Introduction
Pancreatic cancer is one of the most lethal cancers,
with a 5-year overall survival (OS) rate of only 4% (1). Despite developments in diagnostic
imaging, most patients with pancreatic cancer are diagnosed with an
advanced stage of the disease. For example, almost 33% of 100,313
pancreatic cancer patients diagnosed from 1989 through 1995 had
metastasis (2). Moreover, most
patients who undergo curative resection develop incurable local
relapses, liver metastases and/or peritoneal dissemination.
A prospective study of 100 patients with peritoneal
carcinomatosis resulting from non-gynecological malignancies found
that the second most common primary tumor was pancreatic cancer
(20%) (3). A French multicentric
prospective study of 370 peritoneal carcinomatosis patients with
non-gynecological malignancies showed a median OS of 3.1 months,
but was only 2.1 months in those with pancreatic cancer (4). These data suggest that the novel
therapies to control the peritoneal dissemination of pancreatic
cancer may improve patient survival.
Pancreatic cancer is one of the most stroma-rich
cancers and characterized by excessive desmoplasia, which plays a
crucial role in its aggressive behavior (5,6). The
stroma in these tumors is very heterogeneous, consisting of
cellular and acellular components, including fibroblasts,
myofibroblasts, immune cells, blood vessels, extracellular matrix
(ECM) and soluble proteins such as cytokines and growth factors
(7). Stromal myofibroblasts
derived from the primary site of pancreatic cancer were shown to
enhance the progression of pancreatic cancer (8). Moreover, stromal components were
shown to promote the malignant behavior of pancreatic cancer cells,
with myofibroblasts being the especially associated with
cancer-stromal cell interactions in primary tumors (7,9–12).
Few studies, however, assessed cancer-stromal cell interactions at
peritoneally disseminated sites (13–15).
Myofibroblasts are regarded as playing a central
role in pathogenesis of peritoneal fibrosis, which provide a
favorable environment for the dissemination of cancer cells
(13). Although myofibroblasts
have been reported to derive from resident peritoneal fibroblasts,
human peritoneal mesothelial cells (hPMCs) (16,17),
bone marrow progenitor cells or the primary tumor itself (18,19),
the origin of these myofibroblasts has not been clearly
established. Also, during the initial stages of peritoneal
metastasis, cancer cells have been reported to attach to areas of
exposure of collagen-rich connective tissue matrices because of the
lack of mesothelium (20–23). Thus, the interactions between
cancer cells derived from the primary tumor and submesothelium
layer components such as myofibroblasts may be a key to peritoneal
dissemination (24). Hepatocyte
growth factors (HGF) produced by human peritoneal myofibroblasts
have been found to promote the peritoneal dissemination of gastric
cancer (25), and myofibroblasts
in omentum were shown to be activated by tumor cells and to promote
the growth, adhesion and invasiveness of ovarian cancer (14). However, the roles of peritoneal
myofibroblasts and their matrices in the peritoneal dissemination
of pancreatic cancer remain unclear. The development of novel
therapies to control the peritoneal dissemination of pancreatic
cancer requires further understanding of the mechanisms of
induction and the roles of peritoneal myofibroblasts in the process
of dissemination.
In the present study, we established three primary
cultures of human peritoneal myofibroblasts (hPMFs) isolated from
peritoneally disseminated nodules of pancreatic cancer and
investigated the interactions between primary cultures of hPMFs and
pancreatic cancer cells in vitro and in vivo.
Materials and methods
Tissues, cells and culture
conditions
Peritoneally disseminated tissues were obtained from
3 patients who underwent palliative (bypass) operations for
unresectable pancreatic cancer at our institution under an
Institutional Review Board-approved protocol following informed
consent. Human peritoneal myofibroblasts (hPMFs) were isolated from
these fresh surgical specimens using the outgrowth method in our
laboratory (26,27), and the identity of these hPMFs was
confirmed by morphology (spindle-shaped cells) and
immunofluorescence staining for α-SMA, vimentin and cytokeratin 18
(CK18) (8,28). Cells at passage numbers 2–4 were
used for all assays. Two pancreatic cancer cell lines, SUIT-2
(Japan Health Sciences Foundation) and CAPAN-1 (American Type
Culture Collection, Manassas, VA, USA), were maintained as
previously described (29).
Immunohistochemistry and
histopathology
hPMFs isolated from peritoneally disseminated sites
of pancreatic cancer were evaluated by H&E and α-SMA
immunohistochemical staining. Immunohistochemical staining was
performed using a Histofine SAB-PO kit (Nichirei, Tokyo, Japan).
Tissues were sectioned to a thickness of 4 μm and were
incubated with antibody overnight at 4°C. Human tissues were
incubated with mouse monoclonal anti-α-SMA antibody (1:50; Dako,
Glostrup, Denmark) and mouse tissues were incubated with rabbit
polyclonal anti-α-SMA antibody (1:50; Abcam, Cambridge, MA, USA)
(30). Cells were considered
positively stained when either the membrane or cytoplasm was
stained. All slides were evaluated independently by two
investigators blinded to knowledge of the clinical features of each
patient.
Immunofluorescence staining
hPMFs were incubated with by rabbit polyclonal
anti-vimentin antibody (1:100; Abcam), mouse monoclonal anti-CK18
antibody (1:100; sc-6259, Santa Cruz Biotechnology, Santa Cruz, CA,
USA) and anti-α-SMA antibody (1:50; Dako). For comparison, human
mesothelial cells from cancerous ascites of pancreatic cancer were
incubated with antibodies to vimentin and CK18.
Matrigel invasion and migration assays
(indirect co-culture)
Cell invasion was measured by counting the number of
cells that invaded Matrigel-coated Transwell chambers with
8-μm pores (BD Biosciences, Franklin Lakes, NJ, USA), as
previously described (31).
Briefly, Transwell inserts were coated with 20 μg/well
Matrigel (BD Biosciences). Each lower well of a 24-well plate was
seeded with myofibroblasts (2.0×104/well) in 750
μl of DMEM supplemented with 10% FBS or medium alone and
incubated for 24 h. Cancer cells (4.0×104/well) in 250
μl of DMEM supplemented with 10% FBS were seeded into each
upper well. After 69 h of incubation, cells on the lower surface of
the Matrigel-coated membrane were fixed with 70% ethanol, stained
with H&E, and counted in five randomly selected fields at a
×100, magnification under a light microscope. The mobility of
SUIT-2 and CAPAN-1 pancreatic cancer cells was assessed using
uncoated Transwell inserts and incubation times of 24 and 48 h,
respectively. To assess the invasiveness of myofibroblasts, each
lower well was seeded with cancer cells (4.0×104/well)
in 750 μl of DMEM supplemented with 10% FBS or medium alone
and incubated for 24 h. Myofibroblasts (1.5×104/well) in
250 μl of DMEM supplemented with 10% FBS were seeded in each
upper well and incubated for 20 h for the invasion assay and 14 h
for the migration assay. The results were expressed as the mean
number of invaded cells per field. Each experiment was carried out
in triplicate wells and repeated at least 3 times.
Animal models
All experiments with mice were conducted with the
approval of the Ethics Committee of Kyushu University. The model of
intraperitoneal implantation of BALB/c nu/nu mice (5–6 weeks of
age, Kyudo Co.) was used to analyze the dissemination activity of
cancer cells alone and cancer cells plus hPMFs. All animals were
bred in laminar-flow cabinets under specific pathogen-free
conditions. Prior to implanting, the cells were briefly treated
with trypsin/EDTA and washed twice with serum-free medium. The mice
were anesthetized with ether and suspensions of 2×105
SUIT-2 or 1×106 CAPAN-1 cells in 200 μl PBS with
or without 1×106 hPMFs in 200 μl PBS were
transplanted into the peritoneal cavity of groups of 10 mice. All
mice were sacrificed 28 days later, and disseminated nodules >3
mm in size were counted. Each experiment was repeated 3 times.
Green fluorescent protein (GFP)-labeled
myofibroblasts in vivo
The GIPZ non-silencing control vector for shRNA
(Thermo Fisher Scientific, Rockford, IL, USA) was used to express
GFP in hPMFs. 2×105 SUIT-2 in 200 μl PBS and
1×106 GFP-labeled hPMFs in 200 μl PBS were
transplanted into mice as described above. Peritoneally
disseminated nodes were analyzed by stereoscopic microscopy (Zeiss,
SteREO Lumar, Gottingen, Germany).
Flow cytometry analysis
Cultured cells were harvested by exposure to
trypsin/EDTA for 5 min at 37°C, washed in 10% FBS/DMEM, suspended
in ice-cold 1% FBS/PBS solution and analyzed using a flow cytometer
(EC800 Cell Analyzer, Sony) equipped with a laser that provided an
excitation wavelength of 488 nm. Eclipse Analysis software (Sony)
was used to quantify the fluorescent signals and set the logical
electronic-gating parameters. Expression of GFP in original hPMFs
and GFP-labeled hPMFs was analyzed by flow cytometry.
Statistical analysis
For in vitro and in vivo experiments,
all values were expressed as mean ± SD and compared using Student’s
t-tests. All experiments were performed at least three times.
Statistical significance was defined as P<0.05. All statistical
analyses were performed using JMP 9 software (SAS Institute, Cary,
NC, USA).
Results
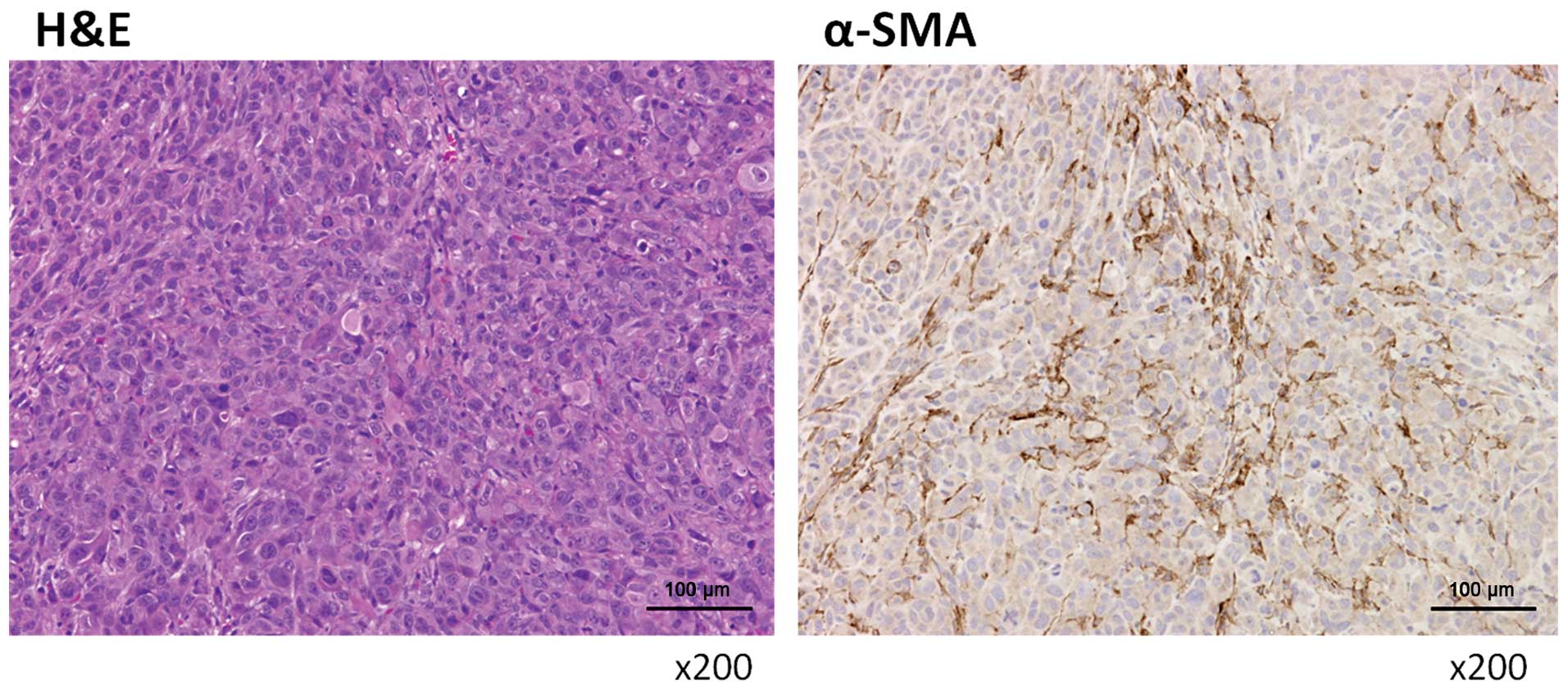
Activated myofibroblasts are abundant in
human peritoneally disseminated nodules
Specimens were obtained from peritoneally
disseminated nodules of human pancreatic cancers. These nodules
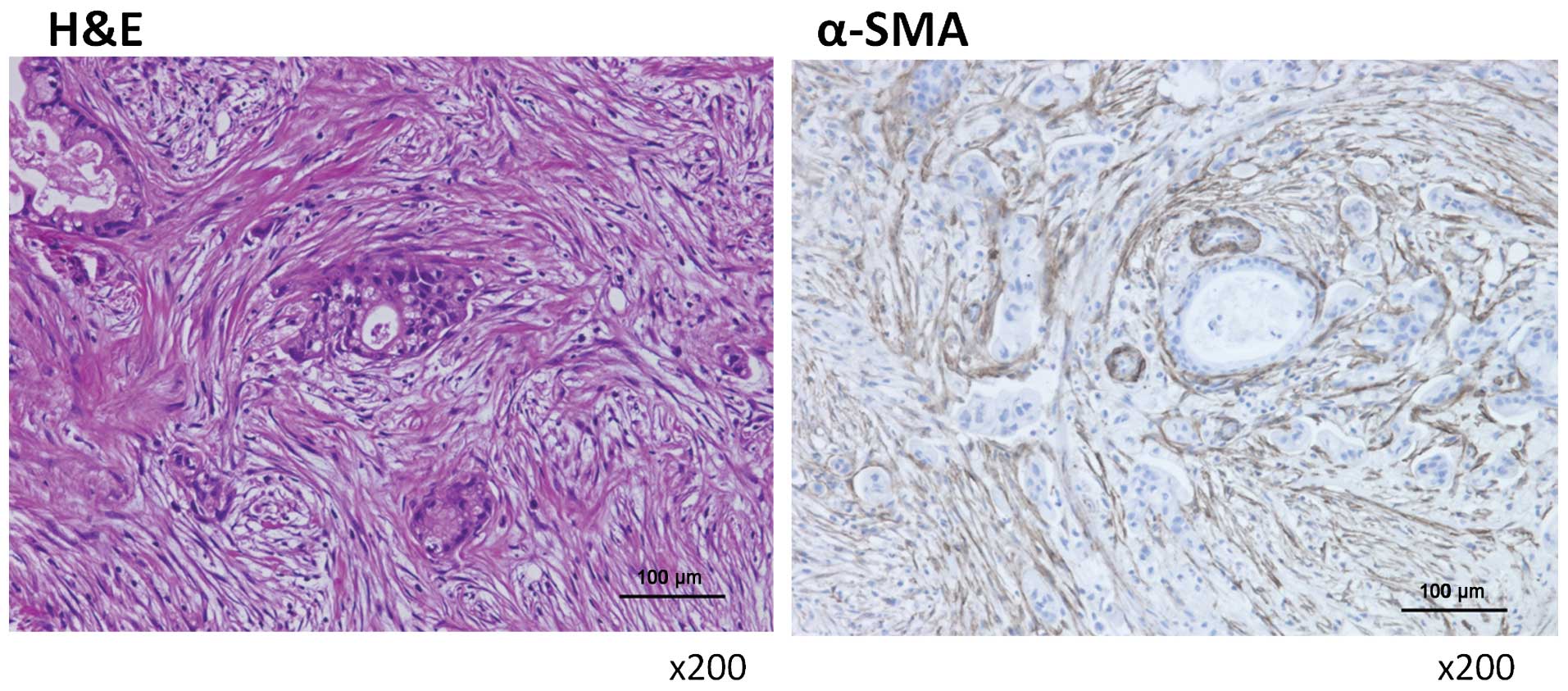
contained many activated hPMFs, as shown by their morphology
(spindle-shaped cells; Fig. 1,
left panel) and their positivity for α-SMA staining (Fig. 1, right panel). Primary cultures of
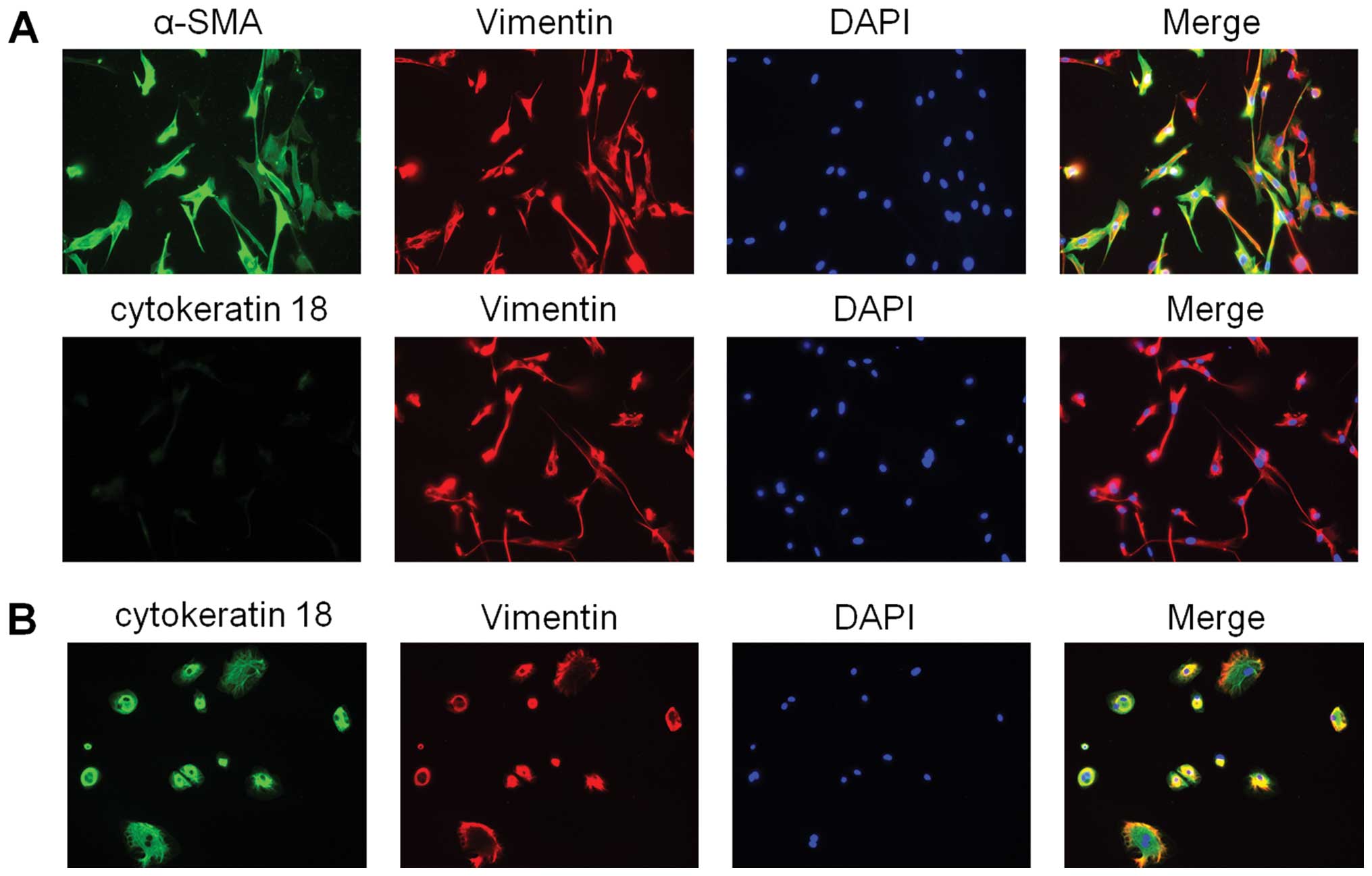
these hPMFs were positive for α-SMA and vimentin and negative for
CK18, suggesting that these cells were activated and were not
contaminated by mesothelial cells (Fig. 2).
Mobility and invasiveness of hPMFs are
enhanced when co-cultured with pancreatic cancer cells
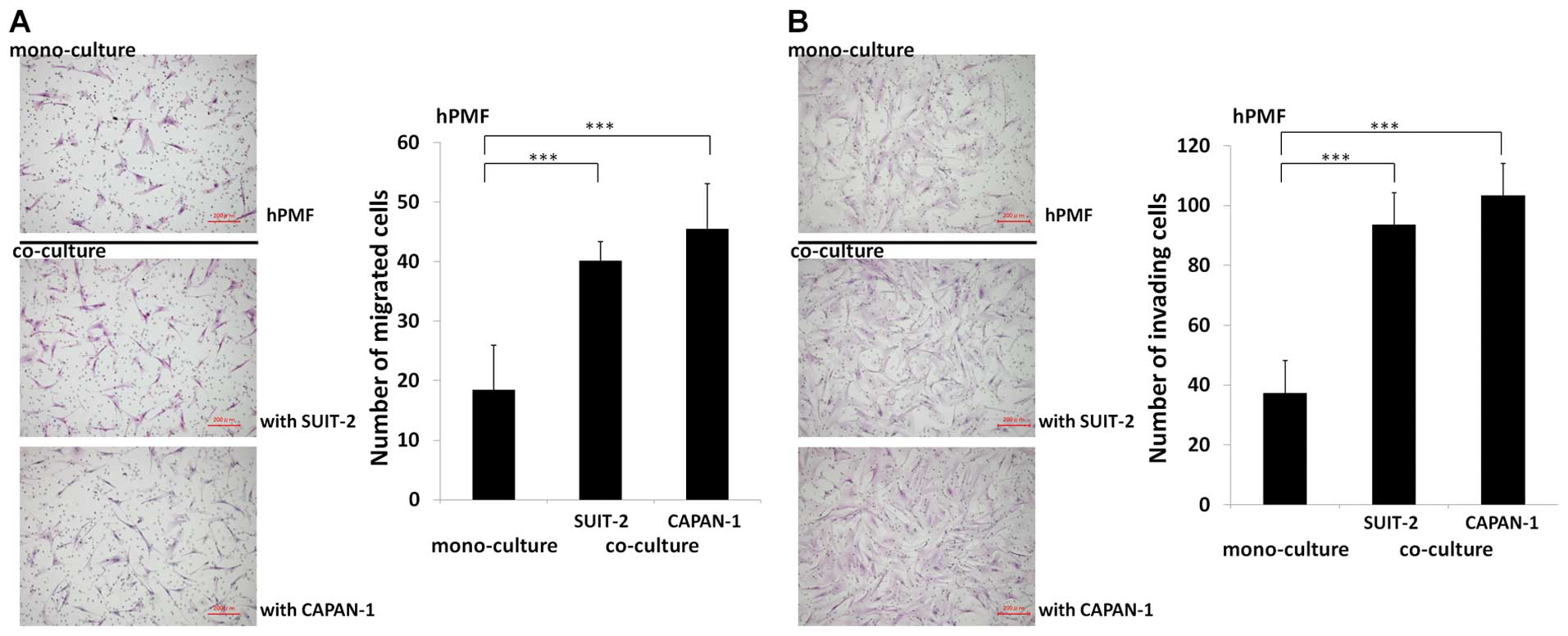
To investigate the cancer-stromal cell interactions
between hPMFs and pancreatic cancer cells, we evaluated the effect
of pancreatic cancer cells on hPMFs migration and invasiveness
using indirect co-cultures. We found that both SUIT-2 and CAPAN-1
pancreatic cancer cells markedly stimulated the migration
(P<0.001) (Fig. 3A) and
invasiveness (P<0.001) (Fig.
3B) of hPMFs.
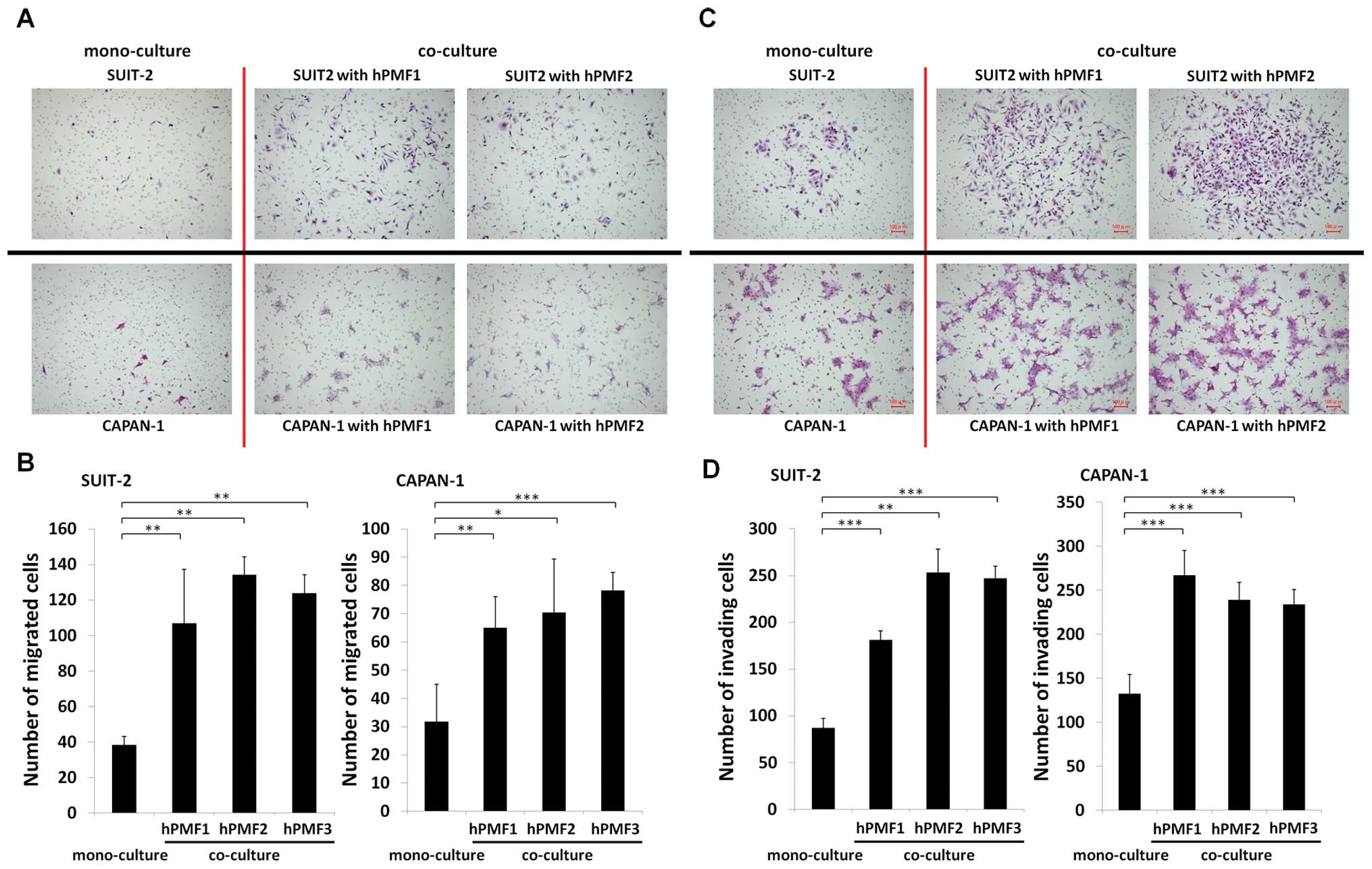
Mobility and invasiveness of pancreatic
cancer cells are enhanced when co-cultured with hPMFs
Similarly, we investigated the effect of co-cultured
hPMFs on the mobility and invasiveness of pancreatic cancer cells.
We found that hPMFs markedly stimulated the migration (P<0.05)
(Fig. 4A and B) and invasiveness
(P<0.01) (Fig. 4C and D) of
SUIT-2 and CAPAN-1 cells.
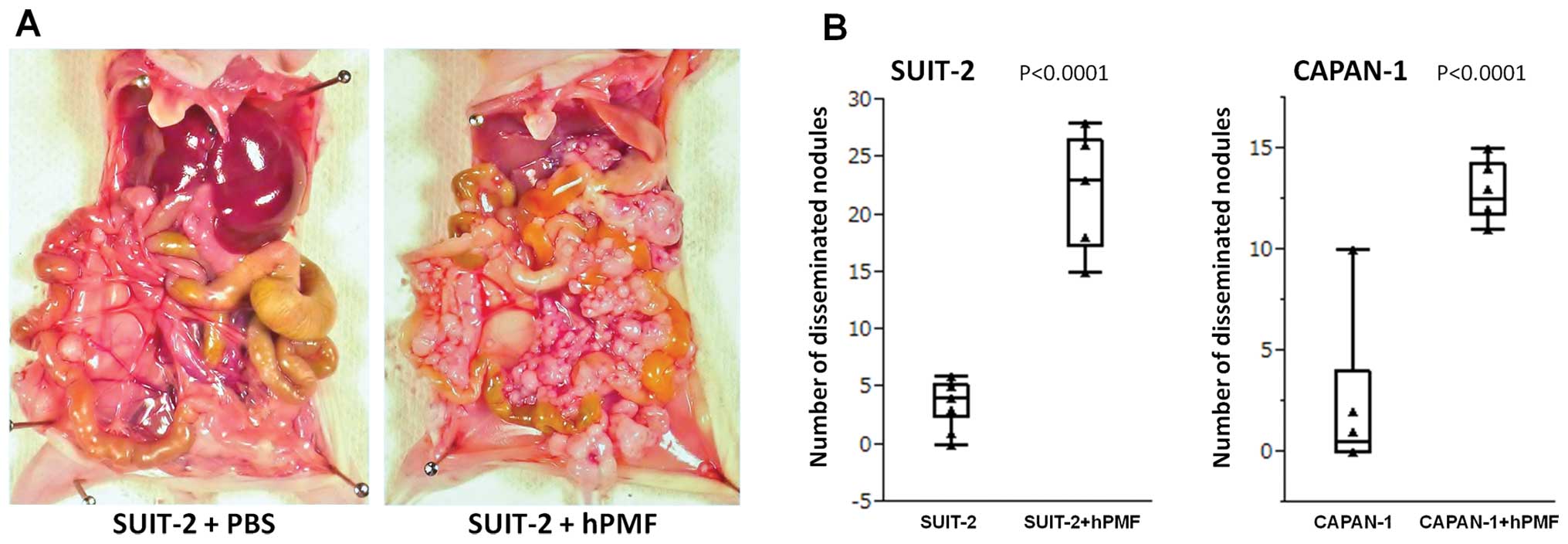
hPMFs promote the peritoneal
dissemination of pancreatic cancer cells in vivo
Intraperitoneal implantation of cells into BALB/c
nu/nu mice, aged 5–6 weeks, was used to compare the dissemination
over 28 days of pancreatic cancer cells alone and with hPMFs.
Intraperitoneal injection of 2×105 SUIT-2 cells plus
1×106 hPMFs (Fig. 5A,
right panel) yielded a mean 22.5±4.9 peritoneally disseminated
nodules >3 mm, compared with 3.8±2.0 nodules in mice injected
with SUIT-2 cells plus PBS (Fig.
5A, left panel), a difference that was statistically
significant (P<0.0001) (Fig.
5B, left panel). Similarly, injection of 1×106
CAPAN-1 cells plus 1×106 hPMFs yielded a mean 12.8±1.5 peritoneally
disseminated nodules, compared with 2.5±4.0 nodules in mice
injected with CAPAN-1 cells plus PBS (P<0.0001) (Fig. 5B, right panel). H&E staining
and immunohistochemical analyses of nodules in mice injected with
SUIT-2 cells plus hPMFs showed the presence of α-SMA positive
myofibroblasts (Fig. 6), similar
to those observed human peritoneally disseminated nodules (Fig. 1).
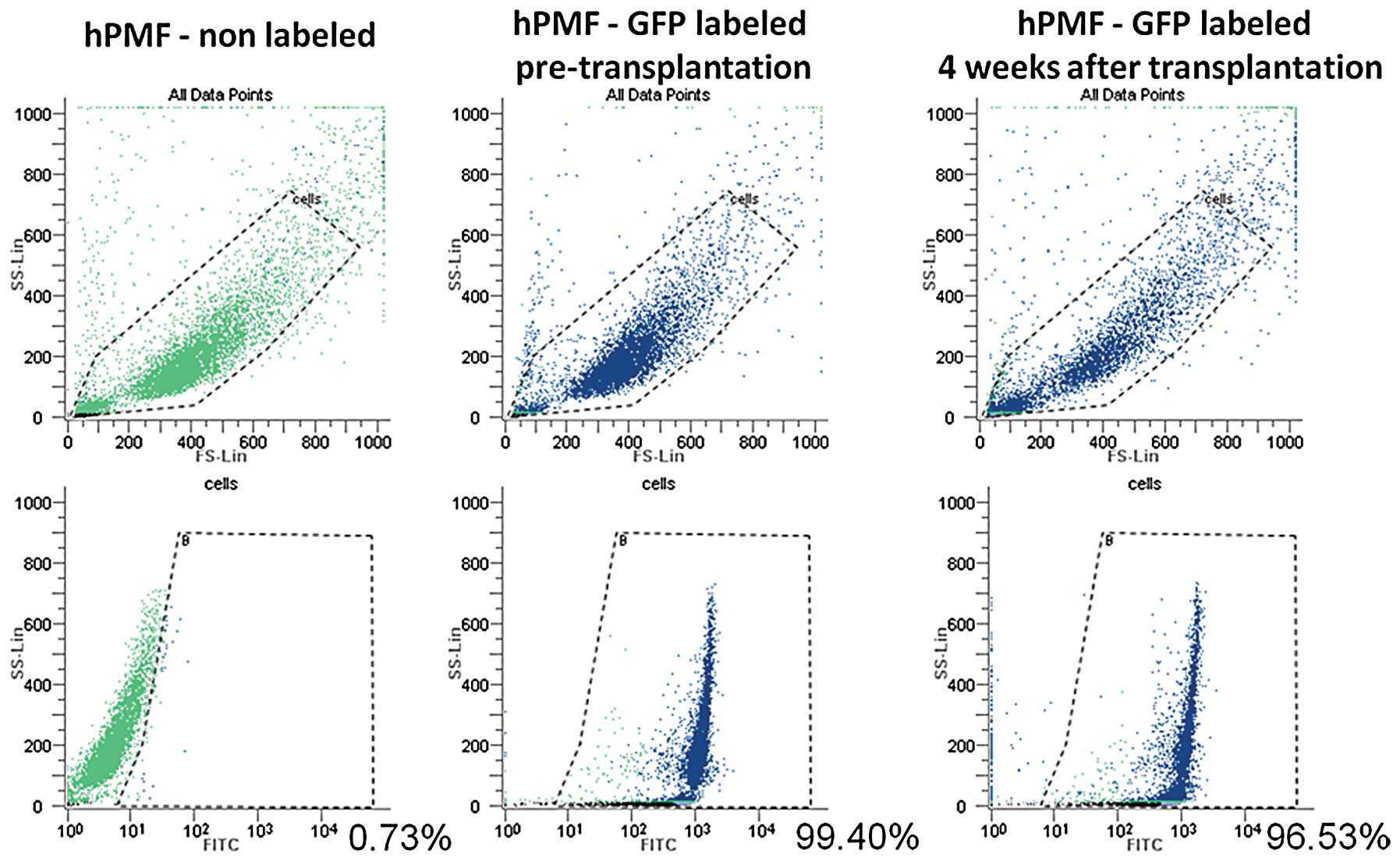
hPMFs survive in peritoneally
disseminated nodules of nude mice only when implanted together with
pancreatic cancer cells
To investigate whether the hPMFs survive and exist
in the peritoneally disseminated nodules after intraperitoneal
implantation with or without pancreatic cancer cells, we used
GFP-labeled hPMFs. Using flow cytometry, we evaluated the
time-dependent changes in GFP expression of cultured hPMFs after
labeling, finding that the expression of GFP in cultured hPMFs
remained constant for 28 days after labeling (Fig. 7).
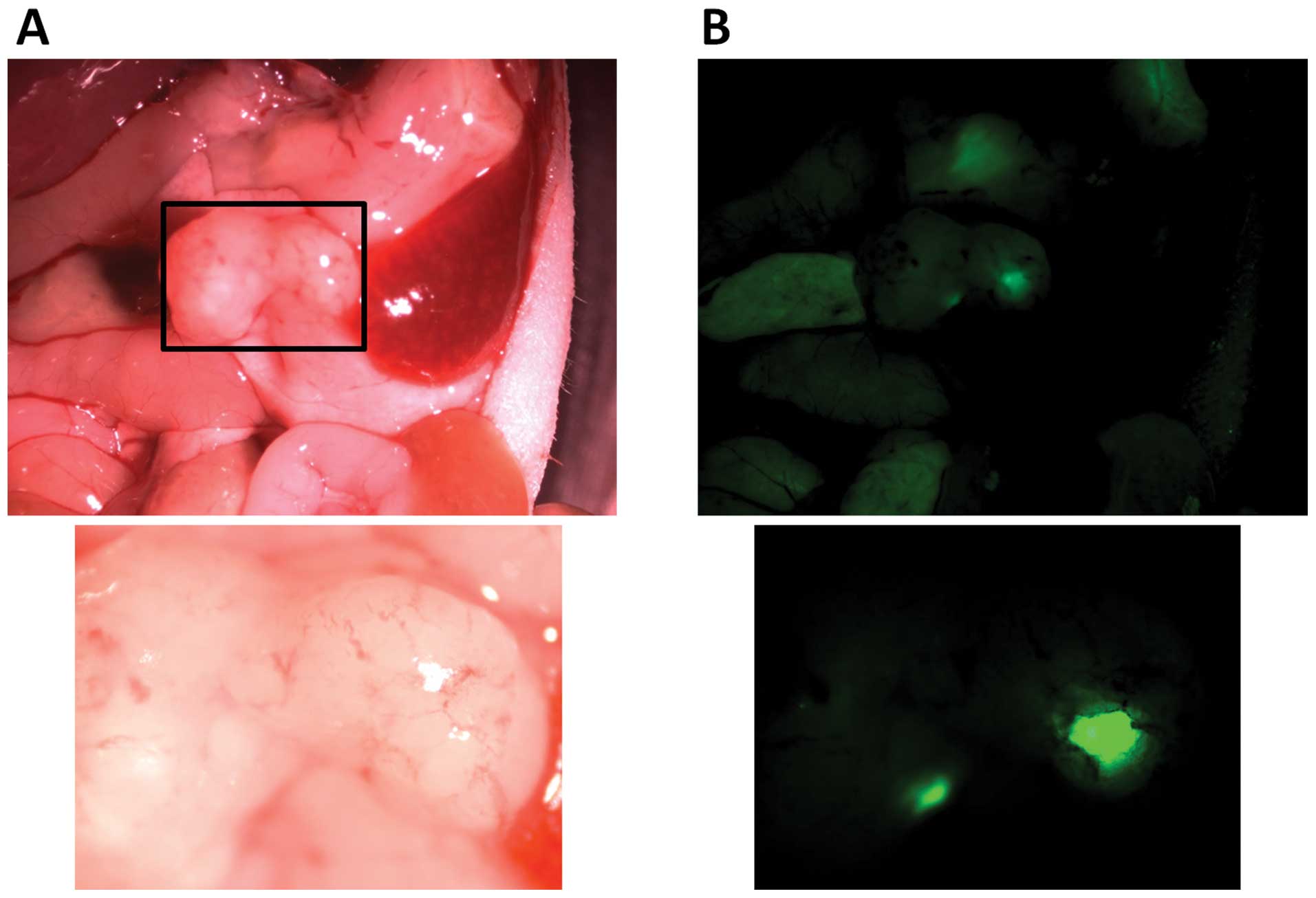
Intraperitoneal transplantation of 2×105
SUIT-2 cells and 1×106 GFP-labeled hPMFs resulted in
peritoneal dissemination in mice (Fig.
8A). Moreover, GFP-labeled hPMFs were observed in these
peritoneally disseminated nodules 28 days after transplantation
with pancreatic cancer cells (Fig.
8B).
Discussion
In the present study, we successfully isolated hPMFs
by outgrowth from peritoneally disseminated nodules of pancreatic
cancer and investigated the interaction between these primary
cultured hPMFs and pancreatic cancer cells. We found that
myofibroblasts were abundant around cancer cells at peritoneally
disseminated sites, similar to primary sites of pancreatic cancer,
and that hPMFs promoted the migration and invasiveness of
pancreatic cancer cells. Conversely, pancreatic cancer cells also
promoted the migration and invasiveness of hPMFs. Our in
vivo results revealed that number of peritoneally disseminated
nodules was significantly greater when cancer cells were
transplanted with hPMFs than when cancer cells were transplanted
alone. In vivo experiments using GFP-labeled hPMFs showed
that peritoneally disseminated nodules contained transplanted human
myofibroblasts. Others also reported that myofibroblasts from human
omentum were shown to promote the peritoneal dissemination of
ovarian cancer cells in nude mice (14), and peritoneal myofibroblasts
promoted the mobility and invasiveness of gastric cancer cells
(13,32). These findings suggest that hPMFs
accelerate the malignant behavior of cancer cells through
cancer-stromal cell interactions during the formation of
peritoneally disseminated nodules.
Although several reports exist on methods of
isolation of peritoneal myofibroblasts or mesothelial cells
(14,28), there is no difference between the
two methods with the exception of the digestion time by
trypsin/EDTA. These reports suggested that primary cultures of
human peritoneal myofibroblasts and mesothelial cells could be
contaminated with other cell types. In contrast, we established
hPMFs from peritoneally disseminated nodules using an outgrowth
method, similar to the method used to isolate human pancreatic
stellate cells (hPSCs) from primary pancreatic cancers (26,27).
Microscopic observation of the primary cultured cells and in
vitro immunofluorescence staining revealed that the isolated
cells were spindle-shaped, positive for α-SMA and vimentin, and
negative for CK18, indicating that these isolated cells are
activated hPMFs and that there was no contamination with hPMCs
(28).
To confirm whether hPMFs, which were co-implanted
with cancer cells, were present in the peritoneally disseminated
nodules in mice, we used GFP-labeled hPMFs and found GFP positive
cells in some disseminated nodules. Similar results were obtained
using GFP-labeled hPSCs derived from primary pancreatic cancers.
hPSCs transplanted into mouse pancreas with pancreatic cancer cells
were shown to be present at peritoneally disseminated sites of
pancreatic cancer (19). In
addition, evaluation of the disseminated nodules derived from
cancer cells co-transplanted with hPSCs in the peritoneal cavity
showed that these hPSCs promoted the dissemination of pancreatic
cancer cells in vivo, with no significant differences
between hPMFs and hPSCs (data not shown). We also found that both
hPMFs and hPSCs were not engrafted in the peritoneal cavity of nude
mice if transplanted without cancer cells (data not shown). These
results suggest that myofibroblasts liberated from their original
tissues could engraft into the peritoneal cavities of mice when
transplanted along with pancreatic cancer cells.
The peritoneum consists of a monolayer of
mesothelial cells supported by a basement membrane that rests on a
layer of connective tissue (24).
Transforming growth factor-β1 (TGF-β1) derived from cancer cells in
the peritoneal micro-environment was shown to activate hPMCs and
transform these cells to myofibroblast-like cells (17). These findings focused on the role
of hPMCs in the early phases of formation of peritoneally
disseminated nodules of cancer cells. We found that hPMFs were
abundant around cancer cells at peritoneally disseminated sites of
pancreatic cancer, but we could not distinguish hPMC-derived
myofibroblasts from other myofibroblasts.
hPMFs may derive from normal fibroblasts in the
sub-mesothelial layer of the abdominal wall or from the
transformation of mesothelial cells by TGF-β1 or HGF (16,17,25,33).
Alternatively, hPMFs may be hPSCs liberated from the pancreas
(19) or mesenchymal stem cells
from the bone marrow (10).
Further efforts are needed to identify the origin of these cells
and the mechanism inducing their activation. These findings may
contribute to the identification of new therapeutic targets to
prevent the peritoneal dissemination of pancreatic cancer.
In conclusion, hPMFs can be isolated as primary
cultures without contamination by hPMCs from disseminated nodules
of pancreatic cancer. The cancer-stromal cell interactions between
pancreatic cancer cells and hPMFs are important in the peritoneal
dissemination of pancreatic cancer cells. Therapy targeting this
interaction may improve the prognosis of patients with pancreatic
cancer.
Acknowledgements
This study was supported in part by a
Grant-in-Aid from the Ministry of Education, Culture, Sports,
Science and Technology of Japan. Grant nos. 24390318, 25670586,
24390319, 25670585, 23390327, 25670584, 25293285 and 25670582.
References
|
1.
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
2.
|
Sener SF, Fremgen A, Menck HR and
Winchester DP: Pancreatic cancer: A report of treatment and
survival trends for 100,313 patients diagnosed from 1985–1995,
using the National Cancer Database. J Am Coll Surg. 189:1–7.
1999.PubMed/NCBI
|
|
3.
|
Chu DZ, Lang NP, Thompson C, Osteen PK and
Westbrook KC: Peritoneal carcinomatosis in nongynecologic
malignancy. A prospective study of prognostic factors. Cancer.
63:364–367. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Sadeghi B, Arvieux C, Glehen O, et al:
Peritoneal carcinomatosis from non-gynecologic malignancies:
results of the EVOCAPE 1 multicentric prospective study. Cancer.
88:358–363. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Armstrong T, Packham G, Murphy LB, et al:
Type I collagen promotes the malignant phenotype of pancreatic
ductal adenocarcinoma. Clin Cancer Res. 10:7427–7437. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mahadevan D and Von Hoff DD: Tumor-stroma
interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther.
6:1186–1197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Feig C, Gopinathan A, Neesse A, Chan DS,
Cook N and Tuveson DA: The pancreas cancer microenvironment. Clin
Cancer Res. 18:4266–4276. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Hwang RF, Moore T, Arumugam T, et al:
Cancer-associated stromal fibroblasts promote pancreatic tumor
progression. Cancer Res. 68:918–926. 2008. View Article : Google Scholar
|
|
9.
|
Apte MV, Park S, Phillips PA, et al:
Desmoplastic reaction in pancreatic cancer: role of pancreatic
stellate cells. Pancreas. 29:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Joyce JA and Pollard JW:
Microenvironmental regulation of metastasis. Nat Rev Cancer.
9:239–252. 2009. View
Article : Google Scholar
|
|
11.
|
Strell C, Rundqvist H and Ostman A:
Fibroblasts - a key host cell type in tumor initiation,
progression, and metastasis. Ups J Med Sci. 117:187–195. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Fujita H, Ohuchida K, Mizumoto K, et al:
Tumor-stromal interactions with direct cell contacts enhance
proliferation of human pancreatic carcinoma cells. Cancer Sci.
100:2309–2317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Fibrosis in the peritoneum induced by scirrhous gastric
cancer cells may act as ‘soil’ for peritoneal dissemination.
Cancer. 77:1668–1675. 1996.PubMed/NCBI
|
|
14.
|
Cai J, Tang H, Xu L, et al: Fibroblasts in
omentum activated by tumor cells promote ovarian cancer growth,
adhesion and invasiveness. Carcinogenesis. 33:20–29. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Peron JM, Bureau C, Gourdy P, et al:
Treatment of experimental murine pancreatic peritoneal
carcinomatosis with fibroblasts genetically modified to express
IL12: a role for peritoneal innate immunity. Gut. 56:107–114. 2007.
View Article : Google Scholar
|
|
16.
|
Yanez-Mo M, Lara-Pezzi E, Selgas R, et al:
Peritoneal dialysis and epithelial-to-mesenchymal transition of
mesothelial cells. N Engl J Med. 348:403–413. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tsukada T, Fushida S, Harada S, et al: The
role of human peritoneal mesothelial cells in the fibrosis and
progression of gastric cancer. Int J Oncol. 41:476–482.
2012.PubMed/NCBI
|
|
18.
|
Epperly MW, Guo H, Gretton JE and
Greenberger JS: Bone marrow origin of myofibroblasts in irradiation
pulmonary fibrosis. Am J Respir Cell Mol Biol. 29:213–224. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Xu Z, Vonlaufen A, Phillips PA, et al:
Role of pancreatic stellate cells in pancreatic cancer metastasis.
Am J Pathol. 177:2585–2596. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Krist LF, Kerremans M, Broekhuis-Fluitsma
DM, Eestermans IL, Meyer S and Beelen RH: Milky spots in the
greater omentum are predominant sites of local tumour cell
proliferation and accumulation in the peritoneal cavity. Cancer
Immunol Immunother. 47:205–212. 1998. View Article : Google Scholar
|
|
21.
|
Mochizuki Y, Nakanishi H, Kodera Y, et al:
TNF-alpha promotes progression of peritoneal metastasis as
demonstrated using a green fluorescence protein (GFP)-tagged human
gastric cancer cell line. Clin Exp Metastasis. 21:39–47. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Sorensen EW, Gerber SA, Sedlacek AL,
Rybalko VY, Chan WM and Lord EM: Omental immune aggregates and
tumor metastasis within the peritoneal cavity. Immunol Res.
45:185–194. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Tsujimoto H, Takhashi T, Hagiwara A, et
al: Site-specific implantation in the milky spots of malignant
cells in peritoneal dissemination: immunohistochemical observation
in mice inoculated intraperitoneally with
bromodeoxyuridine-labelled cells. Br J Cancer. 71:468–472. 1995.
View Article : Google Scholar
|
|
24.
|
Sugarbaker PH: Peritoneum as the
first-line of defense in carcinomatosis. J Surg Oncol. 95:93–96.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Yashiro M, Chung YS, Inoue T, et al:
Hepatocyte growth factor (HGF) produced by peritoneal fibroblasts
may affect mesothelial cell morphology and promote peritoneal
dissemination. Int J Cancer. 67:289–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Bachem MG, Schneider E, Gross H, et al:
Identification, culture, and characterization of pancreatic
stellate cells in rats and humans. Gastroenterology. 115:421–432.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Bachem MG, Schunemann M, Ramadani M, et
al: Pancreatic carcinoma cells induce fibrosis by stimulating
proliferation and matrix synthesis of stellate cells.
Gastroenterology. 128:907–921. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Jorres A, Ludat K, Lang J, et al:
Establishment and functional characterization of human peritoneal
fibroblasts in culture: regulation of interleukin-6 production by
proinflammatory cytokines. J Am Soc Nephrol. 7:2192–2201.
1996.PubMed/NCBI
|
|
29.
|
Ohuchida K, Mizumoto K, Murakami M, et al:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Ikenaga N, Ohuchida K, Mizumoto K, et al:
CD10+ pancreatic stellate cells enhance the progression
of pancreatic cancer. Gastroenterology. 139:1041–1051. 2010.
|
|
31.
|
Sato N, Maehara N, Mizumoto K, et al:
Telomerase activity of cultured human pancreatic carcinoma cell
lines correlates with their potential for migration and invasion.
Cancer. 91:496–504. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Yashiro M, Chung YS, Nishimura S, Inoue T
and Sowa M: Peritoneal metastatic model for human scirrhous gastric
carcinoma in nude mice. Clin Exp Metastasis. 14:43–54. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Lv ZD, Na D, Ma XY, Zhao C, Zhao WJ and Xu
HM: Human peritoneal mesothelial cell transformation into
myofibroblasts in response to TGF-β1 in vitro. Int J Mol
Med. 27:187–193. 2011.PubMed/NCBI
|