J: BMI1’s maintenance of the proliferative capacity of
laryngeal cancer stem cells. Head Neck. 33:1115–1125. 2011.
|
|
18.
|
Guppy M: The hypoxic core: a possible
answer to the cancer paradox. Biochem Biophys Res Commun.
299:676–680. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Tsujinaka S, Soda K, Kano Y and Konishi F:
Spermine accelerates hypoxia-initiated cancer cell migration. Int J
Oncol. 38:305–312. 2011.PubMed/NCBI
|
|
20.
|
Clevers H: The cancer stem cell: premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yoshiba S, Ito D, Nagumo T, Shirota T,
Hatori M and Shintani S: Hypoxia induces resistance to
5-fluorouracil in oral cancer cells via G(1) phase cell cycle
arrest. Oral Oncol. 45:109–115. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Box AH and Demetrick DJ: Cell cycle kinase
inhibitor expression and hypoxia-induced cell cycle arrest in human
cancer cell lines. Carcinogenesis. 25:2325–2335. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Hashimoto O, Shimizu K, Semba S, Chiba S,
Ku Y, Yokozaki H and Hori Y: Hypoxia induces tumor aggressiveness
and the expansion of CD133-positive cells in a hypoxia-inducible
factor-1alpha-dependent manner in pancreatic cancer cells.
Pathobiology. 78:181–192. 2011. View Article : Google Scholar
|
|
24.
|
Soeda A, Park M, Lee D, et al: Hypoxia
promotes expansion of the CD133-positive glioma stem cells through
activation of HIF-1alpha. Oncogene. 28:3949–3959. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Shi G and Jin Y: Role of Oct4 in
maintaining and regaining stem cell pluripotency. Stem Cell Res
Ther. 1:392010. View
Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Schmidt R and Plath K: The roles of the
reprogramming factors Oct4, Sox2 and Klf4 in resetting the somatic
cell epigenome during induced pluripotent stem cell generation.
Genome Biol. 13:2512012. View Article : Google Scholar
|
|
27.
|
Luo W, Li S, Peng B, Ye Y, Deng X and Yao
K: Embryonic stem cells markers SOX2, OCT4 and Nanog expression and
their correlations with epithelial-mesenchymal transition in
nasopharyngeal carcinoma. PLoS One. 8:e563242013. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Takahashi K and Yamanaka S: Induction of
pluripotent stem cells from mouse embryonic and adult fibroblast
cultures by defined factors. Cell. 126:663–676. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Huangfu D, Osafune K, Maehr R, et al:
Induction of pluripotent stem cells from primary human fibroblasts
with only Oct4 and Sox2. Nat Biotechnol. 26:1269–1275. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Kolenda J, Jensen SS, Aaberg-Jessen C,
Christensen K, Andersen C, Brunner N and Kristensen BW: Effects of
hypoxia on expression of a panel of stem cell and chemoresistance
markers in glioblastoma-derived spheroids. J Neurooncol. 103:43–58.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Iida H, Suzuki M, Goitsuka R and Ueno H:
Hypoxia induces CD133 expression in human lung cancer cells by
up-regulation of OCT3/4 and SOX2. Int J Oncol. 40:71–79.
2012.PubMed/NCBI
|
|
32.
|
Covello KL, Kehler J, Yu H, et al:
HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell
function, embryonic development, and tumor growth. Genes Dev.
20:557–570. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Mathieu J, Zhang Z, Zhou W, et al: HIF
induces human embryonic stem cell markers in cancer cells. Cancer
Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Li DW, Zhou L, Jin B, Xie J and Dong P:
Expression and significance of hypoxia-inducible factor-1alpha and
survivin in laryngeal carcinoma tissue and cells. Otolaryngol Head
Neck Surg. 148:75–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Pietras A, Hansford LM, Johnsson AS, et
al: HIF-2alpha maintains an undifferentiated state in neural
crest-like human neuroblastoma tumor-initiating cells. Proc Natl
Acad Sci USA. 106:16805–16810. 2009. View Article : Google Scholar
|
|
36.
|
Sun HC, Qiu ZJ, Liu J, et al: Expression
of hypoxia-inducible factor-1α and associated proteins in
pancreatic ductal adeno-carcinoma and their impact on prognosis.
Int J Oncol. 30:1359–1367. 2007.
|
|
37.
|
Jubb AM, Buffa FM and Harris AL:
Assessment of tumour hypoxia for prediction of response to therapy
and cancer prognosis. J Cell Mol Med. 14:18–29. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Lundgren K, Holm C and Landberg G: Hypoxia
and breast cancer: prognostic and therapeutic implications. Cell
Mol Life Sci. 64:3233–3247. 2007.PubMed/NCBI
|