Introduction
Prostate cancer (PCa) is the most frequently
diagnosed malignant tumor in male and the second leading cause of
cancer deaths in Western countries (1). The main problem arising from PCa is
its propensity to metastasize to bone and raise bone relative
events and death (2). Thus it is
very important to understand the mechanism of metastasis
progression for preventing and developing anti-metastatic
therapies.
Substantial evidence has demonstrated that some
microRNAs (miRNAs), which is a class of small non-coding regulatory
RNAs (19–25 nucleotides), play a pivotal role in most solid tumor
metastasis (3,4). In PCa, a series of miRNAs have been
identified as suppressors of metastasis, such as miR-145, -143,
-205, -34a, -203 and -200c (5–9).
Recently, several studies also found that miR-100 expression is
down-regulated in human PCa tumor tissue compared to normal
prostate (10–12). Importantly, miR-100 level
significantly decreases in the bone metastasis PCa samples compared
with primary PCa (5). Moreover, it
is also downregulated during the PCa progression (10,13,14)
and its downregulation is related with hormone-refractory PCa
(15). However, the importance of
miR-100 in bone metastasis of PCa has not been elucidated to
date.
Epithelial-mesenchymal transition (EMT) plays a key
role in tumor cell metastasis (16) and also has been identified as an
important step in bone metastasis of PCa (2,17).
Furthermore, E-cadherin-mediated cell-adhesion system plays a
critical role in EMT which is regulated by various EMT-inducing
transcription factors including Snail1/2, Twist1/2 and Zeb1/2
(18). Emerging evidence has
demonstrated that cancer stem cells (CSCs) also are the critical
drivers of tumor progression and metastasis (19). Importantly, certain miRNAs directly
regulated EMT and the characteristics of CSCs (3,20,21).
However, it is not known whether and how miR-100 regulates EMT and
the characteristics of CSCs.
The bioinformatics (TargetScan) predicts that
Argonaute 2 (AGO2 or Eif2c2) may be a putative target of miR-100.
AGO2 is the core effector proteins of the miRNA-induced silencing
complex (miRISC) (22) and plays a
role in short interfering RNA-mediated gene silencing (23). Furthermore, AGO2 also is as a
pivotal factor in some miRNA biosynthesis (24) and maturation (25). Notably, Ago2 was overexpressed in
some malignant tumors, including PCa (24,26,27)
and silencing of AGO2 arrested growth and promoted apoptosis of
some malignant tumor cells (25,28).
More importantly, overexpression of Ago2 promotes hepatocellular
carcinoma (HCC) tumorigenesis and metastasis (23). Downregulation of AGO2 also retarded
self-renewal in embryonic stem cells (29) and colony formation of HCC cells
(23). These findings suggest that
overexpression of AGO2 might enhance tumor metastasis. However, it
is unknown whether and how AGO2 is involved in bone metastasis of
PCa. Thus, we hypothesize that miR-100 directly targets AGO2
through which miR-100 negatively regulates metastatic abilities of
PCa cells by modulating migration, invasion, EMT and stemness
properties of cancer cells.
Materials and methods
Cell culture and generation of stable
transfected cell lines
The PC-3 cell line and DU145 cell line of PCa were
purchased from American Type Culture Collection (ATCC). The cells
were grown in Ham’s F-12 culture medium (Hyclone) and DMEM culture
medium (Hyclone), respectively, supplemented with 10% fetal bovine
serum (Hyclone). Cells were grown in a humidified atmosphere of 5%
CO2 at 37°C. The sequence of pri-miR-100, anti-miR-100
and si-h-RNA-AGO2 (a small hairpin RNA targeting AGO2,
5′-CACGGAAGUCCAUCUGAA dTdT-3′) were cloned into pMSCV-puromycin
plasmid. Then, the plasmids were transfected into 293FT cells which
were used as virus-generating host cells by calcium phosphate
precipitation as described previously (30). After incubation at 37°C for 6 h
after transfection, the media were changed and the cells were
incubated overnight. To produce new viruses, the media were
collected three times a day until 293FT cells reached total
confluence. Media containing viruses were used to infect PC-3 cells
and DU145 cells. Twenty-four hours after the addition of viruses,
infected cells were selected by adding puromycin to the growth
medium. Stable cell lines were verified by qRT-PCR.
Plasmids, virus production and infection
of target cells
The different regions of human miR-100 promoter were
generated by PCR amplification from PC-3 cells and cloned into the
KpnI/HindIII sites of the pGL3-basic-luciferase
reporter plasmid, respectively (Promega, Madison, WI, USA). The
full length AGO2-3′-UTR region was generated by PCR amplification
from DNA of PC-3 cells, and subcloned into pEGFP-C3 and a modified
pGL3-control-luciferase vector. To silence endogenous AGO2
expression, RNAi oligonucleotides were synthesizes by Invitrogen
and cloned into the pSuper-retropuro plasmid (Oligoengine, WA,
USA). The sequences of the sense strand for AGO2 was
5′-CACGGAAGUCCAUCUGAA dTdT-3′. The miR-100 mimic and miR-100
inhibitor were purchased from RiboBio (RiboBio Co., Ltd.,
Guangzhou, Guangdong, China). Transient transfection was performed
using the Lipofectamine 2000 reagent (Invitrogen) according to the
manufacturer’s instructions.
Tissue samples and RNA extraction
Tissue samples were collected from 64 primary PCa
patients. The methods of data collection and RNA extraction were as
previously described (5). The
Institutional Ethics Board (IRB) at The First Affiliated Hospital
of Sun Yat-sen University approved the study.
Luciferase assay
Luciferase assays were carried out in 293FT cells.
Cells were co-transfected with miRNAs and luciferase reporter
plasmids in 6-well plates and cultured for 48 h before the cells
were harvested and lysed for luminescence detection. The following
procedure and detection were performed using a luciferase assay kit
(Promega) according to the manufacturer’s protocol. Renilla
luciferase was activated to emitting the primary luminescence, and
firefly luminescence was used for normalization. Each test was
repeated in triplicate.
Quantitative reverse
transcription-PCR
The procedure was performed according to the manual
of All-in-One™ miRNA qRT-PCR Detection kit (GeneCopoeia, USA).
Total RNAs were extracted from cultured cells by using RNeasy kit
(Qiagen), and were reverse transcribed by adding poly-A sequence,
and real-time PCR analysis was performed with specific primer to
hsa-miRNAs which need to be checked (GeneCopoeia). Each sample was
analyzed in triplicate. No template and no reverse transcription
were included as negative controls. U6 snRNA was used as
normalization control. Relative expression levels from three
independent experiments were calculated following the
2−ΔΔCt method of Livak and Schmittgen (31).
Western blotting
For the analysis of expression of related proteins,
western blot assay was carried out. Equal amounts of proteins from
the supernatant were loaded per lane and resolved by
SDS-polyacrylamide electrophoresis. Then, the proteins was
transferred onto PVDF membrane (Millipore), blocked by 5% non-fat
milk for 1 h at room temperature, and probed with probed with
primary antibodies (1:1,000) overnight at 4°C, including mouse
anti-AGO2 (Abcam); anti-ZEB1 (Sigma, USA); rabbit anti-Oct4, c-Myc,
Klf4, CD44 and mouse anti-E-cadherin, vimentin (CST, Cell Signaling
Technology); mouse anti-fibronectin, N-cadherin (BD Biosciences).
Membranes were washed thrice (10 min each) in TBS-T buffer and
incubated for 40 min at room temperature with horseradish
peroxidase-conjugated anti-mouse or anti-rabbit secondary
antibodies. Membranes were washed thrice (10 min each) in TBS-T and
developed using the ECL system. Protein loading was normalized by
reprobing the blots with rabbit anti-β-actin (CST, Cell Signaling
Technology).
Wound healing assay
PCa cells were trypsinized and seeded into 6-well
culture plates 24 h before scratching and grew to reach almost
total confluence in 24 h, followed by non-serum starvation for 24
h. After cell monolayers formed, an artificial homogeneous wound
was scratched onto the monolayer with a sterile tip (Axygen, USA).
After scratching, the cells were washed with PBS and cultured in
10% fetal bovine serum media. Images of PC-3 cells migrating into
the wound were captured at time-points 0, 6 and 12 h by inverted
microscope (×40).
Invasion assay
Invasion assay was conducted by using Transwell
chamber consisting of 8-mm membrane filter inserts (Corning) coated
with Matrigel (BD Biosciences). Briefly, the cells were trypsinized
and suspended in serum-free medium. Then, 1.5×105 cells
were added to the upper chamber, but lower chamber was filled with
1 ml medium with 10% FBS. After incubation for 24–48 h, cells
passed through the coated membrane to the lower surface, in which
cells were fixed, stained, photographed, and quantified by counting
them in five random high power fields (×100).
Colony formation assay
The cells were trypsinized as single cells and
suspended in 10% fetal bovine serum medium. Cells (300 cells) were
seeded into each well of 6-well plates for 10–14 days, and colonies
were dyed with crystal violet. Plating efficiency = number of
colonies (≥50 cells per colony)/per input cells × 100%. To
determine the morphology of the different colonies they were
observed under a light microscope.
Self-renewing spheroid formation
assay
Cells (500 cells/well) were seeded into 6-well Ultra
Low Cluster plate (Corning) and were cultured in suspension in
serum-free DMEM-F12 (BioWhittaker), supplemented with B27 (1:50,
Invitrogen), 20 ng/ml EGF (BD Biosciences), 0.4% bovine serum
albumin (Sigma), and 4 mg/ml insulin (Sigma) for 10–12 days. After
10–12 days, the number of PC-3 cell spheres (tight, spherical,
non-adherent masses >50 μm in diameter) were counted, and
images of the spheres were scored under an inverse microscope.
Sphere formation efficiency = colonies/input cells × 100%.
Statistical analyses
All statistical analyses were carried out using the
SPSS 17.0 statistical software package. Means ± SD was calculated
and two-tailed Student’s t-test or one-way ANOVA was performed
using the data analysis tools provided by the software. The
correlation coefficient (Spearman rank correlation test) was
calculated to estimate the linear correlation between miR-100 and
the clinicopathology in the specimens. p<0.05 was considered
statistically significant.
Results
MiR-100 directly targets AGO2 through
interacting with the 3′-UTR
Since miRNAs regulate gene expression by
sequence-specific binding to the 3′-UTR of targeted mRNA and the
analysis using publicly available algorithms (TargetScan, miRanda)
indicated that the AGO2-3′-UTR region is the theoretical conserved
target of miR-100, we examined whether miR-100 may directly target
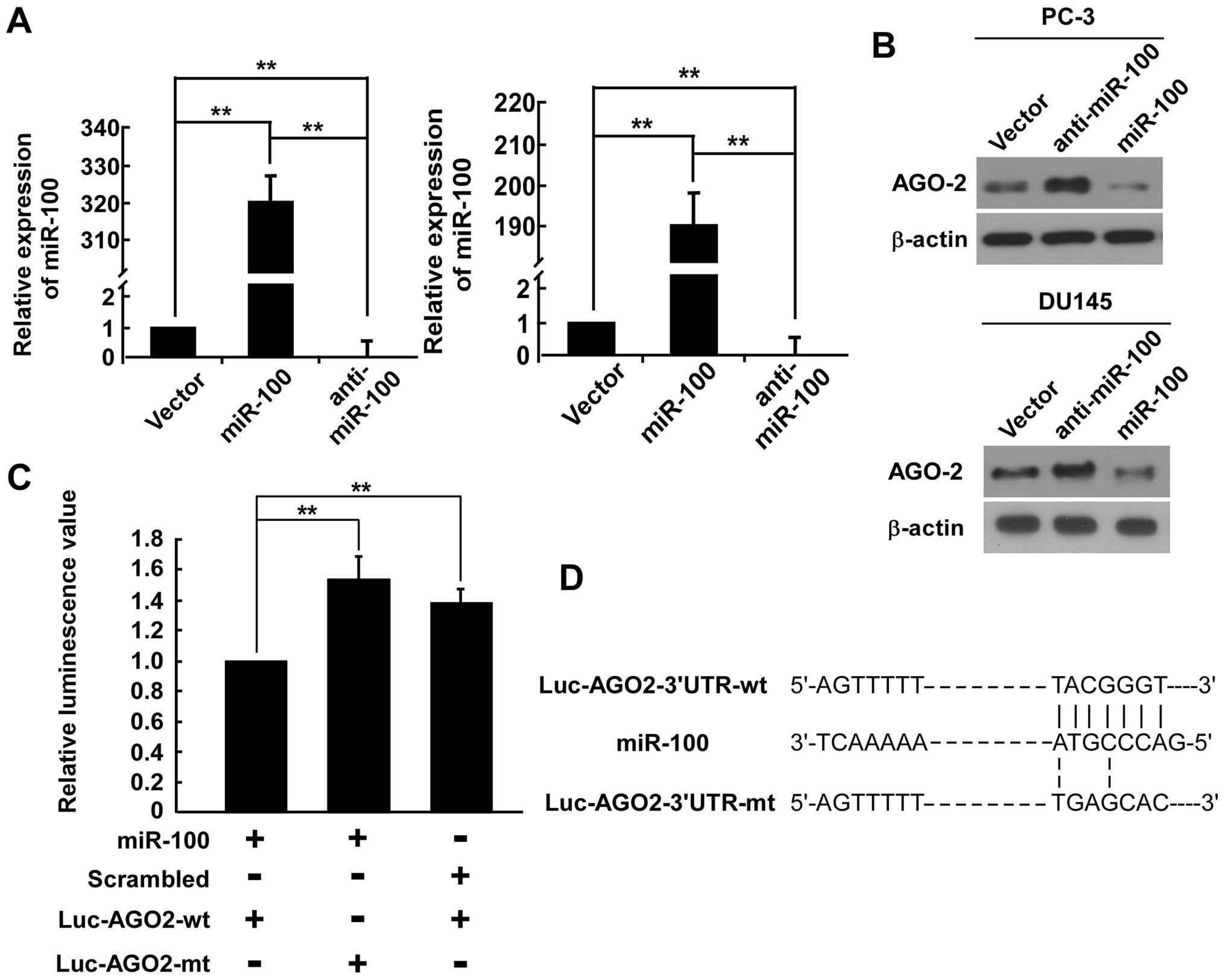
the AGO2-3′-UTR. Western blotting revealed that the expression
level of AGO2 was significantly decreased in cells infected with
miR-100 and increased in cells transfected with miR-100 inhibitor
(Fig. 1A and B), suggesting that
miR-100 may degrade the mRNA of AGO2. We carried out a
dual-luciferase reporter gene assay. Luciferase reporter plasmids
containing the wild-type 3′-UTR (Luc-AGO2-wt) or mutant 3′-UTR
(Luc-AGO2-mt) of AGO2 (Fig. 1D)
were constructed to determine the targeted region. As shown in
Fig. 1C, luciferase activities
were significantly higher in cells transfected with either
Luc-AGO2-mt or scrambled miRNA, with 1.54- and 1.39-fold higher
levels, respectively, than that in cells transfected with
Luc-AGO2-wt. Moreover, the scrambled miRNA base sequence did not
suppress Luc-AGO2-wt, and miR-100 could not bind to the mutant
AGO2-3′-UTR. Thus, these observations indicated that miR-100
directly targeted AGO2 through interacting with the 3′-UTR.
MiR-100 negatively regulates migration,
invasion and EMT in PCa cells
To investigate the role of miR-100 in the
development and progression of PCa metastasis, overexpressing
miR-100 (PC-3/miR-100 and DU145/miR-100) and downexpressing miR-100
(PC-3/anti-miR-100 and DU145/anti-miR-100) cell lines were
established by retrovirus transfection. Blank plasmid transfected
cells (PC-3/vector and DU145/vector) were used as the control
group. The expression levels of miR-100 in all cell lines were
confirmed by real-time PCR (Fig.
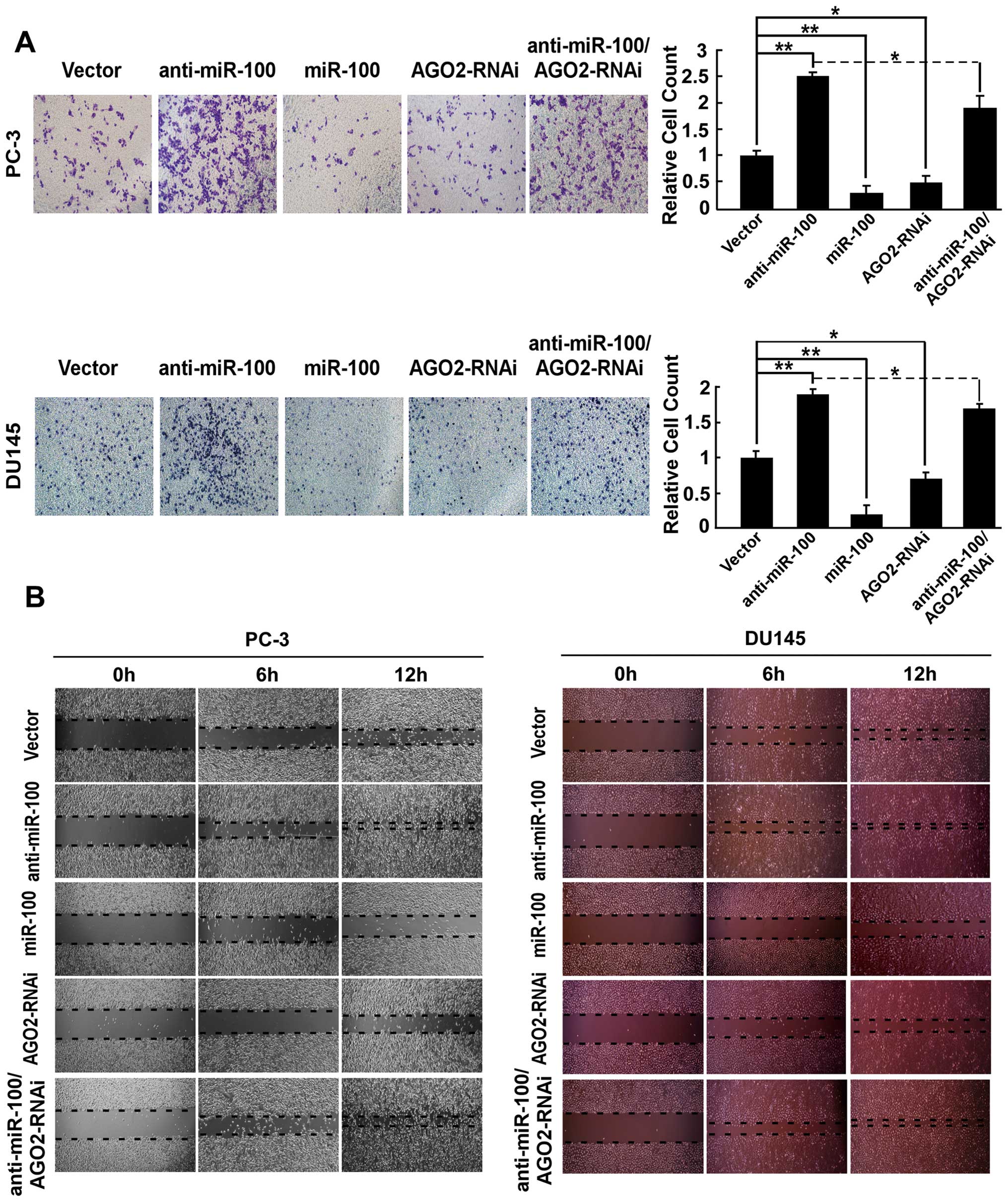
1A). By using wound healing assay to assess cell migration and
trans-well Matrigel invasion assay to assess the invasive ability
of cells, we found that the invasive ability of PC-3/miR-100 and
DU145/miR-100 and the healing speed of the cell wound were
repressed, and inversely, the invasive ability and the healing
speed of the cell wound of PC-3/anti-miR-100 DU145/anti-miR-100
were promoted compared to PC-3/vector and DU145/vector (Fig. 2).
To investigate whether miR-100 inhibited
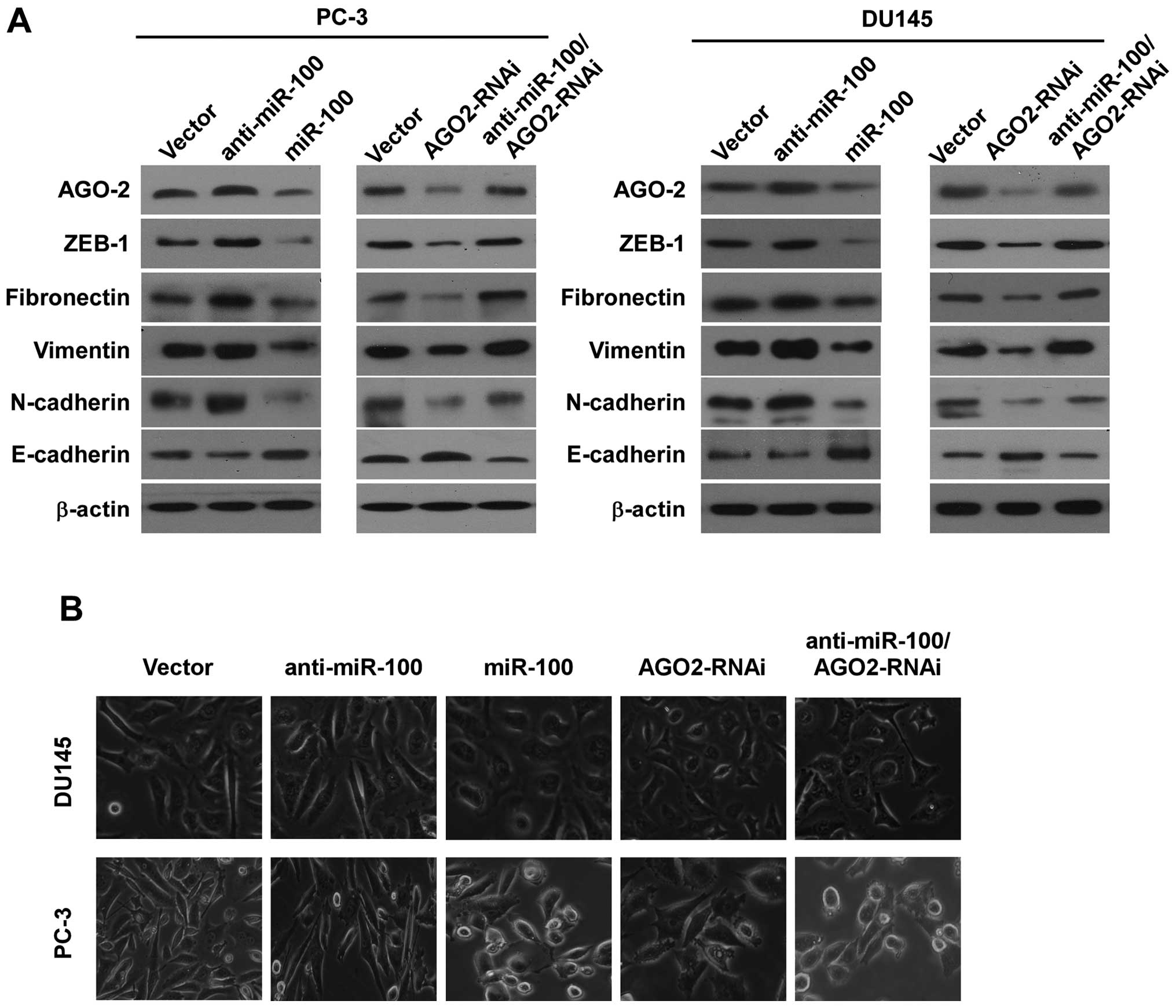
invasiveness by repressing EMT, we examined the influence of
miR-100 on expressions of E-cadherin, N-cadherin, fibronectin,
vimentin and ZEB1 in all cell lines by western blotting. We found
that E-cadherin expression increased in PC-3/miR-100 and
DU145/miR-100, and decreased in PC-3/anti-miR-100 and
DU145/anti-miR-100 compared with PC-3/vector and DU145/vector.
Contrarily, N-cadherin, fibronectin, vimentin and ZEB1 expressions
decreased in PC-3/miR-100 and DU145/miR-100, and increased in
PC-3/anti-miR-100 and DU145/anti-miR-100 (Fig. 3A). We then examined the change of
morphology of all cell lines with specific reference to special
characteristics during EMT. The results showed that overexpressing
miR-100 in PC-3 and DU145 cells converted the predominant
mechenchymal phenotype to an evident epithelial phenotype i.e.,
from the stick-like or long spindle-shaped mesenchymal profile to a
cobblestone-like or a short spindle-shaped epithelial morphology
and downregulation of miR-100 in PC-3 and DU145 cells increased the
long spindle-shaped mesenchymal profile (Fig. 3B).
MiR-100 negatively regulates colony
formation, tumor spheroid formation, CSC marker CD44 and ‘stemness’
factor expression in PCa cells
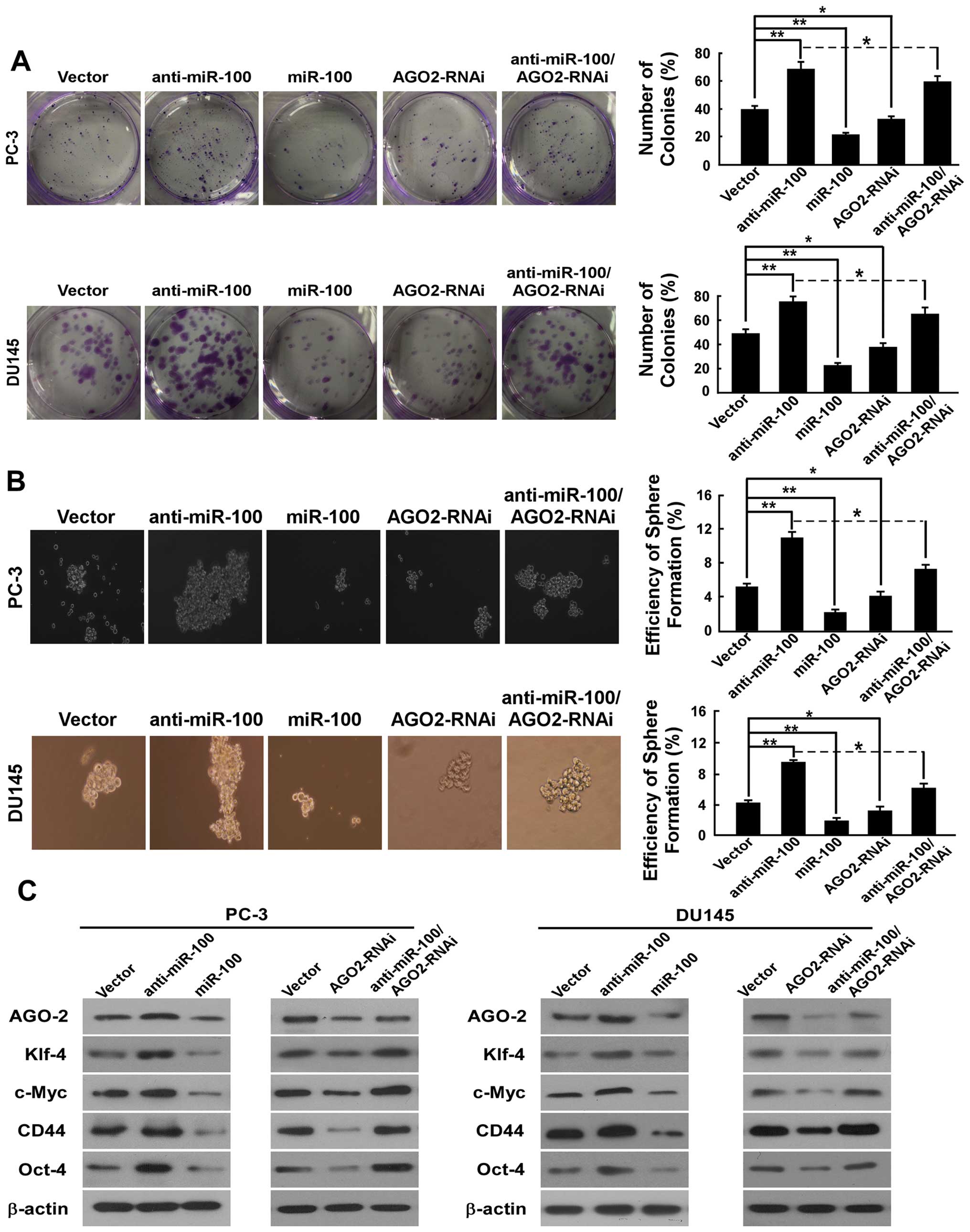
To determine the efficiency of miR-100 in regulating
the stemness of PC-3 and DU145 cells, the colony-forming and
spheroid formation assay were performed. In the colony-forming
assay, we found that the number of colonies (% plating efficiency)
were 22% in PC-3/miR-100, 69% in PC-3/anti-miR-100%, compared with
40% in PC-3/vector, and 23% in DU145/miR-100, 75% in
DU145/anti-miR-100, compared with 49% in DU145/vector (Fig. 4A). These results indicated a
dramatically repressing ability of miR-100 on colony formation. In
spheroid formation assay, there were prostaspheres in all kinds of
cells after culturing for 12 days under non-adherent conditions. As
shown in Fig. 4B, the spheroid
formation efficiency was 2.2% in PC-3/miR-100, 11% in
PC-3/anti-miR-100, compared with 5.2% in PC-3/vector, and 1.9% in
DU145/miR-100, 9.6% in DU145/anti-miR-100, and compared with 4.3%
in DU145/vector.
To determine whether miR-100 also has an influence
on CSC marker and stemness factor expression in PC-3 and DU145
cells, we detected the expression of stem cell
properties-associated factor and marker including CD44, c-Myc,
Oct4, and Klf4. As shown in Fig.
4C, compared with vector, overexpression of miR-100 in PC-3 and
DU145 cells reduced the expression of CD44, which has been
described as a prostate CSC marker based on clinical investigations
and in vitro studies of PCa cell lines, and downregulated
the expression of Oct4, c-Myc and Klf4, which are the key stemness
factors, and are required for maintaining self-renewal and
pluripotency of stem cells. Downregulation of miR-100 in PC-3 and
DU145 cells upregulated the expression of CD44, Oct4, c-Myc and
Klf4.
MiR-100 is negatively correlated with
bone metastasis, the Gleason score and serum PSA level in primary
PCa
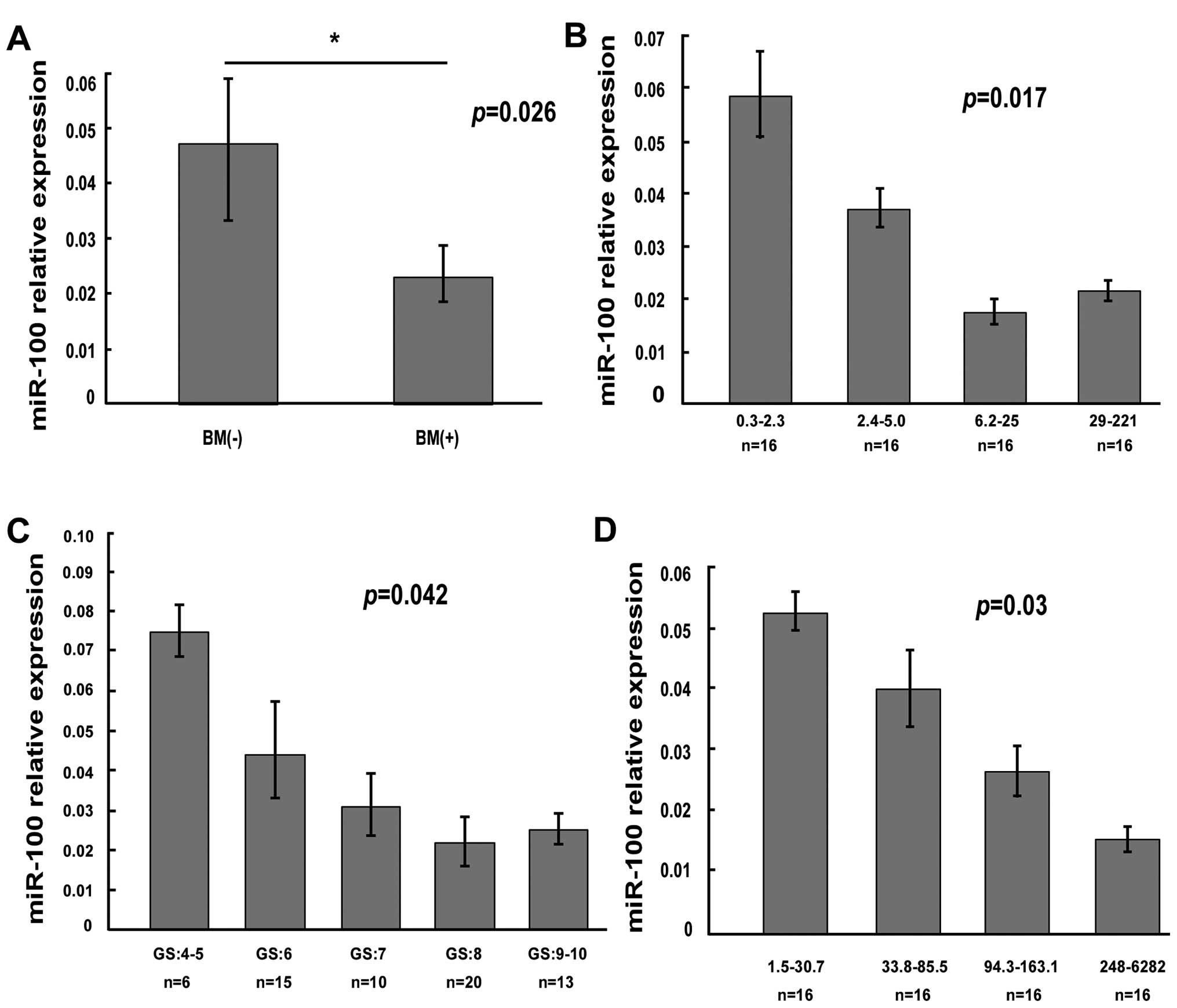
To determine whether the expression of miR-100 was
related to clinicopathology in primary PCa patients, we collected
64 clinical paraffin-fixed PCa samples (the clinical data are
showed in Table I). We detected
expression level of miR-100 by using qRT-PCR. By comparing the
mir-100 level of PCa specimens in 36 patients with bone metastasis
with that in 28 patients without bone metastasis, we found that the
mir-100 level in PCa specimens with bone metastases was
significantly lower than that in PCa specimens without metastases
(Fig. 5A). Next we assessed
whether the mir-100 level was related with Gleason score,
serum-free PSA and total PSA in primary PCa. The results showed
significant inverse correlation between expression of miR-100 and
Gleason score (Fig. 5C), the level
of serum-free PSA (Fig. 5B) and
the level of total PSA (Fig.
5D).
 | Table I.The clinical data of prostate cancer
patients. |
Table I.
The clinical data of prostate cancer
patients.
| No.of cases
(%) |
|---|
| Age (years) | |
| ≤75 | 32 (50.0) |
| >75 | 32 (50.0) |
| Total PSA | |
| ≤85.5 | 32 (50.0) |
| >85.5 | 32 (50.0) |
| Free PSA | |
| ≤5 | 33 (51.6) |
| >5 | 31 (48.4) |
| Gleason score | |
| ≤7 | 31 (48.4) |
| >7 | 33 (51.6) |
| Bone
metastasis | |
| Yes | 36 (56.3) |
| No | 28 (43.7) |
Downregulation of AGO2 represses
invasion, migration and EMT of PCa cells
To demonstrate the effect of AGO2 on the development
and progression of PCa metastasis, AGO2-downregulated cell lines
were established in PC-3 cells (PC-3/AGO2-RNAi) and DU145 cells
(DU145/AGO2-RNAi). The expression level of AGO2 was confirmed by
western blotting (Fig. 3A).
Transwell-Matrigel was used to assess the invasive ability of
cells. We found that downregulating AGO2 decreased the invasive
ability to 27.5% of PC-3/vector and 22.6% of DU145/vector (Fig. 2A). As shown in Fig. 2B, cell migration was observed by
wound healing assay and the healing speed of the cell wound
decreased in PC-3/AGO2-RNAi and DU145/AGO2-RNAi compared with
PC-3/vector and DU145/vector (Fig.
3).
We then investigated whether AGO2 could regulate
invasion and migration by repressing EMT. We examined the influence
of downregulation of AGO2 on expression of E-cadherin, N-cadherin,
fibronectin, vimentin and ZEB1 in PC-3 and DU145 cells by western
blotting, and found that E-cadherin increased in PC-3/AGO2-RNAi and
DU145/AGO2-RNAi, and N-cadherin, fibronectin, vimentin and ZEB1
decreased in PC-3/AGO2-RNAi and DU145/AGO2-RNAi compared with
vector control cells (Fig. 3A). We
also examined the change of morphology of PC-3 cells and DU145. The
results showed that PC-3/AGO2-RNAi and DU145/AGO2-RNAi converted
the predominant mechenchymal phenotype to an evident epithelial
phenotype i.e., from the stick-like or long spindle-shaped
mesenchymal profiles to a short spindle-shaped or a
cobblestone-like epithelial morphology (Fig. 3B).
Downregulation of AGO2 inhibits colony
formation, tumor spheroid formation, CSC marker CD44 and stemness
factor expression in PCa cells
To demonstrate the effect of AGO2 on regulating
stemness of PC-3 and DU145 cells, the colony formation and sphere
formation assays were performed. In the colony-forming assay, we
found that the number of colonies (% plating efficiency) was 33% in
PC-3/AGO2-RNAi compared with 40% in PC-3/vector and 38% in
DU145/AGO2-RNAi compared with 49% in DU145/vector (Fig. 4A). In the sphere formation assay,
there were prostaspheres in all the cell types after culturing for
12 days under non-adherent conditions. As shown in Fig. 4B, the spheroid formation efficiency
was 4.1% in PC-3/AGO2-RNAi and 3.2% in DU145/AGO2-RNAi. Compared to
vector (5.2% in PC-3, 4.3% in DU145), down-regulation of AGO2
significantly decreased the proportion of self-renewal in PC-3 and
DU145 cells.
Further, to demonstrate whether downregulation of
AGO2 also has an impact on stemness factors of PCa cells, we
measured the expression of CD44, c-Myc, Oct4, Klf4 and Sox2. As
shown in Fig. 4C, PC-3/AGO2-RNAi
and DU145/AGO2-RNAi reduced the expression of CD44, Oct4, c-Myc and
Klf4 compared to vectors. These data indicate that down-regulating
AGO2 repressed stemness of PCa cells.
Downregulation of AGO2 reverses the
effects of down-regulated miR-100 promoting migration, invasion,
EMT and stemness in PCa cells
Because AGO2 is directly regulated by miR-100, we
determined whether AGO2 can reverse the effects of miR-100 on
migration, invasion, EMT and stemness in PCa cells. AGO2 expression
was downregulated by sequence AGO2-shRNA in PC-3/anti-miR-100. The
expression of AGO2 was confirmed by real-time PCR and western
blotting. We found that downregulation of AGO2 partially reversed
the effects of miR-100 downregulation in migration speed and
invasive ability in PC-3 and DU145 cells (Fig. 2). AGO2 downregulation also
partially counteracted the effects of miR-100 downregulation of the
expression of E-cadherin, N-cadherin, fibronectin, vimentin and
ZEB1 and cell morphology (Fig. 3).
As shown in Fig. 4, AGO2
downregulation in part counteracted effects of miR-100
downregulation on colony-forming capability, spheroid formation
efficiency and the stemness factors expression in PC-3 and DU145
cells. These data indicate that inhibition of AGO2 at least to some
extent reversed the effects of miR-100 downregulation on migration,
invasion, EMT and stemness in PCa cells.
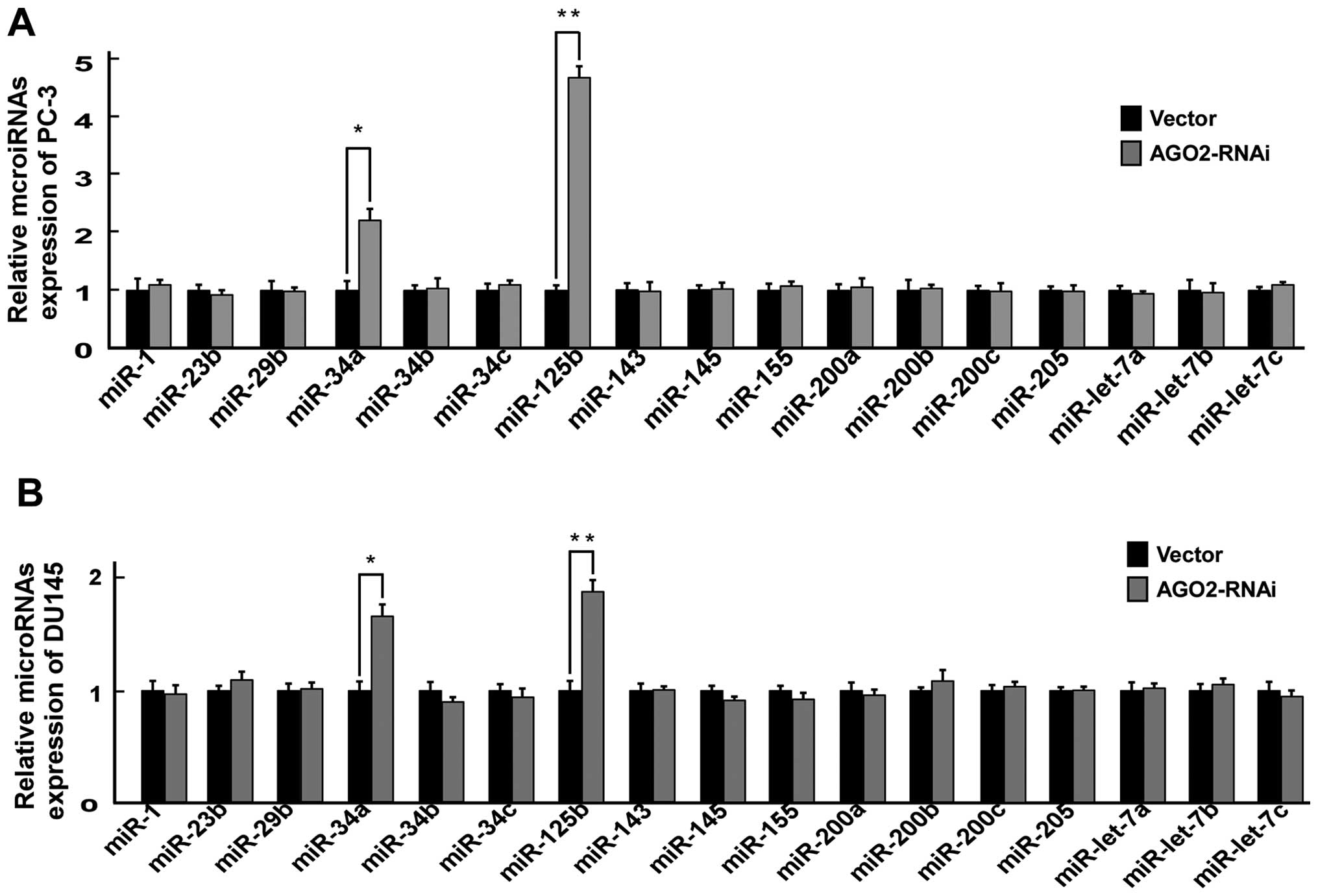
Downregulation of AGO2 promotes miR-125b
and miR34a expression in PCa cells
Previous studies showed that Ago2 directly regulated
certain miRNA biosynthesis (24)
and maturation (25). We attempted
to determine whether Ago2 promoted the metastatic ability of PCs
cells by regulating miRNA expression. We evaluated the expression
levels of 17 miRNAs, which were reported to play a role in
development and progression of PCa metastasis (5–9,32),
in PC-3/AGO2-RNAi, PC-3/vector, DU145/AGO2-RNAi and DU145/vector by
real-time PCR, and found that downregulation of AGO2 significantly
upregulated miR-125b and miR34a expression (Fig. 6).
Discussion
Our previous study found that miR-100 significantly
decreased in bone metastasis tissue compared with primary PCa
(5). In the present study, we
found that the expression of miR-100 was significantly lower in PCa
specimens with bone metastases than that without metastases. Leite
et al (13,14) also reported that miR-100 was
downregulated during the PCa progression, from high grade prostate
intraepithelial neoplasia through metastasis. Moreover, Sun et
al (10) found that miR-100
was downregulated in the more advanced PCa C4-2 cells relative to
the parental LNCaP cells. Importantly, our results showed that
mir-100 negatively regulated migration, invasion EMT and stemness
properties of PCa cells. Therefore, these findings indicate that
mir-100 is a metastatic suppressor in PCa.
However, mir-100 appears to be cancer specific in
regulating tumor metastasis. Huang et al (33) found that miR-100 expression
increased in metastatic pancreatic cancer cells. Furthermore, Wang
et al (34) reported that
miR-100 overexpression strongly associates with advanced tumor
progression in renal cell carcinoma. Moreover, miR-100 was
transiently introduced into A549 cells, NCI-H727 and NCI-H1437
cells, resulting in a significant increase of cell migration
activity (35). The above results
suggest that miR-100 may be a promoter of tumor metastasis. On the
contrary, in clear cell renal cell carcinomas, miR-100 was
downregulated in metastatic tissue samples compared with normal
tissue and was downregulated in metastatic tissue in comparison
with primary tumor tissue (36).
Furthermore, downregulation of miR-100 was significantly associated
with lymph node metastasis and reduced survival in Small cell
carcinoma of the cervix (37).
Thus, these findings supported that miR-100 was a suppressor of
metastasis.
Some previous studies have demonstrated that Ago2 is
a promoter of metastasis in different solid tumors. In
hepatocellular carcinoma (HCC), Ago2 overexpression promoted
migration and metastasis of HCC cells (23). AGO2 was over-expressed in colon
cancer relative to adjacent non-cancer tissue and the expression of
AGO2 appeared to increase in advanced tumors with distant
metastasis, suggesting it may promote tumor invasion (38). In human breast cancer cells, high
level of Ago2 was correlated with an enhanced proliferation and
migration ability (39). In serous
ovarian carcinoma, AGO2 mRNAs were overexpressed in solid
metastases compared with primary carcinomas and higher levels of
AGO2 mRNA was significantly associated with high-grade histology
(40). In head and neck squamous
cell carcinoma cell lines and primary tumors, AGO2 was
overexpressed and was functionally significant in cell lines
(41). In the present study, the
results showed that downregulation of AGO2 repressed migration,
invasion, EMT and stemness of PCa cells. These findings indicated
that AGO2 was a promoter of tumor progression and metastasis.
Furthermore, our results showed that AGO2 is a direct target of
miR-100. Thus, the critical mechanism of miR-100 suppression of
metastasis in PCa cells may be to downregulate AGO2 expression.
However, it remains to be determined how AGO2 regulates
metastasis.
Recent studies have shown that Ago2 directly
regulates miRNA biosynthesis (24). For example, the silencing of Ago2
upregulated miR-125b expression in myeloblastic HL60 cells
(42). In the present study, we
showed that repressing AGO2 in PCa cells enhanced expression of
miR-125b and miR-34a, which were downregulated in human PCa tissues
compared with normal tissues and in bone metastatic PCa tissues
compared to primary tumors (5,7,10,12).
Many studies have demonstrated that miR-100 and miR-125b were
concurrently downregulated in human PCa tissues (10,12)
and in bone metastatic PCa tissue (5). These findings suggested that
downregulation of miR-125b and miR-34a in human primary and
metastatic PCa tissues might be related to upregulation of Ago2,
which was directly regulated by miR-100.
Previous studies have demonstrated that miR-34a and
miR-125b are mainly metastatic suppressors, and play an important
role in inhibiting metastasis of cancer cells, including PCa cells
(7,12,43–48).
The miR-34a was able to directly inhibit EMT regulators Snail1,
ZEB1 and ZEB2 (43,44), and the stemness factor CD44 in PCa
cells (7). Downregulation of
miR-125b was able to promote metastatic oncoprotein expression and
enhance migration, invasion and metastasis (45–48).
ERBB2, a target of miR-125b (49),
played an important role in promoting stemness properties and EMT
(50–52). Therefore, an important mechanism of
AGO2 promoting metastasis of PCa cells may be to enhance
metastasis-promoting proteins, EMT and stemness by repressing
expression of miR-34a and miR-125b.
Recent studies have demonstrated that Ago2 can
directly regulate expression of stemness factors Oct4, Sox2, Nanog,
Klf4 and c-myc following its binding to the regulatory regions of
functional genes (53). Moreover,
in Ago2-knockdown embryos at the blastocyst stage, transcription
levels of Oct3/4, Nanog and Sox2 were decreased (54). In this study, our results showed
that downregulation of AGO2 repressed expression of stemness
factors Oct4, Klf4 and c-myc of PCa cells. Thus another mechanism
of Ago2 regulating metastasis of PCa may be to directly upregulate
stemness gene expression. In addition, Ago2 might have regulatory
functions in CSC self-renewal through the RNA-mediated gene
silencing mechanism as a component of RISC (55).
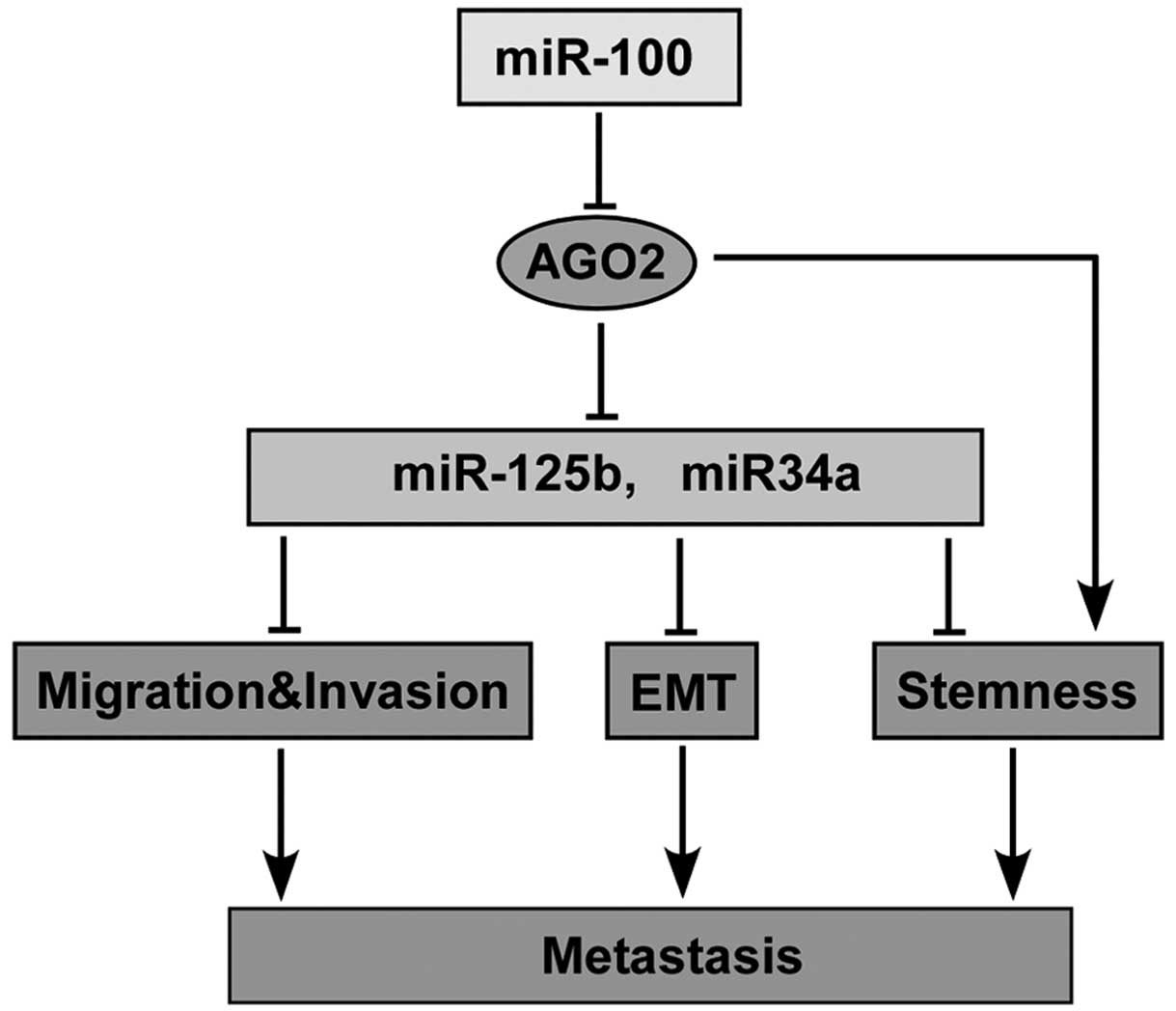
Our results showed that AGO2 was a direct target of
miR-100, and played an opposite role to miR-100 in regulating
migration, invasion, EMT and stemness of PCa cells. Furthermore,
downregulation of AGO2 was able to partially reverse the effects
seen with miR-100 downregulation in PCa cells. Importantly,
downregulation of AGO2 enhanced expression of miR-34a and -125b
which can suppress migration, invasion, EMT and stemness of cancer
cells. Thus, a proposed model of the close interconnections among
miR-100, AGO2 and metastasis is shown in Fig. 7.
In conclusion, our findings indicated that miR-100
negatively regulates the metastatic ability of PCa cells at least
partially by targeting AGO2 which repressed the metastatic
suppressor miR-34a and miR-125b expression, and modulated
migration, invasion, EMT and stemness of cancer cells. Our results
suggest that miR-100/AGO2 may play an important role in regulating
metastasis of PCa and is a potential novel target for prevention
and therapy.
Abbreviations:
|
PCa
|
prostate cancer;
|
|
miRNAs
|
microRNAs;
|
|
EMT
|
epithelial-mesenchymal transition;
|
|
CSCs
|
cancer stem cells;
|
|
Ago2
|
Argonaute 2;
|
|
RISC
|
RNA-induced silencing complex
|
Acknowledgements
This study was supported by grants
from the National Natural Science Foundation of China (no.
81272938).
References
|
1.
|
Nelson WG, De Marzo AM and Isaacs WB:
Prostate cancer. N Engl J Med. 349:366–381. 2003. View Article : Google Scholar
|
|
2.
|
Nauseef JT and Henry MD:
Epithelial-to-mesenchymal transition in prostate cancer: paradigm
or puzzle? Nat Rev Urol. 8:428–439. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Zhang J and Ma L: MicroRNA control of
epithelial-mesenchymal transition and metastasis. Cancer Metastasis
Rev. 31:653–662. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Pencheva N and Tavazoie SF: Control of
metastatic progression by microRNA regulatory networks. Nat Cell
Biol. 15:546–554. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Peng X, Guo W, Liu T, et al:
Identification of miRs-143 and -145 that is associated with bone
metastasis of prostate cancer and involved in the regulation of
EMT. PLoS One. 6:e203412011. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Tucci P, Agostini M, Grespi F, et al: Loss
of p63 and its microRNA-205 target results in enhanced cell
migration and metastasis in prostate cancer. Proc Natl Acad Sci
USA. 109:15312–15317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Liu C, Kelnar K, Liu B, et al: The
microRNA miR-34a inhibits prostate cancer stem cells and metastasis
by directly repressing CD44. Nat Med. 17:211–215. 2011. View Article : Google Scholar
|
|
8.
|
Saini S, Majid S, Yamamura S and Tabatabai
L: Regulatory role of mir-203 in prostate cancer progression and
metastasis. Clin Cancer Res. 17:5287–5298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kong D, Li Y, Wang Z, Banerjee S, Ahmad A,
Kim HR and Sarkar FH: miR-200 regulates PDGF-D-mediated
epithelialmesenchymal transition, adhesion, and invasion of
prostate cancer cells. Stem Cells. 27:1712–1721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Sun D, Lee YS, Malhotra A, et al: miR-99
family of MicroRNAs suppresses the expression of prostate-specific
antigen and prostate cancer cell proliferation. Cancer Res.
71:1313–1324. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Tong AW, Fulgham P, Jay C, et al: MicroRNA
profile analysis of human prostate cancers. Cancer Gene Ther.
16:206–216. 2009.PubMed/NCBI
|
|
12.
|
Giangreco AA, Vaishnav A, Wagner D,
Finelli A, et al: Tumor suppressor microRNAs, miR-100 and -125b,
are regulated by 1,25-dihydroxyvitamin D in primary prostate cells
and in patient tissue. Cancer Prev Res. 6:483–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Leite KR, Sousa-Canavez JM, Reis ST,
Tomiyama AH, et al: Change in expression of miR-let7c, miR-100, and
miR-218 from high grade localized prostate cancer to metastasis.
Urol Oncol. 29:265–269. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Leite KR, Tomiyama A, Reis ST,
Sousa-Canavez JM, Sañudo A, Camara-Lopes LH and Srougi M: MicroRNA
expression profiles in the progression of prostate cancer-from
high-grade prostate intraepithelial neoplasia to metastasis. Urol
Oncol. 31:796–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Sethi S, Macoska J, Chen W and Sarkar FH:
Molecular signature of epithelial-mesenchymal transition (EMT) in
human prostate cancer bone metastasis. Am J Transl Res. 3:90–99.
2010.PubMed/NCBI
|
|
18.
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Monteiro J and Fodde R: Cancer stemness
and metastasis: therapeutic consequences and perspectives. Eur J
Cancer. 46:1198–1203. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Xia H and Hui KM: MicroRNAs involved in
regulating epithelial-mesenchymal transition and cancer stem cells
as molecular targets for cancer therapeutics. Cancer Gene Ther.
19:723–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Huang S, Guo W, Tang Y, Ren D, Zou X and
Peng X: miR-143 and miR-145 inhibit stem cell characteristics of
PC-3 prostate cancer cells. Oncol Rep. 28:1831–1837.
2012.PubMed/NCBI
|
|
22.
|
Janas MM, Wang B, Harris AS, et al:
Alternative RISC assembly: binding and repression of microRNA-mRNA
duplexes by human Ago proteins. RNA. 18:2041–2055. 2012. View Article : Google Scholar
|
|
23.
|
Cheng N, Li Y and Han ZG: Argonaute2
promotes tumor metastasis by way of up-regulating focal adhesion
kinase expression in hepatocellular carcinoma. Hepatology.
57:1906–1918. 2013. View Article : Google Scholar
|
|
24.
|
Cheloufi S, Dos Santos CO, Chong MM and
Hannon GJ: A dicer-independent miRNA biogenesis pathway that
requires Ago catalysis. Nature. 465:584–589. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Zhou Y, Chen L, Barlogie B, Stephens O, et
al: High-risk myeloma is associated with global elevation of miRNAs
and overexpression of EIF2C2/AGO2. Proc Natl Acad Sci USA.
107:7904–7909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Yoo NJ, Hur SY, Kim MS, Lee JY and Lee SH:
Immunohisto-chemical analysis of RNA-induced silencing
complex-related proteins AGO2 and TNRC6A in prostate and esophageal
cancers. APMIS. 118:271–276. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Sand M, Skrygan M, Georgas D, et al:
Expression levels of the microRNA maturing microprocessor complex
component DGCR8 and the RNA-induced silencing complex (RISC)
components argonaute-1, argonaute-2, PACT, TARBP1, and TARBP2 in
epithelial skin cancer. Mol Carcinog. 51:916–922. 2012. View Article : Google Scholar
|
|
28.
|
Naoghare PK, Tak YK, Kim MJ, Han E and
Song JM: Knock-down of Argonaute 2 (AGO2) induces apoptosis in
myeloid leukaemia cells and inhibits siRNA-mediated silencing of
transfected oncogenes in HEK-293 cells. Basic Clin Pharmacol
Toxicol. 109:274–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Shekar PC, Naim A, Sarathi DP and Kumar S:
Argonaute-2-null embryonic stem cells are retarded in self-renewal
and differentiation. J Biosci. 36:649–657. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mo YY and Beck WT: Association of human
DNA topoisomerase IIalpha with mitotic chromosomes in mammalian
cells is independent of its catalytic activity. Exp Cell Res.
252:50–62. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T) (-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Majid S, Dar AA, Saini S, Deng G, et al:
MicroRNA-23b functions as a tumor suppressor by regulating Zeb1 in
bladder cancer. PLoS One. 8:e676862013. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Huang J, Egger M, Grizzle W and McNally L:
MicroRNA-100 regulates IGF1-receptor expression in metastatic
pancreatic cancer cells. Biotech Histochem. 88:397–402. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Wang G, Chen L, Meng J, Chen M, Zhuang L
and Zhang L: Overexpression of microRNA-100 predicts an unfavorable
prognosis in renal cell carcinoma. Int Urol Nephrol. 45:373–379.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Shimamura T, Imoto S, Shimada Y, et al: A
novel network profiling analysis reveals system changes in
epithelial-mesenchymal transition. PLoS One. 6:e208042011.
View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Wotschofsky Z, Liep J, Meyer HA, Jung M,
et al: Identification of metastamirs as metastasis-associated
microRNAs in clear cell renal cell carcinomas. Int J Biol Sci.
8:1363–1374. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Huang L, Lin JX, Yu YH, Zhang MY, Wang HY
and Zheng M: Downregulation of six microRNAs is associated with
advanced stage, lymph node metastasis and poor prognosis in small
cell carcinoma of the cervix. PLoS One. 7:e337622012. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Li L, Yu C, Gao H and Li Y: Argonaute
proteins: potential biomarkers for human colon cancer. BMC Cancer.
10:382010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Adams BD, Claffey KP and White BA:
Argonaute-2 expression is regulated by epidermal growth factor
receptor and mitogen-activated protein kinase signaling and
correlates with a transformed phenotype in breast cancer cells.
Endocrinology. 150:14–23. 2009. View Article : Google Scholar
|
|
40.
|
Vaksman O, Hetland TE, Trope’ CG, Reich R
and Davidson B: Argonaute, Dicer, and Drosha are up-regulated along
tumor progression in serous ovarian carcinoma. Hum Pathol.
43:2062–2069. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Chang SS, Smith I, Glazer C, Hennessey P
and Califano JA: EIF2C is overexpressed and amplified in head and
neck squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec.
72:337–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Iosue I, Quaranta R, Masciarelli S, et al:
Argonaute 2 sustains the gene expression program driving human
monocytic differentiation of acute myeloid leukemia cells. Cell
Death Dis. 4:e9262013. View Article : Google Scholar
|
|
43.
|
Siemens H, Jackstadt R, Hünten S, Kaller
M, Menssen A, Götz U and Hermeking H: miR-34 and SNAIL form a
double-negative feedback loop to regulate epithelial-mesenchymal
transitions. Cell Cycle. 10:4256–4271. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Kim T, Veronese A, Pichiorri F, Lee TJ, et
al: p53 regulates epithelial-mesenchymal transition through
microRNAs targeting ZEB1 and ZEB2. J Exp Med. 208:875–883. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Ferracin M, Bassi C, Pedriali M, et al:
miR-125b targets erythropoietin and its receptor and their
expression correlates with metastatic potential and ERBB2/HER2
expression. Mol Cancer. 12:1302013. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Li Y, Chao Y, Fang Y, et al: MTA1 promotes
the invasion and migration of non-small cell lung cancer cells by
downregulating miR-125b. J Exp Clin Cancer Res. 32:332013.
View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Rajabi H, Joshi MD, Jin C, Ahmad R and
Kufe D: Androgen receptor regulates expression of the MUC1-C
oncoprotein in human prostate cancer cells. Prostate. 71:1299–1308.
2011.PubMed/NCBI
|
|
48.
|
Liang L, Wong CM, Ying Q, et al:
MicroRNA-125b suppressesed human liver cancer cell proliferation
and metastasis by directly targeting oncogene LIN28B2. Hepatology.
52:1731–1740. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Scott GK, Goga A, Bhaumik D, Berger CE,
Sullivan CS and Benz CC: Coordinate suppression of ERBB2 and ERBB3
by enforced expression of microRNA miR-125a or miR-125b. J Biol
Chem. 282:1479–1486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Nair R, Roden DL, Teo WS, et al: c-Myc and
Her2 cooperate to drive a stem-like phenotype with poor prognosis
in breast cancer. Oncogene. Sep 23–2013.(Epub ahead of print).
View Article : Google Scholar
|
|
51.
|
Cheng JC, Qiu X, Chang HM and Leung PC:
HER2 mediates epidermal growth factor-induced down-regulation of
E-cadherin in human ovarian cancer cells. Biochem Biophys Res
Commun. 434:81–86. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Kim IY, Yong HY, Kang KW and Moon A:
Overexpression of ErbB2 induces invasion of MCF10A human breast
epithelial cells via MMP-9. Cancer Lett. 275:227–233. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Kim BS, Im YB, Jung SJ, Park CH and Kang
SK: Argonaute2 regulation for K+ channel-mediated human
adipose tissue-derived stromal cells self-renewal and survival in
nucleus. Stem Cells Dev. 21:1736–1748. 2012.PubMed/NCBI
|
|
54.
|
Shen XH, Han YJ, Cui XS and Kim NH: Ago2
and GW182 expression in mouse preimplantation embryos: a link
between microRNA biogenesis and GW182 protein synthesis. Reprod
Fertil Dev. 22:634–643. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Carmell MA, Xuan Z, Zhang MQ and Hannon
GJ: The Argonaute family: tentacles that reach into RNAi,
developmental control, stem cell ––maintenance, and tumorigenesis.
Genes Dev. 16:2733–2742. 2002.PubMed/NCBI
|