Contents
Introduction
Human papillomavirus (HPV)
Oropharyngeal squamous cell carcinoma (OPSCC)
OPSCC and HPV
An HPV induced epidemic of OPSCC
HPV and OPSCC and treatment
Studies on HPV and other markers in HPV-positive
OPSCC in response to treatment
Prevention of HPV-positive OPSCC
Introduction
A correlation between human papillomavirus (HPV),
besides smoking and alcohol, in the development of oropharyngeal
squamous cell carcinoma (OPSCC) was found and in 2007, this
association was recognized by the International Agency for Research
against Cancer (IARC)(1–4). Furthermore, in many Western countries
a rise in the number of OPSCC cases has been observed, now
attributed to an increase of HPV-positive OPSCC cases (5–17).
Of note, HPV-positive OPSCC has in general a better clinical
outcome than HPV-negative OPSCC and other head neck squamous cell
carcinoma (HNSCC) (80 vs. 40% 5-year disease specific survival with
conventional radiotherapy) (1–3,15–19).
In parallel, due to this development because of its poor prognosis,
HNSCC treatment has become more aggressive with more intensified
chemo-radiotherapy administrations, leading to many additional
acute and chronic adverse side effects. This intensified therapy
may not be necessary for a large majority of patients with
HPV-positive OPSCC that earlier did well already with more
conventional therapy (1–3,15,16,20).
However, not all patients with HPV-positive OPSCC survive, so
before tapering therapy it is important to combine positive
HPV-status with additional biomarkers in the tumors to identify
patients with a very good probability to respond favorably to
therapy. Furthermore, it would be of benefit to find predictive
markers for risk of, or early OPSCC stages, as is done for cervical
cancer, as well as introduce HPV-vaccination of boys in order to
decrease the effects of the upcoming increase of HPV-positive
OPSCC. This review gives an introduction to the field and the
important issues of tailored therapy, prediction and prevention. It
has special focus on the possibility to select patients with the
potential to better respond to therapy and includes some aspects on
early prediction of OPSCC, and prevention.
Human papillomavirus (HPV)
There are over 150 fully sequenced HPV types, with
very many isolated from skin, but also a considerable number in
mucous tissues (21,22). The cutaneous types can potentially
cause skin warts, but their association to skin cancer is unclear,
except for epidermodyplasia vercucciformis patients that are
sensitive to infections with e.g., HPV5 and 8 resulting in
verruca-like papillomatous lesions and multiple skin tumors
(21,22). The mucosal types are separated into
high-risk (HR) types associated with different cancers, e.g.,
cervical, vulvar, vaginal penile, anal and OPSCC; and into low-risk
(LR) types that are seldom observed in cancer, but often found in
benign genital lesions and respiratory papillomas (21,22).
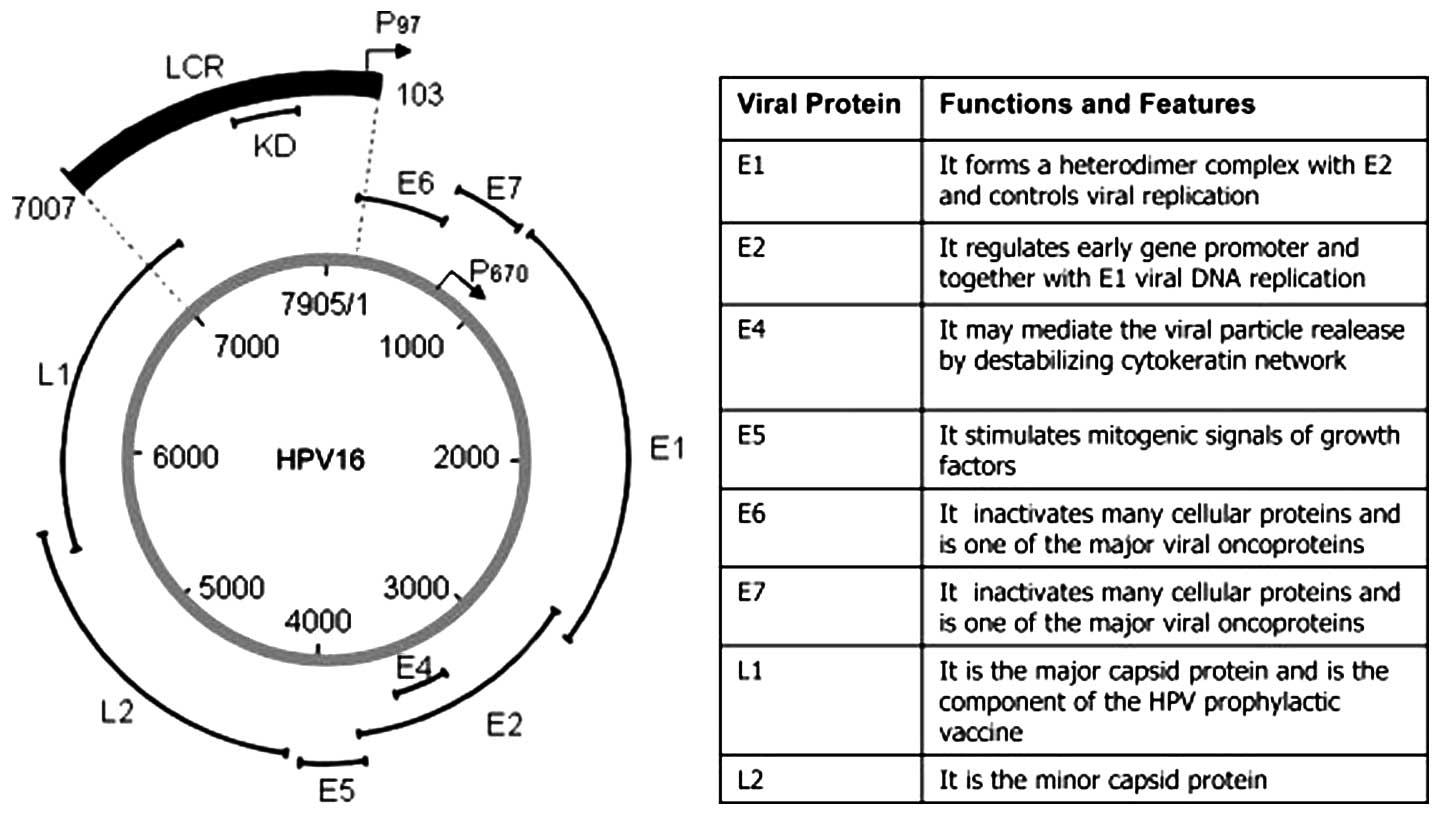
All HPVs are small double stranded circular DNA
viruses with genomes of almost 8 kb. The genome arbitrarily divided
into a non-coding, an early and a late region is contained within a
52–55 nm virion encoding for the non-structural ‘early proteins’
E1-E2, E4-E7, and the two structural viral capsid ‘late proteins’
L1 and L2 (Fig. 1) (21). E1-E2 and E4-E7 are essential for
gene regulation, replication and pathogenesis (21). In HR types the oncogenes E6 and E7,
with high affinity to p53 and pRb, respectively, are important for
immortalization and transformation (21). More specifically, E6 binds to p53
and causes its degradation, while E7 binds Rb and inhibits its
function with deregulation of cell cycle control, also leading to
overexpression of the cyclin-dependent kinase inhibitor
p16Ink4a, the latter sometimes used as a surrogate
marker for presence of HPV in OPSCC (16,21,23).
The L1 major capsid protein contributes to the bulk of the viral
capsid (80–90%) and self-assembles into virus-like particles (VLPs)
under certain conditions. VLPs from HPVs and (other viruses) lack
viral DNA, and are useful as vaccines and vectors (24–27).
Current HPV vaccines consist of VLPs from different HPV types and
both contain HPV16 and 18 VLPs (24,25).
Oropharyngeal squamous cell carcinoma
(OPSCC)
OPSCC comprises tonsil and the base of tongue cancer
(together accounting for 80% of the OPSCC cases) as well as cancer
of the walls of the pharynx and the soft palate (20). Patients with OPSCC, similar to
those with other head neck squamous cell carcinoma (HNSCC) seek
medical care when they have symptoms and by then the tumors are
relatively large. In earlier studies, clinical outcome for OPSCC,
similar to HNSCC at large, was poor with an overall 5-year survival
of approximately 25–40% with conventional radiotherapy and surgery,
and it was difficult to predict clinical outcome despite similar
stage and histology and treatment (1–3,20).
Today, due to the poor prognosis of HNSCC, including that of OPSCC,
its curative treatment is more aggressive, with chemo-radiotherapy
in addition to surgery when necessary, and in some cases epithelial
growth receptor (EGFR) blockers and there has been some improvement
of survival (16,20). As always, the aim is to eliminate
the malignancy, with as little functional and cosmetic impairment
as possible (20). When curative
therapy is impossible palliative therapy is administered to lessen
discomfort.
OPSCC and HPV
In 2000, HPV-positive OPSCC, with >90% of the
cases being HPV16-positive was shown to have a better clinical
outcome compared to HPV-negative OPSCC and other HNSCC (80 vs. 40%
5-year survival) (1–4). Furthermore, HPV-positive and
HPV-negative OPSCC were suggested to likely be different entities
(1–4,15).
Most HPV-positive OPSCC, either with episomal/and or integrated HPV
genomes, exhibited E6 and E7 mRNA expression; with p53 expression
more often, being normal and with 16Ink4a overexpressed
in most cases, in contrast to that observed in HPV-negative OPSCC
(1,18,21,22,28–30).
In addition, HPV-positive OPSCC was generally less differentiated;
more frequently aneuploid compared to HPV-negative OPSCC; and
chromosome 3q often amplified similar to cervical cancer (31,32).
Above all, independent of tumor stage, age, gender,
differentiation, or DNA ploidy, HPV was a favorable prognostic
factor (1–3,31).
Moreover, being a never-smoker indicated an even better clinical
outcome in patients with HPV-positive OPSCC (18,33).
In 2007, the IARC recognized HPV, specially HPV16 as a risk factor
for OPSCC (4).
The definition of HPV-positive status in OPSCC is
not completely convergent. Mostly, formalin-fixed paraffin-embedded
(FFPE) tumor biopsies are used to define HPV status and
16Ink4a overexpression assayed by immunohistochemistry
(IHC) is used as a surrogate marker by some for HPV (23). In situ hybridization,
Southern blots or PCR with general or specific HPV primers for
detection of HPV DNA/RNA in addition to primers for cellular genes
to assay for DNA amplifiability are also used (34–36).
Today many methods including the Hybrid Capture 2; The Roche linear
array HPV genotyping test and a PCR bead based multiplex method are
available for HPV-typing (37–39).
However, most scientists agree that analysis of E6 and E7 mRNA by
RT-PCR should be used as a gold standard, since it is more
indicative of functional HPV expression (18). Still, it has been reported that the
combined presence of HPV DNA tested by PCR and overexpression of
p16 by IHC is nearly as specific and sensitive as employing a gold
standard (40). Notably, HPV
prevalence in OPSCC varies due to methodology used, and due to
time-period of analysis, the material, and geographic location
(1–3,6,9,12,14–16).
In addition, HPV DNA is more often found and of better predictive
value in cancer of the tonsil and base of tongue (the tonsil and
base of tongue accounting for Waldeyers ring of lymphatic tissue)
compared to cancer at other oropharyngeal sites outside the
Waldeyers ring (41).
An HPV induced epidemic of OPSCC
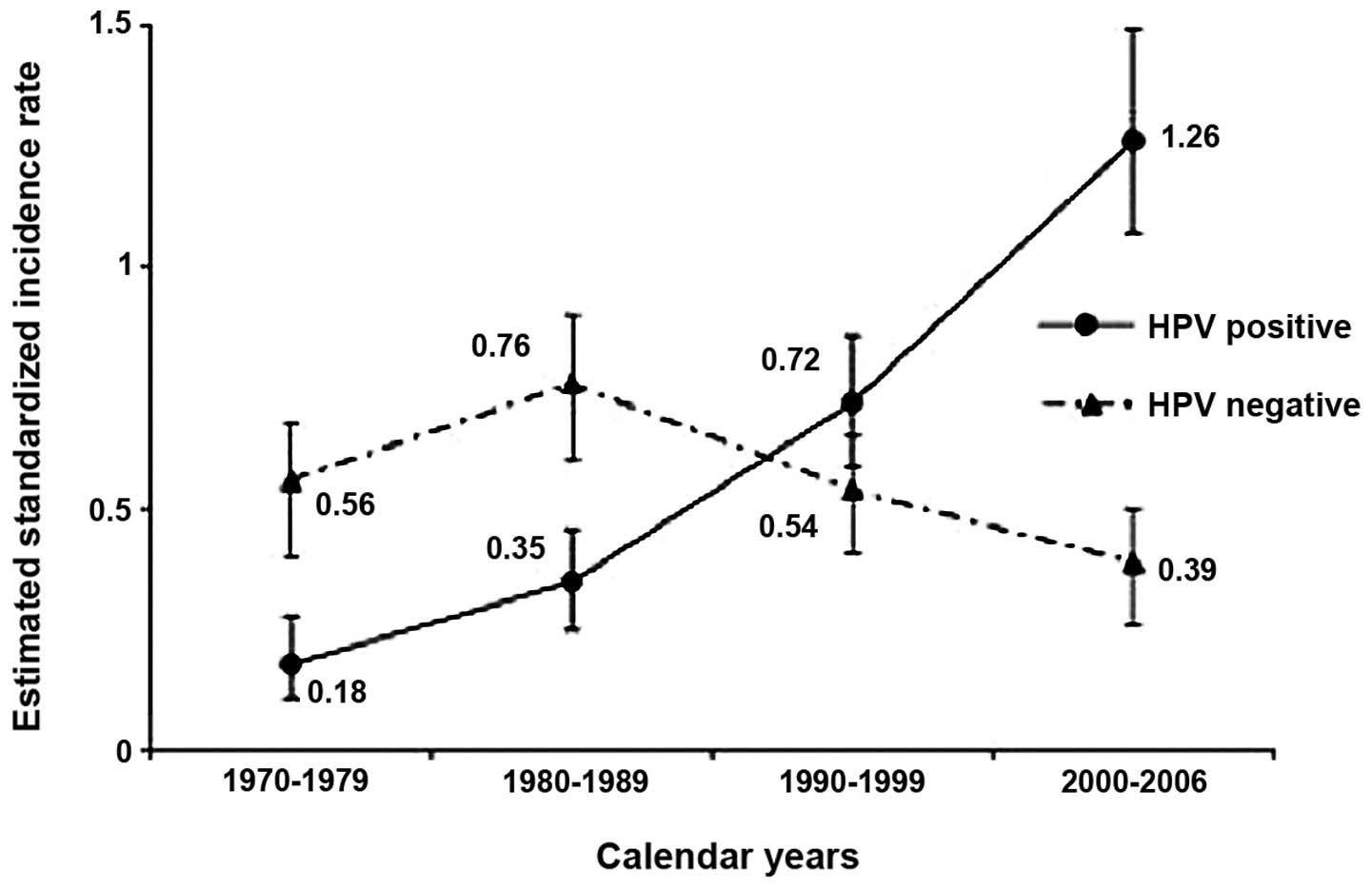
In 2006, a 2.8-fold rise in the incidence of
tonsillar cancer was revealed between 1970–2002 in Stockholm,
Sweden and in parallel, a 2.9 increase in the percentage (23–68%)
of HPV-positive tonsillar cancer was found (6). In 2007, an emerging epidemic of HPV
associated OPSCC was suggested also in the US (9). This was followed by reports in 2009
and 2010 from Sweden showing a 7-fold increase in HPV-positive
tonsillar cancer between 1970–2007 and a decrease of HPV-negative
cancer most likely due to less smoking (Fig. 2), and a similar increase in the
incidence of HPV-positive base of tongue cancer between 1998–2006
(11,13). In 2011, an analogous development
with an increase in incidence of HPV-positive OPSCC and a decline
in HPV-negative OPSCC was also reported in the US (17). Furthermore, during much of the same
period accumulating reports from many Western countries conveyed
both a general increase of OPSCC as well as an increase in the
proportion of HPV-positive OPSCC (6–17).
The main explanation for this development was attributed to changes
in sexual habits with a significant correlation between
HPV-positive OPSCC, early sex debut as well as number of oral or
vaginal partners (42).
Nonetheless, oral- to-oral contact (open-mouth kissing) and oral
HPV-transmission at birth could also account for oral HPV infection
(43,44). To conclude, in many Western
countries there is a presently ongoing epidemic of HPV-associated
OPSCC.
HPV and OPSCC and treatment
New therapeutic and preventive strategies are
required since HPV-positive OPSCC today comprises a larger
proportion of all HNSCC (16). As
stated above, due to its poor prognosis treatment of HNSCC now
includes chemo-radiotherapy, surgery and also EGFR inhibitors with
more side effects and increasing expenses for society and this is
probably not required for 80% of patients with HPV-positive OPSCC
where conventional radiotherapy may be sufficient (16). Nevertheless, to taper therapy,
maintaining excellent survival and improved quality of life, as
well as decreased costs for society, better approaches to select
patients that respond well to therapy are necessary. In some cases,
less intensified radiotherapy has been offered to patients whose
tumors have been sensitive to chemotherapy, but the patients have
not felt confident to comply to this treatment without having
reassurance that this will not affect survival. Therefore it is
important to have more objective biomarkers that together with
positive HPV status can predict response to therapy.
Studies on HPV and other markers in
HPV-positive OPSCC in response to treatment
Numerous studies have focused on following OPSCC
response to treatment based on HPV DNA or RNA status, p16
expression, p53 expression, age gender and smoking as well as the
now more recent studies on other biomarkers (1–3,16,18,33).
As mentioned above, both the presence of HPV DNA/RNA and p16
overexpression are excellent prognostic markers especially combined
together or with being a never smoker (18,33).
In fact, with each package year of smoking, the prognosis
deteriorates (33). However,
several markers have also been analyzed in parallel to HPV status
and have also shown a very good credibility (45–50).
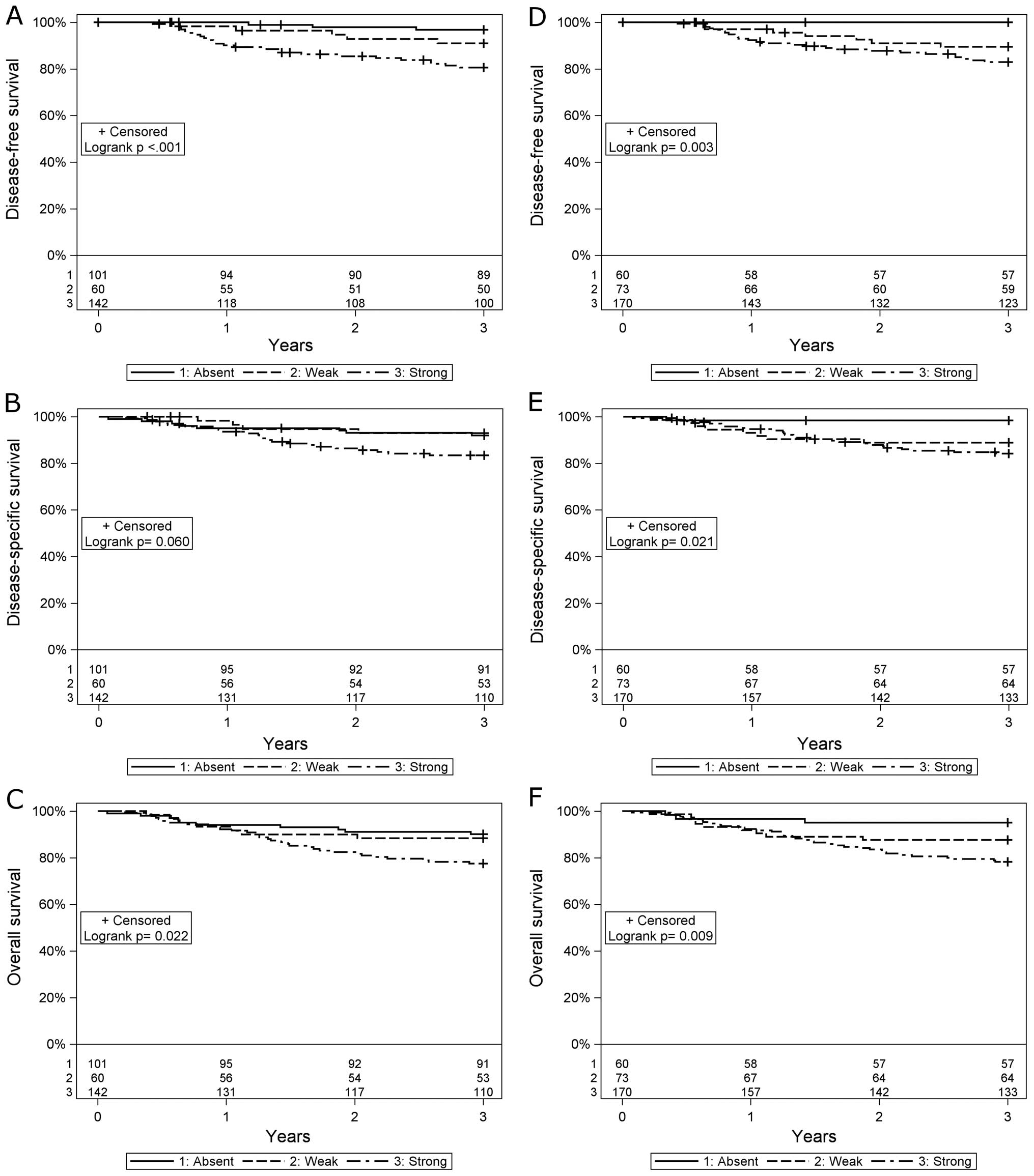
It has been shown that absent/low expression of MHC class I, CD44
or CD98 intensity staining is of very high prognostic value for
patients with HPV-positive OPSCC and e.g., for absent MHC class I
staining indicates a 95–100% probability of a 3-year disease-free
and overall survival (Fig. 3)
(45–49). Furthermore, having high
CD8+ tumor infiltrating lymphocyte (TIL) counts was also
of high prognostic value for patients with HPV-positive OPSCC
(50,51). MHC class II, Cox-2 expression, or
high numbers of CD4+ TILs did not influence prognosis
for patients with HPV-positive OPSCC (46,47,51).
The fact that low MHC class I expression in
HPV-positive OPSCC was a favorable prognostic factor is to some
extent surprising, since downregulation of MHC abrogates the immune
response (45,46). Moreover, there was no increase in
the number of NK-cells in the tumors (46). However, the low MHC class I
expression could be due to high functional HPV activity since both
E5 and E7 contribute to downregulation of MHC class I expression
and that treatment to some extent increases MHC class I expression
enhancing the immune response against these tumors (45,46).
Of note, in this respect in experimental models, HPV-positive
tumors were curable after cisplatin or radiation therapy only in
immunocompetent and not in immunoincompetent mice thus suggesting
that a functional immune response was necessary for final
elimination of the tumors (52).
It is noteworthy that for HPV-negative OPSCC high MHC class I
expression was a favorable prognostic factor as was low CD44
intensity expression and high number of CD8+ TILs
(45,46,48).
The data suggest that some prognostic markers could be specific for
only HPV-positive OPSCC, or HPV-negative OPSCC, while others could
be of more general use.
To obtain more molecular and immunological knowledge
would be valuable. In addition, so far none of the markers above
select all patients with HPV-positive OPSCC with a favorable
clinical outcome. It is therefore important to identify additional
biomarkers that can increase our probability to select as many
patients as possible for randomized clinical trials with lesser
therapy.
Some studies have focused on the role of miRNAs in
HPV-associated cancers and have found that the miRNA profiles in
HPV-positive OPSCC are more similar to those in HPV-positive
cervical cancer as compared to those obtained in HPV-negative OPSCC
(53). However, so far no miRNAs
have been linked to clinical outcome.
In summary, some information is available with
regard to markers e.g., overexpression of 16Ink4a, low
MHC class I, CD44 and CD98 expression, having high CD8+
TIL counts, and being a never-smoker to guide treatment strategy
for patients with HPV-positive OPSCC (18,33,45–51).
In addition, prospective randomized studies including patients with
HPV-positive OPSCC and the above markers, using different therapies
could be of benefit for the patients and to progress towards
better-tailored treatment. Nevertheless, there is still an urgent
need to identify additional markers, since the ones above do not
identify all the patients with a high probability of a good
response to therapy.
Prevention of HPV-positive OPSCC
HPV16 is detected in 80–90% of all HPV-positive
OPSCC indicating that the present HPV vaccines should be able to
eventually counteract the ongoing epidemic increase of HPV-positive
OPSCC, but this could take time (1–4).
Oral HPV prevalence has been stated to be approximately 3–9%, and
transmitted by oral-genital or oral-oral contact, or by maternal
transmission (43,54–58).
Nonetheless, due to the vast production of saliva, oral HPV
prevalence is relatively problematic to screen compared to cervical
HPV prevalence and may therefore yield false negative results more
often. However, based on unpublished data we suggest that a very
high oral HPV signal may indicate the presence of an HPV-positive
OPSCC. Serology has also been attempted to predict HPV-associated
disease (59). Other studies have
focused on cytology, but so far this has not been very useful
(60). Should screening for
HPV-positive OPSCC become necessary, most likely high HPV signals
in mouthwashes could still be the best type of approach.
Finally, since HPV-positive OPSCC is still
increasing in many Western countries, and the HPV vaccine has the
potential to prevent also oral HPV infection (61), it would be of importance to
vaccinate both girls and boys.
Acknowledgements
This study was supported in part by
the Swedish Research Council, the Swedish Cancer Foundation, the
Stockholm Cancer Society, the Stockholm City Council and the
Karolinska Institutet, Sweden.
References
|
1.
|
Gillison ML, Koch WM, Capone RB, Spafford
M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE,
Shah KV and Sidransky D: Evidence for a causal association between
human papillomavirus and a subset of head and neck cancers. J Natl
Cancer Inst. 92:709–720. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Mellin H, Friesland S, Lewensohn R,
Dalianis T and Munck-Wikland E: Human papillomavirus (HPV) DNA in
tonsillar cancer: clinical correlates, risk of relapse, and
survival. Int J Cancer. 89:300–304. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Dahlstrand HM and Dalianis T: Presence and
influence of human papillomaviruses (HPV) in tonsillar cancer. Adv
Cancer Res. 93:59–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
WHO: IARC Monographs on the Evaluation of
Carcinogenic Risk to Humans. International Agency for Research on
Cancer; Lyon: 2007
|
|
5.
|
Robinson KL and Macfarlane GJ:
Oropharyngeal cancer incidence and mortality in Scotland: are rates
still increasing? Oral Oncol. 39:31–36. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Hammarstedt L, Lindquist D, Dahlstrand H,
Romanitan M, Dahlgren LO, Joneberg J, Creson N, Lindholm J, Ye W,
Dalianis T and Munck-Wikland E: Human papillomavirus as a risk
factor for the increase in incidence of tonsillar cancer. Int J
Cancer. 119:2620–2623. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Conway DI, Stockton DL, Warnakulasuriya
KA, Ogden G and Macpherson LM: Incidence of oral and oropharyngeal
cancer in United Kingdom (1990–1999) - recent trends and regional
variation. Oral Oncol. 42:586–592. 2006.PubMed/NCBI
|
|
8.
|
Hammarstedt L, Dahlstrand H, Lindquist D,
Onelöv L, Ryott M, Luo J, Dalianis T, Ye W and Munck-Wikland E: The
incidence of tonsillar cancer in Sweden is increasing. Acta
Otolaryngol. 1279:988–992. 2007. View Article : Google Scholar
|
|
9.
|
Sturgis EM and Cinciripini PM: Trends in
head and neck cancer incidence in relation to smoking prevalence:
an emerging epidemic of human papillomavirus-associated cancers?
Cancer. 110:1429–1435. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Chaturvedi AK, Engels EA, Anderson WF and
Gillison ML: Incidence trends for human papillomavirus-related and
-unrelated oral squamous cell carcinomas in the United States. J
Clin Oncol. 26:612–619. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Nasman A, Attner P, Hammarstedt L, Du J,
Eriksson M, Giraud G, Sparén P, Ye W, Dahlstrand H, Munck-Wikland E
and Dalianis T: Incidence of human papillomavirus (HPV) positive
tonsillar carcinoma in Stockholm, Sweden: an epidemic of
viral-induced carcinoma? Int J Cancer. 125:362–366. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Braakhuis BJ, Visser O and Leemans CR:
Oral and oropharyngeal cancer in The Netherlands between 1989 and
2006: increasing incidence, but not in young adults. Oral Oncol.
45:e85–e89. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Attner P, Du J, Nasman A, Hammarstedt L,
Ramqvist T, Lindholm J, Marklund L, Dalianis T and Munck-Wikland E:
The role of human papillomavirus in the increased incidence of base
of tongue cancer. Int J Cancer. 126:2879–2884. 2010.PubMed/NCBI
|
|
14.
|
Marur S, D’Souza G, Westra WH and
Forastiere AA: HPV-associated head and neck cancer: a virus-related
cancer epidemic. Lancet Oncol. 11:781–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Ramqvist T and Dalianis T: Oropharyngeal
epidemic and human papillomavirus. Emerg Infect Dis. 16:1671–1677.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Ramqvist T and Dalianis T: An epidemic of
oropharyngeal squamous cell carcinoma (OSCC) due to human
papilloma-virus (HPV) infection and aspects of treatment and
prevention. Anticancer Res. 31:1515–1519. 2011.PubMed/NCBI
|
|
17.
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, Liu L, Lynch CF, Wentzensen N, Jordan RC, Altekruse S,
Anderson WF, Rosenberg PS and Gillison ML: Human papillomavirus and
rising oropharyngeal cancer incidence in the United States. J Clin
Oncol. 29:4294–4301. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Lindquist D, Romanitan M, Hammarstedt L,
Nasman A, Dahlstrand H, Lindholm J, Onelöv L, Ramqvist T, Ye W,
Munck-Wikland E and Dalianis T: Human papillomavirus is a
favourable prognostic factor in tonsillar cancer and its oncogenic
role is supported by the expression of E6 and E7. Mol Oncol.
1:350–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Attner P, Du J, Näsman A, Hammarstedt L,
Ramqvist T, Lindholm J, Marklund L, Dalianis T and Munck-Wikland E:
Human papillomavirus and survival in patients with base of tongue
cancer. Int J Cancer. 128:2892–2897. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Licitra L, Bernier J, Grandi C, Merlano M,
Bruzzi P and Lefebvre JL: Cancer of the oropharynx. Crit Rev Oncol
Hematol. 41:107–122. 2002. View Article : Google Scholar
|
|
21.
|
Tommasino M: The human papillomavirus
family and carcinogenesis. Semin Cancer Biol. Dec 4–2013.(Epub
ahead of print). pii: S1044-579X(13)00123-5. View Article : Google Scholar
|
|
22.
|
Zur Hausen H: Papillomavirus infections: a
major cause of human cancer. Infections Causing Human Cancer.
Wiley-VCH Verlag; Weinheim: pp. 145–243. 2006, PubMed/NCBI
|
|
23.
|
Oguejiofor KK, Hall JS, Mani N, Douglas C,
Slevin NJ, Homer J, Hall G and West CM: The prognostic significance
of the biomarker p16 in oropharyngeal squamous cell carcinoma. Clin
Oncol (R Coll Radiol). 25:630–638. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Paavonen J, Jenkins D, Bosch FX, Naud P,
Salmerón J, Wheeler CM, Chow SN, Apter DL, Kitchener HC,
Castellsague X, de Carvalho NS, Skinner SR, Harper DM, Hedrick JA,
Jaisamrarn U, Limson GA, Dionne M, Quint W, Spiessens B, Peeters P,
Struyf F, Wieting SL, Lehtinen MO and Dubin G: HPV PATRICIA study
group: Efficacy of a prophylactic adjuvanted bivalent L1
virus-like-particle vaccine against infection with human
papillomavirus types 16 and 18 in young women: an interim analysis
of a phase III double-blind, randomised controlled trial. Lancet.
369:2161–2170. 2007. View Article : Google Scholar
|
|
25.
|
Future II Study Group: Qvadrivalent
vaccine against human papillomavirus to prevent high-graded
cervical lesions. N Engl J Med. 356:1915–1927. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Ramqvist T, Andreasson K and Dalianis T:
Vaccination, immune and gene therapy based on virus-like particles
against viral infections and cancer. Expert Opin Biol Ther.
7:997–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Dalianis T: Immunotherapy for
polyomaviruses: opportunities and challenges. Immunotherapy.
4:617–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mellin H, Dahlgren L, Munck-Wikland E,
Lindholm J, Rabbani H, Kalantari M and Dalianis T: Human
papillomavirus type 16 is episomal and a high viral load may be
correlated to better prognosis in tonsillar cancer. Int J Cancer.
102:152–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Koskinen WJ, Chen RW, Leivo I, Mäkitie A,
Bäck L, Kontio R, Suuronen R, Lindqvist C, Auvinen E, Molijn A,
Quint WG, Vaheri A and Aaltonen LM: Prevalence and physical status
of human papillomavirus in squamous cell carcinomas of the head and
neck. Int J Cancer. 107:401–406. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mellin Dahlstrand H, Lindquist D,
Bjornestal L, Ohlsson A, Dalianis T, Munck-Wikland E and Elmberger
G: P16(INK4a) correlates to human papillomavirus presence, response
to radio-therapy and clinical outcome in tonsillar carcinoma.
Anticancer Res. 25:4375–4383. 2005.PubMed/NCBI
|
|
31.
|
Mellin H, Friesland S, Auer G, Dalianis T
and Munck-Wikland E: Human papillomavirus and DNA ploidy in
tonsillar cancer -correlation to prognosis. Anticancer Res.
23:2821–2828. 2003.PubMed/NCBI
|
|
32.
|
Dahlgren L, Mellin H, Wangsa D,
Heselmeyer-Haddad K, Björnestål L, Lindholm J, Munck-Wikland E,
Auer G, Ried T and Dalianis T: Comparative genomic hybridization
analysis of tonsillar cancer reveals a different pattern of genomic
imbalances in human papillomavirus-positive and -negative tumors.
Int J Cancer. 107:244–249. 2003. View Article : Google Scholar
|
|
33.
|
Ang KK, Harris J, Wheeler R, Weber R,
Rosenthal DI, Nguyen-Tan PF, Westra WH, Chung CH, Jordan RC, Lu C,
Kim H, Axelrod R, Silverman CC, Redmond KP and Gillison ML: Human
papillomavirus and survival of patients with oropharyngeal cancer.
N Engl J Med. 363:24–35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
de Roda Husman AM, Walboomers JM, van den
Brule AJ, Meijer CJ and Snijders PJ: The use of general primers GP5
and GP6 elongated at their 3’ ends with adjacent highly conserved
sequences improves human papillomavirus detection by PCR. J Gen
Virol. 76:1057–1062. 1995.
|
|
35.
|
Tieben LM, ter Schegget J, Minnaar RP,
Bouwes Bavinck JN, Berkhout RJ, Vermeer BJ, Jebbink MF and Smits
HL: Detection of cutaneous and genital HPV types in clinical
samples by PCR using consensus primers. J Virol Methods.
42:265–279. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
van den Brule AJ, Pol R,
Fransen-Daalmeijer N, Schouls LM, Meijer CJ and Snijders PJ:
GP5+/6+ PCR followed by reverse line blot
analysis enables rapid and high-throughput identification of human
papillomavirus genotypes. J Clin Microbiol. 40:779–787. 2002.
|
|
37.
|
Clavel C, Masure M, Bory JP, Putaud I,
Mangeonjean C, Lorenzato M, Gabriel R and Quereux C: Hybrid Capture
II-based human papillomavirus detection, a sensitive test to detect
in routine high-grade cervical lesions: a preliminary study on 1518
women. Br J Cancer. 80:1306–1311. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Gravitt PE, Peyton CL, Apple RJ and
Wheeler CM: Genotyping of 27 human papillomavirus types by using L1
consensus PCR products by a single-hybridization, reverse line blot
detection method. J Clin Microbiol. 36:3020–3027. 1998.
|
|
39.
|
Schmitt M, Bravo IG, Snijders PJ, Gissmann
L, Pawlita M and Waterboer T: Bead-based multiplex genotyping of
human papillomaviruses. J Clin Microbiol. 44:504–512. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Smeets SJ, Hesselink AT, Speel EJ,
Haesevoets A, Snijders PJ, Pawlita M, Meijer CJ, Braakhuis BJ,
Leemans CR and Brakenhoff RH: A novel algorithm for reliable
detection of human papillomavirus in paraffin-embedded head and
neck cancer specimen. Int J Cancer. 121:2465–2472. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Marklund L, Näsman A, Ramqvist T, Dalianis
T, Munck-Wikland E and Hammarstedt L: Prevalence of human
papillomavirus and survival in oropharyngeal cancer other than
tonsil or base of tongue cancer. Cancer Med. 1:82–88. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Anaya-Saavedra G, Ramirez-Amador V,
Irigoyen-Camacho ME, Garcia-Cuellar CM, Guido-Jimenez M,
Mendez-Martinez R and García-Carrancá A: High association of human
papillomavirus infection with oral cancer: a case-control study.
Arch Med Res. 39:189–197. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
D’Souza G, Agrawal Y, Halpern J, Bodison S
and Gillison ML: Oral sexual behaviors associated with prevalent
oral human papillomavirus infection. J Infect Dis. 199:1263–1269.
2009.PubMed/NCBI
|
|
44.
|
Syrjänen S and Puranen M: Human
papillomavirus infections in children: the potential role of
maternal transmission. Crit Rev Oral Biol Med. 11:259–274.
2000.PubMed/NCBI
|
|
45.
|
Näsman A, Andersson E, Nordfors C, Grün N,
Johansson H, Munck-Wikland E, Massucci G, Dalianis T and Ramqvist
T: MHC class I expression in HPV positive and negative tonsillar
squamous cell carcinoma in correlation to clinical outcome. Int J
Cancer. 132:72–81. 2013.PubMed/NCBI
|
|
46.
|
Näsman A, Andersson E, Marklund L,
Tertipis N, Hammarstedt-Nordenwall L, Nyberg T, Munck-Wikland E,
Masucci GV, Ramqvist T and Dalianis T: HLA class I and II
expression in oropharyngeal squamous cell carcinoma in relation to
tumor HPV status and clinical outcome. PloS One.
8:e77025432013.PubMed/NCBI
|
|
47.
|
Lindquist D, Ahrlund-Richter A, Tarján M,
Tot T and Dalianis T: Intense CD44 expression is a negative
prognostic factor in tonsillar and base of tongue cancer.
Anticancer Res. 32:153–161. 2012.PubMed/NCBI
|
|
48.
|
Näsman A, Nordfors C, Grün N,
Munck-Wikland E, Ramqvist T, Marklund L, Lindquist D and Dalianis
T: Absent/weak CD44 intensity and positive human papillomavirus
(HPV) status in oropharyngeal squamous cell carcinoma indicates a
very high survival. Cancer Med. 2:507–518. 2013.PubMed/NCBI
|
|
49.
|
Rietbergen MM, Martens-de Kemp SR,
Bloemena E, Witte BI, Brink A, Baatenburg de Jong RJ, Leemans CR,
Braakhuis BJ and Brakenhoff RH: Cancer stem cell enrichment marker
CD98: A prognostic factor for survival in patients with human
papillomavirus-positive oropharyngeal cancer. Eur J Cancer. Dec
4–2013.(Epub ahead of print). pii: S0959-8049(13)01005-8.
View Article : Google Scholar
|
|
50.
|
Näsman A, Romanitan M, Nordfors C, Grün N,
Johansson H, Hammarstedt L, Marklund L, Munck-Wikland E, Dalianis T
and Ramqvist T: Tumor infiltrating CD8+ and Foxp3+
lymphocytes correlate to clinical outcome and human papillomavirus
(HPV) status in tonsillar cancer. PLoS One. 7:e387112012.
|
|
51.
|
Nordfors C, Grün N, Tertipis N,
Ahrlund-Richter A, Haeggblom L, Sivars L, Du J, Nyberg T, Marklund
L, Munck-Wikland E, Näsman A, Ramqvist T and Dalianis T: CD8(+) and
CD4(+) tumour infiltrating lymphocytes in relation to human
papillomavirus status and clinical outcome in tonsillar and base of
tongue squamous cell carcinoma. Eur J Cancer. 49:2522–2530.
2013.
|
|
52.
|
Spanos WC, Nowicki P, Lee DW, Hoover A,
Hostager B, Gupta A, Anderson ME and Lee JH: Immune response during
therapy with cisplatin or radiation for human
papillomavirus-related head and neck cancer. Arch Otolaryngol Head
Neck Surg. 135:1137–1146. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Lajer CB, Garnæs E, Friis-Hansen L,
Norrild B, Therkildsen MH, Glud M, Rossing M, Lajer H, Svane D,
Skotte L, Specht L, Buchwald C and Nielsen FC: The role of miRNAs
in human papilloma virus (HPV)-associated cancers: bridging between
HPV-related head and neck cancer and cervical cancer. Br J Cancer.
106:1526–1534. 2012. View Article : Google Scholar
|
|
54.
|
Kreimer AR, Bhatia RK, Messeguer AL,
Gonzalez P, Herrero R and Giuliano AR: Oral human papillomavirus in
healthy individuals: a systematic review of the literature. Sex
Transm Dis. 37:386–391. 2010.
|
|
55.
|
Rautava J and Syrjanen S: Human
papillomavirus infections in the oral mucosa. J Am Dent Assoc.
142:905–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56.
|
Du J, Nordfors C, Ahrlund-Richter A,
Sobkowiak M, Romanitan M, Näsman A, Andersson S, Ramqvist T and
Dalianis T: Prevalence of oral human papillomavirus infection among
youth, Sweden. Emerg Infect Dis. 18:1468–1471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
57.
|
Nordfors C, Grün N, Haeggblom L, Tertipis
N, Sivars L, Mattebo M, Larsson M, Häggström-Nordin E, Tydén T,
Ramqvist T and Dalianis T: Oral human papillomavirus prevalence in
high school students of one municipality in Sweden. Scand J Infect
Dis. 45:878–881. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Steinau M, Hariri S, Gillison ML, Broutian
TR, Dunne EF, Tong ZY, Markowitz LE and Unger ER: Cervical and oral
HPV prevalence among females in the United States. J Infect Dis.
Jan 10–2014.(Epub ahead of print).
|
|
59.
|
D’Souza G, Kreimer AR, Viscidi R, Pawlita
M, Fakhry C, Koch WM, Westra WH and Gillison ML: Case-control study
of human papillomavirus and oropharyngeal cancer. N Engl J Med.
356:1944–1956. 2007.PubMed/NCBI
|
|
60.
|
Fakhry C, Rosenthal BT, Clark DP and
Gillison ML: Associations between oral HPV16 infection and
cytopathology: evaluation of an oropharyngeal ‘pap-test equivalent’
in high-risk populations. Cancer Prev Res. 4:1378–1384.
2011.PubMed/NCBI
|
|
61.
|
Herrero R, Quint W, Hildesheim A, Gonzalez
P, Struijk L, Katki HA, Porras C, Schiffman M, Rodriguez AC,
Solomon D, Jimenez S, Schiller JT, Lowy DR, van Doorn LJ and
Wacholder S: Reduced prevalence of oral human papillomavirus (HPV)
4 years after bivalent HPV vaccination in a randomized clinical
trial in Costa Rica. PLoS One. 8:e683292013.PubMed/NCBI
|