Introduction
Glucosamine (GS) is an amino sugar and has been
widely used as an alternative regimen for joint-related disease,
such as rheumatoid arthritis and osteoarthritis. Many in
vivo studies have implicated that GS has preventive actions on
adjuvant arthritis in rats (1) and
significant symptom-modifying effects on osteoarthritis in human
clinical trials (2). In addition,
results from many in vitro studies have shown that GS
inhibits expression and/or activity of many inflammatory mediators,
including cyclooxygenase-2, inducible nitric oxide synthase, matrix
metalloproteases and nuclear factor-κ B (NF-κB) (3,4),
which further support its anti-inflammatory activity. Moreover,
evidence clearly suggests that GS has strong anticancer effects.
For instance, it has been previously shown that GS inhibits tumor
growth (5,6). We and other investigators also have
demonstrated the ability of GS to induce apoptosis in human cancer
cells, such as prostate (DU145), breast (MDA-MB-231), leukemia
(K562), glioma (U87MG) and tongue (YD-8) (7–12).
Moreover, it is suggested that the mechanisms underlying
GS-mediated anti-proliferative and pro-apoptotic effect on cancer
cells may include translocation of cathepsin D and downregulation
of B-cell lymphoma-extra large (8), inhibition of p70S6 kinase (p70S6K)
(9) and signal transducer and
activator of transcription-3 (STAT-3) (10), induction of autophagy via the
stimulation of endoplasmic reticulum (ER) stress (11), and activation of caspases via the
intrinsic pathway (12). In recent
studies, we and other investigators have also demonstrated the
ability of GS to inhibit expression of HIF-1α, a tumor angiogenic
transcription factor in YD-8 tongue cancer cells (12) and in DU145 prostate cancer cells
(13), which may support its
antitumor property.
HIF-1 protein is composed of an α and a β subunit
(14). In most cells, while
expression of HIF-1α protein is differentially regulated under
normoxic and hypoxic condition, HIF-1β protein is constitutively
expressed regardless of oxygen tension (15). Indeed, numerous studies have
demonstrated that under normoxia HIF-1α protein is unstable and
rapidly destructed via the 26S proteasome-dependent protein
degradation pathway, but HIF-1α protein, under hypoxia, is stable
and the stable HIF-1α binds to HIF-1β (16–19).
The HIF-1α/β dimeric complex then localizes to the nucleus where
the dimeric complex mediates the transcriptional induction of
hypoxia responsive element containing genes that encode products
involved in the hypoxic adaptation and/or new blood vessel
formation (15). However, there is
increasing body of evidence suggesting the hypoxia-independent
upregulation of HIF-1α expression. For instance, it is shown that a
number of factors, including serum, interleukin-1β, insulin,
manganese, and ginsenoside-Rg1, induces expression of HIF-1α
through transcriptional and/or translational upregulation in many
types of cells under normoxia (13,20–23).
Evidence also strongly suggests that activities of many
intracellular signaling proteins, such as phosphoinositide
3-kinase, extracellular-regulated protein kinase-1/2, c-jun
N-terminal kinase-1, S6, p38 mitogen-activated protein kinase,
epidermal growth factor receptor/p70S6K, protein kinase B/mammalian
target of rapamycin-p70S6K, and mTOR/p70S6K/S6, are necessary for
normoxic induction of HIF-1α expression in response to
extracellular stimuli (22,24–28).
Little is known about regulation of HIF-1α and
HIF-1β expressions by GS in cancer cells. In this study, we
investigated whether GS treatment for long-term (24 h) or
short-term (4 h) period differentially regulates expression of
HIF-1α and HIF-1β in serum-treated YD-8 human tongue cancer cells
under normoxic condition and if any, determined the molecular
and/or cellular mechanisms involved.
Materials and methods
Materials
RPMI-1640 medium, fetal bovine serum (FBS), and
penicillin-streptomycin were purchased from Welgene (Daegu, Korea).
Primary antibodies: mouse monoclonal anti-human HIF-1β, rabbit
polyclonal anti-human p-p70S6K (Santa Cruz Biotechnology, Delaware,
CA, USA), rabbit polyclonal anti-human p-S6 (Cell Signaling
Technology, Beverly, MA, USA), rabbit polyclonal anti-human
p-eIF-2α (Epitomics, Burlingame, CA, USA), and mouse monoclonal
anti-human HIF-1α (BD Bioscience, San Jose, CA, USA) were purchased
from the indicated companies. Secondary antibodies: goat
anti-rabbit IgG-HRP and goat anti-mouse IgG-HRP were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). ECL western
detection reagents were purchased from Thermo Scientific (Waltham,
MA, USA). Bradford reagent was bought from Bio-Rad (Hercules, CA,
USA). Plasticware, including 6-well plates, was purchased from SPL
Life Sciences (Gyeonggi-do, Korea). Other reagents, including
GS-HCl, were purchased from Sigma (St. Louis, MO, USA).
Cell culture
YD-8 human tongue cancer cells (Korean Cell Line
Bank, Seoul, Korea) were grown at 37°C in a humidified condition of
95% air and 5% CO2 in RPMI-1640 supplemented with 10%
heat-inactivated FBS, 100 U/ml penicillin and 100 μg/ml
streptomycin.
Preparation of whole cell lysates
To see the effect of GS-HCl on phospho-specific
and/or total expression levels of a variety of cellular proteins,
including HIF-1α, HIF-1β, p70S6K, S6 and eukaryotic translation
initiation factor-2α (eIF-2α), YD-8 cells (0.5×106/2
ml/well) were seeded in 6-well plates the day before GS-HCl
treatment. Cells were treated without or with different
concentrations of GS-HCl for 4 or 24 h. Control or GS-HCl-treated
cells were then washed twice with PBS and exposed to cell lysis
buffer [50 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.1% sodium dodecyl
sulfate, 0.25% sodium deoxycho-late, 1% Triton X-100, 1% Nonidet
P-40, 1 mM EDTA, 1 mM EGTA, proteinase inhibitor cocktail (1X)].
The cell lysates were collected in a 1.5 ml tube and centrifuged
for 20 min at 4°C at 12,000 rpm. The supernatant was saved and
protein concentrations were determined with Bradford reagent.
Western blot analysis
Proteins (50 μg) were separated by SDS-PAGE
(10%) and transferred onto nitrocellulose membranes (Millipore,
Billerica, MA, USA). The membranes were washed with TBS (10 mM
Tris, 150 mM NaCl) supplemented with 0.05% (vol/vol) Tween-20
(TBST) followed by blocking with TBST containing 5% (wt/vol)
non-fat dried milk. The membranes were incubated overnight with
antibodies specific for p-p70S6K (1:2,000), p-S6 (1:2,000), HIF-1α
(1:1,000), HIF-1β (1:1,000), eIF-2α (1:1,000) or actin (1:5,000) at
4°C. The membranes were then exposed to secondary antibodies
coupled to horseradish peroxidase for 2 h at room temperature. The
membranes were washed three times with TBST at room temperature.
Immunoreactivities were detected by ECL reagents. Equal protein
loading was assessed by the expression level of actin protein.
Reverse transcription-polymerase chain
reaction (RT-PCR)
To see the effect of GS-HCl on mRNA expression of
HIF-1α, HIF-1β or actin, YD-8 cells (0.5×106/2 ml/well)
was seeded in 6-well plates the day before GS-HCl treatment. Cells
were treated without or with different concentrations of GS-HCl for
4 or 24 h. Total cellular RNA from control or GS-HCl-treated cells
was isolated with the RNAzol-B (Tel-Test, Friendswood, TX, USA).
Total RNA (3 μg) was reverse transcribed using a random
hexadeoxynucleotide primer and reverse transcriptase. Single
stranded cDNA was amplified by PCR with the following primers. The
sequences of the respective primer are: HIF-1α sense,
5′-CTCAAAGTCGGACAGCCTCA-3′; HIF-1α anti-sense,
5′-CCCTGCAGTAGGTTTCTGCT-3′; HIF-1β sense, 5′-GTG
CGCACACATGCTTCTGT-3′; HIF-1β antisense, 5′-CTTTAT
GGCCAAGTCTCGGGT-3′; actin sense, 5′-GGTGAAGGTC GGTGTGAACG-3′; actin
antisense, 5′-GGTAGGAACACGG AAGGCCA-3′. The PCR conditions applied
were: HIF-1α, 25 cycles of denaturation at 95°C for 30 sec,
annealing at 59°C for 30 sec, and extension at 72°C for 30 sec;
HIF-1β, 25 cycles of denaturation at 95°C for 30 sec, annealing at
56°C for 30 sec, and extension at 72°C for 30 sec; actin, 25 cycles
of denaturation at 95°C for 30 sec, annealing at 56°C for 30 sec,
and extension at 72°C for 30 sec. Expression levels of actin mRNA
was used as an internal control to evaluate the relative mRNA
expression of HIF-1α and HIF-1β.
Measurement of HIF-1α protein
stability
To determine the stability of HIF-1α protein in
control or GS-HCl-treated YD-8 cells, YD-8 cells
(0.5×106 cells in 2 ml/well in a 6-well plate) were
primarily grown in culture medium containing serum (10% FBS) for 4
h under normoxic condition to induce high cellular levels of HIF-1α
protein. Cells were then treated for an additional 0.25, 0.5 or 1 h
without or with GS-HCl in the presence of CHX, a translation
inhibitor, to block ongoing translation. Each time, whole cell
lysates were prepared and subjected to immunoblot analysis for
HIF-1α or actin to measure the amounts of HIF-1α protein remaining
in the cells. Actin was used as an internal control to relatively
compare the level of HIF-1α remaining in the cells.
Results
Time-differential regulation of HIF-1α
and HIF-1β expressions in YD-8 cells by GS-HCl
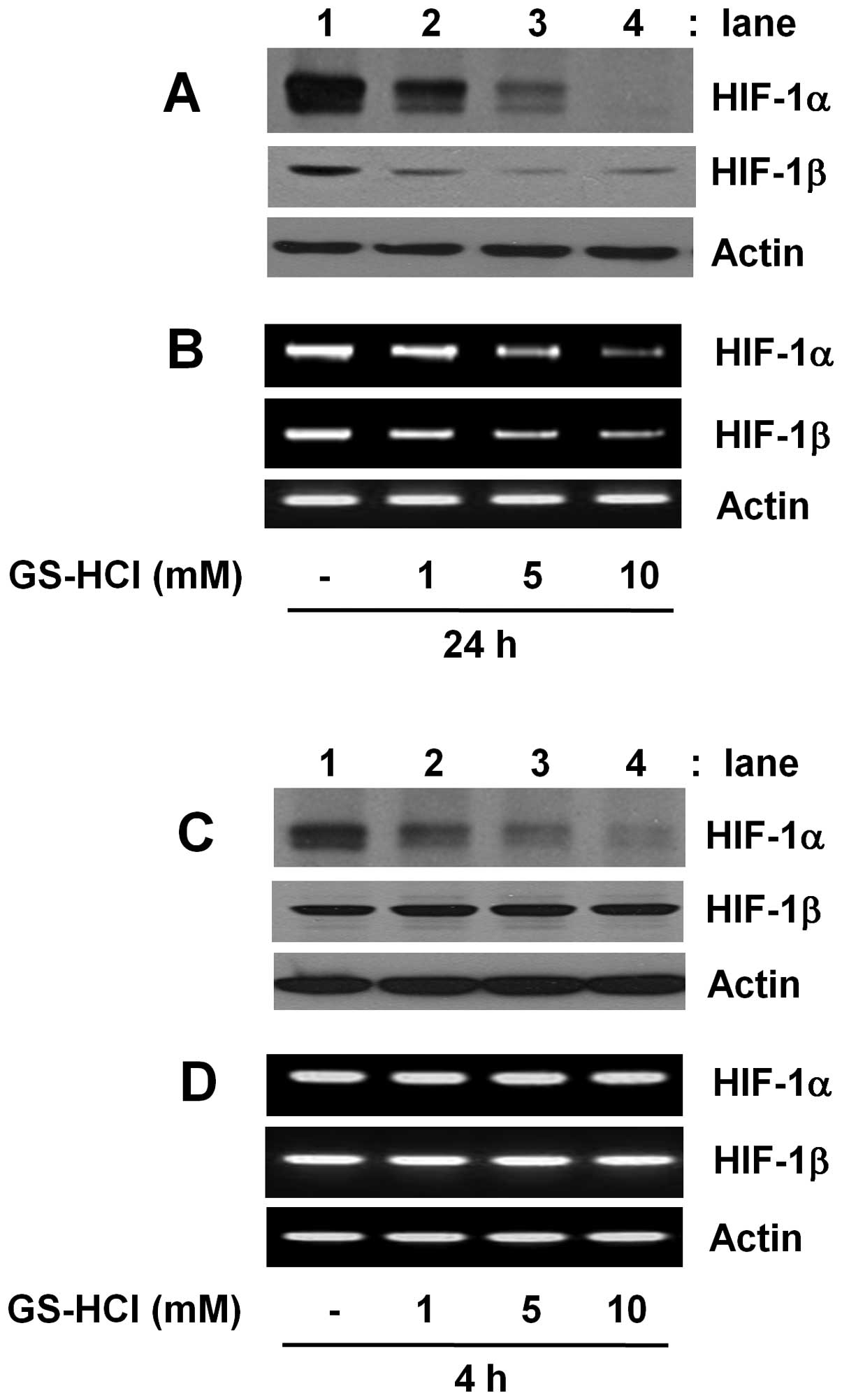
Initially, we investigated whether GS-HCl treatment
for short-term (4 h) or long-term (24 h) period differentially
regulates expressions of HIF-1α and HIF-1β in YD-8 cells grown in
culture media containing serum (10% FBS) under normoxia. As shown
in Fig. 1A and B, compared with
control (lane 1), long-term GS-HCl treatment led to a
concentration-dependent downregulation of HIF-1α at the both
protein and mRNA levels (lanes 2–4). However, there was also a
dose-dependent reduction of HIF-1β protein and mRNA expressions by
long-term GS-HCl treatment. As shown in Fig. 1C and D, compared with control (lane
1), short-term GS-HCl treatment also led to a
concentration-dependent downregulation of HIF-1α protein (lanes
2–4). Short-term GS-HCl treatment at the doses tested, however, did
not affect expression of HIF-1α mRNA, HIF-1β protein and HIF-1β
mRNA. Control actin protein or mRNA expression remained constant
under these experimental conditions (Fig. 1).
Short-term GS-HCl treatment induces
change of the phosphor-ylation levels of p70S6K, S6 and eIF-2α,
translation-related proteins in YD-8 cells
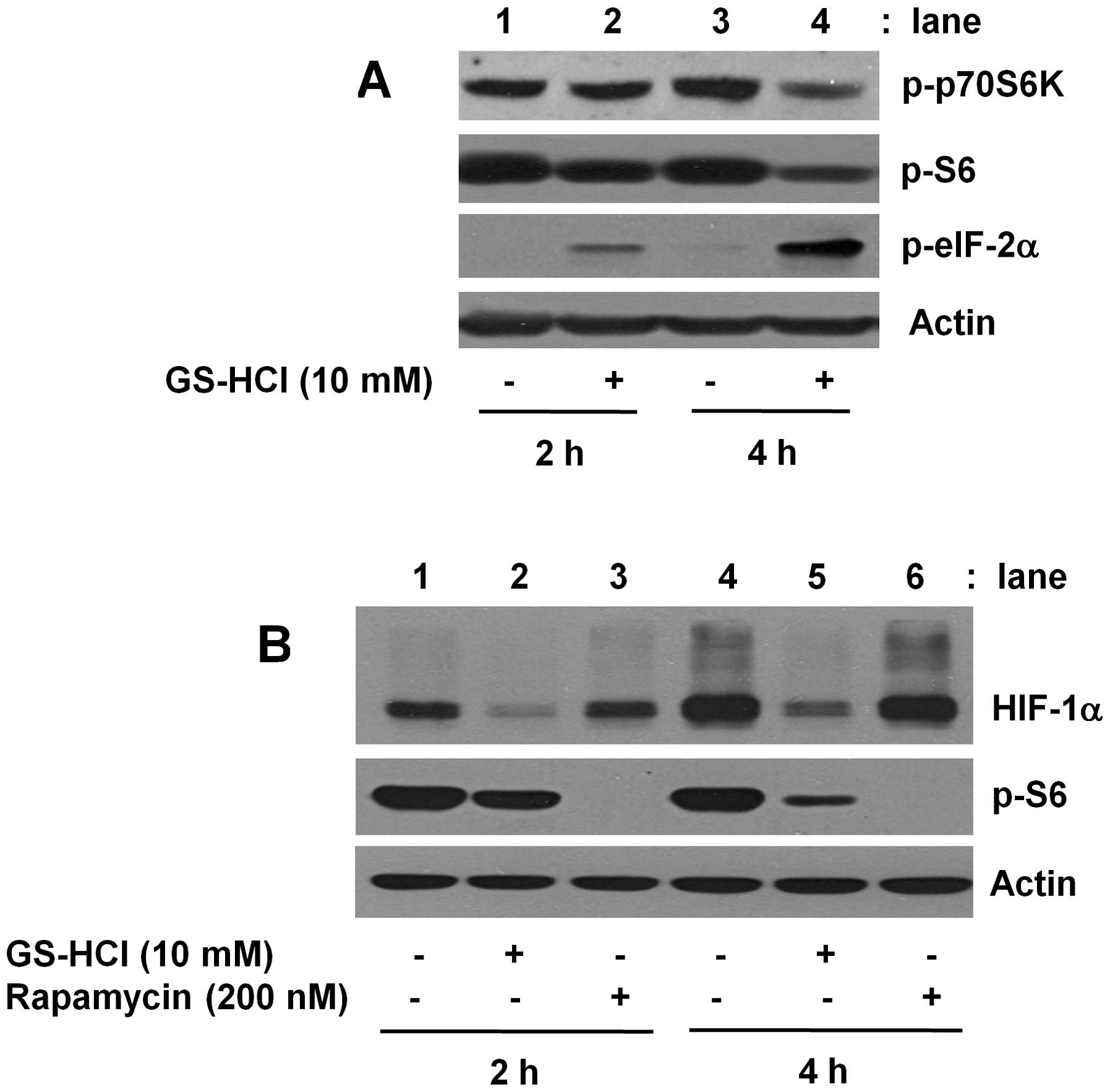
Considering that short-term GS-HCl treatment
inhibits HIF-1α at protein, but not mRNA, level (Fig. 1C and D), we next determined the
effect of short-term GS-HCl treatment on activities of
translation-related signaling proteins, herein p70S6K, S6 and
eIF-2α, in YD-8 cells. As shown in Fig. 2A, in the absence of GS-HCl, there
were high levels of phosphorylated p70S6K and S6 while no or weakly
phosphorylated eIF-2α in YD-8 cells cultured for 2 or 4 h in media
containing serum under normoxia (lane 1 or 3). Notably, short-term
(2 or 4 h) treatment with GS-HCl decreased the amounts of
phosphorylated p70S6K and S6 but increased the levels of
phosphorylated eIF-2α (lane 2 or 4). Rapamycin is an inhibitor of
mTOR kinase complex, a master regulator of protein translation and
has been shown to inhibit phosphorylation and activation of the
mTOR and its downstream targets, p70S6K and S6 (26,29).
Using rapamycin, we next investigated whether HIF-1α protein
downregulation by short-term GS-HCl treatment is due to inhibition
of mTOR/p70S6K/S6 signals. As shown in Fig. 2B, while short-term (2 or 4 h)
GS-HCl treatment that largely blocked S6 phosphorylation (top
panel, lane 2 or 5) strongly suppressed HIF-1α protein expression
(middle panel, lane 2 or 5), treatment with rapamycin for 2 or 4 h
that completely inhibited S6 phosphorylation (top panel, lane 3 or
6) had no effect on expression of HIF-1α protein (middle panel,
lane 3 or 6). Control actin protein expression remained constant
under these experimental conditions (Fig. 2A and B).
The inhibitory effect of short-term
GS-HCl treatment on HIF-1α protein expression in YD-8 cells is the
26S proteasome- and lysosome-independent
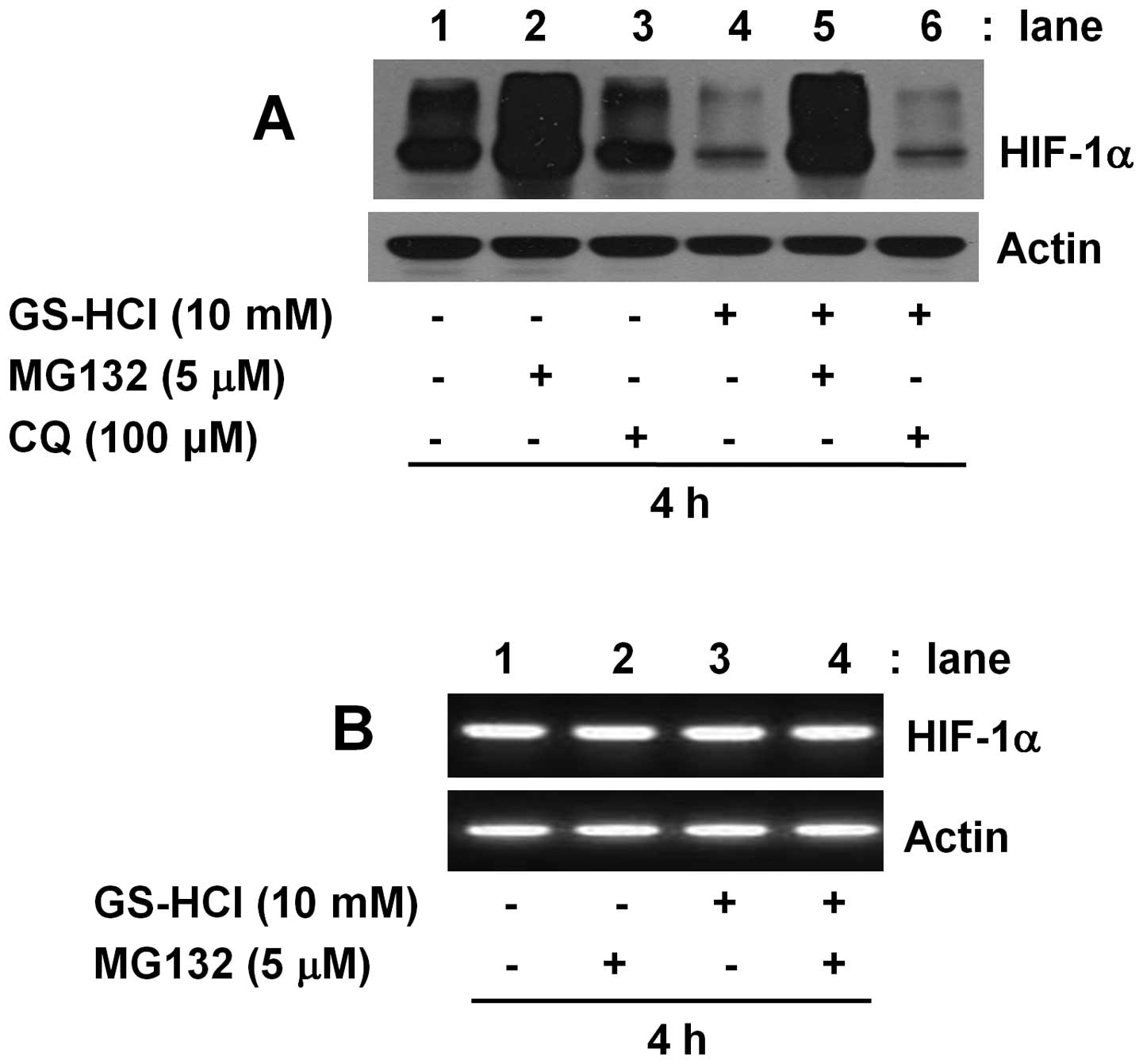
Cellular expression of a protein is largely
influenced by the protein degradation. Protein degradation is often
mediated through the 26S proteosome- and lysosome-mediated
proteolytic pathways. Using MG132, the 26S proteosome inhibitor or
chloroquine (CQ), the lysosomal inhibitor, we next questioned
whether the HIF-1α protein downregulation induced by short-term
GS-HCl treatment is linked to the 26S proteosome and/or lysosome
pathways in YD-8 cells. As shown in Fig. 3A, the inhibitory effect of
short-term GS-HCl treatment on HIF-1α protein expression (lane 4)
was largely blocked by MG132 (lane 5), but not CQ (lane 6).
However, it was found that single treatment with MG132 for 4 h was
enough to strongly increase expression of HIF-1α protein (lane 2).
Single treatment with CQ for 4 h had no enhancing effect on
expression of HIF-1α protein (lane 3). As shown in Fig. 3B, HIF-1α mRNA expression remained
unchanged by 4 h treatment without or with GS-HCl in the absence or
presence of MG132 (lanes 1–4). Control actin protein or mRNA
expression was not affected under these experimental conditions
(Fig. 3A and B).
The inhibitory effect of short-term
GS-HCl treatment on HIF-1α protein expression in YD-8 cells is not
due to alteration of HIF-1α protein stability
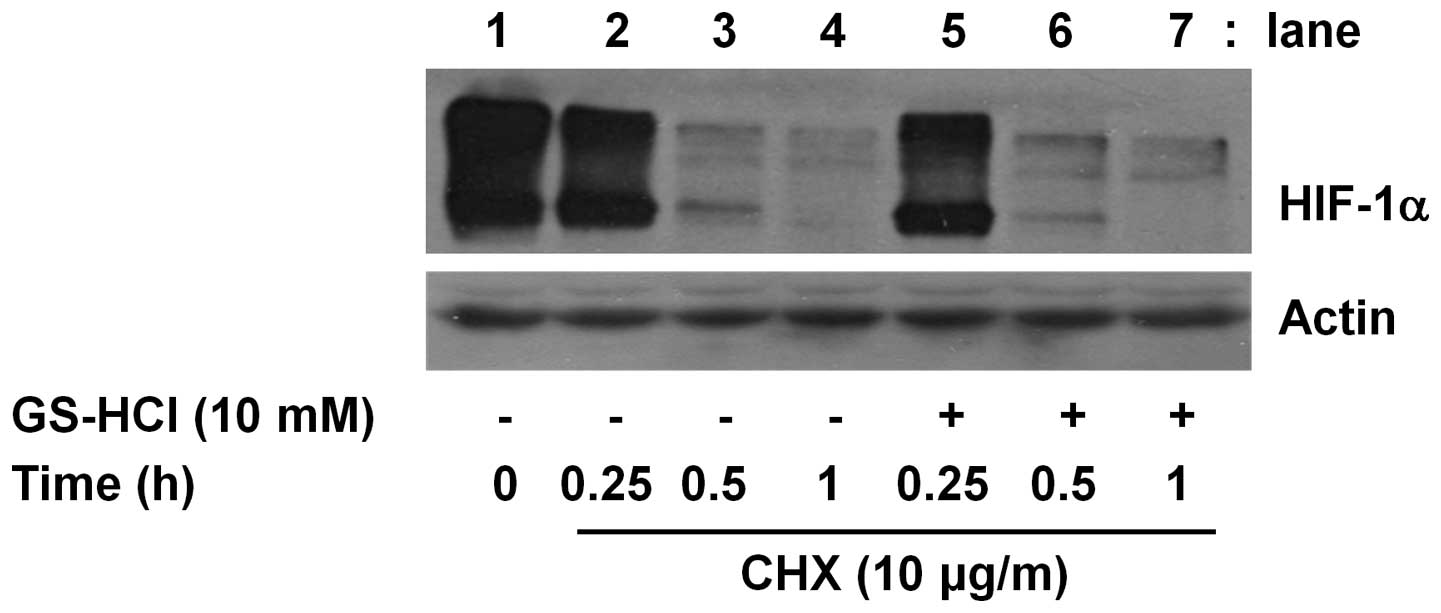
Cellular expression of a protein is largely
influenced by the protein stability. Cycloheximide (CHX) is a
translation inhibitor and has been widely used as a key biochemical
agent in determining the stability of a protein. Using CHX, we
further determined whether HIF-1α protein downregulation induced by
short-term GS-HCl treatment is associated with change of HIF-1α
protein stability. As shown in Fig.
4, in the presence of CHX, there was a sharp decline of the
amounts of HIF-1α protein remained in YD-8 cells (lanes 2–4),
suggesting that when translation is blocked by CHX, HIF-1α protein
is unstable and rapidly degraded in the cells. However, the rapid
degradation of HIF-1α protein was not further enhanced or
accelerated in the presence of GS-HCl at the times tested. Control
actin protein expression remained constant under these experimental
conditions (Fig. 4).
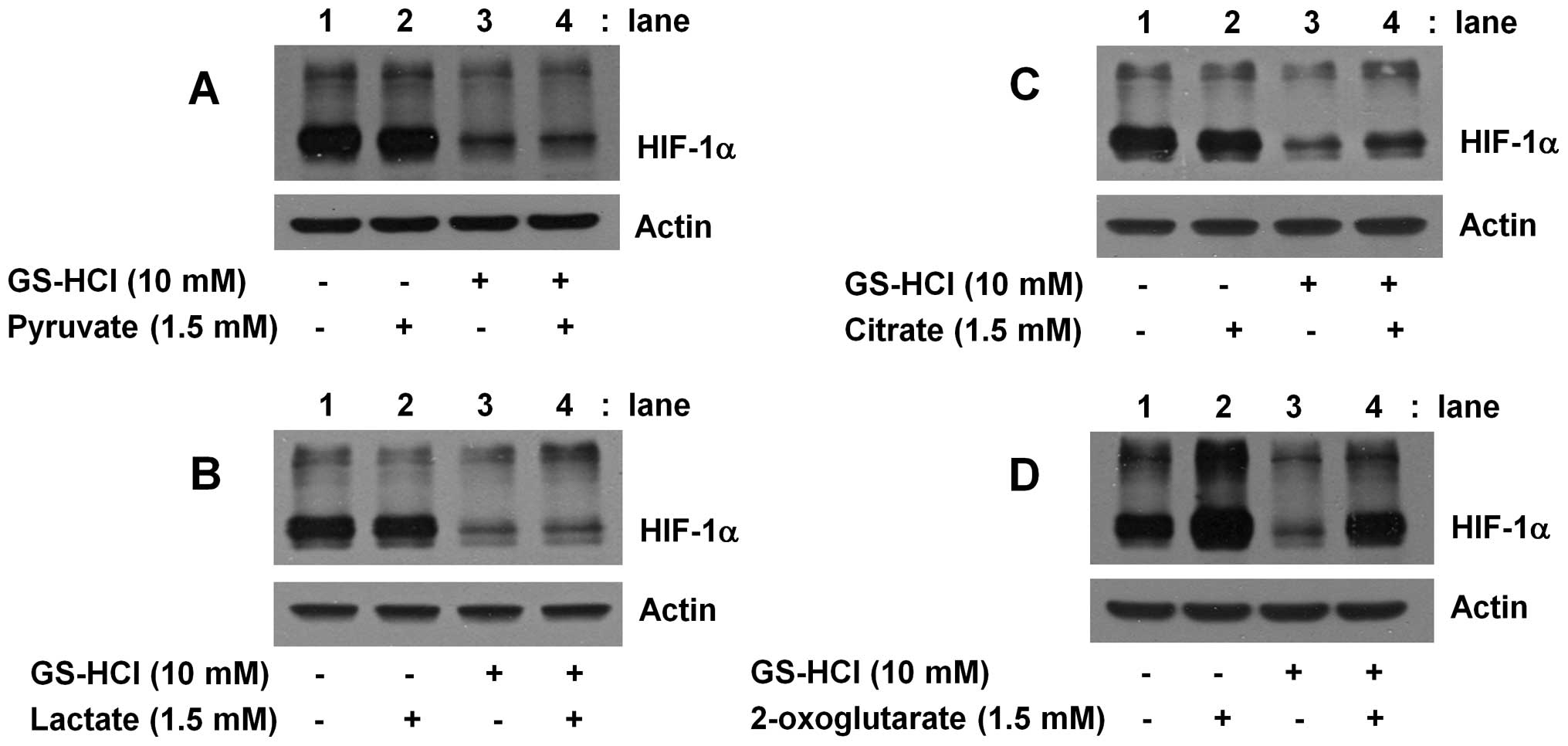
The inhibitory effect of short-term
GS-HCl treatment on HIF-1α protein expression in YD-8 cells is
blunted by exogenous supplementation of the citric acid cycle
intermediates (citrate, 2-oxoglutarate), but not the glycolytic end
products (pyruvate, lactate)
GS is a glucose deprivation mimetic and thus an
inhibitor of glycolysis. It is thus suggested that 4 h exposure of
GS into YD-8 cells may interfere with glucose metabolism, which may
lead to no or less production of the byproducts of glucose
metabolism. Using exogenous supplementation of a number of glucose
metabolites, including lactate, pyruvate (the glycolytic end
products), citrate and 2-oxoglutarate (the citric acid cycle
intermediates), we next investigated whether HIF-1α protein
downregulation by short-term GS-HCl treatment is linked to the
ability of GS-HCl to interfere with glucose metabolism pathway. As
shown in Fig. 5A or B, compared
with control (lane 1), single administration of pyruvate or lactate
did not affect expression of HIF-1α protein (lane 2). Furthermore,
HIF-1α protein downregulation by short-term GS-HCl treatment (lane
3) was not blocked by addition of pyruvate or lactate (lane 4).
Notably, as shown in Fig. 5C,
though single administration of citrate did not affect expression
of HIF-1α protein (lane 2), the inhibitory effect of short-term
GS-HCl treatment on HIF-1α protein expression (lane 3) was in part
blunted by addition of citrate (lane 4). Of further note, as shown
in Fig. 5D, compared with control
(lane 1), single administration of 2-oxoglutarate largely increased
(enhanced) expression of HIF-1α protein (lane 2), HIF-1α protein
downregulation by short-term GS-HCl treatment (lane 3) was not
shown in the presence of 2-oxoglutarate (lane 4).
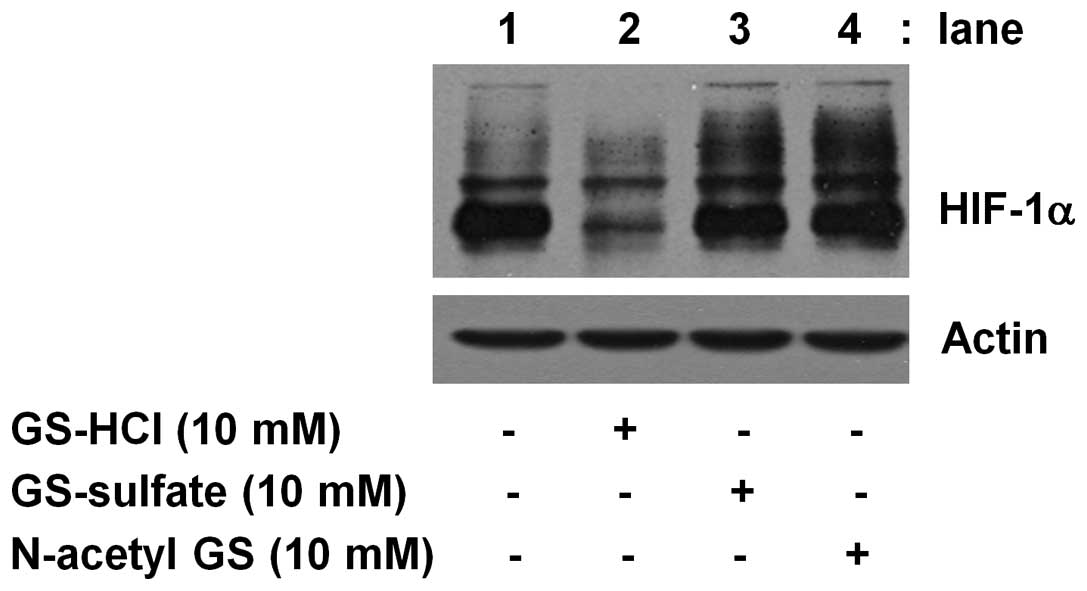
The specificity of short-term GS-HCl
treatment to inhibit expression of HIF-1α protein in YD-8
cells
To evauate the specificity, we next compared the
effect of short-term (4 h) treatment of GS-HCl and other salt form
or derivative of GS, herein GS-sulfate or N-acetyl GS, on
expression of HIF-1α protein in YD-8 cells. As shown in Fig. 6, short-term (4 h) treatment with
GS-HCl led to strong inhibition of HIF-1α protein expression (lane
2), but treatment with GS-sulfate or N acetyl GS for 4 h did not
affect expression of HIF-1α protein (lane 3 or 4).
Discussion
HIF-1 is a tumor angiogenic transcription factor
composed of an α and β subunit and is regarded an interesting
therapeutic target in cancer biology. Little is known about
regulation of HIF-1α and HIF-1β expressions by GS-HCl in cancer
cells. Here, we report for the first time that short-term GS-HCl
treatment selectively downregulates HIF-1α at protein level in YD-8
cells through interference of production of the citric acid cycle
intermediates.
Expression of HIF-1α is regulated at multiple steps,
including transcription, translation and/or post-translation
(17,19,22,30,31).
The present study demonstrates that GS-HCl inhibits expression of
HIF-1α at the protein and mRNA levels in YD-8 cells in the time
differentially. We have shown that long-term GS treatment with
GS-HCl (10 mM) inhibits expression of HIF-1α at the both protein
and mRNA levels in YD-8 cells (Fig. 1A
and B), suggesting HIF-1α transcriptional downregulation.
However, considering the present findings that short-term GS-HCl
treatment inhibits HIF-1α at protein level (Fig. 1C), but it does not influence HIF-1α
mRNA expression (Fig. 1D) and
protein stability (Fig. 3C) in
YD-8 cells, it is likely that short-term GS-HCl treatment represses
HIF-1α protein expression via inhibition of translational process
and/or cellular accumulation of the protein. Aforementioned, HIF-1β
is shown to be ubiquitously expressed in most types of cells
regardless of oxygen tension. We have herein shown that HIF-1β mRNA
and protein are substantially expressed in YD-8 cells under
normoxic condition (Fig. 1),
indicating that expression of HIF-1β is controlled at the levels of
transcription and translation. In this study, however, we show that
long-term GS-HCl treatment inhibits expression of HIF-1β by
transcriptional downregulation while short-term GS-HCl treatment
does not influence expression of HIF-1β at protein and mRNA levels,
which may further strengthen the specificity of short-term GS-HCl
treatment to inhibit expression of HIF-1α protein in YD-8
cells.
Previously, studies have demonstrated the importance
of activities of a number of intracellular signaling proteins
and/or translation-related proteins in normoxic upregulation of
HIF-1α protein in response to extracellular stimuli (22,24–28).
Among these, S6 is a ribosomal protein involved in translation
(32). S6 is shown to be
phosphorylated and activated by the action of an upstream protein
kinase p70S6K (33). Moreover,
there is evidence that PI3K/PKB and mTOR are upstream kinases
responsible for S6K phosphorylation and activation (34). Of interest, there are studies
demonstrating GS-HCl regulation of S6K signaling pathway. For
instance, it is shown that 24 h treatment with GS-HCl (5 mM)
inhibits the activity of S6K in DU145 prostate cancer cells and
MDA-MB-231 breast cancer cells and the inhibition is important for
GS-HCl-induced anti-proliferative effects on these cancer cells
(9). The same group also has
addressed that 10 h treatment with GS-HCl (5 mM) inhibits S6K
signaling pathway and importantly the inhibition is in part linked
to inhibition of HIF-1α at protein level in serum-treated DU145
cells (13). In the present study,
we have shown that short-term (2 or 4 h) treatment with GS-HCl (10
mM) largely blocks phosphorylation of not only S6K but also S6 in
YD-8 cells (Fig. 2A). However, as
deduced from results of pharmacological inhibition studies herein
that treatment with rapamycin, an mTOR/S6K/S6 that completely
blocks S6 phosphorylation does not influence HIF-1α protein
expression in YD-8 cells (Fig.
2B), it appears that no link exists between HIF-1α protein
downregulation and inhibition of mTOR/S6K/S6 signaling pathway in
YD-8 cells in response to short-term GS-HCl exposure. eIF-2α is
another translational regulatory protein (35). It has been shown that
phosphorylation (on Serine 51) of eIF-2α by stress kinases, such as
protein kinase R, leads to its inactivation and inhibition of
global translation (36). There
are previous studies stating that GS inhibits protein, mRNA, DNA
synthesis in mouse leukemic cells L5178Y, which may contribute to
its antitumor effect (6,7). However, it has been shown that
short-term (2 h) treatment with GS-HCl (2 or 5 mM) does not affect
global protein synthesis in DU145 cells (13). In this study, we demonstrated that
short-term (2 or 4 h) GS-HCl treatment increased phosphorylated
forms of eIF-2α in YD-8 cells (Fig.
2A), raising the possibility that short-term GS-HCl treatment
may inactivate eIF-2α leading to inhibition of global translation
in the cells. However, the present findings that short-term GS-HCl
treatment does not affect expression of other proteins, herein
HIF-1β and actin, in YD-8 cells (Fig.
1C and D), and there is no difference of the amounts of total
protein in control or GS-HCl (4 h)-treated YD-8 cells (data not
shown) suggest that though there is eIF-2α inactivation, short-term
GS-HCl treatment does not induce inhibition of global translation
and HIF-1α protein downregulation seems to be not a part of
inhibition of global translation, but a selective event triggered
by short-term exposure of this amino sugar in YD-8 cells.
Increasing evidence suggests that degradation of
HIF-1α at protein level is largely associated with the 26S
proteasome-mediated proteolytic pathways (16,17,19,31,37).
However, the lysosome-mediated degradation of HIF-1α protein also
has been proposed (38).
Interestingly, there is a previous study implicating the 26S
proteasome-independent mechanism of downregulation of HIF-1α
protein induced by GS-HCl in DU145 cells (13). In this study, we demonstrate that
treatment with MG132 (Fig. 3A,
lane 5), but not CQ (lane 6), blocks the repressive effect of
short-term GS-HCl treatment on expression of HIF-1α protein in YD-8
cells (lane 4). However, considering that single treatment with
MG132 strongly upregulates expression of HIF-1α at protein level in
YD-8 cells (lane 2), it is likely that HIF-1α protein
downregulation induced by short-term GS-HCl treatment in YD-8 cells
herein is the 26S proteasome and lysosome-independent. Several
lines of evidence indicate that HIF-1α protein is very labile and
its half-life is less than an hour (16,25).
In agreement with it, the present study demonstrates that when
translation is blocked by CHX, HIF-1α protein induced by serum in
YD-8 cells under normoxia is rapidly destabilized regardless of
presence or absence of GS-HCl, which may further imply that HIF-1α
protein downregulation induced by short-term GS-HCl treatment in
YD-8 cells is not mediated through alteration of HIF-1α protein
stability.
It has been introduced that glucose is an inducer of
HIF-1α expression (accumulation) in human gliomas and other cancer
cell lines under normoxia, which requires the metabolism of glucose
to pyruvate that prevents the aerobic degradation of HIF-1α protein
(39). Furthermore, the same
research group has reported that iodoacetate, an inhibitor of
glyceraldehyde-3-phosphate dehydrogenase completely blocks the
ability of glucose to stimulate aerobic HIF-1α protein accumulation
and the replacement of glucose with the citric acid cycle
intermediates, such as citrate or 2-oxoglutarate, does not
stimulate it. GS is a glucose deprivation mimetic and has been
shown to inhibit glycolysis (39,40).
It also has been demonstrated that both glucose and GS utilize the
same glucose transporter system for import into the cells (41,42),
and GS, through inhibition of glucose transporter, interferes with
cellular glucose uptake (43).
With this in mind, it is assumed that short-term GS-HCl exposure
into YD-8 cells may hinder glucose uptake, inhibit glycolysis,
and/or produce less (or no) glucose metabolites. In this study, we
have shown that HIF-1α protein downregulation by short-term GS-HCl
treatment in YD-8 cells is blunted by exogenous administration of
citrate or 2-oxoglutarate, but not pyruvate or lactate (Fig. 4). These results strongly suggest a
link between HIF-1α protein downregulation and interference of
glucose metabolism pathway (particularly production of the citric
acid cycle intermediates) in YD-8 cells in response to short-term
GS-HCl treatment. An interesting finding in the present study is
the specificity of GS-HCl in downregulating HIF-1α at protein level
in YD-8 cells, as deduced from that short-term treatment with
GS-sulfate or N-acetyl GS does not influence expression of HIF-1α
protein in YD-8 cells (Fig.
5).
Because established cancer cell lines are poor
indicators of the tumor biology, the pathophysiological relevance
of the present finings is unclear at present. Previously, studies
have shown that HIF-1α expression is linked to tumor promotion in
human OSCC (44) and correlates
with the growth and adhesion in human OSCC cells (45). We and others have also recently
demonstrated that HIF-1α protein is highly expressed in YD-8 tongue
cancer cells (12) and DU145
prostate cancer cells (13), and
long-term (24, 48 or 72 h) GS-HCl treatment inhibits proliferation,
decreases survival and/or induces apoptosis in YD-8, DU145 and
MDA-MB231 breast cancer cells (9,12,13).
In view of this, the present study may have importance to address
that i) downregulation of HIF-1α protein induced by short-term
GS-HCl treatment may facilitate anti-proliferative, antisurvival
and pro-apoptotic effects on YD-8 cells triggered by long-term
GS-HCl treatment and ii) short-term treatment with GS-HCl alone
and/or in combination with other anti-cancer therapeutics may be
useful against OSCC and other malignances in which aberrant
expression of HIF-1α protein plays an oncogenic role and/or confers
drug resistance.
Collectively, our data demonstrate for the first
time that short-term GS-HCl treatment specifically reduces HIF-1α
at protein level in YD-8 cells, and the reduction is at least in
part associated with interference of the citric acid cycle and/or
less production of the citric acid cycle metabolites.
Acknowledgements
We greatly thank Professor Ki-Young
Nam for proofreading this manuscript.
References
|
1.
|
Hua J, Suguro S, Hirano S, Sakamoto K and
Nagaoka I: Preventive actions of a high dose of glucosamine on
adjuvant arthritis in rats. Inflamm Res. 54:127–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Reginster JY, Deroisy R, Rovati LC, Lee
RL, Lejeune E, Bruyere O, Giacovelli G, Henrotin Y, Dacre JE and
Gossett C: Long-term effects of glucosamine sulphate on
osteoarthritis progression: a randomized, placebo-controlled
clinical trial. Lancet. 357:251–256. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Largo R, Alvarez-Soria MA, Diez-Ortego I,
Calvo E, Sanchez-Pernaute O, Egido J and Herrero-Beaumont G:
Glucosamine inhibits IL-1beta-induced NFkappaB activation in human
osteoarthritic chondrocytes. Osteoarthritis Cartilage. 11:290–298.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Nakamura M, Shibakawa A, Tanaka M, Kato T
and Nishioka K: Effects of glucosamine hydrochloride on the
production of prostaglandin E2, nitric oxide and metalloproteases
by chondrocytes and synoviocytes in osteoarthritis. Clin Exp
Rheumatol. 22:293–299. 2004.PubMed/NCBI
|
|
5.
|
Quastel JH and Cantero A: Inhibition of
tumor growth by D-glucosamine. Nature. 171:252–254. 1953.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Bekesi JG and Winzler RJ: Inhibitory
effects of D-glucosamine on the growth of Walker 256 carcinosarcoma
and on protein, RNA, and DNA synthesis. Cancer Res. 30:2905–2912.
1970.PubMed/NCBI
|
|
7.
|
Bosmann HB: Inhibition of protein,
glycoprotein, ribonucleic acid and deoxyribonucleic acid synthesis
by D-glucosamine and other sugars in mouse leukemic cells L5178Y
and selective inhibition in SV-3T3 compared with 3T3 cells. Biochim
Biophys Acta. 240:74–93. 1970. View Article : Google Scholar
|
|
8.
|
Wang Z, Liang R, Huang GS, Piao Y, Zhang
YQ, Wang AQ, Dong BX, Feng JL, Yang GR and Guo Y: Glucosamine
sulfate-induced apoptosis in chronic myelogenous leukemia K562
cells is associated with translocation of cathepsin D and
downregulation of Bcl-xL. Apoptosis. 11:1851–1860. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Oh HJ, Lee JS, Song DK, Shin DH, Jang BC,
Suh SI, Park JW, Suh MH and Baek WK: D-glucosamine inhibits
proliferation of human cancer cells through inhibition of p70S6K.
Biochem Biophys Res Commun. 360:840–845. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chesnokov V, Sun C and Itakura K:
Glucosamine suppresses proliferation of human prostate carcinoma
DU145 cells through inhibition of STAT3 signaling. Cancer Cell Int.
9:252009. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Hwang MS and Baek WK: Glucosamine induces
autophagic cell death through the stimulation of ER stress in human
glioma cancer cells. Biochem Biophys Res Commun. 399:111–116. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Jung CW, Jo JR, Lee SH, Park YK, Jung NK,
Song DK, Bae J, Nam KY, Ha JS, Park IS, Park GY, Jang BC and Park
JW: Anti-cancer properties of glucosamine-hydrochloride in YD-8
human oral cancer cells: Induction of the caspase-dependent
apoptosis and down-regulation of HIF-1α. Toxicol In Vitro.
26:42–50. 2012.PubMed/NCBI
|
|
13.
|
Park JY, Park JW, Suh SI and Baek WK:
D-glucosamine down-regulates HIF-1alpha through inhibition of
protein translation in DU145 prostate cancer cells. Biochem Biophys
Res Commun. 382:96–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Wang GL, Jiang BH, Rue EA and Semenza GL:
Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS
heterodimer regulated by cellular O2 tension. Proc Natl
Acad Sci USA. 92:5510–5514. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Semenza GL: Hypoxia-inducible factor 1:
master regulator of O2 homeostasis. Curr Opin Genet Dev.
8:588–594. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Salceda S and Caro J: Hypoxia-inducible
factor 1 alpha (HIF-1alpha) protein is rapidly degraded by the
ubiquitin-proteasome system under normoxic conditions. Its
stabilization by hypoxia depends on redox-induced chages. J Biol
Chem. 272:22642–22647. 1997. View Article : Google Scholar
|
|
17.
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor1alpha is mediated by an
O2-dependent degradation domain via the ubiquitin
proteasome pathway. Proc Natl Acad Sci USA. 95:7987–7992. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Tanimoto K, Makino Y, Pereira T and
Poellinger L: Mechanism of regulation of the hypoxia-inducible
factor-1 alpha by the von Hippel-Lindau tumor suppressor protein.
EMBO J. 19:4298–4309. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ivan M, Kondo K, Yang H, Kim W, Valiando
J, Ohh M, Salic A, Asara JM, Lane WS and Kaelin Jr WG: HIFalpha
targeted for VHL-mediated destruction by proline hydroxylation:
implications for O2 sensing. Science. 292:464–468. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Qian D, Lin HY, Wang HM, Zhang X, Liu DL,
Li QL and Zhu C: Normoxic induction of the hypoxic-inducible
factor-1 alpha by interleukin-1 beta involves the extracellular
signal-regulated kinase 1/2 pathway in normal human cytotrophoblast
cells. Biol Reprod. 70:1822–1827. 2004. View Article : Google Scholar
|
|
21.
|
Doronzo G, Russo I, Mattiello L, Riganti
C, Anfossi G and Trovati M: Insulin activates hypoxia-inducible
factor-1alpha in human and rat vascular smooth muscle cells via
phosphatidylinositol-3 kinase and mitogen-activated protein kinase
pathways: impairment in insulin resistance owing to defects in
insulin signaling. Diabetologia. 49:1049–1063. 2006. View Article : Google Scholar
|
|
22.
|
Shin HJ, Choi MS, Ryoo NH, Nam KY, Park
GY, Bae JH, Suh SI, Baek WK, Park JW and Jang BC:
Manganese-mediated upregulation of HIF-1alpha protein in Hep2 human
laryngeal epithelial cells via activation of the family of MAPKs.
Toxicol In Vitro. 24:1208–1214. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Leung KW, Ng HM, Tang MK, Wong CC, Wong RN
and Wong AS: Ginsenoside-Rg1 mediates a hypoxia-independent
upregulation of hypoxia-inducible factor-1α to promote
angiogenesis. Angiogenesis. 14:515–522. 2011.PubMed/NCBI
|
|
24.
|
Yen ML, Su JL, Chien CL, Tseng KW, Yang
CY, Chen WF, Chang CC and Kuo ML: Diosgenin induces
hypoxia-inducible factor-1 activation and angiogenesis through
estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38
mitogen-activated protein kinase pathways in osteoblasts. Mol
Pharmacol. 68:1061–1073. 2005. View Article : Google Scholar
|
|
25.
|
Jang BC: The fruit juice of Morinda
citrifolia (noni) down-regulates HIF-1α protein expression
through inhibition of PKB, ERK-1/2, JNK-1 and S6 in
manganese-stimulated A549 human lung cancer cells. Int J Mol Med.
29:499–504. 2012.
|
|
26.
|
Tamura K, Yoshie M, Miyajima E, Kano M and
Tachikawa E: Stathmin regulates hypoxia-inducible factor-1α
expression through the mammalian target of rapamycin pathway in
ovarian clear cell adenocarcinoma. ISRN Pharmacol.
30:2795932013.PubMed/NCBI
|
|
27.
|
Xie SR, Wang Y, Liu CW, Luo K and Cai YQ:
Liquiritigenin inhibits serum-induced HIF-1α and VEGF expression
via the AKT/mTOR-p70S6K signalling pathway in HeLa cells. Phytother
Res. 26:1133–1141. 2012.PubMed/NCBI
|
|
28.
|
Jeong JH, Jeong YJ, Cho HJ, Shin JM, Kang
JH, Park KK, Park YY, Chung IK, Chang HW and Magae J: Ascochlorin
inhibits growth factor-induced HIF-1α activation and
tumor-angiogenesis through the suppression of EGFR/ERK/p70S6K
signaling pathway in human cervical carcinoma cells. J Cell
Biochem. 113:1302–1313. 2012.PubMed/NCBI
|
|
29.
|
Laplante M and Sabatini DM: mTOR signaling
in growth control and disease. Cell. 149:274–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Epstein AC, Gleadle JM, McNeill LA,
Hewitson KS, O’Rourke J, Mole DR, Mukherji M, Metzen E, Wilson GD,
Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R,
Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ and Ratcliffe PJ:
C. elegans EGL-9 and mammalian homologs define a family of
dioxygenases that regulate HIF by prolyl hydroxylation. Cell.
107:43–54. 2001. View Article : Google Scholar
|
|
31.
|
Maxwell PH, Wiesener MS, Chang GW,
Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER and
Ratcliffe PJ: The tumour suppressor protein VHL targets
hypoxia-inducible factors for oxygen-dependent proteolysis. Nature.
399:271–275. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Meyuhas O: Physiological roles of
ribosomal protein S6: one of its kind. Int Rev Cell Mol Biol.
268:1–37. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Berven LA and Crouch MF: Cellular function
of p70S6K: a role in regulating cell motility. Immunol Cell Biol.
78:447–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Fang CX, Yang X, Sreejayan N and Ren J:
Acetaldehyde promotes rapamycin-dependent activation of p70(S6K)
and glucose uptake despite inhibition of Akt and mTOR in
dopaminergic SH-SY5Y human neuroblastoma cells. Exp Neurol.
203:196–204. 2007. View Article : Google Scholar
|
|
35.
|
Baird TD and Wek RC: Eukaryotic initiation
factor 2 phosphorylation and translational control in metabolism.
Adv Nutr. 3:307–321. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
De Haro C, Méndez R and Santoyo J: The
eIF-2alpha kinases and the control of protein synthesis. FASEB J.
10:1378–1387. 1996.PubMed/NCBI
|
|
37.
|
Maynard MA and Ohh M: Von Hippel-Lindau
tumor suppressor protein and hypoxia-inducible factor in kidney
cancer. Am J Nephrol. 24:1–13. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Hubbi ME, Hu H, Kshitiz Ahmed I, Levchenko
A and Semenza GL: Chaperone-mediated autophagy targets
hypoxiainducible factor-1α (HIF-1α) for lysosomal degradation. J
Biol Chem. 288:10703–10714. 2013.
|
|
39.
|
Tesoriere G, Vento R and Calvaruso G:
Inhibitory effect of D-glucosamine on glycolysis in bovine retina.
Biochim Biophys Acta. 385:58–67. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Zhang J, Zhao M and Peng S: Synthesis of
mimetic peptides containing glucosamine. Carbohydr Res.
346:1997–2003. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Oguchi M, Miyatake Y, Ayabe J and Akamatsu
N: Phosphorylation of D-glucosamine by rat liver glucokinase. J
Biochem. 77:1117–1121. 1975.PubMed/NCBI
|
|
42.
|
Uldry M, Ibberson M, Hosokawa M and
Thorens B: GLUT2 is a high affinity glucosamine transporter. FEBS
Lett. 524:199–203. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Kim YK, Park JH, Park SH, Lim B, Baek WK,
Suh SI, Lim JG, Ryu GR and Song DK: Protective role of
glucagon-like peptide-1 against glucosamine induced cytotoxicity in
pancreatic beta cells. Cell Physiol Biochem. 25:211–220. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Brennan PA, Mackenzie N and Quintero M:
Hypoxia-inducible factor 1alpha in oral cancer. J Oral Pathol Med.
34:385–389. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Song Y, Wang W, Qu X and Sun S: Effects of
hypoxia inducible factor-1 alpha (HIF-1alpha) on the growth and
adhesion in tongue squamous cell carcinoma cells. Indian J Med Res.
129:154–163. 2009.PubMed/NCBI
|