Introduction
Non-small cell lung cancer (NSCLC) accounts for
approximately 85% of all lung cancers diagnosed (1), with less than 15% of patients
surviving beyond 5 years due to lack of early diagnosis and
effective treatment methods (2,3).
Despite the advances in multimodal neoadjuvant chemotherapy and
radiotherapy in treatment NSCLC, the outcome remains unsatisfactory
because these therapies are toxic and almost never curative of
metastatic NSCLC (4). Thus, this
has highlighted the necessity for new therapeutic modalities,
particularly for patients whose disease does not respond to
conventional therapy.
Human toll-like receptors (TLRs) were first
identified in mammalian immune cells, and belong to type I
transmembrane proteins family consisting of an extracellular domain
with a leucine-rich repeat region and an intracellular domain
homologous to that of the human interleukin (IL)-1 receptor
(5). TLRs have a powerful capacity
to innate immune responses (6)
through recognition of pathogen-associated molecular patterns
(PAMP) expressed by viruses and bacteria, or host-derived PAMPs
(7). Until now, 11 mammalian TLRs
have been identified and found to be involved in the recognition of
PAMPs (8,9). TLR-4 is an important member of type I
transmembrane proteins family. Recently, growing evidence has shown
TLR4 in various tumors (10–12),
including head and neck, lung, gastrointestinal, liver, pancreatic,
skin, breast, ovarian, cervical and prostate cancer (13). Although the TLR-4 profile varies in
different tumor cells, current evidence indicates that the
expression of TLR-4 and signaling cascade are involved in tumor
growth, progression and invasion (14). For example, TLR-4 and signaling
increased COX-2 and PGE2 signaling and early colorectal
carcinogenesis, inhibited apoptosis and promoted angiogenesis
(15). TLR4 increases Nanog gene
expression, which induces liver oncogenesis (16). TLR4-mediated cancer growth involved
in breast tumor progression and downregulation of TLR4 prevented
breast cancer progression and survival (17). TLR4 expressed on human lung cancer
cells is functionally active, and may play important roles in
promoting immune escape of human lung cancer cells by inducing
immunosuppressive cytokines and apoptosis resistance (18). TLR4 acts as a functional receptor
in the pre-metastatic phase in pulmonary metastasis (19). These studies implied that TLR4 are
widely expressed on human tumor cells and may play important roles
in the initiation and progression of cancer. However, to our
knowledge, the effect of TLR4 expression on tumorigenicity in lung
cancer in vivo and in vitro has been insufficiently
reported (18,20), not fully clarifying the of TLR4 in
NSCLC (20).
Therefore, in the present study we investigated the
expression of TLR4 in NSCLC, and analyzed its association with the
occurrence and development of NSCLC. In addition, we also developed
a plasmid vector expressing siRNA-directed against TLR4 to
investigate the key roles in tumor growth in vitro and in
vivo for NSCLC.
Materials and methods
Tissue samples and clinical
information
All patients gave written informed consent to
participate in the study. This study was approved by the Ethics
Committee of Jilin University, Changchun, Jilin Province,
China.
Surgical tissue samples of human tumors and
tumor-free tissues were obtained from patients undergoing surgical
resection at the First Affiliated Hospital of Jilin University
(Changchun, Jilin, China) from March, 2009 to July, 2013 after
consent was obtained from the patients. All samples were
immediately frozen in liquid nitrogen and stored at −80°C until
use. Tumor-free tissues were excised at least 5 cm away from the
tumor border. Fifty tumor tissue and tumor-free tissues of NSCLC
were classified into adenocarcinoma (24/50) and squamous cell
carcinoma (26/50) according to the criteria of WHO (1997). Clinical
information of all patients was collected including age, gender,
smoking, tumor histological type, lymph node metastasis, TNM stage
and differentiation. None of the patients received any prior
radiochemotherapy. Clinical information of study subject is given
in Table I.
 | Table IThe correlation of TLR4 expression
with clinical pathologic features of NSCLC. |
Table I
The correlation of TLR4 expression
with clinical pathologic features of NSCLC.
| Clinical
factor | Positive | Negative | P-value |
|---|
| Age | | | 0.489 |
| <60 (n=29) | 18 | 11 | |
| ≥60 (n=21) | 15 | 6 | |
| Gender | | | 0.356 |
| Man (n=26) | 17 | 9 | |
| Women (n=24) | 16 | 8 | |
| Smoke | | | 0.413 |
| No (n=31) | 20 | 11 | |
| Yes (n=19) | 13 | 6 | |
| Metastasis | | | <0.01 |
| No (n=20) | 8 | 12 | |
| N1–N2 (n=25) | 10 | 15 | |
| M1(n=5) | 5 | 0 | |
| Tumor
differentiation | | | <0.01 |
| Well (n=8) | 1 | 7 | |
| Moderate
(n=26) | 18 | 8 | |
| Poor (n=16) | 14 | 2 | |
| TNM stage | | | <0.05 |
| I–II (n=25) | 8 | 17 | |
| III–IV (n=25) | 17 | 8 | |
| Histological
type | | | 0.347 |
| Adenocarcinoma
(n=24) | 15 | 9 | |
| Squamous cell
carcinoma (n=26) | 18 | 8 | |
Immunohistochemistry
For immunohistological analyses, tissue specimens
were fixed in 10% formalin buffer at pH 7.0 for 24 h and paraffin
embedded. Lung tissue specimens were embedded in paraffin and cut
into 3-μm sections for use in immunohistochemistry. Sections were
dewaxed in xylene, rehydrated in alcohol in descending percentage,
and blocked for endogenous peroxidase and avidin/biotin activities
with 3% bovine serum albumin in 0.01 M phosphate-buffered saline
(PBS, pH 7.2). Sections were incubated with mouse monoclonal
antibody against human TLR4 (Santa Cruz Biotechnology, Santa Cruz,
CA, USA) at a dilution of 1:1,500 overnight at 4°C. Samples were
then washed in 0.01 M PBS (pH 7.2) for 5 min before incubation with
biotin-labelled rabbit anti-mouse antibody (1:1,000; ZSGB-Bio,
Beijing, China) for 2 h at 37°C. After washing with PBS three
times, the immunostain was visualized with a
streptavidin-peroxidase reaction system (Wuhan Boster Biological
Technology Ltd, Wuhan, China), and developed with diaminobenzidine
hydrogen peroxide (Wuhan Boster Biological Technology Ltd).
The intensity of the staining was graded as: 0, if
no immunoreactive cells were observed (negative); 1+, if the
proportion of immunoreactive cells was <25%; 2+, if the
proportion of immunoreactive cells was 25–75%; and 3+, if the
proportion of immunoreactive cells was >75%. Values of 0 and 1
were considered to indicate negative staining, and 2 and 3 were
considered to indicate positive staining.
Cell culture
The human non-small cell lung cancer cell line A549
was purchased from Cell Bank of Type Culture Collection of Chinese
Academy of Sciences, Shanghai Institute of Cell Biology, Chinese
Academy of Sciences (Shanghai, China). A549 cells were cultured in
RPMI-1640 medium (Invitrogen, USA) supplemented with 10% fetal
bovine serum (FBS, Gibco, USA), cells were maintained at 37°C in a
humidified atmosphere containing 5% CO2.
Construction of expressing plasmids
To inhibit the expression of TLR4, two short hairpin
RNA (shRNA) targeting the TLR4 transcript were designed and
synthesized and annealed. The synthesized oligonucleotides contain
specific target sequence, a loop, the reverse complement of the
target sequence, a stop codon for U6 promoter and two sticky ends.
The target sequence in the oligonucleotide for suppressing TLR-4
was as followed: the siRNA1 sequence is: AACTTGTATTCAAGGTC TGGC
(sense); the siRNA2 sequence is: AACTCCCTCCAGG TTCTTGAT (sense).
The scramble sequence is: AATTCTCCG AACGTGTCACGT (sense). The empty
vector (pCDNA-CMV) and scramble sequence had been tested in
multiple cell lines and did not demonstrate any toxicity to cells
as demonstrated by MTT assay after transfection, and had no effect
on the expression of housekeeping genes, GAPDH or β-actin. The
siRNA1, siRNA2 and scramble sequence were cloned into expressing
plasmid pCDNA-CMV, named as p-siRNA1, p-siRNA2 and p-scramble,
respectively.
A549 cells were transiently transfected with empty
vector (pCDNA-CMV), p-siRNA1, p-siRNA2 and p-scramble plasmid using
Lipofectamine™ 2000 reagent (Invitrogen) according to the
manufacturer’s instructions, respectively. After 72 h of
transfection, cells were collected and used for cell proliferation
assays, cell apoptosis assays, western blot analysis, real-time
quantitative PCR analysis, Matrigel invasion assay, caspase
activity assay and cell inflammatory cytokines assay. Empty plasmid
(pCDNA-CMV) acted as control group in the assays.
Quantitative PCR
Total RNA was isolated from A549 cell lines and
NSCLCs tissue using RNeasy mini kit (Qiagen, Valencia, CA, USA)
according to the manufacturer’s instructions. RNA was prepared
using the. RNA was reverse transcribed into cDNA by a Primescript™
RT reagent kit base on the manufacturer’s protocols (Takara, Dalin,
China). Quantitative real-time polymerase chain reaction (qPCR)
assays were performed with SYBR-Green Real-Time PCR Master Mix
(Toyobo, Osaka, Japan) and amplification equipment using specific
primers: TRL4 forward, 5′-CGAGGA AGAGAAGACACCAGT-3′ and reverse,
5′-CATCATCCTCA CTGCTTCTGT-3′; GAPDH forward, 5′-TGTGGGCATCAAT
GGATTTGG-3′ and reverse, 5′-ACACCATGTATTCCGGGT CAAT-3′. The PCR
conditions were as follows: a pre-denaturing at 95°C for 5 min,
followed by 40 cycles of denaturation at 95°C for 20 sec,
annealing/extension at 55°C for 20 sec, final extension 72°C for 10
min. The amplification specificity was checked by melting curve
analysis. The 2−ΔΔCT method (21) was used to calculate the relative
abundance of target gene expression generated by Boineer Exicycler™
analysis software (Bioneer Corp., Daejeon, Korea). The expression
of TLR4 were determined by normalization of the threshold cycle
(Ct) of these genes to that of the control GAPDH. Each sample was
run in triplicates.
Western blot analysis
After 72 h of transfection, A549 cells were washed
twice with PBS and lysed in radio immune precipitation assay buffer
for 30 min on ice. Cell lysates were clarified by centrifugation at
10,000 × g for 15 min, and protein concentrations were determined
using the Bradford reagent (Sigma Chemical Co., St. Louis, MO,
USA). Lysates were separated on 8 or 15% SDS-PAGE; proteins were
transferred to Immobilon membrane (Millipore, Bedford, MA, USA)
immunoblotted with specific primary antibodies and incubated with
corresponding horseradish peroxidase- conjugated secondary
antibody. Protein bands were visualized with enhanced
chemiluminescence reagent (ECL, Amersham, GE Healthcare,
Velizy-Villacoublay, France). The primary antibodies used in the
western blots were as follows: antibodies against TLR4, β-actin,
MMP-2 and MMP-9 (Santa Cruz Biotechnology); Akt, phosphorylated
(p-) Akt, PI3K and p-PI3K (Sigma Chemical Co.). Secondary Abs used
for immunodetection were: HRP-conjugated goat anti-mouse IgG and
goat anti-rabbit IgG (Amersham Biosciences, Uppsala, Sweden).
MTT assay
The cell density of A549 cells was adjusted to
5×104/ml, and cells were added to a 96-well plate (100
μl/well). In the blank controls, 100 μl of medium alone was added.
At 24 h after culture, cells were transfected with the indicated
plasmid. At 72 h after culture, 20 μl of
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT,5
mg/ml) (Sigma Chemical Co.) was added to each well followed by
incubation at 37°C for 48 h. Then, centrifugation was performed at
2,000 × g for 10 min. The supernatant was removed, and 200 μl of
DMSO was added to each well followed by shaking for 10 min.
Absorbance was measured at 570 nm test wavelength with an ELISA
multi-well spectrophotometer (Molecular Devices Corp., Sunnyvale,
CA, USA). The mean proliferation of cells without any treatment was
expressed as 100%. Growth inhibition was calculated with the
following formula (22):
inhibition rate (%) =[1−(average absorbance of experimental
group/average absorbance of blank control group)] × 100%.
Apoptosis analysis
A549 cells were cultured in 6-well plates in
RPMI-1640 medium containing 10% FBS and were treated with indicated
plasmid for 72 h. The cover slips were washed three times with PBS
(pH 7.2), then cells were stained with 100 μg/ml acridine orange
(AO) and 100 μg/ml ethidium bromide (EB) for 1 min. Cells were
observed under a fluorescence microscope (Olympus, Tokyo, Japan).
At least 200 cells were counted and the percentage of apoptotic
cells was determined. We also detected caspase-3 and caspase-8
activity by ELISA as an additional indicator of apoptosis.
Caspase activity assay
The activity of caspase-3 and caspase-8 was measured
using caspases colorimetric protease assay kits (Millipore
Corporation, Billerica, MA, USA) according to the manufacturer’s
instructions. In brief, A549 cells were treated with indicated
plasmid for 24 h. After treatment, cells were washed twice with
ice-cold PBS (pH 7.2) and harvested by centrifugation at 700 × g
for 10 min. The cell pellets were then lysed in 150 μl buffer
provided in the kit. Protein concentrations of lysates were
determined using the Lowry method (23). Then, an aliquot of lysates (80 μl)
was incubated with 10 μl substrate of each caspase at 37°C for 2 h.
Samples were analyzed at 405 nm in a microplate reader (Thermo
Fisher Scientific Inc., Waltham, MA, USA). The relative caspase
activity of the control group was taken as 100. Each sample was run
in triplicates.
Migration assay
Cell migration was determined using a scratch assay
as previous described (24). In
brief, the transfected cell lines were seeded on a 24-well plate
and allowed to reach confluence. After scratching the bottom of the
well with a pipette tip, then the monolayer of cells was washed
three times with PBS (pH 7.2) to remove the detached cells. The
remaining adherent cells were incubated in RPMI-1640 medium
containing 1% FBS for 24 h; this medium was then replaced with
RPMI-1640 medium containing 10% FBS. After 48 h, cell migration was
evaluated using bright-field microscopy. The experiments were
performed in triplicate.
Invasion assay
Invasion assay were performed using BD BioCoat™
Matrigel invasion chambers (Becton-Dickinson Labware, Bedford, MA,
USA) according to the manufacturer’s instructions. RPMI-1640 was
added to the interior of the bottom and top chamber of inserts and
allowed to hydrate for 2 h at 37°C with 5% CO2. Albumin
at 100 ng/ml was added to the bottom chamber. Next,
5×104 A549 cells transfected with indicated plasmids
were added to the top chamber of inserts and incubated at 37°C and
5% CO2 for 24 h. After incubation, cells at the bottom
surface of the insert were fixed with purity methanol for 2 min,
stained for 2 min in 1% toluidine blue supplemented with 1% borax
(all from Sigma Chemical Co.), and rinsed twice with deionized
water (distilled H2O). The cells that had invaded to the
lower side of the filter were observed under a Nikon phase-contrast
microscope and counted in >10 fields of view at ×200
magnification. The assay was done in triplicate. We also detected
MMP-2 and MMP-9 by western blot analysis as an additional indicator
of migration and invasion.
Human inflammatory cytokine assay
IL-6, IL-8 and TNF-α presence in the supernatant of
transfected cells were detected according to the instruction of
human inflammatory cytokine kit (BD™ Cytometric Bead Array,
Becton-Dickinson Labware). FACScan flow cytometer (BD) was used to
analyze samples.
Tumor xenograft assay
About 6–8 weeks old female BALB mice were obtained
from the Institute of Laboratory Animal Science, Jilin University
(Changchun, China), and were maintained under specific
pathogen-free conditions and provided with food and water ad
libitum. All animal experiments were performed in accordance
with institutional guidelines, following a protocol approved by the
Ethics Committees of the Disease Model Research Center, Jilin
University (Changchun, China).
All the animals were fed with a normal pellet diet
one week prior to the experimentation. A549 cells in exponential
growth phase were harvested and single-cell suspensions
(2×106 cells in 100 μl PBS) were injected subcutaneously
(s.c.) into the right dorsal flank of nude mice. Tumor size was
measured every 2 to 3 days, and tumor volume calculated as 0.5236 ×
width2 × length. When tumors grew to an average volume
of 75 mm3, mice were randomly divided into siRNA,
scramble group and control group (n=10 in each group), and
inoculated with 30 μg/50 μl per mouse via i.t. injection of
indicated plasmids one time a week for 21 days, respectively. Mice
were sacrificed 7 days after the final plasmid injection. Tumor
tissue was excised, measured volume and weighed. Some of the tissue
was snap-frozen immediately for immunoblotting.
Statistical analysis
Statistical analyses were undertaken using the
SPSS® statistical package, version 16.0 (SPSS Inc.,
Chicago, IL, USA) and the GraphPad Prism version 5.01 (GraphPad
Software, San Diego, CA, USA) for Windows®. All data are
expressed as mean ± SD. Statistical analysis between two samples
was performed using Student’s t-test. Statistical comparison of
more than two groups was performed using one-way ANOVA followed by
a Tukey’s post hoc test. Pearson’s correlation coefficients were
used to determine whether two prognosis related factors were
correlated to each other over all cases. P<0.05 was considered
significant.
Results
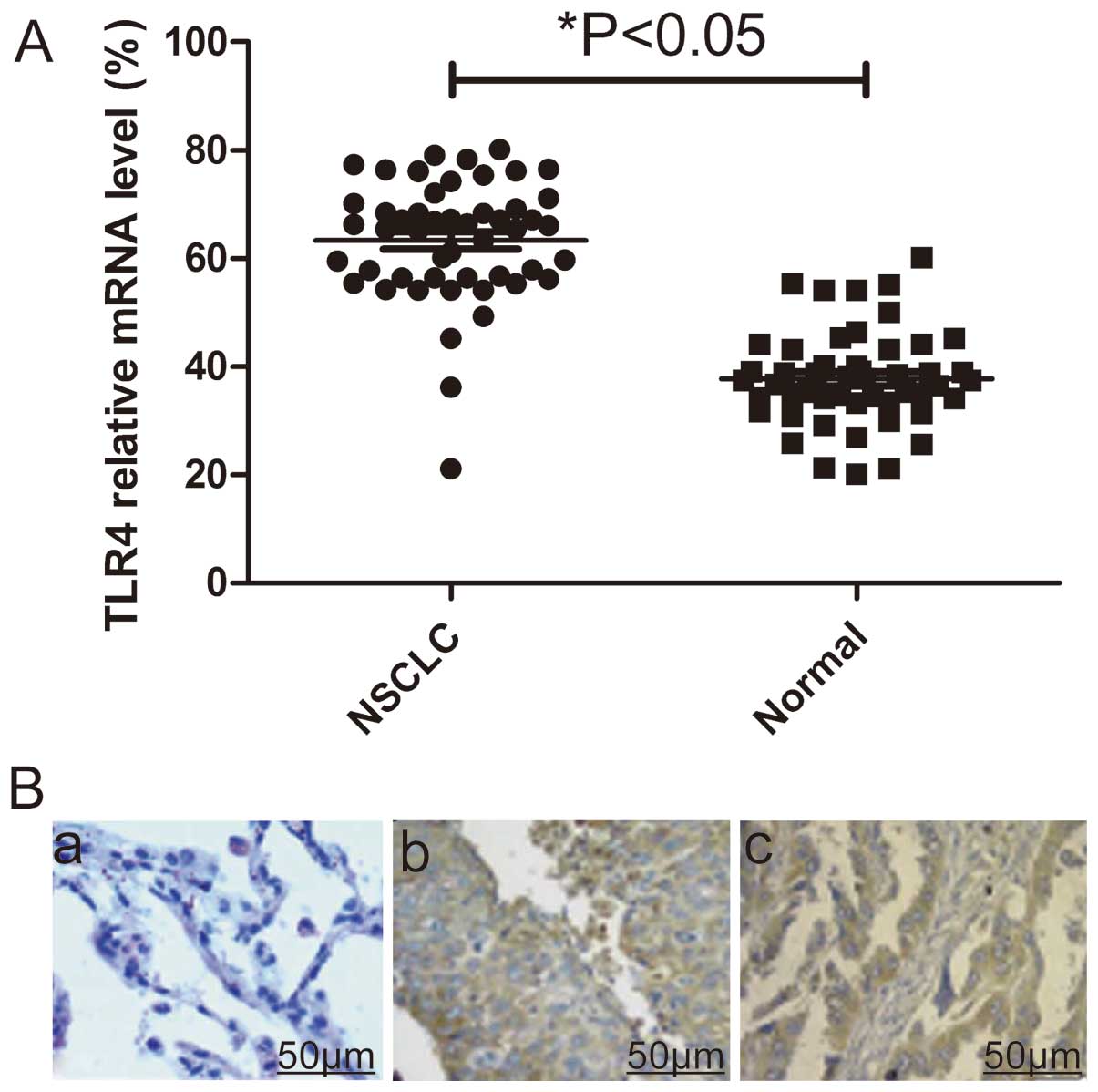
TLR4 is upregulated in NSCLC and
correlates with clinical features of patients with NSCLC
To identify the potential roles of TLR4 in the
development and progression of NSCLC, we assessed its mRNA
expression level and protein expression level in 50 pairs of
matched lung tissue samples by real-time polymerase chain reaction
(qPCR) and immunohistochemistry, respectively. Real-time polymerase
chain reaction (qPCR) assay showed that mRNA expression levels of
TLR-4 were significantly higher in NSCLC tumors compared with their
normal lung counterparts (P<0.05, Fig. 1A). At protein level, elevated
levels of TLR4 protein were found in NSCLC tumors compared with the
paired normal tissues from the same patients as shown by
immunochemical staining (Fig.
1B).
Testing the association between TLR4 immunostaining
with the clinicopathological parameters of the patients with NSCLC
showed no significant differences with regard to patient gender,
age and smoking history. The TLR4-positive tumors were of larger
size, were poorly differentiated, had a higher TNM stage and were
more likely to have metastasis than the TLR4-negative tumors
(P<0.05, Table I).
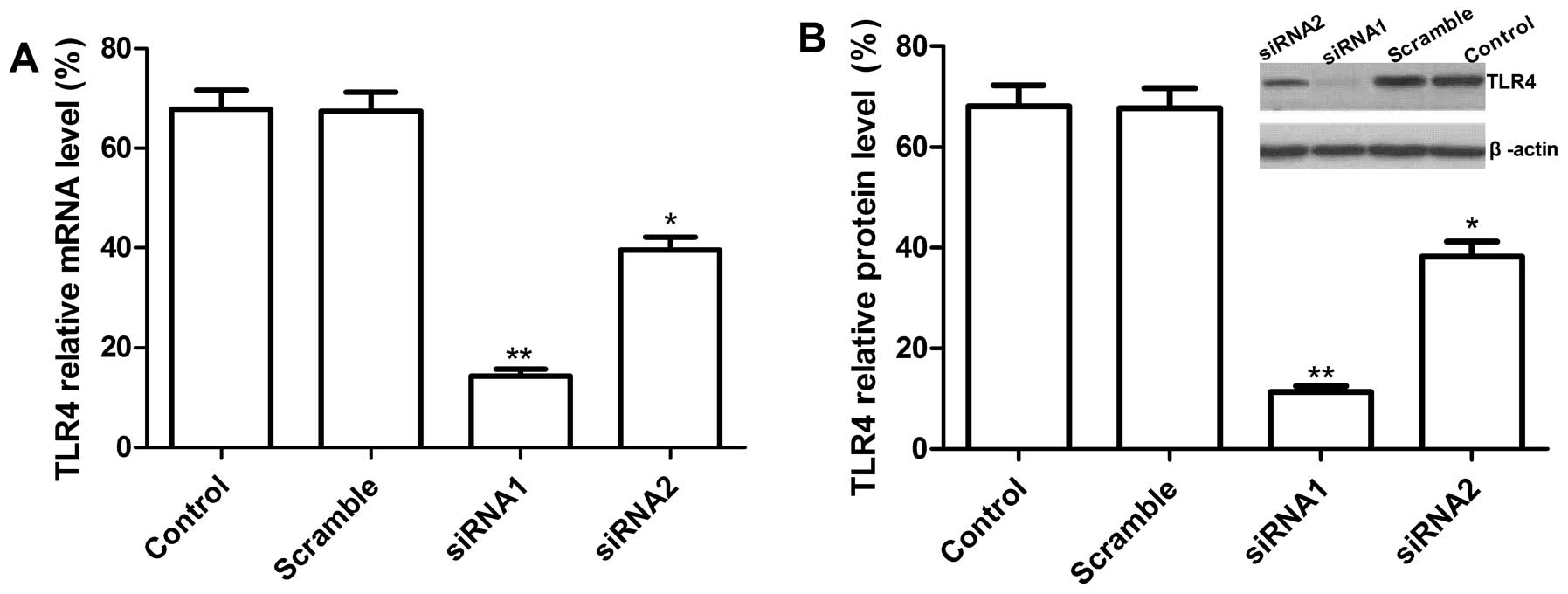
Knockdown of TLR4 gene using siRNA in
human A549 lung cancer cells
To study the biological role of TLR4 in NSCLC
progression, we first constructed pcDNA3-CMV vectors expressing two
small hairpin siRNA oligonucleotides targeting TLR4 (GenBank:
NM-138554.3) to selectively reduce TLR4 gene expression in A549
cells, then the vectors expressing TLR4 siRNA or scramble siRNA
were transfected into human lung cancer A549 cells. To determine
the effect of siRNA on the endogenous expression of TLR4, the mRNA
and protein levels of TLR4 were analyzed with real-time RT-PCR and
western blot analysis, respectively. As shown in Fig. 2A, at mRNA level, there were
different reductions in siRNA1, siRNA2 transfected cells and the
decreased expression of TLR4 at mRNA levels for siRNA1, siRNA2 was
75.1±8.5, 46.2±4.7% as compared to vector control (P<0.05).
However, no significant difference was observed in scramble and
control group (empty group) (P>0.05). At protein level, western
blot analysis results showed that two independent target sequences
siRNA1 and siRNA2 markedly decreased protein expression of TLR4
compared with the scramble and control group (P<0.05, Fig. 2B) and siRNA1 had high reduction
ratio as compared to siRNA2. Therefore, siRNA1 was chosen for use
in subsequent functional assay.
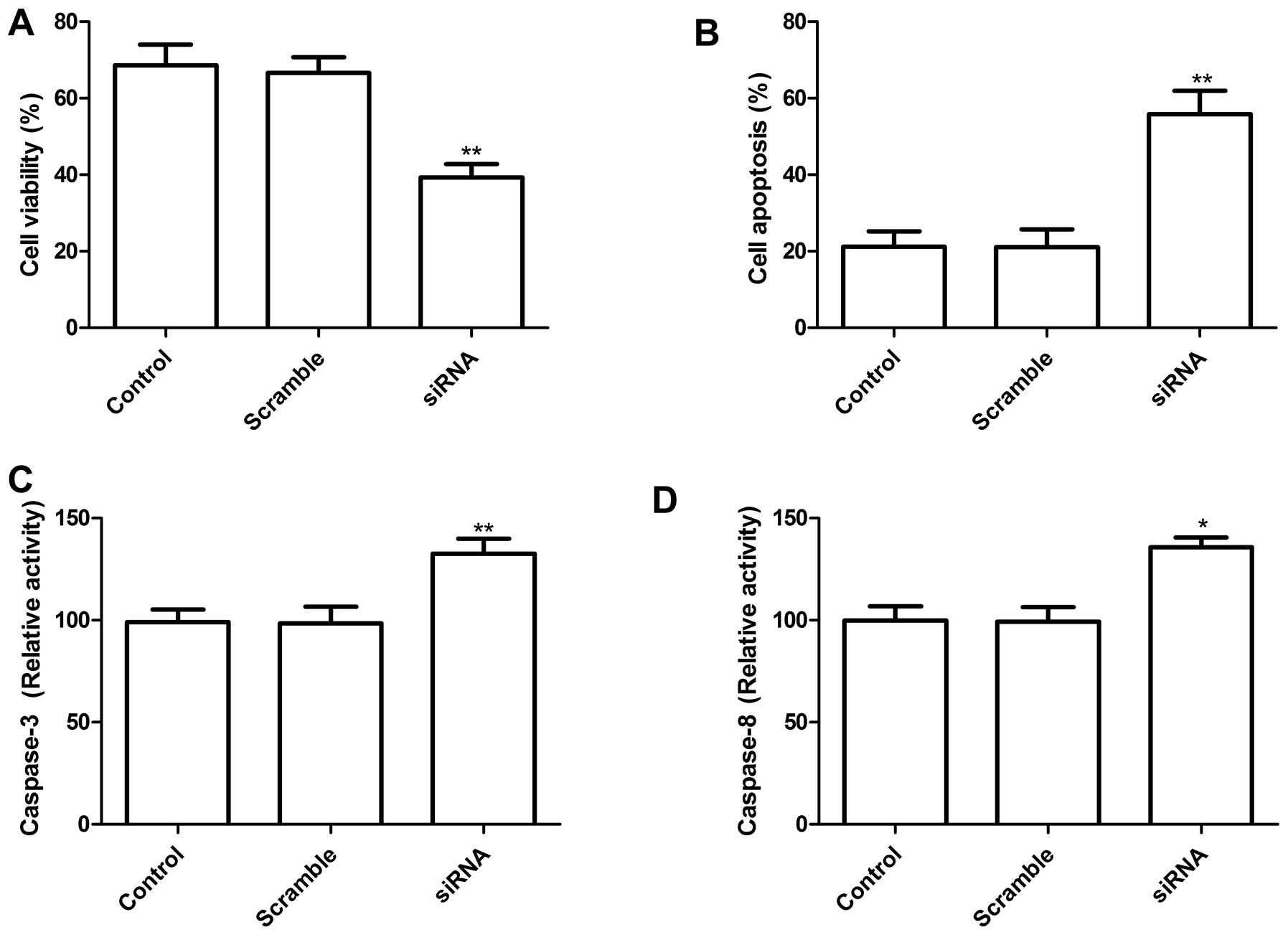
Knockdown of TLR4 in lung cells reduces
proliferation and induction of apoptosis
To determine the potential effects of siRNA-mediated
TLR4 silencing on cell proliferation and survival, MTT analysis was
performed 72 h after transfection with siRNA targeting TLR4. The
results clearly show that transfection of A549 cells with siRNA
targeting TLR4 significantly inhibited cell proliferation as
compared to control group and scramble group (P<0.01, Fig. 3A). Next, the effects of the
knockdown TLR4 by siRNA on lung cancer cell apoptosis were
assessed. As shown in Fig. 3B,
knockdown of TLR-4 induced cell apoptosis compared to control group
and scramble group (P<0.01).
To determine the potential mechanism of cell growth
inhibition in vitro, caspase-3 and caspase-8 activity was
detected using ELISA. Caspase-3 and caspase-8 activity was
significantly decreased in knockdown TLR4 treatment groups,
compared to the controls and scramble siRNA groups (P<0.05;
Fig. 3C and D). These results
suggest that reducing TLR4 levels may inhibit cell proliferation
and induce cell apoptosis in lung cancer cells.
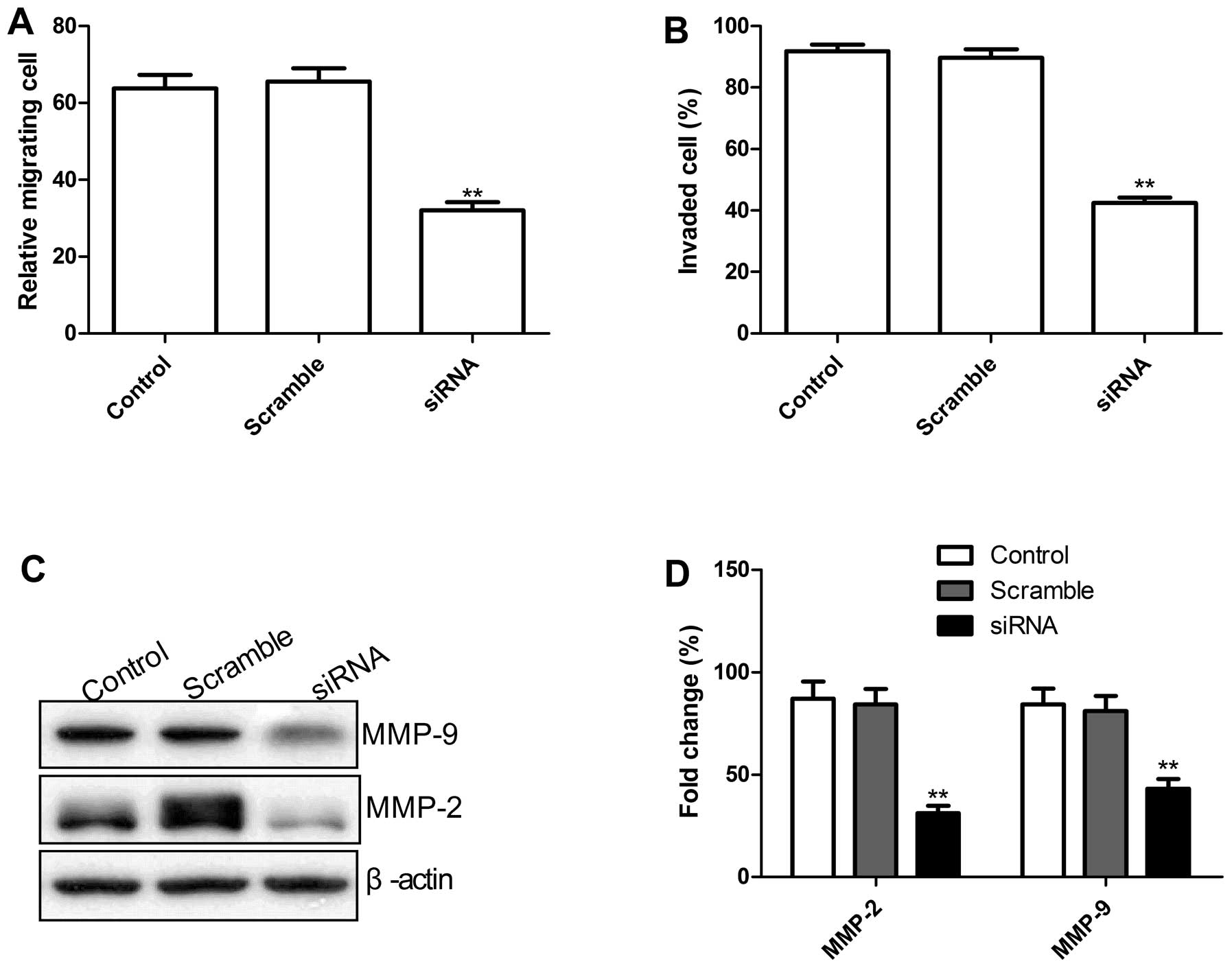
Knockdown of TLR4 in lung cells inhibits
cell migration and cell invasion
To ascertain the inhibitory effect of knockdown of
TLR4 on lung cancer on cell motility in vitro, scratch assay
was performed to investigate their effects on the migration
potential of A549 cells. As shown in Fig. 4A, knockdown TLR4 by siRNA
significantly reduced the migration capacity in A549 tumor cells
(P<0.01).
To evaluate the impact of the TLR4 knockdown on
invasion of human lung cancer cells A549, invasion assay using the
siRNA-transfected cells was performed. Our results showed that cell
invasion ability in the knockdown TLR4 group was significantly
decreased compared with controls and scramble groups, when assessed
after 48 h by the modified Boyden chamber assays (Fig. 4B).
Migration and invasion play a crucial role in tumor
metastasis. To determine the potential mechanism of the TLR4
knockdown on the inhibition of cell migration and invasion in
vitro, MMP-2 and MMP-9 protein expression was examined using
western blots. Western blot analysis displayed a significant
decrease in MMP-2, and MMP-9 proteins in the knockdown TLR4 group
infected A549 cells compared to control and Scramble groups
(Fig. 4C and D). Taken together,
these results suggest that reduction of TLR-4 on the inhibitory
effect of metastasis of lung cancer was at least partially mediated
by the downregulation of MMP-9 and MMP-2. These data indicate that
TLR4 plays an important regulatory role in tumor cell migration and
invasion.
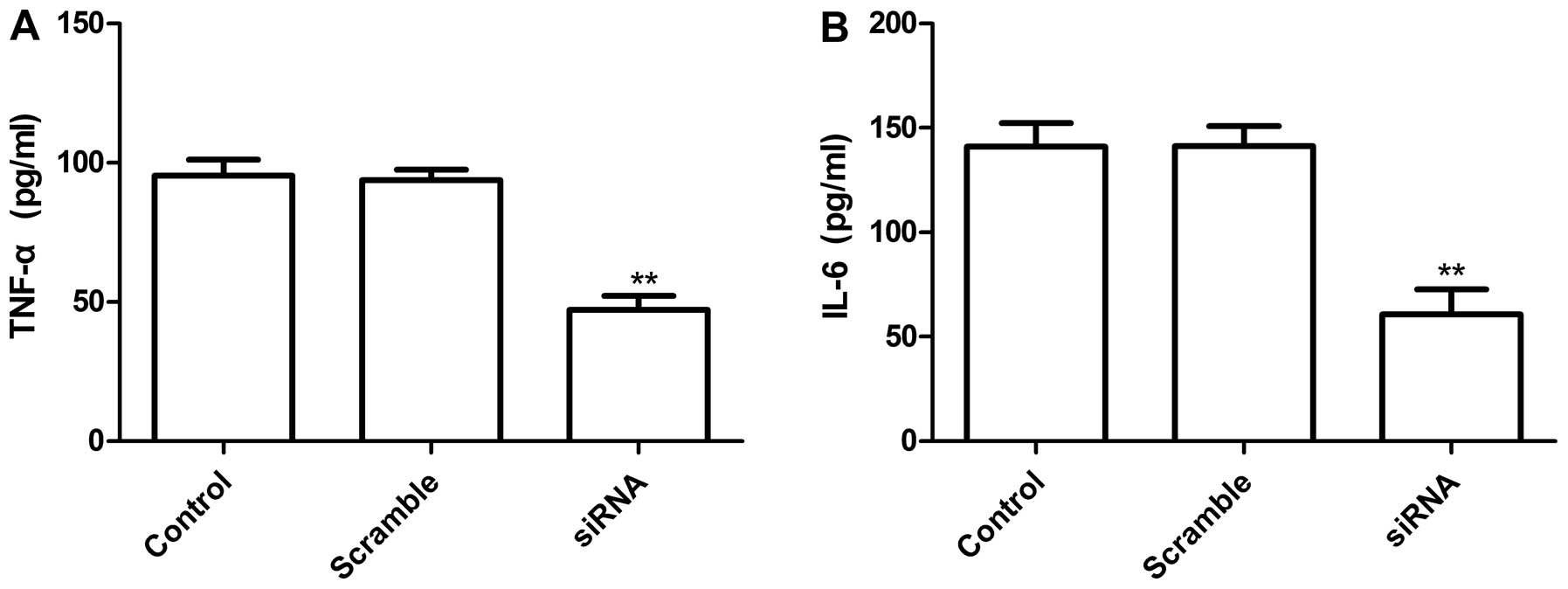
TLR4 knockdown in lung cells inhibits
TNF-α and IL-6
To examine the status of the TLR4-related
inflammatory cytokines in the lung cell line A549 with TLR4 gene
knockdown, ELISA assay was performed. Our results demonstrated that
IL-6 and TNF-α were markedly depressed in the supernatant of
silenced cells. The inhibition ration of cytokine IL-6 and TNF-α
was 45.3±3.6 and 46.1±3.5%, respectively, when compared with vector
control (P<0.05, Fig. 5A and
B), no significant difference occurred in control group and
scramble group (Fig. 5A and B).
These results suggested that decreased TLR4 levels in tumor cells
might reduce inflammatory cytokines.
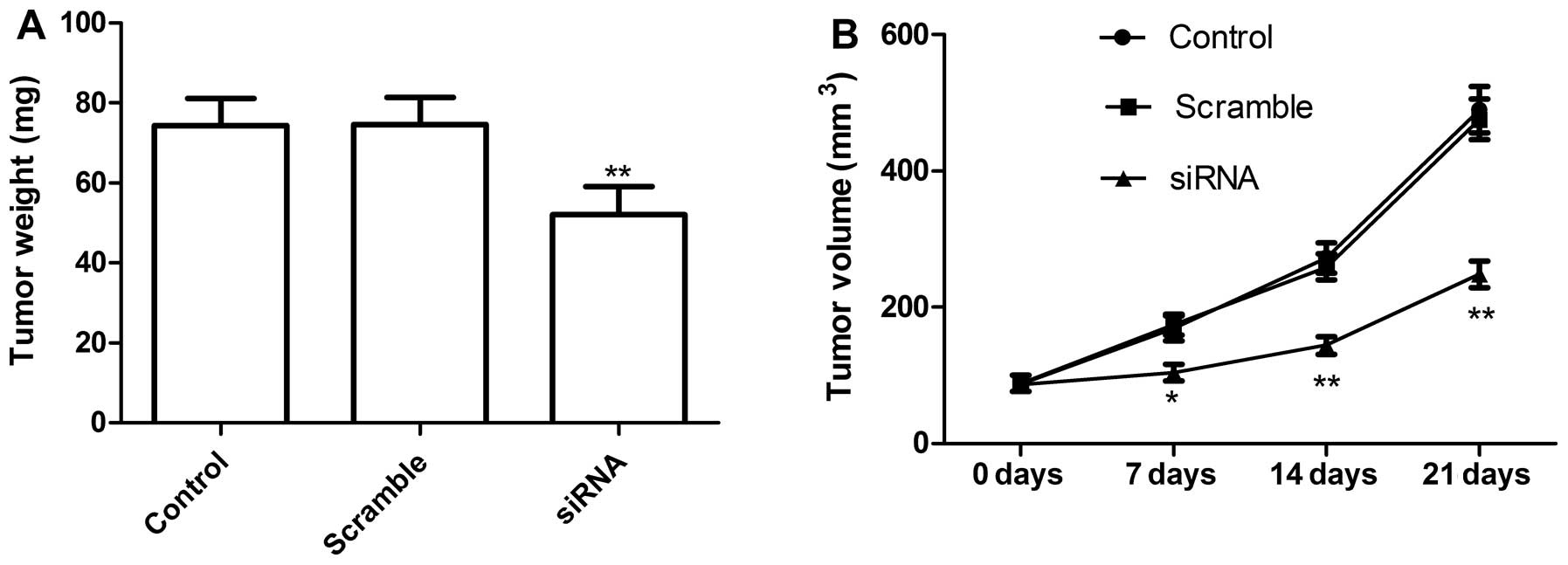
TLR4 knockdown in lung cells inhibits
cell migration and cell invasion
We next determined if knockdown of TLR4 could
inhibit tumor growth by a xenograft tumor model. At 7 days after
the end of treatment, mice were sacrificed and tumor weights were
measured. The tumor weight was significantly lower in the knockdown
TLR4 group than in control and scramble groups (P<0.01; Fig. 6A). In addition, tumor volume also
was determined at different time. Tumor volume in knockdown TLR4
group groups were significantly diminished when compared with the
scramble and control group (Fig.
6B). These data demonstrated that knockdown of TLR4 suppressed
tumor growth of NSCLC in vivo.
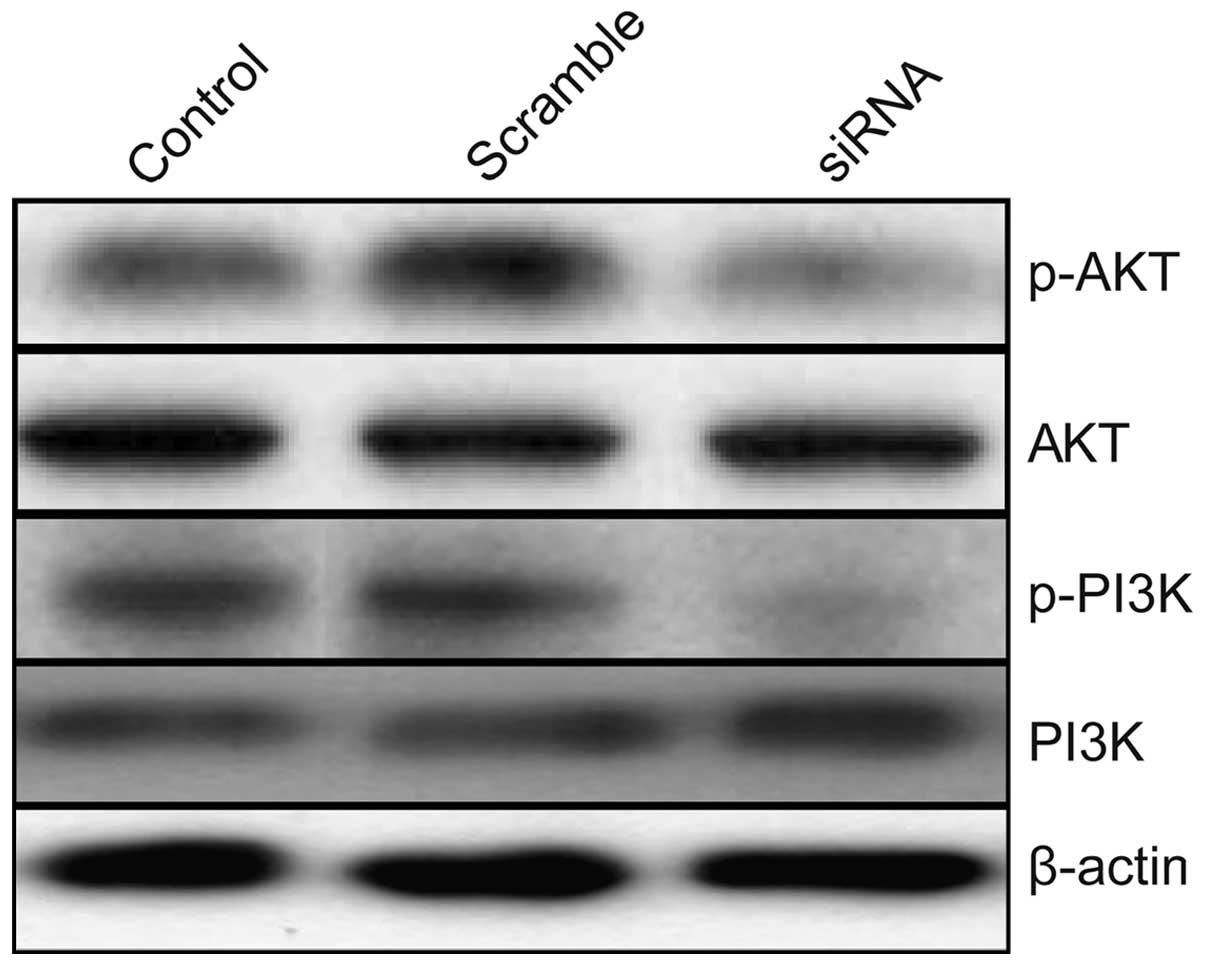
Effects of TLR4 on PI3K/AKT signaling
pathway in A549 cells
To clarify the molecular mechanisms involved due to
downregulation of TLR4 inhibition the growth of human lung tumor
in vitro and in vivo, we focused on the effects of
knockdown of TLR4 on the activation of PI3K/AKT signaling pathway,
which participate in the main intracellular signaling pathway
required for cell proliferation and survival. As shown in Fig. 7, compared with control group and
scramble group, knockdown of TLR4 by siRNA resulted in a
significant suppression of phosphorylation of Akt and PI3K. These
results indicate that reduction of TLR4 by siRNA inhibits lung
tumor cell growth, to some extent, by suppressing the PI3K/AKT
signaling pathway.
Discussion
Lung cancer is one of the leading causes of
cancer-related mortality worldwide (25). Lung cancer mortality rates have
been rising in recent decades. It has been shown that chronic
inflammatory disease is a risk factor for lung cancer (26). Since TLR-4 is also actively
involved in the immune response against cancers, some researchers
have suggested that TLR-4 exerts both a defensive role in normal
cells and a negative role in cancer cells. However, the available
evidence is still not conclusive on the link between TLR-4 and lung
cancer. Bauer et al found that mutated TLR-4 in mice had
less lung capillary permeability, less weight loss, leukocyte
inflammation, and primary tumor formation (27), and that TLR-4 activation could
protect the lungs from being inflamed during any potential
tumorigenesis (28). This finding
suggests the potential role of TLR-4 for airway inflammation and
lung cancer progression. Yet, growing evidence has also found that
TLR-4 is constantly expressed and upregulated in human lung cancer
cells (18,20). He et al (18) found that the level of TLR-4 was
significantly linked with the production of immunosuppressive
cytokines, production of proangiogenic chemokine and with
resistance to apoptosis by lung cancer cells. Zhang et al
(20) showed that TLR4 expression
was increased in patients with NSCLC, and TLR4 expression levels
correlated with malignancy. In this study, our result showed that
TLR-4 expression was elevated in most patients with NSCLC, and its
expression level correlated with key pathological characteristics,
such as, tumor differentiation, stage and metastasis, which was
similar with previous results (20). These results imply that the
presence of TLR4 in cancer cells may possess a negative role in
lung cancer progression and metastasis.
A study on a larger number showed that TLR4
expression was increased in cancer cell or highly malignant tissues
(20,29,30),
and that activation of the TLR4 signaling transduction pathway
promoted tumor progression and resistance to apoptosis (31). Hua et al showed that
upregulation of TLR4 in human prostate cancer cells correlated
positively with tumor metastasis (31). Kelly et al found that
activation of the TLR4 signaling transduction pathway promoted
tumor progression and chemo-resistance of epithelial ovarian cancer
cells (11). Similar results were
also observed in human head and neck squamous cell carcinoma and
breast cell carcinoma where stimulation of TLR4 enhanced (29,32).
In addition, growing evidence has shown that knockout TLR4 could
inhibit cancer cell proliferation, cell metastasis and induce
cancer cell apoptosis (11,17,29,32).
Therefore, knocking down TLR4 from cancer cells could reduce tumor
metastasis whereas stimulation of TLR4 on cancer cells would
enhance the development of aggressive tumors. In the present study,
we constructed pcDNA3-CMV vectors expressing two small hairpin
siRNA oligonucleotides targeting TLR4 and transfected A549 cells to
study the role of TLR4 in lung cancer and found that downregulation
of TLR4 expression using RNA silencing approach in A549 tumor cells
significantly suppressed cell proliferation, migration and
invasion, and induced tumor apoptosis in vitro, and
suppressed tumor growth in vivo.
An opinion that chronic inflammation promotes tumor
development and progression has been supported by many
epidemiological studies and experimental findings (33,34).
It has been reported that when tumor cells are stimulated with
lipopolysaccharides (LPS), a ligand for TLR4, the proinflammatory
factors such as nitric oxide, IL-6 and IL-12 are expected to be
released from tumor cells, attracting and activating inflammatory
cells. Moreover, these factors play crucial roles in resistance of
tumor cells to cytotoxic T lymphocyte (CTL) and natural killer cell
(NKC) attack and facilitate evasion from immune surveillance
(30). He et al found that
knockdown of TLR4 in vitro lead to TLR4-related inflammatory
cytokines being markedly depressed and so it could weaken the
ability to the resistance of MDA-MB-231 to CTL and NKC attack and
facilitate evasion from immune surveillance. In this study, our
result showed that downregulation of TLR4 expression significantly
decreased TNF-α and IL-6 levels, which was consist with previous
results (18). These results may
indicate that TLR4 knockdown in vivo inhibited the growth
and promoted the death of lung tumors.
It has been reported that TLR-4 is involved in
signal pathway regulation. It has been shown that the expression of
higher levels of TLR-4 on human prostate adenocarcinoma (DU-145)
cells and its activation, lead to NF-κB and proinflammatory
cytokine production through the MyD88-dependent pathway (35). Another study showed
TLR-4/MyD88-dependent signaling pathway involvement in laryngeal
carcinoma progression (36).
Hartmann et al showed that activated TLR-4 expression could
enhance cancer cell growth, NF-κB translocation, and activated
phosphatidylinositol 3-kinase/Akt pathway (37). Furthermore, He et al found
that TLR4 activation contributes to active p38MAPK pathway, which
is necessary for increased VEGF and IL-8 production (18). Hua et al study revealed
evidence of a multifaceted signaling network operating downstream
of TLR4-mediated tumor cell invasion, proliferation and survival
(31). The phosphatidylinositol
3-kinase (PI3K)/AKT signaling pathway plays an important role in
survival when cells are exposed to various kinds of apoptotic
stimuli (38). Recent reports have
indicated that the activation of Akt pathway is implicated in
conferring resistance to conventional chemotherapy and multiple
chemotherapeutic agents on cancer cells (39). Therefore, in the present study, we
mainly focus on effect of TLR4 on the PI3K/AKT signaling pathway by
western blot assay. Our results showed that downregulation of TLR4
expression using RNA silencing suppressed phosphorylation of Akt
and PI3K, which indicate that TLR4 silencing inhibits tumor cell
growth, to some extent, by suppressing activation of the PI3-K/Akt
pathway signaling.
In conclusion, the present study demonstrated that
TLR4 was elevated in most NSCLC and its expression level correlated
with key pathological characteristics including clinical stage and
metastasis and that knockdown of TLR4 could actively inhibit
proliferation and survival of lung cancer cells in vitro and
in vivo. Taken together, our results suggest RNAi-directed
targeting of TLR4 may be a beneficial strategy for lung cancer
therapy.
Acknowledgements
This study was supported by Science and Technology
Research and Innovation Team funded of Jilin province
(JL2013018).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Reungwetwattana T, Weroha SJ and Molina
JR: Oncogenic pathways, molecularly targeted therapies, and
highlighted clinical trials in non-small-cell lung cancer (NSCLC).
Clin Lung Cancer. 13:252–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Schiller JH: Small cell lung cancer:
defining a role for emerging platinum drugs. Oncology. 63:105–114.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roy M, Luo YH, Ye M and Liu J: Nonsmall
cell lung cancer therapy: insight into multitargeted small-molecule
growth factor receptor inhibitors. Biomed Res Int.
2013:9647432013.PubMed/NCBI
|
|
5
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll
protein signals activation of adaptive immunity. Nature.
388:394–397. 1997. View
Article : Google Scholar
|
|
6
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar
|
|
7
|
Medzhitov R and Janeway CA Jr: Decoding
the patterns of self and nonself by the innate immune system.
Science. 296:298–300. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elson G, Dunn-Siegrist I, Daubeuf B and
Pugin J: Contribution of Toll-like receptors to the innate immune
response to Gram-negative and Gram-positive bacteria. Blood.
109:1574–1583. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
De Bouteiller O, Merck E, Hasan UA, et al:
Recognition of double-stranded RNA by human toll-like receptor 3
and downstream receptor signaling requires multimerization and an
acidic pH. J Biol Chem. 280:38133–38145. 2005.PubMed/NCBI
|
|
10
|
Goto Y, Arigami T, Kitago M, et al:
Activation of Toll-like receptors 2, 3, and 4 on human melanoma
cells induces inflammatory factors. Mol Cancer Ther. 7:3642–3653.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kelly MG, Alvero AB, Chen R, et al: TLR-4
signaling promotes tumor growth and paclitaxel chemoresistance in
ovarian cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fukata M, Chen A, Vamadevan AS, et al:
Toll-like receptor-4 promotes the development of colitis-associated
colorectal tumors. Gastroenterology. 133:1869–1881. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
O’Neill LA, Bryant CE and Doyle SL:
Therapeutic targeting of Toll-like receptors for infectious and
inflammatory diseases and cancer. Pharmacol Rev. 61:177–197.
2009.PubMed/NCBI
|
|
14
|
Yu L and Chen S: Toll-like receptors
expressed in tumor cells: targets for therapy. Cancer Immunol
Immunother. 57:1271–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garza-Gonzalez E, Bosques-Padilla FJ,
Mendoza-Ibarra SI, Flores-Gutierrez JP, Maldonado-Garza HJ and
Perez-Perez GI: Assessment of the toll-like receptor 4 Asp299Gly,
Thr399Ile and interleukin-8 −251 polymorphisms in the risk for the
development of distal gastric cancer. BMC Cancer.
7:702007.PubMed/NCBI
|
|
16
|
Wang JP, Zhang Y, Wei X, et al:
Circulating Toll-like receptor (TLR) 2, TLR4, and regulatory T
cells in patients with chronic hepatitis C. APMIS. 118:261–270.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang H, Zhou H, Feng P, et al: Reduced
expression of Toll-like receptor 4 inhibits human breast cancer
cells proliferation and inflammatory cytokines secretion. J Exp
Clin Cancer Res. 29:922010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hiratsuka S, Watanabe A, Sakurai Y, et al:
The S100A8-serum amyloid A3-TLR4 paracrine cascade establishes a
pre-metastatic phase. Nat Cell Biol. 10:1349–1355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang YB, He FL, Fang M, et al: Increased
expression of Toll-like receptors 4 and 9 in human lung cancer. Mol
Biol Rep. 36:1475–1481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
22
|
Campo E, Merino MJ, Liotta L, Neumann R
and Stetler-Stevenson W: Distribution of 72KD type IV collagenase
in nonneoplastic and neoplastic thyroid tissue. Hum Pathol.
23:1395–1401. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lowry OH, Rosebrough NJ, Farr AL and
Randall RJ: Protein measurement with the Folin phenol reagent. J
Biol Chem. 193:265–275. 1951.PubMed/NCBI
|
|
24
|
Zhang D, Chen ZG, Liu SH, et al:
Galectin-3 gene silencing inhibits migration and invasion of human
tongue cancer cells in vitro via downregulating beta-catenin. Acta
Pharmacol Sin. 34:176–184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mendez M, Custodio A and Provencio M: New
molecular targeted therapies for advanced non-small-cell lung
cancer. J Thorac Dis. 3:30–56. 2011.PubMed/NCBI
|
|
26
|
Yao H and Rahman I: Current concepts on
the role of inflammation in COPD and lung cancer. Curr Opin
Pharmacol. 9:375–383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauer AK, Dixon D, DeGraff LM, et al:
Toll-like receptor 4 in butylated hydroxytoluene-induced mouse
pulmonary inflammation and tumorigenesis. J Natl Cancer Inst.
97:1778–1781. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bauer AK, Fostel J, Degraff LM, et al:
Transcriptomic analysis of pathways regulated by toll-like receptor
4 in a murine model of chronic pulmonary inflammation and
carcinogenesis. Mol Cancer. 8:1072009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szczepanski MJ, Czystowska M, Szajnik M,
et al: Triggering of Toll-like receptor 4 expressed on human head
and neck squamous cell carcinoma promotes tumor development and
protects the tumor from immune attack. Cancer Res. 69:3105–3113.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang B, Zhao J, Li H, et al: Toll-like
receptors on tumor cells facilitate evasion of immune surveillance.
Cancer Res. 65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hua D, Liu MY, Cheng ZD, et al: Small
interfering RNA-directed targeting of Toll-like receptor 4 inhibits
human prostate cancer cell invasion, survival, and tumorigenicity.
Mol Immunol. 46:2876–2884. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ahmed A, Wang JH and Redmond HP: Silencing
of TLR4 increases tumor progression and lung metastasis in a murine
model of breast cancer. Ann Surg Oncol. 20(Suppl 3): S389–S396.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kusmartsev S and Gabrilovich DI: Immature
myeloid cells and cancer-associated immune suppression. Cancer
Immunol Immunother. 51:293–298. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gatti G, Quintar AA, Andreani V, et al:
Expression of Toll-like receptor 4 in the prostate gland and its
association with the severity of prostate cancer. Prostate.
69:1387–1397. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Starska K, Forma E, Brys M, et al: The
expression of TLR pathway molecules in peripheral blood mononuclear
cells and their relationship with tumor invasion and cytokine
secretion in laryngeal carcinoma. Adv Med Sci. 57:124–135. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hartmann E, Wollenberg B, Rothenfusser S,
et al: Identification and functional analysis of tumor-infiltrating
plasmacytoid dendritic cells in head and neck cancer. Cancer Res.
63:6478–6487. 2003.PubMed/NCBI
|
|
38
|
Oka N, Tanimoto S, Taue R, et al: Role of
phosphatidylinositol-3 kinase/Akt pathway in bladder cancer cell
apoptosis induced by tumor necrosis factor-related
apoptosis-inducing ligand. Cancer Sci. 97:1093–1098. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kai K, D’Costa S, Sills RC and Kim Y:
Inhibition of the insulin-like growth factor 1 receptor pathway
enhances the antitumor effect of cisplatin in human malignant
mesothelioma cell lines. Cancer Lett. 278:49–55. 2009. View Article : Google Scholar : PubMed/NCBI
|