Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor, mainly arising in the metaphysis of long
bones of adolescents and young adults. OS has been characterized by
a high propensity for lung metastasis, the second highest cause of
cancer-related death in the pediatric age (1). The 5-year survival rate of patients
with OS was very low before the 1970s when treatment for OS
patients was mainly limb amputation. By now, the standard treatment
associates both neoadjuvant and adjuvant chemotherapies and
surgical resection of the primary tumor. Most chemotherapy regimens
applied for OS are based on methotrexate, cisplatin, doxorubicin
and ifosfamide. With well scheduled treatments, long-term survival
rate of OS has improved to ~70%, but long-term survival in both
localized and metastatic OS has stagnated in the last decades
(2). Therefore, it is necessary to
explore and develop more effective anticancer drugs for OS.
Traditional Chinese Medicine (TCM) plays an increasingly important
role in the prevention and treatment of tumors. In particular, the
combination of TCM with tranditional chemotherapy agents has
greatly improved the prognosis of some cancers.
Oridonin (ORI), a diterpenoid isolated from
medicinal herb Rabdosia rubescens, has drawn attention of
cancer biologists due to its remarkable antitumor activities
(3). ORI has been reported to
induce apoptosis in a variety of cancer cells, such as lymphoma
cells (4), colon cancer cells
(5), breast cancer cells (6) and leukemia cells (7). Recently, Jin et al reported
that ORI can inactivate Akt, extracellular signal-regulated kinase
(ERK), activate p38 mitogen-activated protein kinases (MAPK) and
c-Jun N-terminal protein kinase (JNK) signaling pathways in human
OS cells, resulting in the suppression of proliferation and
apoptosis (8). Nonetheless, it
remains unknown whether any other molecular mechanisms are involved
in the anti-proliferation effect of ORI on OS cells.
Wnt/β-catenin signaling has been identified as one
of the critical signalings in development, regulating cell growth,
motility and differentiation (9).
Aberrant activation of Wnt signaling is a major trait of a variety
of bone and soft-tissue sarcomas (10–13).
It was reported that several Wnt ligands, receptors and
co-receptors are highly expressed in OS cell lines, whereas Wnt
inhibitors are suppressed (14–18).
As a result, the Wnt/β-catenin signaling pathway has been
considered as a target for developing novel anticancer agents for
OS. Although ORI shows a strong antitumor activity in various
cancers, it still remains unknown whether the exact mechanism
underlaying its anticancer function is associated with
Wnt/β-catenin signaling. Recently, it was found that ORI treatment
activates GSK3β, a negative regulator of Wnt/β-catenin signaling,
by decreasing the phosphorylation level of GSK3β in OS cell lines
(8). Additionally, a previous
study indicated that ORI can induce apoptosis and senescence in
colorectal cancer cells partly through suppressing the expression
of c-Myc (19), a downstream
target of Wnt/β-catenin signaling pathway. These findings suggest
that ORI may exert its antitumor activity though mediating
Wnt/β-catenin signaling transduction.
In this study, we investigated the
anti-proliferation effect of ORI in OS cells, and unveiled the
possible mechanism responsible for the proliferation inhibitory
effect of ORI in OS cells. Our results indicate that ORI can
inhibit the OS cells proliferation, and this effect may be mediated
by downregulating Wnt/β-catenin signaling transduction through
upregulating the expression of Dkk-1 and/or increasing the function
of GSK3β.
Materials and methods
Chemicals and drug preparations
ORI was purchased from Hao-xuan Bio-tech Co., Ltd.
(Xi’an, China). OS cell line 143B was purchased from American Type
Culture Collection. ORI was dissolved in dimethyl sulfoxide (DMSO)
for in vitro test, or prepared with 0.4%
carboxymethylcellulose sodium (CMC-Na) as suspension for in
vivo experiments. Antibodies were purchased from Santa Cruz
Biotechnology. Cells were maintained in the Dulbecco’s modified
Eagle’s medium (DMEM) with 10% fetal bovine serum (FBS), 100 U/ml
of penicillin and 100 μg/ml of streptomycin at 37°C in 5%
CO2.
Crystal violet viability assay
Crystal violet assay was conducted as described
(20). Briefly, 143B cells were
seeded in 24-well plates and treated with different concentrations
of ORI. At the scheduled time-points, cells were washed carefully
with cold (4°C) phosphate-buffered saline (PBS) and stained with
0.5% crystal violet formalin solution at room temperature to
visualize the cell viability. For quantification, crystal violet in
the stained cells was extracted with 1 ml 20% acetic acid at room
temperature for 20 min with shaking. A total of 100 μl was taken
and added to 1 ml ddH2O. Absorbance at 570 nm was
measured. Each assay was done in triplicate.
Construction of the recombinant
adenovirus
Recombinant adenoviruses expressing Dkk-1
(Ad-Dkk-1), β-catenin (Ad-BC) and small interfering RNA (siRNA)
fragments targeting β-catenin (Ad-siBC) were generated previously
using the AdEasy technology, as described (21–23).
Flow cytometric analysis for apoptosis
and cell cycle arrest
Sub-confluent 143B cells were seeded in 6-well
plates. For apoptosis assay, cells were treated with different
concentrations of ORI or DMSO for 48 h. Then, cells were collected
and washed with cold (4°C) PBS, followed by incubating with Annexin
V-EGFP and propidium iodide (PI) as described in the instructions
of the kit (KeyGen Biotech, Nanjing, China). The stained cells were
analyzed by fluorescence activated cell sorting (FACS). For cell
cycle analysis, 143B cells were treated with different
concentrations of ORI for 24 h. Then, cells were harvested, washed
with PBS, fixed with cold (4°C) 70% ethanol, washed with 50 and 30%
ethanol, and PBS finally; stained with 1 ml of 200 mg/ml PI
containing RNase (10 mg/ml) in PBS for 30 min followed by FACS for
cycle analysis. Each assay was done in triplicate.
Annexin V-EGFP staining
Sub-confluent 143B cells were seeded in 24-well
plates and treated with various concentrations of ORI for 12 h.
Cells were washed with PBS twice and incubated with 500 μl of
binding buffer and 2 μl of Annexin V-EGFP fusion protein (KeyGen
Biotech) each well for 5 min, followed by washing with PBS twice.
Green fluorescent protein signal was detected under a fluorescence
microscope.
Western blot assay
Sub-confluent 143B cells were seeded in 6-well
plates and treated with different concentrations of ORI or DMSO for
24 h. For total protein level assay, cells were washed with cold
PBS and lysed in 300 μl lysis buffer. For nucleus fraction protein
extraction, the protein was harvested with Nuclear and Cytoplasmic
Protein Extraction kit (Thermo, no. 78833) according to the
manufacturer’s instructions. Cell lysates were boiled for 10 min,
and then subjected to SDS-PAGE and transfered to polyvinylidene
fluoride (PVDF) membranes. The membranes were immunoblotted with
various primary antibodies, followed by incubating with HRP
conjugated second antibodies. The proteins of interest were
visualized by using the SuperSignal West Pico Substrate (Pierce,
Rockford, IL, USA). Each assay was done in triplicate.
Reverse transcription and polymerase
chain reaction analysis (RT-PCR)
Sub-confluent 143B cells were seeded in T25 flasks
and treated with different concentrations of ORI or DMSO for 24 h.
Total RNA was isolated using TRIzol reagents (Invitrogen, Carlsbad,
CA, USA) and used to generate cDNA templates by RT reaction. Then,
the cDNAs were used as templates for detecting the expression level
of interesting genes by PCR. The primers used were as following:
GAPDH, forward 5′-CAACGAATTTGGCTACAGCA-3′, reverse
5′-AGGGGAGATTCAGTGTGGTG-3′; Dkk-1, forward
5′-CCTTGGATGGGTATTCCAGA-3′, reverse 5′-GGCAAGACAGACCTTCTCCA-3′;
β-catenin, forward 5′-CCCACTAATGTCCAGCGTTT-3′, reverse
5′-AACGCATGATAGCGTGTCTG-3′. Each assay was done in triplicate.
Luciferase reporter assay
Sub-confluent 143B cells were seeded in T25 flask
and transfected with 2 μg per flask of β-catenin/Tcf4 luciferase
reporter (pTop-luc) (21–23) with Lipofectamine (Invitrogen),
replacing the medium 4 h later with fresh complete medium. After
incubating for 12 h, cells were seeded in 24-well plates and then
treated with different concentrations of ORI or DMSO. At 24 h after
treatment, cells were lysed and subjected to luciferase assays
using luciferase assay kit (Promega, E1500). Each assay was done in
triplicate.
Xenograft tumor model of human OS
All animal experiments were approved by the
Institutional Animal Care and Use Committee (IACUC) of Chongqing
Medical University. Athymic nude mice (female, 4–6-week old,
5/group) were ordered from the Animal Centre of Chongqing Medical
University (Chongqing, China). 143B cells were collected and
resuspended in cold PBS to a final density of 2×107
cells/ml. Cells in 50 μl of cold PBS were injected into the
proximal tibia of athymic mice. At 3 days after injection, animals
were treated with either different doses of ORI (50 and 100 mg/kg)
or solvent by intragastric administration once a day. Five weeks
after injection, the animals were sacrificed and the tumor samples
were retrieved for histological evaluation.
Histological evaluation and
immunohistochemical staining
Retrieved tumor masses were fixed in 10% formalin
and embedded in paraffin. Serial sections of the embedded specimens
were stained with hematoxylin and eosin (24). For immunohistochemical staining,
slides were deparaffinized and then rehydrated in a graduated
manner. The deparaffinized slides were subjected to antigen
retrieval and probed with an anti-proliferating cell nuclear
antigen (PCNA) antibody or anti-β-catenin antibody, or isotype IgG
as control, followed by incubation with biotinylated secondary
antibodies and streptavidin conjugated horseradish peroxidase. The
proteins of interest was visualized by DAB staining and examined
under a microscope as described (24).
Statistical analysis
All quantitative experiments were performed in
triplicate. Data are expressed as mean ± SD. Statistical
significance between vehicle treatment versus drug treatment was
determined by the Student’s t-test. A value of p<0.05 was
considered statistically significant.
Results
ORI inhibits the proliferation in OS
cells
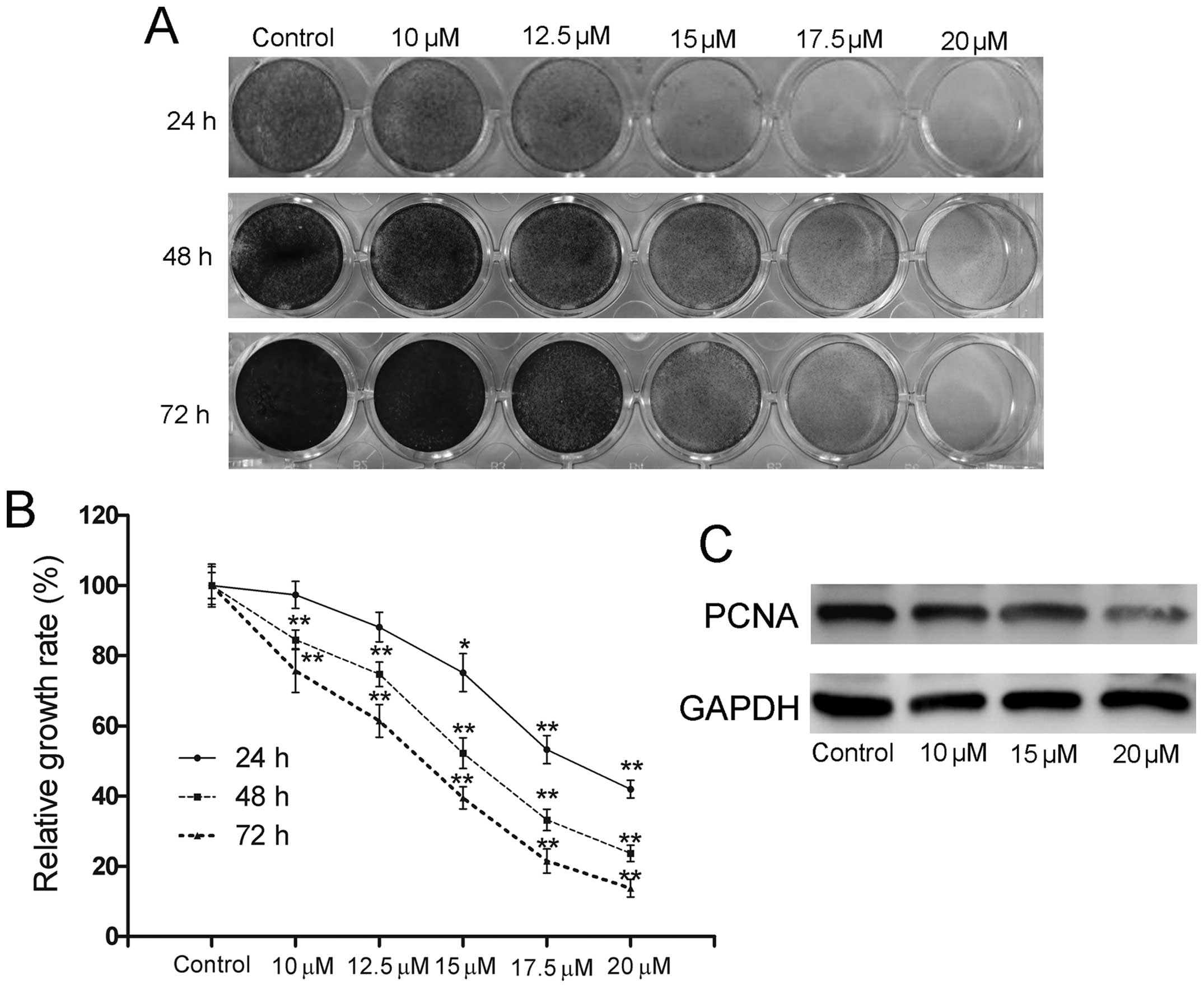
Using crystal violet staining, we firstly explored
the proliferation inhibitory effect of ORI on 143B cells to
validate whether ORI can be used as a novel chemotherapeutic agent
for human OS. It was shown that ORI effectively inhibited the
proliferation of 143B cells in a time- and concentration-dependent
manner (Fig. 1A and B). As shown
in Fig. 1C, ORI also significantly
suppressed the expression of proliferating cell nuclear antigen
(PCNA), a marker for the assessment of OS growth (25).
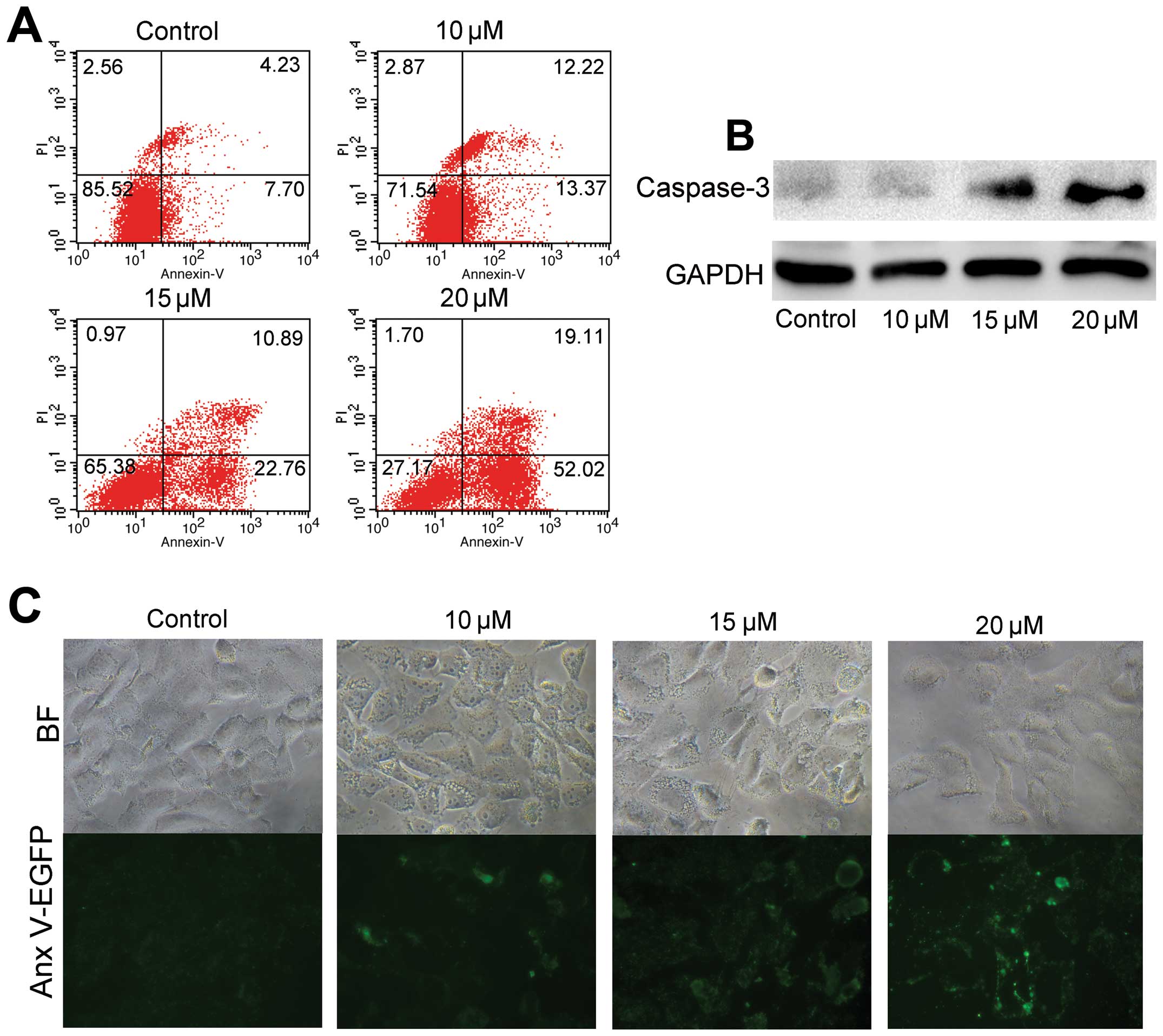
ORI induces apoptosis in OS cells in
vitro
We investigated whether ORI can induce OS cells to
undergo apoptosis. The 143B cells were treated with indicated
concentrations of ORI or DMSO for 24 or 48 h. Then, cells were
subjected with FACS analysis (Fig.
2A), or were lysed and subjected to western blot analysis for
detecting caspase-3 protein level (Fig. 2B). The results showed that ORI can
induce apoptosis in 143B cells, and the protein expression of
caspase-3 increased at 24 h in a concentration-dependent manner.
Furthermore, 143B cells were also stained with Annexin V-EGFP
fusion protein after treatment with different concentrations of ORI
for 12 h. We found that ORI induced EGFP staining in a
concentration-dependent manner (Fig.
2C), indicating that ORI can effectively induce the
translocation of phosphatidylserines in cell membrane phospholipids
from the inner surface to the outer surface during the early stages
of apoptosis. Taken together, these results suggest that ORI can
induce apoptosis in OS cells.
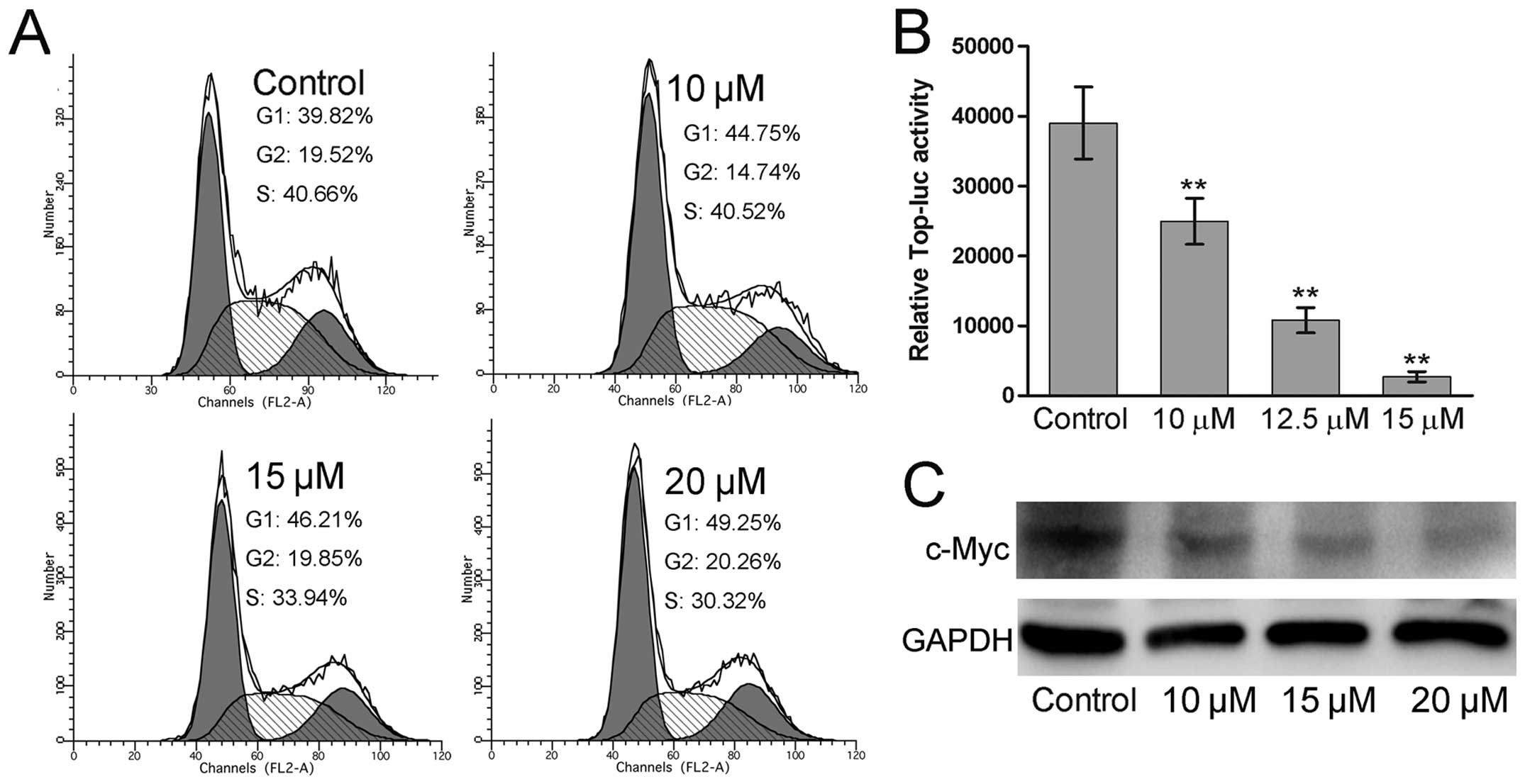
ORI arrests the cell cycle at G1 phase
and inhibits the Wnt/β-catenin signaling in human OS cells
To explore the mechanism of ORI-induced
proliferation inhibition and apoptosis in human OS cells, we tested
whether these functions were associated with the cell cycle arrest.
It was shown that the cells in G1 phase of ORI treated groups
increased compared to that of the control group
concentration-dependently (Fig.
3A), suggesting that ORI can arrest the cell cycle at G1 phase
in human OS cells. Cell cycle control is a pivotal event controlled
by many essential signaling pathways. Wnt/β-catenin signaling is
one of these pathways (26).
Therefore, we investigated whether ORI can target Wnt/β-catenin
signaling to exert its anticancer activity in 143B cells. Using
luciferase reporter assay, we examined the effect of ORI on the
β-catenin/Tcf4-responsive reporter and found that ORI effectively
inhibited the reporter activity (Fig.
3B). In addition, we further examined the expression of the
known target of Wnt/β-catenin signaling, c-Myc, in response to ORI
treatment. The result showed that c-Myc expression was decreased in
ORI-treated 143B cells in a concentration-dependent manner
(Fig. 3C). Taken together, these
results suggest that ORI can inhibit Wnt/β-catenin signaling
transduction.
ORI inhibits β-catenin expression in
human OS cells
Given that stabilization and nucleus translocation
of β-catenin are the key events in the transduction of the
canonical Wnt/β-catenin signaling, we conducted western blot
analysis to explore whether ORI can suppress β-catenin protein
level in the cytoplasm, nucleus and the whole cell to investigate
how ORI inactivates Wnt/β-catenin signaling. The results indicated
that ORI can decrease the β-catenin protein level not only in the
nucleus, but also in the cytoplasm and the whole cell after the
treatment with ORI for 24h (Fig.
4A). To validate the role of β-catenin in the proliferation
inhibitory effect of ORI in OS cells, we tested the effects of
exogenous expression or knockdown of β-catenin on the proliferation
inhibitory effect of ORI in OS cells. We found that exogenous
expression of β-catenin attenuated the growth inhibitory function
of ORI, while knockdown of β-catenin enhanced this function in 143B
cells (Fig. 4B), suggesting that
downregulation of β-catenin plays a critical role in the function
of ORI in OS cells. To explore the mechanism by which ORI inhibits
the β-catenin protein expression in 143B cells, we determined
whether ORI can decrease the expression of β-catenin at mRNA level
by RT-PCR analysis. However, the result showed that ORI did not
affect the mRNA expression of β-catenin (Fig. 4C), suggesting that other cytokines
involved in Wnt/β-catenin signaling may participate in ORI-induced
downregulation of β-catenin.
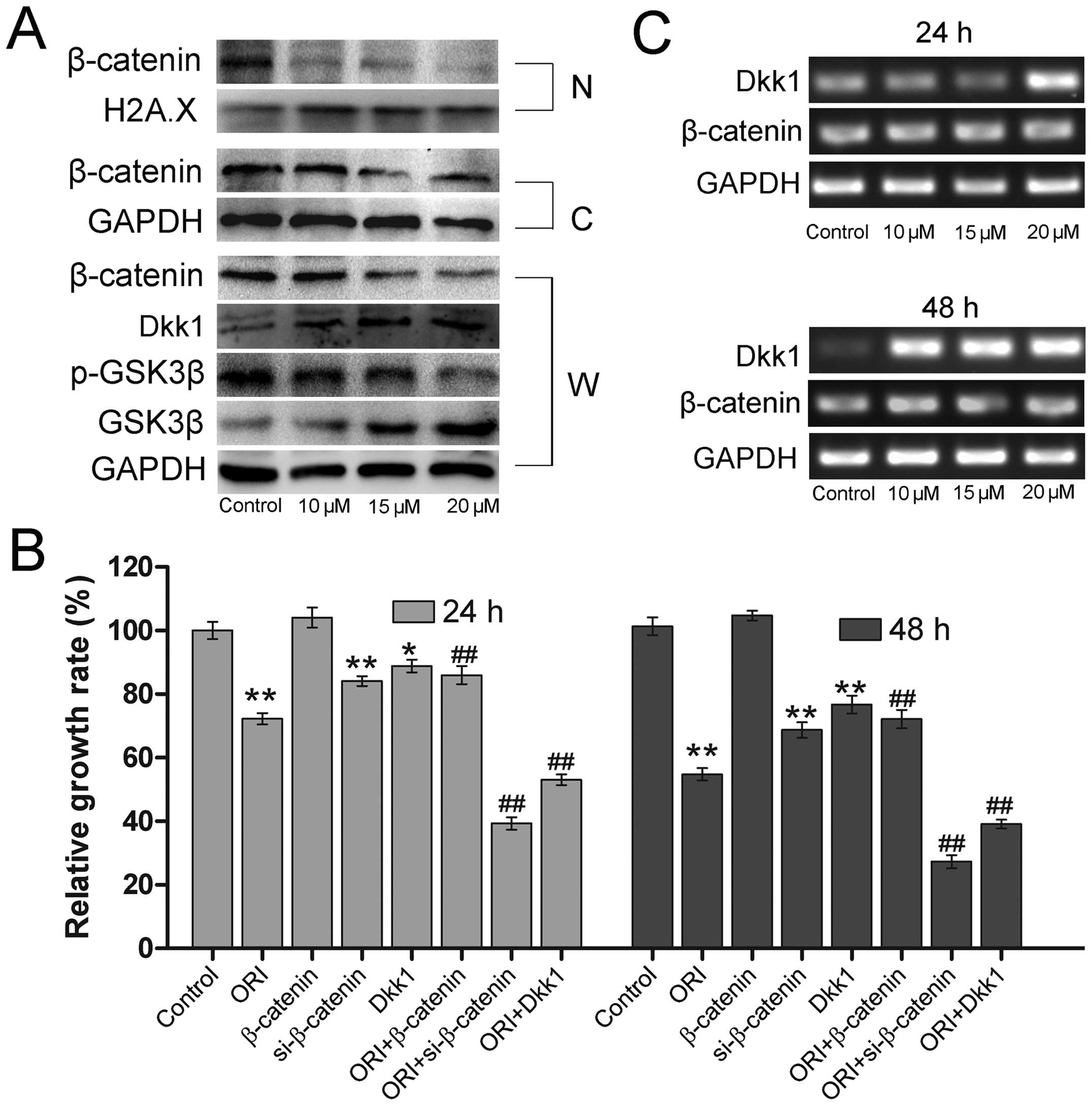 | Figure 4ORI targets the Wnt/β-catenin
signaling for the proliferation inhibitory effect in human OS
cells. (A) Western blot assay results show the effect of ORI on
p-GSK3β (Ser 9), GSK3β, and β-catenin in the nucleus, cytoplasm and
the whole cell (N, nucleus; C, cytoplasm; W, whole cell). GAPDH was
used as loading control. The 143B cells were seeded in 6-well
plates and treated with the indicated concentrations of ORI for 24
h and then harvested for western blot assay. (B) The effect of
β-catenin and Dkk-1 on the proliferation inhibitory effects of ORI
on OS cells. The 143B cells were seeded in 24-well plates and
infected with Ad-BC, Ad-siBC or Ad-Dkk-1 in the presence or absence
of 15 μM ORI. At 24 and 48 h after treatment, the cells were
stained with crystal violet and growth rate was quantified. The
assay was performed in triplicate. *p<0.05, compared
with control; **p<0.01, compared with control;
##p<0.01, compared with ORI. (C) The effect of ORI of
mRNA expression of β-catenin and Dkk-1. 143B cells were treated
with the indicated concentrations of ORI for 24 or 48 h, and then
semiquantitative RT-PCR was performed to assess the expression of
β-catenin and Dkk-1 in gene level. GAPDH was used as loading
control. |
The stability of β-catenin in cells is tightly
regulated by the Axin/APC/GSK3β complex. Phosphorylation of
β-catenin by GSK3β results in its degradation, which leads to the
inactivation of Wnt/β-catenin signaling (27,28).
Thus, we explored whether ORI can affect the expression of GSK-3β,
the negative regulator of Wnt/β-catenin signaling. We found that
its protein expression level was markedly increased in response to
ORI (Fig. 4A). Previous studies
have demonstrated that phosphorylation of GSK3β at Ser9 can lead to
GSK3β inactivation (29,30), we therefore also examined the
effect of ORI on the phosphorylation of GSK3β. As shown in Fig. 4A, the phosphorylation level of
GSK3β was reduced under the action of ORI. In addition to GSK3β, we
also found that the expression of another canonical Wnt inhibitor,
Dickkopf-1 (Dkk-1), was significantly improved upon ORI treatment
(Fig. 4A and C). Overexpression of
Dkk-1 enhanced the proliferation inhibitory effect of ORI in 143B
OS cells (Fig. 4B). The above
results suggest that ORI may regulate Wnt/β-catenin signaling
through enhancing the function of GSK3β and/or upregulation of
Dkk-1 to exert its anticancer activity in OS cells.
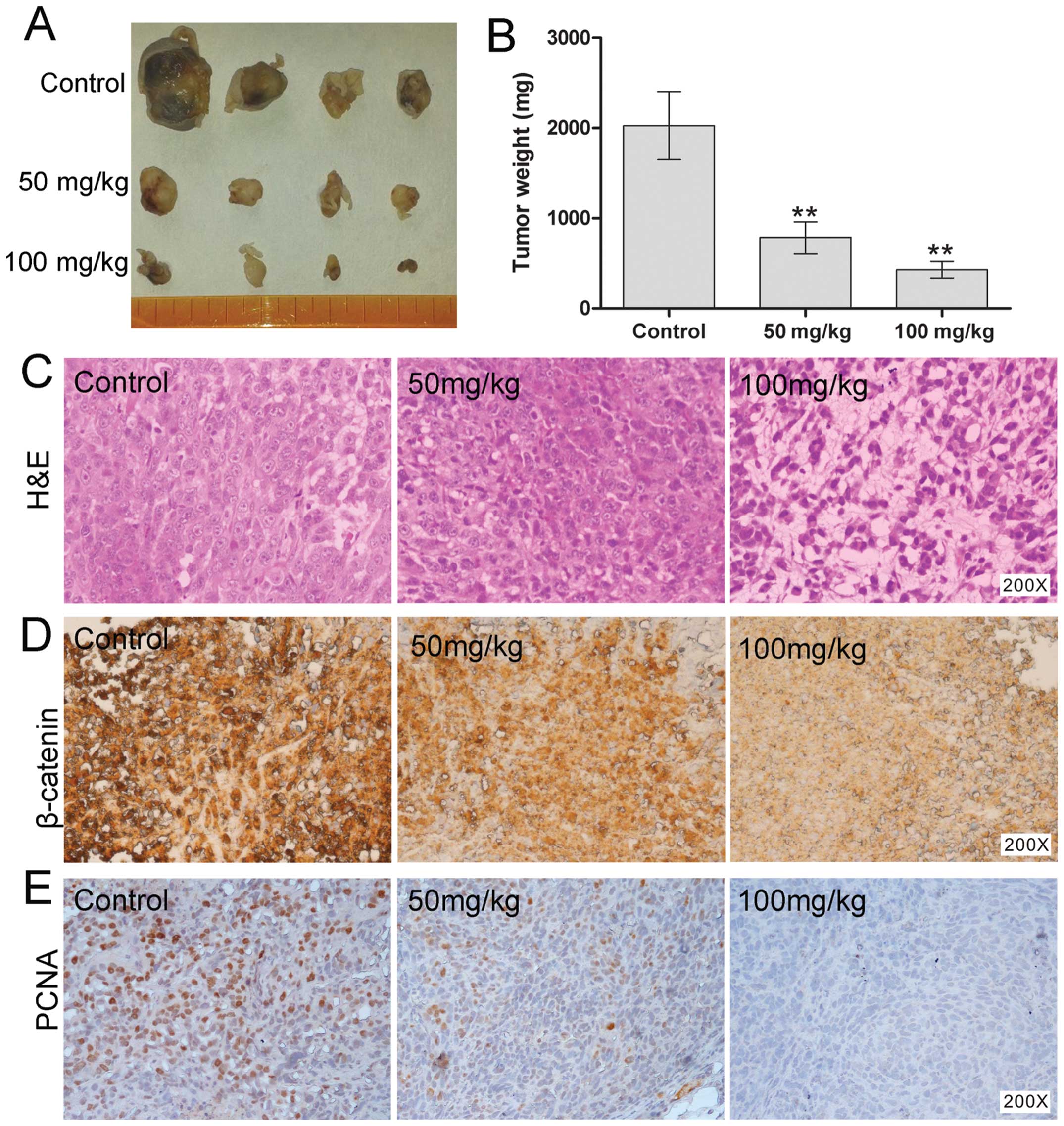
ORI inhibits tumor growth in the
xenograft model of human osteosarcoma
We further assessed the in vivo
anti-osteosarcoma effects of ORI. Using a xenograft tumor model, we
injected the 143B cells subcutaneously into the flanks of athymic
nude mice. At one week after injection, animals were given
different doses of ORI (50 or 100 mg/kg) or solvent as control by
intragastric administration, once a day up to 4 weeks. The result
showed that treatment with ORI resulted in significant suppression
of tumor growth in nude mice dose-dependently, compared with the
solvent control group (Fig. 5A and
B). We subsequently conducted histologic evaluation of the
tumor samples. Hematoxylin and eosin (H&E) staining showed that
ORI treatment group exhibited more necrotic cells than those of
solvent control group (Fig. 5C).
Moreover, we also examined the expression pattern of β-catenin
protein. Consistent with our in vitro result, β-catenin was
dramatically decreased in ORI-treated groups (Fig. 5D). PCNA-positively stained cells
were significantly decreased in the ORI treatment group, compared
with control group (Fig. 5E).
Collectively, these in vivo results further indicate that
the inhibitory effect of ORI on OS cells may result from the
inactivation of Wnt/β-catenin signaling.
Discussion
ORI is a diterpenoid compound extracted from the
Chinese traditional medicine herb Rabdosia rubescens
(3). It has been reported that ORI
can inhibit proliferation and induce apoptosis in various cancer
cells (4). However, the effect of
ORI on the proliferation of OS cells remains unclear, as well as
the exact mechanism underlaying this function. In the present
study, our results demonstrated that ORI can inhibit the
proliferation and induce apoptosis in 143B OS cells.
Mechanistically, we found that the anti-proliferation activity of
ORI in 143B OS cells may be mediated by downregulating
Wnt/β-catenin signaling transduction through upregulating the
expression of Dkk-1 and/or promoting the activity of GSK3β.
ORI has been shown to be able to target several
crucial genes and signaling pathways that are responsible for
regulating apoptotic cell death and the cell cycle (31), a few studies have attempted to
clarify the possible molecular mechanism by which ORI exerts its
anticancer activities. For example, ORI can inactivate Akt and ERK,
and activate p38 MAPK and JNK signaling pathways in OS cells,
resulting in the suppression of proliferation and induction of
apoptosis (8). However, another
study showed a converse observation that ERK served as a tumor
suppressor and linked mitochondrial-related apoptotic pathway to
MAPK-mediated pathways in ORI-treated human melanoma A375-S2 cells
(32). Furthermore, it was also
evidenced that ORI could induce cell cycle arrest and apoptosis
through activating ERK-p53 apoptotic pathway and inhibiting
PTK-Ras-Raf-JNK survival pathway in murine fibrosarcoma L929 cells
(33). These findings suggest that
the regulatory effect of ORI on MAPK signaling is cell
type-specific. In addition to MAPK signaling, ORI was reported to
be able to inhibit proliferation and induce caspase-dependent
apoptosis via downregulation of PI3K/Akt pathway in cervical
carcinoma HeLa cell line (34).
Treatment of prostate cancer cells with ORI also caused the
upregulation of P21, autophagy and apotosis (35). Although these studies have provided
important insights into the molecular mechanism through which ORI
exhibits anticancer activity, it is still conceivable that other
signaling pathways may also participate in the anticancer activity
of ORI.
It has been well demonstrated that Wnt/β-catenin
signaling pathway plays a critical role in various cancers. In the
canonical Wnt/β-catenin signaling, Wnt ligands bind to the dual
receptor complex comprised of frizzled and low-density lipoprotein
receptor-related protein 5/6 (LRP5/6). This leads to inactivation
of the β-catenin destruction complex, Axin/APC/GSK-3β, thus
relieving the critical mediator β-catenin from its constitutive
proteosomal degradation. β-catenin subsequently accumulates in the
cytoplasm and translocates into the nucleus, where it associates
with transcription factors to regulate the downstream target genes
(36,37). Deregulation of Wnt signaling has
been implicated in the development and pathogenesis of a wide range
of cancers (38), including OS. A
previous study has evidenced that increased cytoplasmic and/or
nuclear accumulation of β-catenin protein is a common occurrence in
human OS that is implicated in the pathogenesis of OS (39). Additionally, it was also reported
that the OS often expresses the Wnt co-receptor LRP5 which
significantly correlates with metastaic disease in human OS
(14). Moreover, the Wnt
inhibitory factor 1 (WIF1), which encodes an endogenous secreted
Wnt pathway antagonist, was reported to be epigenetically silenced
in human OS; targeted deletion of mouse WIF1 could accelerate
osteosarcomagenesis in vivo (18). Conversely, overexpression of WIF1
and dominant negative mutation of LRP5 effectively decreased
tumorigenicity and metastasis of OS in vivo (40,41).
Based on these observations, the compounds from Chinese herbal
medicine targeting Wnt/β-catenin signaling can be considered as
attractive candidates for OS treatment.
Up to now, athough ORI can mediate various signaling
pathways to exert its anticancer activities in OS, the effect of
ORI on Wnt/β-catenin signaling still remains to be elucidated.
Given that ORI could activate GSK3β activity and suppress the
expression of c-Myc (8,19), we speculated that the anticancer
effect of ORI on OS cells may result from targeting Wnt/β-catenin
signaling. In this study, our data showed that ORI can reduce the
protein level of β-catenin in both nucleus and cytoplasm in a
concentration-dependent manner. Overexpression of β-catenin can
attenuate the function of ORI, while knockdown of β-catenin can
enhance the growth inhibitory effect of ORI. Using a xenograft
tumor model of human OS, we demonstrated that ORI can also inhibit
cancer cell proliferation in vivo. The histologic
examination result has revealed a decreased staining intensity of
β-catenin in ORI treatment group. Therefore, these results suggest
that the inhibitory effect of ORI on OS is at least resulted from
reducing nuclear translocation of β-catenin protein. However, the
exact mechanism through which ORI downregulates β-catenin is still
unclear. Herein, we examined the β-catenin mRNA expression level in
143B cells and found that ORI has no effect on the mRNA expression
of β-catenin, suggesting that ORI may target some other components
of Wnt/β-catenin signaling pathway to promote the degradation of
β-catenin.
The stability of β-catenin in cells is tightly
regulated by the Axin/APC/GSK3β complex. The phosphorylation of
β-catenin by GSK3β results in ubiquitin-mediated degradation of
β-catenin leading to the inactivation of Wnt/β-catenin signaling.
Therefore, GSK3β has been identified as a tumor suppressor that is
frequently inactivated in various tumors (42). However, studies have provided
evidence that the exact role of GSK3β in tumorigenesis of human OS
is controversial (43–46). In the present study, we explored
the effect of ORI on GSK-3β and found that the total GSK-3β protein
level was significantly elevated in response to the treatment with
ORI. Moreover, the phosphorylation of GSK3β at Serine 9 was
diminished. This result is supported by another study in which ORI
decreased the phosphoralation level of GSK-3β in U2OS, SaOS-2 and
MG63 OS cell lines (8). Our data
indicate that downregulation of β-catenin protein in OS cells may
result from the degradation initialized by GSK-3β, at least. In
addition to GSK-3β, other natural Wnt antagonists have been
identified, including the Dickkopf (Dkk) family consisting of four
secretory proteins (Dkk-1, Dkk-2, Dkk-3, and Dkk-4) (47,48).
Dkk-3 has been shown to have a negative impact on the progression
of OS (49,50). To further explore the mechanism
underlying the regulatory role of ORI in Wnt/β-catenin signaling,
we examined whether Dkks could be influenced by ORI. Though we
found no changes in the mRNA expression pattern of Dkk2, Dkk3 and
Dkk4 (data are not shown), ORI dramatically upregulated the
expression of Dkk-1. Moreover, overexpression of Dkk-1 potentiated
the anti-proliferative effect of ORI in OS cells. It suggests that
upregulation of Dkk-1 by ORI may also relate to the anticancer
activities of ORI by inactivation of Wnt/β-catenin signaling in OS
cells.
Taken together, our data suggest that ORI can be
used as an effective chemotherapy agent for human OS. The
anticancer effect of ORI in OS cells may result from inactivating
Wnt/β-catenin signaling transduction through increasing GSK-3β
activity and/or upregulation of Dkk-1. Future studies should be
directed to the identification of more ORI target proteins, which
is essential to elucidate the molecular mechanism underlying ORI
anticancer activity. On the other hand, more strict and robust
pre-clinical examinations should also be performed to ensure the
safety of ORI before developing ORI to an antitumor drug.
Acknowledgements
We thank Dr T.-C. He (University of Chicago Medical
Center, USA) for generously providing all recombinant adenoviruses
and pTOP-luc reporter plasmid. This study was supported in part by
research grants from the Natural Science Foundation of China (NSFC,
81071462 and 81372120 to B.C.H.; 31000434 to L.C.).
References
|
1
|
Longhi A, Errani C, De Paolis M, Mercuri M
and Bacci G: Primary bone osteosarcoma in the pediatric age: state
of the art. Cancer Treat Rev. 32:423–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ando K, Heymann MF, Stresing V, Mori K,
Redini F and Heymann D: Current therapeutic strategies and novel
approaches in osteosarcoma. Cancers. 5:591–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abelson PH: Medicine from plants. Science.
247:5131990. View Article : Google Scholar
|
|
4
|
Liu YQ, Mu ZQ, You S, Tashiro S, Onodera S
and Ikejima T: Fas/FasL signaling allows extracelluar-signal
regulated kinase to regulate cytochrome c release in
oridonin-induced apoptotic U937 cells. Biol Pharm Bull.
29:1873–1879. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu Y, Xie L, Chen G, Wang H and Zhang R:
Effects of oridonin on proliferation of HT29 human colon carcinoma
cell lines both in vitro and in vivo in mice. Pharmazie.
62:439–444. 2007.PubMed/NCBI
|
|
6
|
Hsieh TC, Wijeratne EK, Liang JY,
Gunatilaka AL and Wu JM: Differential control of growth, cell cycle
progression, and expression of NF-kappaB in human breast cancer
cells MCF-7, MCF-10A, and MDA-MB-231 by ponicidin and oridonin,
diterpenoids from the chinese herb Rabdosia rubescens.
Biochem Biophys Res Commun. 337:224–231. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhou GB, Kang H, Wang L, et al: Oridonin,
a diterpenoid extracted from medicinal herbs, targets AML1-ETO
fusion protein and shows potent antitumor activity with low adverse
effects on t (8;21) leukemia in vitro and in vivo. Blood.
109:3441–3450. 2007. View Article : Google Scholar
|
|
8
|
Jin S, Shen JN, Wang J, Huang G and Zhou
JG: Oridonin induced apoptosis through Akt and MAPKs signaling
pathways in human osteosarcoma cells. Cancer Biol Ther. 6:261–268.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Behrens J and Lustig B: The Wnt connection
to tumorigenesis. Int J Dev Biol. 48:477–487. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barker N and Clevers H: Mining the Wnt
pathway for cancer therapeutics. Nat Rev Drug Discov. 5:997–1014.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin YC, You L, Xu Z, et al: Wnt signaling
activation and WIF-1 silencing in nasopharyngeal cancer cell lines.
Biochem Biophys Res Commun. 341:635–640. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Weeraratna AT, Jiang Y, Hostetter G, et
al: Wnt5a signaling directly affects cell motility and invasion of
metastatic melanoma. Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wissmann C, Wild PJ, Kaiser S, et al:
WIF1, a component of the Wnt pathway, is down-regulated in
prostate, breast, lung, and bladder cancer. J Pathol. 201:204–212.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hoang BH, Kubo T, Healey JH, et al:
Expression of LDL receptor-related protein 5 (LRP5) as a novel
marker for disease progression in high-grade osteosarcoma. Int J
Cancer. 109:106–111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iozzo RV, Eichstetter I and Danielson KG:
Aberrant expression of the growth factor Wnt-5A in human
malignancy. Cancer Res. 55:3495–3499. 1995.PubMed/NCBI
|
|
16
|
Lu BJ, Wang YQ, Wei XJ, et al: Expression
of WNT-5a and ROR2 correlates with disease severity in
osteosarcoma. Mol Med Rep. 5:1033–1036. 2012.PubMed/NCBI
|
|
17
|
Ma Y, Ren Y, Han EQ, et al: Inhibition of
the Wnt-beta-catenin and Notch signaling pathways sensitizes
osteosarcoma cells to chemotherapy. Biochem Biophys Res Commun.
431:274–279. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kansara M, Tsang M, Kodjabachian L, et al:
Wnt inhibitory factor 1 is epigenetically silenced in human
osteosarcoma, and targeted disruption accelerates
osteosarcomagenesis in mice. J Clin Invest. 119:837–851. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao FH, Hu XH, Li W, et al: Oridonin
induces apoptosis and senescence in colorectal cancer cells by
increasing histone hyperacetylation and regulation of p16, p21, p27
and c-myc. BMC Cancer. 10:6102010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He BC, Chen L, Zuo GW, et al: Synergistic
antitumor effect of the activated PPARgamma and retinoid receptors
on human osteosarcoma. Clin Cancer Res. 16:2235–2245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu K, Yang Q, Mu Y, et al: Berberine
inhibits the proliferation of colon cancer cells by inactivating
Wnt/beta-catenin signaling. Int J Oncol. 41:292–298.
2012.PubMed/NCBI
|
|
22
|
He TC, Zhou S, da Costa LT, Yu J, Kinzler
KW and Vogelstein B: A simplified system for generating recombinant
adenoviruses. Proc Natl Acad Sci USA. 95:2509–2514. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang N, Song WX, Luo J, et al:
BMP-9-induced osteogenic differentiation of mesenchymal progenitors
requires functional canonical Wnt/beta-catenin signalling. J Cell
Mol Med. 13:2448–2464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
He BC, Gao JL, Luo X, et al: Ginsenoside
Rg3 inhibits colorectal tumor growth through the down-regulation of
Wnt/β-catenin signaling. Int J Oncol. 38:437–445. 2011.PubMed/NCBI
|
|
25
|
Park HR and Park YK: Expression of p53
protein, PCNA, and Ki-67 in osteosarcomas of bone. J Korean Med
Sci. 10:360–367. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davidson G and Niehrs C: Emerging links
between CDK cell cycle regulators and Wnt signaling. Trends Cell
Biol. 20:453–460. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lustig B and Behrens J: The Wnt signaling
pathway and its role in tumor development. J Cancer Res Clin Oncol.
129:199–221. 2003.PubMed/NCBI
|
|
28
|
Nakamura T, Hamada F, Ishidate T, et al:
Axin, an inhibitor of the Wnt signalling pathway, interacts with
beta-catenin, GSK-3beta and APC and reduces the beta-catenin level.
Genes Cells. 3:395–403. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cross DA, Alessi DR, Cohen P, Andjelkovich
M and Hemmings BA: Inhibition of glycogen synthase kinase-3 by
insulin mediated by protein kinase B. Nature. 378:785–789. 1995.
View Article : Google Scholar
|
|
30
|
Bikkavilli RK, Feigin ME and Malbon CC:
p38 mitogen-activated protein kinase regulates canonical
Wnt-beta-catenin signaling by inactivation of GSK3beta. J Cell Sci.
121:3598–3607. 2008. View Article : Google Scholar
|
|
31
|
Li CY, Wang EQ, Cheng Y and Bao JK:
Oridonin: an active diterpenoid targeting cell cycle arrest,
apoptotic and autophagic pathways for cancer therapeutics. Int J
Biochem Cell Biol. 43:701–704. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang CL, Wu LJ, Zuo HJ, Tashiro S,
Onodera S and Ikejima T: Cytochrome c release from oridonin-treated
apoptotic A375-S2 cells is dependent on p53 and extracellular
signal-regulated kinase activation. J Pharmacol Sci. 96:155–163.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cheng Y, Qiu F, Ye YC, Tashiro S, Onodera
S and Ikejima T: Oridonin induces G2/M arrest and apoptosis via
activating ERK-p53 apoptotic pathway and inhibiting PTK-Ras-Raf-JNK
survival pathway in murine fibrosarcoma L929 cells. Arch Biochem
Biophys. 490:70–75. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu HZ, Yang YB, Xu XD, et al: Oridonin
induces apoptosis via PI3K/Akt pathway in cervical carcinoma HeLa
cell line. Acta Pharmacol Sin. 28:1819–1826. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li X, Li X, Wang J, Ye Z and Li JC:
Oridonin up-regulates expression of P21 and induces autophagy and
apoptosis in human prostate cancer cells. Int J Biol Sci.
8:901–912. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Polakis P: Wnt signaling and cancer. Genes
Dev. 14:1837–1851. 2000.
|
|
37
|
Baron R and Kneissel M: WNT signaling in
bone homeostasis and disease: from human mutations to treatments.
Nat Med. 19:179–192. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haydon RC, Deyrup A, Ishikawa A, et al:
Cytoplasmic and/or nuclear accumulation of the beta-catenin protein
is a frequent event in human osteosarcoma. Int J Cancer.
102:338–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rubin EM, Guo Y, Tu K, Xie J, Zi X and
Hoang BH: Wnt inhibitory factor 1 decreases tumorigenesis and
metastasis in osteosarcoma. Mol Cancer Ther. 9:731–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo Y, Rubin EM, Xie J, Zi X and Hoang BH:
Dominant negative LRP5 decreases tumorigenicity and metastasis of
osteosarcoma in an animal model. Clin Orthop Relat Res.
466:2039–2045. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mishra R: Glycogen synthase kinase 3 beta:
can it be a target for oral cancer. Mol Cancer. 9:1442010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wu J, Liao Q, He H, Zhong D and Yin K:
TWIST interacts with beta-catenin signaling on osteosarcoma cell
survival against cisplatin. Mol Carcinog. Dec 31–2012.(Epub ahead
of print). View Article : Google Scholar
|
|
44
|
Xia JJ, Pei LB, Zhuang JP, et al:
Celecoxib inhibits beta-catenin-dependent survival of the human
osteosarcoma MG-63 cell line. J Int Med Res. 38:1294–1304. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Cai Y, Mohseny AB, Karperien M, Hogendoorn
PC, Zhou G and Cleton-Jansen AM: Inactive Wnt/beta-catenin pathway
in conventional high-grade osteosarcoma. J Pathol. 220:24–33. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang QL, Xie XB, Wang J, et al: Glycogen
synthase kinase-3beta, NF-kappaB signaling, and tumorigenesis of
human osteosarcoma. J Natl Cancer Inst. 104:749–763. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zorn AM: Wnt signalling: antagonistic
Dickkopfs. Curr Biol. 11:R592–R595. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kawano Y and Kypta R: Secreted antagonists
of the Wnt signalling pathway. J Cell Sci. 116:2627–2634. 2003.
View Article : Google Scholar
|
|
49
|
Lin CH, Guo Y, Ghaffar S, et al: Dkk-3, a
secreted wnt antagonist, suppresses tumorigenic potential and
pulmonary metastasis in osteosarcoma. Sarcoma.
2013:1475412013.PubMed/NCBI
|
|
50
|
Hoang BH, Kubo T, Healey JH, et al:
Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma
cells by modulating the Wnt-beta-catenin pathway. Cancer Res.
64:2734–2739. 2004. View Article : Google Scholar : PubMed/NCBI
|