Introduction
Colorectal cancer (CRC) is a major burden to
healthcare systems worldwide accounting for approximately one
million of new cancer cases (1).
The mortality associated with CRC remains high despite advances
made in adjuvant and neoadjuvant therapy (2). Search for new and effective
anti-cancer treatments is needed and an attractive avenue of
research is gene intervention therapy for cancer.
The klotho (KL) gene is a classical ‘aging
suppressor’ gene. The role of KL was first demonstrated in the
pathology of chronic kidney diseases. The physiological and
pathological function of KL shows it can be used as a regulator of
oxidative stress and senescence (3). Moreover, recent studies show that KL
is involved in the progression of several types of human cancers,
and plays an important role in tumorigenesis, proliferation,
survival, autophagy and resistance to antitumor therapies (4). KL is inactivated through promoter
hypermethylation and potentially functions as a tumor suppressor
gene in human cancers (5,6), suggesting loss of KL may serve as an
early marker for breast tumorigenesis (7). KL is also decreased in small cell
lung cancer (SCLC) and hepatocellular carcinoma (HCC), predictive
of a favorable outcome following resection in SCLC and HCC patients
(8,9).
The function of KL in cancer is characterized by
participating in infiltration and proliferation of cancer cells
through multiple signaling pathways. KL suppresses renal fibrosis
and cancer metastasis through transforming growth factor (TGF)
signaling (10), while loss of KL
increases Wnt5A expression and enhances melanoma cell motility
(11). KL is downregulated in
pancreatic adenocarcinoma, reduces growth of pancreatic cancer
cells through inactivation of the IGF-I and the bFGF pathways
(12). Overexpression of KL
inhibits cell proliferation, motility and invasion, and induces
apoptosis in lung and cervical cancers (13–15),
suggesting the use of KL as potential strategy for the development
of novel therapeutic interventions for cancer.
However, some studies show that KL is highly
expressed in ovarian cancer (16)
and HCC (17) and is associated
with increased risk of disease progression and death (16), but KLB-silencing in HCC cells
decreases cell proliferation and suppresses FGFR downstream
signaling (17). In order to
expound the expression and function of KL in cancer, we investigate
the expression of KL in human colon cancer by immunohistochemistry
(IHC) assay, and assessed the tumor growth and invasion in colon
cancer cells in vitro and in vivo. We hypothesized
that overexpression of KL might function as a tumor suppressor in
colon cancer.
Materials and methods
Materials
The colon cancer cell lines (SW480 and HT-29) used
for experiments were obtained from the Institute of Biochemistry
and Cell Biology (Shanghai, China). Lentivirus-mediated KL vector
(Lv-KL), negative control vector, and virion-packaging elements
were purchased from Genechem (Shanghai, China). Human colon cancer
tissues and the corresponding ANCT were collected from Department
of Colorectal Surgery of Fudan University. The tissue microarray of
colon cancer was made by Shanghai Outdo Biotech Co. Ltd (Shanghai,
China). All the antibodies were purchased from Cell Signaling
Technologies (Boston, MA, USA). KL primer was synthesized by ABI
(Framingham, MA, USA).
Drugs and reagents
Dulbecco’s modified Eagle’s medium (DMEM) and fetal
bovine serum (FBS) were purchased from Thermo Fisher Scientific
Inc. (Waltham, MA, USA); TRIzol Reagent and Lipofectamine 2000 were
obtained from Invitrogen (Carlsbad, CA, USA); M-MLV Reverse
Transcriptase was purchased from Promega (Madison, WI, USA);
SYBR-Green Master Mix was obtained from Takara (Otsu, Japan); and
the ECL Plus Kit was obtained from GE Healthcare (Piscataway, NJ,
USA).
Clinical samples and data
Tissue microarray was prepared for IHC. Human colon
cancer tissues and the corresponding ANCT were obtained from biopsy
in a total of 68 consecutive colon cancer cases admitted in our
hospital from January, 2006 to December, 2011. The baseline
characteristics of the patients before neo-adjuvant chemotherapy
were summarized. The study was approved by Medical Ethics Committee
of Fudan University and written informed consent was obtained from
the patients or their parents before sample collection. Two
pathologists independently reviewed all of the cases.
Tissue microarray
The advanced tissue arrayer (ATA-100, Chemicon
International, Tamecula, CA, USA) was used to create holes in a
recipient paraffin block and to acquire cylindrical core tissue
biopsies with a diameter of 1 mm from the specific areas of the
‘donor’ block. The tissue core biopsies were transferred to the
recipient paraffin block at defined array positions. The tissue
microarrays contained tissue samples from 68 formalin-fixed
paraffin-embedded cancer specimens with known diagnosis, and
corresponding ANCT from these patients. The block was incubated at
45°C for 20 min to allow complete embedding of the grafted tissue
cylinders in the paraffin of the recipient block, and then stored
at 4°C until microtome sectioning.
Immunohistochemical staining
Tissue microarray sections were processed for IHC
analysis of KL protein as follows. Immunohistochemical examinations
were carried out on 3-mm thick sections. For anti-KL
immunohistochemistry, unmasking was performed with 10 mM sodium
citrate buffer, pH 6.0, at 90°C for 30 min. For anti-KL
immunohistochemistry, antigen unmasking was not necessary. Sections
were incubated in 0.03% hydrogen peroxide for 10 min at room
temperature, to remove endogenous peroxidase activity, and then in
blocking serum (0.04% bovine serum albumin, A2153, Sigma-Aldrich,
Shanghai, China; and 0.5% normal goat serum X0907, Dako
Corporation, Carpinteria, CA, USA; in PBS) for 30 min at room
temperature. Anti-KL antibody was used at a dilution of 1:200. The
antibody was incubated overnight at 4°C. Sections were then washed
three times for 5 min in PBS. Non-specific staining was blocked
with 0.5% casein and 5% normal serum for 30 min at room
temperature. Finally, staining was developed using diaminobenzidine
substrate, and sections were counterstained with hematoxylin.
Normal serum or PBS was used to replace anti-KL antibody in
negative controls.
Quantification of protein expression
The expression of KL was semiquantitatively
estimated as the total immunostaining scores, which were calculated
as the product of a proportion score and an intensity score. The
proportion and intensity of the staining was evaluated
independently by two observers. The proportion score reflected the
fraction of positive staining cells (0, none; 1, ≤10%; 2, 10 to
≤25%; 3, >25 to 50%; 4, >50%), and the intensity score
represented the staining intensity (0, no staining; 1, weak; 2,
intermediate; and 3, strong). Finally, a total expression score was
given ranging from 0 to 12. Based on the analysis in advance, KL
expression was categorized into two groups: low-level KL expression
(score 0–3) and high-level KL expression (score 4–12). The scoring
was independently assessed by two pathologists.
Cell culture and transfection
Colon cancer cell lines were cultured in DMEM medium
supplemented with 10% heat-inactivated FBS, 100 U/ml of penicillin,
and 100 μg/ml of streptomycin. Cells in this medium were placed in
a humidified atmosphere containing 5% CO2 at 37°C. Cells
were subcultured at a 1:5 dilution in medium containing 300 μg/ml
G418 (an aminoglycoside antibody, commonly used stable transfection
reagent in molecular genetic testing). On the day of transduction,
colon cancer cells were replated at 5×104 cells/well in
24-well plates containing serum-free growth medium with polybrene
(5 mg/ml). When reached 50% confluence, cells were transfected with
recombinant experimental virus or control virus at the optimal MOI
(multiplicity of infection) of 50, and cultured at 37°C and 5%
CO2 for 4 h. Then supernatant was discarded and serum
containing growth medium was added. At 4 days of post-transduction,
transduction efficiency was measured by the frequency of green
fluorescent protein (GFP)-positive cells. Positive and stable
transfectants were selected and expanded for further study. The
Lv-KL vector-infected clone and the negative control
vector-infected cells were named as Lv-KL group and NC group.
Quantitative PCR
To quantitatively determine the mRNA expression
level of KL in colon cancer cells, real-time PCR was performed.
Total RNA was extracted from each clone using TRIzol according to
the manufacturer’s protocol. Reverse transcription was carried out
using M-MLV and cDNA amplification was performed using the
SYBR-Green master mix kit according to the manufacturer’s
guidelines. The KL gene was amplified using a specific
oligonucleotide primer and the β-actin gene was used as an
endogenous control. Data were analyzed using the comparative Ct
method (2−ΔΔCt). Three separate experiments were
performed for each clone.
Western blot assay
Colon cancer cell lines were harvested and extracted
using lysis buffer (Tris-HCl, SDS, mercaptoethanol and glycerol).
Cell extracts were boiled for 5 min in loading buffer, and then an
equal amount of cell extract was separated on 15% SDS-PAGE gels.
Separated protein bands were transferred onto polyvinylidene
fluoride (PVDF) membranes, which were subsequently blocked in 5%
skim milk powder. Primary antibodies against KL, p-IGF1R, p-PI3K,
p-AKT, PCNA and MMP-2 were diluted according to the manufacturer’s
instructions and incubated overnight at 4°C. Subsequently,
horseradish peroxidase-linked secondary antibodies were added at a
dilution of 1:1,000 and incubated at room temperature for 2 h. The
membranes were washed 3 times with PBS, and the immunoreactive
bands were visualized using the ECL Plus Kit according to the
manufacturer’s instructions. The relative protein levels in
different cell lines were normalized to the concentration of
β-actin. Three separate experiments were performed for each
clone.
Cell proliferation assay
Cell proliferation was analyzed using the MTT assay.
Briefly, cells infected with Lv-KL virus were incubated in
96-well-plates at a density of 1×105 cells per well with
DMEM medium supplemented with 10% FBS. Cells were treated with 20
μl of MTT dye at 0, 24, 48 and 72 h, and subsequently incubated
with 150 μl of DMSO for 5 min. The color reaction was measured at
570 nm using an Enzyme Immunoassay Analyzer (Bio-Rad Laboratories,
Hercules, CA, USA). The proliferation activity was calculated for
each clone.
Colony formation assay
Colon cancer cells infected with Lv-KL were counted
and seeded in 12-well plates (in triplicate) at 100 cells per well.
Fresh culture medium was replaced every three days. Colonies were
counted only if they contained more than 50 cells, and the number
of colonies was counted from the 6th day after seeding and then the
cells were stained using crystal violet. The rate of colony
formation was calculated with the equation: colony formation rate =
(no. of colonies/no. of seeded cells) × 100%.
Transwell invasion assay
Transwell filters were coated with Matrigel (3.9
μg/μl; 60–80 μl) on the upper surface of a polycarbonate membrane
(diameter, 6.5 mm; pore size, 8 μm). After incubating at 37°C for
30 min, the Matrigel solidified and served as the extracellular
matrix for analysis of tumor cell invasion. Harvested cells
(1×105) in 100 μl of serum-free DMEM were added into the
upper compartment of the chamber. A total of 200 μl of conditioned
medium derived from NIH3T3 cells was used as a source of
chemoattractant, which was placed in the bottom compartment of the
chamber. After 24 h of incubation at 37°C with 5% CO2,
the medium was removed from the upper chamber. The non-invaded
cells on the upper side of the chamber were scraped off with a
cotton swab. Cells that had migrated from the Matrigel into the
pores of the inserted filter were fixed with 100% methanol, stained
with hematoxylin, then mounted and dried at 80°C for 30 min. The
number of cells invading through the Matrigel was counted in 3
randomly selected visual fields from the central and peripheral
portion of the filter by using an inverted microscope (×200
magnification). Each assay was repeated 3 times.
Subcutaneous tumor model and gene
therapy
Six-week-old female immune-deficient nude mice
(BALB/c-nu) were bred at the laboratory animal facility (Institute
of Chinese Academy of Sciences, Shanghai, China), and were housed
individually in microisolator ventilated cages with free access to
water and food. All experimental procedures were performed
according to the regulations and internal biosafety and bioethics
guidelines of Fudan University and the Shanghai Municipal Science
and Technology Commission. Three mice were injected subcutaneously
with 1×108 colon cancer cells (HT-29) in 50 μl of PBS
pre-mixed with an equal volume of matrigel matrix
(Becton-Dickinson). Mice were monitored daily for development a
subcutaneous tumor. When the tumor size reached approximately 5 mm
in length, they were surgically removed, cut into 1–2
mm3 pieces, and re-seeded individually into other mice.
When tumor size reached approximately 5 mm in length, the mice were
randomly assigned as NC group and Lv-KL group. In the treatment
group, 15 μl of Lv-KL was injected into subcutaneous tumors using a
multi-site injection format. Injections were repeated every other
day after initial treatment. The tumor volume every three days was
measured with a caliper, using the formula volume = (length ×
width)2/2.
Statistical analysis
SPSS 20.0 was used for the statistical analysis.
Kruskal-Wallis H test and χ2 test were used to analyze
the expression rate in all groups. One-way analysis of variance
(ANOVA) was used to analyze the differences between groups. The LSD
method of multiple comparisons was employed when the probability
for ANOVA was statistically significant. Statistical significance
was set at P<0.05.
Results
The expression of KL in colon cancer
tissues
The expression of KL protein was evaluated using IHC
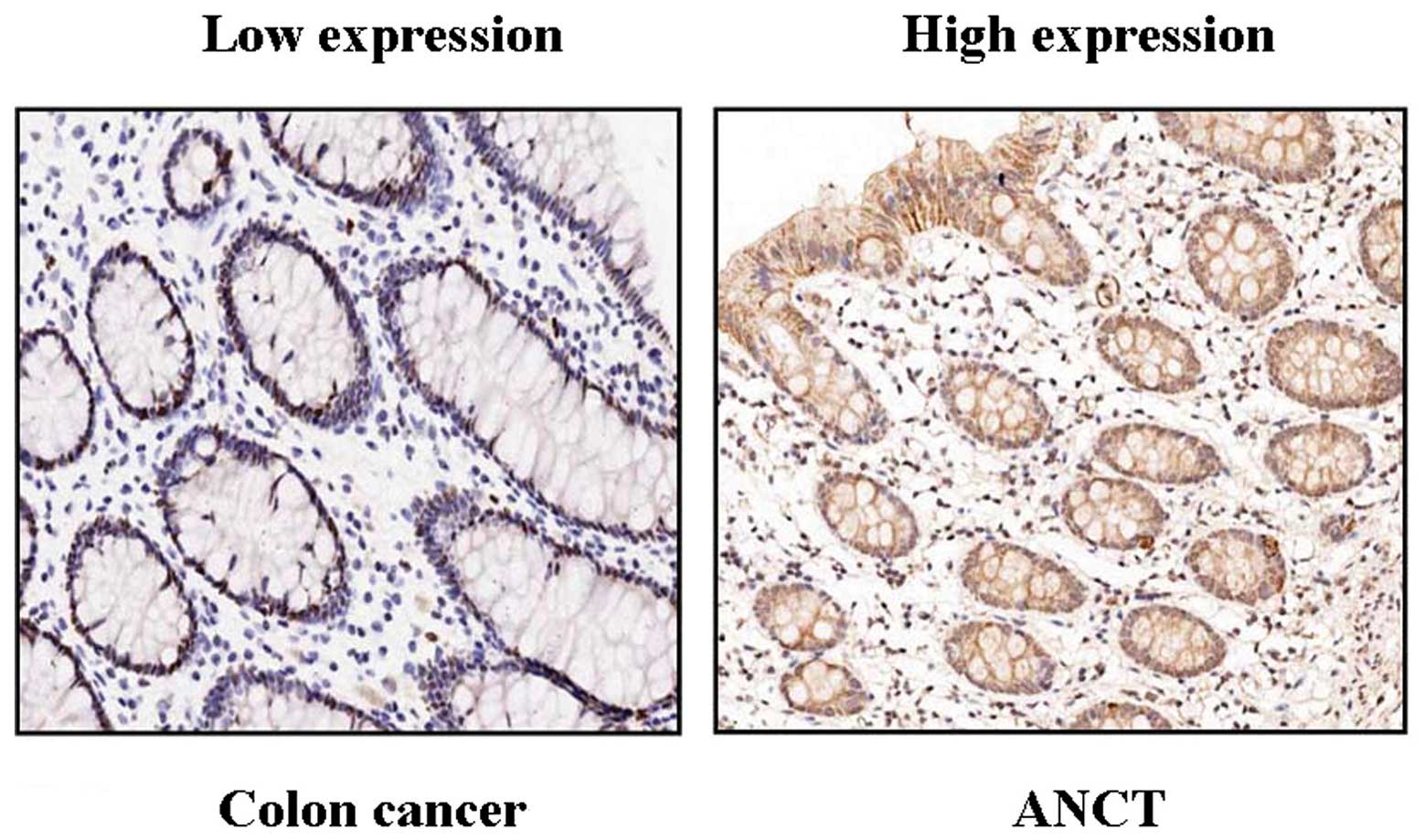
staining in colon cancer tissues. As shown in Fig. 1, different levels of positive
expression of KL protein were detected in colon cancer and ANCT
tissues. Positive KL immunostaining was mainly localized in the
cytoplasm of colon cancer tissue cells. According to the KL
immunoreactive intensity, the positive expression of KL in colon
cancer tissues was significantly decreased compared with that in
ANCT (P<0.001) (Table I).
 | Table IThe expression of KL protein in colon
cancer tissues. |
Table I
The expression of KL protein in colon
cancer tissues.
| Variables | Group | Cases (n=68) | Expression levels
(n=68) | Positive rate
(%) | χ2 | P-value |
|---|
|
|---|
| − | + | ++ | +++ |
|---|
| KL | Cancer | 68 | 27 | 24 | 12 | 5 | 60.3 | 5.263 | 0.022 |
| ANCT | 68 | 15 | 27 | 17 | 9 | 77.9 | | |
Correlation of KL expression with
clinicopathological parameters
According to the KL immunoreactive intensity, 27
(39.71%) patients were classified as KL-negative group, and 41
(60.29%) patients were classified as KL-positive group. We then
analyzed the association between KL expression and the
clinicopathological data of patients with colon cancer. As
summarized in Table II, we found
that decreased expression of KL was closely correlated with the
Dukes staging (P=0.034) and invasive depth of colon cancer
(P=0.008), but did not relate with the other clinicopathological
factors including age, gender, tumor size, degree of
differentiation and CEA level (each P>0.05).
 | Table IIAssociation between KL expression and
clinicopathological characteristics of patients with colon
cancer. |
Table II
Association between KL expression and
clinicopathological characteristics of patients with colon
cancer.
| | KL expression | | |
|---|
| |
| | |
|---|
| Variables | Cases (n=68) | (n=27) (−) | (n=41) (+) | χ2 | P-value |
|---|
| Age | | | | 0.117 | 0.732 |
| ≤60 | 42 | 16 | 26 | | |
| >60 | 26 | 11 | 15 | | |
| Gender | | | | 2.034 | 0.154 |
| Male | 35 | 11 | 24 | | |
| Female | 33 | 16 | 17 | | |
| Tumor size
(cm) | | | | 3.006 | 0.083 |
| ≤5 | 39 | 12 | 27 | | |
| >5 | 29 | 15 | 14 | | |
| Dukes staging | | | | 4.480 | 0.034 |
| A+B | 36 | 10 | 26 | | |
| C+D | 32 | 17 | 15 | | |
| Depth of
invasion | | | | 7.046 | 0.008 |
| Within serosa | 41 | 11 | 30 | | |
| Beyond serosa | 27 | 16 | 11 | | |
| Degree of
differentiation | | | | 0.254 | 0.881 |
| Well | 23 | 9 | 14 | | |
| Moderately | 28 | 12 | 16 | | |
| Poorly | 17 | 6 | 11 | | |
| CEA (ng/ml) | | | | 0.675 | 0.411 |
| ≥5 | 31 | 10 | 20 | | |
| <5 | 37 | 16 | 21 | | |
The effect of KL overexpression on the
expression of IGF1R, PI3K and AKT
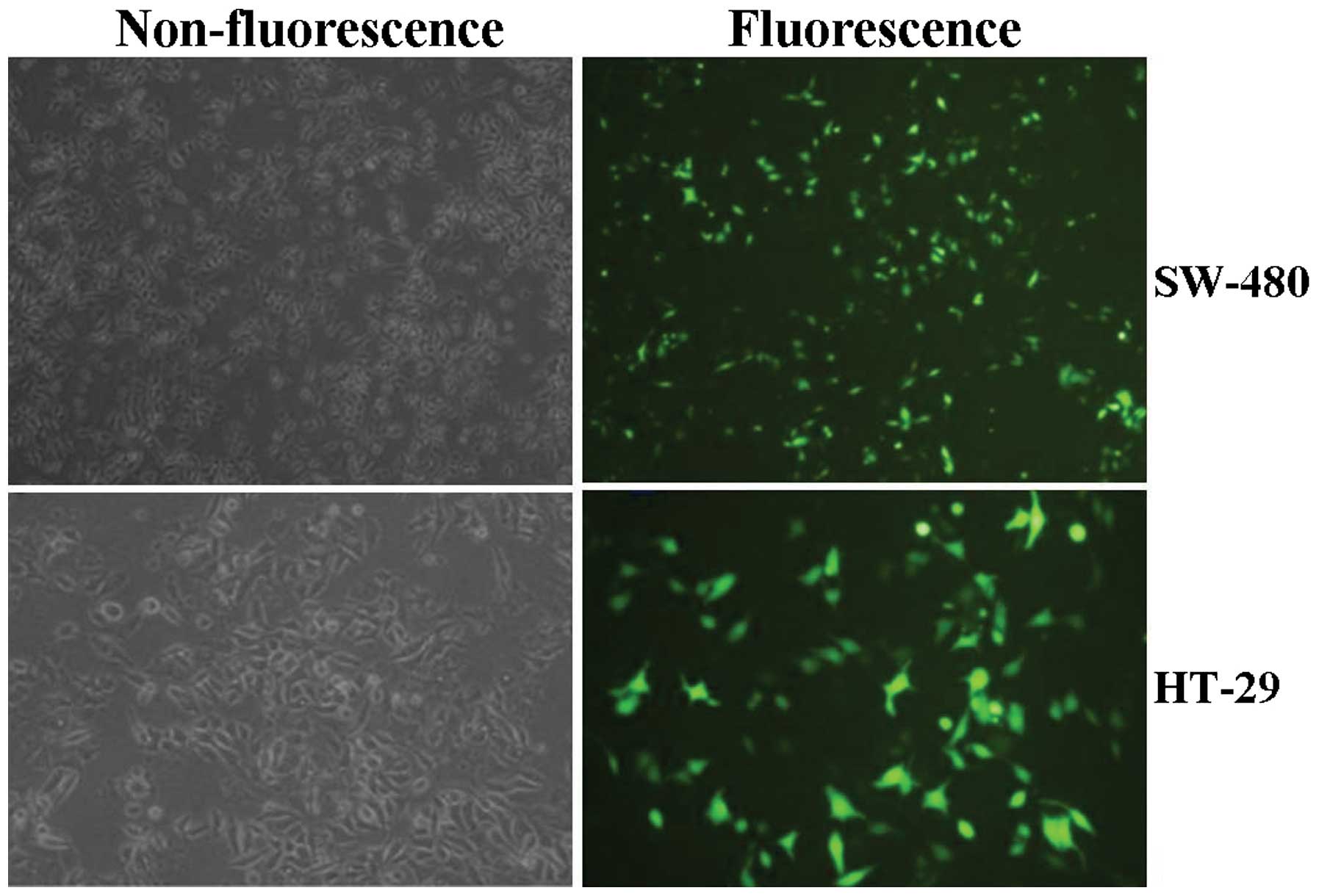
First, lentiviruses of different multiplicity of
infection (MOI) were used to transfect into colon cancer cell lines
(SW480 and HT-29), and the transfection efficiency of Lv-KL
(MOI=50), observed by fluorescence microscopy was high, reaching
more than 70% (Fig. 2). Then Lv-KL
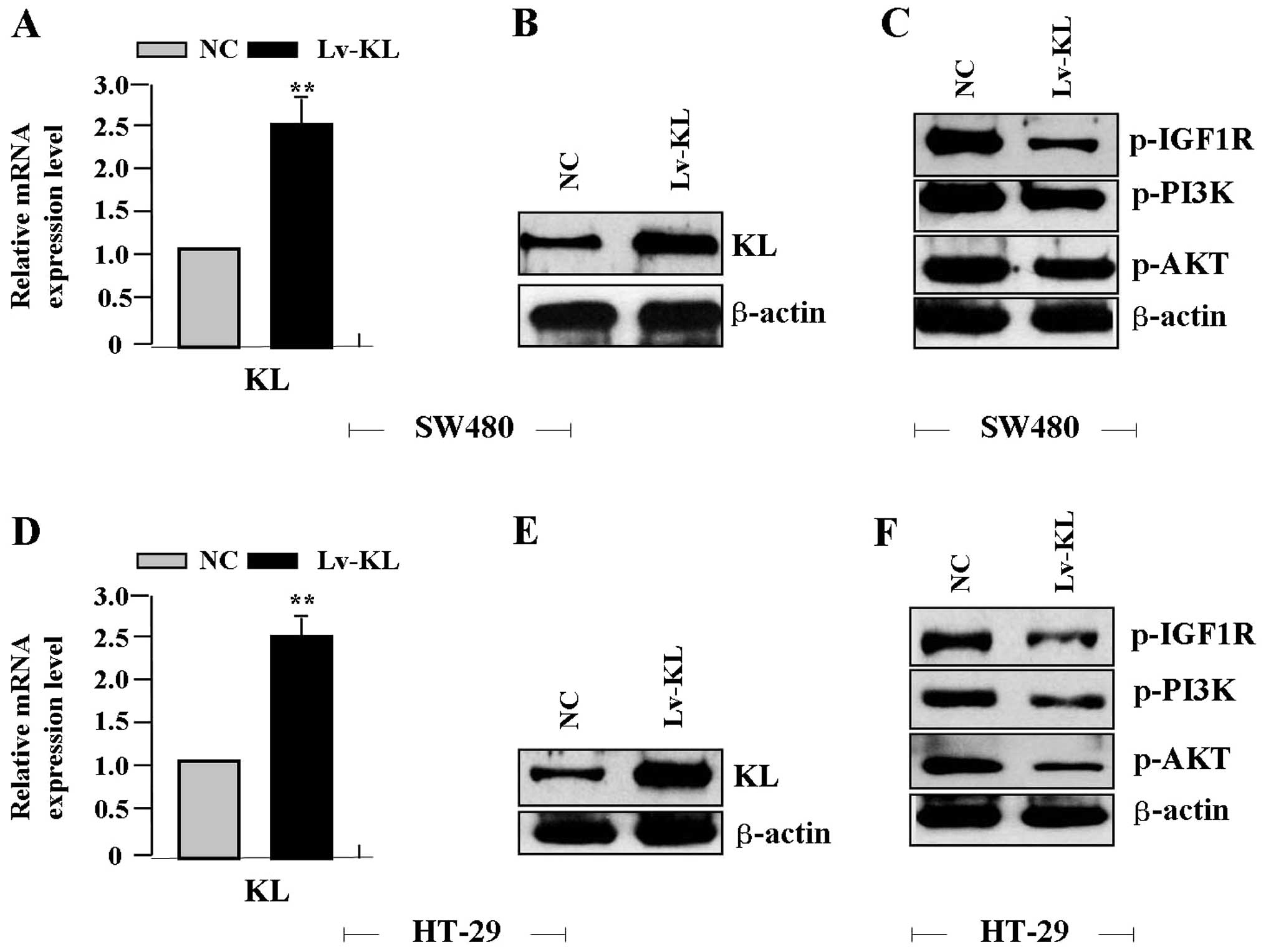
(MOI=50) was transfected into colon cancer cell lines for 24 h, the
expression levels of KL mRNA (Fig. 3A
and D) and protein (Fig. 3B and
E), p-IGF1R, p-PI3K and p-AKT proteins (Fig. 3C and F) were detected by real-time
PCR and western blot assays, indicating an obvious increase of KL
expression, but a significant decrease of p-IGF1R, p-PI3K and p-AKT
expression in Lv-KL group compared with the NC group.
The effect of KL overexpression on cell
proliferation and independent growth
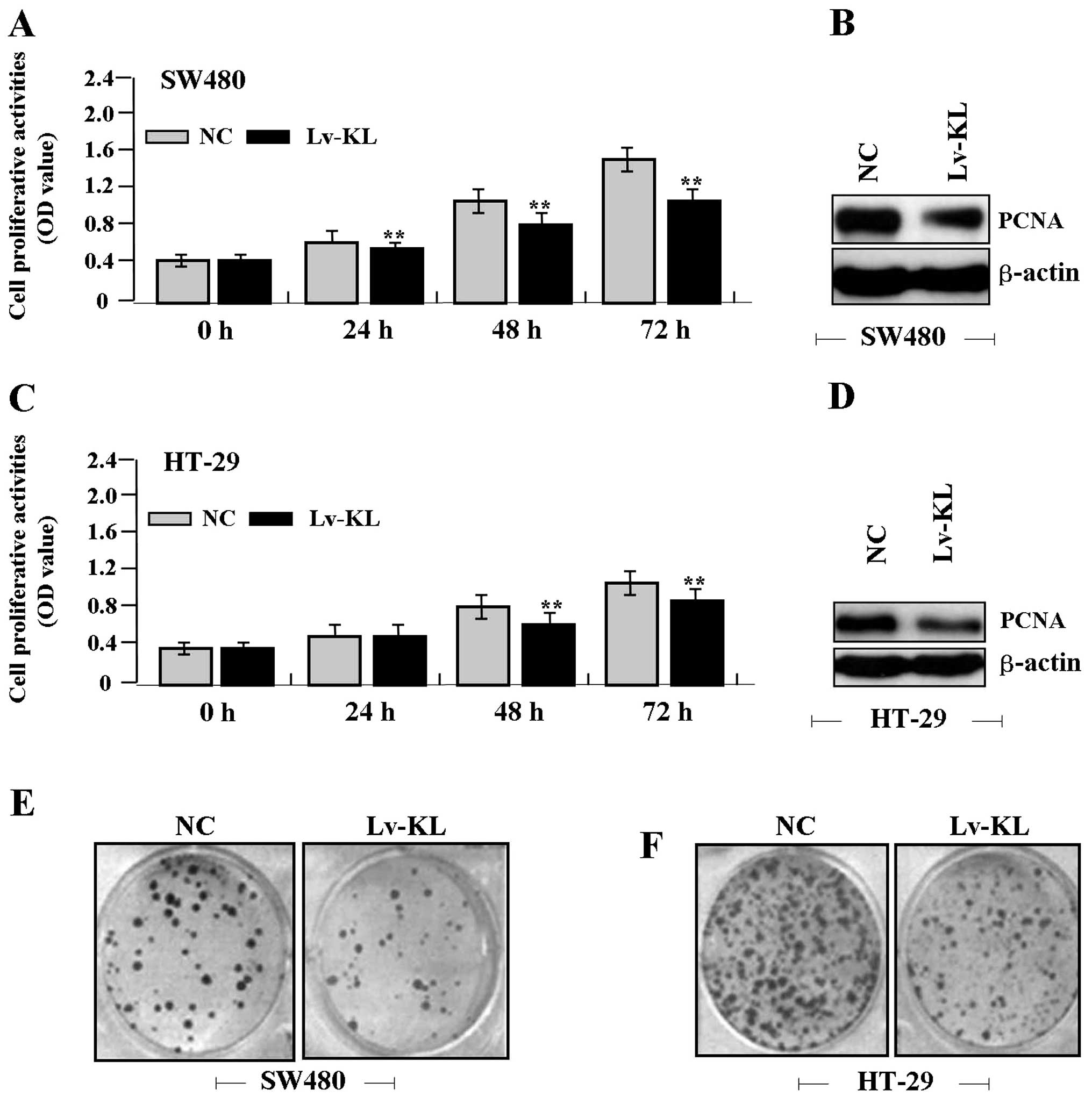
Deregulated cell proliferation is a hallmark of
cancer (18). To verify the effect
of KL overexpression on tumor growth in colon cancer cells, we
examined cell proliferative activities by MTT and colony formation
assays. The results showed that KL overexpression markedly
diminished the proliferative activities of colon cancer cells in a
time-dependent manner compared to the NC group (Fig. 4A and C). Colony formation rate of
cancer cells was markedly lower in Lv-KL group than the NC group
(Fig. 4E and F). In addition, the
expression level of PCNA protein, examined by western blot assay
(Fig. 4B and D), was significantly
downregulated in Lv-KL group compared with the NC group.
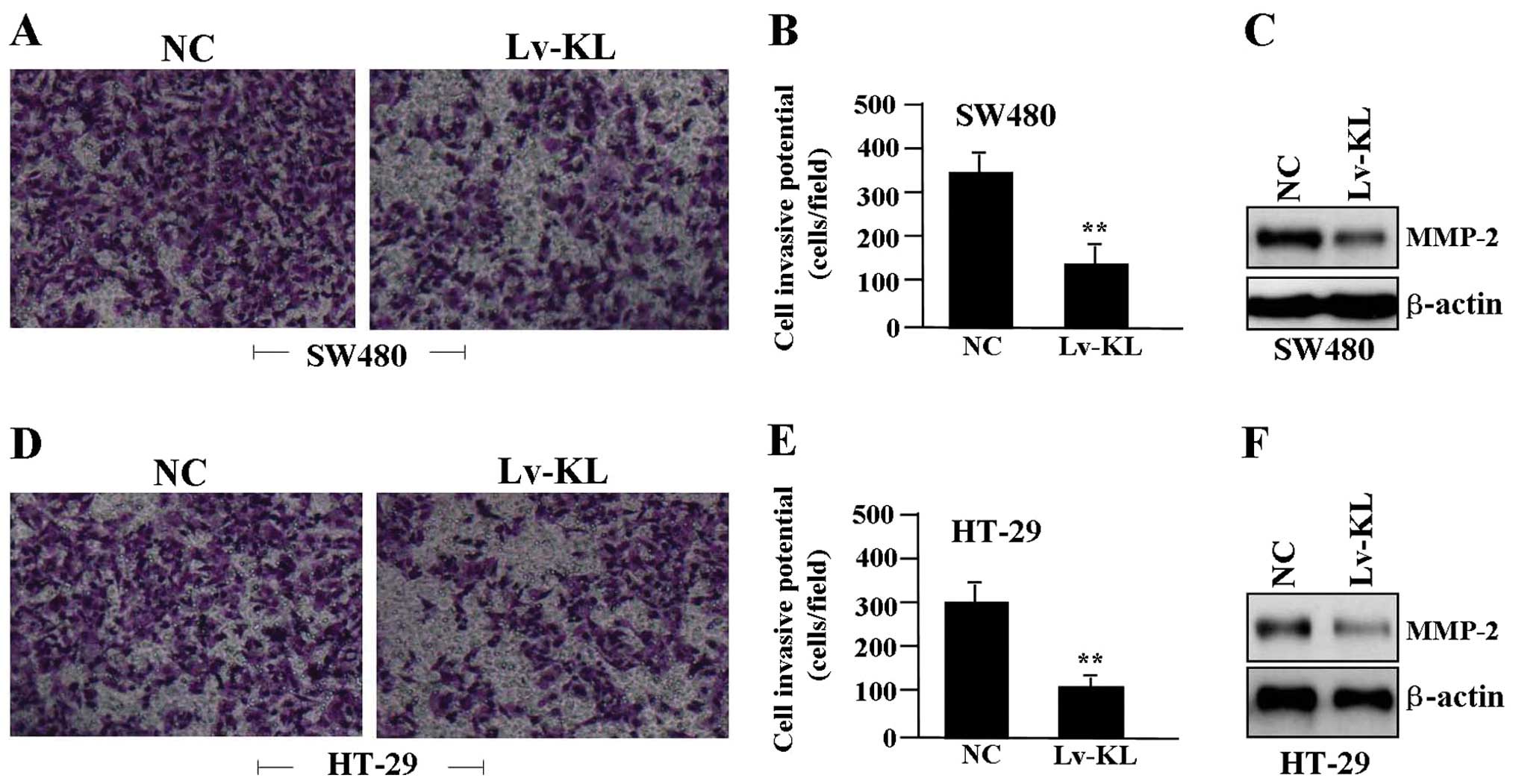
The effect of KL overexpression on cell
invasion
To determine the effect of KL overexpression on cell
invasion, a Transwell assay was performed. The invasive potential
of tumor cells in Transwell assay was determined by the ability of
cells to invade a matrix barrier containing laminin and type IV
collagen, the major components of the basement membrane.
Representative micrographs of Transwell filters can be seen in
Fig. 5A and D. We found that the
invasive potential of colon cancer cells was apparently decreased
in Lv-KL group compared to NC group (P<0.01) (Fig. 5B and E). In addition, the
expression level of MMP-2 protein, examined by western blot assay
(Fig. 5C and F), was significantly
downregulated in Lv-KL group compared with the NC group.
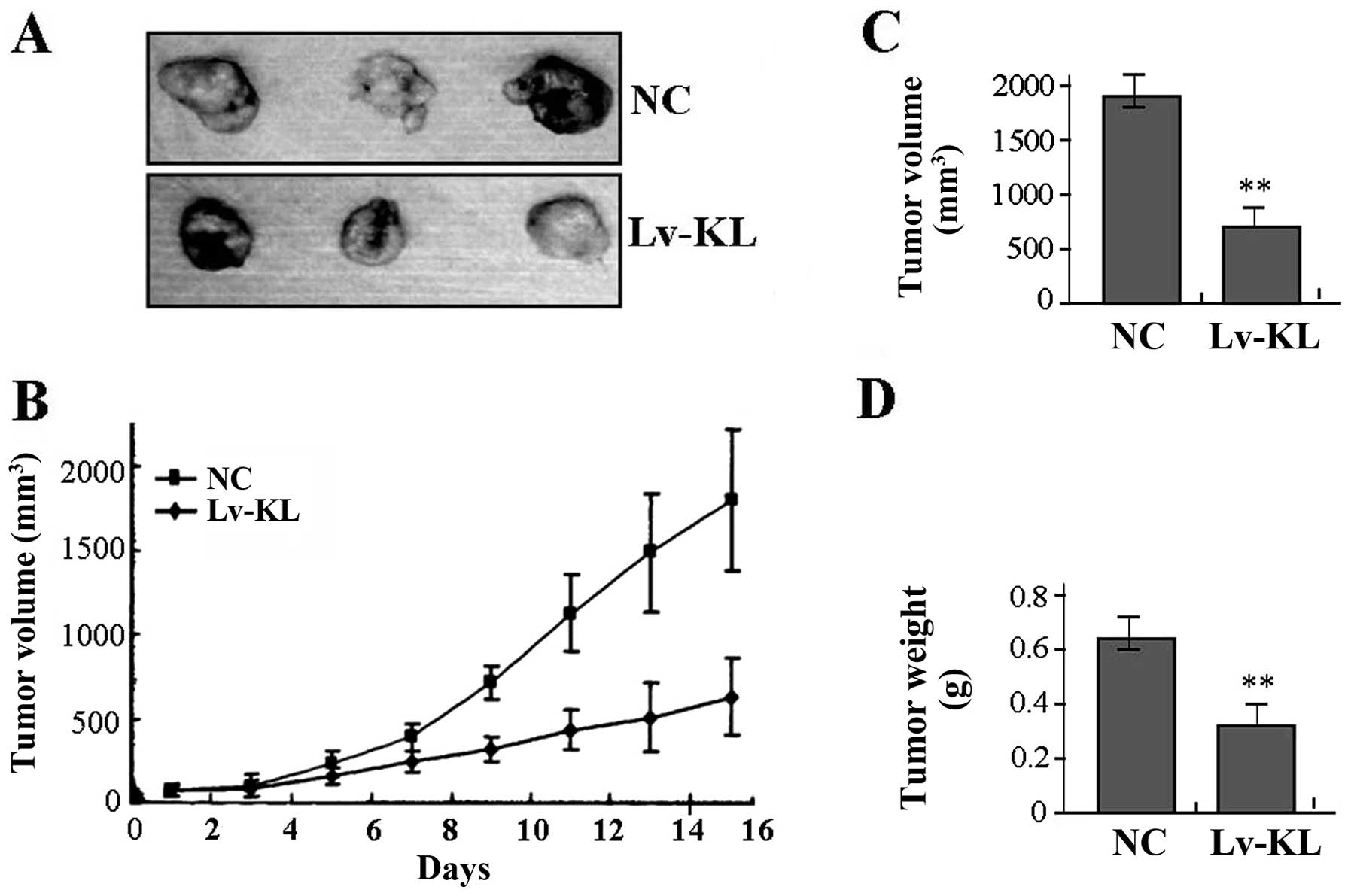
The effect of KL overexpression on
xenograft tumor growth
Our in vitro experiments demonstrated the
inhibitory effect of KL overexpression on tumor growth of colon
cancer cells. Therefore, it was necessary to further investigate
the effect of KL overexpression on xenograft tumor growth in
vivo. The mean volume of tumors in the experimental mice before
treatment was 71.45±19.33 mm3. During the whole tumor
growth period, the tumor growth activity was measured. The tumors
treated with Lv-KL grew substantially slower compared to the NC
group (Fig. 6A and B). When the
tumors were harvested, the average weight and volume of the tumors
in Lv-KL group were significantly smaller than those of the NC
group (Fig. 6C and D), suggesting
that KL overexpression could suppress growth of colon cancer
cells.
Discussion
The KL gene was originally identified as a putative
aging-suppressor gene in mice and supplementation of KL can be a
novel therapeutic strategy for many age-related diseases (19). KL is expressed most abundantly in
the liver, followed by the small intestine, colon, spleen and
kidney tissues, and especially the expression of KL in tumors is
lower than that in non-tumor regions, suggesting loss of KL
expression may be linked to the malignant formation in certain
cancers (20). Though few studies
indicate KL as the tumor-promoting factor (16), more evidence supports that
decreased expression of KL is significantly associated with a good
outcome of resected carcinoma patients (21). Our present study and Pan et
al (6) demonstrated that KL
was markedly downregulated in the cytoplasm of colon cancer tissues
when compared to the adjacent non-tumor tissues, and negatively
associated with Dukes staging and tumor invasion, suggesting that
loss of KL might represent a new biomarker involved in the
development of colon cancer.
Overexpression of KL not only functions as an ‘aging
suppressor’, but also regulates cell survival and proliferation,
and serves as a potential tumor suppressor implicated in cancer
metastasis (22). Exogenous KL
gene expression significantly inhibits cell growth and invasion,
and induces cell apoptosis and autophagy in HCC (23–25).
Inversely, some studies have revealed a novel oncogenic function of
KL of in promoting tumor migration and invasion via activation of
VEGFR2/PAK1 signaling (26). Thus,
to further clarify the role of KL in cancer, we assessed the
function of KL on its the biological behavior in colon cancer
cells, and found that overexpression of KL suppressed growth and
invasion of colon cancer cells in vitro and in vivo,
indicating that KL might play an important role in the development
of colon cancer.
Furthermore, a few studies have focused on the
molecular regulatory mechanisms of KL in cancer. KL participates in
the progression of several types of human cancers, and functions as
a tumor suppressor by inhibiting multiple pathways including
insulin/IGF1, p53/p21 and Wnt signaling pathways (4). In addition, it is shown that KL can
inhibit epithelial-mesenchymal transition and tumor migration and
invasion, and improve the resistance of cancer cells to cisplatin
based chemotherapy through inhibition of the PI3K/Akt/GSK3β/Snail
pathway in renal cell carcinoma and lung cancer (27,28).
IGF1R simulates colon cancer cell growth via activation of the
PI3K/AKT pathway (29). Our
present study also demonstrated that overexpression of KL could
downregulate the expression of IGF1R, p-PI3K and p-AKT in colon
cancer cells, suggesting that KL might regulate IGF1R and
subsequently activate the downstream PI3K/Akt signaling pathway
(23,25).
PCNA is a nuclear protein expressed in proliferating
cells and may be required for maintaining cell proliferation, used
as a marker for cell proliferation of colon cancer (30). MMP-2 is thought to be a key enzyme
involved in the degradation of type IV collagen and high level of
MMP-2 in tissues is associated with tumor invasion and metastasis
(31). It has been reported that
blockade of PI3K/AKT pathway inhibits growth and metastasis of
malignant tumor cells via inhibition of PCNA and MMP-2 expression
(32). Moreover, in our study, it
was found that overexpression of KL downregulated the expression of
IGF1R, p-PI3K, p-AKT, PCNA and MMP-2 in colon cancer cells,
suggesting that overexpression of KL might inhibit growth and
invasions of colon cancer cells through IGF1R/PI3K/AKT
pathway-mediated regulation of PCNA and MMP-2 expression.
In conclusion, our findings indicate that KL is
downregulated in human colon caner and correlates with tumor
invasion, while overexpression of KL blocks growth and invasion of
colon cancer cells through inhibition of IGF1R-mediated PI3K/AKT
pathway, suggesting that KL may serve as a potential therapeutic
target for the treatment of colon cancer.
References
|
1
|
Antonic V, Stojadinovic A, Kester KE, et
al: Significance of infectious agents in colorectal cancer
development. J Cancer. 4:227–240. 2013. View Article : Google Scholar
|
|
2
|
Young PE and Womeldorph CM: Colonoscopy
for colorectal cancer screening. J Cancer. 4:217–226. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kuro-o M: Klotho as a regulator of
oxidative stress and senescence. Biol Chem. 38:233–241. 2008.
|
|
4
|
Xie B, Chen J, Liu B, et al: Klotho acts
as a tumor suppressor in cancers. Pathol Oncol Res. 19:611–617.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Wang X, Wang X, et al: Klotho is
silenced through promoter hypermethylation in gastric cancer. Am J
Cancer Res. 1:111–119. 2011.PubMed/NCBI
|
|
6
|
Pan J, Zhong J, Gan LH, et al: Klotho, an
anti-senescence related gene, is frequently inactivated through
promoter hypermethylation in colorectal cancer. Tumour Biol.
32:729–735. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rubinek T, Shulman M, Israeli S, et al:
Epigenetic silencing of the tumor suppressor klotho in human breast
cancer. Breast Cancer Res Treat. 133:649–657. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Usuda J, Ichinose S, Ishizumi T, et al:
Klotho predicts good clinical outcome in patients with
limited-disease small cell lung cancer who received surgery. Lung
Cancer. 74:332–337. 2011. View Article : Google Scholar
|
|
9
|
Xie B, Zhou J, Yuan L, et al: Epigenetic
silencing of Klotho expression correlates with poor prognosis of
human hepatocellular carcinoma. Hum Pathol. 44:795–801. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Doi S, Zou Y, Togao O, et al: Klotho
inhibits transforming growth factor-beta1 (TGF-beta1) signaling and
suppresses renal fibrosis and cancer metastasis in mice. J Biol
Chem. 286:8655–8665. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camilli TC, Xu M, O’Connell MP, et al:
Loss of Klotho during melanoma progression leads to increased
filamin cleavage, increased Wnt5A expression, and enhanced melanoma
cell motility. Pigment Cell Melanoma Res. 24:175–186. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Abramovitz L, Rubinek T, Ligumsky H, et
al: KL1 internal repeat mediates klotho tumor suppressor activities
and inhibits bFGF and IGF-I signaling in pancreatic cancer. Clin
Cancer Res. 17:4254–4266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen B, Ma X, Liu S, et al: Inhibition of
lung cancer cells growth, motility and induction of apoptosis by
Klotho, a novel secreted Wnt antagonist, in a dose-dependent
manner. Cancer Biol Ther. 13:1221–1228. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen B, Wang X, Zhao W, et al: Klotho
inhibits growth and promotes apoptosis in human lung cancer cell
line A549. J Exp Clin Cancer Res. 29:992010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang B, Kim J, Jeong D, et al: Klotho
inhibits the capacity of cell migration and invasion in cervical
cancer. Oncol Rep. 28:1022–1028. 2012.PubMed/NCBI
|
|
16
|
Lu L, Katsaros D, Wiley A, et al: Klotho
expression in epithelial ovarian cancer and its association with
insulin-like growth factors and disease progression. Cancer Invest.
26:185–192. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poh W, Wong W, Ong H, et al: Klotho-beta
overexpression as a novel target for suppressing proliferation and
fibroblast growth factor receptor-4 signaling in hepatocellular
carcinoma. Mol Cancer. 11:142012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hanahan D and Weinberg RA: The hallmarks
of cancer: the next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kuro-o M: Klotho in health and disease.
Curr Opin Nephrol Hypertens. 21:362–368. 2012. View Article : Google Scholar
|
|
20
|
Yahata K, Mori K, Arai H, et al: Molecular
cloning and expression of a novel klotho-related protein. J Mol Med
(Berl). 78:389–394. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Usuda J, Ichinose S, Ishizumi T, et al:
Klotho is a novel biomarker for good survival in resected large
cell neuroendocrine carcinoma of the lung. Lung Cancer. 72:355–359.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dërmaku-Sopjani M, Kolgeci S, Abazi S, et
al: Significance of the anti-aging protein Klotho. Mol Membr Biol.
30:369–385. 2013.
|
|
23
|
Shu G, Xie B, Ren F, et al: Restoration of
klotho expression induces apoptosis and autophagy in hepatocellular
carcinoma cells. Cell Oncol (Dordr). 36:121–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ye X, Guo Y, Zhang Q, et al: βKlotho
suppresses tumor growth in hepatocellular carcinoma by regulating
Akt/GSK-3β/cyclin D1 signaling pathway. PLoS One. 8:e556152013.
|
|
25
|
Xie B, Zhou J, Shu G, et al: Restoration
of klotho gene expression induces apoptosis and autophagy in
gastric cancer cells: tumor suppressive role of klotho in gastric
cancer. Cancer Cell Int. 13:182013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen L, Liu H, Liu J, et al: Klotho endows
hepatoma cells with resistance to anoikis via VEGFR2/PAK1
activation in hepatocellular carcinoma. PLoS One. 8:e584132013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu Y, Xu L, Zhang J, et al: Klotho
suppresses tumor progression via inhibiting PI3K/Akt/GSK3β/Snail
signaling in renal cell carcinoma. Cancer Sci. 104:663–671.
2013.PubMed/NCBI
|
|
28
|
Wang Y, Chen L, Huang G, et al: Klotho
sensitizes human lung cancer cell line to cisplatin via PI3k/Akt
pathway. PLoS One. 8:e573912013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
do Lim Y, Cho HJ, Kim J, et al: Luteolin
decreases IGF-II production and downregulates insulin-like growth
factor-I receptor signaling in HT-29 human colon cancer cells. BMC
Gastroenterol. 12:92012.PubMed/NCBI
|
|
30
|
Risio M: Cell proliferation in colorectal
tumor progression: an immunohistochemical approach to intermediate
biomarkers. J Cell Biochem (Suppl). 16G:79–87. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liabakk NB, Talbot I, Smith RA, et al:
Matrix metalloproteinase 2 (MMP-2) and matrix metalloproteinase 9
(MMP-9) type IV collagenases in colorectal cancer. Cancer Res.
56:190–196. 1996.
|
|
32
|
Fu Y, Zhang Q, Kang C, et al: Inhibitory
effects of adenovirus mediated Akt1 and PIK3R1 shRNA on the growth
of malignant tumor cells in vitro and in vivo. Cancer Biol Ther.
8:1002–1009. 2009. View Article : Google Scholar
|