Introduction
Tanshinone IIA (TSIIA,
1,6,6-trimethyl-6,7,8,9-tetrahydrophenanthro [1,2-b]
furan-10,11-dione) is the most abundant diterpene quinine isolated
from Salvia miltiorrhiza, known as ‘Dan-Shen’. This plant
has been safely used for more than 2,000 years in traditional
Chinese medicine for treating a large variety of cardiovascular
diseases including atherosclerosis, coronary artery disease, angina
pectoris, myocardial infarction and hypertension (1). TSIIA and the other two tanshinones,
cryptotanshinone (CTS) and tanshinone I (TSI), are the three major
active ingredients, the potent antitumor properties of which have
been extensively investigated both in vitro and in
vivo in recent years (2).
Previous studies have suggested that CTS mainly inhibited
angiogenesis (3), while TSI mainly
inhibited migration, invasion and metastasis (4). Whereas, TSIIA significantly induced
cell apoptosis on a panel of human tumor cell lines, such as breast
cancer (5–7), glioma (8), lung cancer (9,10),
ovarian cancer (11), gastric
cancer (12), hepatoma (13), leukemia (14), prostate cancer (15), renal cell carcinoma (16), cervix carcinoma (17) and colon cancer (18). Therefore, TSIIA might have
therapeutic potential in cancer therapy. However, the molecular
mechanisms of TSIIA-induced apoptosis still remain elusive.
Lung cancer is the leading cause of cancer mortality
in USA and worldwide (19,20). It is estimated that about 585,720
Americans will die from cancer in 2014, corresponding to about
1,600 deaths per day with more than one-quarter due to lung cancer.
Approximately 87% of lung cancer cases are non-small cell lung
cancer (NSCLC) and the majority of patients presents with advanced
stage disease at diagnosis (20,21).
The available chemotherapeutics is often limited due to undesirable
drug resistance and side-effects (22). The cell line A549 was first derived
from the patient with lung cancer in 1972 (23). A549 cells are adenocarcinomic human
alveolar basal epithelial cells, which were commonly employed as
the in vitro model of NSCLC. In 2010, Chiu and Su showed
that TSIIA induced apoptosis in A549 lung cancer cells in
vitro, due to inducing the reactive oxygen species (ROS)
release and upregulating the Bax/Bcl-2 ratio (9). In 2012, Liu et al demonstrated
that TSIIA significantly inhibited tumor growth of A549 xenografts
in vivo mediated by inhibiting NQO1, an emerging and
promising therapeutic target in cancer therapy (24). As TSIIA exerted great antitumor
activity in cellular and animal lung cancer models, it might be a
promising new drug for the treatment of NSCLC.
Currently, three basic apoptotic signaling pathway
have been established: mitochondria, endoplasmic reticulum, and
death receptor, changes in which can provide rational targets for
new anticancer drugs (25).
Mitochondria could be considered as a novel target for
chemotherapies. A variety of key events in apoptosis focus on
mitochondria, including the release of caspase activators (such as
cytochrome c), changes in electron transport, loss of
mitochondrial membrane potential, and participation of pro- and
anti-apoptotic Bcl-2 family proteins (26). Furthermore, evidence suggests that
the c-jun N-terminal kinase (JNK) cascade participates in the
mitochondria-mediated death pathway in response to various chemical
and physical stresses (27). The
experiments related to some of these factors were carried out in
this study. Our data demonstrate for the first time that TSIIA
might induce the activation of JNK pathway, mediate the release of
cytochrome c, and trigger the activation of caspase-9 and
caspase-3 caspase cascade in the mitochondrial-induced apoptotic
pathway.
Materials and methods
Chemicals and reagents
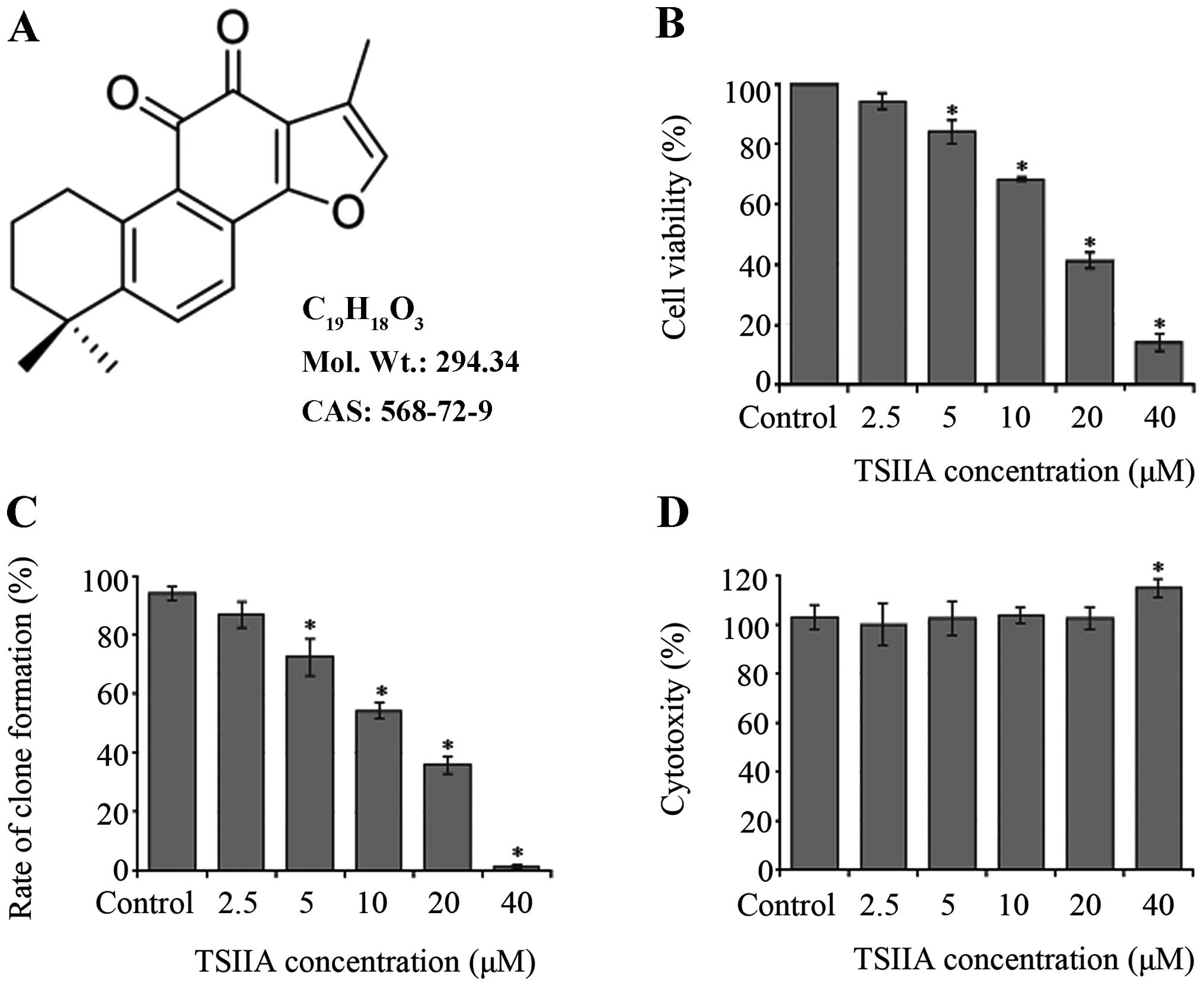
TSIIA (Fig. 1A,
>97% HPLC) was purchased from Sigma-Aldrich Chemical Co.
(Shanghai, China). A stock solution of 40 mM TSIIA was prepared in
sterilized DMSO and further diluted to appropriate concentrations
with cell culture medium immediately before use. DMSO [0.1% (v/v)]
was used as a vehicle control throughout the study.
RPMI-1640 medium, heat-inactivated fetal bovine
serum (FBS), penicillin and streptomycin were acquired from Gibco
Co., USA. Cell Counting Kit-8 (CCK-8, #CK04) was obtained from
Dojindo Laboratories (Kumamoto, Japan). Lactate dehydrogenase (LDH)
assay kit (#A020) was purchased from Jiancheng Bioengineering
Institute (Nanjing, China). JNK inhibitor SP600125 (#8177S) was
purchased from Cell Signaling Technology (Danvers, MA). Hoechst
Staining Kit (#C0003), Mito-Tracker Green (#C1048), Mitochondrial
membrane potential assay kit with JC-1 (#C2006), Cell Mitochondria
Isolation Kit (#C3601), Caspase-3 Activity Assay Kit (#C1115),
Caspase-8 Activity Assay Kit (#C1151), Caspase-9 Activity Assay Kit
(#C1157), BCA Protein Assay Kit (#P0012), BeyoECL Plus kit
(#P0018), Alexa Fluor 555-labeled goat anti-mouse IgG (H+L)
(#A0459), and all other antibodies for cytochrome c, Bax,
β-actin, and goat anti-rabbit and anti-mouse secondary antibodies
were purchased from Beyotime Institute of Biotechnology (Haimen,
China). All other chemicals were of analytic grade and commercially
available.
Cell culture
A549 human lung cancer cells were obtained from Cell
Library of Committee on Type Culture Collection of Chinese Academy
of Sciences. Cultures were maintained in 95% air and 5%
CO2 at 37°C in RPMI-1640 with 10% FBS, 2 mM L-glutamine,
100 U/ml penicillin and 100 U/ml streptomycin. To determine the
effects of JNK inhibitor (SP600125) on TSIIA-induced apoptosis in
A549 cells, cell culture was pre-incubated for 2 h with SP600125
(10 μM) before the addition of TSIIA.
CCK-8 assay
A549 cells (1×105 cells/ml in 96-well
culture plates) were treated with DMSO (0.1%) or TSIIA (2.5, 5, 10,
20 and 40 μM) for 48 h. The medium (90 μl) was incubated with 10 μl
of CCK-8 solution for 2 h at 37°C. Absorbance was read at 450 nm on
a microplate reader (Bio-Rad Model 550, Bio-Rad Laboratories,
Hercules, CA). Cell viability (%) was calculated as (experimental
absorbance - background absorbance)/(control absorbance -
background absorbance) ×100% (28). IC50 value, the
concentration of TSIIA inhibiting 50% of the cell growth at 48 h,
was calculated by the method of Reed and Muench (29).
Clone formation assay
A549 cells seeded at 500 cells/well in 6-well
culture plates were treated with DMSO (0.1%) or TSIIA (2.5, 5, 10,
20 and 40 μM) for 48 h and then maintained in the routine medium.
After 10 days incubation, cell colonies consisting of more than 50
cells stained with crystal violet were counted and pictures were
taken by a digital camera (30).
The ratio of clone formation was calculated following the equation:
rate of clone formation (%) = (clone amount/500) ×100%. The
IC50 value was then calculated.
LDH measurement
After A549 cells were exposed to DMSO (0.1%) or
TSIIA (2.5, 5, 10, 20 and 40 μM) for 48 h, each culture medium was
centrifuged at 250 × g for 10 min. Supernatant was transferred to a
96-well culture plate to determine the amount of LDH according to
the manual of the LDH assay kit (31). LDH activity is reported as
percentage relative to control level. Absorbance of samples was
measured at 450 nm.
Detection of mitochondrial
fluorescent
After treated with DMSO (0.1%) or TSIIA (20 μM) for
48 h, respectively, A549 cells were washed twice with pre-chilled
PBS. The mitochondrial green fluorescent probe (Mito-Tracker Green)
was added to a final concentration of 100 nm, and the cells were
then cultured in the dark for 30 min (32). After being washed three times with
PBS, the cells were imaged by a reflected fluorescence microscope
(Nikon MF30 LED, Japan) with excitation wavelength of 490 nm and
emission wavelength of 516 nm.
Measurement of mitochondrial membrane
potential (MMP)
After labeling cells using a Mito-Tracker Green, we
measured the change in mitochondrial membrane potential of
TSIIA-treated A549 cells. After treatment, cells were incubated
with JC-1 (2.5 μg/ml) at 37°C in dark for 20 min. After washing
twice with ice-cold dyeing working buffer, the samples were
collected and placed on ice using a method previously described
(33). The MMP changes were
evaluated by a FACSCalibur Flow Cytometer (Becton-Dickinson, San
Jose, CA). High membrane potential was associated with emission at
590 nm (red), and low membrane potential at 530 nm (green) when
excited at 488 nm. MMP was determined by a ratio of fluorescence
intensity at 590 nm to that at 530 nm.
Immunofluorescent labeling
A549 cells were seeded on sterile cover glasses
placed in the 6-well plates the day before treatment with DMSO
(0.1%) or TSIIA (20 μM) for 48 h. After fixation with 4%
formaldehyde, cells were permeabilized for 15 min in 1% Triton
X-100 in PBS. The cells were incubated with the mouse
anti-cytochrome c antibody overnight at 4°C and then
incubated with Alexa Fluor 555-labeled goat anti-mouse IgG (H+L)
antibody for 2 h at room temperature. The nucleus was stained with
Hoechst 33258 for 20 min in the dark (34). Images were observed under a
reflected fluorescence microscope (Nikon MF30 LED, Japan).
Mitochondrial and cytosolic
fractionation
After treatment with DMSO (0.1%) or TSIIA (20 μM)
for 48 h, respectively, A549 cells were incubated in 100 μl
ice-cold mitochondrial lyses buffer on ice for 10 min. Cell
suspension was then taken into a glass homogenizer and homogenized
for 30 strokes using a tight pestle on ice. The homogenate was
centrifuged at 600 × g for 10 min at 4°C to remove nuclei and
unbroken cells. Then the supernatant was collected and centrifuged
again at 12,000 × g for 30 min at 4°C to obtain the cytosol
(supernatant) and mitochondria (deposition) fraction (35). Protein concentrations were
determined using BCA Protein Assay Kit.
Western blot analysis
Release of cytochrome c from the mitochondria
to cytosol and translocation of Bax from cytosol to mitochondria
were measured by western blot analysis as previously described
(36). The signal was visualized
using a BeyoECL Plus kit. The level of β-actin was used to
normalize the amount of the protein of interest. The density of the
band was expressed relative to the density in vehicle control
cells.
Caspase activity measurement
Lysates of A549 cells were prepared after treatment
with TSIIA (10 or 20 μM) for 48 h. In some cases, cells were
pretreated for 2 h with SP600125 (10 mM) before TSIIA treatment.
Caspase-3, -8, -9 activity assays were performed on 96-well
microplates by incubating 10 μl cell lysate in 80 μl reaction
buffer containing 10 μl caspase substrate (2 mM). Lysates were
incubated at 37°C for 4 h. Samples were measured with a microplate
reader at an absorbance of 405 nm. Caspase activity was expressed
as the ratio of treated to vehicle control cells (37).
Statistical analysis
All data were obtained from three independent assays
(n=3). Values were expressed as mean ± standard deviation (SD).
Statistical analyses of the data between treated and control cells
were performed by Student’s t-test and one-way analysis of variance
(ANOVA). A p-value <0.05 was considered statistically
significant.
Results
TSIIA inhibits the proliferation of A549
cells
The number of viable cells was assessed by CCK-8
assay. Treatment with TSIIA (2.5, 5, 10, 20 and 40 μM) resulted in
a dose-dependent suppression of cell viability (Fig. 1B, p<0.05). IC50 value
of TSIIA on A549 cells was 16.0±3.7 μM at 48 h.
Also, clone formation assay was done to determine a
long-term effect of TSIIA on A549 cell growth. The results of
colony images showed that the rate of clone formation was reduced
in a dose-dependent manner (Fig.
1C, p<0.05). IC50 value was 14.5±3.3 μM with 10
days incubation after TSIIA treatment for 48 h.
Leakage of LDH to the cell culture medium indicates
cell membrane damage. Our LDH assay detects the amount of LDH
released by cells with damaged membranes as indicator of necrosis.
Except 40 μM, treatment with TSIIA (2.5, 5, 10 and 20) did not
affect the concentration of LDH in the supernatant of culture
medium (Fig. 1D, p>0.05). It
suggested that the anti-proliferative effect of TSIIA on A549 cells
was not due to cytotoxicity at the TSIIA dose of 10 and 20 μM, and
these concentrations were used in further analysis.
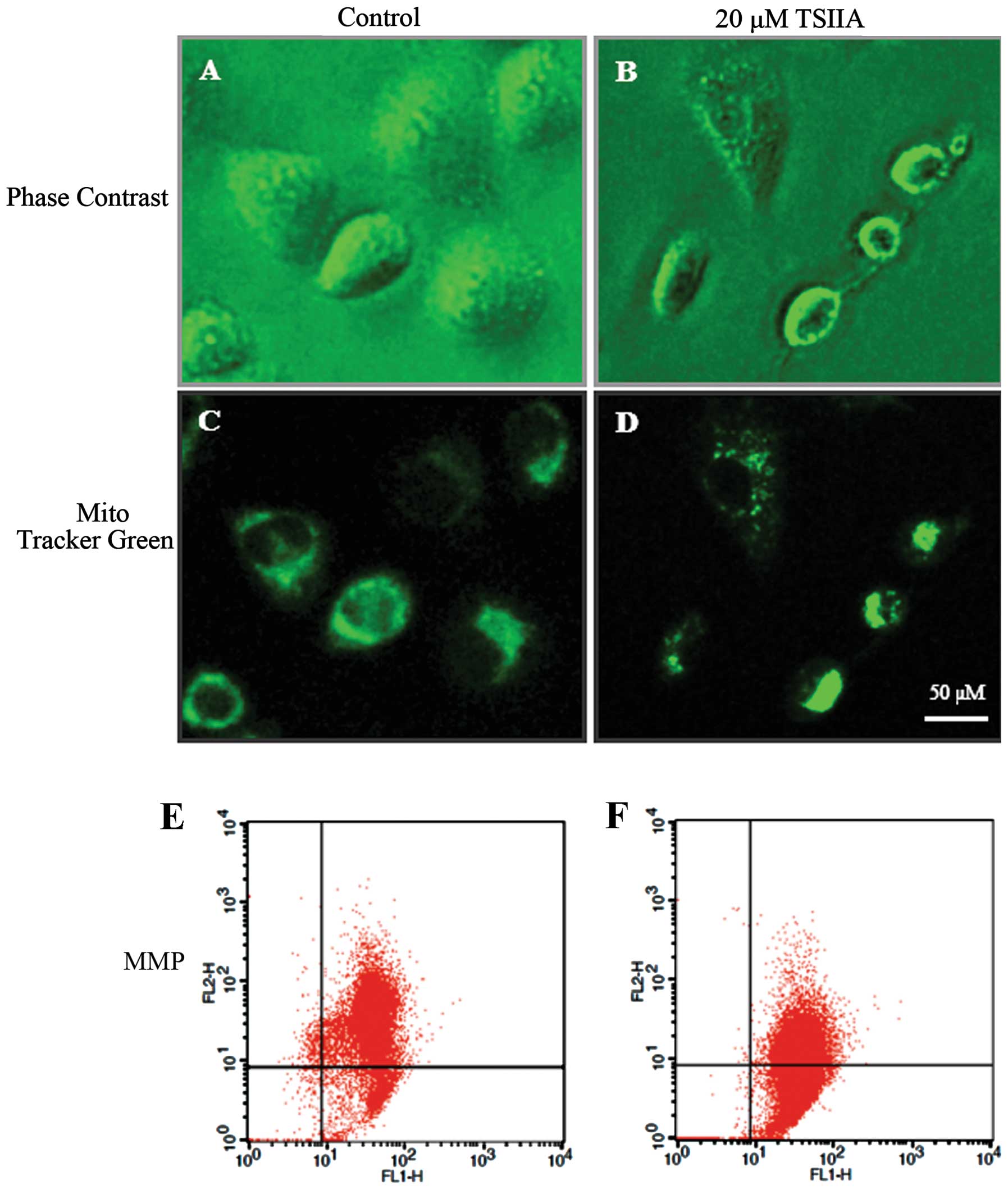
TSIIA causes mitochondrial morphological
changes and MMP loss
The change of mitochondrial morphology and
decreasing of MMP are associated with mitochondrial damage linked
to apoptosis (38). Thus, we next
evaluated the effect of TSIIA on mitochondria. Cell shrinkage and
cytoplasm vacuolization were observed in TSIIA-treated A549 cells
(Fig. 2A and B). As one of three
results shown in Fig. 2D and F,
after treatment with DMSO (0.1%) or TSIIA (20 μM), the JC-1
red/green fuorescent ratio in A549 cells was 5.83±0.862 and
1.14±0.156, respectively, suggesting that TSIIA induces a marked
decrease of MMP in A549 cells (p<0.05). Compared to JC-1, the
mitochondrial fluorescent probe Mito-Tracker Green, could track
mitochondria independent of the MMP. It was observed that after
TSIIA treatment, some mitochondria changed their filamentous
staining pattern to aggregates, some lost the green fluorescence,
which was different from the control group (Fig. 2C and D). As previous studies showed
that TSIIA significantly induced cell apoptosis on a panel of human
tumor cell lines, we thought that TSIIA could induce NSCLC A549
cell apoptosis through the mitochondria pathway.
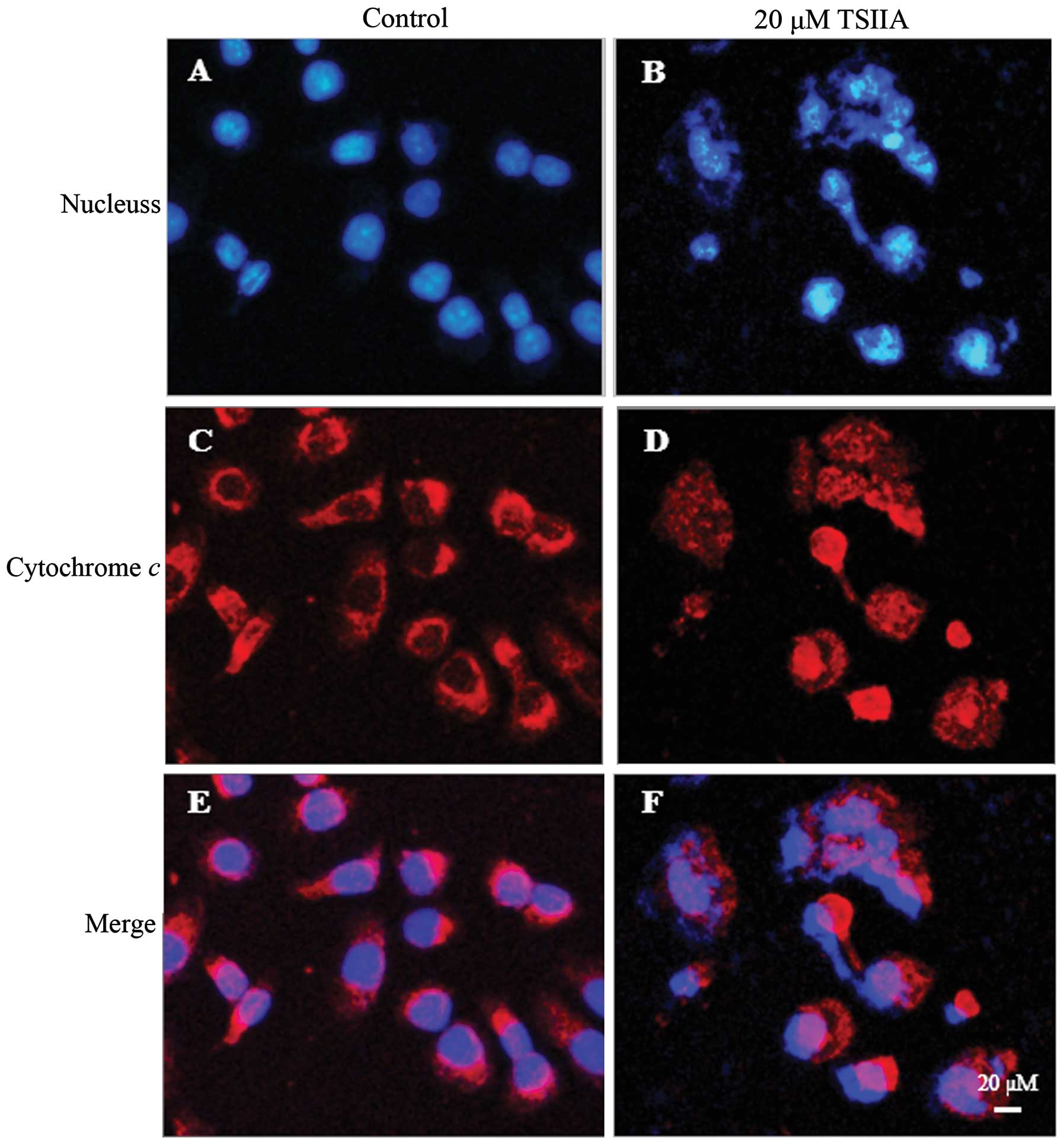
TSIIA induces cytochrome c release into
the cytosol and Bax translocation into mitochondria
The release of cytochrome c from mitochondria
into the cytosol is one of the major apoptosis pathways (39). To elucidate whether cytochrome
c release in TSIIA-induced apoptosis, we determined the
subcellular localization of cytochrome c by
immunofluorescent labeling. As shown in Fig. 3, the staining pattern became
diffuse in most cells treated with 20 μM TSIIA for 48 h, consistent
with a translocation of cytochrome c into the cytosol,
whereas, cytochrome c displayed a dotted pattern in
untreated cells, consistent with its location within the
mitochondria. The condensation and fragmentation of the chromatin
of the nucleus was also observed by Hoechst 33258 in these cells
with diffuse cytochrome c staining.
It has been well documented that during
mitochondria-mediated apoptosis, Bax can translocate from cytosol
to mitochondria, causing the release of cytochrome c
(40). By cell fractionation-based
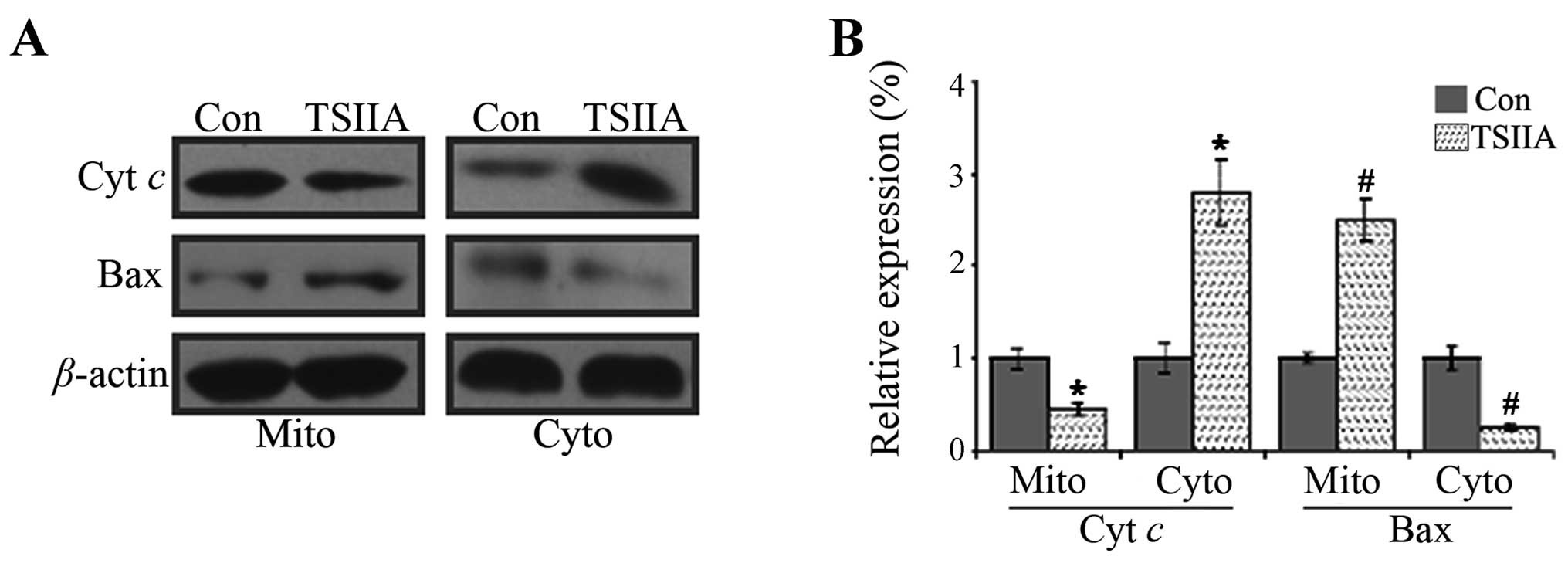
western blot assay, the results showed that Bax level reduced and
cytochrome c level increased in the cytosol fraction, while
Bax level increased and cytochrome c level decreased in the
mitochondrial fraction in A549 cells treated with 20 μM TSIIA for
48 h (Fig. 5, p<0.05).
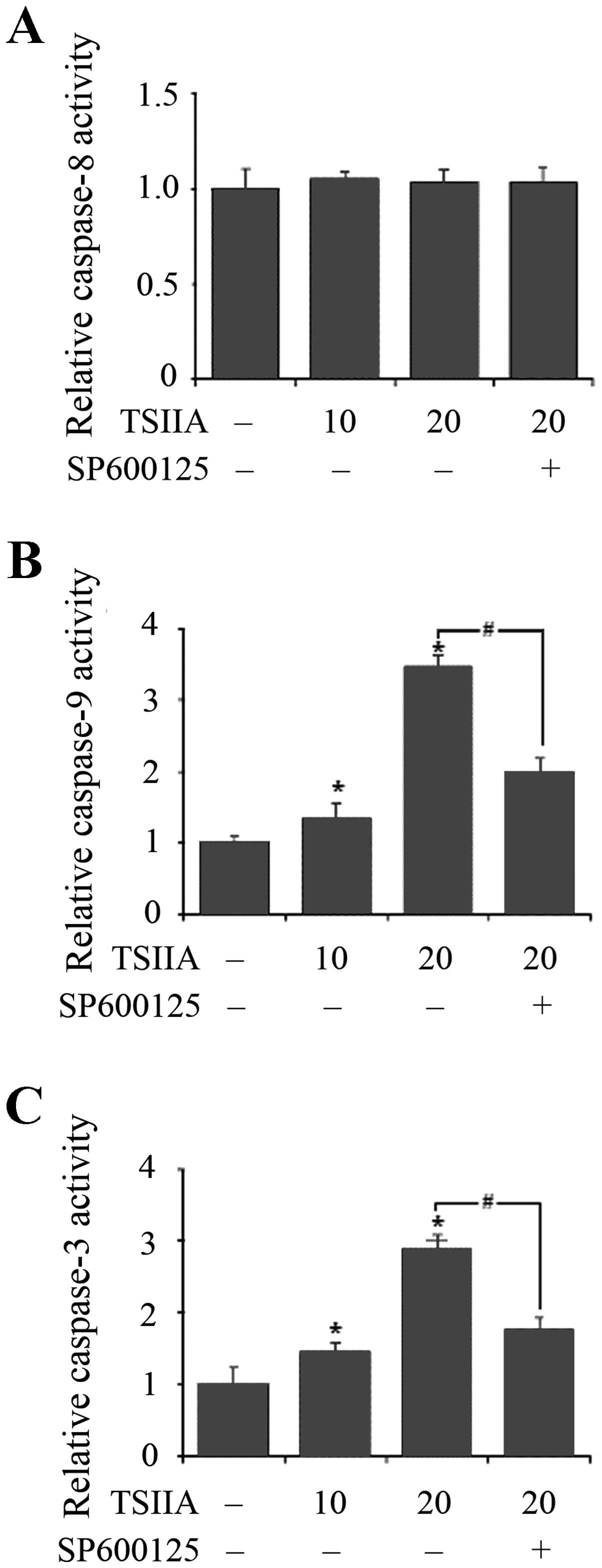
TSIIA activates the caspase-9 and
caspase-3 cascade
The caspase cascade is crucial for apoptotic signal
transduction. Cytosolic cytochrome c induces
caspase-9-dependent activation of caspase-3 (41). The working principles of Caspase-3,
-8 or -9 Activity Assay Kit are based on the cleavage of the
following substrates: acetyl-Asp-Glu-Val-Asp p-nitroanilide
(Ac-DEVD-pNA), acetyl-Ile-Glu-Thr-Asp p-nitroanilide (Ac-IETD-pNA)
and acetyl-Leu-Glu-His-Asp p-nitroanilide (Ac-LEHD-pNA). Activity
of caspase-9 and caspase-3 was shown to be dose-dependently
increased with treatment of TSIIA for 48 h in A549 cells
(p<0.05), but activity of caspase-8 was not significantly
induced (p>0.05).
The JNK pathway plays a pivotal role in stress
responses and induces apoptosis in response to various stimuli and
the absence of JNK caused a defect in the mitochondrial death
signaling pathway, including the failure to release cytochrome
c (42). To detect whether
TSIIA-induced mitochondria apoptosis related to JNK pathway,
effects of SP600125 (10 μM, JNK inhibitor) on TSIIA-induced caspase
activation were also assessed. The results showed that inhibition
of JNK signaling by adding SP600125 significantly blocked caspase-9
and caspase-3 activation in A549 cells with TSIIA treatment
(Fig. 5, p<0.05). This strongly
suggested that JNK pathway performed a crucial function in
TSIIA-induced apoptosis in A549 cells, accompanied by the
activation of caspase-9 and caspase-3.
Discussion
TSIIA was originally isolated and identified from
the traditional Chinese medicinal herb Dan-Shen. More than 40
tanshinones and their analogs have been isolated since the 1930s,
which exhibit various biological activities of
anti-atherosclerosis, cardioprotection, neuroprotection and
antitumor effects (43). Studies
in the past decade suggest that TSIIA hits the model of
‘one-drug-multi-target-multi-disease’, which is shared by
traditional Chinese medicines (TCM), such as berberine (44). TSIIA exhibited broad antitumor
activity towards cancer cell lines of different origins, including
H146 (10), A549 (9), COC1/DDP (11), BEL-7402 (13), U-937 (14), MDA-MB-231 (7), 786-O (16), LNCaP (15) and CaSki (17). However, the precise mechanism of
the antitumor activity of TSIIA has remained unclear. For new drug
development to treat human lung cancer, we evaluated the mechanism
of apoptosis of TSIIA on NSCLC A549 cells in vitro.
Consistent with previous studies, the proliferation
of A549 cells was significantly inhibited by exposure to various
concentrations (2.5, 5, 10, 20 and 40 μM) of TSIIA in a
dose-dependent manner by both CCK-8 assay (Fig. 1B) (9) and clone formation assay (Fig. 1C). The IC50 value
(14.5±3.3 μM) obtained by clone formation assay was lower than that
of CCK-8 assay (16.0±3.7 μM). In contrast to CCK-8 assay, clone
formation is a very convenient and highly sensitive assay. With
this long-term cell clonogenic ability assay, we revealed that
TSIIA-treated A549 cells might perform an irreversible loss of
long-term growth ability. Also, the results of LDH assay (Fig. 1D), show that the concentrations of
TSIIA with 10 and 20 μM were without any obvious necrosis.
Mitochondria, which generate 80% of the energy
required for cellular activities, are important sites of
respiration and oxidative phosphorylation in the cell. To gain
insight into the molecular mechanism involving TSIIA-induced
apoptosis in A549 cells, some key factors in mitochondria-mediated
apoptosis were assessed. MMP is an important parameter of
mitochondrial function and has been used as an indicator of cell
health. The loss of MMP is a hallmark for apoptosis (26). In this study, after 48 h with TSIIA
(20 μM), the mitochondria morphological damage of A549 cells was
present and the MMP was decreased (Fig. 2).
Accumulating evidence indicates that mechanism by
which TSIIA induced apoptosis associated with expression of the
Bcl-2 family proteins (9). We
focused our efforts on one cardinal member of this family that are
known to be pro-apoptotic Bax. In our study, Bax, localized in the
outer mitochondrial membrane, was downregulated. This event might
result in losing mitochondrial membrane potential and releasing
cytochrome c from mitochondria into the cytosol, which can
lead to irreversible commitment of A549 cells to apoptosis through
the mitochondrial pathway (Figs. 3
and 4).
Furthermore, whether TSIIA could trigger apoptosis
in the caspase cascade through the release of cytochrome c,
activities of some important caspases were studied. Caspase-8 and
caspase-9 are the key initiator caspases of the death receptor
pathway and the mitochondrial pathway, respectively, that cleave
and activate downstream effector caspases such as caspase-3
inducing apoptosis (26). Our
results showed that TSIIA dose-dependently triggered the activity
of caspase-3 and caspase-9 without significantly inducing the
activity of caspase-8, which led to apoptosis in A549 cells through
the mitochondrial pathway (Fig.
5).
Finally, we assessed whether TSIIA can induce
apoptosis in the JNK-dependent pathway or the JNK-independent
pathway. Previous research showed that Bax was essential for
apoptotic signal transduction by JNK (45,46)
and the release of cytochrome c from mitochondria was
involved in apoptosis induced by JNK activation (42). We confirmed TSIIA induced apoptosis
with cytochrome c release from mitochondria and Bax
translocation to mitochondria. However, the relationship between
TSIIA-induced apoptosis and JNK pathway has not been reported. In
our study, SP600125, an inhibitor of JNK pathway, inhibited the
TSIIA-induced activity of caspase-9 and caspase-3 (Fig. 5B and C). Although our present data
did not confirm whether another pathway performed this function,
our findings here indicated that in response to TSIIA, the JNK
pathway might give rise to apoptosis of A549 cells.
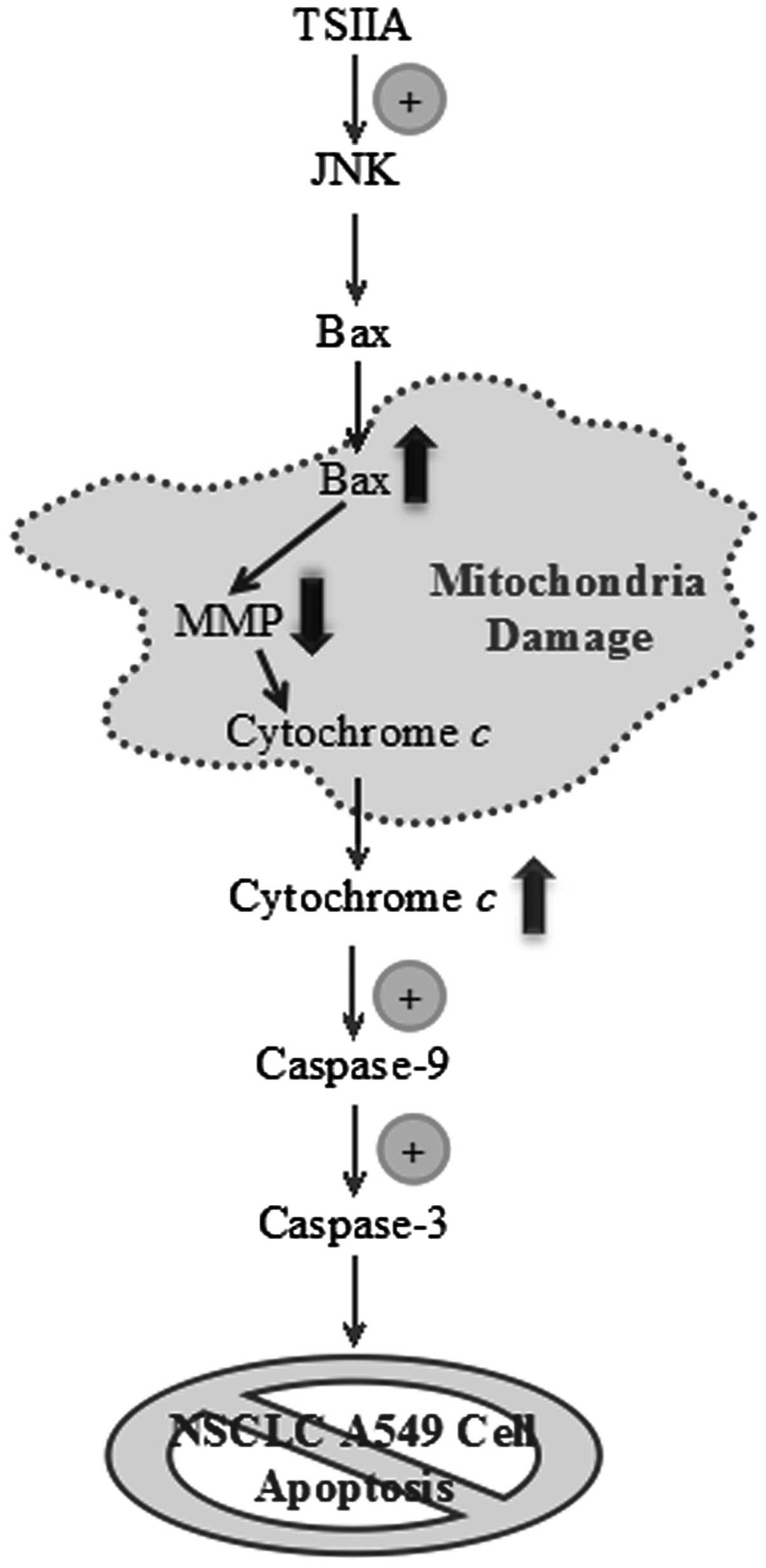
Altogether these studies revealed for the first time
a regulatory mechanism of TSIIA-induced apoptosis, which was
associated, at least in part, with the activation of JNK signaling
and caspase cascade mediated by the release of cytochrome c
(Fig. 6). Given the fact that
TSIIA has been safely used in the clinic (mostly in China)
(2,47), TSIIA may be a novel therapeutic for
treating NSCLC.
Acknowledgements
This study was supported by the research fund of
Harbin Medical University, P.R. China.
References
|
1
|
Xu S and Liu P: Tanshinone II-A: new
perspectives for old remedies. Expert Opin Ther Pat. 23:149–153.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian XH and Wu JH: Tanshinone derivatives:
a patent review (January 2006 - September 2012). Expert Opin Ther
Pat. 23:19–29. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hur JM, Shim JS, Jung HJ and Kwon HJ:
Cryptotanshinone but not tanshinone IIA inhibits angiogenesis in
vitro. Exp Mol Med. 37:133–137. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee CY, Sher HF, Chen HW, et al:
Anticancer effects of tanshinone I in human non-small cell lung
cancer. Mol Cancer Ther. 7:3527–3538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang X, Wei Y, Yuan S, et al: Potential
anticancer activity of tanshinone IIA against human breast cancer.
Int J Cancer. 116:799–807. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lu Q, Zhang P, Zhang X and Chen J:
Experimental study of the anticancer mechanism of tanshinone IIA
against human breast cancer. Int J Mol Med. 24:773–780.
2009.PubMed/NCBI
|
|
7
|
Su CC, Chien SY, Kuo SJ, Chen YL, Cheng CY
and Chen DR: Tanshinone IIA inhibits human breast cancer MDA-MB-231
cells by decreasing LC3-II, Erb-B2 and NF-κBp65. Mol Med Rep.
5:1019–1022. 2012.PubMed/NCBI
|
|
8
|
Wang J, Wang X, Jiang S, et al: Growth
inhibition and induction of apoptosis and differentiation of
tanshinone IIA in human glioma cells. J Neurooncol. 82:11–21. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chiu TL and Su CC: Tanshinone IIA induces
apoptosis in human lung cancer A549 cells through the induction of
reactive oxygen species and decreasing the mitochondrial membrane
potential. Int J Mol Med. 25:231–236. 2010.PubMed/NCBI
|
|
10
|
Cheng CY and Su CC: Tanshinone IIA may
inhibit the growth of small cell lung cancer H146 cells by
upregulating the Bax/Bcl-2 ratio and decreasing mitochondrial
membrane potential. Mol Med Rep. 3:645–650. 2010.PubMed/NCBI
|
|
11
|
Jiao JW and Wen F: Tanshinone IIA acts via
p38 MAPK to induce apoptosis and the downregulation of ERCC1 and
lung-resistance protein in cisplatin-resistant ovarian cancer
cells. Oncol Rep. 25:781–788. 2011.PubMed/NCBI
|
|
12
|
Chen J, Shi DY, Liu SL and Zhong L:
Tanshinone IIA induces growth inhibition and apoptosis in gastric
cancer in vitro and in vivo. Oncol Rep. 27:523–528.
2012.PubMed/NCBI
|
|
13
|
Dai ZK, Qin JK, Huang JE, et al:
Tanshinone IIA activates calcium-dependent apoptosis signaling
pathway in human hepatoma cells. J Nat Med. 66:192–201. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu C, Li J, Wang L, et al: Analysis of
tanshinone IIA induced cellular apoptosis in leukemia cells by
genome-wide expression profiling. BMC Complement Altern Med.
12:52012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Won SH, Lee HJ, Jeong SJ, Lu J and Kim SH:
Activation of p53 signaling and inhibition of androgen receptor
mediate tanshinone IIA induced G1 arrest in LNCaP prostate cancer
cells. Phytother Res. 26:669–674. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wei X, Zhou L, Hu L and Huang Y:
Tanshinone IIA arrests cell cycle and induces apoptosis in 786-O
human renal cell carcinoma cells. Oncol Lett. 3:1144–1148.
2012.PubMed/NCBI
|
|
17
|
Pan TL, Wang PW, Hung YC, Huang CH and Rau
KM: Proteomic analysis reveals tanshinone IIA enhances apoptosis of
advanced cervix carcinoma CaSki cells through mitochondria
intrinsic and endoplasmic reticulum stress pathways. Proteomics.
13:3411–3423. 2013. View Article : Google Scholar
|
|
18
|
Liu M, Wang Q, Liu F, et al:
UDP-glucuronosyltransferase 1A compromises intracellular
accumulation and anti-cancer effect of tanshinone IIA in human
colon cancer cells. PLoS One. 8:e791722013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
20
|
Siegel R, Ma J, Zou Z and Jemal A: Cancer
statistics, 2014. CA Cancer J Clin. 64:9–29. 2014. View Article : Google Scholar
|
|
21
|
Novello S, Milella M, Tiseo M, et al:
Maintenance therapy in NSCLC: why? To whom? Which agent? J Exp Clin
Cancer Res. 30:502011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu CP, Ohnuma S and Ambudkar SV:
Discovering natural product modulators to overcome multidrug
resistance in cancer chemotherapy. Curr Pharm Biotechnol.
12:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giard DJ, Aaronson SA, Todaro GJ, et al:
In vitro cultivation of human tumors: establishment of cell lines
derived from a series of solid tumors. J Natl Cancer Inst.
51:1417–1423. 1973.PubMed/NCBI
|
|
24
|
Liu F, Yu G, Wang G, et al: An
NQO1-initiated and p53-independent apoptotic pathway determines the
anti-tumor effect of tanshinone IIA against non-small cell lung
cancer. PLoS One. 7:e421382012. View Article : Google Scholar
|
|
25
|
Danial NN and Korsmeyer SJ: Cell death:
critical control points. Cell. 116:205–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Green DR and Reed JC: Mitochondria and
apoptosis. Science. 281:1309–1312. 1998. View Article : Google Scholar
|
|
27
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000. View Article : Google Scholar
|
|
28
|
Wen M, Wang H, Zhang X, et al:
Cytokine-like 1 is involved in the growth and metastasis of
neuroblastoma cells. Int J Oncol. 41:1419–1424. 2012.PubMed/NCBI
|
|
29
|
Devignat R: Calculation of Reed and
Muench’s 50 percent point in survival time measured in a recording
cage. Ann Inst Pasteur (Paris). 83:372–380. 1952.
|
|
30
|
Franken NA, Rodermond HM, Stap J, et al:
Clonogenic assay of cells in vitro. Nat Protoc. 1:2315–2319. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang CC, Aronstam RS, Chen DR and Huang
YW: Oxidative stress, calcium homeostasis, and altered gene
expression in human lung epithelial cells exposed to ZnO
nanoparticles. Toxicol In Vitro. 24:45–55. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu F, Yu H, Liu J and Cheng L:
Pyrroloquinoline quinone inhibits oxygen/glucose
deprivation-induced apoptosis by activating the PI3K/AKT pathway in
cardiomyocytes. Mol Cell Biochem. 386:107–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao J, Chen X, Lin W, et al: Total
alkaloids of Rubus aleaefolius Poir inhibit hepatocellular
carcinoma growth in vivo and in vitro via activation
of mitochondrial-dependent apoptosis. Int J Oncol. 42:971–978.
2013.
|
|
34
|
Gao LW, Zhang J, Yang WH, Wang B and Wang
JW: Glaucocalyxin A induces apoptosis in human leukemia HL-60 cells
through mitochondria-mediated death pathway. Toxicol In Vitro.
25:51–63. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Z, Wang S, Qiu H, Duan C, Ding K and
Wang Z: Waltonitone induces human hepatocellular carcinoma cells
apoptosis in vitro and in vivo. Cancer Lett. 286:223–231. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Viedma-Rodriguez R, Baiza-Gutman LA,
Garcia-Carranca A, Moreno-Fierros L, Salamanca-Gomez F and
Arenas-Aranda D: Suppression of the death gene BIK is a critical
factor for resistance to tamoxifen in MCF-7 breast cancer cells.
Int J Oncol. 43:1777–1786. 2013.
|
|
37
|
Zhao D, Lin F, Wu X, et al: Pseudolaric
acid B induces apoptosis via proteasome-mediated Bcl-2 degradation
in hormone-refractory prostate cancer DU145 cells. Toxicol In
Vitro. 26:595–602. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chipuk JE, Bouchier-Hayes L and Green DR:
Mitochondrial outer membrane permeabilization during apoptosis: the
innocent bystander scenario. Cell Death Differ. 13:1396–1402. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Newmeyer DD and Ferguson-Miller S:
Mitochondria: releasing power for life and unleashing the
machineries of death. Cell. 112:481–490. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Finucane DM, Bossy-Wetzel E, Waterhouse
NJ, Cotter TG and Green DR: Bax-induced caspase activation and
apoptosis via cytochrome c release from mitochondria is inhibitable
by Bcl-xL. J Biol Chem. 274:2225–2233. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Budihardjo I, Oliver H, Lutter M, et al:
Biochemical pathways of caspase activation during apoptosis. Annu
Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar
|
|
42
|
Tournier C, Hess P, Yang DD, et al:
Requirement of JNK for stress-induced activation of the cytochrome
c-mediated death pathway. Science. 288:870–874. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong Y, Morris-Natschke SL and Lee KH:
Biosynthesis, total syntheses, and antitumor activity of
tanshinones and their analogs as potential therapeutic agents. Nat
Prod Rep. 28:529–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tillhon M, Guaman Ortiz LM, Lombardi P and
Scovassi AI: Berberine: new perspectives for old remedies. Biochem
Pharmacol. 84:1260–1267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsuruta F, Sunayama J, Mori Y, et al: JNK
promotes Bax translocation to mitochondria through phosphorylation
of 14–3-3 proteins. EMBO J. 23:1889–1899. 2004.PubMed/NCBI
|
|
46
|
Lei K, Nimnual A, Zong WX, et al: The Bax
subfamily of Bcl2-related proteins is essential for apoptotic
signal transduction by c-Jun NH(2)-terminal kinase. Mol Cell Biol.
22:4929–4942. 2002. View Article : Google Scholar
|
|
47
|
Bi HC, Zuo Z, Chen X, et al: Preclinical
factors affecting the pharmacokinetic behaviour of tanshinone IIA,
an investigational new drug isolated from Salvia
miltiorrhiza for the treatment of ischaemic heart diseases.
Xenobiotica. 38:185–222. 2008. View Article : Google Scholar : PubMed/NCBI
|