Introduction
The prostate is a major exocrine gland in males,
which is involved in sexual developments (1). The prostate organ grows gradually
during puberty by stimuli of male hormone androgen in the body
(2). Dihydrotestosterone (DHT) is
the critical hormone responsible for prostate growth (3). Diagnosis of prostate cancer formation
is increasing and has become a common cancer in males (4). Various factors to promote cancer in
human have been supposed to be obesity, smoking, and age (5). The mechanism of the key process in
prostate cancer has not been clarified yet (6).
Some reports have assumed that the imbalance in
hormones such as androgens and estrogens is the main cause of
prostate cancer. Estrogens are known to be potential factors in the
progression of breast cancer and ovarian cancer expressing estrogen
receptors (ERs). These facts suggest that estrogen-responsive
organs can be adenocarcinoma by overactivation of ERs signaling and
are asserted with evidence of laboratory and clinical research
(7). Estrogen-induced signaling
directly contributes to modify gene expression to alter normal
biological mechanism (8).
Especially, the distinct roles of ERs, ERα and ERβ, in relation
with androgen receptor (AR) have been focused on prostate disease
(9). In addition, it has been
reported that growth factors or cytokines are involved in the
progression of prostate cancer (10) and applied for therapeutic targets
to conquer cancer and metastasis into other secondary site
(11).
Among growth factors, the growth regulatory proteins
of transforming growth factor-β (TGF-β) family are endogenous
inhibitors of cell growth (12).
After binding of three types of TGF-β with their receptors, Smad2/3
proteins are phosphorylated and form a complex with Smad4. Smad
complex translocates to the nucleus and regulates transcriptional
expression of downstream genes by binding DNA as a transcription
factor (13). Cancer is
characterized by uncontrolled cell proliferation compared to normal
cells (14). TGF-β is a
multifunctional cytokine, which regulates cell proliferation,
differentiation and apoptosis of cells in most tissues. For
example, null mouse experiments for TGF-β have suggested that TGF-β
plays a role in inflammation. Other studies also showed that TGF-β
is linked to carcinogenesis (15).
Variation of mRNA levels of three TGF-β correlates
with progression of human cancer such as glioma, as determining
tumor growth by modification of microenvironment surrounding
cancer. TGF-β may cause an activation of signaling pathways of
other growth factors such as vascular endothelial growth factor
(VEGF) and plasminogen activator inhibitor-1 (PAI-1) (16). Carcinogenesis is affected by TGF-β
as well as the family of Smad. The TGF-β/Smad signaling pathway,
which is activated in prostate cancer, has a regulatory effect on
the cell cycle. TGF-β signaling pathway is closely associated with
p21, p27, c-myc and c-fos in cancer (17). c-myc is a cellular
proto-oncogene, with a potential in modification of proliferation
and apoptosis of human cancer cells (18). c-myc and c-fos reportedly control
TGF-β signaling and cell cycles (19). Overexpression of c-myc is
associated with cancer initiation and metastasis (20). Family of myc is important in the
transformational changes to cancer (21). Myc plays central roles in the
activity of cyclin D1-Cdk4 during G0 to S transition in
the cell cycle (22). Abnormality
of the cancer cell cycle results in production of cyclin (23) which degrades p21 and p27 (24).
Expressions of the cell cycle-related genes, c-myc,
cyclin D and p21, were altered in cancer by endocrine disrupting
chemicals (EDCs) via steroid hormone receptor signaling pathways in
our previous studies (25–27). EDCs are environmental synthetic
chemicals that disrupt the endocrine system (28,29).
Accumulation of EDCs in the body may lead to severe reproductive
problems in humans (30). As a
result of the development of industry, many synthetic chemicals
have been identified as EDCs causing the human health problems
(31,32). For example, some pesticides are
known as EDCs (33). Among EDCs,
humans are exposed to phthalate in the environment (34). Phthalates are similar diesters of
phthalic acid used as plasticizer to make plastics soft (35). Recently, the production volume of
phthalates has increased due to wide applications for plastics
(36). Therefore, humans may be
easily exposed to phthalate through the plastics, clothes and
bottles (25). The impact of
phthalate exposure on human health have also been focused on due to
toxicity on the reproductive system via androgen and estrogen
receptor signaling pathways.
In this study, we determined whether exposure to
phthalate may promote prostate cancer in in vitro and in
vivo through molecular crosstalk between estrogen receptor (ER)
signaling and TGF-β signaling pathway. We employed prostate cancer
LNCaP cells, which express ERα, ERβ and ARs. Therefore, these cells
are a useful model to study estrogen receptors signaling in
prostate cancer.
Materials and methods
Reagents and chemicals
17β-estradiol (E2), di-n-buthyl phthalate (DBP) and
ICI 182,780 were purchased from Sigma-Aldrich Corp. (St. Louis, MO,
USA). All chemicals were dissolved in 100% dimethyl sulfoxide
(DMSO; Junsei Chemical Co., Tokyo, Japan) and corn oil (Junsei
Chemical Co.) 3 days before treatment.
Cell culture and media
LNCaP cells were cultured in Dulbecco’s modified
Eagle’s medium (DMEM; HyClone Laboratories Inc., Logan, UT, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
HyClone Laboratories Inc.), 1% penicillin G and streptomycin
(Cellgro; Mediatech, Inc., Manassas, VA, USA), and HEPES
(Invitrogen Life Technologies, Carlsbad, CA, USA) at 37°C in a
humidified atmosphere with 5% CO2–95% air.
Ablation of estrogenic components
To exclude the effects of estrogenic components in
DMEM and FBS, cells were also cultured in phenol red-free DMEM
supplemented with 5% charcoal-dextran stripped FBS (CD-FBS). Cells
were detached with 0.05% trypsin/0.02% EDTA in
Mg2+/Ca2+-free Hanks’ balanced salt solution
(PAA Laboratories, Pasching, Austria) before the DBP and E2
treatment.
Cell viability assay
To evaluate the effect of E2 or DBP on LNCaP cell
proliferation, cell viability assay was performed as previously
described (32). Cells were seeded
at a density of 8,000 cells/100 μl of phenol red-free DMEM with 5%
CD-FBS medium per well of 96 well culture plates. After an
incubation for 24 h, the cells were washed and treated with various
concentrations of chemicals in phenol red-free DMEM supplemented
with 0.1% DMSO for 5 days. DMSO was used as a vehicle and a
negative control and E2 as a positive control. Cell viability was
detected with the addition of
3-(4-,5-dimethylthiazol-2-yl)-2,5-dyphenyltetrazolium bromide (MTT;
Sigma-Aldrich) solution. MTT (10 μl of 5 mg/ml solution) was added
to each well and the plates were incubated for 4 h at 37°C.
Supernatants were removed and 100 μl of DMSO was added to each well
to dissolve the resultant formazan crystals. The optical density
(OD) of each well was measured at 540 nm using an ELISA reader
(Molecular Devices, Sunnyvale, CA, USA) and used to calculate the
number of viable cells. All experiments were done at least three
times.
Semi-quantitative reverse transcription
(RT) PCR
Cells were seeded at a density of 5.0×105
cells per well in a 6-well plate, and then treated with either
DMSO, E2 or DBP. Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies) according to the manufacturer’s
instructions. cDNA was synthesized from total RNA by reverse
transcription (RT). The reaction mixture contained murine leukemia
virus reverse transcriptase (M-MLV RT), 200 pM nonamer random
primer, dNTPs, RNase inhibitor and RT buffer (all from Intron
Biotechnology, Sungnam, Korea). cDNA synthesis was performed at
37°C for 1 h and 95°C for 5 min. To analyse the expression of p21,
cyclin D1, c-myc and GAPDH, cDNA was amplified by PCR with specific
forward and reverse primers, Taq polymerase, PCR buffer and dNTP
mixture, and each cDNA template as previously described. The
following primers (Bioneer Co., Daejeon, Korea) were used: for
cyclin D1, forward (F)-TCTAA GATGA AGGAG ACCAT C and reverse
(R)-TGACA GGTCC ACATG GTCTT CC; for p21, F-AGGCA CCGAG GCACT CAGAG
and R-TGACA GGTCC ACATG GTCTT CC; and for GAPDH, F-ATGTT CGTCA
TGGGT GTGAA CCA and R-TGGCA GGTTT TTCTA GACGG CAG. The PCR products
were separated on a 1.5% agarose gel and the size of each gene band
was estimated by comparison with 100-bp size ladders (Intron
Biotechnology). The gels were scanned and the band densities were
quantified using Gel Doc 2000 (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). All experiments were done at least three
times.
Western blot analysis
To detect protein expression of cyclin D1 and Smad
in LNCaP, cells were cultured to a density of 2.0×106
cells per of 100-mm dish and then treated with DMSO, E2 or DBP.
After treatment, the cells were suspended in 100 μl of 1× RIPA
buffer (50 mM Tris-HCl; pH 8.0, 150 mM NaCl, 1% NP-40, 0.5%
deoxycholic acid and 0.1% SDS). Total protein concentrations were
determined by bicinchoninic acid (BCA; Sigma-Aldrich Corp.) and 50
μg of total protein was then separated by SDS-polyacrylamide gel
electrophoresis (SDS-PAGE). The proteins were transferred to a
polyvinylidene difluoride (PVDF) membrane (Bio-Rad Laboratories,
Inc.), and the membranes were blocked with 5% bovine serum albumin
(BSA; Sigma-Aldrich Corp.) for 2 h at room temperature. The
membranes were incubated with mouse monoclonal anti-Smad (1:500;
Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit monoclonal
anti-pSMAD3 (1:200 dilution; Santa Cruz Biotechnology), mouse
monoclonal anti-cyclin D1 or anti-p21 (1:3,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), or mouse monoclonal anti-GAPDH
(1:1,000; Santa Cruz Biotechnology) antibodies overnight at room
temperature. The membranes were subsequently probed with anti-mouse
IgG HRP-conjugated secondary antibody (1:1,000; Santa Cruz
Biotechnology) for 4 h at room temperature. Target proteins were
detected with a West-Q Chemiluminescent Substrate Plus kit
(Gendepot, Barker, TX, USA). All experiments were done at least
three times.
Establishment of xenograft prostate
cancer models
LNCaP cells (6×106) were mixed with
Matrigel (BD Biosciences, Bedford, MA, USA) and injected
subcutaneously (s.c.) into male nude balb/c mice (5-week-old). Mice
were monitored for tumor growth every week, and the tumor volumes
were measured using a caliper and expressed by length × width ×
height × 0.5236 (mm3). Once tumor reached 50
mm3, the mice were surgically castrated under anesthesia
using avertin (Sigma-Aldrich Corp.). The animal experiment was
performed according to the protocols approved by the Animal Care
Committee of Chungbuk National University. Mice were reinstated for
1 week after surgery, grouped into three groups, and injected s.c.
with corn oil (vehicle, n=5), E2 (n=5; 20 μg/kg/body weight), or
DBP (n=5; 200 mg/kg/body weight) every 2 days for 5 weeks (Fig. 1). Cancer tissues were obtained from
mice after treatment with chemicals, fixed in 4% formalin and
embedded in paraffin for immunohistochemical analysis.
Immunohistochemistry and
bromodeoxyuridine (BrdUrd) incorporation assay
Immunohistochemistry for p21 and bromodeoxyuridine
(BrdUrd) was performed for the specimen slides of tumor sections
obtained from the mice. Antigen retrieval was achieved in a
microwave using 0.01 M citrate buffer, and the slides were treated
sequentially with 0.3% H2O2, blocking buffer
and first antibodies. For detection of expression of target
proteins in tissue, biotinylated-mouse anti-goat IgG (1:1,000
dilution, Vector Laboratories, Inc., Burlingame, CA, USA) was used
as a secondary antibody. The primary antibodies used in this assay
were a mouse monoclonal antibody against p21, which is used in the
same condition as in western blot analysis, and a mouse monoclonal
antibody against BrdUrd (1:100 dilution, Thermo Scientific,
Rockford, IL, USA). The tissues were counterstained with
hematoxylin (Sigma-Aldrich) and observed using the BX51 microscope
(Olympus, Center Valley, PA, USA) for digital photography.
Statistical analysis
All data were analyzed with GraphPad Prism software
(San Diego, CA, USA). Data are presented as the mean ± SD.
Statistical analyses were performed using a one-way ANOVA;
Dunnett’s multiple comparison or Student’s t-test. p-values
<0.05 were considered statistically significant.
Results
Stimulation effects of phthalate on
prostate cancer
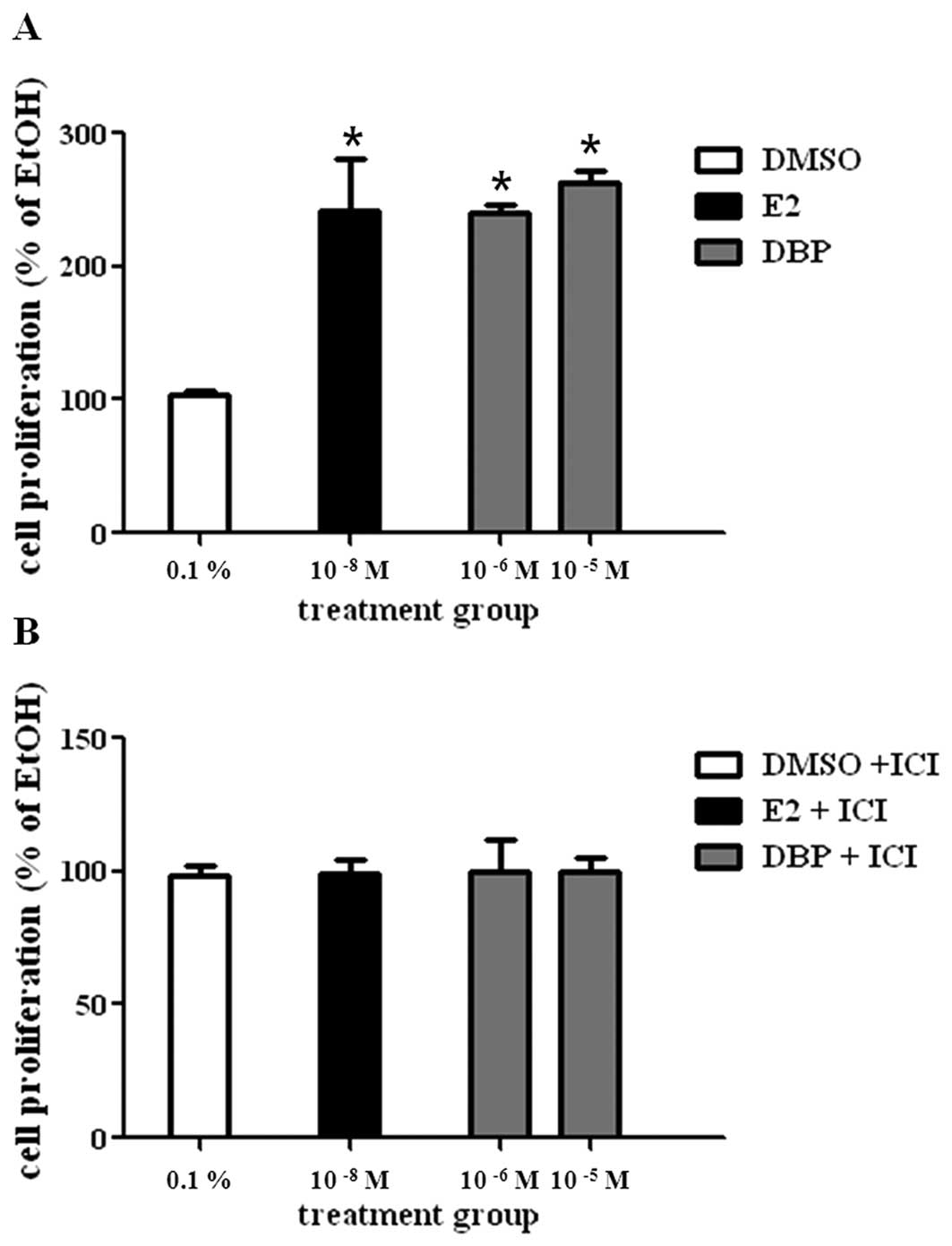
To evaluate the effect of E2 or DBP on cell
proliferation of LNCaP, cells were cultured with vehicle (0.1%
DMSO, control), E2 (10−8 M), or DBP (10−6 M)
for 5 days. E2 statistically increased the growth of prostate
cancer cells compared to DMSO as shown in Fig. 1A. DBP showed a cell proliferation
effect similar with E2 at concentrations of 10−6 and
10−5 M. DBP-induced cell proliferation was reduced by
ICI 182,780 (Fig. 1B), an ER
antagonist. This fact may suggest proliferative effect of DBP on
LNCaP cells via ER signaling.
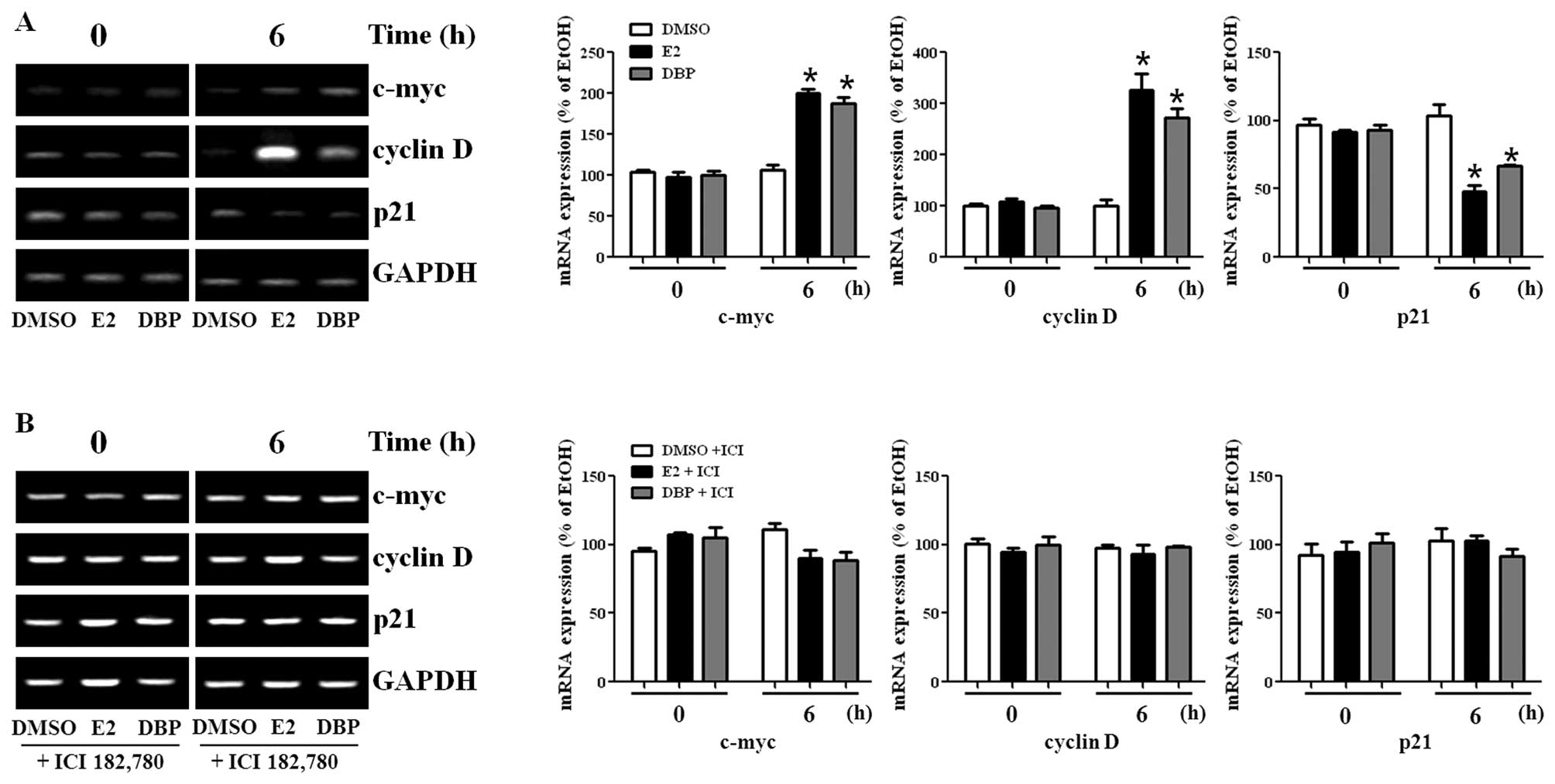
Disruption of mRNA expression regulation
of genes by DBP
To perform semi-quantitative RT-PCR on total RNA
samples, RNA was isolated from the cells treated with these agents
and amplified by PCR. First, mRNA levels of c-myc and cyclin D1
were significantly increased by treatment with E2 or DBP for 6 and
24 h, while expression of p21 was markedly decreased compared to
the control (Fig. 2A). Cyclin D1
and c-myc are promoting effectors, while p21 is an inhibitor in
cell cycle progression. Based on MTT assay results, the breakdown
of ER signaling pathway by the ER antagonist, ICI 182,780, reversed
the effects of DBP in these cancer cells as seen in Fig. 2B. These results show that
phthalates may alter expression of genes related with both TGF-β
signaling and ER signaling pathway.
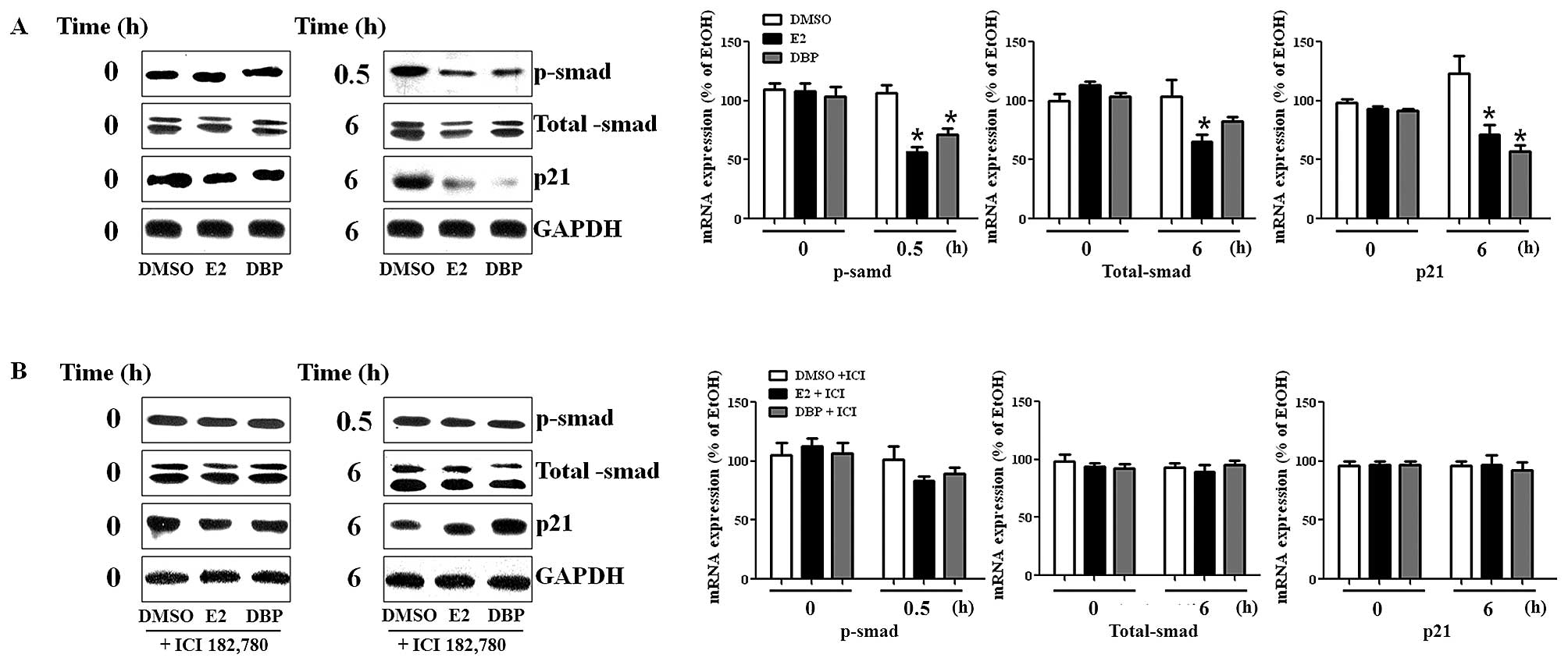
Altered protein expression of smad and
p21 in LNCaP cells by exposure to DBP
To detect protein expression of genes, we performed
western blot analysis using protein isolated from LNCaP cells. The
expression of p-smad was reduced by DBP as shown in Fig. 3A. The protein expression of p-smad
was disrupted following treatment with DBP (10−6 M). In
addition, p21 protein was markedly reduced in LNCaP cells exposed
to DBP compared to DMSO, as shown in RT-PCR analysis. However, the
suppressive effect of DBP on the expression of these genes in LNCaP
was reversed by the addition of ICI 182,780 as demonstrated in
Fig. 3B. This fact may imply
crosstalk between ER and TGF-β signaling pathway because the
expression of smads (total smad and p-smad) was influenced by the
treatment of E2 and an ER antagonist in prostate cancer cells
expressing ER.
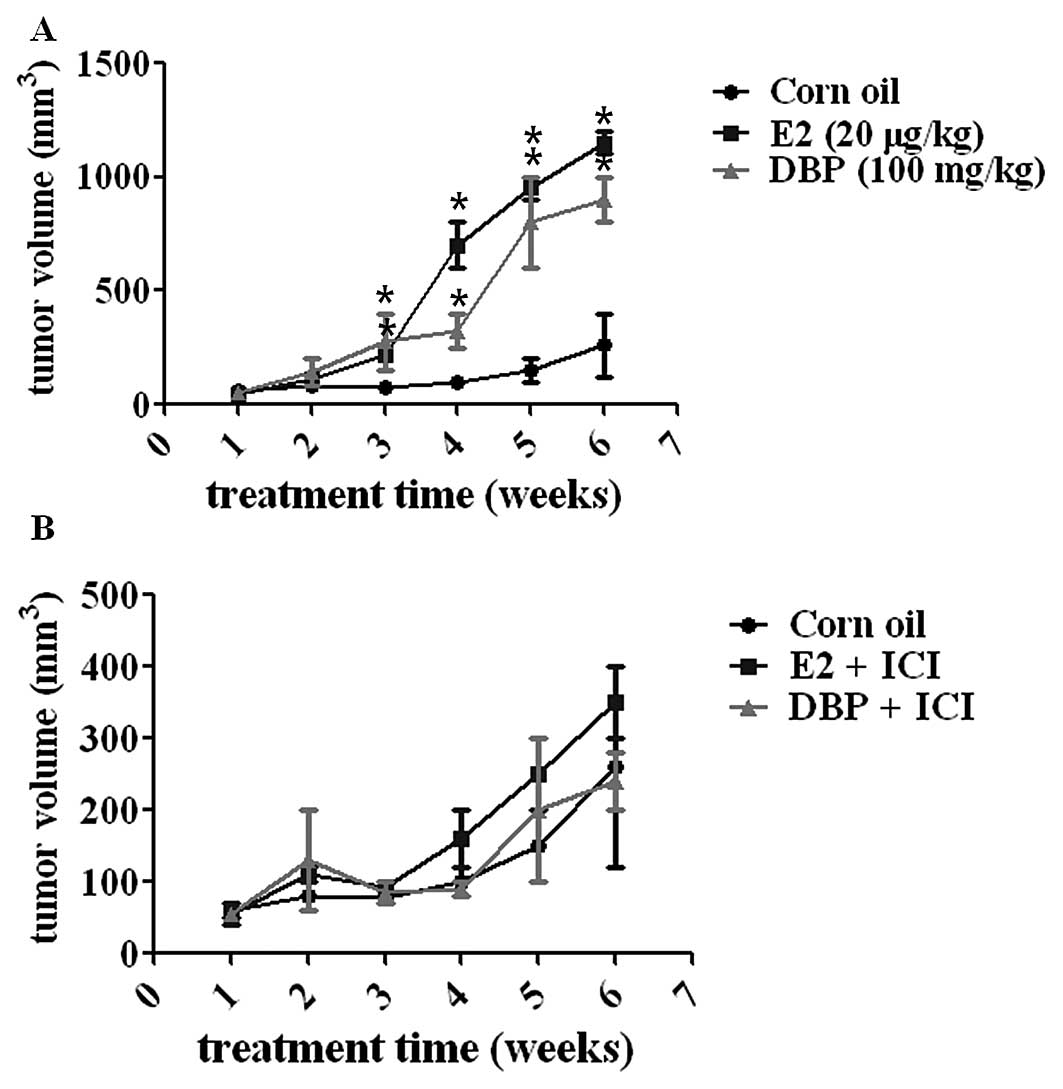
Effect of DBP on tumor growth in
vivo
To evaluate the ability of DBP to promote cancer
growth, the xenografted male mice transplanted with LNCaP were
injected s.c. with DBP, E2 or corn oil every other day as indicated
in Materials and methods. E2 (a positive control) and DBP markedly
stimulated tumor volume compared to corn oil group (a negative
control) as shown in Fig. 4A
(p<0.05). However, the pre-treatment of the mice with ICI
182,780 reversed the DBP- or E2-induced increase in tumor volume of
prostate cancer xenografted mice (Fig.
4B). This fact demonstrated a critical role of ER-dependent
signaling in tumor growth in the mouse models in vivo.
Analysis of cell cycle transition in
prostate cancer
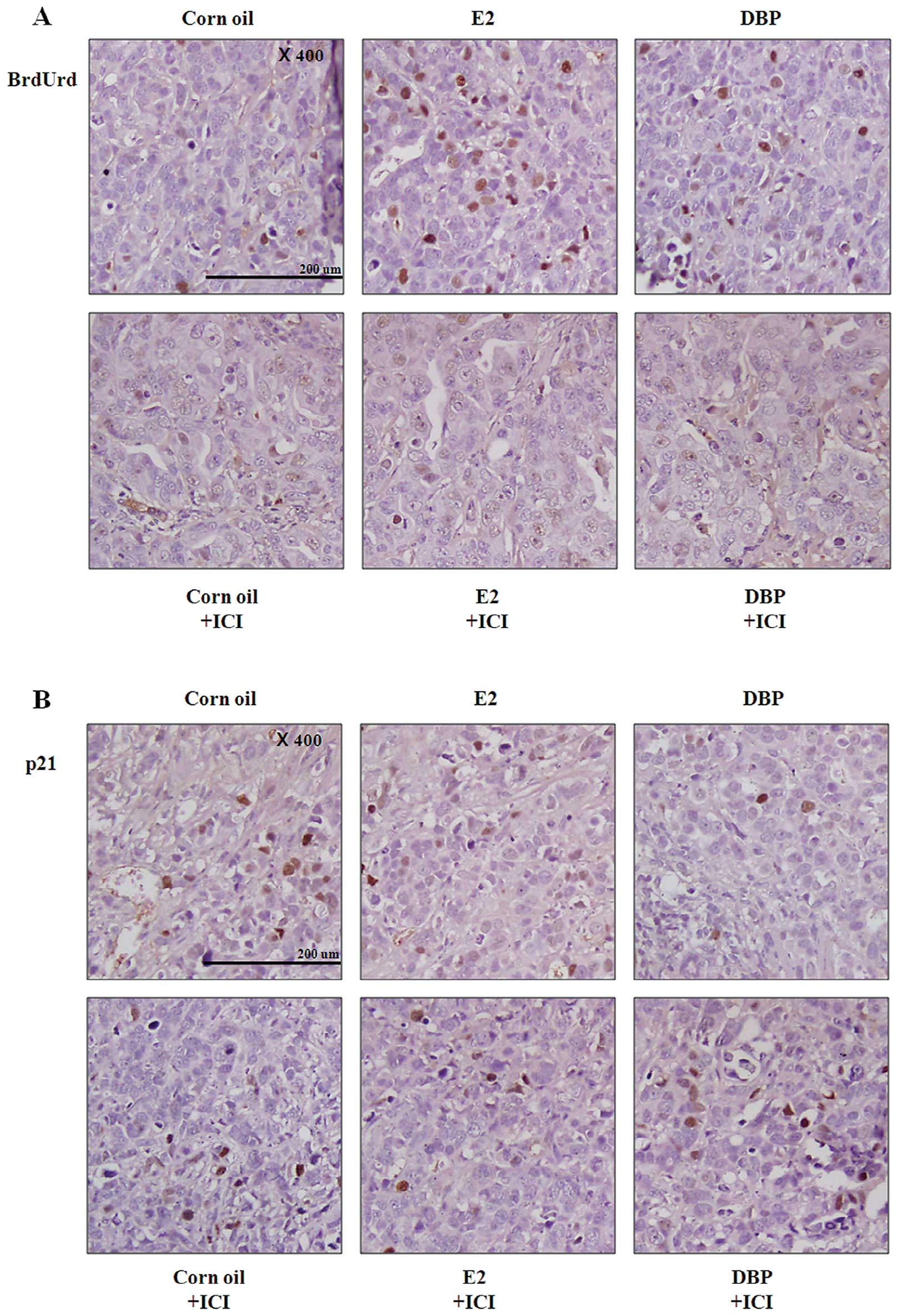
The xenografted male mice were injected
intraperitoneally (i.p.) with BrdUrd before euthanization and the
amount of BrdUrd incorporated into nuclei including the S-phase DNA
was detected using immunohistochemistry (Fig. 5). In both E2 and DBP groups,
tissues showed significantly increased number of BrdUrd positive
nuclei compared with corn oil group (Fig. 5A). These results demonstrated that
DBP promotes the growth of prostate cancer as promoting the cell
cycle transition. However, pretreatment with ICI 182,780 led to
reduced number of BrdUrd positive nuclei in cancer tissue (Fig. 5A). The expression of p21 was
reduced in cancer tissue by exposure to DBP similarly to in
vitro results (Fig. 5B).
DBP-induced expression changes of p21 were reversed by inhibition
of ER signaling pathway (Fig. 5B).
These results suggest that the effects of DBP on prostate cancer
are dependent on the ER signaling pathway.
Discussion
Prostate cancer is the most common malignancy
diagnosed in men. Androgen signaling is required to affect
apoptosis and proliferation of prostate cancer (37,38).
Among the factors responsible for formation of cancer, EDCs may
adversely impact human health through complex mechanisms, via
disruption to endogenous hormone receptor binding (39). In humans, a relation with increased
abundance of EDCs and cancer has been reported to focus on the
hormone-dependent pathway in cancer formation, especially for
estrogen (40).
Phthalates, chemicals for plastic resin, mimic
estrogen signaling, resulting in disrupting the action of steroid
receptors (26). Humans easily
absorb phthalates through the skin or ingest them if food wrapped
with plastic film is eaten because phthalates are widely spread in
various plastic products as plasticizers. This fact would increase
the danger of phthalates that have been reported to have weak
estrogenic activity and to compete with endogenous estrogen
(26).
An estrogen signaling pathway activates
transcription of target genes such as c-myc, cyclin D1 and p21
(41). They are regulated to
address the G0/G1 and G1/S
transition in cells in response to stimuli (42). Furthermore, c-myc may affect other
growth factor signaling such as TGF-β and ras signaling (43). Both c-myc and cyclin D1 are
required for activation of cell growth (44). In addition, c-myc may lead to
over-expression of cyclin D1 and down-regulation of p21 (45). In this study, we focused on
crosstalk between TGF-β and estrogen signaling by DBP in prostate
cancer. That LNCaP prostate cancer cells express both ER and AR
suggests the critical role of not only AR but also ER signaling
pathway during cancer progression (46). Research has shown clinical evidence
that estrogen may play an important role in human prostate cancer,
as well as in rodent (47).
However, the mechanism of estrogen signaling pathway has not been
clarified due to its complexity (48).
We performed MTT assays, RT-PCR and western blot
analysis using the cellular models in LNCaP cells. Many research
groups have used LNCaP cells exposed to E2 (49) showing that estrogen affects
epigenesis and cancer (50), and
suggesting that ER signaling can be a target for therapy (51). In this study, DBP was shown to
promote cell proliferation in LNCaP, while it does not have this
effect under the inhibition of ER signaling by ICI 182,780. In
addition, c-myc and cyclin D1 expressions were increased and p21
expression was decreased, resulting in cell proliferation. This
suggests that DBP may induce cell proliferation by upregulating the
gene expression of c-myc and cyclin D1 and by downregulating p21
expression in prostate cancer. The concentrations of DBP in this
study were higher compared to E2 because the binding affinity of
DBP to ERs appears to be 1,000 times lower compared to that of
E2.
An interaction between ER and TGF-β signaling
pathway was found by the effect of E2 and ER antagonist on the
expression of smad, which is an intracellular protein that
transduces extracellular signals from TGF-β ligands to the nucleus.
In western blot analysis, the expression of p-smad was reduced by
E2 as well as DBP, while this effect was reversed by the treatment
of ICI 182,780, implying that TGF-β signaling is affected by ER
signaling, and the DBP mimics E2 action in this interaction.
Although the mechanism is not clearly investigated, the result of
this study implies that molecular crosstalk between TGF-β and ER
signaling pathway may have an effect on the stimulation of prostate
cancer progression and provides a pathway on which phthalate can
act.
The effect of E2 and DBP on prostate cancer growth
was confirmed in experimental animals. Tumor volume of mice exposed
to E2 and DBP was increased compared to a negative control. These
results coincide with the immunohistochemical observations in which
the number of cells in S phase was increased by E2 and DBP, while
the expression of p21 was reduced in the tissues of E2 and
DBP-treated mice. However, the effects of E2 and DBP disappeared in
breakdown of ER signaling pathway following the treatment with ICI
182,780, a typical ER antagonist. The amount of injected DBP
greatly exceeded E2 in the present study, because EDCs may have an
accumulative effect in the body due to continuous exposure and
ingestion from an environment unlike endogeneous E2.
In conclusion, DBP, a type of phthalate, may have
the potential to promote LNCaP prostate cancer proliferation
similarly to E2. Moreover, our results demonstrated that a
phthalate acts on crosstalk between TGF-β and ER signaling pathway
in inducing the growth of prostate cancer. Therefore, this
crosstalk can be a target for therapeutic treatment of prostate
cancer that can be induced by endogenous hormones or EDCs,
including phthalates.
Acknowledgements
This study was supported by the Priority Research
Centers Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(MEST) of Korea government (2009-0094035). In addition, this study
was also supported by a grant from the Next-Generation BioGreen 21
Program (no. PJ009599), Rural Development Administration, Republic
of Korea.
References
|
1
|
Parnes HL, House MG and Tangrea JA:
Prostate cancer prevention: strategies for agent development. Curr
Opin Oncol. 25:242–251. 2013.PubMed/NCBI
|
|
2
|
Powers GL and Marker PC: Recent advances
in prostate development and links to prostatic diseases. Wiley
Interdiscip Rev Syst Biol Med. 5:243–256. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arai S, Shibata Y, Nakamura Y, et al:
Development of prostate cancer in a patient with primary
hypogonadism: intratumoural steroidogenesis in prostate cancer
tissues. Andrology. 1:169–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dietrich D, Hasinger O, Banez LL, et al:
Development and clinical validation of a real-time PCR assay for
PITX2 DNA methylation to predict prostate-specific antigen
recurrence in prostate cancer patients following radical
prostatectomy. J Mol Diagn. 15:270–279. 2013. View Article : Google Scholar
|
|
5
|
Meeks JJ and Schaeffer EM: Genetic
regulation of prostate development. J Androl. 32:210–217. 2010.
View Article : Google Scholar
|
|
6
|
Lin D, Bayani J, Wang Y, et al:
Development of metastatic and non-metastatic tumor lines from a
patient’s prostate cancer specimen-identification of a small
subpopulation with metastatic potential in the primary tumor.
Prostate. 70:1636–1644. 2010.
|
|
7
|
Onita T, Igawa T, Hisamatsu H, Sakai H and
Kanetake H: Secondary endocrine therapy with oral estrogen for
relapsed prostate cancer. Hinyokika Kiyo. 55:595–598. 2009.(In
Japanese).
|
|
8
|
Sissung TM, Danesi R, Kirkland CT, et al:
Estrogen receptor alpha and aromatase polymorphisms affect risk,
prognosis, and therapeutic outcome in men with castration-resistant
prostate cancer treated with docetaxel-based therapy. J Clin
Endocrinol Metab. 96:E368–E372. 2011. View Article : Google Scholar
|
|
9
|
Fromont G, Yacoub M, Valeri A, et al:
Differential expression of genes related to androgen and estrogen
metabolism in hereditary versus sporadic prostate cancer. Cancer
Epidemiol Biomarkers Prev. 17:1505–1509. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Holt SK, Kwon EM, Fu R, et al: Association
of variants in estrogen-related pathway genes with prostate cancer
risk. Prostate. 73:1–10. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vitkus S, Yeh CR, Lin HH, et al: Distinct
function of estrogen receptor alpha in smooth muscle and fibroblast
cells in prostate development. Mol Endocrinol. 27:38–49. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kaminska B, Wesolowska A and Danilkiewicz
M: TGF beta signalling and its role in tumour pathogenesis. Acta
Biochim Pol. 52:329–337. 2005.PubMed/NCBI
|
|
13
|
Li X, Placencio V, Iturregui JM, et al:
Prostate tumor progression is mediated by a paracrine
TGF-beta/Wnt3a signaling axis. Oncogene. 27:7118–7130. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Assinder SJ, Dong Q, Kovacevic Z and
Richardson DR: The TGF-beta, PI3K/Akt and PTEN pathways:
established and proposed biochemical integration in prostate
cancer. Biochem J. 417:411–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jones E, Pu H and Kyprianou N: Targeting
TGF-beta in prostate cancer: therapeutic possibilities during tumor
progression. Expert Opin Ther Targets. 13:227–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lenferink AE, Cantin C, Nantel A, et al:
Transcriptome profiling of a TGF-beta-induced
epithelial-to-mesenchymal transition reveals extracellular
clusterin as a target for therapeutic antibodies. Oncogene.
29:831–844. 2009. View Article : Google Scholar
|
|
17
|
Danielpour D: Functions and regulation of
transforming growth factor-beta (TGF-beta) in the prostate. Eur J
Cancer. 41:846–857. 2005. View Article : Google Scholar
|
|
18
|
Miller DM, Thomas SD, Islam A, Muench D
and Sedoris K: c-Myc and cancer metabolism. Clin Cancer Res.
18:5546–5553. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bockelman C, Koskensalo S, Hagstrom J,
Lundin M, Ristimaki A and Haglund C: CIP2A overexpression is
associated with c-Myc expression in colorectal cancer. Cancer Biol
Ther. 13:289–295. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bouchalova K, Cizkova M, Cwiertka K,
Trojanec R and Hajduch M: Triple negative breast cancer - current
status and prospective targeted treatment based on HER1 (EGFR),
TOP2A and C-MYC gene assessment. Biomed Pap Med Fac Univ Palacky
Olomouc Czech Repub. 153:13–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Long X, Hu S, Cao P, Liu Z, Zhen H and Cui
Y: The expression of oncogene c-myc and its role on human laryngeal
cancer. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi.
23:1127–1129. 2009.(In Chinese).
|
|
22
|
Liu M, Casimiro MC, Wang C, et al: p21CIP1
attenuates Ras- and c-Myc-dependent breast tumor epithelial
mesenchymal transition and cancer stem cell-like gene expression in
vivo. Proc Natl Acad Sci USA. 106:19035–19039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Du YP, Peng JS, Sun A, Tang ZH, Ling WH
and Zhu HL: Assessment of the effect of betaine on p16 and c-myc
DNA methylation and mRNA expression in a chemical induced rat liver
cancer model. BMC Cancer. 9:2612009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang KA, Kang NH, Yi BR, Lee HR, Park MA
and Choi KC: Genistein, a soy phytoestrogen, prevents the growth of
BG-1 ovarian cancer cells induced by 17β-estradiol or bisphenol A
via the inhibition of cell cycle progression. Int J Oncol.
42:733–740. 2012.PubMed/NCBI
|
|
25
|
Lee HR, Hwang KA, Park MA, Yi BR, Jeung EB
and Choi KC: Treatment with bisphenol A and methoxychlor results in
the growth of human breast cancer cells and alteration of the
expression of cell cycle-related genes, cyclin D1 and p21, via an
estrogen receptor-dependent signaling pathway. Int J Mol Med.
29:883–890. 2012.
|
|
26
|
Park MA, Hwang KA, Lee HR, Yi BR, Jeung EB
and Choi KC: Cell growth of BG-1 ovarian cancer cells is promoted
by di-n-butyl phthalate and hexabromocyclododecane via upregulation
of the cyclin D and cyclin-dependent kinase-4 genes. Mol Med Rep.
5:761–766. 2012.PubMed/NCBI
|
|
27
|
Park MA, Hwang KA, Lee HR, Yi BR, Jeung EB
and Choi KC: Benzophenone-1 stimulated the growth of BG-1 ovarian
cancer cells by cell cycle regulation via an estrogen receptor
alpha-mediated signaling pathway in cellular and xenograft mouse
models. Toxicology. 305:41–48. 2013. View Article : Google Scholar
|
|
28
|
Geier R, Adler S, Rashid G and Klein A:
The synthetic estrogen diethylstilbestrol (DES) inhibits the
telomerase activity and gene expression of prostate cancer cells.
Prostate. 70:1307–1312. 2010.PubMed/NCBI
|
|
29
|
Lee HR, Jeung EB, Cho MH, Kim TH, Leung PC
and Choi KC: Molecular mechanism(s) of endocrine-disrupting
chemicals and their potent oestrogenicity in diverse cells and
tissues that express oestrogen receptors. J Cell Mol Med. 17:1–11.
2013. View Article : Google Scholar
|
|
30
|
Hess-Wilson JK: Bisphenol A may reduce the
efficacy of androgen deprivation therapy in prostate cancer. Cancer
Causes Control. 20:1029–1037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kang NH, Hwang KA, Kim TH, Hyun SH, Jeung
EB and Choi KC: Induced growth of BG-1 ovarian cancer cells by
17β-estradiol or various endocrine disrupting chemicals was
reversed by resveratrol via downregulation of cell cycle
progression. Mol Med Rep. 6:151–156. 2012.
|
|
32
|
Lee HR and Choi KC: 4-tert-Octylphenol
stimulates the expression of cathepsins in human breast cancer
cells and xenografted breast tumors of a mouse model via an
estrogen receptor-mediated signaling pathway. Toxicology.
304:13–20. 2013. View Article : Google Scholar
|
|
33
|
Derouiche S, Warnier M, Mariot P, et al:
Bisphenol A stimulates human prostate cancer cell migration
remodelling of calcium signalling. Springerplus. 2:542013.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mnif W, Hassine AI, Bouaziz A, Bartegi A,
Thomas O and Roig B: Effect of endocrine disruptor pesticides: a
review. Int J Environ Res Public Health. 8:2265–2303. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Prins GS, Tang WY, Belmonte J and Ho SM:
Developmental exposure to bisphenol A increases prostate cancer
susceptibility in adult rats: epigenetic mode of action is
implicated. Fertil Steril. 89:e412008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wetherill YB, Fisher NL, Staubach A,
Danielsen M, de Vere White RW and Knudsen KE: Xenoestrogen action
in prostate cancer: pleiotropic effects dependent on androgen
receptor status. Cancer Res. 65:54–65. 2005.PubMed/NCBI
|
|
37
|
Dulinska-Litewka J, McCubrey JA and
Laidler P: Increased Akt signaling resulting from the loss of
androgen responsiveness in prostate cancer. Curr Med Chem.
20:144–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee SO, Ma Z, Yeh CR, et al: New therapy
targeting differential androgen receptor signaling in prostate
cancer stem/progenitor vs. non-stem/progenitor cells. J Mol Cell
Biol. 5:14–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hess-Wilson JK, Webb SL, Daly HK, et al:
Unique bisphenol A transcriptome in prostate cancer: novel effects
on ERbeta expression that correspond to androgen receptor mutation
status. Environ Health Perspect. 115:1646–1653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim SM, Jung EM, An BS, et al: Additional
effects of bisphenol A and paraben on the induction of
calbindin-D(9K) and progesterone receptor via an estrogen receptor
pathway in rat pituitary GH3 cells. J Physiol Pharmacol.
63:445–455. 2012.PubMed/NCBI
|
|
41
|
Pries R, Hogrefe L, Xie L, et al:
Induction of c-Myc-dependent cell proliferation through toll-like
receptor 3 in head and neck cancer. Int J Mol Med. 21:209–215.
2008.PubMed/NCBI
|
|
42
|
Serra JM, Gutierrez A, Alemany R, et al:
Inhibition of c-Myc down-regulation by sustained extracellular
signal-regulated kinase activation prevents the antimetabolite
methotrexate- and gemcitabine-induced differentiation in
non-small-cell lung cancer cells. Mol Pharmacol. 73:1679–1687.
2008. View Article : Google Scholar
|
|
43
|
Guo J, Xiao B, Liu Q, Gong Z and Le Y:
Suppression of C-myc expression associates with anti-proliferation
of aloe-emodin on gastric cancer cells. Cancer Invest. 26:369–374.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang H, Mannava S, Grachtchouk V, et al:
c-Myc depletion inhibits proliferation of human tumor cells at
various stages of the cell cycle. Oncogene. 27:1905–1915. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Morrish F, Neretti N, Sedivy JM and
Hockenbery DM: The oncogene c-Myc coordinates regulation of
metabolic networks to enable rapid cell cycle entry. Cell Cycle.
7:1054–1066. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nakamura Y, Felizola SJ, Kurotaki Y, et
al: Cyclin D1 (CCND1) expression is involved in estrogen receptor
beta (ERβ) in human prostate cancer. Prostate. 73:590–595.
2012.
|
|
47
|
Gross M, Ramirez C, Luthringer D, et al:
Expression of androgen and estrogen related proteins in normal
weight and obese prostate cancer patients. Prostate. 69:520–527.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nicolaiew N, Cancel-Tassin G, Azzouzi AR,
et al: Association between estrogen and androgen receptor genes and
prostate cancer risk. Eur J Endocrinol. 160:101–106. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Chae YK, Huang HY, Strickland P, Hoffman
SC and Helzlsouer K: Genetic polymorphisms of estrogen receptors
alpha and beta and the risk of developing prostate cancer. PLoS
One. 4:e65232009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Szendroi A, Speer G, Tabak A, et al: The
role of vitamin D, estrogen, calcium sensing receptor genotypes and
serum calcium in the pathogenesis of prostate cancer. Can J Urol.
18:5710–5716. 2011.PubMed/NCBI
|
|
51
|
Celhay O, Yacoub M, Irani J, Dore B,
Cussenot O and Fromont G: Expression of estrogen related proteins
in hormone refractory prostate cancer: association with tumor
progression. J Urol. 184:2172–2178. 2010. View Article : Google Scholar
|