Introduction
Oral squamous cell carcinoma (OSCC) is the most
common malignancy in the head and neck region with approximately
389,000 new cases presented yearly (1,2).
Despite recent advances in surgical, radiotherapy, and chemotherapy
treatment protocols, the 5-year survival rate of patients with OSCC
has remained at <60% (3). This
highlights the urgent need to develop novel approaches for the
prevention and treatment of OSCC.
It is well known that immune system functions as a
host defensive mechanism protecting against invading pathogens and
transformed cells such as cancer (4). However, increasing evidence suggests
that the prolonged presence of a host immune response brought on by
chronic infection can also lead to malignant transformation
(5,6). For example, individuals with
ulcerative colitis, a chronic inflammatory disease of the colon,
have a 10-fold higher likelihood of developing colorectal carcinoma
(7). Similarly, inflammatory
conditions of the liver, such as chronic hepatitis and cirrhosis,
are well-established risk factors for the development of
hepatocellular carcinoma (8,9).
Understanding the relationship among the immune cell community, the
tumor cell community, and the tumor microenvironment then becomes a
powerful tool in the development of new targeted therapies.
Manipulating the Toll-like receptors (TLRs), i.e.,
pathogen-recognition molecules in cancer cells, provides a method
of uncovering the pathways that likely contribute to this
relationship (10). TLRs are
members of the interleukin-1 receptor superfamily and play a
crucial role in the activation of innate immunity and the
subsequent inflammatory process (11,12).
Recently, carcinogenesis mediated by chronic inflammation was found
to be closely tied to abnormal TLR-9 expression. In our previous
study, we reported that over expression of TLR-9 in inflammatory
oral mucosa and OSCC tissues, as well as CpG-ODN induced TLR-9
stimulation increases tumor cell proliferation through increased
cyclin D1 expression (13,14). However, given the recent evidence
suggesting TLRs can also be involved in extracellular signaling,
our focus shifted to the effects of TLR-9 signaling on the T-cell
immune response and the underlying molecular mechanisms.
The purpose of this study is to investigate the
effect of TLR-9 signaling in human OSCC cells on human T-cell
immune response. More specifically, we aim to identify which
cytokines are key players in the signaling pathway and elucidate
the molecular mechanism of cytokine production in OSCC. With regard
to the mechanism downstream of TLR-9 activation, two main
inflammation-related pathways were investigated: NF-κB and AP-1
pathways.
Materials and methods
Reagents and cell culture.
Unmethylated phosphorothioate modified, human
specific CpG-ODN 2006 (5′-TCGTCGTTT TGTCGTTTTGTCGTT-3′) was
purchased from InVivoGen (San Diego, CA, USA) and dissolved into
endotoxin-free sterile distilled deionized H2O according
to the manufacturer's suggestion and used at the indicated
concentrations. The anti-TLR-9 antibody was purchased from Imgenex
(CA, USA). Both AP-1 specific inhibitor, curcumin, and the NF-κB
specific inhibitor, pyrrolidinedithiocarbamate (PDTC), were
obtained from Calbiochem (San Diego, CA, USA).
Human immortalized oral epithelial cell line, HIOEC
cells, and cancerous cell line, HB cells, in the cellular
carcinogenesis model of oral squamous cell carcinoma OSCC were used
as previously described (15,16).
Normal oral epithelial cells were obtained from surgical resections
of non-cancer patients and cultured routinely. All cells were
cultured in a humidified atmosphere of 5% CO2 at
37°C.
T-cell isolation and culture
T-cells were isolated from peripheral blood
collected from healthy human donors. Blood was layered over
Ficoll-Hypaque and centrifuged for 15 min at 2,000 rpm. The
peripheral blood mononuclear leukocytes were then removed, plated
in 24-well plates, and allowed to adhere for 2 h at 37°C to remove
macrophages/monocytes. Non-adherent T-cells were collected, washed
and plated in 96-well, round-bottom plates at a density of
2.5×105 cells per well on immobilized anti-CD3 (R&D
Systems, Minneapolis, MN, USA). T-cells were maintained in RPMI
culture medium with 10% heat-inactivated FBS, 200 U/ml penicillin
G, 200 μg/ml streptomycin sulfate, 500 μg/ml
amphotericin B, 5×10−5 M 2-mercaptoethanol
(Sigma-Aldrich, St. Louis, MO, USA) and 10 U/ml recombinant human
IL-2 (R&D Systems).
T-cell treatments
HIOEC and HB cells were first treated with CpG-ODN
(0.8 μM) for 24 h. Supernatants from CpG-ODN-treated HIOEC
or HB cells were collected and used to treat freshly isolated
T-cells for indicated duration. Treatment medium contained a range
of the collected supernatant from 0 to 40%. After the treatment
period, T-cells were washed and new medium was added for an
additional 24 h of incubation.
T-cell immune response assays
T-cell proliferation was assessed by 3-(4,
5-dimethylthiazol-2-yl)-5-
(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS)
analysis (Promega, Madison, WI, USA). MTS in its reduced form was
detected spectrophotometrically (absorbance at 492 nm) and assessed
at the indicated time-points.
Flow cytometric analysis
T-cell intracellular cytokine levels were measured
by flow cytometric analysis of immunostained cells. T-cells were
treated with monensin (GolgiStop) for 2 h prior to antibody
staining. Anti-CD16/CD32 monoclonal antibodies against the
FcγII/III receptors and mouse serum were used to block non-specific
binding. Cell surface antigen staining was performed using anti-CD4
and anti-CD8 monoclonal antibodies. After staining, cells were
washed twice, fixed and permeabilized with Cytofix/Cytoperm. Cells
were then stained with anti-IFN-γ, anti-granzyme B or anti-perforin
antibodies. Marker channels were set using isotype control
antibodies. Flow cytometric analysis was performed on a BD
FACSCanto flow cytometer using FACS Diva flow cytometry analysis
software. All flow cytometry reagents were obtained from BD
Biosciences (San Jose, CA, USA).
ELISA analysis
IFN-γ, IL-1α, IL-4, IL-6, IL-8, CM-GSF and VEGF in
cell supernatants were measured using a fluorometric analysis kit
according to the manufacturer's recommendations (Chemicon,
Temecula, CA, USA). The samples were analyzed with a plate reader
by optical density (OD) at a wavelength of 405 nm. Readings were
conducted in triplicate.
Western blot analysis
The procedure was performed as previously described
(17). The following antibodies
were used: (from Santa Cruz Biotechnology, Santa Cruz, CA, USA)
anti-IL-1α antibody (dilution 1:150), anti-IL-4 antibody (dilution
1:200), anti-IL-6 antibody (dilution 1:300), anti-IL-8 antibody
(dilution 1:200), anti-GM-CSF antibody (dilution 1:300), anti-VEGF
antibody (dilution 1:150), anti-p50 antibody (dilution 1:200),
anti-p65 antibody (dilution 1:200), anti-IκBα antibody (dilution
1:300), anti-phospho-IκBα antibody (dilution 1:200), anti-c-jun
antibody (dilution 1:200), anti-jun-B antibody (dilution 1:150) and
anti-jun-D antibody (dilution 1:200), (from Oncogene Science, USA),
anti-c-fos (dilution 1:200), anti-fos-B antibody (dilution 1:250),
and (from Sigma, USA) anti-β-actin antibody (dilution
1:10,000).
Small interfering RNA preparation and
cell transfection
Chemically synthesized human TLR-9-specific siRNAs
(sense CUGUCCUUCAAUUACCAAAtt; antisense GUAAUUG AAGGACAGgt) and the
control non-silencing siRNA (sense UUCUCCGAACGUGUCACGUtt, antisense
ACGUGACAC GUUCGGAGAA) were purchased from MWG (Ebersberg, Germany).
For siRNA transfection, 3×105 HB cells/well were plated
in 6-well plate and transfected by using Amaxa Nucleofector™
(Amaxa, Köln, Germany) according to the manufacturer's protocol
(Nucleofector™ Solution V, Nucleofector™ program G-16) with 2
μg siRNA per 106 cells. After 48 h of
transfection, TLR-9 expression was analyzed by western blot
analysis.
Nuclear extract and electrophoretic
mobility shift assay (EMSA)
HB cells were treated with 0.8 μM CpG-ODN for
the indicated time period (0–24 h), and nuclear extracts were
prepared as described previously (18). The sequences of the
oligonucleotides used were 5′-CGCTTGATGAGTCAGCCG GAA-3′ and
5′-AGTTGAGGGGACTTTCCCAGG-3′ for AP-1 and NF-κB, respectively.
Chromatin immunoprecipitation (ChIP)
assays
Chromatin immunoprecipitation (ChIP) assays and
subsequent real-time PCR analysis was performed as described
(19). The PCR primers specific to
the AP-1 binding region of the human IL-6 promoter were:
5′-GAACTGACCTGACTTACATA-3′ and 5′-TTGAGACTCA-TGGGAAAATCC-3′. The
PCR primers specific to the NF-κB binding region of the IL-6
promoter were: 5′-TAGAGCTTCTCTTTCGTTCCCGGT-3′ and 5′-TGT
GTCTTGCGATGCTAAAGGACG-3′.
Statistical analyses
Data are presented as mean ± standard errors from at
least three independent experiments. The ANOVA test was used to
evaluate the differences among the groups treated with each
concentration of CpG-ODN or supernatants from CpG-ODN-treated HB
cells, and the differences between two groups were assessed using
Student's t-test. Statistical significance was defined as P<0.05
for all tests.
Results
Supernatants from CpG-ODN-treated HB
cells stimulate T-cell proliferation
We first examined the effect of supernatants from
CpG-ODN-treated cells (NO or normal epithelial cells, HIOEC and HB
cells) on T-cell proliferation. The assay was performed 24 h after
the removal of the supernatant. As shown in Fig. 1A, T-cells treated with normal
epithelial cell supernatant (40% for 24 h) show a slight increase
in proliferation compared to the control (0% for 24 h). However,
when treated with supernatants from CpG-ODN-treated HIOEC or HB
cells (40% for 24 h); T-cells exhibited a significantly greater
increase in proliferation compared to their respective control
groups (0%, 24 h). This effect was confirmed in both a
dose-dependent (from 0 to 40% supernatant composition) and
time-dependent (0–24 h) manner (Fig.
1B and C). T-cell proliferation increased ∼30 and 50% with
treatments of 20 and 40% supernatants (CpG-ODN-treated HB cell, 24
h) respectively. With regard to T-cell subtype, CD4+ and
CD8+ T-cell counts were both higher in the treatment
groups compared to the no-treatment group by flow cytometric
analysis (Fig. 1D).
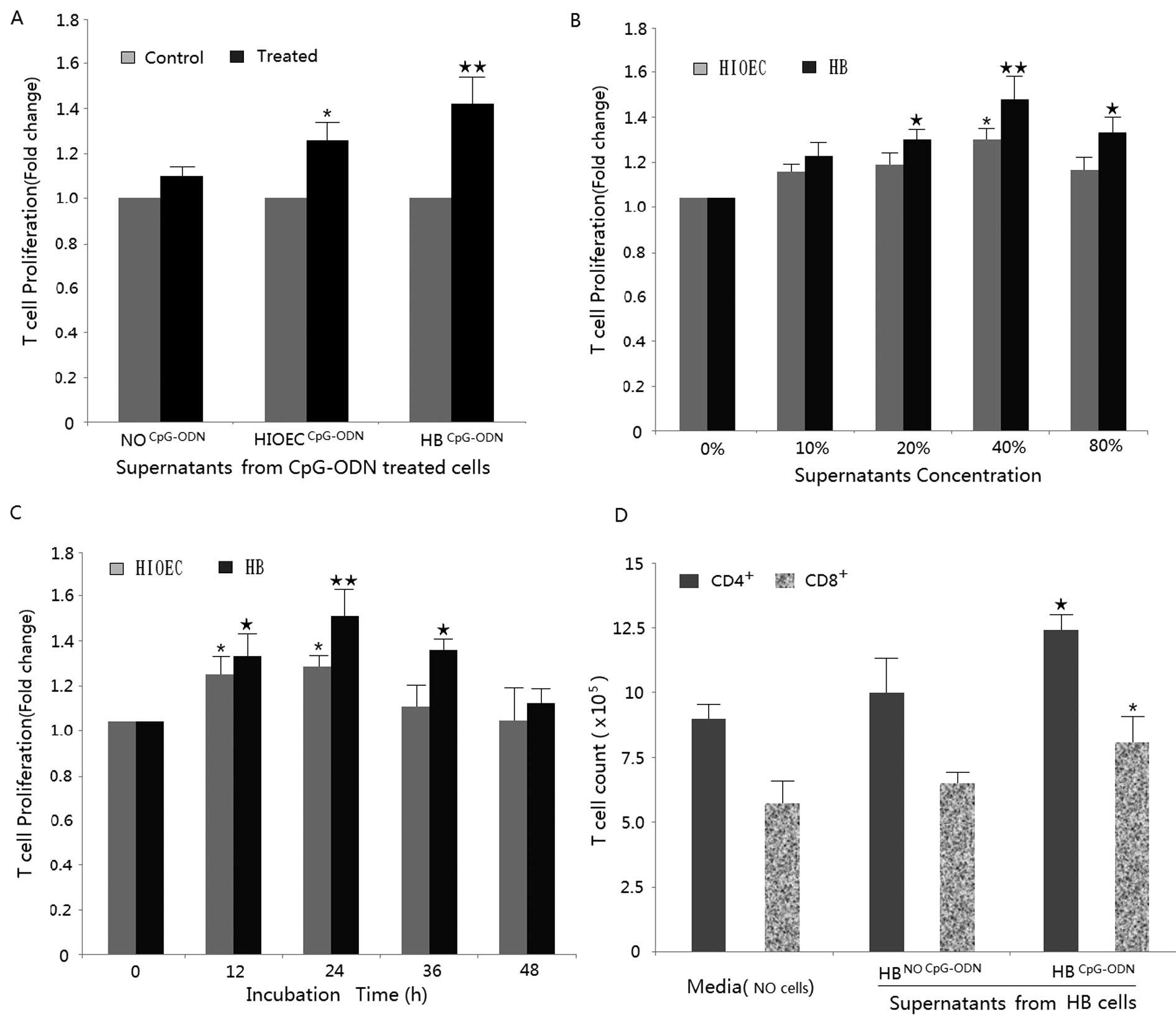 | Figure 1.Supernatants from CpG-ODN-treated
HIOEC and HB cell lines inhibit T-cell proliferation. (A) Normal
oral epithelial cells, HIOEC cells and HB cells were first treated
with 0.8 μM CpG-ODN for 24 h. Then, healthy donor T-cells
were cultured for 24 h with 40% supernatants from CpG-ODN treated
cells. Proliferation was assessed by MTS analysis in response to
anti-CD3 stimulation. Data show supernatants from CpG-ODN treated
HIOEC cells and HB cells enhanced proliferation of T-cell compared
with the control (non-treated) group (*P<0.05,
compared with the control HIOEC; ★★P<0.01, compared
with the control HB). (B) T-cells were treated with various
concentrations of (0, 10, 20, 40 and 80%) supernatants from CpG-ODN
treated HIOEC/HB cells for 24 h and analyzed by MTS assay
(*P<0.05; **P<0.01, compared with the
control HIOEC) (★P<0.05; ★★P<0.01,
compared with the control HB). (C) Time-dependent effects on T-cell
proliferation in the presence of supernatants from CpG-ODN treated
HIOEC/HB cells by MTT assay (*P<0.05; compared with
the control HIOEC) (★P<0.05; ★★P<0.01,
compared with the control HB). (D) Proliferative response of
CD4+ and CD8+ T-cells were calculated based
on flow cytometric analysis, more CD4+ and
CD8+ T-cells are generated after treatment of 40%
supernatants from CpG-ODN treated HB cells for 24 h
(★P<0.05, compared with panel 1;
*P<0.05, compared with panel 2). The data are
presented as mean ± SD of three repeats from one independent
study. |
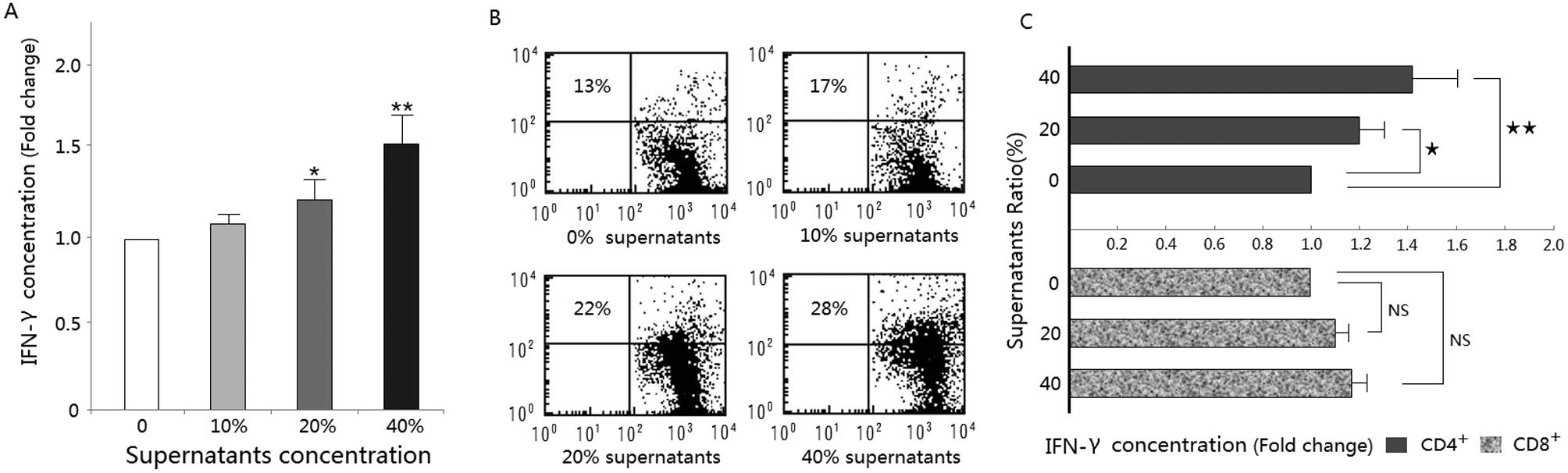
Supernatant from CpG-ODN-treated HB cells
promotes T-cell IFN-γ production
Production of IFN-γ increased in a dose-dependent
manner with supernatant composition and that of the 40% trion of
IFN-γ increased dose-dependently with supernatant composition and
that of the 40% treatment group produced a significantly greater
amount of IFN-γ than the control group (Fig. 2A). Flow cytometric analysis of
intra-cellular IFN-γ expression confirmed that treatment with the
supernatants increased the percent of total T-cells immunostaining
positive for IFN-γ (Fig. 2B).
Further analysis showed that the CD4+ T-cell population
was primarily responsible for the observed changes in IFN-γ
production (Fig. 2C).
IL-6 is involved in the evaluated T-cell
immune responses
To determine which cytokine(s) may be involved in
inducing the T-cell immune response, we harvested the supernatant
of CpG-ODN-treated HB cells after 24 h of culture. Cytokines
believed to play a critical role both in chronic inflammation and
T-cell activity, including IL-1α, IL-4, IL-6, IL-8, GM-CSF and
VEGF, were analyzed by ELISA. Data show that the IL-6
concentrations in the supernatant of CpG-ODN-treated HB cells were
significantly higher than that in the control group (P<0.01)
(Fig. 3A). This effect was
confirmed by western blot analysis (Fig. 3B and C). Neutralization of IL-6
using monoclonal IL-6 antibody resulted in a significant decrease
in T-cell proliferation and IFN-γ production (Fig. 3D). Therefore, the enhanced T-cell
immune response following treatment is at least partially mediated
through the increased secretion of IL-6.
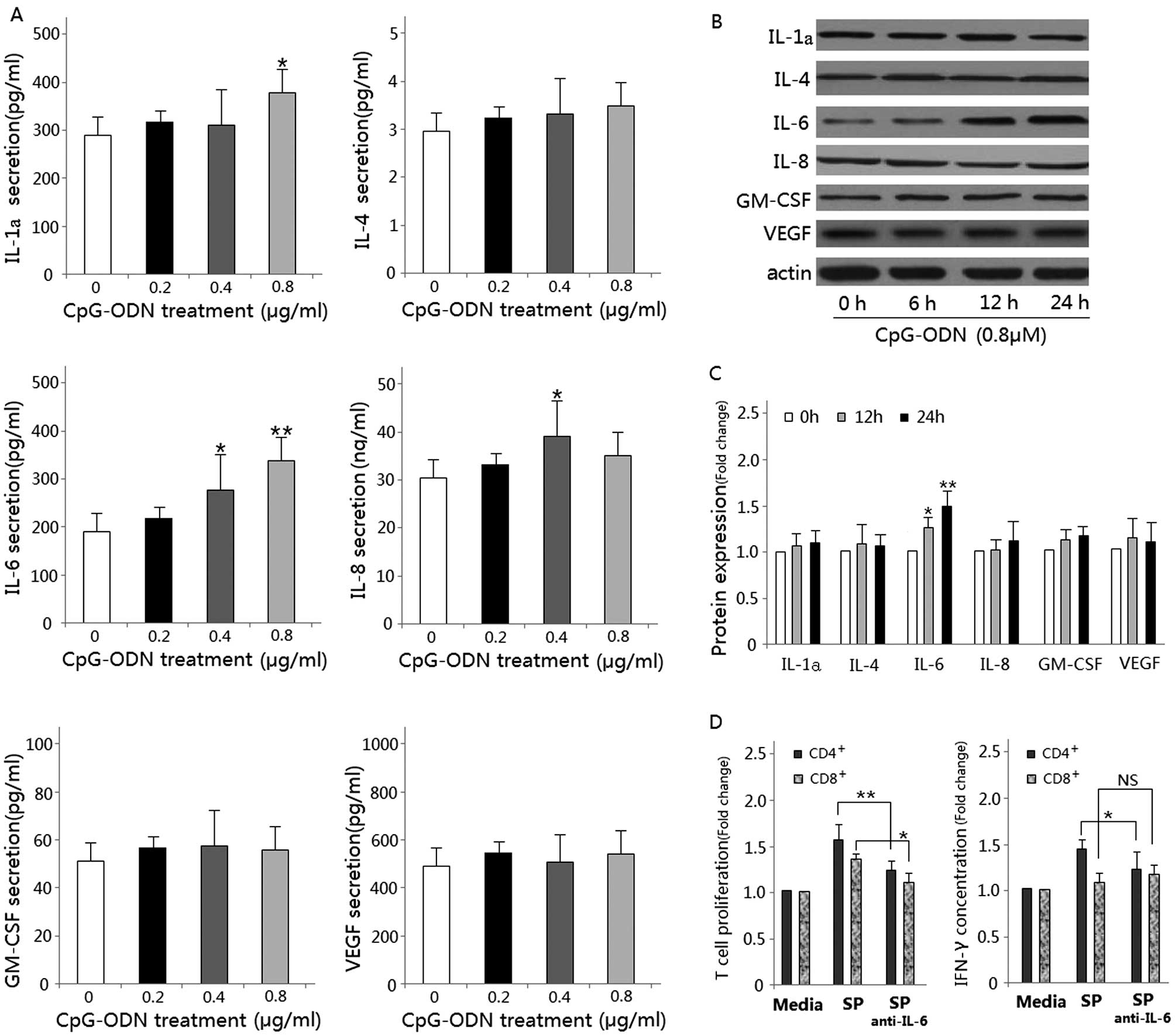 | Figure 3.Detection of cytokines secreted by
CpG-ODN treated HB cells. (A) IL-1α, IL-4, IL-6, IL-8, GM-CSF and
VEGF were measured by ELISA after HB cells culture for 24 h in
culture medium contain 0–0.8 μM CpG-ODN
(*P<0.05; **P<0.01, compared with panel
1). (B) Western blot analysis for IL-1α, IL-4, IL-6, IL-8, GM-CSF
and VEGF using lysates from HB cells treated with 0.8 μM
CpG-ODN for the indicated time periods (0–24 h). Immunoblotting for
each protein was done at least three times using independently
prepared lysates with similar results. (C) Changes in protein
levels compared with control as determined by densitometric
scanning of the immunoreactive bands (*P<0.05;
**P<0.01, compared with the control). (D)
Neutralization of IL-6 using monoclonal IL-6 antibody resulted in a
significant decrease in T-cell proliferation and IFN-γ expression
by flow cytometric analysis (*P<0.05;
**P<0.01). |
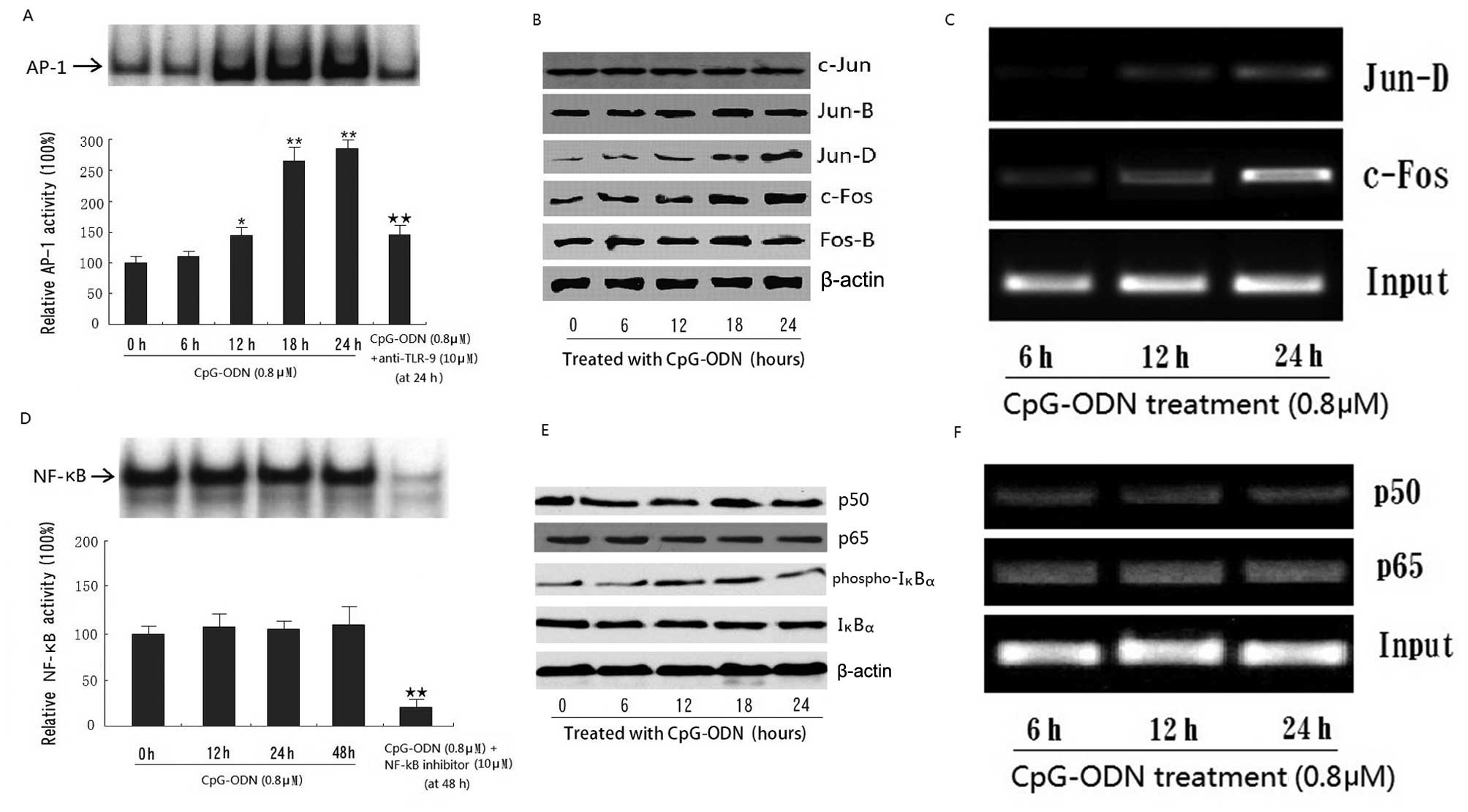
CpG-ODN enhances IL-6 secretion via the
AP-1 pathway
To examine changes in IL-6 expression at the
transcriptional level, we employed the NF-κB and AP-1 EMSA assays.
Results show that treatment with CpG-ODN significantly increased
the AP-1 activity in HB cells (Fig.
4A). To determine the altered subunits (c-Jun, Jun-B, Jun-D,
c-Fos and Fos-B), western blot analysis was performed on nuclear
extracts. Results show that treatment with CpG-ODN significantly
increased the expression of c-Fos and Jun-D, indicating that the
complex composed of these two subunits may play a more important
role in AP-1 activity in response to TLR-9 activation in HB cells
(Fig. 4B). ChIP analysis also
demonstrated that CpG-ODN treatment enhanced the DNA-binding
activity of AP-1 (c-Fos/Jun-D) to the promoter of IL-6 in a
time-dependent manner (Fig. 4C).
However, treatment with CpG-ODN did not produce any significant
changes in NF-κB activity by western blot analysis, EMSA or ChIP
analysis (Fig. 4D–F).
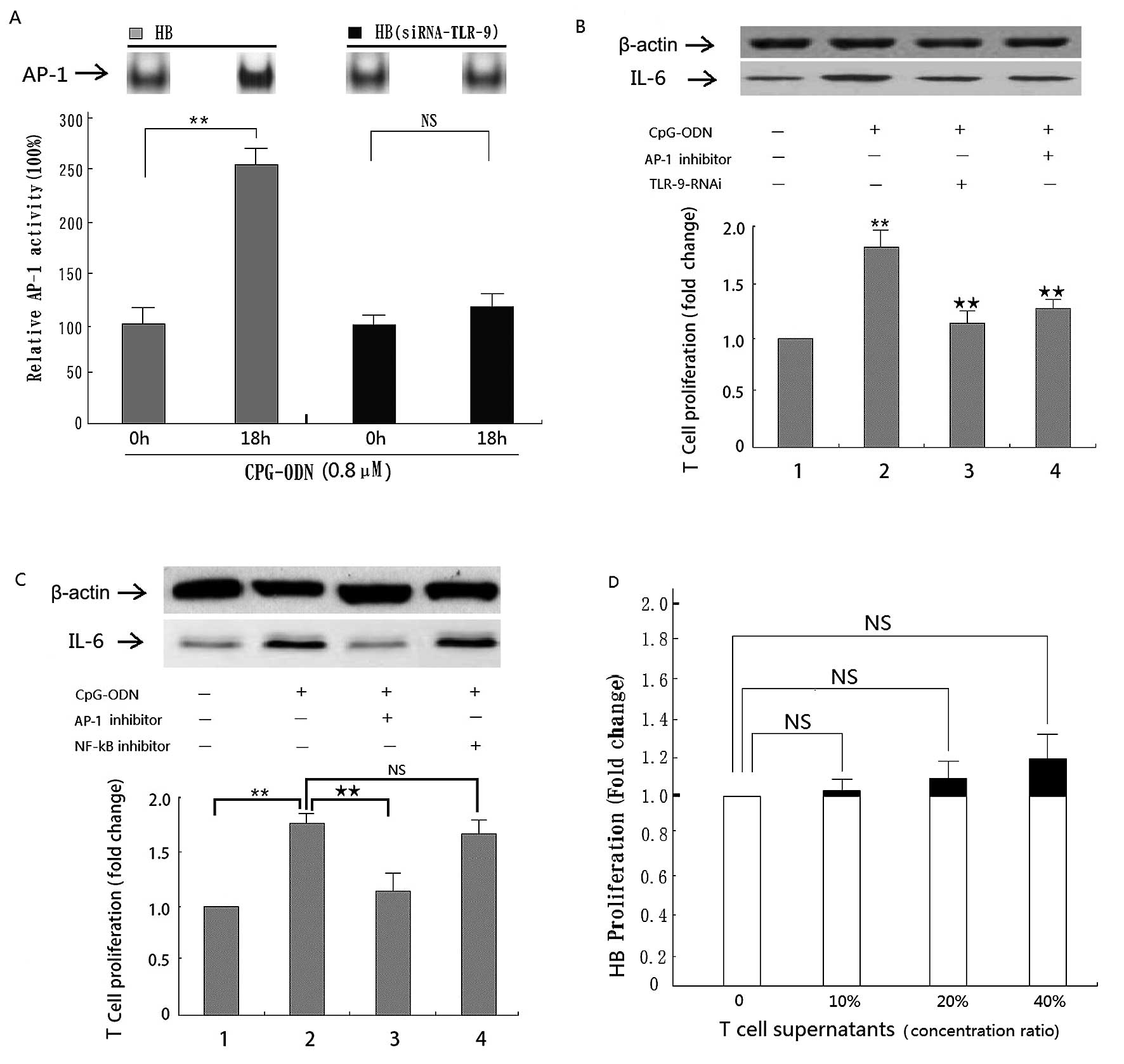
The role of TLR-9/AP-1 pathway in IL-6
mediated T-cell immune response promotion
To confirm the role of the TLR-9/AP-1 pathway in
promoting the IL-6 mediated T-cell immune response, TLR-9 activity
was inhibited using an antibody and siRNA in two separate assays.
Results show that inhibition by either method significantly
decreased AP-1 activity in CpG-ODN treated HB cells (Figs. 4A and 5A). In addition, siRNA inhibition of
TLR-9 significantly decreased IL-6 expression and, accordingly,
T-cell proliferation in CpG-ODN treated HB cells (Fig. 5B). Moreover, the same effect was
seen in cells treated additionally with AP-1 inhibitor curcumin
(Fig. 5B). However, there was no
significant effect in response to the NF-κB inhibitor (Fig. 5C).
Supernatants from treated T-cells
slightly promote HB cell proliferation
In order to assess whether the T-cells are in turn
inducing a response in the tumor cells, we treated HB cells with
T-cell supernatant. Although no significant changes were found it
is interesting to note that proliferation of HB cells increased in
a dose-dependent manner with the treatment (Fig. 5D). This phenomenon gives us some
insight into the ability of T-cells to manipulate the tumor
microenvironment and thus affecting tumor development.
Discussion
A growing body of evidence acknowledges a
pro-tumorigenic role for chronic inflammation in carcinogenesis by
promoting several pathways that induce proliferation and influence
the immune system (5,6). Based on the results of our previous
study (13,14), wherein stimulation of TLR-9 in oral
cancer cells resulted in increased proliferation, we initially
hypothezised that increased expression of TLR-9 may suppress the
immune response. Thus, we expected to observe inhibition of T-cell
proliferation and/or IFN-γ production in response to treatment with
supernatant from TLR-9 stimulated HIOEC and HB cells. Contrary to
our hypothesis, the treated cells increased T-cell proliferation
and IFN-γ production. In order to identify the effectors within the
supernatant, six cytokines (IL-1α, IL-4, IL-6, IL-8, GM-CSF and
VEGF) believed to play a critical role both in chronic inflammation
and T-cell activity were further investigated. Only IL-6 was
detected at a significantly high lever in response to TLR-9
stimulation. These results suggest that supernatants from
CpG-ODN-treated HB cells may enhance T-cell immune response via
increased IL-6 secretion.
IL-6 is a classic pro-inflammatory cytokine that is
important in normal cell inflammatory processes, host immune
responses and modulation of cellular growth. However, some cancer
cell lines, for example, oral cancer cells, also secrete IL-6
(20). The data become clinically
significant in light of the fact that IL-6 was detected at higher
concentrations in the serum of patients with oral squamous cell
carcinoma compared with gender- and age-matched disease-free
subjects (21). Moreover, IL-6
secreted by oral cancer cells plays a significant role in lymph
node metastasis and bone invasion, and has also been linked with
radioresistance and chemoresistance of OSCC patients (22–24).
Our data suggest that it also plays a role in local tumor
progression by recruiting a T-cell community with the ability to
manipulate the tumor microenvironment. Through activating the
production of IL-6, the tumor cells effectively kick the host's
immune system into action; hence, gives off a ‘find me’ signal
(25). By promote T- and/or B-cell
immune response, they can formulate a much better microenvironment
in which there are plenty of pro-inflammatory cytokines that
produced by these immune cells, which ultimately lead to further
tumor cell proliferation, angiogenesis, metastasis and immunologic
tolerance (4–6,26).
The IL-6 promoter contains two important
transcriptional elements, which are the binding sites of AP-1 and
NF-κB (27,28). As the most frequently involved
inflammatory signal transduction pathway, both NF-κB and AP-1 were
demonstrated to participate in the development of OSCC (29,30).
The present study shows that CpG-ODN can only lead to a tenuous
change of NF-κB activity in HB cells with no statistical
significance. In support of this result, inhibition of NF-κB
activity with pyrrolidinedithiocarbamate (PDTC) did not
significantly decrease TLR-9 stimulated IL-6 expression nor did it
decrease T-cell proliferation in subsequent experimentation. In
colon cancer cells, IL-6 secretion is upregulated through the
activation of AP-1 signal transduction pathway (31). We hypothesized that IL-6 secretion
in HB cells might also be caused by AP-1 binding. TLR-9 stimulation
was found to increase AP-1 DNA binding activity, and, moreover,
this effect was reversed with the use of an anti-TLR-9 antibody. In
addition, blockage of AP-1 activity with a specific inhibitor
significantly reduced IL-6 expression and subsequent T-cell
proliferation in response to treatment.
An interesting finding in this study is that HB
cells show a slightly increased proliferation when treated with
supernatants from treated T-cell. In fact, during carcinogenesis,
the innate immune system emerges as ‘double-edged sword’,
describing its ability on the one hand to fight tumor pathogens and
on the other to produce autoimmunity. This metaphor applies to the
immune system's relationship to cancer - the immune system can
destroy tumors, and yet paradoxically also promotes and sustains
(32,33). Clinical observations recorded
through the centuries also have pointed to the strong association
of chronic inflammation caused by immune response and cancer,
including gastric and, colon cancer (6,7). Our
novel finding suggests that pro-inflammatory cytokine secreted by
tumor supernatants activated T-cells may contribute to the
establishment of tumor microenvironment and ultimately lead to
tumor cell proliferation, angiogenesis and metastasis, and this
vicious circle may help to explain the clinical observation that
some young patient with better immune system always conversely have
a worse prognosis (34).
Similarly, a phase III clinic trial also demonstrated that
utilization of PF-3512676 (a special TLR-9 agonist) did not improve
survival of patients with advanced NSCLC but did increase toxicity
and suggested that this regimen cannot be recommended for treating
patients with advanced NSCLC (35).
In conclusion, the present study provides evidence
that TLR-9 activation promotes a T-cell immune response at least
partly via AP-1 activated IL-6 secretion in human oral squamous
cell carcinoma HB cells. In addition, activated T-cells could
slightly promote HB cells proliferation in reverse via producing
plenty of tumor-stimulating cytokines. These results provide not
only new insight into the precise mechanisms of cross talk between
tumor cells and the host immune system, but also a new therapeutic
target in the prevention and treatment of OSCC.
Acknowledgements
We thank Dr Andrew Owe for commenting
on the manuscript, and we also thank Professor Guoqing Zhang for
statistical analysis. This study was supported by the National
Natural Science Foundation of China (grant no. 81102049), the
Natural Science Foundation of Shanghai (grant no. 11ZR1420600) and
Shanghai Leading Academic Discipline Project (project no.
S30206).
References
|
1.
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar
|
|
2.
|
Ferlay J, Shin HR, Bray F, Forman D and
Mathers C: GLOBOCAN 2008, cancer incidence and mortality worldwide:
IARC Cancer Base No. 10. International Agency for Research on
Cancer. http://globocan.iarc.fr.
Accessed July 20, 2012.
|
|
3.
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, et al: Concurrent chemotherapy and radiotherapy for
organ preservation in advanced laryngeal cancer. N Engl J Med.
349:2091–2098. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Melief CJ: Cancer: immune pact with the
enemy. Nature. 450:803–804. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Colotta F, Allavena P, Sica A, Garlanda C
and Mantovani A: Cancer-related inflammation, the seventh hallmark
of cancer: links to genetic instability. Carcinogenesis.
30:1073–1081. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Greten FR, Eckmann L, Greten TF, Park JM,
Li ZW, et al: IKKbeta links inflammation and tumorigenesis in a
mouse model of colitis-associated cancer. Cell. 118:285–296. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Maeda S, Chang L, Li ZW, Luo JL, Leffert
H, et al: IKKbeta is required for prevention of apoptosis mediated
by cell-bound but not by circulating TNFalpha. Immunity.
19:725–737. 2003. View Article : Google Scholar
|
|
9.
|
Rakoff-Nahoum S, Paglino J,
Eslami-Varzaneh F, Edberg S and Medzhitov R: Recognition of
commensal microflora by toll-like receptors is required for
intestinal homeostasis. Cell. 118:229–241. 2004. View Article : Google Scholar
|
|
10.
|
El-Omar EM, Ng MT and Hold GL:
Polymorphisms in Toll-like receptor genes and risk of cancer.
Oncogene. 27:244–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
O'Neill LA: Signal transduction pathways
activated by the IL-1 receptor/Toll-like receptor super family.
Curr Top Microbiol Immunol. 270:47–61. 2002.
|
|
12.
|
Takeda K and Akira S: Toll-like receptors
in innate immunity. Int Immunol. 17:1–14. 2005. View Article : Google Scholar
|
|
13.
|
Ruan M, Zun Z, Siyi L, Wenjun Y, Lizheng
W, et al: Increased expression of Toll-like receptor-9 has close
relation with tumour cell proliferation in oral squamous cell
carcinoma. Arch Oral Biol. 56:877–884. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Min R, Siyi L, Wenjun Y, Shengwen L, Ow A,
et al: Toll-like receptor-9 agonists increase cyclin D1 expression
partly through activation of activator protein-1 in human oral
squamous cell carcinoma cells. Cancer Sci. 103:1938–1945. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Sdek P, Zhang ZY, Cao J, Pan HY, Chen WT,
et al: Alteration of cell-cycle regulatory proteins in human oral
epithelial cells immortalized by HPV16 E6 and E7. Int J Oral
Maxillofac Surg. 35:653–657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Zhong LP, Pan HY, Zhou XJ, Ye DX, Zhang L,
et al: Characteristics of a cancerous cell line, HIOEC-B(a)P-96,
induced by benzo(a)pyrene from human immortalized oral epithelial
cell line. Arch Oral Biol. 53:443–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Ruan M, Ji T, Yang WJ, Duan WH, Zhou XJ,
et al: Growth inhibition and induction of apoptosis in human oral
squamous cell carcinoma Tca-8113 cell lines by Shikonin was partly
through the inactivation of NF-κB pathway. Phytother Res.
22:407–415. 2008.PubMed/NCBI
|
|
18.
|
Kaomongkolgit R, Cheepsunthorn P, Pavasant
P and Sanchavanakit N: Iron increases MMP-9 expression through
activation of AP-1 via ERK/Akt pathway in human head and neck
squamous carcinoma cells. Oral Oncol. 44:587–594. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Toualbi-Abed K, Daniel F, Güller MC,
Legrand A, Mauriz JL, et al: Jun D cooperates with p65 to activate
the proximal κB site of the cyclin D1 promoter: role of PI3K/PDK-1.
Carcinogenesis. 29:536–543. 2008.PubMed/NCBI
|
|
20.
|
Woods KV, El-Naggar A, Clayman GL and
Grimm EA: Variable expression of cytokines in human head and neck
squamous cell carcinoma cell lines and consistent expression in
surgical specimens. Cancer Res. 58:3132–3141. 1998.PubMed/NCBI
|
|
21.
|
St John MA, Li Y, Zhou X, Denny P, Ho CM,
et al: Interleukin 6 and interleukin 8 as potential biomarkers for
oral cavity and oropharyngeal squamous cell carcinoma. Arch
Otolaryngol Head Neck Surg. 130:929–935. 2004.PubMed/NCBI
|
|
22.
|
Nagata M, Fujita H, Ida H, Hoshina H,
Inoue T, et al: Identification of potential biomarkers of lymph
node metastasis in oral squamous cell carcinoma by cDNA microarray
analysis. Int J Cancer. 106:683–689. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Okamoto M, Hiura K, Ohe G, Ohba Y, Terai
K, et al: Mechanism for bone invasion of oral cancer cells mediated
by interleukin-6 in vitro and in vivo. Cancer. 89:1966–1975. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
De Schutter H, Landuyt W, Verbeken E,
Goethals L, Hermans R, et al: The prognostic value of the hypoxia
markers CA IX and GLUT 1 and the cytokines VEGF and IL 6 in head
and neck squamous cell carcinoma treated by radiotherapy +/−
chemotherapy. BMC Cancer. 5:422005.PubMed/NCBI
|
|
25.
|
Okamoto M, Lee C and Oyasu R:
Interleukin-6 as a paracrine and autocrine growth factor in human
prostatic carcinoma cells in vitro. Cancer Res. 57:141–146.
1997.PubMed/NCBI
|
|
26.
|
Smith HA and Kang Y: The
metastasis-promoting roles of tumor-associated immune cells. J Mol
Med. 91:411–429. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Beetz A, Peter RU, Oppel T, Kaffenberger
W, Rupec RA, et al: NF-kappaB and AP-1 are responsible for
inducibility of the IL-6 promoter by ionizing radiation in HeLa
cells. Int J Radiat Biol. 76:1443–1453. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Romano M, Sironi M, Toniatti C,
Polentarutti N, Fruscella P, et al: Role of IL-6 and its soluble
receptor in induction of chemokines and leukocyte recruitment.
Immunity. 6:315–325. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Mishra A, Bharti AC, Saluja D and Das BC:
Transactivation and expression patterns of Jun and Fos/AP-1
super-family proteins in human oral cancer. Int J Cancer.
126:819–829. 2010.PubMed/NCBI
|
|
30.
|
Rao SK, Pavicevic Z, Du Z, Kim JG, Fan M,
Jiao Y, et al: Pro-inflammatory genes as biomarkers and therapeutic
targets in oral squamous cell carcinoma. J Biol Chem.
285:32512–32521. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Lin CM, Chen YH, Ma HP, Wang BW, Chiu JH,
et al: Silibinin inhibits the invasion of IL-6-stimulated colon
cancer cells via selective JNK/AP-1/MMP-2 modulation in vitro. J
Agric Food Chem. 60:12451–12457. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Houghton AN, Uchi H and Wolchok JD: The
role of the immune system in early epithelial carcinogenesis:
B-ware the double-edged sword. Cancer Cell. 7:403–405. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Huang B, Zhao J, Unkeless JC, Feng ZH and
Xiong H: TLR signaling by tumor and immune cells: a double-edged
sword. Oncogene. 27:218–224. 2008. View Article : Google Scholar
|
|
34.
|
Anders CK, Hsu DS, Broadwater G, Acharya
CR, Foekens JA, et al: Young age at diagnosis correlates with worse
prognosis and defines a subset of breast cancers with shared
patterns of gene expression. J Clin Oncol. 26:3324–3330. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Hirsh V, Paz-Ares L, Boyer M, Rosell R,
Middleton G, et al: Randomized phase III trial of
paclitaxel/carboplatin with or without PF-3512676 (Toll-like
receptor 9 agonist) as first-line treatment for advanced
non-small-cell lung cancer. J Clin Oncol. 29:2667–2674. 2011.
View Article : Google Scholar : PubMed/NCBI
|